IN THE FIRST CHAPTER, where we started from the empty plate that the cook seeks to fill, I barely touched upon the question of consistency, ignoring gases and considering only liquids and solids. But these are crude notions. Cooks know perfectly well how to make things that are much more interesting than ordinary liquids or common solids: emulsions, sauces of various kinds, pastries, and so on. Between solid and liquid an entire continent remains to be explored—an almost infinite number of variations waiting to be discovered by anyone who is willing to go to the trouble of producing them. This is a truly magnificent opportunity, for we have seen that a dish is created by assembling masses having not only different shapes, but also different consistencies. The art of combining them in a harmonious whole accounts for a large part of the happiness we feel when we eat food that has been expertly prepared.
Recall that our senses evolved in such a way as to perceive contrasts. Earlier, in discussing shape and form, I should perhaps have mentioned that David Hubel and Torsten Wiesel were awarded the Nobel Prize in Physiology in 1984 for their work exploring the mechanisms of visual recognition and, in particular, for explaining how the brain is able to recognize edges in images—a topic of more than passing interest to cooks. In the chapters that follow, we shall look more closely at taste, odor, trigeminal sensations, and color. For the moment, however, let us examine the culinary prohibition against serving a dish that has a homogeneous or uniform consistency. The reason for this, of course, is that the crispness of the skin of a roasted chicken is indispensable if the tenderness of the meat itself is to be fully appreciated; so, too, carving a roasted leg of lamb at an angle perpendicular to the bone, in the French manner, presents a whole palette of contrasting colors and textures, from the crispy brown of the exterior to the bloody red of the meat nearest the bone. But there is much more to the matter than meets the eye.
It is perhaps not surprising that it is mainly scientists studying the appearance of foods who maintain that this aspect is preponderant in the experience of eating (measurably so, some of them would even argue). Most cooks would say that the odor of a roasted chicken that permeates the kitchen precedes the sight of the crispy golden skin and that we salivate when we smell the odor of baked cheese, not when we look at it. This may well be true. But if the color of a wine or the visible texture of a food leads us to anticipate a taste or a flavor that turns out not to be present (or, in the extreme case, makes us believe that something soft is really hard), the confusion lasts only for a few moments. During the course of tasting, whatever false expectations we may have formed on the basis of visual inspection are quickly dispelled as a result of the perception of consistency by the teeth (which sense degrees of pressure) and by the tongue. Together they set us straight by identifying the nature of the object being consumed and, crucially, by releasing sapid and odorant molecules. Indeed, when a dish is really good, one closes one’s eyes in sheer delight, dispensing with visual perception altogether and concentrating on exactly that part of the flavor that is devoid of it. Consistency is an essential element of our appreciation of food, as cooks well know. This is why they are so concerned with cooking times: leave a pan on the stove a moment too long, or remove it from the flame a moment too soon, and the dish will have the wrong consistency.
A word or two of explanation may be in order here. Some readers will wonder why I have so far spoken mainly of “consistency” rather than of “texture.” The short answer is that these terms refer to different things. Because we modify a food as we eat it, a single consistency can assume a number of different textures. The situation may be likened to what happens when you dive into a swimming pool. If the entry is clean, almost noiseless, you pierce the water, causing it to part smoothly in front of you. But if the dive is misjudged, your body lands on top of so great a surface that the water doesn’t have time to part, because water cannot move faster than the speed of sound in water—a bit like an airplane that cannot break the sound barrier. In either case, however, whether the dive is perfectly or disastrously executed, water is water, and its consistency remains unchanged. Yet the same water, which seems liquid in the case of a clean entry, seems hard as cement in the case of a belly flop. We must distinguish, then, between the consistency of water, a characteristic that is independent of our perception of it, and our perception of the water’s consistency, which is to say its texture.
The same thing is true of foods. Here again we must distinguish between consistency, which is a consequence of their molecular structure, and what we actually perceive, which is their texture. Mayonnaise, for example, seems thick if it is eaten slowly, whereas it seems fluid if we wolf it down. In this chapter it is consistency that we are concerned with—and, in particular, the question whether consistency is a primary, or instead a secondary, component of the pleasure of eating. Almost thirty-five years ago, in 1979, two American physiologists, H. G. Schutz and O. L. Wahl, published the results of a study purporting to show that consistency is unimportant. Readers should have been skeptical from the first. After all, why should cooks have worked so hard for so many hundreds of years to get the consistency of roasted meats just right? Why should they have taken such great pains to invent and prepare so many subtly different sauces?
Respondents in Schutz and Wahl’s study were asked to rank the relative importance of the characteristics of appearance, flavor, and consistency (which they called “texture,” but which should, for the reasons I have just given, be called “consistency”) for ninety-four products. These products had been chosen with reference to four criteria: they were easily obtainable in supermarkets; they were available in a form that made them easy to consume; they were widely consumed; and they were consumed without any preparation or admixture with other foods. The study was done in and around Sacramento, California, by means of questionnaires sent by mail: after two reminders, 994 of 1,000 questionnaires sent had been returned, but only 420 of them had been correctly filled out. This alone is enough to suggest how doubtful the results really were. Once the results had been tabulated, it became clear that odor and flavor were unambiguously ranked ahead of appearance and consistency: more than nine persons out of ten assigned greater importance to flavor than to any of the other criteria for each of the ninety-four products.
But wasn’t this a foregone conclusion? Since we are incapable of discriminating between the various sensations that make up the flavor of the foods we eat, flavor is the only thing we have to go on. A study establishing the preponderance of flavor in gustatory perception is quite ridiculous: it amounts only to demonstrating that flavor is something more than its various components—that the whole is greater than any one of its parts.
The reason we are unable to isolate the various sensations we detect while we are eating is that these sensations interact with one another. A simple experiment will illustrate the point. Put on a headset with earphones and a microphone, then connect the headset to a computer so you can hear the sounds that are transmitted and processed by the volume-control program. Now eat a flaky, freshly baked croissant, for example, adjusting the volume control as you chew: the croissant will seem to have more of a crunch to it the higher the volume, less of a crunch the lower the volume—proof that our perception of a food’s consistency, its texture rather than the consistency itself, depends in part on the noise a food makes when it is chewed.
The study by Schutz and Wahl is vulnerable to another objection, that it was uncontrolled by any experiment that might have served as a safeguard against baseless conjecture and reckless misinterpretation. (The wonderful thing about the experimental sciences, by the way, is that they prevent us from claiming whatever we like in the absence of corroborating evidence.) A study of consistency done without any product actually being chewed, and without any qualitative analysis of the results of mastication, can hardly be taken seriously. Worse still, Schutz and Wahl failed to control for a well-known bias in sensory research. Subjects who take part in tasting studies often answer the questions put to them by experimenters in a way that conforms to their own preconceptions. If, for example, they believe that consistency is less important than flavor, they are likely to give more weight to flavor in their responses. But let’s face it: the reality is that people do in fact like and buy many products—crackers, pâtés, and so on—for their consistency.
How much importance should be attached to consistency, then? For a long while it was accepted that solid foods act on appetite by means of a sort of digestive conditioning: because they take longer to be digested and absorbed than an equivalent amount of liquid derived from them, we have the impression that they satisfy physiological needs more fully. Experimental tests of this hypothesis were few, however, and most of these suffered from the same methodological defect I just mentioned (technically known as omitted variable bias). A 1978 study comparing an orange and a comparable quantity of orange juice, for example, concluded that the orange was more filling. Alas, the authors failed to take into account the fact that an orange contains forty times more pectin, which reduces gastric evacuation and thereby contributes to the sensation of satiety. To do the experiment properly, they would have had to compare an orange and a quantity of juice having the same pectin content.
A far better-designed study was conducted more recently by Hélène Rosier-Labouré at the European Centre for Taste, Food, and Nutrition Sciences in Dijon. Rosier-Labouré and her team studied the short- and long-term effects in rats of consuming two versions of a soup consisting of meat, beans, cream starch, and water. The soup was presented in two “textures” (more accurately, as I say, consistencies), one a rough mixture of meat and vegetable matter, the other a purée of the same ingredients. Over the course of three weeks the rats consumed three times more of the chunky version than of the puréed version: not only did they eat the first kind more frequently, but their consumption per meal was greater as well.
This short-term result seemed to contradict the idea that foods are perceived to be more filling the more chewing they require. But it might also be the case that chunky soups are more palatable than puréed soups. Because postingestive effects are typically observed that do not appear in short-term studies, however, the experiment was repeated for a period of six weeks, this time comparing the behavior of rats offered only the chunky soup with ones that were offered only the puréed soup or else the soup of their choice. Over this longer term, the rats fed with the puréed soup ate more than the rats fed with the chunky soup, whose intake declined beginning in the third week.
Are these same effects found in humans? In a related set of experiments, Rosier-Labouré studied the feeding patterns of twelve subjects, which seemed to indicate that the hedonic effect (the appeal of the meal in and of itself, which is to say its flavor) was negligible, whereas there were significant differences in the duration of eating, with the chunky soup being chewed for a longer time—logically enough—than the puréed soup. This observation could be interpreted just the opposite way, however. It may be that the longer chewing time corresponded to the detection of greater flavor.
All of this is very well and good, you may say, but surely cooks do not need food science and technology to tell them that the flavor of foods depends in part on their consistency—it should be obvious to anyone who knows what these words mean!
Let us therefore start all over again, this time by eating an apple. We discover that a variety of sensations come together: taste, temperature, texture, odor, color, and so on. But while we do indeed perceive each of these things, we find it very hard to isolate them. We register a synthetic, or general, sensation: the apple’s flavor (or, more precisely, the flavor of this particular apple). We can try to try to determine if the taste is sweet or sour, for example, but we cannot consider it independently of the odorant compounds that are released by the destructuring of the apple’s fleshy tissue when we chew, rising up from the rear of the mouth into the nose via the retronasal passages (the same passages that carry water from the mouth to the nose when accidentally we get a gulpful while swimming). When we eat, in other words, we detect odors; we do not perceive taste alone, we also perceive a food’s smell. The same is true for consistency, the perception of which is complicated by taste and smell.
It is therefore a very clever person who can say what the true consistency of a food is. Texture presents no problem: it is whatever we perceive when we bite into something. But consistency is something different. To say that the flavor of foods depends on their consistency is trivial, then, a sort of pleonasm. Nevertheless it is true that meat juices acquire a special flavor when they are thickened to make a sauce. It is true, too, that a sorbet may taste sharp or dull depending on whether it is made with an ice cream maker or with the aid of liquid nitrogen, that a sour mayonnaise loses its harsh edge with additional beating, and that jelly loses its flavor when it becomes sticky—that is, when it has too much pectin or other gelling agent, which is what causes it to harden.
For centuries, mastering consistencies was a matter of trial and error. Traditional cooking long ago discovered that the gelatin extracted by heating bones and meats in water was useful in making stocks that themselves could be reduced further to form glazes. Without knowing it, cooks did three essential things: they dissolved the protein known as collagen, which gives meats their toughness; dissociated the three molecular strands that make up collagen; and then concentrated these strands. In this way it became possible to impart viscosity to liquids, bind odorant molecules together, trigger reactions that produce such molecules, and emulsify the fatty matter (usually butter or cream) used to thicken sauces. If all these wonderful discoveries were able to be made through the patient accumulation of empirical knowledge alone, how many more are there waiting to be made by cooks who have a theoretical understanding of the physicochemical basis of these phenomena?
Pastry chefs, for their part, learned long ago to cook fruits in order to extract pectin, which they then used to make jams and jellies set. But how much better these same jams and jellies were once the underlying chemistry was known! Pectin molecules are present in fruits that have reached a certain stage of ripeness. Along with cellulose molecules and other polysaccharides, they constitute the cell walls of most plants. Heating fruits in liquid causes the pectin molecules to dissolve, forming a hot syrup; as the syrup cools, the molecules extracted in this way become linked together and form a gel so long as the sugar concentration remains within a range of 55 to 75 percent. The practical expertise developed by jam makers over the centuries nevertheless falls short of what we want to know. Modern chemists discovered that different pectins have different gelling properties. It had long been understood, of course, that when jams are made in copper pans, the copper produces a firmer gel by binding the pectin molecules together (over time copper becomes toxic, however, and can be substituted for by calcium, which has the additional advantage of strengthening our bones). Commercial research on thickening, gelling, and texturizing additives in recent years has shown that some pectins are esterified, which is to say that they contain methyl groups (groups of one carbon atom and three hydrogen atoms) that modify gelling properties. More remains to be learned about these properties.
But do we have the right, as purists who truly love food, to add pectins to jams? By all means! Cookbooks have long recommended doing just this, particularly in connection with certain kinds of cherry jam: in the event the jam fails to set, the cook is advised to put a muslin or cheesecloth bag containing apple skins and seeds (which have a high pectin content) in the pan. The very same expedient is resorted to today in the commercial manufacture of gelling agents, where the skin extract of apples and citrus fruits is used to produce pectins of various qualities for both home cooks and the food-processing industry.
Pectins and gelatins are not the only possible gelling agents. Manufacturers of emulsifying, thickening, and gelling additives have refined traditional methods for modifying textures, with the result that the dairy industry is now able to make Chantilly creams and other products that withstand the rigors of long-distance transport far better than they did in the past. With the addition of emulsifying compounds to augment the expansive (that is, foaming) properties of cream, and of thickening compounds to stabilize the cream, dairy products that are shaken in the course of handling and shipping no longer separate.
Today the variety of such additives is rather extensive: gum arabic, extracted from the acacia tree; carob gum and guar gum, extracted from seeds (polysianes, which prevent infants from spitting up, are derived from carob gum, used for this purpose since ancient times); fruit pectins; alginates and carrageenans, extracted respectively from brown and red algae; gelatins, extracted from animal bones and hides; various emulsifiers obtained from natural fats by distillation or from natural fats and sugars by chemical reaction, or by extraction from soybeans; xanthan gum, secreted by bacteria; and so on. The opportunity of creating new and varied consistencies that all these compounds present is one that cooking today can no longer afford to neglect, especially in view of the abiding obsession with making foods lighter—chiefly by increasing their water and air content. Nothing, after all, is lighter than air!
The roots of this tendency go back to the early twentieth century, when sauciers began to replace flour, disliked for its blandness and heaviness, by pure vegetable starches, which do a better job of thickening cold sauces. And yet these starches do not wholly remove the problem of starch retrogradation, as it is known, which occurs when moisture seeps out from a sauce as the starch molecules slowly combine, forming a crystal that displaces the water in the starch. A little later chefs perfected the art of making a sabayon, where the thickening action of the egg yolk depends on the formation of microscopic aggregates in the liquid (classically, a sweet wine) in which it is heated. Then came the fashion of nouvelle cuisine, in which sauces were thickened still more gently, particularly ones whose viscosity in traditional preparations is due to the high concentration of gelatin, slowly extracted by long cooking from meat and the bones to which it is attached.
Let’s be frank: for quite a long time now, French cuisine has been beating around the bush. Why shouldn’t we go directly to the heart of the matter and thicken sauces by using the various compounds that are now available to us in order to obtain new and, above all, more varied consistencies than before? Why shouldn’t we harness recent advances in food technology, which already has managed to resolve a number of long-standing problems? In the past, cooks often found that in trying to make solid foods from liquid milk, the milk turned sour. Today, by selecting more suitable gelling agents than gelatin, this form of disaster can be avoided. The same goes for the gelification of wines, which no longer become cloudy once gelatin is replaced by agar-agar, for example. There is no reason that culinary artists should be deprived of simple tools of this sort for creating new dishes. As Brillat-Savarin famously and rightly said, “The discovery of a new dish does more for human happiness than the discovery of a star.”
To say that note-by-note cooking does nothing more than combine compounds is therefore mistaken. One also hears it said that this style of cooking has no consistency, that it’s liquid. The same criticism was leveled, no less unjustly, against molecular cooking, which some food critics reproached for its reliance on gels and foams. Understanding why this objection is groundless will help us avoid falling into still more serious errors later.
To accuse molecular cooking of being soft because it uses gels is a sign of great ignorance, because meats, fish, vegetables, and fruits are gels. Gels, by definition, are solid systems that contain a dispersed liquid. Animal tissues are mainly (50 to 90 percent) made up of water, which does not leak out because it is trapped in cells known as fibers. The fibers are aggregated in bundles, which themselves are grouped together in larger bundles, and so on in still larger and larger bundles until they form muscles. Trapped in muscle fibers, the water in meat is released only when, in the course of chewing, our teeth disorganize the tissue—allowing us to perceive juiciness. The same is true of fruits and vegetables, whose tissues are composed of more or less parallelepipedal cells that trap a considerable amount of liquid as well. Meat, fish, vegetables, and fruits, whether they are raw or cooked, are gels; indeed, virtually all traditional cooking is done with gels. This is why I say that some critics of molecular cooking are uninformed.
They are right, however, to have complained that some chefs, in their boundless enthusiasm for whipping siphons and gelling agents, have abused foams and gels, just as in the era of nouvelle cuisine there were those who delighted in undercooking vegetables and serving unreasonably small portions, forgetting (as Brillat-Savarin also remarked) that the appeal of even the most delicious rarity is less the less of it there is. Still, not all molecular cooks are maniacal proponents of these novel devices and ingredients, and I can testify from personal experience that there is quite enough to chew on at the tables of those moderns who know how to manipulate consistencies in interesting ways. Besides, in the unending quarrel between ancients and moderns, the moderns are bound to win because the ancients will always be the first to die. Molecular cooking is in any case not going to go away anytime soon: alginates, carrageenans, and other gelling compounds are now widely available; siphons are sold even in supermarkets today; and as for gels, well, it would not be going too far to say that they are our daily bread—bread, from the physiochemical point of view, is an expanded gel.
Whether what we are making is a gel or something else is less important than exploring different consistencies, hard and soft, tender and tough, moist and dry, flaky and sticky, crunchy and crispy, and so on. The mistaken belief that note-by note cooking must be soft, even liquid, seems to have something to do with the fact that, in the expression “mixture of compounds,” the term mixture makes some people think of a substance that is not structured. And yet mixing cement and sand makes a very hard mortar; in the kitchen, mixing the white and yolk of an egg makes something solid once it has been cooked. There is no reason why mixing powders and liquids must necessarily produce a liquid. If you mix sugar—sucrose—and water, a liquid syrup forms, but if this syrup is heated (note-by-note cooking, no less than traditional cooking, depends on the mastery of fire), the water evaporates; and once the cooking temperature exceeds 127°C (about 260°F), drizzling the hot syrup onto an unheated marble surface produces a very hard solid. Does anyone think that a dragée made with hardened sugar is either liquid or soft?
It must be admitted, however, that the expression “mixture of compounds” fails to convey the idea that the intelligent cook is obliged to order, organize, and build. The great Marie-Antoine Carême (1784–1833), cook of emperors and emperor of cooks, saw that cooking has much in common with architecture—even if culinary constructions are to be eaten, not merely admired.
With note-by note cooking, the methods developed by Carême have at last been fully extended to the molecular level: the truly modern cook will construct his materials, organize the materials he has constructed, and, finally, assemble and arrange these materials in the form of dishes. We are now going to see how.
There are many sorts of consistency, ranging from nonviscous liquids to perfectly hard solids. But even though this may seem to suggest the existence of a continuum of states, consistency cannot be reduced to a degree of hardness that becomes progressively greater or less, for consistency exists in several dimensions.
Keep in mind, to begin with, that the majority of traditional foods are made of colloidal material in which various phases are dispersed in other phases. The meaning of the terms
colloidal and
phase will become clear if we consider the air in a sealed container. Air is a transparent phase (or type) of matter, invisible to the naked eye. Let’s therefore use a magnifying glass, then a microscope—if possible, a mesoscope as well, even a nanoscope—to examine our sample at ever greater scales of magnification. Ultimately what we find is a gas that is rather empty, but yet not altogether without molecular motion. A few numbers will help to focus our thinking. A liter of air at ambient pressure and temperature contains about 1.25 grams of matter, mainly dinitrogen, each molecule of which consists of two nitrogen atoms. These molecules are perpetually in motion, a little like billiard balls, and, on average, any two molecules are a little more than twenty molecular diameters apart from each other.
A liquid phase such as water is more condensed than air, which is to say that the molecules in it are closer to each other. Most solid phases are denser still, with a greater number of possible molecular structures, as we will see later on. For a physicist, the chief difference between liquids and solids has to do with their respective flow properties: a solid preserves its shape when it is set on a surface, whereas a gas or a liquid flows, assuming more or less quickly the shape of the container in which it is placed.
PURE SOLIDS
Here, since we are interested mainly in consistency, let’s consider solids with regard to their behavior in the mouth. A solid, from this point of view, is a piece of matter that is more or less easily deformed and fragmented. The simplest solids to study are those that are deformed in proportion to the force that is applied to them.
The phrase “in proportion to” may seem clear enough, but to a physicist it is hopelessly vague. From everyday experience, we know that the greater the pressure applied to a solid, the more it will be deformed. A physicist, however, has something more specific in mind: if one applies twice the pressure, the deformation is exactly two times greater; three times the pressure, it is exactly three times greater; and so on. When a physicist speaks of proportional behavior, he really means it. Perfectly proportional behavior is nowhere to be found, of course, at least not in the world we know. But certain solids are nevertheless more perfect than others in this respect. Two basic kinds may be distinguished, Hookean solids and non-Hookean solids.
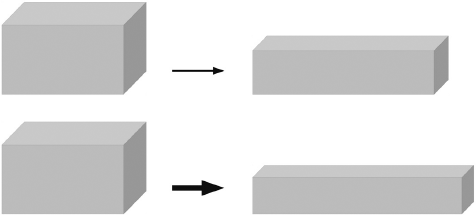
Hookean solids are ideal solids named after the seventeenth-century English physicist Robert Hooke (1635–1703), who studied the deformation of solid objects, in particular elastic solids that revert to their original shape after being deformed. Not only are Hookean solids deformed in proportion to the force that is applied to them, they also recover their initial form when compression or extension ceases. Their behavior under compression can be described by a single number, the so-called modulus of elasticity (also known as Young’s modulus after another English physicist who came after Hooke, Thomas Young). This number expresses the limit of proportionality between stress and strain—that is, between the amount of force applied and the degree of deformation.
Examples of Hookean solids in cooking are at once innumerable and exceedingly rare: innumerable because every (or almost every) solid behaves in Hookean fashion when a small amount of force is applied to it; and exceedingly rare because the force exerted by our teeth in chewing is so great that it actually tears apart the solids that we put in our mouth. Moreover, the food we eat seldom has the form of a solid compressible block. The few Hookean solids we do eat are typically broken up into particles, if not actually crushed into powder, so that we do not break our teeth on them. Sugar is a classic example.
If Hookean solids are rare, then naturally non-Hookean solids must be common. Gelatin gels, meats, and vegetable tissues give a partial idea of the range of possible behaviors, from a solid that is more and more easily deformed the more pressure is applied it to one that, by contrast, is deformed less and less easily as the force of compression increases. If you find it difficult to imagine some of these behaviors, think back to the perfect dive and the belly flop: what is true of water is true of solids to a certain extent, especially in the case of gels, many of which consist chiefly of water. (For a physicist, by the way, an aqueous solution—obtained, for example, by dissolving a pinch of salt in a glass of water—is “water.” It is not pure water, of course, but the amount of salt is so small that the behavior of the liquid, from the mechanical and rheological point of view, resembles that of water. We may think of it as flavored water. Similarly, melting a fatty substance produces what a cook is accustomed to think of as “oil.” I therefore use the term oil to refer to any melted fat.)
An entire book might usefully be written about the deformability of solids in the mouth and the way in which they are broken up, for this is what happens when solid matter is placed between the teeth: it is divided, divided again, and then again and again until an equilibrium is finally established between the size of the fragments and the release of saliva, which coats the fragments, facilitating their passage from the mouth to the esophagus. Note that flavor has its origin in this fragmentation. Before a food is swallowed, some of the water-soluble compounds it contains diffuse in the saliva and dissolve (saliva being an aqueous solution of proteins and other compounds). A fruit jelly, for example, which consists mainly of sucrose dissolved in water that has been converted into a gel by the addition of pectins, releases sucrose molecules that dissolve in the saliva. Once these dissolved molecules reach the surface of the taste buds (technically known as papillae), they bind with the molecules of a particular class of proteins known as receptors. The binding of a sucrose molecule to a receptor causes electrical currents—nerve signals—to be propagated to the brain, where they are associated with the sensation of sweetness.
Compounds that are immiscible (that is, not mixable) in water follow a different path. Citral, which has a lovely lemon odor, is one such water-insoluble compound. Instead of dissolving in saliva, its molecules evaporate in the form of gas in the mouth. From there they rise up into the nose, where they bind with olfactory receptors. In either case, the longer one chews, the more intense the sensation. This is why people who eat quickly take less enjoyment from the bioactive compounds present in foods than people who eat more slowly. Their loss—if the food is good!
For the same reason, the friability (or crumbliness) of the solid foods we eat is important. A piece of food that is broken up into a myriad of smaller fragments, each of which releases sapid and odorant compounds, will seem more flavorful than one in which these compounds have a harder time escaping, unless we go on chewing for a longer time. I shall have more to say about all this when the time comes to discuss what may well be called, following Carême, the “architecture of flavor.”
Why is a solid more or less solid? So far we have looked at Hookean and non-Hookean solids from the point of view of the physicist, who sees only one aspect of the world. At this juncture it will be helpful to have the perspective of the chemist, for whom the world cannot be reduced to a relatively small number of general laws. The chemist sees it instead as a vast collection of physical systems, each with its own distinctive pattern of atomic and molecular behavior. To oversimplify: the physicist, who believes in the existence of overarching laws that apply urbi et orbi, finds to his dismay that he is contradicted from time to time by the particular behavior of different kinds of matter; the chemist, on the other hand, starts from just this, the particular, on which he performs experiments, without, however, always managing to formulate the sort of grand theory that would earn him the fame that is reserved for the most eminent scientists. These two points of view can be reconciled in the form of physical chemistry, which moves from the macroscopic to the microscopic level, starting with a solid object, for example, and proceeding to analyze its molecular and atomic chemistry.
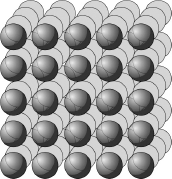
Let’s start by looking at a small heap of granulated sugar. It consists of tiny objects that can be clearly seen only with the aid of a magnifying glass. Individually, the grains are transparent: it is the reflection of white light on the faces that makes them appear white; the smaller the grain, the more numerous the reflections. In speaking once again of “faces,” I am referring to the fact that the magnifying glass allows us to see that the grains are not rounded, but instead have flat surfaces. An even better way of viewing these facets is to make a large single crystal (or “monocrystal”). Stick a sugar crystal to the end of a thread, dip it in a solution of sweetened water, and let the moisture slowly evaporate. After a few days, you’ll wake up to find large a sugar monocrystal with quite visible and regular faces. This monocrystal is formed by the accretion of sucrose molecules along each of the three spatial dimensions, stacked on top of one another like the squares of a series of superimposed checkerboards.
Now dissolve some sucrose in water and heat it in order to eliminate the water. Then, without degrading the sucrose molecules, drizzle the syrup onto a room-temperature marble surface. The abrupt cooling prevents the molecules from moving rapidly enough to become regularly stacked: they remain fixed in the same disordered positions they occupy in the liquid, only now the liquid is a solid. The result is a substance not unlike ordinary glass in a window pane. Because the sugar glass obtained from concentrated syrups crystallizes after a certain time, candy manufacturers use anticrystallants such as glucose to prevent this from happening.
The fact that solids can be either crystalline (exhibiting a regular molecular structure in three dimensions) or amorphous (exhibiting a random molecular structure) does not explain why solids are hard or soft, however. To answer this question we must consider the forces that operate among individual molecules. In a sugar crystal, for example, the sucrose molecules are held together by forces much stronger than the ones that hold triglyceride molecules together in crystals of cooled oil (think of the crystals that appear in bottles of oil stored in unheated rooms during the winter).
Sugar crystals are a type of molecular solid, made of stacked molecules. Salt crystals form another type, the so-called ionic solids. In these crystals, chlorine and sodium atoms are very powerfully bound together, having exchanged an electron (the sodium atom, which normally has eleven such charged particles, gives up one of them to the chlorine atom, which normally has seventeen). The effect of this exchange is to create a stacked molecular structure in which atoms of each kind alternate.
Then there is the class of substances known as polymers, in which so-called covalent bonds assemble the atoms of small molecules into very large, macroscopic molecules. Polymers have a number of interesting and distinctive properties that can be exploited by cooks to create contrasting consistencies. One example is a dish to which I gave the name “Sens dessus dessous” (after Jules Verne’s story “The Earth Turned Upside Down”). It is based on a very simple idea, that when we eat we are aware chiefly of the surface of foods. (I applied it to the case of a liquid foam and two solids, but it works with two juxtaposed solids as well.) Begin by making a sort of sandwich consisting of some whipped cream placed between two thin pieces of chocolate. When you bite into it, your teeth come into contact first with the hard chocolate layers. Since our teeth are pressure sensors, we have a sensation of hardness despite the gradual softening of the chocolate by the cream. Now, using the same ingredients, see what happens when you put a piece of chocolate in between two layers of cream. Your initial impression is that the preparation is soft—an impression that is not contradicted when you finally bite into the chocolate. The surface of a food is what captures our attention. Even if cooks limit themselves to solids, they will have no shortage of opportunities for experimentation. After all, there are so many different kinds of hardness!
PURE LIQUIDS
Liquids are no less varied and numerous than solids. Everyone knows that some liquids flow easily (water is the most obvious example), and others less easily (think of honey). What is less well appreciated is that viscosity, a familiar characteristic that physicists have transformed into a quantitative concept, is something more than a parameter of proportionality between gravity (that is, the force that causes a liquid to flow) and the rate of flow. Once again this notion of proportionality—unavoidable in categorizing a great many physical behaviors!
Fill a container with liquid, and on the surface put a thin plate. If you push the plate sideways, the liquid on which it is floating is carried along with it on account of the bonds formed between the molecules of the plate and the molecules of the liquid, as well as among the molecules of the liquid themselves. Obviously if the liquid is nonviscous, like water, friction among the molecules of the liquid is minimal, and only a weak force is needed to move the plate downward. In the case of honey, a stronger force is needed.
Recall once again the comparison between a perfect dive and a belly flop. The rate of speed at which we try to drag the plate is a factor: if the speed is great enough, the water’s behavior is no longer that of a liquid, but that of a solid. In other words, while proportionality obtains so long as the plate is dragged over the surface at low speeds, proportionality is lost past a certain threshold. Still, the behavior of most liquids, when they are made to move at very low speeds, can reasonably be called “perfect” or “Newtonian.”
TEMPERATURE (°C) |
DENSITY |
KINEMATIC VISCOSITY
10-6 m2/s |
|
5 |
1.000 |
1.520 |
10 |
1.000 |
1.308 |
15 |
0.999 |
1.142 |
20 |
0.998 |
1.007 |
25 |
0.997 |
0.897 |
30 |
0.995 |
0.804 |
35 |
0.993 |
0.727 |
40 |
0.991 |
0.661 |
50 |
0.990 |
0.556 |
65 |
0.980 |
0.442 |
|
Source: Hydraulic Models (ASCE Manual of Engineering Practice No. 25), reproduced in Ranald V. Giles, Jack B. Evett, and Cheng Liu, Fluid Mechanics and Hydraulics, 3rd ed. (New York: McGraw-Hill, 1994).
NEWTONIAN LIQUIDS
In the kitchen, as elsewhere, Newtonian liquids (more or less sugary syrups are perhaps the most common instances) are defined as liquids for which deformation is proportional to force. This simplicity is rapidly complicated in cooking, however, because viscosity varies with temperature. In the case of water, for example,
table 2.1 shows how density (which makes it possible in principle to create layered cocktails and other liquid preparations, with the denser layers lying beneath the less dense ones) and viscosity (more formally, kinematic viscosity) vary.
Here the temperature is given in degrees Celsius, a unit familiar to most cooks. Density has no unit because it is determined in relation to the density of water at a temperature of 4°C (39.2°F), and the unit of viscosity has no importance in the present context. Only the observed variation matters: when water at room temperature is heated to more than 60°C (140°F), the viscosity is roughly halved. In cooking, the same phenomenon is encountered in many other liquids apart from water, as every chef is well aware.
NON-NEWTONIAN LIQUIDS
Alongside Newtonian liquids there are more complex liquids, such as mayonnaise and (among nonfood products) modern paints. Mayonnaise is fluid in the mouth because it is made of liquid oil droplets dispersed in a slightly acidified aqueous solution. At rest the sauce is firm, however, to the point that a spoon inserted in it stands up, and a dollop of mayonnaise put on a plate seems solid (even if it flows when stirred). The same property is exploited in paint manufacture. Paints today are made to flow easily enough that they can be smoothly rolled on a wall, for example, but not so easily that they drip when rolled on a ceiling.
Mayonnaise and modern paints are examples of what are called rheofluidifying liquids: the prefix
rheo- means to flow, and
fluidifying means that the liquid becomes less viscous as it flows; indeed, the more rapidly such liquids are made to flow, the more fluid they become. This is obviously a wonderful property in cooking because in the mouth a quasi-solid suddenly acquires a marvelously smooth quality. Cooks are nevertheless wise not to rely on this effect too often. We also like to eat foods that we can bite into, something with a crunch to it, which is why they should also investigate the properties of rheothickening liquids. Here again
rheo- refers to the fluid behavior of the liquid, only now it becomes thicker when it is shaken or stirred. In cooking, this effect may be produced by mixing corn starch with water, for example. In sufficiently great concentration (a few tablespoons of water for 100 grams of corn starch), the liquid behaves in a quite remarkable way. If you happen to have a wading pool and enough corn starch, see what happens when you pour some in the pool: walk slowly through the mixture, and it behaves like an ordinary liquid—your feet sink into it; try to run, however, and the pressure exerted by your legs is now much greater, causing the mixture to seem like an impenetrable solid—you have the feeling you’re walking on water! Or try shaping a rheothickening liquid into a ball and throwing it up into the air: if you catch it and then immediately throw it back up in the air, it behaves like a solid; so long as it is kept constantly in motion (in a game of catch, for example, or by juggling), its appearance remains that of a ball. But if you throw it to a friend without warning him beforehand what sort of ball it is, he will catch it without throwing it back, and the ball will suddenly turn back into a liquid and run all over his fingers!
Not all liquids are as simple as Newtonian liquids, then. Let’s leave it at that for the moment, while taking due note of the fact that they can be used to make striking contrasts. Compare a cup of normal drip-brew coffee and a cup of espresso: the presence of a foam on the surface of the espresso by itself makes an immense difference, even though the foam is made of a gas and a liquid.
COLLOIDS AND DISPERSED SYSTEMS
In the preceding section the temptation to complicate the discussion of liquids was great—and indeed I succumbed to it twice: first by mentioning mayonnaise, in which droplets of oil, a liquid, are dispersed in a liquid phase constituted by egg yolk and vinegar (no mustard in a mayonnaise, by the way, since in that case it becomes a remoulade), and then by imagining a game of catch with a ball made of corn starch mixed with water.
We saw that the behavior of these systems corresponds to that of a liquid or of a solid depending on the proportions of the various phases. If a mayonnaise is liquid when the proportion of oil is small, as you begin to make it, it becomes firm enough to cut with a knife as it approaches the limit at which the emulsion breaks down (because the egg yolk can’t absorb any more oil). Let’s take a closer look, starting with a pure liquid—that is, a collection of identical molecules in which attractive forces are not strong enough to hold them in place. Then let’s dissolve more or less large objects in it, everything from ions (as in the case of salt) to small molecules (sucrose, for example) and then large molecules (not only the soluble component of starch, amylose, but also proteins). These larger molecules are of special interest because they don’t quite dissolve; instead they disperse in the form of particles of differing size. As in the case of corn starch, the consistency will completely change depending on the various phases of a system and on the nature and size of the dispersed particles.
Particles? Flour consists of particles—granules. Oil droplets in an emulsion are no different. Now, when such objects are dispersed in a liquid, one obtains a colloid. Historically, the term colloid has been used to refer to gels. We shall soon see, however, that gels, emulsions, foams, and aerosols are all closely related. And just as liquids display behaviors whose variety makes the world of sauces a realm of marvels, and just as pure solids exhibit a great diversity of mechanical characteristics, so too colloids inhabit their own world of physical properties. With regard to the fluid mechanics of colloidal substances, in particular, there is a whole range of behaviors between the viscous and the elastic—whence the term viscoelasticity.
In exploring this world it will be useful to follow the example of the French philosopher Abraham Moles (1920–1992), who devised a general method of analysis based on what he called “invention matrices,” or tables. A table is a marvelous thing, because each cell is an empty space asking to be filled. Up until now we have considered solids, liquids, and gases. The following
table (2.2), in which the forward slash / means “dispersed in,” summarizes the result of dispersing each of these phases in another phase.
Although it may not be apparent at first sight, the information in this table needs to be restated. Consider the advances in mathematics made possible by the new formalism introduced by Descartes and Leibniz in the seventeenth and eighteenth centuries. Before them, in dealing with equations, it was customary to say, for example, “The square of the unknown multiplied by five and added to the unknown multiplied by three and to the constant two is equal to zero.” Today any high school student knows how to express this idea more concisely: 5
x2 + 3
x + 2 = 0. Similarly, the father of modern chemistry, Antoine Laurent de Lavoisier (1743–1794), simplified the language used to describe reactions. Instead of writing, “Ferrous chloride reacts with sodium hydroxide to form ferrous hydroxide, which precipitates,” one now writes, more concisely: FeCl
3 + 3NaOH Fe(OH)
3 + 3Na
+ + 3Cl
−.
We can do much the same thing. Rather than waste time spelling out the word
gas, let’s simply uppercase the initial letter (G); so too in the case of the terms
liquid (L) and
solid (S), as in
table 2.2B. Furthermore, the example of mayonnaise reminds us that we should distinguish water and water-based liquids (W) from oil and other melted fats (O). Using these extra letters, we can enrich the informational content in
table 2.2A, giving a more precise description where before there was only L.
By a simple manipulation of symbols, then, it becomes clear that in
table 2.2A we had omitted to indicate that aerosols can be made from both oil-based and aqueous solutions, that gels can be made from both oil and water, that suspensions can be created in both oil and water, and so on.
Moreover, dispersions of different phases in a single phase can be indicated using the plus sign, as in the expression (G + O)/W, which means that both gas bubbles and oil droplets have been dispersed in an aqueous solution. How? For example, by beating an egg white (an aqueous solution that is roughly 90 percent water and 10 percent proteins) with oil: the whisk divides the oil into droplets while at the same time introducing air bubbles. Thus one obtains a system that might be called either “foamy emulsion” or an “emulsified foam.” Neither one of these names is quite appropriate, however, since each assumes a principal system and a secondary system. The formula (G + O)/W does not have this disadvantage: the order of the terms within the parenthesis is determined by an arbitrary rule of alphabetical sequence.
The symbols / and + are called operators. For purposes of geometrical description, it will be helpful also to use the symbol @ to designate inclusion, the symbol σ to designate superposition, and the symbol × to designate imbrication (which is to say the interpenetration of two continuous phases, as in a gelatin gel or a jam). Gels of this kind can be described either as a solid network (or “matrix”) extended in an aqueous solution or as water trapped in a solid network. Ought one therefore write S × W or W × S? Here again let’s apply the rule of alphabetical sequence and write S × W in referring to such systems.
What value does this theoretical exercise have in cooking? Since we have no difficulty in recognizing solids, liquids, and gases, let us start with these materials—and look to painters for inspiration. How do they grind colors? Using the modern equivalent of a pestle and mortar, they form a paste by dispersing pigments in a siccative oil, so called because it has the property of drying (unlike the oil used in cooking, which remains liquid). In the kitchen, then, begin with sugar and salt since they are solids (even in powdered form, each granule is a solid). If you put the sugar and salt in a mortar and use a pestle to grind them together with oil (a flavored oil, for example), you obtain a liquid suspension or paste. Evidently it is possible to produce either an oil-based paste or a water-based paste (so long as the solid is not water soluble). Alternatively, instead of grinding salt and sugar, one might grind titanium dioxide, a pigment used by pastry chefs to color the surface of white cakes. There is no technical obstacle in this case (although the challenge of reducing titanium dioxide to particles that are nanometric in size—that is, on the order of a billionth of a meter—is apt to give pause to even the most intrepid culinary experimentalist), but there may be a legal obstacle one day. For the moment, under the regulations that govern the food industry in the European Union and Switzerland, titanium dioxide is an approved coloring agent (identified by the code E171). But who can be sure that the health police, in their overzealous determination to turn the world of cooking into a sort of antiseptic retirement home, will not yet succeed in preventing us from using such additives?
Still, we ought not be too pessimistic. Think how much practical knowledge cooks have already accumulated over the centuries! Chocolate makers, for example, have long known that solid particles cease to be perceptible in the mouth if they are too small (strictly speaking, when their diameter is smaller than 15 micrometers, or fifteen thousandths of a millimeter). This, as it turns out, is roughly the size of the particulate matter yielded by conching, a process in which cocoa butter and sugar are slowly ground into a fine paste.
THE PARTICULAR CASE OF EMULSIONS
From the culinary point of view, one of the most important phenomena responsible for giving consistency to liquids, and particularly to water, which accounts for the bulk of vegetable and animal tissues, is emulsification. When it is harnessed by the cook in the proper way, so that the droplets of the dispersed phase are either very finely divided or highly concentrated, emulsification produces consistencies that do not appear to be liquid. We saw earlier that a borderline liquid, which is to say one that flows only beyond a certain threshold, is very close to being a solid.
Whereas aqueous solutions are, well, aqueous solutions, we shall see that oil-based solutions may assume several different forms. Still, let’s begin with aqueous solutions in order to familiarize ourselves with some of the compounds that can be used in note-by-note cooking. In traditional cooking, the juices and liquids derived from fruits, vegetables, and meats—wine, broth, stock, and so on—are all aqueous solutions, laden to one degree or another with sapid, odorant, and nutritive compounds. Wine, for example, is an aqueous solution containing about 10 percent ethanol (the alcohol of wine and brandies), mineral salts, tartaric acid, various amino acids, and other organic substances such as phenolic compounds, often wrongly called “tannins” (certain phenolic compounds are in fact used for tanning purposes and so qualify as true tannins, but others aren’t and therefore don’t deserve the name). What about beef broth and other kinds of bouillon? They consist in the main of water and amino acids, which come from the slow degradation of gelatin, itself obtained by the degradation of collagenic tissue. Bouillon obviously contains many other compounds as well, such as glucose, extracted during the cooking of meat in water, but for the most part it consists of amino acids. All cooks should make a point, by the way, of tasting monosodium glutamate, which is nothing other than the “salt” (as chemists call it) produced by combining glutamate and sodium. Glutamate is an ionized form (obtained, as we saw earlier, through the exchange of an electron) of glutamic acid—an amino acid that is present in meat and has the flavor of bouillon!
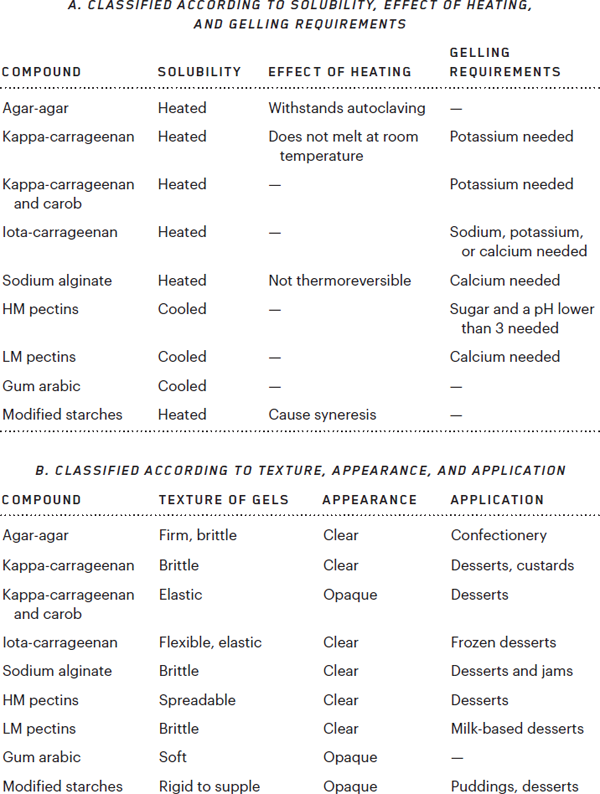
Oils exhibit greater diversity, not least because the name “fat” (or “fatty matter”) is given to almost any compound that is not soluble in water. Many fats are made of triglycerides. This is true not only of cooking oils, but also of solid fats (animal fats such as lard, milk, cream, butter, and so on). The class of fats nonetheless includes other compounds, such as lecithins, which compose a good part of an egg’s yolk, and monoglycerides and diglycerides, which the food industry has long manufactured for use in making emulsifiers.
Lecithins are found in the membranes of human cells, and indeed in the membranes of all animal and vegetable cells. An egg yolk contains many kinds of lecithin, but industrial producers prefer to extract them from soybeans. The commercial use of these products is regulated by law. In Europe, lecithins constitute a class of approved additives (identified by the code E322), and, with the triumph of science over fear represented by molecular cooking, they have now found their way into the kitchen. Still today, however, too few chefs make use of the sodium, potassium, and calcium salts of fatty acids or of monoglycerides and diglycerides or of emulsifiers falling between E470 and E483 on the list of authorized additives. These products have been used in industrial food processing for many years now, as I say, but cooks have scarcely begun to experiment with them. A whole constellation of consistencies is waiting to be explored.
Let me conclude this very brief discussion of a rather immense topic by pointing out that Albert Einstein himself contributed to the investigation of dispersed systems. Einstein reckoned that the viscosity of hard spherical particles dispersed in a liquid is equal to the viscosity of the liquid plus two and a half times the proportion of the dispersed phase in relation to the total volume—a calculation that has been refined since. Do cooks really need to worry about such details? Perhaps not. At a minimum, however, cooks must know that an emulsion cannot absorb more than 95 percent of the dispersed phase: beyond that, sauces break. Two further observations. First, assuming constant proportions of oil and water, vigorously whisking an emulsion makes it easier to smell the odorant compounds dissolved in the oil, whereas in the same emulsion, whisked less vigorously, water-soluble odorant compounds are more easily detected. Second, contrary to what Einstein’s calculation seems to suggest, the viscosity of an emulsion completely changes depending on the size of the droplets: a mayonnaise that has been beaten very vigorously is firmer than the same mayonnaise beaten less vigorously.
Although emulsions are indispensable in cooking, they have the disadvantage of enlarging the love handles that naturally grow on our hips with age! The idea of replacing the fatty matter in emulsions with air opens up another vast world for us to explore: foams.
The best known of the foams used in cooking is obtained by vigorously beating an egg white. Whisking disperses the air bubbles, while at the same time breaking them apart into smaller bubbles. When the bubbles are so tightly packed that there is no more room left, they become polyhedric, and the foam firms up. If we add some sugar to the egg white, whisking dissolves the sugar in the aqueous phase that constitutes the walls of the bubbles, and the diameter of the bubbles diminishes as we go on beating the mixture—by much more than it would in the absence of sugar. The technical explanation of this phenomenon is a bit involved, but pastry chefs have long known that it has something to do with the smoother texture that comes from beating sweetened egg whites, similar to the texture that one hopes to achieve in a meringue. Sweetened or not, foams, like emulsions, display a certain threshold behavior; that is, they do not flow easily because the water is trapped between gas pockets by the walls of these pockets. For the moment, to keep things simple, we may speak simply of “capillarity.”
Note that compounds other than sugar can be added to the aqueous phase of a foam. Citric acid, for example, contributes a nice lemony flavor; anthocyanins, which give red and black fruits their colors, impart a pleasing hue. The possibilities are immense once you know how to use hydrosoluble compounds. Hydrophobic compounds are a different story. They cannot be dissolved in a water-based foam because, by definition, they are not soluble in water. Why not then disperse droplets of these compounds in the foam’s aqueous phase? Nothing is simpler than to beat an egg white until it is stiff and then, still whisking, add to it drops of a flavored oil, an essential oil. This time the foam will have flavor from the molecules dissolved in the aqueous phase, and odor from the compounds present in the oil phase. Another option, of course, is to use hydrophobic compounds in oil-based foams.
Keep in mind, finally, that foams can be cooked. For example, beat a mixture of water and egg proteins (known to pastry chefs as egg-white powder) until it is stiff, then whisk in some beet juice and heat the enriched mixture in a microwave oven for a few seconds, and you will see the foam expand—a sign that the temperature at which water boils (100°C) has been reached. At this temperature, the egg proteins coagulate, and the foam becomes a solid that can be left to cool like a meringue.
PARTICULAR CASES OF GELS
We ought to take a closer look at gels since, as we saw earlier, they are at the heart of traditional cooking: vegetable and animal tissues are essentially dispersions of a liquid in various solids (fruits, vegetables, meat, fish). Using the formalism I have just described, we may refer to these gels by the formula L/S.
We saw earlier, too, that liquids are of two types: water (W) and oil (O). From the formula L/S, then, two others can be derived: O/S and W/S. Now, if we can write down these formulas, we can also put them to use in the kitchen. A gel corresponding to the first of these two formulas is obtained by dispersing some oil (flavored, by all means) in an egg white and then heating the resulting emulsion in a microwave oven as before. After a few seconds it will expand, a sign not only that the water is beginning to evaporate, but also that the temperature has reached 100°C and the egg proteins have coagulated. Take the cooked emulsion out of the oven; this is what I call a “gibbs.” Left to dry, it makes an oil gel, O/S, which I call a “graham.”
To apply the second formula, let’s use sodium alginate. Start with tomato juice and add to it a calcium salt (in this case calcium citrate) recovered from the liquid that coats a clean egg shell after it has been dipped in lemon juice and left to effervesce, which is to say to give off gas bubbles. Next, dissolve some sodium alginate in a bowl of pure water, using an immersion blender to thoroughly dissolve the powder (allowing about 10 grams for 100 grams of water). Now drip the calcium-salted tomato juice into the aqueous alginate solution: beads of liquid form, similar in appearance to salmon eggs, with the liquid interior being sealed by a layer of jelly. Finally, coating these “pearls” with a gelatinous liquid so that they stick together, we obtain an artificial vegetable tissue corresponding to the formula W/S, to which I have given the less cumbersome (and, it seems to me, rather prettier) name “conglomèle.”
Another group of gels having two interpenetrating phases is designated by the formula L × S. Here again, two types of liquids can be distinguished, corresponding to the formulas O × S and S × W. The second has long been familiar in the form of gelatin gels and jams. The first, by contrast, is new in the history of cooking.
Still more possibilities will come into view at this point if we introduce the concept of dimension. The basic idea is quite simple. Remember the sort of cube we all played with as children? If you put the cube in front of you and then change your own position in relation to it—moving from the left near corner by a certain distance to the right, then by another distance to the rear, and, finally, by a third distance toward the top—you can locate any point inside the cube by reference to three numbers. Three numbers, three dimensions. To locate all the points on a sheet of paper, however, only two numbers are needed because the sheet has two dimensions—assuming that it is infinitely thin (a crucial assumption—otherwise we would find ourselves back in the previous case, with one dimension, thickness, being smaller than the two others). An actual sheet of paper does have a thickness, of course. But isn’t it “almost” zero? Everything depends on what are known as orders of magnitude. Suppose we have a sheet that is square, with sides 20 centimeters in length, and whose thickness is a millimeter—200 times less than 20 centimeters. A number a is said to be smaller than another number A by an order of magnitude if the ratio A/a is equal to or greater than 10. For our sheet the ratio is 200, so the thickness is smaller than the length of the side by two orders of magnitude.
The notion of an order of magnitude makes it possible to understand what is meant by the dimensionality of an object. In the case of a sheet of paper, because the dimension corresponding to thickness is smaller than the other two dimensions by more than an order of magnitude, the sheet is conventionally said to be of dimension two. Similarly, a string—in the kitchen, a spaghetti noodle, for example—is of dimension one because two of its dimensions are smaller than the third dimension by at least an order of magnitude. Thus, too, a grain of rice may be said to be of dimension zero if the plate on which it sits is taken as the basis for measurement.

Let us now go back to the operator /, which signifies random dispersion. We could, of course, limit our attention to a formula such as W/S, in which a liquid is dispersed in a solid. But we can speak more precisely by considering the dimensions of gases, liquids, and solids: instead of indicating simply the nature of the substance dispersed, we can note its dimensionality (D) as well. One might write Dj/Dc, for example, or Da/Dc or Db/Dc. What do these formulas mean? The first, Dj/Dc, refers to small, zero-dimensional objects dispersed in a three-dimensional mass. (In the case of a gel, the dispersed zero-dimensional objects are liquids.) The second formula, Da/Dc, corresponds to strings dispersed in a volume, and the third, Db/Dc, to sheets dispersed in a volume.
How does one go about producing such objects in the kitchen? It will be obvious that the first formula, Dj/Dc, describes vegetable tissues—natural and artificial alike. Earlier, I described making conglomèles in the form of liquid-filled sacs (or “pearls”), one by one, but there are many ways of doing this more quickly. For example, you could stack small pieces of frozen liquid (lobster bisque, say) in a box and then fill it with a gelatinous liquid (grapefruit-flavored chicken stock, perhaps, in which some gelatin has been dissolved). The frozen liquid will melt as the stock gels, and you will be left with a mass of pearls dispersed in the gel.

How about dispersing a fatty substance, rather than an aqueous solution, in a solid matrix? It would then be possible to obtain the preparations to which I have given the names “liebig” and “gibbs,” respectively, by emulsifying a fatty liquid in a solution enriched with gelatin or by emulsifying it in an egg white (in which case the emulsion is heated).
As you can see, giving material form to a formula is quite straightforward. The second of the three formulas mentioned, Da/Dc, is also simply applied. For example, if the one-dimensional structures are liquid, then the formula describes animal muscle tissues (meat, fish). These physical systems can be recreated with the aid once again of alginate. Take a tube of calcium-enriched aqueous solution and let it gel in a tray containing water in which sodium alginate has been dissolved. This will give you long strings filled with water. If you then arrange the strings lengthwise and form them into a cohesive bundle, you will obtain a piece of artificial meat, to which I have given the name “fibré.” Here, too, other methods are possible. For example, the first time I made a fibré, I put long hollow noodles in a glass and then filled it to the top with a hot aqueous solution in which some gelatin had been dissolved.
Alternatively, one might cut a lattice of cylindrical shafts in a solid substance (using, say, a board of wood with long protruding nails that, when pressed into the solid, make a matrix of holes in one go), then fill the shafts with a liquid and plug them.
THE MECHANISMS OF COAGULATION
Up to this point, I have considered the structure of gels, but not their molecular aspect. We need first to distinguish two principal types of gel, physical and chemical. To make the first type, dissolve some gelatin in warm water; as the solution cools, the gelatin forms a lattice that traps the water. The water no longer flows, and we are left with a particular kind of solid, a gel. Once heated it melts and again forms a liquid that, on cooling, will reassume the form of a gel. The temperature at which this transition takes place is rather low, about 36°C (97°F). In other words, not much energy is needed to dissociate the molecules of a gel of this type because the bonds between gelatin molecules are weak. The gel is thermoreversible: it is a physical gel.
Another example, this time a chemical gel. An egg white is composed of water in which proteins are dissolved (accounting for about 10 percent of the total mass). The liquid solidifies when it is heated, but the more it is heated, the harder the gel becomes. Moreover, the gel is thermoirreversible; that is, it cannot be converted back to its original state through heating because the proteins degrade before separating. (Some years ago, I showed how to “uncook” an egg by separating the protein molecules, but this process requires a much more energy-intensive method than simply heating to 36°C.) Eggs, moreover, are not the only culinary ingredients containing proteins that coagulate to form a chemical gel. Meats, like fish, contain great quantities of protein. This is why we can make terrines: ground meat (which can be stretched by adding water) forms a gel when it is heated.
The use of proteins to structure liquids will become clearer if we examine a cousin of muscle tissue, the artificial fish-based food product known as surimi. Manufacturing surimi involves two basic operations. The first is carried out at sea, on board a factory longliner (the industry name for a commercial trawler equipped with a processing plant), where fish are caught, headed, and gutted. The filets are then thrown together, washed several times in fresh water, and pressed. Eliminating the soluble proteins, blood, fat, and connective tissues yields “surimi base”—a white tasteless paste, rich in proteins and poor in lipids. Compounds are then added to improve the proteins’ resistance to cold (polyphosphates, sugar, sorbitol), and the paste is cut up into 10-kilogram slabs that are then frozen to −30°C (−22°F). When the factory longliner returns to land, the second phase of processing takes place. The frozen surimi base is thawed on arrival at the production facility and inspected for whiteness, gel cohesion, microbiological content, and so on. Various substances are then added to it: starch (potato or wheat), egg white, oil, salt, sorbitol, calcium sulfate, as well as natural or artificial fragrances (crab, shrimp, lobster) and coloring agents (paprika is often used to give the final product an orange tint on the outside). The paste is then kneaded and rolled out to form a thin layer and steamed. Finally, it is shaped (usually rolled and scored), vacuum packed, and pasteurized.
Is surimi any good? Without quibbling over semantics (“good” is, in any case, a very personal judgment), it must be admitted that surimi is highly variable in quality. Selecting the right ingredients is important: if a low-quality fragrance is used, the odor will be uninviting; if an excessive amount of starch is used, the texture will be doughy. Everything depends on how much intelligence and labor are put into making it—and also how much money (“The cost is forgotten, but the quality remains,” as the gangsters say in the old French film
Les Tontons flingueurs [Crooks in Clover, 1963]). From the chemist’s point of view, the wonderful thing about surimi is that it shows how easily proteins can be strung together to produce quite extraordinary consistencies. What’s more, it is just one possibility among a vast number—further proof that note-by-note cooking is not limited to making jellies, jams, and gelatins!
More than once in our exploration of consistencies we have found ourselves following in the footsteps of the food industry, which in turn was following in the footsteps of cooks. Cooks learned long ago how to make emulsions using eggs. Scientists subsequently investigated the properties of eggs. From scientists the food industry learned that proteins and lecithins are good emulsifiers and then found a way to isolate lecithins from inexpensive vegetable matter such as soybeans. Fittingly, then, molecular cooks have closed the circle by substituting artificial emulsifiers when, for one reason or another, the flavor of an egg yolk is unwanted. So, too, scientists succeeded in explaining why cooking down veal shanks makes it possible to make aspics and desserts such as Bavarian cream: the collagenous tissue is dissociated when it is heated in water, releasing gelatin, a natural gelling agent. Today, commercial food manufacturers make the like of these products, originally obtained through a long and costly process of cooking, only now much more efficiently and cheaply by using gelling agents extracted from algae.
To protect citizens (you will forgive my use of a political term here—it’s simply that I refuse to call people “consumers”), governments regulate the use of novel products of this sort. In France, regulation is based on a 1905 law stipulating that food products must be “wholesome, genuine, and saleable.” It is forbidden, for example, to sell damaged fruits and vegetables or to sell a product under a false or misleading name. All this is as it should be. But it is a sad state of affairs, in my view, when we are led to fear an entire class of compounds that impart not only color and flavor to foods, but also consistency. The desire to give different consistencies to foods is by no means new. Cooks have long made use of egg, meat, and fish proteins for just this purpose, as we have seen. One might even regard traditional cooking as a perpetual oscillation between stronger and weaker forms of consistency. To make a terrine, for example, meat is ground up until it forms a soft paste and then cooked until it forms a hard paste. Carrots, which in their natural state are hard, are softened by cooking, but then become firm again when they are used to make a carrot flan. Are these pointless operations? Not at all—they are ways of creating flavor!
Gelification and thickening are therefore matters of some importance. Over many decades the food industry created a range of products that today are known as texturing agents. Again, there is nothing new about the basic idea. Cooks have long been accustomed to thicken sauces with flour. They have long known that cooking meat and fish (with or without their bones) yields bouillons that gel, which is to say liquids that can be converted into solids. People who live near the sea have found many uses for algae, wrack, and lichens, whose polysaccharides are the source of various modern texturing agents. Alginates were first used by the cosmetics industry and later introduced in food processing, along with carrageenans (extracted from red algae), xanthan gum (produced by the bacterial species
Xanthomonas campestris), and so on. Many such agents figure today on the list of officially approved additives, which are divided into a number of categories.
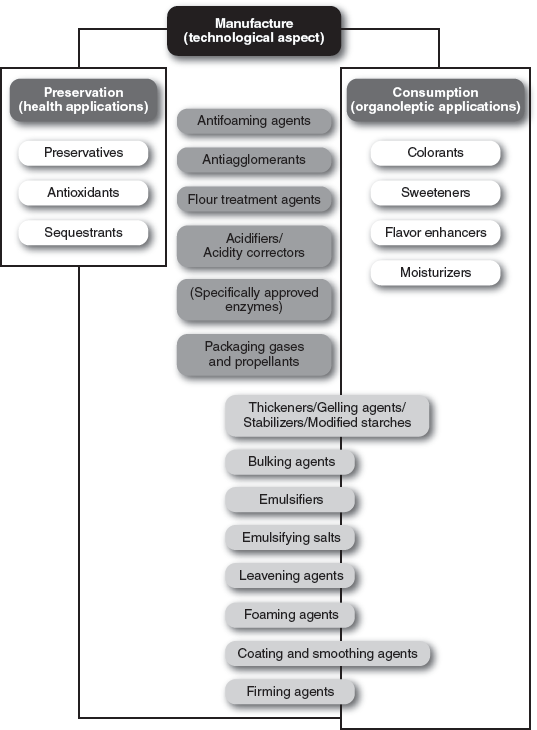
The left-hand and right-hand columns of
figure 2.9 need not detain us here. The middle column is the one we need to examine, in particular the lower part, which catalogs the various types of compounds that are useful in cooking. Emulsifiers make it possible to make emulsions, foams, suspensions; emulsifying salts are useful for modifying the consistency of milk-based gels; leavening agents, by releasing gas, produce foams; foaming agents induce expansion; firming agents make food products less soft, thickening agents make liquids more viscous. Let’s take a closer at these substances in the order of their E number, the numerical code assigned to food additives approved for use within the European Union and Switzerland.
E400 alginic acid (emulsifier/thickener and gelling agent)
E401 sodium alginate (emulsifier/thickener)
E402 potassium alginate (emulsifier/thickener)
E403 ammonium alginate (emulsifier/thickener)
E404 calcium alginate (emulsifier/thickener)
All of these substances are extracted from brown algae of the class Phaeophyceae (a group that contains the Fucaceae, Laminariaceae, Alliaceae, and Lessoniaceae families). Molecularly, alginates are linear polymers of alpha-l-guluronate acid and beta-d-mannuronate acid.
The last part is a mouthful, I know. Let’s take it one step at a time, beginning at the beginning—with glucose. Glucose, as we saw earlier, is a compound. In its pure form, at room temperature, it is a crystallized or amorphous powder. In white light, glucose is white: the light is reflected by its solid granules, which individually are transparent. Glucose has a very mild flavor, not really sweet. And it’s literally in our blood, which carries it as fuel for cells throughout the body. From the chemical point of view, glucose comes under the head of sugars (and, more precisely, of monosaccharides), first systematically studied by the brilliant German chemist Emil Fischer (1852–1919) in the late nineteenth and early twentieth centuries.
Like glucose, fructose is a sugar, but it is much sweeter. Both glucose and fructose are found in vegetable tissues, particularly in the elaborated sap that descends from the leaves of plants, where saccharides and, in smaller amounts, amino acids are synthesized. Unsurprisingly, these sugars are also found in the underground organs of certain vegetables (the roots of the carrot and the tubers of the potato plant, for example), since these organs serve primarily to store energy. Glucose and fructose are classified as “simple” sugars, or monosaccharides, because they can be linked together to form larger molecules. Sucrose, for example, is formed by joining glucose and fructose. In onions, somewhat larger sugars called oligosaccharides are formed by several fructose molecules linked to a sucrose molecule.
Glucose and fructose are not the only basic molecular components, or building blocks. Two other elementary sugars, guluronic acid and mannuronic acid, can also form chains. There are many other such compounds as well. When a molecule is made up of fewer than a hundred or so repeating units (known as monomers), it is called an oligomer (from the Greek oligo-, meaning “few”). By contrast, when the number of monomers is higher than a hundred, the molecule is known as a polymer (from the Greek poly-, “many”). Note, too, that elementary sugars can be joined together at their ends to form a ring, in which case the polymer is said to be “linear,” or arranged in the shape of a tree, in which case one speaks of a “branched” polymer.
With regard to alginates, there are three further things to keep in mind: first, that in an acidic environment they precipitate in the form of alginic acid, the initial item on the preceding list (E400); second, that the molecular constitution of a particular alginate in a given marine substance determines its exact properties as well as the appropriate manufacturing technique; and third, that alginate molecules can be tightly bound together with the aid of calcium ions, as we saw earlier with liquid-filled pearls.
E406 agar-agar (emulsifier/thickener)
Agar-agar is not a very new substance, but just the same it appears in the category of food additives because it is indeed “a substance added in small quantities to foods, in the course of their preparation, for a precise, technical purpose,” as the official definition stipulates. Agar-agar (often abbreviated as “agar”) is extracted from red algae of the class Rhodophyceae. From the molecular point of view, it is a linear polymer whose basic pattern (the elementary ring of the chain) is composed of two galactose residues, more or less modified by various atoms. Agarose, the principal constituent of agar-agar, is composed of beta-d-galactose and a 3.6 anhydro-l-galactose. The length of each molecule depends on its actual source and the particular extraction procedure employed. Extraction is performed by solubilization in water at a temperature of 100°C for several hours in the presence of a base. Separation is done by freezing.
Sorry, I forgot to introduce galactose—a monosaccharide found particularly in milk sugar, lactose, which is a disaccharide like sucrose. Whereas sucrose is made of glucose linked with fructose, lactose is made of glucose linked with galactose.
E407 carrageenan (emulsifier/thickener)
Like agar-agar, carrageenans are a naturally occurring polysaccharide isolated from red algae of the class Rhodophyceae, but this time the basic pattern is a sulfated beta-d-galactose linked with an alpha-d-galactose, sulfated or not, sometimes in 3.6 anhydro form.
If anyone is wondering, by the way, whether the mention of a “sulfated” component here should arouse fears of copper sulfate poisoning, the answer is emphatically no. Human cartilage also contains sulfate groups, composed of one sulfur atom linked with four oxygen atoms. Moreover, it is mainly the copper in copper sulfate that is toxic, as you can readily see by means of a simple experiment. Let some water stand in two bottles, one of which contains copper turnings. After a few weeks or months, you will see green algae in the bottle without copper, but none in the other bottle because the copper turnings poisoned the algae before they could become established.
To come back to carrageenans. I use the plural since conventionally one distinguishes between iota-, kappa-, and lambda-carrageenans according to the degree of sulfation and the position of the sulfated carbon atoms. These compounds do not share the same properties, nor are they easily substituted for one another. For example, whereas kappa-carrageenan forms rigid, brittle, slightly opaque gels, iota-carrageenan forms elastic, clear gels, and lamb-da-carrageenan does not form gels at all, though it does promote viscosity. Preparation of all these products is the same as in the case of agar-agar, and recovery is done by drying the residue of a solution that has been filtered by alcohol precipitation (microballs, formed with the addition of potassium chloride [KCl], can be used instead of alcohol).
E410 carob gum (emulsifier/thickener)
Carob gum (also known as locust bean gum) is obtained from the seeds of the carob tree (Ceratonia siliqua), native to the Mediterranean littoral (Spain, Portugal, Greece, Morocco). It is a galactomannan—a linear polymer composed of alpha-d-mannose units, with lateral branching of a single alpha-d-galactose unit. Carob gum comprises on average one galactose unit for four mannose units. (Mannose, as everyone will have guessed by now, is another simple sugar, a monosaccharide.)
Carob seeds are reduced by a series of mechanical procedures (separation of the endosperm, removal of the seed hull, milling) that yield a flour consisting very largely (95 percent) of galactomannans.
E412 guar gum (emulsifier/thickener)
Guar gum comes from the seeds of the guar tree (Cyamopsis tetragonolobus). It is produced mainly in India and Pakistan, and to a smaller extent in Texas. Made up of galactomannans, like carob, guar gum is characterized by the repetition of one galactose unit for every two mannose units.
E413 tragacanth gum (emulsifier/thickener)
Also known as adragante gum, tragacanth gum is obtained from several species of Astragalus, shrubs of the Leguminosae family. It is a mixture of two polymers: tragacanthic acid, which is a polymer of d-galacturonic acid that also contains d-galactose, d-xylose, and small quantities of l-rhamnose; and a neutral polymer, tragacanthin, which is a highly branched arabinogalactan, the principal chains being constituted of d-galactose residues and branched l-arabinose residues.
E414 acacia gum (thickener/stabilizer)
Better known perhaps as gum arabic, acacia gum is produced in many regions of Africa, generally from several species of the acacia tree, Acacia sene-gal (or Acacia verek), Acacia seyal, and Acacia laeta. It is constituted by densely branched polymers, with a principal chain consisting of linked beta-d-galactose residues, with substituted beta-d-galactose units having arabinose and d-galacturonic acid endings. Already in the nineteenth century, cookbooks mentioned the use of gum arabic as a stabilizer for whipped creams—nothing very new here on the industrial side, then.
E415 xanthan gum (thickener)
An extracellular polyoside, xanthan gum is produced by aerobic fermentation of the microorganism Xanthomonas campestris on a glucidic substrate (typically molasses) under specific conditions: pH between 6 and 7.5 (pH is a measure of acidity, ranging from 0, very acidic, to 14, very alkaline) at temperatures between 18° and 31°C (64.4° and 87.8°F). The gum is recovered at the end of the fermentation process by alcohol precipitation, then dried and ground.
From the molecular point of view, xanthan gum is a polymer composed of a linear chain of beta-d-glucose with lateral branching at every other glucose unit. These side chains contain an alpha-d-mannose, a glucuronic acid, and a terminal beta-d-mannose.
E417 tara gum (stabilizer)
Tara gum is obtained from the seeds of the tara tree (Caesalpinia spinosa), found mainly in the South American sierra (especially in Peru) and harvested chiefly for the tannins extracted from its pods. The gum is a by-product of this operation.
The polymer in this case is formed by a linear chain of beta-d-mannose, with only single-unit branching of alpha-d-galactose residues.
E418 gellan gum (thickener/stabilizer)
An extracellular polyoside produced by aerobic fermentation of the microorganism Pseudomonas elodea, gellan gum is obtained by a method similar to the one employed in the production of xanthan gum. Commercial processing uses two forms of the product: a native, acetylated form and a deacetylated form. The native form is a linear molecule whose basic unit is a chain of glucose residues, glucuronic acid, glucose, and rhamnose. In half of the units, an acetyl group (one carbon atom linked to three hydrogen atoms and to another carbon atom that is itself linked to two hydrogen atoms) is linked to one of the glucose residues. Nevertheless the gelling properties of the esterified form remain limited, and it is the deacetylated form that is more commonly used.
E440 pectins (emulsifier/thickener)
Pectins are complex mixtures extracted from the cell wall of plants. It is these substances that cause jams and jellies to set: cooking fruits and sugars extracts pectins and dissolves them in the acidic liquid released by the fruits as they are broken down; then, on cooling, the extracted pectins join together to form a gel that traps the liquid from the fruit along with bits of its flesh. The food industry found it profitable to look for ways of extracting various kinds of pectin individually, rather than let what was left over from the pressing of fruits (citrus fruits and apples in particular) and the processing of sugar beets go to waste. Pectins are first extracted by solubilization in a heated acidic medium and then, after purification and concentration, precipitated with alcohol, dried, and finally ground.
The major molecular constituent of pectins is galacturonic acid, with a few inserted rhamnose residues. Side chains are attached to a backbone, forming “hairy” regions that alternate with “smooth” regions. The smooth (or homogalacturonic) regions are made up exclusively of linked galacturonic acid units and account for 80 to 90 percent of the molecular structure of pectins.
Varieties of pectin are distinguished because they can be partially esterified to one degree or another, which is to say linked in different proportions with methanol, an alcohol related to ethanol (the alcohol found in brandies, wine, and beer). The relevant parameter in classifying the gelling properties of pectins is the degree of esterification (DE)—that is, the number of carboxylic acid groups in a pectic chain esterified by methanol per hundred galacturonic units. In this way high methyl-ester (HM) pectins, having a DE greater than 50 percent, are distinguished from low methyl-ester (LM) pectins, whose DE falls between 25 percent and 45 percent. Depending on whether more or less sugar is combined with more or less calcium, one can obtain gels of different degrees of firmness. A word to the wise cook: choose your pectins well!
E460 microcrystalline cellulose, powdered cellulose (emulsifier/thickener)
E461 methyl cellulose (emulsifier/thickener)
E462 ethyl cellulose (emulsifier/thickener)
E463 hydroxypropyl cellulose (emulsifier/thickener)
E464 hydroxypropyl methylcellulose (emulsifier/thickener)
E465 ethyl methyl cellulose (emulsifier/thickener)
E466 carboxymethyl cellulose (emulsifier/thickener)
Cellulose is the most abundant organic polymer on earth. Its chemical inertia and insolubility in water make it indispensable in textile manufacturing: if it were to dissolve in hot water, our shirts and blouses would vanish when we wash them. (The cotton used in making clothes is virtually pure cellulose.) Where does it come from? From vegetable matter: vegetable tissues are made of cells cemented to one another by a wall that is made up of cellulose molecules—great pillars, in effect, held in place side by side by a web of pectin molecules.
Cellulose itself holds little interest in connection with thickening or gelling. But some of its derivatives, generally obtained by simple modifications that make cellulose molecules hydrosoluble, have surprising properties. Although most cellulose derivatives are thickeners, there are exceptions: methyl cellulose, for example, gels on being heated and reliquifies on being cooled—the opposite of gelatin and pectins!
E1200 polydextrose (stabilizer/thickener)
Let us now turn our attention to another substance that is naturally very abundant on earth—starch, found in wheat, rice, potatoes, corn, and so on. The name “starch” is given to grains that are composed chiefly of two types of compound: amyloses and amylopectins (the latter have nothing to do with pectins). Amyloses are linear polymers, and amylopectins are branched polymers; in each case the basic repeating unit is glucose.
Why are these polymers present in the vegetable kingdom? A teleological reply to such questions, which assumes a purpose in the evolution of living things, is generally to be avoided (since otherwise one ends up saying that our nose and ears, for example, were created so that we could wear glasses). Nevertheless it is true that glucose, which is synthesized in the leaves of plants, is a water-soluble compound and so liable to being washed out by rain, whereas starch, a water-insoluble substance produced in the course of seed germination, very usefully remains embedded in roots, seeds, and tubercles as an internal reserve of energy.
Cooks have long been adept at thickening soups and sauces with flour, kneaded butter, roux, starchy foods such as potatoes and rice, and so on. The problem with using plant (or native) starches is that they give sauces, especially, a sticky consistency. Sauces containing amylose, such as ones that are thickened with corn starch or wheat starch, likewise form opaque and rigid gels when cooled; and if they are kept warm for a certain time (in a bain-marie during restaurant service, for example), they expel water—a phenomenon known as syneresis, in which moisture beads up on the surface of a sauce because the amylose molecules released during cooking reassociate and form a gel.
In order to avoid these inconveniences, which cooks have found various ways of minimizing over the centuries, the food industry devised a method, known as reticulation, for modifying starches. Reticulated starches, in the form of either distarch adipates or distarch phosphates, are characterized by the presence of cross-linked polymer chains that strengthen the cohesiveness of starch grains, prevent syneresis, and make gels less viscous.
Reviewing the list of thickening and gelling additives, one item at a time, may seem a bit tedious, but only if you do not stop to consider their culinary uses! The compounds I have mentioned so far yield very different gels and are used for a variety of purposes. Their properties are summarized in
table 2.4.
Surely the information contained in this table justifies molecular cooking’s introduction of such products. Why should cooks be condemned to use only native pectins and the gelatin extracted from veal shanks when so many new alternatives are available? It is as though musicians had limited themselves to the triangle, without touching the piano that was offered to them; as if painters resolved to draw only with a charcoal pencil, even though a whole range of colors had been placed at their disposal!
In recent years, fearmongers—for that, frankly, is what they are—have shamelessly sought to take advantage of a certain mood of ignorant naturalism in order to make people believe that additives are dangerous. But isn’t there more danger in a barbequed piece of meat than in xanthan gum? The answer, unquestionably, is yes. And yet, as I know myself from personal experience, arguing on the basis of examples even as telling as this one is useless. The only way of convincing people of the truth is to explain the facts as carefully and as impartially as possible, without seeking to convince. There is a paradox in this, I admit. But there you are. Anyone who wishes to go on cooking as people did in the Middle Ages should by all means feel free to do so. Other, less timid souls will gladly adopt the compounds I have just described. They will throw themselves into note-by-note cooking, seeing in it, as I do, the source of a new kind of connoisseurship. Over time, I predict, the success of note-by-note cooking will erase the altogether arbitrary distinction that is made today between meat, fish, fruits, and vegetables, on the one hand, and compounds (not only additives, but all such examples of chemical ingenuity) on the other. And with it, I pray, will come the end of the obligation that presently falls upon the regulatory agencies of the state to introduce legal niceties that have no rightful place in food and cooking. May the very notion of additives disappear as soon as possible, in fact—because these compounds, far from being mere technical accessories, form the basis of the very foods we eat!
In his first note-by-note dish, Pierre Gagnaire created what I had the idea of calling “péligot disks,” after the nineteenth-century French chemist Eugène-Melchior Péligot (1811–1890), a sort of glucose caramel that he stacked in thin round slices, one on top another. The oral sensation produced by three superimposed disks is wholly unlike the sensation produced by a single disk: one perceives not so much crunchiness as crispiness, the result of a cascade of tiny crackles. By itself, this example shows that the basic operations of dispersion, inclusion, superposition, and interpenetration must be reconceived if we are to obtain sensory effects in the mouth that will improve upon the flat, vapid, dull quality of a simple gel.
We already have had an opportunity to appreciate the usefulness of employing various symbols, known as operators, in analyzing the relations among objects of different dimensions. By defining a restricted set of symbols that, together with the rules governing their use, constitutes a formalism for describing dispersed systems, it becomes possible to imagine a vast field of culinary innovation in terms of consistency alone.
Let’s begin by asking how many options are available to us if we wish to create a dish having only two components. The range of possibilities contemplated by the Dispersed Systems Formalism I proposed several years ago is given in
tables 2.4 through
2.7. Each table considers two objects of a particular dimension and describes the structural consequences of subjecting them to one of the four basic operations just mentioned—dispersion, inclusion, superposition, and interpenetration. (Obviously, there is no particular reason why a dish should be limited to two objects, and so one would have to go on to draw up tables for three objects, four objects, and so on.) Once again there is a risk that surveying the range of possible combinations will grow tiresome, but there is the chance of reward as well: just as molecular cooking proudly took the siphon bottle and liquid nitrogen as its emblems, so it may be that new consistencies will prove to be the distinguishing marks of note-by-note cooking. Nevertheless we must be careful: just as molecular cooking cannot be reduced to a few simple preparations, so note-by-note cooking cannot be limited to a small class of elementary combinations. A flag is not the same thing as the people it stands for!
Even so, molecular cooking has already shown that progress has followed upon attempts to devise practical recipes. When I proposed using alginates to make liquid pearls, cooks were not so much reluctant to experiment as unfamiliar with the technique involved. Once they understood that they had only to dissolve 5 grams of alginate for every 100 grams of liquid and 5 grams of calcium lactate for every 100 grams of liquid, things went smoothly. The same will be true in the case of note-by-note cooking—which is why I have collected a number of recipes in the appendix to this book. It is also why I beg you to indulge me as I comment, occasionally at some length, on the combinations summarized in
tables 2.4 through
2.7.
Neither random dispersion (
table 2.4) nor inclusion (
table 2.5) has been systematically explored by traditional cooking. There has been some interest in investigating primitive forms of superposition (
table 2.6), but only in a rather desultory way. As for the interpenetration (or imbrication) of two continuous networks (
table 2.7), a somewhat more complicated exercise from the technical point of view, virtually nothing has been done. All the more useful, then, will it be to have a schematic inventory of possibilities to hand, from which one can readily choose without having to mentally recreate the list again and again, at the risk of omitting one or more of the panels each time.
Off we go, then—bearing in mind, as I say, that however large the number of possible combinations of only two objects may be, many more exist with three objects, still more with four, and so on. In fact, the situation is far more complicated still. For each of the possibilities listed in these tables, nothing whatever is said about the exact shape of the objects assembled: from the dimensional point of view, a square sheet and a disk are identical; a pyramid and a cube are indistinguishable. And there are infinitely many such forms!
Figura, Ludger O., and Arthur A. Teixeira. Food Physics: Physical Properties—Measurement and Applications. New York: Springer, 2007.
Foster, K. D., J. M. V. Grigor, J. Ne Cheong, M. J. Y. Yoo, J. E. Bronlund, and P. Morgenstern. “The Role of Oral Processing in Dynamic Sensory Perception.” Journal of Food Science 76, no. 2 (2011): R49–R61.
Guérard, Michel. La grande cuisine minceur. Paris: Robert Laffont, 1976; partly translated in Michel Guérard, Cuisine for Home Cooks. New York: Morrow, 1984.
Hargittai, István, and Magdolna Hargittai. Symmetry Through the Eyes of a Chemist. 2nd ed. New York: Plenum Press, 1995.
Hubel, David H. Eye, Brain, and Vision. New York: Scientific American Library, W. H. Freeman, 1988.
Thibault, Jean-François, and Marie-Christine Ralet. “Pectins: Their Origin, Structure, and Functions.” In Barry V. McCleary and Leon Prosky, eds., Advanced Dietary Fibre Technology, 369–379. Oxford: Blackwell, 2001.
This, Hervé. “Formal Descriptions for Formulation.” International Journal of Pharmaceutics 344, nos. 1–2 (2007): 4–8.
——. Science, technologie, technique [culinaires]: Quelles relations? Cours de gastronomie moléculaire no. 1. Paris: Belin-Quae, 2009.