NO ONE WILL DISAGREE that aromatic plants have an aroma, that wines have a bouquet, that flowers have a fragrance—and that meats have an odor. In reality, however, meats, like all foods, have two odors: an “orthonasal” odor that is perceived by smelling and a “retronasal” odor that appears when the odorant compounds released by chewing pass through the air inside the buccal cavity and then rise up into the nose through the retronasal passages.
But why aren’t retronasal and orthonasal odors the same? After all, the odorant compounds are the same in each case. There are two other things to consider, however. First, that certain compounds are released only when a food is destructured. Second, that the perceptible proportion of each odorant compound varies depending on whether it is smelled or chewed because chewing heats the food to a temperature of 37°C (98.6°F) or so, which modifies the released compound’s odor profile. This profile is what we recognize when we eat.
An odor can be thought of as resembling the shape of a tent. The way in which poles of different heights are placed to support the tent fabric is what gives the tent its shape. If you change the number or the position or the height of the poles, you get another shape—another odor. Thus if you add a little orange blossom water (one group of poles) to a strawberry (another group of poles), you get a new smell, the smell of wild strawberries. So, too, if you add a drop of pastis to a small amount of coffee, an odor of licorice appears. Sensory physiology provides a wealth of evidence to suggest that this phenomenon is responsible for the discovery of many classic combinations in cooking: chestnut and fennel, carrot and orange, and so on.
Nothing is more human than the urge to combine different fragrances and, more generally, the passion for modifying the basic flavors of foods. Since antiquity, cooks and perfumers have experimented with herbs, spices, flowers and other parts of plants, and various animal substances. It is sometimes said that our ancestors were drawn to these products because they have antiseptic properties analogous to those of modern preservatives. It is true, for example, that rosemary contains rosmarinic acid, which is a good preservative; also that thyme contains thymol, that clove contains eugenol, that hot peppers contain extremely pungent capsaicinoids that kill microscopic mushrooms, that many vegetable terpenes have antibacterial properties, and so on. Nevertheless there are good grounds for supposing that humans were attracted in the first place by the odor of these ingredients. Exactly why is still unknown, however. Why do we like the smell of roses? This remains a mystery, but molecular biologists are now beginning to shed light on the question through the study of pheromones, which trigger specific behaviors in animals, and of the role odors play in the immune system.
All this is a very complicated story that has little immediate relevance to cooking. We need only assume for present purposes that our earliest ancestors didn’t have to be terribly astute to notice that foods take on the odor of vegetable and animal tissues that are added to them. It was only much later, probably in the Neolithic period, that techniques were invented for making nutritive oils, some of which have very distinctive flavors. One thinks in particular of oils made from hazelnuts, walnuts, pistachios, and olives. Maceration, infusion, and other types of decoction probably came after. And yet here again it would have readily been appreciated that the odor of fragrant substances is released in both water and oil. Enfleurage, a technique that is still used today to make perfumes from delicate flowers, involves solid fats, but the basic idea is the same. I shall come back to it in due course.
Distillation is more complex. It seems to have first appeared with the Chaldeans around the sixth millennium B.C.E. Once more we encounter an invention that demanded not so much genius as well-developed powers of observation. It would not have escaped anyone’s notice that heating fragrant substances gives off fragrant fumes, for example, or that placing a cold solid above boiling water causes the vapor to condense into water, which can be collected from the surface of the solid by tilting it. A closed vessel designed for this latter purpose would have been an early prototype of the modern distillation device known as a retort. The procedure seems to have been improved in the ninth century by the Arabs, to whom we owe the word alembic, but not much more progress was made until the thirteenth century, when pharmacists began to prepare medicinal oils and ointments. Many essential oils used still today by perfumers and aromaticians (who combine such oils with herbs, spices, and various synthetic compounds to improve the taste and smell of foods) were first distilled by pharmacists in the sixteenth and seventeenth centuries. In the first half of the nineteenth century, artisanal manufacture of such products gave way to industrial production.
Improved distillation columns made it possible to separate mixtures of odorant compounds. We need not dwell on the details here, but even as brief a survey of odorant compounds as this one cannot omit to mention the discovery by chemists in the nineteenth century that simple organic compounds have agreeable odors, whether they are synthesized or extracted from natural substances. In 1834, Eugène-Melchior Péligot and another French chemist, Jean-Baptiste Dumas (1800–1884), isolated cinnamaldehyde from cinnamon oil. (If you use an atomizing sprayer to lightly coat toasted flour with cinnamaldehyde, professional cooks will tell you that they smell cinnamon.) Three years later, in 1837, the German chemists Justus von Liebig and Friedrich Wöhler isolated benzaldehyde from the oil of bitter almonds. The first aromatic oils were synthesized in the 1840s from short-chain fatty-acid esters and various alcohols that had attracted attention because of their fruity odor. Methyl salicylate (artificial wintergreen oil) was introduced in 1859, and benzaldehyde was synthesized in 1870. With the industrial synthesis of vanillin in 1874 by Haarmann & Reimer GmbH, and then of coumarin in 1878, a new sector of commercial production opened up. New developments have been regularly forthcoming ever since with regard not only to the isolation of natural compounds, but also to the synthesis of compounds known to exist in nature as well as previously unknown compounds.
I have already mentioned a few of the techniques used by chemists and perfumers to produce odorant compounds, whether by extraction from natural substances or by synthetic preparation. Over the centuries these methods have been refined to one degree or another. The most recent advance is due to the use of carbon dioxide in a so-called supercritical state—that is, a state of matter that may assume the form of either a gas (a highly fluid substance that is introduced into the animal or vegetable tissues from which one wishes to extract odorant compounds) or a liquid (in which such compounds are dissolved). Each extract has its own distinctive characteristics. For the moment, however, let’s focus our attention on more traditional methods of extraction and then quickly take a first look at processed products and synthesized compounds.
Ointments and pomades are examples of a fatty preparation containing odorant compounds that has been produced by cold or hot enfleurage. In hot enfleurage, fragrant substances are directly immersed in a hot liquid obtained by heating solid fats; in cold enfleurage, the procedure is more involved since it is necessary first to cover a neutral fat with a fragrant substance, then wait until the odorant molecules pass into the air and from the air into the fat. Industrial production in each case was inaugurated in the south of France in the nineteenth century.
Essential oils, or essences, may likewise be obtained by either cold or hot methods. The former involve a series of mechanical procedures (the washing and pressing of citrus peels, for example); the latter involve a process known as steam distillation, in which steam is injected into an aromatic substance to trigger the release of odorant compounds; then, once the vapor has condensed, the oil is separated from the aqueous phase. Using steam distillation, the yield is somewhere between 0.1 and 1 percent. Although the essential oils extracted in this way are concentrates of aromatic substances, they need not have all the properties of cooking oil. A drop on a piece of paper, for example, does not always leave a stain.
Essential oils are to be distinguished from distillates, which contain ethanol (ethyl alcohol) and are obtained by distilling vegetable matter with ethanol or with a hydroalcoholic solution. The principle is the same in either case: evaporating a solvent produces odorant compounds, which are collected by allowing the vapor to condense.
Concrete essences (concretes), such as oleoresins, are prepared by extraction using nonpolar solvents (toluene, hexane, petroleum ether). The odorant compounds are subsequently recovered with the elimination of the solvent by means of filtering and distillation.
Absolute essences (absolutes) are obtained by heating and stirring concretes with an alcohol, typically ethanol. Fractional distillation of this mixture yields a wax-free absolute once the ethanol has been eliminated.
Resinoids are the result of treating the exudates of vegetable tissues with solvents such as methanol, ethanol, and toluene. They are not to be confused with oleoresins, which are concentrates prepared by solvent extraction, often from spices.
Tinctures, or infusions, are solutions in which ethanol or a hydroalcoholic solution is used as a solvent to produce liquid concentrates (typically of fruits) with the aid of various filtration techniques, sometimes accompanied by the recovery of very volatile compounds.
Alcoholic distillates, infusions, macerations, percolates, and the like have been used since ancient times. Pure substances (menthol, for example) are sometimes obtained by crystallization.
PROCESSED PRODUCTS
If the food industry produces fragrances and flavoring agents through the extraction of naturally occurring odorant compounds, it also creates them artificially by processing vegetable and animal matter in various ways. The phenomenon of Maillard reactions, for example, can be exploited to impart the smells of grilled and other kinds of cooked foods. Enzymes can be used to hydrolyze various substances. Fermentation yields a vast range of odors. Thus, for example, the equivalent of vanillin can be obtained by fermenting pine needles with the aid of microorganisms that are likewise found in nature.
SYNTHESIS
Finally, alongside compounds extracted from natural substances or created on the basis of such substances, there are synthesized compounds. Once again, let us be clear about what is meant by the term synthesized: synthesized (or synthetic) compounds may be identical to natural compounds or they may be altogether new compounds.
The manufacture of perfumes, which many people consider a frivolous indulgence, nothing more, has nevertheless become a very sizeable and prosperous industry that attracts some of the best young minds in organic chemistry. Several Nobel prizes have already been awarded in recognition of advances in this domain, and attempts to create new fragrances have led to the discovery of mechanisms of molecular rearrangement (“chemical reactions,” in the more familiar phrase) having a variety of applications in fields beyond perfumery. More than ten thousand odorant compounds have been identified so far—and we have not yet begun to approach the limits of molecular synthesis!
After mentioning benzaldehyde and vanillin, I broke off my historical précis of naturally occurring odorant compounds that can also be synthesized. The procedure for making such substances in the laboratory is so simple that they are within the reach of any trained herbal chemist. And yet despite the current vogue for “natural” products, the ability to synthesize compounds that are in every respect identical to compounds extracted from plants or otherwise present in the environment poses problems, at least from the regulatory point of view.
Once again, we need to be clear at the outset about the meaning of the terms we use. Things are properly said to be “natural” if they have not been transformed by human activity; things that have been transformed by human activity are said to be “artificial.” This means that cultivated plants (carrots, onions, and so on) are not natural, nor are the compounds extracted from plants. Myristicin, for example, which gives nutmeg a significant part of its flavor, is natural only so long as it resides in the kernel of the nutmeg tree’s fruit, not if it has been extracted from a nutmeg pod. Nor is an essential oil prepared by human hands natural, even if a government regulatory agency says it is. The molecules that constitute synthesized myristicin, by contrast, are exactly the same as those that constitute the myristicin found in a nutmeg pod. This is what is meant when synthetic myristicin is said to be indistinguishable from natural myristicin—“same as natural” in the conventional shorthand.
And yet there is nothing wrong with the term
synthetic. Indeed, in debating such matters and in drafting regulations, we would do well to use it, for it has the virtues of both accuracy and transparency. No matter that myristicin is still myristicin whether it is synthesized or extracted, citizens in a democratic society have a right to be fully informed about the nature and provenance of the products they buy. Benzaldehyde, if it is obtained by using specially selected reagents to cause a chemical reaction under specific laboratory conditions, is a synthetic compound. It is therefore artificial as well, and there is no reason why the manner in which it was produced should not be plainly stated.
So long as these terms are used ambiguously, however, it is understandable that people should regard the “aromas” created by the “aromatics” industry (misnomers both, for the reasons I gave earlier) with suspicion. All such products are artificial, in the strict sense of the word, because they have been prepared by human beings. Some of them contain compounds extracted from plants and animals; others contain synthesized compounds. Public trust in the wholesomeness of processed food products would be greater, I believe, if we were to speak instead of “fragrant preparations”—or, better still, of “odorigenic compositions,” as I shall explain in due course.
Just as sapid compounds must be soluble in water, since they have to pass through saliva in the mouth in order to reach the papillary receptors, so too odorant compounds must be able to be released into the air. This is what is called “volatility,” which depends both on the particular chemical characteristics of the atoms present in the molecules and on the size of these molecules.
It used to be believed that olfaction is a rather straightforward business: once released, odorant molecules bind directly with receptors, one by one. But the simple picture of an odorant molecule as a key that fits into a receptor lock, which we looked at in
chapter 3, has to be modified in light of the recent discovery of proteins in nasal mucus that serve as intermediaries between odorant molecules and receptors. Current research may be expected to further revise our understanding of this phenomenon. Whatever the exact mechanism of olfaction turns out to be, however, it will be useful for note-by-note cooks to be familiar with the concept of the threshold perception of an odorant compound. This is the smallest concentration in which the odor of a compound can be perceived under a specified set of experimental conditions. For certain compounds (1-p-menthene-8-thiol, for example, which is found in grapefruit), the threshold is very low—so low, in fact, that a teaspoonful in enough water to fill a million swimming pools would still be detectable!
The power of odorant compounds acts also as a deterrent, preventing us from ingesting them in excessive quantities. We have seen that some compounds are truly dangerous. Estragole, for instance, found is tarragon and basil, is converted by the human body into hydroxyestragole, a carcinogenic and teratogenic substance even in very small doses. The fact that odorant compounds are typically found in very small concentrations in vegetable matter reduces their noxious effects, though the synthetic preparation of these compounds in concentrated form is evidently not without its own dangers. Cooks must therefore choose between learning the art of distillation or turning to professional distillers who can do the job for them. Many qualified experts recommend that odorant-compound concentrations not exceed ten to twenty parts per million. What does that mean as a practical matter? It means that a teaspoonful of an odorant compound—a few grams—will need to be dissolved in a million grams (roughly a ton) of an inert solvent to yield a tolerably safe dose. Not all inert solvents are suitable, by the way. Water, for example, isn’t a good choice for this purpose. Cooking oils are frequently used, but a variety of alcohols, including ethanol and propylene glycol, may also be considered.
A word of advice for cooks who are looking to buy dilutions of odorant compounds: the raw material itself is relatively cheap (twenty euros, or about twenty-seven dollars, for a kilogram of limonene, for example); it is mainly the dilution process that drives up the total cost, which also includes minor expenses associated with packaging, labeling, and compliance with the relevant regulatory standards.
The art of imparting fragrance, usually (and, in my view, unfortunately) called “aromatization,” needs an adequate technical vocabulary. The short list I give in this section is an attempt to group sensations on the basis of what might be thought of as family resemblances, which owe nothing to biology and everything to subjective—and, in many cases, culturally influenced—perceptions. Note-by-note cooks will be wise to make these terms their own, adding to them in case they notice any omissions, because perfumery is something quite different from cooking.
ALDEHYDIC the characteristic smell of heated iron, sea water, fat, sweat
ANIMAL musk, civet, ambergris
BALSAMIC a heavy, sweet odor associated with chocolate, vanilla, cinnamon
CAMPHORATED impregnated with the smells of camphor and similarly scented products
CITRUS a refreshing smell, with hints of lemon and orange
EARTHY the odors of rich, damp soil
FATTY redolent of streaky bacon and lard
FLORAL fragrances evoking the scents of flowers
FRUITY reminiscent of the scents of fruit
GREEN the familiar fragrances of cut grass and leaves
HERBACEOUS a complex odor combining notes of grass, sage, menthol, eucalyptus
MEDICINAL the singular odor of disinfectants (phenolic, cresolic, and the like)
MENTHOLATED fragrances evoking the scent of menthol
METALLIC an odor noticed near metal surfaces
OAKMOSS the scent of undergrowth and seawater
POWDERY reminiscent of the scent of talc
RESINOUS odors of pine sap and other arboreal exudates
SPICY scents of spices
WAXY the odor of candle wax
WOODY the many scents of wood
When we try to describe odorant compounds, the question arises whether they should be grouped in terms of their characteristic odors, with the aid of the preceding glossary, or in terms of their molecular structure. The first method has the advantage of generating a series of lists, but these would be cumbersome and endlessly long. The second has the disadvantage of making it necessary to organize compounds according to a rather elaborate system of classification, but this is offset by the advantage of showing the sheer diversity of the world of odors and, perhaps more importantly, of introducing new terms that the cooking of tomorrow will soon make familiar. Besides, the basic principles of classification are easy to understand. Let’s get started, then, beginning with the simplest molecules and moving on to more complex ones.
Molecules in aliphatic compounds exhibit a straight chain (or “backbone”) of bonded carbon atoms to which many hydrogen atoms are attached, along with a few oxygen, nitrogen, and other atoms. There are a number of different types.
 Hydrocarbons
Hydrocarbons. These are the simplest organic compounds in a sense because, as their name indicates, they are composed only of carbon and hydrogen atoms. They include gaseous substances used as fuel, such as methane, ethane, propane, and butane. With the addition of more carbon atoms, these compounds are no longer gaseous at ambient temperature and pressure, but liquid or solid as well as inert and odorless.
Modifications of molecular structure need to occur in order for odors to appear, particularly when neighboring carbon atoms are held together by two chemical bonds rather than by one bond (in which case the compounds are said to be “unsaturated”). Whether hydrocarbons are saturated or unsaturated, linear or branched, they are abundant in edible substances, but few of them contribute significantly to the odor of foods. Examples of odorigenic hydrocarbon compounds include 1,3-trans-5-cis-undecatriene and 1,3-trans-5-trans-undecatriene, which are largely responsible for the smell of galbanum resin (and the oil extracted from it).
 Alcohols
Alcohols. Replace a hydrogen atom in a hydrocarbon by a hydroxyl group, in which an oxygen atom is bonded to a hydrogen atom, and you get an alcohol. Saturated alcohols are common in foods, notably in fruits. Since their odor is mild, perfumers do not often use them, but the food industry frequently does in producing compositions and extracts (linear alcohols with four to ten carbon atoms, for example, and isoamyl alcohol). The list of such compounds is extensive. To name just a few:
3-octanol, a colorless liquid that has an odor of earth and undergrowth found in mushrooms, is used to make—you guessed it!—mushroom odors.
2,6-dimethyl-2-heptanol, a colorless liquid with a delicate scent reminiscent of freesia, a South african iris, has not yet been found in natural substances.
Trans-2-hexen-1-ol, found in many fruits, has a green smell, milder than cis-2-hexen1-ol, which it very closely resembles (compounds having the same molecular description but nonetheless different molecular structures are known as isomers).
Cis-3-hexen-1-ol is a colorless liquid with the characteristic smell of freshly cut grass that accounts for 30 percent of the essential oil of green tea.
1-octen-3-ol, our old friend octenol, passes off in the water vapor of mushrooms. Also found in the essential oil of lavender, it has an intensely earthy odor, redolent of undergrowth.
9-decen-1-ol, with a fresh rose smell, is used to make rose-scented soaps.
10-decen-1-ol has a slightly lemony, green, fatty smell and imparts a fresh note to the strongest floral fragrances.
2-trans-6-cis-nonadien-1-ol, with a smell of violet leaf, is found in cucumber, violet leaf, and violet blossom oils. The food industry values it for its fresh, green scent of cucumber.
 Aldehydes and acetals
Aldehydes and acetals. The class of aldehydes is molecularly similar to alcohols. Instead of having a hydroxyl group, aldehydes have a single oxygen atom bonded to a carbon atom. Aliphatic aldehydes are essential in perfumery. Aldehydes whose molecules contain a small number of carbon atoms (acetaldehyde, isobutyraldehyde, isovaleraldehyde, 2-methylbutyraldehyde) are used to give fruity and roasted odors. Aldehydes with between eight and thirteen carbon atoms are widely used in preparing what I propose be called “odorigenic” (rather than aromatic) extracts and compositions, or OECs for short. (More than once in the preceding pages I have criticized the use of the words
aroma and
aromatic in connection with synthetic fragrances. Here, more positively, I am pleased to be able to recommend a new and more accurate term.) The intensity of the odor of these compounds diminishes with their molecular size.
Acetals are formed by combining alcohol and aldehyde molecules. Acetals derived from aliphatic aldehydes have characteristic odors that resemble those of aldehydes, though they are less concentrated. They are frequently used in alcoholic beverages, but seldom in foods because they are unstable. This class of compounds includes:
Hexanal (more formally, caproaldehyde)—found in apples, strawberries, oranges, and lemons—has a green, fatty odor reminiscent of unripe fruit.
Octanal (caprylalehyde) is naturally present in several citrus oils, notably in the oil extracted from oranges. Its strong odor acquires a citrus note when diluted.
Nonanal (pelargonaldehyde) has a rose smell and is found in citrus and rose oils.
Decanal (capraldehyde) is found in many essential oils and citrus-peel oils. It has a strong odor of orange peel, which is transformed into a fresh citrus odor by means of dilution.
Undecanal (undecyl aldehyde) is also found in citrus essential oils. Its odor is flowery, waxy, fresh.
Dodecanal (lauraldehyde or lauric aldehyde) has a waxy odor that evokes the smell of violet when it is concentrated. Found in citrus fruits and pine needles, it is used to impart citrus notes in OECs.
Tridecanal (tridecanaldehyde), present in lemons and cucumbers, has a waxy, citrus odor and gives fresh top notes.
2-methyldecanal has not been observed in nature. It has an odor of citrus peel.
2-methylundecanal is not yet known to exist in nature either. It has a fatty odor with notes of incense.
Trans-2-hexanal is found in essential oils and the green leaves of many plants. Its grassy odor, sharp and rather pungent, becomes green and pleasant when encountered in more diluted form, recalling the scent of apple.
Cis-4-heptanal is a so-called trace compound (one that can be smelled even though its presence is not detectable by analytical techniques) found in many processed foods. It has a fatty odor, slightly fishy with creamy nuances.
10-undecanal has an odor that is at once flowery, heavy fatty, green, slightly metallic.
 Ketones
Ketones. Members of this class of aliphatic compounds have quite different odors from the ones already cited. Ketones have an oxygen atom double-bonded to a carbon atom in the middle of the chain, not to a terminal carbon. I will content myself with mentioning but two examples from a long list:
3-hydroxy-2-butanone (acetoin) has a buttery odor used to add flavor to margarines.
2,3-butanedione (diacetyl), present in many fruits and in butter, is added to both processed butters and margarines.
 Acids and esters
Acids and esters. Let us conclude this brief survey by looking at carboxylic acids and at esters, the compounds these acids form when they react with alcohols. Carboxylic acids are seldom used in either perfumery or the OEC sector of the food industry, but esters (especially acetates, which is to say esters containing acetic acid) are commonly used in both to enhance fruit odors. These compounds include:
Ethyl formate, an ester of formic acid (so called because it is present in the urticating liquid released by many kinds of ants), has a fruity, ethereal, rather assertive odor.
Cis-3-hexenyl formate has been identified in tea. Its green and fruity odor is used to impart green notes to a variety of commercial preparations.
Ethyl acetate has a fruity odor and is found in many kinds of fruits.
Butyl acetate has a strong fruity odor of the sort that is especially pronounced in apples.
Isoamyl acetate is the compound principally responsible for the odor of bananas.
Hexyl acetate has a smell evoking that of peaches.
3,5,5-trimethylhexyl acetate, not found in nature, has a woody scent.
Trans-2-hexenyl acetate, present in fruits and mint essential oil, has a fruity, green odor.
Ethyl propionate, found in fruits, has an odor reminiscent of rum.
Ethyl butyrate, present in fruits and cheese, has a fruity odor recalling that of grapefruit.
Butyl butyrate is present in fruits and honey and contributes to their odors.
Isoamyl butyrate, which has a strong fruit odor, occurs in bananas.
Hexyl butyrate likewise has a strong fruit odor.
Cis-3-hexenyl isobutyrate, present in mint oil, has a fruity, green scent.
Ethyl isovalerate has an odor of blueberry.
Ethyl 2-methylbutyrate, present in citrus fruits, has a green odor redolent of apple.
Ethyl hexanoate and 2-propenyl hexanoate have an odor of grapefruit.
Ethyl heptanoate has scents of cognac.
Ethyl octanoate has a floral, fruity odor.
Ethyl 2-trans-4-cis-decadienoate, identified in pears, has an odor reminiscent of pear brandy.
By now, and probably not for the first time, it will have become clear to you that the number of fresh opportunities for culinary creativity presented by compounds is simply staggering. What the note-by-note cook must keep on reminding himself is that I have so far only scratched the surface of the world of odorant compounds. I have not yet even mentioned terpenes, whose immense diversity is associated with the fact that their basic molecular structure is repeatedly encountered in the multifaceted process of plant synthesis. This time, rather than scroll through a long list, I give only a few examples of compounds that are already well known in the world of perfumery:
Geraniol (3,7-dimethyl-trans-2,6-octadien-1-ol), found in many essential oils, is a colorless liquid with a flowery, slightly soapy odor. In odorigenic compositions, a small quantity accentuates the citrus note.
Nerol (3,7-dimethyl-cis-2,6-octadien-1-ol) is generally found in the company of geraniol. It, too, is a colorless liquid, but with a fresher rose scent.
Linalool (3,7-dimethyl-1,6-octadien-3-ol) is likewise present in many essential oils. It is the principal constituent (60–70 percent) of coriander essential oil, for example. It has a fresh odor, redolent of lily, and is highly volatile, giving a “natural” quality to top notes.
Myrcenol (2-methyl-6-methylene-7-octen-2-ol) has been isolated in essential oils. It accentuates notes of citrus and lavender.
Citronellol (3,7-dimethyl-6-octen-1-ol), usually produced by mixing together two enantiomers, is a colorless liquid with a soft rose odor.
Citral (3,7-dimethyl-2,6-octadien-1-al), one of the most widely used of all odorant compounds, is typically a mixture of two isomers that appears as a nearly colorless liquid, slightly yellowish, with a lemony odor. It is found in citronella oil and is used in a great variety of citrus compositions.
CYCLIC TERPENES
Up to this point I have spoken of compounds whose chains of carbon atoms are linear or branched, but not cyclic (or ring shaped). I now turn to cyclic compounds for several reasons, not least because of the occasion they provide to pay tribute to the English physicist and chemist Michael Faraday (1791–1867), who discovered benzene, regarded today as the prototype “aromatic” compound. The soft, agreeable smell of benzene (a carcinogen, as it happens) is a molecular characteristic determined by the bonds among its carbon atoms—yet another reason not to speak of “aroma” in note-by-note cooking. We will avoid confusion by speaking of “odor” instead.
The basic structure of cyclic compounds is quite simple. If you take a chain of carbon atoms and then loop it back on itself so that the atoms at each extremity are now attached, you obtain what chemists call a ring. Cyclic compounds may contain rings composed of three, four, five, six, or more carbon atoms. Some may also include oxygen, nitrogen, and other atoms.
Certain terpenes, found in many essential oils, are cyclic. Limonene (1,8-p-menthadiene), a liquid with a lemony odor, occurs in citrus peels in the form of its (+) isomer in concentrations as high as 90 percent; the (−) isomer is found in mints and conifers. Menthol exists in several forms: (−)-menthol, (+)-neomenthol, (+)-isomenthol, and (+)-neoisomenthol, the first being the most frequent; the principal compound of mint essential oils, menthol is also refreshing. Carvone (1,8-p-menthadiene-6-one) also exists in several liquid forms, all of them slightly yellowish, but with very different odors: in the (+) form, carvone is the principal compound of caraway and dill essential oils; in the (−) form, it is present in mint. Fenchone (1,3,3-trimethylbicyclo[2.2.1] heptan-2-one) is a compound whose (−) isomer has an odor of fennel. Ion-ones, produced by the degradation of carotenoids—yellow, orange, and red pigments that are cousins of the beta-carotene found in carrots—are present in many essential oils.
OTHER AROMATIC COMPOUNDS
Not all cyclic compounds are terpenes, of course. Other aromatic compounds—I would be happier, as I say, if they were called odorigenic compounds—are cyclic as well. For example, p-cymene is present in many essential oils; in its pure form, it has a citrus scent. Benzaldehyde has a bitter almond odor. Cinnamaldehyde (3-phenyl-2-propenal), a somewhat spicy liquid in which the trans isomer is dominant, is the principal compound of cinnamon.
PHENOLS AND PHENOLIC DERIVATIVES
When aromatic compounds contain a hydroxyl group, they become phenolic compounds, a class of organic compounds in which a hydroxyl group is bound directly to a carbon atom in a benzene ring. This class includes the following compounds:
Anethole (1-methoxy-4-[1-propenyl]benzene) composes 80 to 90 percent of the essential oil of anise, star anise, and fennel. It is this molecule, soluble in alcohol but insoluble in water, that causes the cloudiness one observes when water is poured into pastis. (The proof that water is dangerous, by the way—as alphonse allais, inventor of the frosted-glass aquarium for shy goldfish, famously remarked—is that a single drop is enough to cloud the purest pastis.
Eugenol (2-methoxy-4-allylphenol), also a yellowish liquid with a spicy odor, is the principal constituent of several essential oils, notably clove and cinnamon.
Isoeugenol (2-methoxy-4-[1-propenyl]phenol), a viscous, yellowish liquid with an odor of clove, is often found together with eugenol in essential oils.
Thymol (2-isopropyl-5-methylphenol), the principal constituent of thyme and oregano essential oils, forms colorless crystals.
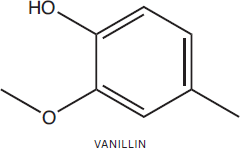
Vanillin (4-hydroxy-3-methoxylbenzaldehyde) is a colorless solid with a vanilla odor and the principal constituent of vanilla essential oil.
4-(4-hydroxyphenyl)-2-butanone has a strong raspberry odor.
O-, N-, S-HETEROCYCLES
I spoke earlier of cyclic compounds in which a carbon atom is replaced by an oxygen, nitrogen, or sulfur atom. By way of conclusion, here are a few examples that I hope will inspire note-by-note cooks to use heterocycles, as these cyclic compounds are called, albeit in suitably diluted form:
2-furaldehyde has an odor of freshly baked bread.
2-methylfuran-3-thiol has an odor of roasted beef.
Eucalyptol (1,8-epoxy-p-menthane) is used to impart a note of freshness.
Maltol (3-hydroxy-2-methyl-4H-pyran-4-one) occurs in the form of colorless needles having an odor of caramel or freshly baked cake. It is used as a flavor enhancer in fruity compositions, especially ones in which a note of strawberry is predominant.
Gamma-decalactone has a peach odor. Delta-decalactone is used for creams and butters.
Nature does, of course, provide us with an abundance of fragrant products that are easy to use—herbs and spices, for example—but these things do not always make a very powerful impression, for the odorant compounds they contain have to migrate from the ingredient to the food before then passing more or less completely into the mouth, as we saw in connection with tastes (recall the matrix effects discussed in
chapter 3). OECs, by contrast, so long as they are dispersed on the surface of foods, make their effects felt at once and without limitation. What’s more, such preparations do not spoil, nor are they sensitive to the vagaries of the weather. The cook who hopes to experiment successfully with these products will nevertheless need to keep in mind three essential points: he must choose a substance that is well suited to his purpose, incorporate it in his dish at the right moment, and use it in the right amount.
With regard to proper dosage, first, the note-by-note cook will profit by learning from perfumers, who take care to distinguish between top notes, the first notes to be sensed; middle notes, the next ones to be perceived; and bass notes, which are recognized last. Top notes are often derived from compounds with the smallest and most volatile molecules, but both molecular structure and the environment into which compounds are introduced need to be reckoned with. A compound is released more rapidly when it is pure than when it is dissolved in a fatty substance, for example.
The art of perfumery can also teach cooks that there must be a kind of balance, or equilibrium, between top and middle notes. Aromaticians—counterparts to perfumers in the food industry, who specialize in OECs—are keenly aware that there must be a more general equilibrium between odor and taste as well. Aerated products, for example, serve to make top notes more prominent: the longer a foam can be kept in a stable state before being consumed, the more time there is for the leading compounds to pass into the air inside the bubbles that make up the foam before being released in the mouth, where they are immediately recognized by the organs of taste and smell.
Basic physical chemistry suggests many other rules of thumb. The cook must, for instance, take into account the absorbent properties of gums and other fatty substances that constitute the chemical environment of an odorant compound: the more thoroughly the compound is absorbed, the less of it is released; conversely, the greater the absorption, the longer it takes for the compound to be precipitated (or evolved as a gas). This is why some products (recall once again the matrix effects we examined in connection with taste) accentuate the perception of certain compounds, whereas others make them seem less assertive. Milk, for example, has a powerful masking effect, unlike alcohol, which heightens the taste and smell of foods, giving them color and sparkle. The temperature at which a dish is consumed is no less important, as makers of processed pork products well know. The same thing is true in the case of odor, for heating causes the most volatile compounds to evaporate. A food eaten cold will therefore have to contain a larger quantity of odorant compounds than the same food eaten hot if it is to have a comparable sensory effect.
There is an important but so far poorly understood class of sensations whose receptors, like the ones responsible for our perception of odors and tastes, are located in the nose and the mouth but are not therefore reducible to odors and tastes. They are associated with a specific neural pathway, the trigeminal nerve, which runs down from the back of the skull and divides into three branches—hence the nerve’s name. The receptors at the ends of these branches register both fresh, cooling sensations and the sensations of pungency and heat produced by many spices. These receptors seem also to play an important role in the perception of pain, although in this connection their functioning is even less well understood. There’s no point belaboring the fact of our ignorance. We must step aside and let science get on with its work.
The food industry spends a great deal of time and money investigating the properties of coolness and heat. Commercial secrecy is intense. Manufacturers of compounds and preparations that stimulate trigeminal sensations jealously guard the results of their research, and still today note-by-note cooks do not have ready access to information of the sort I have just given regarding odorant compounds. Here and there I have mentioned in passing a certain freshening compound, such as menthol (extracted from mint), eugenol (from clove), and xylitol (from various fruits). The hard work of discovering exactly how their effects are produced nevertheless remains to be done—and not only by scientists. A step forward would be taken, for example, if an online discussion forum were to be set up where cooks could post tasting notes about refreshing and pungent ingredients. Head colds are not a disability in this enterprise, by the way, because they allow distinctions to be made that will be of value to sensory physiologists. The next time you come down with a cold, taste some mustard, a hot pepper, some ground black pepper, watercress, a raw chanterelle mushroom, some raw garlic, and then try to describe the sensations. Why these particular ingredients? Because they all are hot or pungent in one way or another. Do the same thing with ingredients that impart a cooling, refreshing sensation (or at least are popularly supposed to do so), once again keeping in mind that some people perceive food sensations mainly through the mouth, others mainly through the nose; and also that the trigeminal system, though it is not reducible to tastes and odors, is not unrelated to them either, for a compound that is cool or hot often has a distinctive odor or taste, or both.
Many people have heard it said that the pungency of black pepper is due to piperine and that the pungency of hot peppers is due to capsaicin. And so they are. But these compounds are only two among a great many more or less similar compounds that have trigeminal effects. Recent studies of vanilla have shown that the pods also contain, in addition to the vanillin compound I mentioned earlier, velvety-tasting compounds. Are these compounds associated with a presently unknown trigeminal sensation? No one knows. But chemists have long been aware that the world of natural products—the good Lord’s rich storehouse, as it is referred to in the Bible—is far more vast than the part of it that is known to us today. It is high time that cooks were aware of this as well.
Borysik, A. J., L. Briand, A. J. Taylor, and D. J. Scott. “Rapid Odorant Release in Mammalian Odour Binding Proteins Facilitates Their Temporal Coupling to Odorant Signals.” Journal of Molecular Biology 404, no. 3 (2010): 372–380.
Calvino, B., and M. Conrat. “Pourquoi le piment brûle.” Pour la science 366, no. 4 (2008): 54–61.
Charles, M., S. Lambert, P. Brondeur, J. Courthaudon, and E. Guichard. “Influence of Formulation and Structure of an Oil-in-Water Emulsion on Flavor Release.” In D. D. Roberts and A. J. Taylor, eds., Flavor Release, 342–354. Washington, D.C.: American Chemical Society, 2000.
Labbe, D., F. Gilbert, N. Antille, and N. Martin. “Sensory Determinants of Refreshing.” Food Quality and Preference 20, no. 2 (2009): 100–109.
Surburg, Horst, and Johannes Panten. Common Fragrance and Flavor Materials: Preparation, Properties, and Uses. 5th ed., completely revised and enlarged. Weinheim, Germany: Wiley-VCH, 2006.



