8
THE INTERNAL CLOCK
It’s two A.M. Why am I hungry?
Have you ever woken in the middle of the night and found yourself ravenously hungry? Or maybe you’ve felt hyperactive just before bedtime. Maybe you fell asleep at an inappropriate time, while at a meeting or a concert (perhaps even while reading a book about aging and the brain). If you’ve experienced any of these, you’ve experienced a deregulation of your circadian rhythms. The word circadian was coined by scientists in the 1950s, from the Latin words circa (approximately) and diem (day): a rhythm that operates on a cycle of around 24 hours.
Circadian rhythms are the product of biological clocks, an evolutionary adaptation in response to the earth’s twenty-four-hour rotation period. They allow our bodies and minds to predict what will come next, so as to be better prepared for different situations and circumstances. For example, predicting when the sun will rise allows the brain to release wake-up chemicals (such as orexin, dopamine, and norepinephrine) and to suppress the drowsiness chemicals (such as melatonin, adenosine, and GABA) that might make us want to roll over and go back to sleep. The inner clock lets us wake up in the morning refreshed and ready to go.
Biological clocks evolved early in evolutionary history, when most cells were light-sensitive. Ultimately, these light-sensitive cells became embedded in plants, fungi, bacteria, and all multicellular animals. Clocks have also been found in a bread mold, Neurospora crassa, where they release spores at the appropriate time of day when spore-bearing moisture in the air is at its peak. Clocks have also been discovered in the eye of the aplysia, a marine snail (or sea slug), whose ancestors diverged from ours 500 million years ago. The aplysia’s clocks modulate its memory and locomotor activity in synchrony with light-dark cycles. The clocks are wired into the genes. And across hundreds of millions of years of evolution, similar genes have been found to control cellular clocks in all these organisms, from bacteria, plants, and fruit flies to fish, birds, mammals, and humans. In the aplysia, scientists have found genes that are remarkably similar to those in humans, including those related to Parkinson’s and Alzheimer’s disease.
Plants use photosensitive internal cellular clocks to detect the length of days. When they sense the shorter days of autumn, the clocks activate genes that signal the plant to produce seeds and drop its leaves. When the clocks sense longer days in the spring, the plants grow back their leaves, along with flowers or fruit. Biological clocks help plants prepare for sunrise by raising their leaves, tilting them toward the sun, and preparing their internal factories to perform photosynthesis, converting sunlight into nutrients. At night, clocks orchestrate the opening and closing of leaf pores and the nighttime folding of leaves to prevent water loss. If the clocks are regulating plants, mold, and mollusks, you can imagine the complexity of the various roles they play in our functioning. They also exert a large influence on aging—so much so that when tissue from the biological clocks of young animals has been transplanted into older animals, the older animals live longer.
The Master Clock
In mammals, circadian rhythms rely on three separate processes:
-
an input system that takes in information from the environment, consisting of cues such as light-dark cycles and food consumption that are received via peripheral oscillators;
-
a central, master oscillator, or clock, that keeps track of the time of the input events and can generate a consistent rhythmic signal; and
-
output pathways that allow the master clock to influence and synchronize the various peripheral oscillators that govern physiological operations, such as digestion, sleep-wake cycles, core body temperature, hunger, and alertness.
Thus, circadian rhythms are hierarchically organized, and different parts of the clock system communicate and modify one another, using feedback and feed-forward loops. All cells in the brain and body are sensitive to time of day, and genes (such as PER1, BMAL1, CLK1, DBT, and most famously, CLOCK) activate proteins according to a more or less twenty-four-hour cycle. I say more or less because these cells function like a cheap watch—they tend to drift, to speed up and slow down. To regulate them, we evolved a master clock. In humans, this is located within the hypothalamus, in a structure called the suprachiasmatic nucleus, or SCN, a group of about twenty thousand neurons that oscillate according to an almost twenty-four-hour rhythm. The starting time or phase of that rhythm can be reset by input from light and other time givers (called zeitgebers, the German translation for the phrase time givers).
The SCN is like the stationmaster at a busy railroad station, keeping all the trains on schedule so that they don’t run into one another, and so that people who need to get somewhere get there on time. It’s less like the atomic clocks that governments set their official times to, because atomic clocks function with little or no outside interference. Our SCN, on the other hand, is sensitive to inputs from the retina and from additional photosensitive cells on the skin to distinguish daytime from nighttime. It also takes input from various metabolic processes. The SCN communicates time-of-day information to various brain regions and to peripheral organs including the heart, lungs, liver, and endocrine glands. Tissues in the liver and pancreas regulate metabolic rhythms for stable glucose levels, lipid metabolism, and the removal of foreign compounds from the body and blood (xenobiotic detoxification). So, for example, when we eat and digestive juices are released, the SCN finds out about it and uses that information to regulate digestive cycles.
The developmental science approach seeks to understand the interaction between genes, culture, and opportunity. Biological clocks are a fascinating example of this. They function by taking input from the environment—primarily light, but also your eating schedule and your own activity cycle, which are influenced by culture. Light, whether it arrives from the rising sun or that little blue light on your cell phone charger, can turn particular genes on and off, changing the timing of when they produce the proteins that influence the biological clock and our circadian rhythms. Daylight or lack of it can speed up or slow down the circadian rhythms.
All of this interacts with your own aging process in a variety of ways. Here’s just one example. Light—and blue light in particular—is necessary to program the biological clock. Cataracts, which accompany aging, are yellow and so they tend to block out blue light. Consequently, cataracts can restrict the amount of blue light that reaches the retina and in turn diminish important neuronal signaling to the pineal gland and SCN. In some cases, cataract surgery has restored sleep quality to older adults by allowing more blue light in during the day and thus restoring a healthy melatonin release schedule. But blue light near bedtime, such as from a cell phone, alarm clock, or computer, can stimulate the pineal gland and make it more difficult to fall asleep. (Perhaps the engineers who designed alarm clocks should have checked with neuroscientists before they chose the LED colors they did.)
When May Be as Important as What
What you eat, how much you exercise, and how much you sleep are important, but in the past few years, neuroscientists and chronobiologists (people who study biological clocks) have come to understand that when you eat, when you exercise, and when you sleep may be just as important. This is particularly true of older adults.
The twelfth-century philosopher and physician Moses Maimonides understood the importance of when to eat, as well as how much. His advice for living a healthy life was to “eat like a king in the morning, a prince at noon, and a peasant at dinner.” In contemporary times, the field of chrono-nutrition strives to synchronize our ingestion of calories with the body’s circadian rhythms. The timing of meals can have profound effects on a number of physiological processes, including sleep-wake cycles, core body temperature, peak performance, and alertness. Eating at different times each day, or at times that are out of sync with your circadian rhythms, can lead to obesity, metabolic syndrome, diabetes, and other problems. Your mother was right when she told you to eat a big breakfast and to have dinner at a fixed time.
The gastrointestinal tract has a powerful circadian clock of its own and is also home to billions of microbiota, also called the microbiome. Everyone’s microbiome is unique, and the organisms that make it up have their own clocks. Research on the microbiome is still in its infancy, but early evidence points to the possibility that what and when you eat can influence the microbiomic clock (the circadian rhythms of your microbioata) and, further, that your microbiome can send information up to the master clock in your SCN to influence its timing. In addition, the SCN (suprachiasmatic nucleus in the hypothalamus) can regulate the microbiome through triggering production of cyclic glucocorticoids, insulin, and other timing- and rhythmic-altering substances.
As you probably know, people differ in terms of the time of day when they are most fresh and alert. I wake up at five thirty A.M. and I’m raring to go—I get most of my writing and research done before ten A.M. My wife, on the other hand, is most productive in the afternoon and evening—if she didn’t have an eight A.M. neuroscience class to teach, she’d probably stay up all night working, and do so very efficiently. In popular parlance, I’m an early bird; my wife is a night owl. We have different chronotypes. Different chronotypes have a genetic basis, but they also interact with environment and experience. Years of staying up late, consistently, and exposure to blue light beyond sundown can cause changes in gene expression that shift your chronotype at the genetic level. But this doesn’t always happen—a great many shift workers live in conflict with their inherent chronotype, and this is responsible for accidents, depression, and loss of productivity.
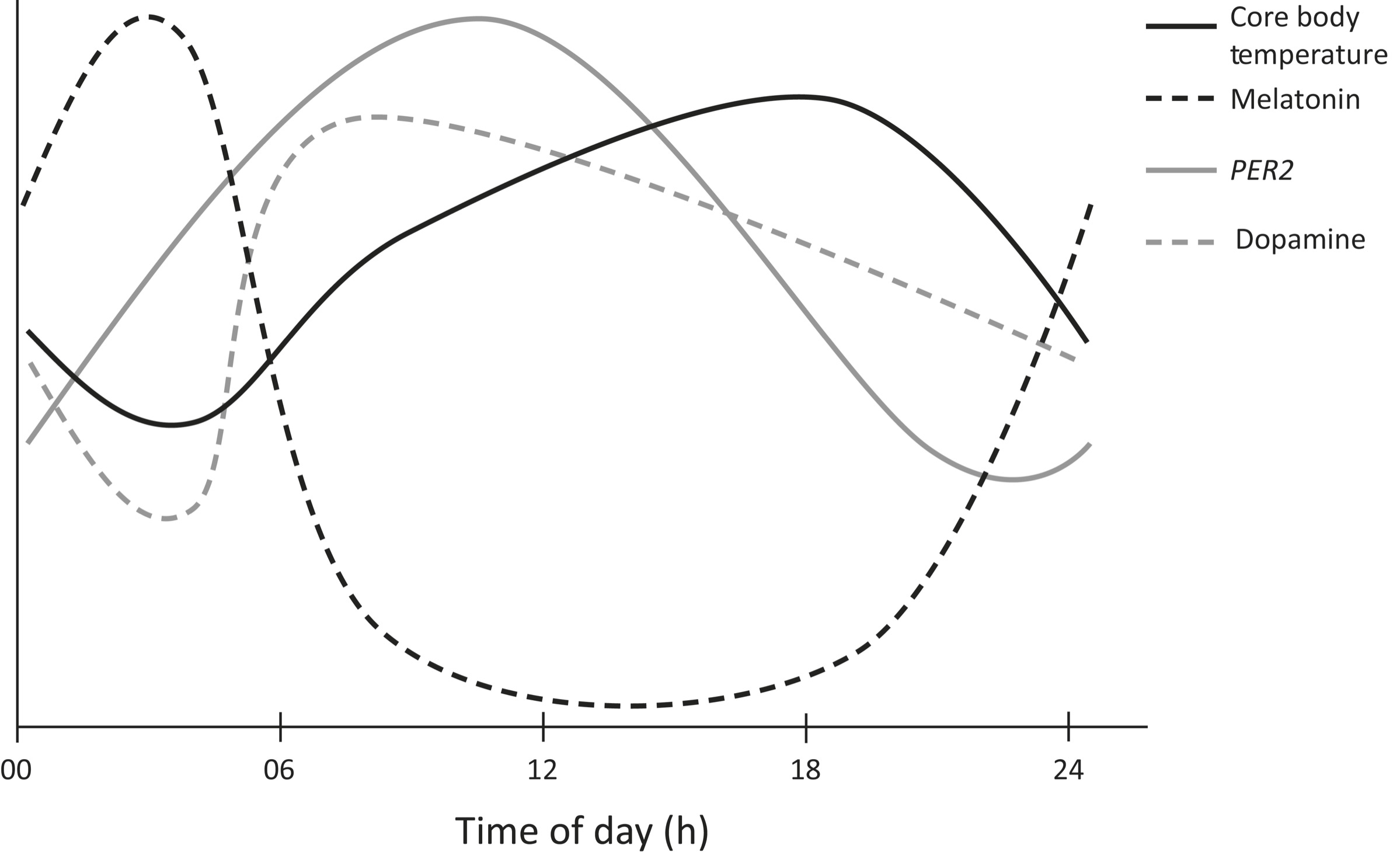
Four of the different circadian rhythms are shown on the previous page. The y-axis represents individual, scaled units for each cycle, with increasing values from bottom to top. Dark solid line: Core body temperature starts rising from the nighttime low of approximately 36.5°C about three hours before wakening, reaches 37.2°C by nine A.M., keeps rising slowly to a peak of 37.4°C at about eight P.M., and then falls to reach the initial level of 36.5°C at four A.M. Dark dashed line: Circadian rhythm of melatonin. Gray solid line: Temporal regulation of the PER2 gene. Gray dashed line: Plasma dopamine levels throughout a twenty-four-hour period. There can be marked individual variation in the amplitude and extent of each waveform. (This graph is based on scaled averages and so the units across different systems are not identical.)
The invention of bright, artificial lights more than a century ago has caused a problem that humankind had never experienced before: the possibility of tricking the SCN into thinking it’s daytime when it’s not, and the possibility of allowing our circadian rhythms to become fashioned to our own will. Unfortunately, much of the time, all the lights do is disrupt our million-year-old cycles, and that can have serious health consequences. I’m not advocating a return to homes without artificial light—just a better understanding of the effects they have on us so that we can create better home environments. The use of home lighting, and most recently of computer screens, clocks, and various devices that emit blue light, has created a population of night owls. Currently only 30 percent of the population sleep best by going to bed before midnight. That means 70 percent of the population can’t get to work by eight or nine A.M. without waking their body up before it is biologically ready. And teenagers experience a particular shift in their sleep schedules for reasons we don’t fully understand but are related to the sudden influx of pubertal hormones. A nascent movement in the United States to delay the starting times of high schools is gaining traction. Businesses that run around the clock and employ workers outside of the normal nine-to-five workday unfortunately tend to assign workers to shifts indiscriminately, without regard to the workers’ individual biological clocks. This can result in great inefficiencies and lead to sleep deprivation, work days lost to illness, and serious accidents.
As just one example, chronobiologist Till Roenneberg and his colleagues conducted an experiment at the ThyssenKrupp steel factory in Germany. ThyssenKrupp is one of the largest steel producers in the world, and its products include high-speed trains, elevators, and ships. The scientists identified those workers who were early birds versus night owls and gave them different shifts so that their work schedules aligned with their internal clocks. Once their chronotypes were aligned with their duty shifts, workers enjoyed 16 percent more sleep, almost a full night’s length over the course of the week. The study did not last long enough to collect data on workplace accidents or errors, but a mountainous literature shows that sleep deprivation is responsible for some of the worst industrial disasters in modern times, including the Exxon Valdez oil spill, the Chernobyl nuclear accident, and the Bhopal methyl isocyanate gas leak disaster. The US National Highway Traffic Safety Administration reports that one in six traffic fatalities in the United States is caused by drowsy drivers.
Why do individuals have different chronotypes? To quote Shakespeare, “Some must watch while some must sleep.” From an evolutionary standpoint, consider what life was like for our ancestors ten or twenty thousand years ago. Sleep was necessary for survival, and yet it was a time when we were especially vulnerable to attack by animal predators and violent humans, as well as the occasional hurricane or erupting volcano. The sentinel hypothesis is that when living in groups, animals share the task of nighttime vigilance, some watching over those who sleep.
Chronotype variation is found across many different species, and that’s the origin of the colloquial terms that we use to describe humans: early birds and night owls. For humans, these labels represent the extremes of the distribution—most people are somewhere in the middle, with earlyish or latish tendencies. There are also sex differences, with males more likely to stay up late than females. (There isn’t any research yet on why—or on the sleep patterns of trans individuals, or gender nonbinary/nonconforming individuals. Because gender identity is hormonally and biologically based, it is likely that chronotype follows gender, not biological birth sex.)
Chronotype is heritable, and a number of genes have been identified that contribute to variability across individuals. In a comprehensive new study, researchers analyzed the genomes of seven hundred thousand Britons and discovered more than 350 genes that contribute to chronotype. Additional variation comes from the fact that circadian cycles are advanced in older adults who tend to go to sleep earlier and wake earlier. Such age-related variation also may have been an evolutionary adaptation: It might have been a survival advantage for the older people, whose hunting skills had diminished, to stand on guard at night so that the younger, sharper hunters could get a good night’s sleep. This has led one group of researchers to propose the “poorly sleeping grandparent hypothesis.”
If the sentinel hypothesis is true, we’d predict that in ancient times, only rarely would all members of a living group be asleep at the same time. Alternatively, if all individuals were asleep at the same time, it would discredit the sentinel hypothesis.
The sentinel hypothesis was recently confirmed by anthropologists studying a contemporary group of hunger-gatherers, the Hadza of north-central Tanzania. The Hadza are a group of about twelve hundred people who live around Lake Eyasi in the central Rift Valley and in the neighboring Serengeti plateau. Anthropologists consider them an important window into the lifestyle of our Pleistocene ancestors. They have not adopted the herding or farming practices of other Tanzanians nearby, and we believe that they are living today as they did for many thousands of years.
The researchers found that, over a twenty-day period, there were only eighteen minutes in total when everyone in the group was asleep. At any given time during the night, about one-quarter of the group were awake, serving as sentinels. And they’ve survived.

The Aging Clock
As we age, the signaling to and from the SCN degrades. Part of this signaling deficit is due to loss or degradation of the nervous system’s myelin sheath, and part due to an overall age-related depletion of neurochemicals and hormones. This is partly why older adults can have trouble getting to sleep, wake up at five in the morning, and want their dinner at four thirty in the afternoon. Lose the master clock and you’re living like a ninety-year-old in Boca Raton.
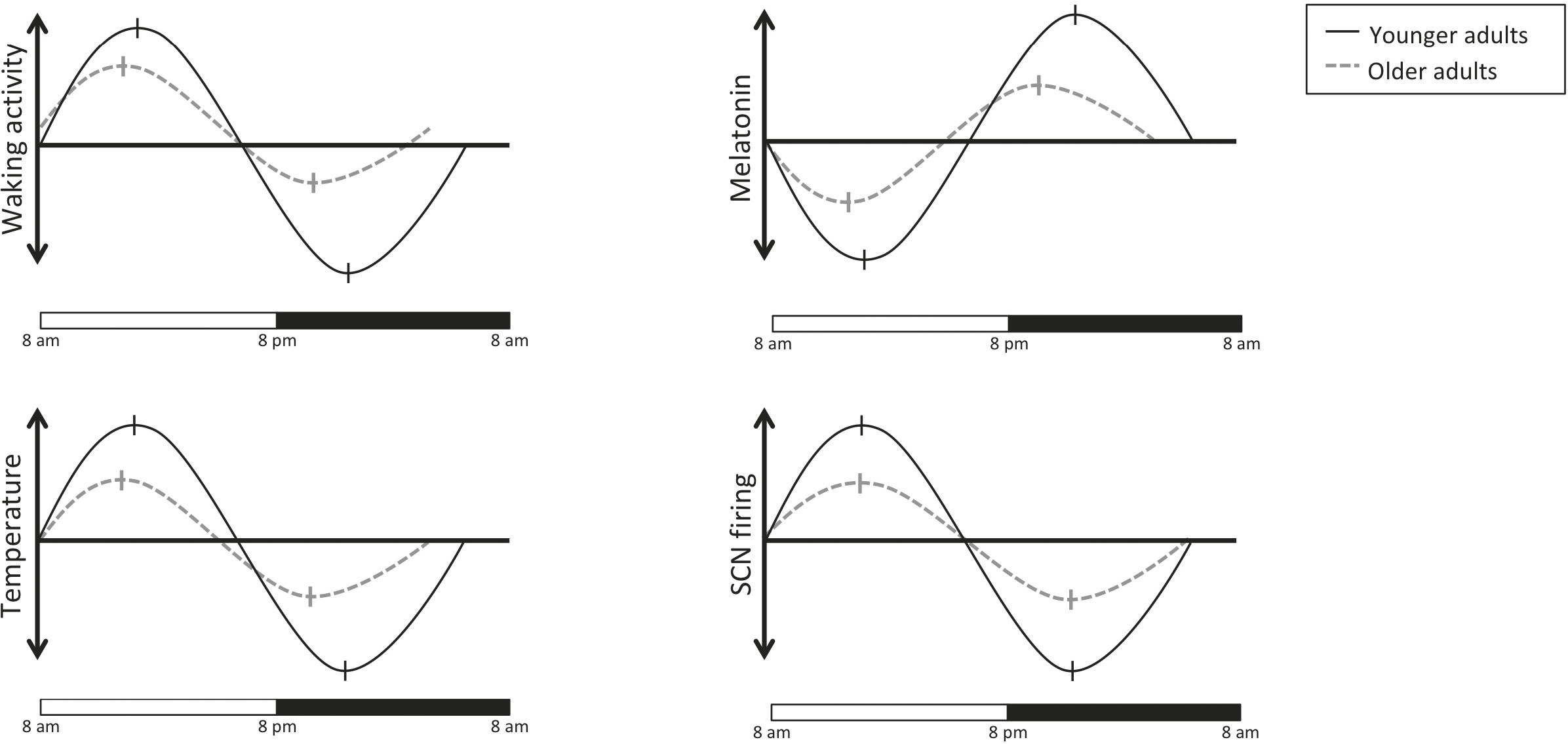
With increasing age, the amplitude of SCN signaling decreases and shifts leftward (advancing phase, the dashed lines) which accounts for changes in sleep, waking, and eating cycles among older adults. (As with the previous graph, these are based on scaled averages and so the units are not identical across graphs.)
Some evidence indicates that the problem is in fact with the integrity of neurons in the SCN itself—transplanting young tissue into the SCN of hamsters improved the clock synchrony of aging hamsters and increased their life span. This suggests that good circadian rhythm may be related to longevity. We’re a long way off from being able to do such transplants in humans, but the results of these experiments help us to understand the vulnerabilities of the aging clock—and offer directions for longevity-increasing research.
Older adults tend to experience a shift in their chronobiological cycles (see figure on the previous page) called a phase advance; as they age, people are more likely to become morning types as they age. After age sixty, the rhythms of the genes PER1 and PER2 in the human orbitofrontal cortex are flattened and phase-advanced by approximately four to six hours, and the expression of CRY1 becomes arrhythmic, compared to adults under forty years of age. Degradation of vasopressin signaling is responsible for increased fragmentation of the sleep-wake-activity cycle and for disruption of core body temperature rhythms, and more frequent urination—all lead to poor sleep quality and quantity, resulting in an increase in daytime drowsiness. The functioning of the biological clock is disrupted to an even greater degree in dementia. Postmortem studies of dementia patients’ brains show stark degeneration of the hypothalamus’s SCN.
This affects more than just eating and sleeping. There are time-of-day effects for alertness and performance that become starkly emphasized when we age. Adults after the age of sixty or so begin to show performance differences on a range of neuropsychological tests—memory, problem solving, spatial intelligence, reasoning, fine motor coordination, and athletic performance. Test them in the morning and they are normal; test them in the mid- to late afternoon and they show reduced performance, compared to forty- or fifty-year-olds (as we saw in Chapter 4, on the problem-solving brain). The differences become even more pronounced after age seventy. Give a standard memory test in the morning and they appear fine. But after noon, the decrements can be large. The take-home message is this: Make important decisions, those about finances, health, and the like, before noon. Your thinking is better. And if you’re going to exercise and there might be a fall, do it earlier in the day, when you’re sharper. This is why George Shultz and Vicente Fox, for example—highly productive people who are past nominal retirement age—show up at work early in the morning and tend to take the afternoons off, or at least not schedule crucial tasks for the afternoons and evenings.
The detrimental effects of disrupted cycles tend to be subtle and far less pronounced among younger adults. But at some point, it’s likely that you’ll begin to notice the effects. That time point is variable—it could be age fifty, sixty, seventy, or eighty, depending on the interaction of genes, culture, and environment. Disrupted circadian rhythms without a clearly identifiable external cause are an early warning sign of Parkinson’s, Alzheimer’s, and Huntington’s diseases, chronic inflammation, and cancers.
Repetitive disturbances of the circadian rhythm, particularly from frequent time-zone shifts and irregular exposure to light, have now been implicated in a variety of diseases, including metabolic syndrome, immune deficiencies, bone and muscle weakness, cardiovascular disease, cancer, and a shortened life span. Sundowner’s syndrome, the tendency for people with Alzheimer’s disease to show confusion and poor memory in the early-evening hours, may well be the result of circadian rhythm disturbances—patients who can successfully restore a normal sleep-wake pattern can stay longer with their families and postpone the need to be housed in special care facilities.
Travel
One of the most well-known functions of the biological clock is to control the release of melatonin to promote sleep. When there is less light—for example, at night, or in polar latitudes in winter—your body makes less melatonin. Jet lag occurs when the ordinary zeitgebers that you are accustomed to—sunrise, intensity of light, length of the day, food intake, activity levels—are changed. This happens most typically when you cross time zones and the local time at your destination is different from the local time where you started out. The sun may come up earlier or later than your biological clock expects. The clock tries to reset itself, but this can take several days.
When we’re young, the biological clock is more flexible, malleable, and it can react to environmental changes quickly. As we get older, resetting the biological clock can take longer—much longer. Phase advances—a shift to waking up earlier and going to bed earlier than usual—are more difficult for older adults to recover from; phase delays show no difference between older and younger adults. Older adults will tend to find it easier to adjust to a new time zone when traveling west, but not east, because the advanced phase shift accords with tendencies already present in their biological clocks.
Generally, it takes your body one day of recovery or planning per one hour of time-zone shift when traveling east, and half a day per one hour of time-zone shift when traveling west. This is a best case scenario. As you get older, it may well take longer. Before traveling east, start advancing your body clock as many days before your trip as the number of time zones you’ll be crossing. Get into sunlight early in the day, or use a sunlight lamp. Once you’re on the plane eastbound, wear eyeshades to cover your eyes two hours or so before sunset in your destination city, to acclimate yourself to the new “dark” time.
Winter months also pose a potential problem because they rob us of the most important zeitgeber, light. The cold weather can also cause us to overeat, and that can throw off the time markers associated with eating. Jet lag can also occur with north-to-south travel, even if time zones don’t shift, because the length of the day can change considerably. The farther you are from the equator, the more extreme will be the difference in light between summer and winter months.
Sleep Hygiene
For most people, the sleep-wake cycle creates an energy dip between two and four A.M. (when they’re asleep) and another one between about one and three P.M. (postlunch). If you’re sleep-deprived—which refers not just to the amount of sleep you’ve gotten over the past few days but also to the quality of that sleep—you will tend to notice the postlunch dip more. And you’ll notice it more as you age.
Sleep hygiene involves avoiding bright lights before bed, sleeping in a completely dark room (get blackout curtains if you need to!), and going to bed and waking at the same time every day. Now that we know about these circadian rhythms, this makes sense—your biological clock expects you to go to sleep at a certain point within the twenty-four-hour cycle. It adjusts your core body temperature, slows digestion, releases melatonin, represses dopamine, and oversees dozens of other regulations. If you go to sleep earlier or later than usual, these cycles are a little out of sync with the fact that you’re sleeping. Sleep quality is impaired.
Diet is important too: Eating within two hours of bedtime can decouple the central circadian rhythms from those in the liver, stomach, and intestine. What you eat can also be a factor: Alcohol is known to disrupt sleep cycles and circadian rhythms, and high-fat diets tend to advance the clock—a practical implication of this is that if you need to stay up late, eat fatty foods like those pictured in virtually every late-night TV commercial. (Coincidence? I think not.)
Light therapy and melatonin treatments are the most effective means of resetting the circadian clock, especially in the aged. They are also effective in people with Alzheimer’s-related or mild cognitive impairment. It’s possible that these treatments may also prevent or delay the onset of Alzheimer’s disease itself. In lab studies, melatonin interacts with beta-amyloid protein to inhibit the formation of those dangerous amyloid fibrils, and a link between disrupted circadian rhythms and Alzheimer’s is well-established. In one review, melatonin use in early-stage Alzheimer’s supported findings that sleep quality was improved, sundowning was reduced, and the progress of cognitive decay was slowed. In four studies of melatonin treatment, cognitive performance improved, and agitated behavior was reduced. The efficacy of therapeutic melatonin in late-stage Alzheimer’s disease is likely limited by the strongly diminished number and density of melatonin receptors in the SCN.
Light-therapy lamps that simulate a gentle dawn are readily available and cost less than one hundred dollars. Increasing light intensity and optimizing the wavelength of light can compensate for some of the organic deterioration of the SCN and related chemical circuits that accompany aging. But the light therapy must be done at the correct time of day—upon waking—and it must be done consistently. Everyone is different, but by experimenting with different intensities and durations of light exposure, you can arrive at the most effective treatment. You may have heard of seasonal affective disorder (SAD), which causes mood and concentration disturbances and is triggered by the shorter, grayer days of winter. Many of the lamps sold for light therapy are branded and marketed as SAD lamps. Don’t be fooled by the acronym—to my way of looking at it, the research shows that using them can make you HAPPY (healthy, asymptomatic, peppy, perceptive, and youthful).
Melatonin is available over the counter and can be used to help you entrain to a more regular sleep cycle. There are noticeable individual differences in how melatonin is processed by the body, and the best treatment would involve working with a physician who can test the dim-light melatonin-onset levels in your blood every thirty to sixty minutes throughout the late afternoon and evening and then prescribe a dose and a time to take melatonin. Sleep-medicine specialist Alfonso Padilla at UCLA recommends taking just 0.25–0.5 mg to resynchronize your biological clock. This amount mimics the psychological levels that your body naturally releases (when everything is going well). Melatonin’s sleep-promoting action works as a step function, meaning that if you get enough of it, more won’t help (and could be harmful). Although over-the-counter products commonly available often contain 5–10 mg (and at least one manufacturer sells 60 mg tablets), overdosing can cause extreme drowsiness the next day and disrupt your sleep cycle for a week or more. Remember: it is not a sleeping pill—it just resets your biological clock, which is not the same thing.
Melatonin levels tend to rise about fourteen hours after waking. If you get eight hours’ sleep every night and wake at six A.M., that means your melatonin levels will naturally rise around eight P.M., and you’ll start to get sleepy and go to bed two hours after that, around ten P.M. If it takes about an hour for a melatonin pill to be absorbed into your bloodstream, that means taking one about three hours before bedtime.
The next most effective treatment, after light therapy and melatonin, is moderate late-afternoon or early-evening exercise, like going outside for a walk. The combination of all three is the best.
Caffeine
Caffeine is one of the most widely consumed mind-altering substances in the world. For many people, it enhances wakefulness, alertness, and focus and can assist in establishing or changing circadian rhythms, particularly when crossing time zones.
The extent to which caffeine interferes with the human circadian clock, if at all, is not known yet. It has been shown to lengthen the daytime activity rhythm in fruit flies, sea snails, bread mold, and algae. The detrimental effects of caffeine on sleep are well-known, and they include delaying the onset of nighttime sleep, reducing total sleep time, impairing sleep efficiency, and worsening perceived sleep quality. Tina Burke, a young scientist at the Walter Reed Army Institute of Research in Boulder, Colorado, approached this question with converging evidence from different methods: genetics, pharmacology, and human experiments. Using cultured cells in vitro, she showed that caffeine does interfere with a number of chemical processes that contribute to circadian timekeeping and the resetting of the clock, by delaying the timing of these rhythms. Then she gave volunteers a double espresso (caffeinated or decaffeinated, unknown to the volunteers) three hours before bedtime. The caffeinated double espresso delayed the melatonin cycle by forty minutes, and she discovered further that the delay appears to be dose-dependent. Older adults may find that they have become more sensitive to the effects of caffeine on sleep than when they were younger.
Peak Performance
At the beginning of the book, I introduced the concept of health span versus disease span—the amount of time you are able to avoid declining health and live a fully productive, self-sufficient life. There’s a parallel concept called productivity span that can apply not just to the arc of your life but also to the arc of a single day: There are times of day when you are at your best and other times when you are not. I mentioned this earlier in the discussion of chronotypes and the fact that older adults tend to perform better in the morning (whenever that subjective morning is—the first six hours of being awake). All of us, I think, would prefer to have longer stretches of time during the day when we are at our peak and shorter times of being in a trough. This is true regardless of how we spend our time, whether we place the most value on using our brains, our emotions, our social skills, or our physical skills. The effects of circadian rhythms on peak performance have been studied extensively in professional athletes, for obvious reasons—there is a lot of money at stake.
Top athletes often have to travel across time zones to compete, and the time of the competition can’t be adjusted to suit their own particular chronobiology. Studies report conflicting findings on whether professional athletes reach their peak performance in the late afternoon/early evening or in the morning. The most obvious explanation for this is that there are individual differences among athletes and differences in the demands of various sports. The available evidence is that elite athletes tend to select, and excel in, sports that suit their chronotype. Sports that typically conduct early-morning training, such as rowing and track, tend to attract those individuals with an early-morning chronotype. Sports that are typically done in afternoons and evenings, such as water polo, volleyball, cricket, hockey, and soccer, attract late-chronotype athletes. Because chronotypes fall along a continuum, some individuals are considered neither type (neither early-morning nor late-evening), and there is evidence that these individuals can shift their chronotype simply by practicing consistently at a particular time of day.
Some studies show that peak performance in grip strength, running, jumping, oxygen uptake, and muscle function tends to occur between four P.M. and eight P.M. in the athlete’s home time zone. Similar peaks have been found for football, swimming, and cycling. The differences between elite athletes’ on-peak and off-peak performance, though relatively small in terms of numbers, have very large effects in the world of professional sports. An elite runner who has to perform on the other side of the world—twelve time zones away from home—could lose two seconds in the fifteen-hundred-meter race and seventy-five seconds in a marathon. For elite women hockey players traveling from Australia to Europe, crossing six time zones, full recovery time on sprinting tests took eight days. Even relatively short flights of three hours (across three time zones) have been shown to affect performance in American football, basketball, hockey, and baseball.
I’ve touched on the ways in which the effectiveness of eating, exercise, and sleep in maintaining optimal health and vigor is dependent on our bodies’ natural rhythms. You can see that they are connected through circadian rhythms; their decoupling can cause problems, especially in old age, when our bodies are less resilient. In the next three chapters, I’ll double-click on these three key components of our daily lives to reveal more of what we know about optimizing and tweaking them to give us maximum benefit.