7
PAIN
It hurts when I do this
One of the most common features of old age is aches and pains—it seems as though, like in an old car, parts just start to wear out. Pain accounts for 80 percent of trips to the doctor in the United States—it is the number one presenting complaint when people go to the doctor. When the doctor says, “Why are you here?” almost all doctor visits are for a patient saying, “It hurts here,” or a variant, “It hurts when I do this.”
My friend Michael and I have been comparing notes now for about ten years on our inventory of the physical markers of aging. They seem to make their presence known slowly, one at a time, and each one seems manageable by itself, but they add up. Some respond to treatment. Some require a change in behavior. Some you just have to live with.
Mostly what we talk about is how lucky we are. We have both known people who suffer debilitating, life-threatening pain. I met J. D. Buhl in 1984 when he was a singer-songwriter, and I was working as a record producer. By age twenty-five, he had already led two successful bands, J. D. Buhl and the Believers and the Jars. When we met he was launching a career as a solo artist. I produced some records for him, we performed together, and we became friends. I admired his talent, and I also admired his encyclopedic knowledge of records . . . he knew who wrote every song, the year that the record came out, the name of every musician who played on the session. Among my other friends, I had always been the king of this sort of trivia. But with J.D. I was a novice, and I loved him for it. In the 1990s and 2000s, we went on to do other things—we both became teachers—and we stayed in touch. In 2013, J.D. called me up and said he’d been diagnosed with terminal cancer. He wanted to write and record one last album, and he wanted me to produce it. Six months of chemotherapy had left him tired and worn-out, but the cancer was in remission and he was feeling upbeat. So we started. Slowly.
By 2016 the cancer had returned, and he asked to go back into the studio to record four new songs. The pain was now severe and there didn’t seem to be anything he could do to relieve it. He’d made plans to enter a hospice. In Oakland, where he lived, the hospice workers helped him set himself up to end his life when the pain became unbearable. Anxiety has been shown to demonstrably increase pain, and knowing you can’t do anything about the pain can be especially anxiety provoking. In addition, J.D. had anxiety about his dwindling finances, his loss of mobility, and his diminishing energy.
One day in the summer of 2017 at age fifty-seven, he called me and said it was time. The pain was too much. He awoke every morning from a rolling, restless half-sleep riddled with painful sensations throughout his body, inside and out, knowing that the new day held nothing but more pain with no release. He had the embarrassment of a colostomy bag and a drawn, sunken appearance. He hadn’t been blessed with many romantic partners in his life, and realized he’d never have another. He lacked the energy to play music or even listen to records. “I have very little to look forward to,” he said. I phoned him on the evening of August 14, 2017, to say good-bye, and the next morning he administered a special pharmaceutical cocktail to himself and was gone.
Pain is a formidable opponent and even the spectacular advances made in medical science are no match for it. We tend to think of medical science in terms of how much it can prolong life, and as a society, we pour a great deal of money and other resources into trying to cure diseases that shorten life span—such as cancer. But we haven’t solved the problem of how to eradicate pain. In other words, medical science tends to focus on life span rather than disease span.
At any given time, 30 percent of the population is experiencing chronic pain—which means they’ve been in pain and they’ve had that pain for more than three months. For older adults the number is closer to 40 or 50 percent. The odds of experiencing chronic pain at some point in your life are one in two. More people are in chronic pain at this minute than have cancer, heart disease, or diabetes combined.
The Global Burden of Disease Project is a statistical, epidemiological look at the various diseases and injuries affecting people worldwide. Produced by the World Health Organization, the project provides an interactive map that allows you to look at a number of variables, including causes of death by country and state, age, and sex. An innovative feature is the reporting of YLDs—years living with (or lost to) disability—what I described as disease span.
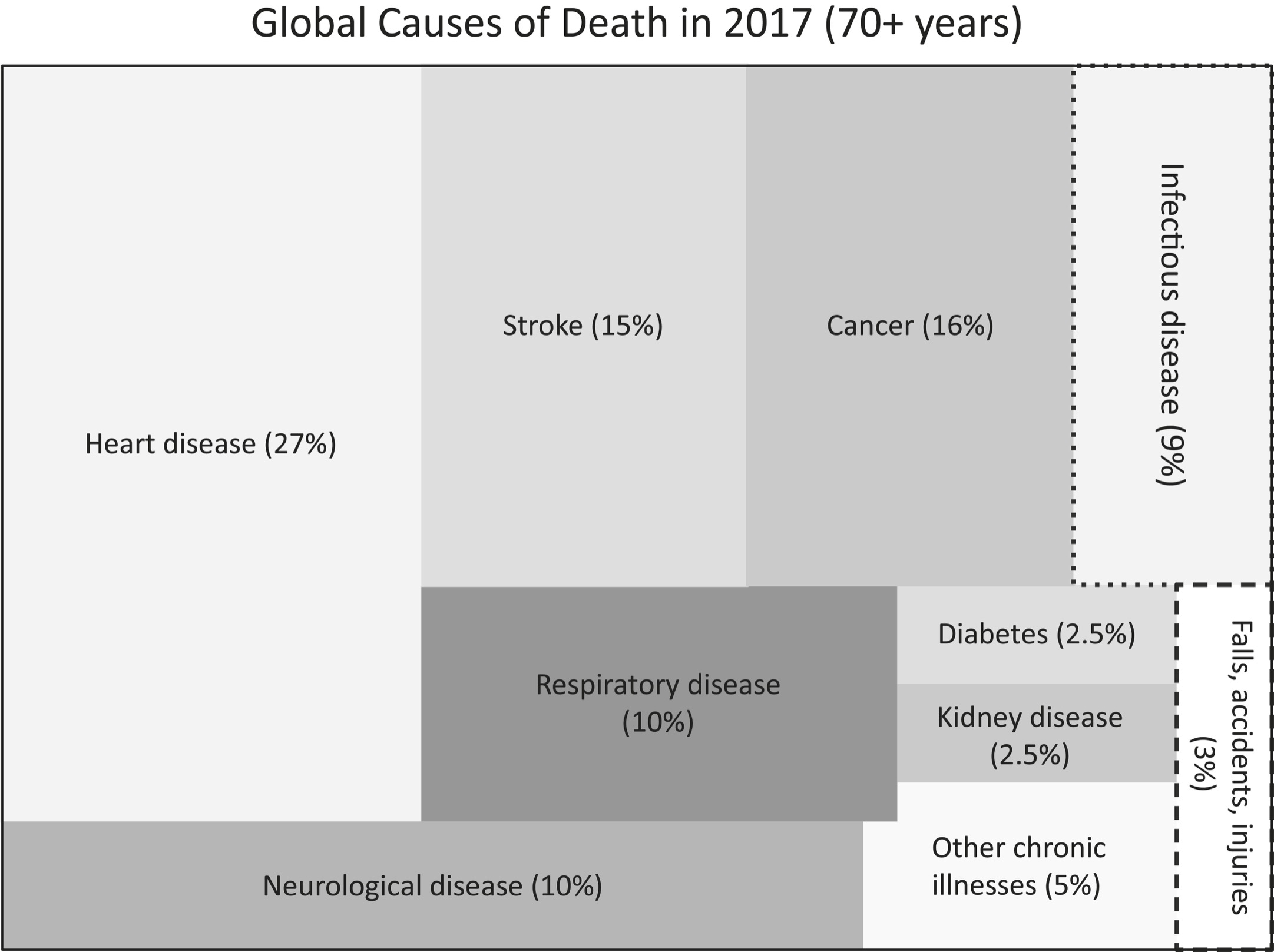
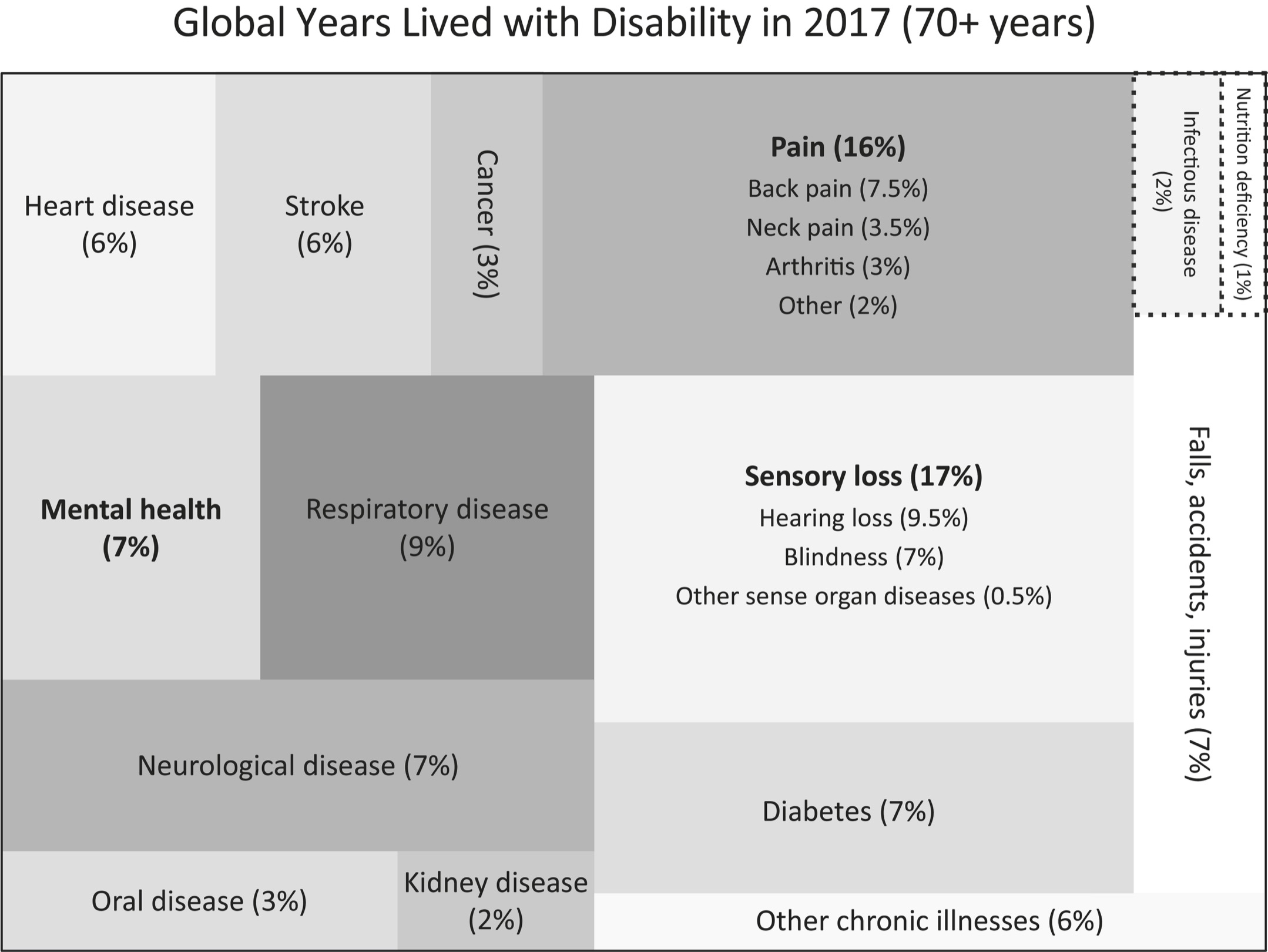
Consider the two visualizations (redrawn from World Health Organization data) on the following page. The first picture shows cause of death for seventy-plus-year-olds. The second shows YLD for seventy-plus-year-olds. The point here is to show that the things that disable us are different from the things that kill us.
As the top chart shows, stroke and cancer account for 15 percent and 16 percent of deaths in people over seventy, respectively. Chronic pain accounts for that same proportion of YLDs—years within the disease span portion of your life span. (Headache accounts for around 1 percent and is grouped into “other chronic illnesses.”) Note also that of people who are in chronic pain, nearly half have back pain. About one person in five has neck pain and another one in five has arthritis. And compare the magnitude of cancer as a cause of death versus a cause of disability: Cancer (at 16 percent) is a major cause of death, but in terms of disability it is a tiny 3 percent compared to, say, falls, which account for 7 percent of disability. It isn’t that we shouldn’t be trying to cure cancer and heart disease, but that an outsized amount of money gets spent on life span research compared to what is spent on health span research. The costs of treating chronic pain alone account for more than $635 billion in expenditures annually in the United States and can lead to catastrophic unforeseen consequences, possibly including the current opioid epidemic. Pain research receives just a tiny sliver of the funding devoted to medical research, perhaps because of the widely held belief that “pain hurts, but it doesn’t kill you.” But in fact, it can. Chronic pain creates a 1.57 increased mortality risk. What that means is that for every ten years you are in chronic pain, your life span is lowered by one year. Put another way, chronic pain confers on you a risk-adjusted age of six years higher than your chronological age. If you’ve got chronic pain at age seventy-four, you are effectively eighty.
Many people assume that pain simply gets worse as we age, but this isn’t true—it peaks and then falls off. Chronic pain increases and peaks in our fifties and sixties, and then declines in our seventies and older. These numbers could arise because older adults become more stoic and stop complaining about it, or it could be that they simply don’t have it anymore.
The sensation of pain is actually generated in the brain, even though we usually experience it in a specific part of the body. In other words, your toe may hurt, but the “hurt” is occurring in the part of your brain map that represents your toe. This is why if we shut down your brain, through sleep, loss of consciousness, or certain drugs, the pain goes away. Or why, if we block the transmission of neural firings from your toe to your brain, the pain goes away. This might be done with a topical anesthetic or an intravenous nerve-blocking agent. Either way, no distress signal from the sensory receptor reaches the brain, and so no pain is felt. As we saw in the perception chapter (Chapter 3), you can experience pain even when there is no sensory input, such as with an amputee’s phantom limb pain. Thus, pain is a brain-based phenomenon.
Pain isn’t simply an automatic reaction you feel when you’re injured. In a paper published just after World War II, Lieutenant Colonel Henry Beecher wrote, “There is a common belief that wounds are inevitably associated with pain, that the more extensive the wound, the worse the pain.” He found that this is not always true after he observed a strange phenomenon: Soldiers in battle can experience a terrible injury, such as a bullet wound or severed limb, and not feel any pain until much later.
Pain also has an emotional, affective component; that is, we don’t consider it pain unless we experience it as unwanted and undesirable. The same sensation of someone pressing aggressively on your neck is interpreted differently if it’s from an attacker or a massage therapist. As Beecher wrote, “There was no dependable relation between the extent of a pathological wound and the pain experienced. No significant difference was found between the pain of sudden injury and that of chronic illness. The intensity of suffering is largely determined by what the pain means to the patient.”
The reason that soldiers can be injured but not feel pain is due to stress-induced analgesia. Basically, the brain is telling the spinal cord, “Don’t bother me now, I have more important things to worry about—I’m just trying to keep us alive.”
McGill University in Montreal, where I’ve spent much of my career, is one of the leading centers for the study of pain. As a native Californian, I attribute this to the punishingly cold winters there. (My Canadian colleagues, who seem to like the cold, were quick to point out that Siberia, Alaska, Mount Everest, and the Yukon are also very cold but produce no game-changing pain research.)
One of the big contributions to pain research was made by McGill researcher Ronald Melzack in the 1960s. We tend to think that our peripheral nerves tell us when we’re in pain. We stub a toe or the knife slips while chopping an onion and we cut ourselves, and voilà—pain. But Melzack showed that it is the brain that decides whether we experience pain or not. His theory, along with the British physiologist Patrick Wall’s, called the gate control theory of pain, accounts for a number of real life experiences.
In particular, Melzack showed that the brain can override everything going on in the spinal cord, increasing or decreasing the sensation of pain. If the brain is sensitized or on alert to expect pain, normal run-of-the-mill sensory inputs can end up being perceived by the brain as pain signals even though the spinal cord isn’t sending any. This may be what chronic pain is: You’ve had some injury and it’s been repaired, but just touching the region causes the feeling of pain. (This is called allodynia.)
The neuroscientific view is that pain is an emotional-motivational condition that tells you to either do something, like rubbing or licking a wound, or refrain from doing something, like putting your hand on a hot stove. But not all experiences that tell you to do something are perceived as pain. For example, dysesthesia, that pins-and-needles feeling you get when your foot’s asleep, may make you want to jump up and wiggle or rub the offending limb, but that’s not usually considered pain. Paresthesia is the general term that applies to any kind of abnormal skin sensation, including numbness, tingling, chilling, or heat sensations that are not painful. When they are uncomfortable, they are called dysesthesia.
What about gut-wrenching psychological suffering, such as you experience when your boyfriend or girlfriend leaves you—is that a kind of physical pain? We talk of a broken heart as pain, metaphorically. Yet mental anguish is related to pain. Grief, for example, can be felt quite physically, and stress or sadness can lead to migraines, fatigue, gastric upset, and so on. Because all pain is ultimately brain-based, there is no scientific reason to separate mental, emotional pain from physical pain.
There are also a number of aversive, unpleasant sensory experiences that are not pain, such as eating spoiled food, hearing dripping water while you’re trying to sleep, or hearing fingernails scratching on a chalkboard. Disgusting, annoying, unpleasant, perhaps, but not the same as pain.
Ronald Melzack also advanced the way that we talk about and treat pain, when he introduced the McGill Pain Questionnaire. The next time you need to see your doctor for a pain, it would be helpful to think about your pain in these descriptive terms:
0 = No pain
1 = Mild
2 = Discomforting
3 = Distressing
4 = Horrible
5 = Excruciating
|
Temporal |
Spatial |
Punctate Pressure |
Incisive Pressure |
Constructive Pressure |
|
Flickering |
Jumping |
Pricking |
Sharp |
Pinching |
|
Quivering |
Flashing |
Boring |
Cutting |
Pressing |
|
Pulsing |
Shooting |
Drilling |
Lacerating |
Gnawing |
|
Throbbing |
Stabbing |
Cramping |
||
|
Beating |
Lancinating |
Crushing |
||
|
Pounding |
||||
|
Brief |
||||
|
Momentary |
||||
|
Transient |
||||
|
Rhythmic |
||||
|
Periodic |
||||
|
Intermittent |
||||
|
Continuous |
||||
|
Steady |
||||
|
Constant |
|
Traction Pressure |
Thermal |
Brightness |
Dullness |
Misc. Sensory |
|
Tugging |
Hot |
Tingling |
Dull |
Tender |
|
Pulling |
Burning |
Itching |
Sore |
Taut |
|
Wrenching |
Scalding |
Smarting |
Hurting |
Rasping |
|
Searing |
Stinging |
Aching |
Splitting |
|
|
Heavy |
|
Tension |
Autonomic |
Fear |
Punishment |
Misc. Affective |
|
Tiring |
Sickening |
Fearful |
Punishing |
Wretched |
|
Exhaustive |
Suffocating |
Frightful |
Grueling |
Blinding |
|
Terrifying |
Cruel |
|||
|
Vicious |
||||
|
Killing |
|
Misc. words |
||||
|
Spreading |
Tight |
Cool |
Nagging |
Annoying |
|
Radiating |
Numb |
Cold |
Nauseating |
Troublesome |
|
Penetrating |
Drawing |
Freezing |
Agonizing |
Miserable |
|
Piercing |
Squeezing |
Dreadful |
Intense |
|
|
Tearing |
Torturing |
Unbearable |
||
Note that some of the words describe sensory events (tingling, hot), some describe “feelings” (fearful, wretched), and some are cognitive-evaluative (annoying, nagging). The distinctions between the sensory components of pain and the feeling-related components are reflected in two different pathways that the pain signal takes through the thalamic nuclei of the brain. From there, the signals that we experience as sensory go to the somatosensory cortex, a part of the brain that contains a kind of map of your body, with different parts of the body represented in different chunks of this cortex. This neurological map shows where in your brain the sensations from different parts of your body are represented. It should be noted that different parts of the body have different amounts of brain matter assigned to them, and the relative amounts of brain matter are not related to the size of the body part. For example, a large body part like the torso is assigned a much smaller chunk of the somatosensory cortex than the thumb. This is because our evolutionary ancestors needed to develop sensitive thumbs to feel for food and use tools, while the torso was just a container for a few internal organs. This is shown in the picture below, which was originally conceived by Wilder Penfield at McGill. You’re looking at a side view of the brain, with all its folds, and the sizes of the related body parts are roughly proportional to how many neurons are used to represent sensations from them.
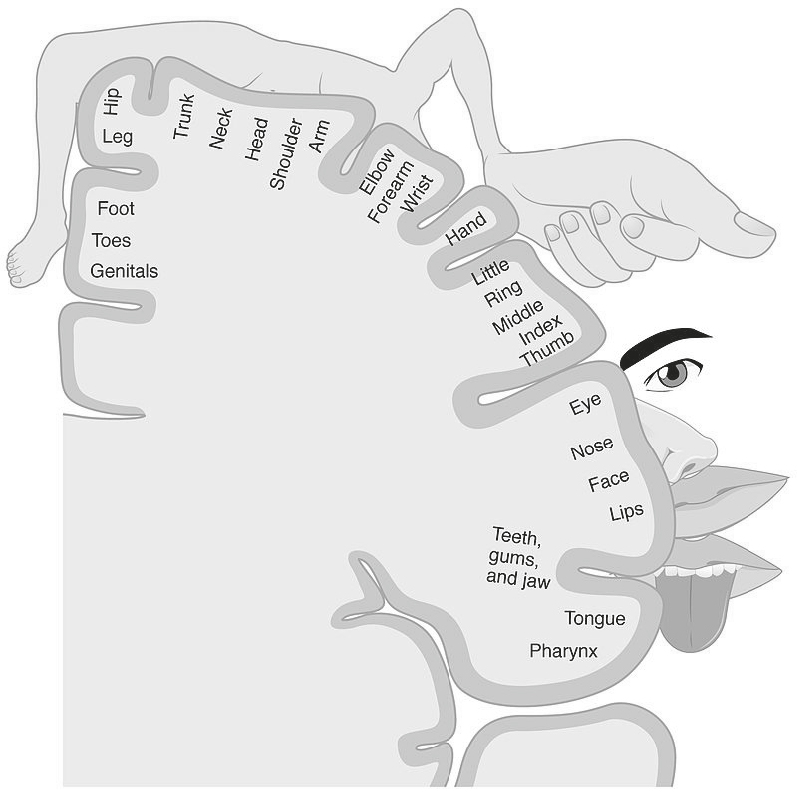
You may have noticed that you have lower resolution for distinguishing touch in different parts of your body. For instance, if you’ve been bitten by a mosquito near your elbow, you may feel an itch sensation but have trouble locating it, trouble knowing exactly where to scratch. This is because the number of neurons representing sensation in the elbow is relatively low, and so this area has lower sensation resolution than a bite on your face, which has a much greater number of neurons representing it.
The different types of pain in Melzack’s chart—for example, stinging, burning, or aching—map to different brain regions. The pain signals that we experience as emotional (affective-motivational) travel from the thalamus to the anterior cingulate and the insula, parts of the limbic system. The pain signals we interpret cognitively are handled in different circuits of the frontal lobe, in conjunction with the limbic system. The somatosensory cortex tells you how much it hurts, where it hurts, and how long it’s been hurting. The limbic system tells you how unpleasant it is and motivates you to do something about it. The cognitive system helps you to analyze, contextualize, and appraise the injury. A practical consequence of all this is that a lesion in the brain, such as due to stroke, could cause a deficit in one of these three pain systems but not the others, and we have seen patients develop indifference to pain (affective-emotional) while still noticing it (sensory) and being able to evaluate it (cognitive).
All these systems interact. For example, our perception of pain is altered through empathy—our pain sensitivity is increased when we observe a loved one in pain, compared to observing a stranger. This appears to be mediated by mirror neurons, specialized brain cells that allow us to mentally simulate the world of action. I think of them as our “monkey-see, monkey-do” neurons, because of the way they were discovered. One monkey, watching another peel a banana, started having neural activity in just the part of his brain that would move his hands to do the same thing, even though he wasn’t physically making any movements at all—it was just his brain running a neural simulation. Similarly, when we see someone else get hurt, even from a scene in a movie, we wince as if we, too, are being harmed. The evolutionary basis for this may be to help us learn about aversive things without having to go through them ourselves.
Pain can have a negative effect on emotions and on cognitive function, putting us in a bad or impatient mood and impairing attention, memory, and decision making. In the other direction, a negative emotional state can lead to increased pain and a positive emotional state can reduce pain. Also, cognitive assessments of pain can increase or reduce it. The brain is wired in such a way that cognition, emotion, and pain can all interact with one another bidirectionally.
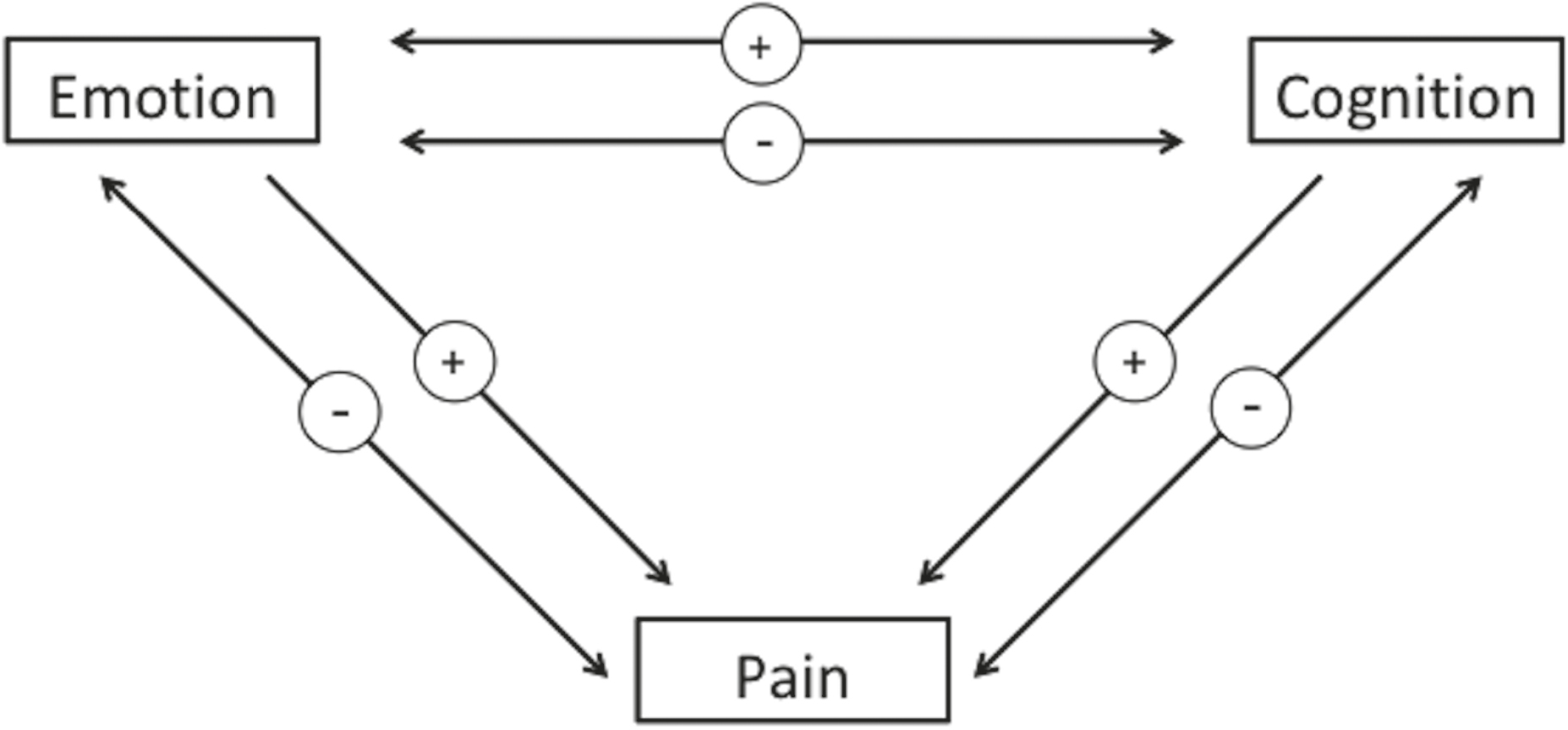
Minus signs indicate a negative effect, plus signs indicate a positive effect.
Catherine Bushnell at McGill and the National Institutes of Health has shown that cutaneous pain (on the skin, also called somatic pain) and visceral pain (internal organs—the viscera, also called epicritic pain) are experienced very differently. We tend to subjectively rate our visceral pain as being more unpleasant than our cutaneous pain. Neurologically measured, the pain intensity of a cut finger could equal that of a stomachache. But, subjectively, we would rate the stomachache as being more unpleasant. Having a dentist file or scrape your teeth may be grossly unpleasant, but it’s not usually described as painful. A foot massage using acupressure or reflexology can be painful but pleasant in a weird way. So networks in our brains can separate pain from unpleasantness.
The distinction between cutaneous and visceral sensations, and between pain and unpleasantness, probably has an evolutionary origin. Sensory perception for the part of you interacting with the outside world has evolved to be very sensitive to where on your body an injury occurred. Interior pain doesn’t usually require such precision. Accordingly, cutaneous pain is usually experienced as local and precise, and people are much better at discriminating different intensities of cutaneous pain. In contrast, visceral pain is more difficult to localize. Most of the nerve fibers that communicate between the internal organs and the brain are unmyelinated, and they are more sparsely distributed. This leads to a certain imprecision. For example, esophageal pain is often confused for heart trouble, and indigestion is often described as heartburn.
This evolutionary history has created distinct brain circuits for the two types of pain. Cutaneous pain activates the ventrolateral (underneath, and toward the sides) prefrontal cortex to a greater degree than visceral pain. Visceral pain in turn elicits greater activation from the somatosensory cortex, the part of the brain shown in the previous drawing, along with the anterior cingulate and the motor cortex. Why the motor cortex? The regions of the motor cortex that are activated by visceral pain control the face, tongue, and gag reflex. Researchers figured this out by inserting, and then inflating, a balloon inside the participants’ esophagus. This simulated the kind of discomfort and pain experienced if we ingest harmful food or drink, and we need to prepare to close our mouths, spit out anything remaining, salivate to dilute what’s left, and possibly regurgitate the harmful material. Hence the motor cortex activation of the face, tongue, and gag reflex.
The words we use to describe these two sources of pain, and the way they feel to us, are very different. We use more precise words for describing cutaneous pain and are very specific about it. Because visceral pain is not as well localized, when describing it we gesture or point. We describe it as dull or throbbing. Often we’re not sure where it’s coming from. Patients use more words overall, and more emotional words, to describe visceral pain.
You may remember from the previous chapter that the hallucinogenic drug ketamine can reduce social anxiety, a condition that is influenced by glutamate levels in the brain. Administration of ketamine to people in pain has differential effects on cutaneous and visceral pain, with minimal side effects. For visceral pain, ketamine reduces both pain and unpleasantness. For cutaneous pain, ketamine reduces only unpleasantness. And really, this is what anxiety is: a feeling of unpleasantness and the apprehension that it will continue in the future.
The differences in the experience of these two types of pain is especially important for older adults, and for treatment options. Older adults are more likely to experience visceral pain than younger adults, due to aging of internal organs and the systems that support them. Reductions in the effectiveness of kidneys, liver, lungs, heart, digestive tract, and gallbladder can all cause significant pain, and the coming years hold the promise of increasingly differentiated therapies for these internal pains.
The anticipation of pain lights up many of the same neural regions as actual pain, regions that are important for pain sensation, pain affect, pain modulation, and pain-associated anxiety. Similarly, the sensory cortex and the anterior cingulate are activated both during tickling and during the anticipation of tickling. (Tickling is very interesting from an evolutionary standpoint. In effect it is a simulated false threat—someone touching you in a place that is vulnerable [e.g., belly, neck]. This is why tickling only “works” if it is someone you trust doing the tickling—otherwise it is aversive. Nonhuman primates love tickles as much as human infants, and a dog’s joy having a belly rub is probably related to this.)
Why We Have Pain
The most obvious reason we have pain is that for thousands of years it has given us a survival advantage—it causes us to protect that part of our bodies that has been injured, giving it a better chance to heal. The layer of skin that stretches over our bodies from the tips of our toes up to the tops of our heads serves to hold in vital fluids and organs that might otherwise come in contact with harmful things in the environment. When that skin barrier is breached, we need to know. Similarly, we have pain sensors inside our bodies to signal when something is going wrong—a stomachache, for example, after eating bad food, that discourages us from eating that food again. Pain serves as an essential warning signal.
Just consider what life is like for people with the disorder HSAD (hereditary sensory autonomic neuropathy, also called congenital insensitivity to pain, or CIPA), who don’t feel any pain. Although rare (there are only fifty-six reported cases in the world), the disorder entered popular culture when Stieg Larsson wrote about it in the trilogy beginning with The Girl with the Dragon Tattoo, giving it to the character Ronald Niedermann. (It was also featured in an episode of the television drama Grey’s Anatomy, and in House, in which a sixteen-year-old patient, Hannah, has it.) In reality, toddlers with HSAD/CIPA have trouble becoming toilet trained because they are unable to recognize the feelings associated with going to the bathroom. As children they may fall and break bones or cut themselves, or bite their cheek without knowing it. Many bite off the tip of their tongue while chewing. They may not sense that food is too hot and burn their mouth or esophagus. Foreign objects in their eyes go undetected and lead to infections and corneal damage. In one particularly horrific case, a six-month-old bit off the tips of his fingers and his thumb. Without pain perception, children with HSAD develop intractable bedsores because there is no motivation to shift positions as they sleep. The life expectancy of such people is short, around twelve years—they tend to die of hypothermia and complications from multiple bone fractures and infected sores. Twenty percent of people with this disorder die before age three, and it is uncommon to find patients over twenty-five years old. In a cruel trick of nature, they still feel emotional pain, however, just like anyone else.
One type of HSAD is caused by a random mutation in the SCN9A gene, which provides instructions for making sodium channels in the brain and is on the long arm of chromosome 2. Sodium channels allow positively charged sodium ions to be transported into neuronal cells, where they play a key role in the neuron’s ability to transmit signals. The SCN9A gene encodes for one subpart of a sodium channel called NaV1.7, which governs the functioning of pain receptors in the peripheral nervous system. The reason this disorder doesn’t cause a complete and total breakdown of cell signaling is that NaV1.7 isn’t the only sodium channel—our physiology abounds with these kinds of redundancies, back-up systems, developed through evolution, to enhance our chance of survival.
Another type of HSAD leaves patients with an ability to feel pain, but they are entirely indifferent to it. That is, an injury that we might experience as painful isn’t accompanied for them by any negative emotions, and so they have no motivation to alter their behavior.
For the rest of us, our reaction to pain typically follows a sequence: escape the painful stimulus, limit further damage (often accomplished by the site of injury becoming sensitive), seek safety and relief, and allow time for healing. So, we know why we have pain, but why does it have to be so darn unpleasant and make us so miserable? The short answer is because that unpleasantness is what motivates us to do something about the source of the pain, to learn from mistakes, to go to the doctor, to stop putting pressure on that compressed joint, to alter the repetitive movement patterns that are grinding down the cartilage in one of our hips, to rest and take it easy.
While acute, short-term pain has survival value, what about chronic pain? Chronic, disabling backaches or arthritis can last for years, even for the rest of one’s life, and are not easily treated or cured. It doesn’t function as a warning because there’s nothing you can do about it. What could be the biological benefit of that? We don’t know. It is one of several still-unsolved mysteries in neuroscience.
A recent study of squid and its natural predator, the black sea bass, puts us closer to an answer. Neurobiologist Robyn Crook and her colleagues wondered if in addition to the protective effects of pain, the purpose of chronic pain might also be to produce a kind of sensitization, and hypervigilance, to predators. Squid are good for this sort of study because their defensive behaviors are easy to track—they either change color (to match their environment) or squirt ink. Squid have eight arms and two tentacles. The researchers applied a minor injury to one of the arms by cutting off the tip of it. It was enough to cause pain (science can be brutal) but it didn’t impair the squids’ ability to swim and maneuver. They then put a group of these injured squid in a tank with some hungry black sea bass and put other, uninjured squid in a different tank with some other hungry black sea bass.
“The injured squid were really touchy,” said Crook. “They responded more strongly to visual stimuli than normal squid. So an encounter that a normal squid might just want to keep an eye on caused the injured squid to start up their defense mechanisms.” These injured squid paid far more attention to subtle visual cues from the black sea bass. That is, the injury made their sensory systems hypervigilant, just as Crook had hypothesized. As a control condition, the researchers anesthetized a separate group of squid before snipping the tip of one arm; these squid did not experience traumatic pain. Like the group of uninjured squid, these guys were not hypervigilant in the presence of black sea bass.
Interestingly, although the researchers couldn’t discern which squid had been injured and which hadn’t just by looking at their mobility and activity, the hungry fish could—they were much more likely to hunt an injured squid than a noninjured squid, regardless of whether the squid had been anesthetized or not. In this case, the experience of pain meant the difference between squid life and squid death. (Memo to squid: avoid scientists.) So although squid evolved a set of behaviors to make them more vigilant when injured, the black sea bass evolved an ability to detect squid injuries, even when squid don’t know they have them and aren’t feeling pain. Evolution is an arms race. (Or a tentacle race.)
What about the human experience of chronic pain? We know that humans in pain can be more attentive to their external environment, just like the squid. Think about it: If you’ve just been injured, it strongly suggests that the environment you’re in isn’t as safe as you thought it was. Cranking up sensory vigilance seems like a good idea. Any biological mechanism that is as prevalent as chronic pain probably served an important function in our ancestors, which is why it has persisted through millions of years of evolution.
The Culture, Genes, and Cognition of Pain
We don’t all experience pain the same way. Pain is influenced by cultural, environmental, historical, and cognitive factors. Our culture defines what is acceptable and taboo in how we deal with pain. There are distinctive variations in how people of different ethnicities experience and communicate pain, and what their expectations are. Many cultural rituals involve what you or I might consider pain but the participants don’t. Piercings, tattoos, and ornamental surgical procedures that outsiders would consider mutilation are not typically regarded as being in the same sensory-emotional category as arthritis or migraine. In many cultures, soldiers, warriors, and hunters who have survived a painful ordeal are admired.
Also the microculture of the family influences how we, as children, learn to think about, experience, and cope with pain. Parents may encourage a more stoic view (suck it up and tough it out) or a more medicalized view (wow, you seem much better after that pill and that little bit of rest). This stoicism versus medicalizing is influenced by the culture both within a family and the culture of communities. In the emergency room, medical personnel will typically ask a patient to describe their pain on a scale of one to ten, with one being no pain and ten being the worst pain imaginable. Emergency room doctors are trained to be alert to the fact that some patients will say, “Eight,” and yet the doctors don’t need to act right away, because as a generalization, people from some cultures tend to ascribe high numbers to relatively moderate pain levels and to make public displays of their discomfort. On the other hand, if members of other cultures report that their pain level is a “four” doctors might rush to prep the operating room because members of that group are known to be highly reserved in their public displays of pain.
The cultural backdrop in which we experience pain is one of the factors that contribute to the psychology of pain and to the attribution of that pain. The way people are injured influences their neuropsychological state, which in turn affects the way they recover. Soldiers who were shot might see their injuries as heroic and part of a noble cause. Convenience store clerks who were shot might have no such positive framing—they might see themselves as victims. The store clerks would be more likely to suffer from depression and far more likely to become addicted to opioids. Context matters. As psychological scientist Steven Linton says, “I have personally witnessed a man stick a rusty nail through his arm without the slightest complaint or flinch, while as a child I experienced a simple injection as excruciating.”
For more evidence that pain is heavily influenced by psychological factors, you need look no further than the enormous success of placebos, which are nearly as successful as actual drugs. In one pain study, a placebo was effective in 35 percent of patients, and opioids in only 36 percent. Another study (funded by pharmaceutical company Eli Lilly) found that for patients with chronic knee osteoarthritis, placebo worked in 47 percent of patients—the same number that responded to Eli Lilly’s Cymbalta, a nerve pain medication that is also used as an antidepressant and antianxiety drug. There are even placebo effects in acupuncture, where sham needling at nonacupuncture insertion points is as effective as actual acupuncture, with effects lasting up to one year.
Taking a placebo—an inert pill or procedure that you are unaware is inert—releases the brain’s natural painkillers, endogenous opioids. One of the ways we know this is that administering naloxone, a drug that blocks the receptors for opioids, undoes the placebo effect. Neuroimaging shows that the pain-relieving aspects of placebos are recruiting brain circuits in the anterior cingulate, nucleus accumbens, and middle frontal gyrus—the same regions that generate our endogenous opioids.
There are also genetic factors to pain perception, apart from the inherited insensitivity (or indifference) to pain. Genes are complex, and a given gene can influence the development of quite different attributes or phenotypes. For decades, medical personnel noted that people with red hair were harder to anesthetize. This was well-known and even taught in medical schools, but no one could figure out why. Surely redheadedness wasn’t a culture, like being from Japan or being Hindu. Who would have thought that the gene that confers red hair also yields radical increases in pain sensitivity? But it does. This was discovered by Jeffrey Mogil at McGill University, and it might, someday soon, lead to a separate line of analgesics on your drugstore shelf with the label Specially Formulated for Redheads. Geneticists such as Mogil don’t think that having red hair causes increased pain sensitivity any more than that increased pain sensitivity causes red hair. It’s just that the same DNA sequence affects both traits for reasons we don’t yet understand, or just by chance. And we may never understand it. With only twenty thousand genes or so and a near-infinite number of traits, it must be the case that individual genes do a great many unrelated things and that a single gene usually cannot be considered a sole or deterministic cause.
As we’ve seen, the amount of pain we experience is related to our upbringing—family models and culture as they interact with the other two parts of the developmental triad, genes and opportunity. By opportunity I mean the particular circumstances that you’re exposed to. If you grew up in an environment in which someone experienced a debilitating accident or disease, or with friends and family members coming back from military conflicts with injuries, your environment exposed you to how people in your family or culture react to pain and injury. On the other hand, if you grew up in a family with no aches and pains, you didn’t have this exposure.
Another factor is that your genetic makeup can predispose you to being clumsy or fumble-fingered or to have poor balance, in which case you’re more apt to experience injury. Or your genome can predispose you to having unusual pain sensitivity, such as the redheads mentioned earlier. A striking finding shows that the chemicals the body produces in response to stress and pain can be passed to infants through mother’s milk, affecting the infants’ own lifelong responses, even when the nursing mother is not the biological mother.
There are temporal factors to pain perception as well, and like the red hair–to–pain sensitivity link, they are counterintuitive. You would be perfectly reasonable, for example, to assume that people would rather have pain last for a shorter time period than a longer time period. Consider two different situations. (1) Pain stays constant at a level of eight (on some scale) for thirty minutes. We can graph it this way (the figure on the left). (2) Pain is constant at a level of eight for thirty minutes and then is reduced to a level of three for another twenty-five minutes (the figure on the right).
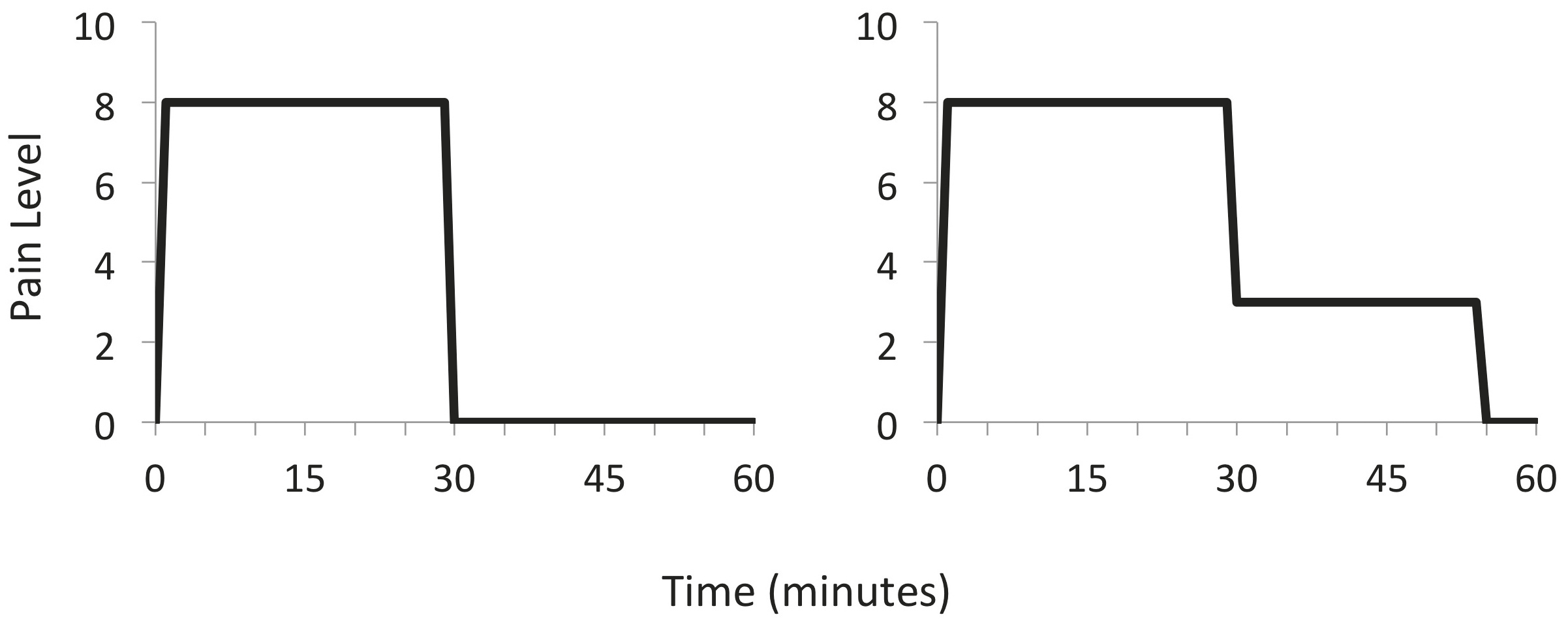
Clearly scenario 2 should be more aversive—you’re experiencing pain for fifty-five minutes rather than thirty, and moreover, you’re experiencing all the pain of scenario 1 and then some. (If you enjoy math, you could use integral calculus or plane geometry to calculate the total amount of pain experienced in each case and conclude that scenario 1 yields a total pain-time index of just under 240 and scenario 2 yields a total pain-time index of 315.) But research by Nobel Prize–winning psychologist Daniel Kahneman shows this isn’t the way we react at all. When they undergo these procedures, most people rate scenario number 2 as less painful than scenario 1 and would be more likely to recommend the procedure to a friend or undergo it again themselves. This is not because all the people Kahneman studied were masochists! It’s because of the nature of human memory. More pain is preferred to less pain when the most recent experience of it is lower. Rather than evaluating the total amount of time we’ve been in pain, the brain selectively remembers only the end of a painful episode. The duration of pain plays a very small role in our overall assessment of the pain; our discomfort is dominated by the worst and the final moments of the episode. The practical implications for this are that pain can perhaps become bearable if we can have periods of relative respite from it. A concern in medicine is that the amount of highly addictive opioids necessary to completely relieve terrible pain leads to a host of other medical problems. Perhaps alternating periods of light doses and heavier doses of such medications will prove to be more effective over the long term.
What Can We Do about It?
A central goal of modern medical practice and research is to relieve pain. Although medical science has advanced enormously in the past few decades, there have been few advances in pain technology. Opioids are still the most reliable and effective way of reducing pain, but as the recent North American opioid epidemic has revealed, pharmaceutical opioids are highly addictive and overdoses can too easily occur. Under carefully controlled conditions they may be safe, but these conditions are too often not met. Multiple pain pathways are involved in pain processing. This redundancy is important to the warning system and survival-enhancing roles of pain. But this very redundancy is one of the reasons that pain is so difficult to treat.
For inflammatory pain, such as insect bites, arthritis, sprains, and some headaches, anti-inflammatory drugs can work well. In order to increase tolerability of long-term use of anti-inflammatories in older patients, there has been a movement over the last decade to switch from oral to topical analgesics, particularly nonsteroidal anti-inflammatory agents (NSAIDs). But they remain underutilized, as physicians fall into the habit of prescribing the same things over and over again.
Fifty percent of modern NSAID prescriptions are for osteoarthritis, the most common form of arthritis, and the most prevalent pain condition in the United States, affecting around 30 million people. The most used NSAID worldwide is diclofenac and it is available as a pill, a gel, a cream, and a patch; it is currently the most effective treatment for osteoarthritis, with nearly 100 percent of patients reporting at least moderate relief from arthritic pain. The availability of a diclofenac patch has signaled a new promise for patients. The gels and creams are meant only to be absorbed by the skin, and after an hour or two, there is no more to be absorbed. The oral version of diclofenac is responsible for serious gastrointestinal problems, including stomach bleeding, in 30 percent of patients over the age of sixty, whereas the patch is associated with none. I’ve found the gel version to be very effective for insect bites.
Acetaminophen (aka Tylenol) has been found to be the least effective for arthritis, but that makes sense—it is not an anti-inflammatory and is not interchangeable with, for example, ibuprofen (e.g., Advil and Motrin). Acetaminophen is a pain reliever and reduces fever. If you have a swollen ankle, it probably won’t help as much as an anti-inflammatory.
Glucosamine is commonly taken by older adults for arthritis, but the scientific results are actually inconclusive. In the United States it is considered a “dietary supplement,” not a drug, and so its manufacture and use are not regulated, and it is illegal to advertise it for medical conditions. As with many supplements, its popularity is based on unsubstantiated claims infused with a lot of technical jibber-jabber that only superficially resembles scientific language and is carefully designed to persuade naive consumers. Glucosamine is a natural compound found in cartilage—the tissue that cushions joints. Some cases of osteoarthritis involve a breakdown of cartilage, so why not take cartilage in a pill? Well, there is no evidence that ingesting glucosamine actually affects its levels in joints. It might, or it might not. It’s sort of like saying that if you have liver disease, you should just eat a lot of calf’s liver and it will restore your own. The body doesn’t work like that. Nevertheless, aggressive advertising and claims surrounding glucosamine have made it a $15-billion industry in the United States alone.
There is some early evidence that yoga can bring real and lasting pain relief. Yoga practice enlarges the insula, giving patients greater ability to tolerate pain. Mild exercise can reduce pain too—as Jeffrey Mogil says, “Exercise is the best analgesic we know of by a wide margin. The problem is that when you’re in pain it hurts to exercise. But if you can get past that, it really helps.”
Neuropathy affects nearly 8 percent of older adults and is among the most common complications of type 2, adult-onset diabetes. Neuropathy is kind of a catchall term for conditions in which the nerves that carry messages from the body to the brain and spinal cord are damaged—this includes nerves on the skin as well as inside the body organs and viscera. You may hear the term peripheral neuropathy, referring to damage to the peripheral nervous system, but it is just a fancy way of saying neuropathy. The only other nervous system is the one in your brain and spinal cord, and that’s called the central nervous system. Central neuropathy is caused by a lesion, injury, or other damage to the brain or spinal cord. It includes things like multiple sclerosis, Parkinson’s disease, and cerebral palsy.
Peripheral neuropathy is often effectively treated by amitriptyline, duloxetine, and the gabapentinoids, and some people have success with topical NSAIDs. Central neuropathy requires a different approach. If it is caused by lesions to the brain or spinal cord, surgeries are often performed, but there is no evidence such surgeries are effective. Analgesics and opioids are not typically successful for central neuropathy, but antidepressants and anticonvulsants have had some limited success, although the reason they work is not well understood.
Remember our friend neurokinin, mentioned in the context of social isolation in the previous chapter? A related neurochemical, called substance P (for pain), is found in sensory nerves and in the brain and is believed to be responsible for pain and inflammation. If you’re thinking that blocking substance P could relieve pain, you’re not alone—this was an active area of research for twenty years, until a number of clinical trials failed in 1999. The problem turned out to be that substance P also does many helpful and necessary things, such as regulating mood, anxiety, and stress, the growth of new neurons, wound healing, and the growth of new cells, so blocking it can be worse than doing nothing.
Another issue that starts to crop up in old age is pain hypersensitivity, or hyperalgesia. The skin is an organ. Pain in one part of the skin can cause pain sensitivity in another, uninjured part of the skin. Or all over the skin—part of the hypervigilance reaction we learned about from the poor squid. Imagine that you’ve got a nasty spider bite on your leg. It itches like crazy. So you apply Benadryl cream or cortisone cream, which eases the pain. But later, your arm starts to itch for no apparent reason. Or your back. Sensory-pain receptors in the skin (they’re called nociceptors) become activated from pain or itch in one site, and—because they’re all part of the same organ and are communicating electrochemically with one another—the activation spreads. Paradoxically, the chronic use of opioids to reduce pain can cause hypersensitivity. Allodynia can occur, in which you experience a painful response to something that is ordinarily not painful, such as a light touch.
Much pain comes about from nerves in the neck or spinal cord being pinched. That old neck injury from when you were in your twenties can come back to haunt you in your sixties in the form of aches and pains. There’s one particularly insidious ailment that I’ll mention because it is often undiagnosed and untreated, and because it sounds both comic and tragic. Picture that part of your back, between your shoulder blades, that is just out of reach. Now imagine that you get an itch there. You can’t scratch it easily because, well, it’s out of reach. If you manage to rub against a tree like a grizzly bear, or get one of those bamboo back-scratching tools, or a good friend to scratch it for you, you get some relief. The itch doesn’t go away after several months. You try every anti-itch agent and cream you can think of, and they don’t help. You try lidocaine patches, which numb the skin, but the spot continues to itch, and now, because the skin is numb, scratching provides no relief. At some point, hypersensitivity kicks in and even normally pleasurable touch experiences, like getting a back rub, having your back scratched, or holding hands with a loved one become uncomfortable. This is not a made-up disease—it is called notalgia paresthetica. It is maddening. There is no known cure; however, some patients have reported success with anti-inflammatory gels, such as diclofenac (mentioned earlier for osteoarthritis).
One case report shows some relief from notalgia paresthetica through exercise. The patient tended to work at a computer all day with her shoulders rounded and facing inward. This protracted and elevated her scapulae and flexed her head and spine, causing her spinal nerve angles to become more severe. Through exercise, she strengthened her rhomboids and latissimus dorsi muscles and stretched the pectoral muscles. This changed her posture and she experienced a decreased sensation of itch, although not 100 percent relief.
Coping Strategies
If we can manage not to think about a particular pain, it tends to bother us less; distraction is one of the most effective ways to alleviate pain. The brain is bombarded with millions of input stimulus packets every hour and we pay attention to only a small proportion of them—if we could structure things so that pain was not at the forefront of our attentional systems, we’d be more comfortable.
People who are in enriched environments—with lots of things to see, listen to, and do—experience less pain than those in simpler environments, and this sort of distraction diminishes pain signals in the insula and primary sensory cortex. Effective distraction while in pain includes exercise, hobbies, interesting conversation, practicing yoga, meditation, socializing, listening to soothing music, or immersing yourself in nature. Even when the distracting activity is forced on an individual in pain, it leads to a reduction in pain and an increase in the body’s production of its own organic opioid analgesics.
The more interesting experiences we can have in the external world, the less time we focus on our internal world, which is where pain resides. Steven Linton describes the role of an enriched environment with his grandmother, which yielded an 80 percent reduction in her pain.
My grandmother at one time lived in a sterile, gray housing unit with little to do during the day but stare at the walls. . . . Upon visiting her in the evening, she had a long list of pain-related complaints that could take between thirty to sixty minutes to describe. Fortunately she was able to move to a housing unit for the elderly where there were other people to visit with, planned social activities, and regular visits by personnel. . . . There were significantly more things happening to divert her attention to the external world. While my grandmother still had pain complaints, I was impressed that they were far fewer and took no more than five or ten minutes to describe.
Separate from distraction, if we are in a good mood, pain is less likely to get us down. If we are already in a bad mood, a bit of pain is all it takes to put us in a worse mood. Remember also that memory is mood-state dependent. If you’re in a bad mood, you tend to have easier access to memories of other times you were in a bad mood or were sad, or times when things didn’t go right, and it’s easy to fall into a despondency cycle of “this pain is just going to get worse and worse . . . this always happens to me.” If you’re in a good mood, your mind tends to recall happy events, and you predict a more positive future. This good mood can lead to a virtuous cycle in which the positive-mood neurochemicals help with healing and you do actually get better more quickly. This is why mood-enhancing drugs, such as SSRIs, are often prescribed to patients in pain.
Psychological factors play a major role in pain, as mentioned previously. If you’re hiking or working out and your muscles start to ache, your brain may encode this as a good thing—it means you’re getting exercise and building up your muscles. That kind of framing shifts your understanding of the pain and distracts you from the discomfort, unlike, for example, getting a bee sting or having a rock in your shoe.
One common coping style is prayer or meditation. Some people pray for recovery and release from the pain, and in some forms of praying, they put responsibility for the pain in the hands of a higher power. Other fans of prayer give thanks for the opportunity to “show what they’re made of.” That is, if they attribute the pain they’re experiencing to a test of their resolve, or an opportunity to draw on their spiritual strength, it can transform their subjective reactions to it into a positive challenge—possibly activating a completely different part of the brain.
Special Problems in Treating Pain in Old Age
Chronic Pain
Patients tend to react to short-term (acute) pain differently than they do to long-term (chronic) pain. With short-term pain, we think we can treat it with medication. We might also curtail our activities and seek help from others.
Chronic pain is much more difficult to treat. Patients with chronic pain often report that a stimulus that should seem innocuous is painful. This lowering of the general pain threshold, or increased sensitivity, occurs in many patients with chronic pain disorders, including irritable bowel syndrome, back pain, and fibromyalgia. Their brain scans show abnormal activation patterns in brain regions involved in pain regulation, especially the anterior cingulate. Structural brain changes have been observed in people with chronic pain, including loss of gray-matter and white-matter volume, not just in the anterior cingulate (which is part of the pain circuit), but also in the dorsolateral prefrontal cortex, the region of the brain responsible for decision making, working memory, cognitive flexibility, planning, inhibition, and abstract reasoning. Neurochemical systems are affected as well, including reductions in dopamine production, opioid receptor binding, and modulation of the GABA and glutamate systems. If you’ve ever been in pain and felt you weren’t thinking clearly, these are the reasons why.
One piece of bright news in pain research in the last year is the availability of the new migraine drugs Aimovig, Emgality, and Ajovy. We still aren’t sure what causes migraines, but these drugs have been life changing for migraine sufferers—they only have to be administered once a month and they act as a preventative.
On the horizon, the next biggest development is that tanezumab (who names these things?) might gain FDA approval soon; it’s an antibody against nerve growth factor that treats pain and is effective against bone cancer pain, arthritis, and chronic low back pain. In phase 2 clinical trials, tanezumab was effective, but a side effect showed up: Joints began to degenerate more quickly, and so the FDA suspended further testing. Pfizer, one of the developers of the drug, looked very closely at the data and concluded that the joint degeneration was due to an interaction with the NSAIDs that people were taking at the same time. In theory, Pfizer said, if patients take only tanezumab there shouldn’t be a problem. The FDA was convinced, and so they lifted the hold on further testing. Jeffrey Mogil observes, “The original Phase 2 study was actually the most impressive clinical trial data I’d ever seen. In the Phase 2 the drug was beating placebo by 40 points out of 100, which is absolutely unheard of because you can get FDA approval with 10 points. And now in the larger Phase 3 trials it’s looking more like it’s beating placebo by the usual 10–15. It seems to be reliably doing that. Beating placebo by 15 points doesn’t sound like much, but for a subset of the population, that ends up being a big deal.”
Untreated, chronic pain disrupts sleep patterns, which in turn can cause profound deficits in memory and mood. Societal attitudes about pain in older people are often at fault. Health-care providers assume (without evidence) that pain is “just a normal part of aging.” Older patients often don’t report pain because they are afraid of their doctors’ dismissive responses, or due to the false belief that “good” patients don’t complain. You don’t have to be that sort of patient. It’s okay to speak up.
Polypharmacy
One of the things that’s important to speak up about is the drugs and supplements you’re taking. The average number of prescription medications taken by older adults often exceeds ten. This situation is called polypharmacy. It results from doctors wanting to please patients by writing a quick prescription that might address a single symptom—then doing it over and over and over again. This poses huge problems, creating very complex drug interaction effects. From a scientific standpoint, we have lots of data on how drugs interact pairwise, because studies of two drugs administered at the same time are easy to do. But it’s woefully difficult and impractical to study every possible interaction among a combination of ten drugs. So we simply don’t have data on these complex interactions. Some drugs mask or neutralize the effects of other drugs; some drugs are dangerously incompatible with others; side effects can multiply quickly. Some drugs are contraindicated for various conditions that affect the elderly disproportionately, such as heart and circulatory problems, or organ deterioration.
As just one example, the natural course of aging can lead to decreased gastric secretions. Meanwhile the loss of vitamin D receptors leads to loss of appetite, which in turn can lead to malnutrition and decreased bone density. Drugs with the side effect of decreasing appetite are compounding an existing problem. The natural course of aging can also lead to increased thickening and rigidity of the arteries, which increases cardiovascular risk. Decreased elasticity in the lungs makes us susceptible to pulmonary disorders. Changes in the kidneys compromise filtration, increasing the body’s accumulation of toxic materials. The digestive system functions less efficiently, leading to chronic constipation. Any drug with a side effect that amplifies or exacerbates these preexisting conditions can cause patients a nightmare of discomfort.
Often, these different, powerful medications have been prescribed by different doctors with no single doctor “in charge.” The side effects of polypharmacy can create conditions in which you think that you have a disease but don’t, and moreover, some drugs and drug interactions can mask early signs of illness. To add to all of this, polypharmacy persists because few doctors want to take somebody off of a drug they’ve been on for fear of something bad happening that they’d be blamed for. So they just keep on adding drugs without ever reevaluating the big picture.
It turns out that the primary cause of confusion, disorientation, and delirium among older adults is not Alzheimer’s disease—it’s adverse effects from medications or from drug interactions. There are a number of cases of older adults being shunted off to an old age home not because they have become mentally incapacitated, as well-intentioned family and friends might think, but because of polypharmaceutical complications.
An important part of successful aging is for each of us to take responsibility for our own health care by informing our doctors and pharmacists of all the different things we’re taking—including over-the-counter remedies (which are often as powerful and as subject to complex interactions as prescription drugs). That’s just the conscientious thing to do.