Energy, entropy and the search for perpetual motion
The exact origins of the phrase “no such thing as a free lunch” are unclear, but most sources say it began life as the pithiest summary of economics. It appeared in Pierre Dos Utt’s 1949 monograph TANSTAAFL: a Plan for a New Economic World Order, where Dos Utt tells of a king seeking economic advice. His advisers, looking for ever-simpler ways to get their message across, conclude with the now-classic version of the phrase: “There ain’t no such thing as a free lunch.”
It is doubtful this would have been enough to motivate economists to usher in a new world order, and the physicists of the time would have certainly been unimpressed. The idea of something for nothing had long been a goal of inventors trying to get a free lunch by coming up with “perpetual motion machines” that would do work without the need for any external power. Physicists had long been telling them this was impossible.
There is no such thing as a free lunch because you simply can’t get something for nothing: someone, somewhere always has to pay. Physicists have enshrined this principle as a fundamental law of physics. So you need to think hard before you start looking for a free lunch, because you are battling against the way the universe runs. Perhaps the great artist, visionary and inventor Leonardo da Vinci put it best. He took a keen interest in perpetual motion, investigating designs, and coming up with a few of his own. But he was skeptical about them all: one of his notebooks contains a detailed analysis of a popular kind of machine, showing why and how it could not work. “O you researchers of perpetual motion,” Leonardo wrote, “how many harebrained ideas have you created in this search. You may as well join the alchemists.”
“O you researchers of perpetual motion, how many harebrained ideas have you created in this search. You may as well join the alchemists.”
LEONARDO DA VINCI
There are two kinds of perpetual motion machines. The first supplies an endless output of work despite the fact that there is no input of fuel or any other form of energy. The second converts heat to mechanical work with perfect efficiency. Both, it should be made clear, are wishful thinking—and physics tells us why.
As with alchemy, the search for perpetual motion engaged some of the finest minds that have graced the Earth. The dream has been around since at least AD 624, when the Indian mathematician and astronomer Brahmagupta described a wheel whose hollow spokes could be filled with mercury. The mercury would shift weight around the wheel as it rotated. As a result, Brahmagupta wrote, “the wheel rotates automatically forever.”
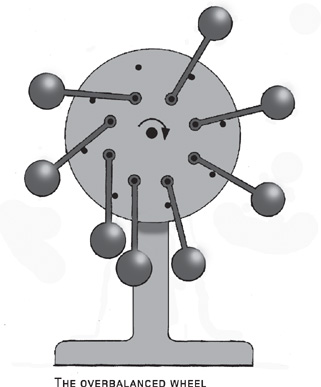
The idea was repeated numerous times. In 1235, Villard de Honnecourt, a French artist and inventor, produced his own version. De Honnecourt was no fool: he drew the first known plans for a mechanical escapement mechanism that would keep time. But de Honnecourt’s “overbalanced wheel” still doesn’t work. Here, a series of hinged weights are attached around the circumference of a wheel, their motion limited by pins. As the wheel turns, an imbalance in the distribution of weights causes the wheel to turn. As it turns, the elevated weights drop onto their pins, and the transfer of weight keeps the wheel turning.
The fact that the perpetually rotating wheel is a running theme in the search for perpetual motion can only mean that very few people tried to build these kinds of machines. Build one and you soon learn that they just don’t work. Take de Honnecourt’s overbalanced wheel, for example. What is needed for this to carry on forever is for the uppermost rod to flip over as it reaches the top of the wheel, maintaining the imbalance. Unfortunately, this doesn’t happen: the weight distribution is such that it doesn’t quite flip. After one revolution, the weights return to their initial position, and everything is back exactly where it started—including the stationary wheel.

To be fair to de Honnecourt, the reason for this was not clear until well after his time. The problem is that energy is transformed between two different forms. Because the rods have the potential to fall under the influence of gravity, they are said to have “potential energy.” If the wheel turns, some of this converts to the “kinetic energy” of movement. However, after one cycle, the rods return to their initial position, and therefore must have exactly the same potential energy (which is due to their position) as before. Since there is no external source of energy, and the rods have the same potential energy at every turn, there is nothing to put energy into turning the wheel.
By 1775, the Royal Academy of Sciences in Paris had had enough of perpetual motion. It issued a statement declaring that the Academy “will no longer accept or deal with proposals concerning perpetual motion.” And in 1841, scientists finally found a scientific principle to throw at perpetual motion seekers: the first law of thermodynamics.
It was the first explicit statement of the conservation of energy. Leonardo da Vinci had suggested that, “Falling water lifts the same amount of water, if we take the force of the impact into account,” but it took the German physicist Julius Robert Von Mayer to explore the matter properly and issue an edict. Energy, he said, cannot be created or destroyed.
Not that he was taken seriously straight away: Von Mayer was told, for instance, to find some experimental evidence to back up this strange idea. This he did, by showing that the kinetic energy of vibration could be transferred to water molecules, manifesting as an increase in temperature. Once the point was proven, the principle was quickly accepted by physicists, and used to keep perpetual motion at bay. Motion takes energy, and the conservation of energy principle tells us that you can’t get more energy out of a closed system than is there in the first place. Since friction affects any and every mechanism, dissipating some of that energy as heat and sound, inventing perpetual motion machines of the first kind became a fool’s errand. Not that this put the perpetual motion seekers off. Around this time, the science of thermodynamics was giving them a whole new lease of life. Their goal? Perpetual motion machines of the second kind.
The second kind of perpetual motion machine is something that extracts heat energy from a reservoir, such as the air or the ocean, and converts it into mechanical energy. It certainly seems like a good idea. The oceans are so vast a resource that, if we could extract heat that would cause a one degree drop in ocean temperatures, it would supply something like the energy needs of the United States for half a century.
The plausibility of this kind of machine is enticing. Indeed, creating an efficient steam-powered engine has been a human obsession since Hero of Alexandria created the “aeolipile” in AD 1. This ball, that was set rotating by jets of steam, had no particular uses. However, subsequent inventions used steam turbines to turn spits, pump water from mines and power grinding pestles. None of them got anywhere near a truly useful efficiency, however. That efficiency came with James Watt’s steam engine, first demonstrated in 1765. It was a development of the engine invented by Thomas Newcomen, and raised the efficiency enough to kick off the Industrial Revolution. The theory behind such engines, though, was still very much in development. The builders of steam engines were working on hunch and intuition, not scientific theory.
It wasn’t until 1824 that the French military scientist Sadi Carnot published On the Motive Power of Fire. Even then, this primary work in the field went largely unnoticed for a decade. But the scientific principles behind the steam engine were now in place. And, as a bonus, Carnot had worked out the principle that denies a free lunch to perpetual motion machines of the second kind.
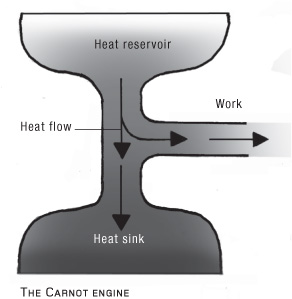
There is a good reason why you can’t get useful work out of a room temperature heat source. It is called the second law of thermodynamics, and it says, essentially, that you can’t take the heat from something then turn all the heat into mechanical work. Some of that heat has to be passed on to a “heat sink” at a lower temperature. It is the temperature difference between the heat source and the heat sink that determines how much work you’ll get out of this “heat engine.” Carnot showed that creating a perfectly efficient heat engine is impossible.
To see why, let’s imagine an engine. Any engine seeking to perform work requires energy, which we will consider to come in the form of heat. Heat flows from a hot source to a colder one (this principle seems so obvious it was only formalized as the “zeroth law” of thermodynamics long after the other laws were laid down), so both reservoirs are required; work can be extracted as heat flows from a hot “reservoir” to a cold one.
The work extracted in this situation is the difference between the heat flowing out of the hot reservoir and the heat flowing into the cold reservoir. A perfect efficiency would have zero heat flowing into the cold reservoir so that all of the heat energy is used for the work you want to do.
Now let’s consider, as Carnot did, the practicalities of the engine. Carnot imagined a piston engine much like the cylinder of a car engine, where the heat is used to expand gas that pushes on a piston. The gas is then compressed, and the cycle begins again. By considering the gas laws that relate pressure, temperature and volume, Carnot showed that the efficiency of an engine depends upon the ratio of the temperatures of the hot and cold reservoirs. No matter what fluid or gas is being used to power the engine, the ratio of the two temperatures is everything. And here is the problem with this free lunch.
The average diesel engine operates at around 550 Celsius. The exhaust gases exit to the outside temperature. The maximum efficiency possible, according to Carnot’s work, is around 60 percent. In reality, a diesel-powered car converts around 50 percent of its fuel’s chemical energy into energy that can move the car along the road. The rest is wasted as heat (which is why cars need cooling systems). Gasoline engines are significantly less efficient.
What if we operate the two reservoirs at the extremes of temperature? In theory, the hot reservoir can operate at infinitely high temperatures. But the cold reservoir cannot be colder than absolute zero. Even dumping the heat in outer space would give a cold reservoir temperature of 3 K, or –270 Celsius. Because you can’t get lower than zero, and an infinitely hot reservoir does not exist (at least not one that we know about), a perfectly efficient engine is impossible. You cannot convert heat into work without wasting some of the heat. And that means that, to continue the cycle, you always have to put in energy. No free lunch, in other words.
Carnot’s work led directly to the formulation of the second law of thermodynamics. As phrased by the English physicist Lord Kelvin and the German physicist Max Planck, it states that an engine operating in a cycle cannot transform heat into work without some other effect on its environment. Thanks to the second law, not only can you not get a free lunch, you can’t even keep your lunch cool in the refrigerator for free. Refrigeration, it turns out, is nothing more complicated than the Carnot engine working in reverse.
“An engine operating in a cycle cannot transfer heat from a cold reservoir to a hot reservoir without some other effect on its environment.”
RUDOLPH CLAUSIUS
In 1850, the German physicist Rudolph Clausius rephrased the second law to read, “An engine operating in a cycle cannot transfer heat from a cold reservoir to a hot reservoir without some other effect on its environment.” A refrigerator, in other words, needs energy put into it. This arises from the natural tendency of energy to flow “downhill”: from hot to cold. Keeping the inside of your refrigerator below the temperature of your kitchen involves the same process of expanding and contracting, heating and cooling gases as running your car engine, and it all takes energy. This time, though, you need a compressor rather than an expander for the gas.
As mentioned, Carnot’s work involved consideration of the pressure, temperature and volume of the gas. The process that Carnot uncovered led to another revelation for physicists: the notion of entropy. The whole universe, it turns out, is spiraling into ever-more disorder. It was Clausius who classified this disorder as “entropy,” a word derived from the Greek for “transformation.” In 1865, he wrote a mathematical treatise on the work that the atoms do on one another in a gas. The result, Clausius showed, is that the second law can be expressed in a new way: the entropy, or disorder, of a closed system either stays the same or increases—it never decreases.
That doesn’t mean you’ll never see entropy increase on a small scale. Your lunch inside the refrigerator will get cold, for instance, decreasing the disorder in its constituent molecules. But don’t be fooled that this breaks the second law of thermodynamics. The inside of your fridge is not a closed system—the molecules of refrigerant gas take the heat away, and their disorder increases as they do. As the heat is transferred to the air in your kitchen, the disorder in your house increases too.
This kind of thing is happening throughout the universe as the processes of nature unfold. It creates, in physicists’ view, the irreversibility of natural processes: the arrow of time is just another way of expressing the second law of thermodynamics. The wasted energy of Carnot’s engine cycle is the slow unraveling of the universe in microcosm.
Together, the first and second laws of thermodynamics put up a brick wall to any claims for the generation of a free lunch. So well proven are they, in fact, that the US Patent Office warns anyone submitting a patent for a perpetual motion machine that they should think carefully; they will most likely lose their money. “The views of the Patent Office are in accord with those scientists who have investigated the subject and are to the effect that such devices are physical impossibilities,” the office’s official statement says. “The position of the Office can only be rebutted by a working model…. The Office hesitates to accept fees from applicants who believe they have discovered Perpetual Motion, and deems it only fair to give such applicants a word of warning that fees cannot be recovered after the case has been considered by the Examiner.” So not only is there no such thing as a free lunch; even looking for one could end up costing you money.