Chapter 1
The Four Secrets The Drug Companies Don’t Want You To Know
Pretending to care about our health is often just a part of the drug and other medical industries’ overall strategy to increase their sales.
—Dr. John Abramson, Overdosed America
A comic strip entitled “Off the Mark” (written by Mark Parisi) depicted the following: The Tin Man comes upon Dorothy and the Cowardly Lion in the woods. At their feet are the remains of the Scarecrow. Dorothy, who, along with the Lion, is eating the last bits of straw, says to the horrified Tin Man: “Dr. Oz said to eat more whole wheat. . . .”
Admittedly, this cartoon takes the influence of Dr. Oz to the extreme. But its premise is not too far from the truth. Dr. Oz has become America’s physician. He has the endorsement of Oprah Winfrey and his own daily show (twice a day in some markets!). In 2011, he won two Daytime Emmy awards, one for best informative talk show and the other for best talk-show host. His show on average is watched by over 4 million people each day. He is clearly beloved by his followers. He is better known and, when it comes to dispensing medical advice, he is more influential than the US Surgeon General (Dr. Regina Benjamin at the time of this writing).
But this fame and influence puts an onus on him to be especially accurate in his comments and opinions. When I appeared on Dr. Oz’s show with Dr. John Abramson, we debated the value of statin drugs to prevent heart attacks and strokes: Dr. Abramson took the view that statins are unnecessarily prescribed while I defended the fact that statins have saved countless lives. Dr. Abramson said that he often took his patients off statins and I was stunned by this. I turned to Dr. Oz and asked: “Dr. Oz, you’re a cardiologist, how do you prescribe statins?” He replied: “I am usually the one taking them off statins,” at which point the audience broke into applause.
Dr. Oz then said that he doesn’t have a vendetta against statins and that: “There are people whose lives are saved every day with statins.” However, I am afraid that these later points were lost to both the audience and the millions who watched the show. What many people actually heard from Dr. Oz’s pronouncement was that millions of people are needlessly taking statins. This is evident from subsequent summaries of this discussion that now appear on various websites. These summaries highlight the fact that Dr. Oz takes patients off statins and don’t mention his points about how statins do save lives.
Like Dr. Oz, I am a big believer in making lifestyles changes, such as watching your diet or exercising, as a way of warding off disease and also preventing the need for medications. But I believe that doctors don’t haphazardly prescribe statins to their patients. They prescribe these drugs knowing their patients’ health history and medical profiles. When a patient hears someone as prominent as Dr. Oz saying that medicines like statins are overprescribed, many are likely to stop taking these drugs—resulting in potential dire downstream consequences.
Dr. Oz does a great job with certain things such as warning his audience about the perils of too much sugar in their diet. He is a great teacher of good nutrition and the benefits of eating fruits and vegetables. He often provides sound advice and information. But Dr. Oz is in a unique position among daytime hosts. His words are gospel to the American public. Thus, he has an enormous responsibility when it comes to commenting on medicines that members of his own profession prescribe. Unfortunately, there will be patients who will act like Dorothy and take his words a bit too literally.
It was, therefore, concerning to me that he took on the pharmaceutical industry from a position that can be viewed as inflammatory. Here were his “Four Secrets That Drug Companies Don’t Want You to Know”:
1. Drug companies underestimate dangerous side effects.
2. Drug companies control much of the information your doctor gets.
3. You’re often prescribed drugs that you don’t need.
4. Drugs target the symptoms, not the cause.
It was amazing to watch the reaction of the audience as these topics were discussed. Right from the outset of each discussion, the audience was clearly in agreement with Dr. Oz as evidenced by the nodding of heads and applause for some of his comments. To be clear, there were points that Dr. Oz made that I openly agreed with. In fact, each one of his “secrets” was rooted in a valid starting point. But overall, his views paralleled many preconceived incorrect notions, which continue to haunt the pharmaceutical industry. These need to be corrected before the pharmaceutical industry can regain trust as an innovator against disease.
Drug Companies Underestimate Dangerous Side Effects
Dr. Oz believes that all drugs have dangerous side effects. He is absolutely correct. It is impossible to discover and develop a drug that will be universally safe in millions of people around the world: males and females; old and young; large and small; all members of different ethnic groups. Think about people with peanut allergies who can die by ingesting a single nut, or consider others who are lactose-intolerant. If basic foods are not tolerated by all, how can it be expected that a medicine, which is specifically designed to interact with critical biological processes in one’s body, be totally safe for every person?
When a company wants a new medicine approved by the FDA, it files a New Drug Application (NDA). The NDA contains all relevant data from tests done with the potential new drug, starting with the earliest animal studies through all the human studies done to prove the efficacy and safety of the medicine. Thus, the FDA has access to all data generated with the drug, and they will determine, in conjunction with outside experts, the benefits of the drug along with its risks. If the drug is intended for use in patients for whom good treatment options already exist, the risks need to be relatively minimal because there is no need to expose patients to a new drug with added risks over existing, well-understood therapy. However, if the new drug is for a condition, especially a life-threatening condition, for which no good treatment option exists, the FDA will be tolerant of a certain degree of side effects in order to get such an important drug to patients in need. Thus, the risk–benefit profile of a drug is not evaluated via a fixed equation. It is based on evaluating the need of the new medicine as measured against the negative effects it might have for the patient.
So, if the FDA has such a rigorous system, how is it that drugs get approved that are later found to have more serious side effects than were originally known? In spite of the years devoted to studying a new drug in patients (often in excess of a decade) and the enormous costs involved (estimates will vary based on the nature of the drug, but in general anywhere from $1 to $3 billion is spent on a single new medicine), usually each NDA has test data from 2000 to 20,000 patients. Once the NDA is approved, it is likely that millions of patients will be prescribed the medicine. Thus, if the drug causes a rare side effect, say 1 in 50,000 patients, it may not be discovered until it has been broadly used. However, neither the drug maker nor the FDA is ignorant of such an occurrence. Every company has a pharmacovigilance program, the purpose of which is to monitor any unusual safety issues that may arise with both experimental and approved drugs. The FDA also maintains an Adverse Event Reporting System, a computerized database designed to monitor the safety of all medicines sold in the United States. The database contains reports submitted by manufacturers, health-care professionals, payers, and consumers, and these reports get scrutinized by FDA officials to determine if further investigation is warranted.
Despite this system, Dr. Oz is right in saying that we don’t know the full profile of a new drug. However, his solution is to use more generic drugs:
Use generic drugs, because they’ve been around for a long time, they’re old drugs, and they’ve been used by so many people that we know about their side effects. And, they’re cheaper.
Ironically, Dr. Oz opened his show talking about recent safety concerns that have arisen over the use of Alli, a generic drug. Clearly, Dr. Oz’s suggestion that generic drugs are safer is an oversimplification. His contradiction highlights the fact that the public needs to be better informed about what a generic drug is.
When a drug company invents a new medicine and has it approved by the FDA, it has a patent on the new medicine that allows it a period of time (generally 10–12 years) during which it is the only company that can sell the drug. Once the patent for this medicine expires, it “goes generic”—that is, anyone can seek approval from the FDA to make and sell this drug—provided that their version of the drug is bioequivalent to the original medicine (that is, the drug behaves identically). In fact, the vast majority of prescriptions written every year are for generic drugs.
But just because a drug reaches generic status doesn’t automatically make it universally safe. It just means that its patent has expired and it can be made by a lot of other companies. Because it has been on the market for a number of years, more is understood about the risk–benefit profile of the medication at this point than when it was first marketed. However, the safety profile of the compound doesn’t change. As a generic, it still has the same side effects it had when it was a branded drug. A great example of this is acetaminophen, the active ingredient in Tylenol. There is no difference between Tylenol, the branded drug, and acetaminophen branded by CVS, Rite Aid, or another pharmacy. Acetaminophen has been taken by hundreds of millions of people around the world over the last 50 years. You can buy it in any drug store and most supermarkets. More is known about it than about most prescription medications. And yet acetaminophen is the leading cause of calls to Poison Control Centers every year (>100,000). Acetaminophen accounts for more than 56,000 emergency room visits, 2600 hospitalizations, and an estimated 458 deaths annually as a result of acute liver failure. These incidents are largely due to overdoses of this drug, both intentional and unintentional.
Perhaps the best explanation of the dangers of an old drug comes from David Stipp’s article in Fortune magazine entitled: “Take Two Possibly Lethal Pills and Call Me in the Morning.”1
In it, Stipp describes a medicine that is “. . . commonly given to patients for non-fatal conditions such as mild inflammation. Yet, studies suggest that it and several drugs like it are fatal to at least 10,000 Americans a year. The victims die grisly deaths, typically from internal bleeding.” This drug is acetylsalicylic acid, also known as aspirin. This drug has been available over a century and has probably been ingested by over a billion people. And yet, if it were discovered in a pharmaceutical R&D laboratory today, it might never have been developed. The reason is simple. In standard animal testing, gastric lesions and bleeding are observed very quickly. Such toxicity might not be accepted by companies or regulators today because it would lead to thousands of patients being hospitalized from gastrointestinal bleeding. Thus, scientists would likely abandon aspirin and look for a safer compound that provided pain relief without this toxicity.
So, the bottom line is that every medicine can cause a safety problem in people. People should only turn to medicines as a last resort. Lifestyle changes, diet, and exercise are the sorts of things people should do before turning to pills. And when people do need to turn to medicines to alleviate their illness, they should discuss with their doctor the potential side effects that the prescribed medication has. Yet, despite all of these caveats, medicines save lives, prevent serious debilitating diseases, and improve the lives of millions of people each year.
After reading all of this, a person may be turned off from taking any medicine, be it prescription or generic. Why ingest anything that might cause harm? However, people do get sick, get headaches, sprain ankles, and so on. Interestingly, a number of people turn to herbal remedies for these situations.
There is no denying that many drugs have their beginnings in traditional medicines. Tales abound of the healing properties of Chinese herbal medicines or plant treatments used by Native American healers. Hundreds of years ago, people knew that chewing the bark of a willow tree relieved their pain. In the 1800s, salicylic acid was identified as the active component in willow bark; and, in 1899, scientists at Bayer synthesized a new form of salicylic acid called acetylsalicylic acid, the aforementioned aspirin. This is not an isolated example.
The modern drug industry may owe its roots to early traditional medicine, and certainly the end goal is the same now as it was hundreds of years ago. But the continued grip of herbal medicines on the general population is astounding. The New York Times2 reported that in 2011 the US sales of dietary supplements purported to be used for health benefits came to $28.1 billion, a jump from $21.3 billion in 2005. Consumers of these products are driven to them because of the expectation that natural products are inherently safer than medicines derived from drug companies. Furthermore, there is a belief that centuries of use ensure efficacy, a belief echoed somewhat in Dr. Oz’s views of generic medicines. However, studies show that this is not always true.
Ginkgo biloba is marketed as an herbal medicine that enhances memory, and it may be the most widely consumed herbal treatment used to prevent age-related cognitive decline. Its popularity is attested to by its US sales, which exceed $250 million annually. In 2008, the Journal of the American Medical Association (JAMA) published the initial results of the Gingko Evaluation of Memory (GEM) trial,3 a randomized, double-blind, placebo-controlled clinical trial designed to test Ginkgo biloba for preventing dementia. GEM was conducted in five academic medical centers in the United States between 2000 and 2008 with 3069 volunteers aged 75 or older with normal cognition or mild cognitive impairment. Half of this population received a standardized extract of Ginkgo biloba and half received a placebo; they were followed for an average of 6.1 years. At the end of this time, there was no statistically significant difference in the occurrence of either dementia or Alzheimer’s disease between the two groups. In other words, Ginkgo biloba had no beneficial effect with respect to these parameters.
Saw palmetto is extracted from the fruit of Serenoa repens. Native Americans use the saw palmetto berries to treat urinary problems, and it has grown popular among older men as a treatment for benign prostatic hyperplasia (BPH). In 2006, the results of the Saw Palmetto Treatment for Enlarged Prostates (STEP) trial were published.4 This was another multicenter double-blind, randomized, placebo-controlled trial comparing 160 mg twice a day of saw palmetto versus a placebo. Despite looking at a variety of parameters (prostate size, residual volume after voiding, PSA score, or quality of life), after one year there was no difference between the results seen with saw palmetto and placebo. In addition, the results of yet another trial, this one published in JAMA,5 showed that doubling and then tripling the dose used in the STEP trial for two years also had no effect. Saw palmetto is no more effective than placebo in treating BPH.
The following headlines are pretty impressive:
“Smokers quit with cheap Bulgarian remedy. For as little as $6, there may be a smoking-cessation remedy that actually works.”
“Soviet-era pill from Bulgaria helps smokers quit.”
“Smokers get chance to beat the habit with 12-pence tablets.”
This publicity was generated by a paper in the New England Journal of Medicine (NEJM) entitled “Placebo-Controlled Trial of Cytisine for Smoking Cessation.”6 In this double-blind study, 740 smokers were equally divided into two groups: one taking a cytisine preparation sold in Bulgaria as Tabex, the other given placebo. After 4 weeks of dosing, treatment was stopped and the patients were monitored for a year, after which time 8.4% (31 participants) on cytisine and 2.4% (9 participants) on placebo had quit smoking. How does this compare with other smoking cessation regimens? A similar type of smoking cessation study published in JAMA7 showed that Chantix (varenicline) provided 23% continuous abstinence, buproprion 14.6%, and placebo 10.3%. (In terms of full disclosure, Chantix was discovered and developed at Pfizer during my tenure as head of research.)
The cytisine study is noteworthy in that, while its anti-smoking effects have been known for over 40 years, this is the first reported clinical trial done in a double-blind placebo-controlled manner as required by regulatory agencies like the FDA. But the real driver and interest in this work is the fact that the Tabex brand of cytisine is cheap and available online. The leader of the NEJM study, Professor Robert West, predicted that “the publicity surrounding his findings would trigger a surge in people turning to websites to obtain it.” Costs of smoking cessation products vary from country to country; but, as the study authors point out, the cost of a course of cytisine therapy is about 10–20% that of Chantix. That difference could mean big savings to agencies like Britain’s National Health Service and Medicare in the United States.
So should we rush to get this drug? I would argue not just yet. There are still a few issues that need to be addressed. First of all, how effective is cytisine compared to the currently marketed agents? Unfortunately, the NEJM study does not include a “positive comparator,” either Chantix or buproprion, so that one can get a sense of how the efficacy of a current treatment compares directly with cytisine in the same study. Looking at the data, it doesn’t seem that cytisine is as effective as the other compounds. If cytisine is only half as effective in causing people to quit smoking, that should cause payers, physicians, and patients to think twice about replacing other methods with cytisine.
However, there is a much bigger issue facing cytisine use: safety. While Tabex has been available in all former socialist countries since the early 1960s, it was withdrawn from the market in many of these countries when they joined the European Union. Safety concerns could possibly have been one reason. The Tabex preparation originates from the plant Cytisus laborinum L. There are reports of people getting poisoned with the seeds of this plant. J. F. Etter, in the review article entitled “Cytisine for Smoking Cessation,”8 states that “Poisoning in children who eat laborinum seeds is frequent” and that “in an average summer over 3000 children are admitted to hospitals in England and Wales because of laborinum poisoning.” The symptoms, which include nausea, abdominal pain, respiratory stimulation, and muscle weakness, are consistent with poisoning symptoms with nicotine, which is related chemically to cytisine. Clearly, eating these seeds results in a large overdose of cytisine, and the Tabex dose levels of cytisine are much lower. But this points out the need for extensive safety studies of cytisine. The side effects of Chantix and bupropion are well known. But these adverse effects were found as a result of the extensive data safety monitoring that has been accumulated by the manufacturers over the years these drugs have been on the market. To my knowledge, such an adverse event monitoring system has not been in place for Tabex.
Peter Hajek, director of the Tobacco Dependence Unit at Queen Mary University Hospital in London, said the following to the Associated Press:
It is possible that extensive bureaucracy and over cautious regulations will prevent its (cytisine’s) use in the U.S. and Europe.
One would hope that a person in his position would be concerned with the safety and efficacy of a drug before advocating its use.
There is no doubt that cytisine, in the form of Tabex, is a cheap way to help some people stop smoking. But is it more effective and safer than existing medicines? That can’t be answered with the current data; more studies are needed. Furthermore, by the time these types of clinical trials are carried out, it is possible that a generic form of Chantix, varenicline, will be available, making the cost arguments moot.
One may ask: What’s the harm in these herbal remedies? These extracts aren’t overtly toxic, and so people should be free to take whatever they’d like. Maybe taking these herbal medicines gives them peace of mind. However, because herbal drugs aren’t nearly as well studied as prescription drugs, there could be issues with them that are unrecognized. For example, does taking either Ginkgo biloba, saw palmetto, or cytisine interfere with the metabolism of other medications that one is taking? This idea isn’t far-fetched. It is known, for example, that St. John’s wort, a controversial herbal medicine taken by people to treat their depression, limits the effectiveness of a variety of medications, including antiviral agents, birth control pills, and some anticancer medications.
If a person wants to take herbal medications, that’s his or her personal choice. But patients should be aware that these medications haven’t been subjected to the rigorous regulatory agency scrutiny that all prescription medicines must receive. Buyer beware.
Drug Companies Control Much of the Information Your Doctor Gets
Another topic closely related to the safety of drugs is the claim that drug companies hide negative data on their experimental medicines. These could be data that show that the drug works poorly or that the drug has unreported side effects. This is a tenet of Dr. Abramson, who made the following comment during our discussion:
About 85% of clinical trials are now funded by the drug industry. They own that data. The docs don’t understand that they are getting a selected, filtered version of what the information is.
Some of Dr. Abramson’s charges have a factual basis. In fact, most clinical trials are funded by the drug companies. They are the ones studying the investigational new drug, and so they are obliged to pay for the costs of running these trials. The trials themselves, however, are run by independent physicians at various academic centers and teaching hospitals around the world. Thus, it is safe to assume that they are rigorous in the conduct of the studies as well as in reporting all of the beneficial and harmful effects that the new medicine may cause. As was stated earlier, these data get reported to the supervising regulatory agency.
Furthermore, it is no longer possible to hide clinical trials from the public. As part of the established principles for good conduct of clinical trials, a summary of the protocol of each trial must be made available both while the study is being recruited and while the study is ongoing. The study results then must be made available to the public in a timely fashion. All of this occurs by making use of a government hosted website: www.ClinicalTrials.gov. All companies that run a trial, be it with a drug or a device, must register the trial on this website, making the clinical activities of a company very transparent.
So why is Dr. Abramson still concerned about this? He would like all clinical trial results published on ClinicalTrials.gov and in peer-reviewed journals. This, he believes, would make these data more readily available without the need to go searching for them. The problem is that companies don’t publish all of their results. This is not because they are trying to hide data. Rather, not all trials are worthy of scientific publication, and the decision whether or not to publish a manuscript rests with the medical journal’s editorial staff. A great example exists in the field of depression—interestingly, a topic also discussed by Drs. Oz, Abramson and myself on the show.
Major depression is a serious disease. In 2005, the New England Journal of Medicine published Dr. J. John Mann’s excellent review entitled “The Medical Management of Depression.”9 In this piece, Dr. Mann states: “Major depressive disorder accounts for 4.4% of the global disease burden, a contribution similar to that of ischaemic heart disease. . . .” Equally concerning is that: “Patients who have diabetes, epilepsy, or ischaemic heart disease with concomitant major depression have poorer outcomes than do those without depression.”
One would think, therefore, that those concerned with medical care would be grateful for the availability of drugs to treat depression. Yet, there are many who challenge the value and the need for antidepressants. Dr. Abramson, in his Overdosed America, says the following in his book: “. . . new antidepressants were found to be not even 10% more effective than placebos: symptoms of depression improved by 30.9% in the people who took placebos and by 40.7% of people who took the newer antidepressants.” Dr. Abramson’s implication is that these drugs offer little value over sugar pills.
Dr. Marcia Angell, a noted pharmaceutical industry critic and a former editor of the New England Journal of Medicine, has also expressed a number of concerns about antidepressants, not the least of which is that drug companies rarely publish negative data on these drugs. In her article entitled “The Epidemic of Mental Illness—Why?,” which appeared on June 23, 2011 in the New York Review of Books, Dr. Angell states the following:
When drug companies seek approval from the FDA to market a new drug, they must submit to the agency all clinical trials they have sponsored. . . . If two trials show that the drug is more effective than a placebo, the drug is generally approved. But companies may sponsor as many trials as they like, most of which could be negative—that is, fail to show effectiveness. . . . For obvious reasons, drug companies make very sure that their positive studies are published in medical journals and doctors know about them, while the negative ones often languish unseen within the FDA. . . .
Factually speaking, Dr. Abramson and Dr. Angell are correct. Antidepressant drug trials usually show only modest superiority of the experimental drug over the placebo.
And yes, drug trials where there is no difference in the efficacy of the drug versus the placebo normally aren’t published. However, such statements don’t tell the whole story in the battle to treat psychiatric disorders.
In doing clinical studies, there are some medical areas where it is easy to measure whether a drug is working or not. If you have a new antibacterial to treat an infection, you can take blood samples to measure the effect that the drug is having on the eradication of the bacteria from the patient. Similarly, with a drug to treat high blood pressure, you can treat a patient with a new drug and take real-time blood pressure measurements to quantify the drug’s effects.
Psychiatric diseases are different. In clinical studies, patients are given a standardized test, such as the Hamilton Rating Scale for Depression (HRSD), which involves answering a variety of questions about a patient’s mood, anxiety, ability to sleep, and so on. The patients are then randomized to receive either the placebo or the test drug. Each week, the patient returns and is seen by the psychiatrist to determine if any improvements are evident. Generally, these studies last for about two months. Such studies are notorious for the high efficacy response rates seen in patients who turn out to be taking the placebo. In an excellent 2002 paper on this subject, “Placebo Response in Studies of Major Depression,”10 analysis of 75 clinical trials showed that the response to the placebo across the trials ranged from 10% to more than 50%. While the authors couldn’t point to one definitive reason why such high placebo rates occur, they offered a few possibilities:
1. Patients in these trials, whether on the placebo or on the drug, are usually allowed to take sedatives and anti-anxiety medication, so the placebo responses encompass the effects of these drugs.
2. The weekly physician visits contribute to the patient’s great sense of improvement.
3. It’s likely that these studies included patients with milder, briefer, and more responsive forms of depression, thereby enhancing the chances of either the placebo or the drug being effective.
Thus, it is well established that there are high placebo efficacy rates in clinical trials in psychiatric disorders. As a result, studies where the efficacy of the placebo is equal to that of the drug being tested are rarely accepted for publication by reputable scientific journals. It is not that companies are hiding data. Rather, such a result is of little interest. Most skilled in the science behind the results realize that seeing the benefit of a placebo over an experimental drug is a hazard of this type of study. However, it is big news when a new antidepressant does, in fact, show statistically significant efficacy over the placebo. This instance is deemed very important by the scientific community; and, therefore, medical journals are very willing to publish such results. In other words, Dr. Angell’s concerns are unjustified.
The placebo effect is not unique to depression. Shirley Wang, in an excellent Wall Street Journal article entitled “Why Placebos Work Wonders,”11 gives the following examples, one in weight loss and another in fertility.
Hotel-room attendants who were told they were getting a good workout at their jobs showed a significant decrease in weight, blood pressure, and body fat after four weeks, in a study published in Psychology Science in 2007 and conducted by Alia Crum, a Yale graduate student, and Ellen Langer, a professor in the psychology department at Harvard. Employees who did the same work but weren’t told about exercise showed no change in weight. Neither group reported changes in physical activity or diet.
Fertility rates have been found to improve in women getting a placebo, perhaps because they experience a decrease in stress. A recent randomized trial of women with polycystic ovarian syndrome found that 15%, or 5 of 33, got pregnant while taking placebo over a six-month period, compared with 22%, or 7 of 32, who got the drug—a statistically insignificant difference. Other studies have demonstrated pregnancy rates as high as 40% in placebo groups.
Given this background, it becomes clear that, while the results of all clinical trials need to be registered and filed with regulatory agencies, it is hard to justify publishing all of them. This is not to say that publishing negative data for a medicine isn’t of value. Studies that show a drug doesn’t work in certain indications are just as important as those that show a benefit. But the fact is that the results of a lot of studies don’t merit publishing in major medical journals, be it due to placebo effects or other reasons. This isn’t burying negative data. It is simply publishing meaningful data, be it positive or negative, that can help guide physicians in properly treating their patients.
However, Drs. Abramson and Angell have a valid criticism in terms of the timing of publishing the study results. It is great to have every clinical trial registered on ClinicalTrials.gov. at the time the first patient is enrolled in a study. However, once the study is completed and fully analyzed, it is crucial for the results to be reported in a timely fashion. There are two articles in the British Medical Journal (BMJ) that suggest that this is not happening. In 2007, the FDA began to require that summary results for all trials be posted on ClinicalTrials.gov within 12 months. This was made mandatory for trials started or ongoing as of September 2007. So, how are companies doing?
In the first BMJ paper,12 the authors looked at studies that were already registered on ClinicalTrials.gov and completed between January 1 and December 31, 2009. They found that only 22% had reported results within one year of completion (another 10% were not subject to mandatory reporting). The second BMJ paper13 looked at NIH funded (academic-based research) trials. Interestingly, these authors found that for academic trials carried out from September 30, 2005 until December 31, 2008, only 46% had published results 30 months after the completion of the trial. Clearly, both the industry and academic groups are not distinguishing themselves in the timely publication of trial results. The fact that both research segments are having difficulties complying with this FDA mandate leads one to speculate that there may be unforeseen inherent issues with the specific time requirements.
Nevertheless, this is clearly a black eye for an industry trying to regain the public’s trust. In an accompanying BMJ editorial, Richard Lehman and Elizabeth Loder make the following statement.
What is clear from the linked studies is that past failures to ensure proper regulation and registration of clinical trials, and a current culture of haphazard publication and incomplete data disclosure, make the proper analysis of the harms and benefits of common interventions almost impossible for systematic reviewers. Our patients will have to live with the consequences of these failures for many years to come.
What is at stake here is the credibility of the industry. Pharmaceutical companies claim to be committed to transparency, and perhaps there is no greater place to improve the pharmaceutical company image than by the timely publication of clinical trials data. Not doing so gives critics a major target because it enables them to continue the charge that companies are hiding negative data on their new medicines. I don’t believe that is the case. But this problem needs to be corrected if the industry is going to have any credibility on this topic.
You’re Often Prescribed Drugs That You Don’t Need
A major criticism of drug companies is that they try to encourage people to take pills that often aren’t needed or for indications that are nonimportant. As was stated earlier, high on the radar screen for Drs. Abramson and Oz are statin drugs—compounds like Lipitor (atorvastatin) and Zocor (simvastatin), which are designed to lower LDL cholesterol, the so-called bad cholesterol, which is associated with atherosclerosis leading to heart attacks and strokes. They feel that these are far too often prescribed to patients who are basically healthy.
Drs. Abramson and Oz are not unique in their view. Some physicians and cardiovascular experts believe that statins are lifesaving drugs. Yet, there are those who believe they should only be used in cases when heart disease is clearly evident. The evidence, however, clearly demonstrates the value of statins and clearly measures their risk–benefit profile.
First of all, while you will see that I am a big proponent of statins, I am also a big believer in diet and exercise as a first step in everyone’s personal health regimen. This is not true just in treating high cholesterol. The benefits of a healthy diet and an active lifestyle extend to diabetes, osteoarthritis, osteoporosis, and even psychological disorders like depression. But oftentimes, diet and exercise are not sufficient to reduce the risk of these diseases; and, at some point, specific medicines may be required to restore a person’s health or to prevent long-term consequences of the disease. Furthermore, I practice what I preach. I work out for an hour every day. I do this for a variety of reasons, not the least of which is that I have a strong family history of heart disease. I believe that my exercise regimen will minimize my cardiovascular risk.
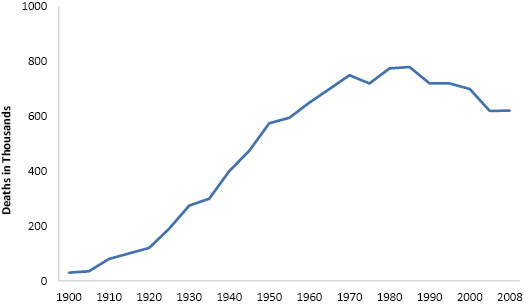
The American Heart Association has been monitoring deaths due to cardiovascular disease (CVD) in the United States for over a century. While the CVD death rate grew steadily for most of the twentieth century, it leveled off and then began to drop somewhat over the past 25 years (Figure 1.1). Nevertheless, CVD is still the leading cause of death in the United States, with 600,000 people dying annually, which accounts for more than 25% of all deaths in the country. The direct costs associated with treating heart disease amount to over $80 billion/year, and indirect costs attributed to loss of productivity exceed $60 billion/year.
Despite the progress made in moderating the CVD death rate, it is still a major disease. Furthermore, as the obesity epidemic continues in the U.S., recent headway made in the CVD death rate is liable to be counteracted by the increase in obesity, which is already resulting into a concomitant increase in type 2 diabetes, a precursor to heart disease (Figure 1.2). Even with improvements in diagnosis and treatment, better understanding of risk factors, reductions in smoking, etc., CVD is going to remain a major health problem for decades.
Yet, diet and exercise are not always a panacea. Despite the obvious benefits, two studies in the New England Journal of Medicine suggest that for the majority of the obese population, just diet and exercise won’t be enough.
The papers are entitled “Comparative Effectiveness of Weight-Loss Interventions in Clinical Practice” and “A Two-Year Randomized Trial of Obesity Treatment in Primary Care Practice.”14,15 Essentially, these papers are similar in that they look at the effectiveness of different interventions in primary care practices by physicians who were trying to help their obese patients gain better control of their health. On average, the patients in these studies had a body mass index (BMI) of about 35 (e.g., a height of 5′7″ and a weight of 220 pounds) and had at least one cardiovascular risk factor (high blood pressure, plasma glucose, or cholesterol). Both studies had control groups who received usual physician care. In the behavioral intervention study, besides the control group, one group received additional face-to-face counseling and the other group received advice remotely (telephone or email). In the obesity treatment study, the control group was compared to two groups: those who received monthly lifestyle counseling and those who received enhanced counseling plus meal replacements and weight-loss medications.
The good news is that, in both studies, those patients who were getting enhanced treatment, be it extra counseling on behaviors, more frequent sessions with their doctors or physician assistants, or enhanced lifestyle counseling, all had sustained statistically significant weight loss after two years. The amount of weight loss wasn’t trivial—around 5%. Even if the counseling was done remotely, the results were meaningful. Thus, extra time spent by primary care physicians and their associates can make a difference in helping their patients lose weight.
But the disappointing news is that even with the loss of 5% of body weight, these patients are still obese. Someone who loses 5% from his or her 220-pound frame now weighs 209 pounds with a BMI of 34—still well in the obese range. When you consider that the Center for Disease Control statistics for 2010 show that there are now 12 states where more than 30% of the adult population is obese, the loss of 5% body weight is just a small step to where we must go in order to improve the nation’s health.
The impact of obesity on the future health of the United States cannot be trivialized. Lifestyle changes are very important and can’t be minimized. But for millions of Americans, this isn’t nearly enough.
One might argue that these people may be obese; but, if they are feeling fine, why should they be put on medications? Chances are that even if they haven’t progressed to diabetes, these people have high blood pressure and high cholesterol; and, according to work published in the New England Journal of Medicine,16 if at age 45 you have two or more of either elevated blood pressure, cholesterol, diabetes or smoking, and if you are a man, you have a 50–50 chance that you will have a stroke or a heart attack at some point in your remaining lifespan. Even a 45-year-old woman with two of these risk factors has a 30% chance of such a cardiovascular event in her lifetime.
So, let’s say these data convince you to watch what you eat, go for vigorous walks a few times a week, and stop smoking. You’re feeling better and you are no longer obese but you are still overweight and your cholesterol is still high. Some physicians will prescribe a low-dose statin for you, particularly if you have a family history of heart disease. But not all will. Why?
Physicians reluctant to prescribe statins tend to have two fundamental questions: Are their protective benefits maintained over many years of use? Are patients exposed to these drugs for long periods susceptible to other diseases? Over the past few years, studies have shown that statins like Lipitor (atorvastatin) can, in fact, reduce the occurrence of heart attacks and strokes in patients with heart disease. A study reported in The Lancet from the Heart Protection Study (HPS) Collaborative Group provides answers to these questions.
The trial design was pretty simple. This group of patients at high risk of heart attacks and/or strokes was given either 40 mg of simvastatin or placebo for slightly over 5 years, and then post-trial follow-up occurred for another 5+ years, bringing the duration of the study to 11 years. Not surprisingly, those patients on simvastatin had an average decrease in LDL cholesterol of 40 mg/dL and, more importantly, a decrease of 23% in major vascular events. This benefit continued throughout the post-trial follow-up period. Equally important was the fact that there was no evidence of “emerging hazards” (e.g., cancer) resulting from long-term simvastatin use.17
The lead author of the HPS, Dr. Richard Bulbulia of the University of Oxford, commented: “All of those at increased vascular risk should start taking statins early and continue taking them long-term.” He also commented that these results should provide reassurance to patients and physicians about the safety of statins and that the results should translate to other members of this class of medicines such as atorvastatin and Crestor.
The HPS has provided valuable results at a time when health-care providers are struggling with increasing rates of obesity, diabetes, and heart disease. But this study is important for another reason. Medications designed for controlling a chronic disease that patients will need to take for decades—not only heart disease, but also diseases like osteoporosis, depression, or even cancer—will need this type of long-term outcome study, not just to provide patients and physicians with all-important risk–benefit data, but also to justify to payers the value of new medications. These studies add time to the development of a new drug and greatly increase development costs. But they are invaluable for teaching physicians and patients the long-term implications of taking a drug like a statin.
Despite these results from the Heart Protection Study group, patients may seek a more “natural” medication to treat their cholesterol abnormalities. A way one can do this is by using niacin. Niacin, also known as vitamin B3, is known to raise HDL, the so-called good cholesterol, by about 25% as well as modestly lower both LDL and triglycerides. It has been used for decades to treat dyslipidemia based on results from the Coronary Drug Project (CDP). Carried out in the late 1960s, the CDP study tested niacin versus placebo in men who had a previous heart attack, over a period of five years. Interestingly, niacin showed no difference from placebo in the death rate of the men in this study, but fewer patients on niacin had a nonfatal heart attack or stroke, by 26% and 24% respectively. This study is the basis of the use of niacin in cardiovascular (CV) disease.
In fact, I tried niacin as a way to control my cholesterol levels. Despite having a normal BMI, normal blood pressure, and daily exercise, I have high LDL cholesterol. Given my family history of heart disease, I decided to try niacin to get my LDL levels to the recommended American Heart Association levels.
My experience with niacin was pretty typical. There were modest reductions of both total cholesterol and LDL (∼15%), but these changes weren’t maintained over time. But I also experienced the major niacin side effect, flushing. This irritation wasn’t minor. The flushing was intense and was accompanied by itching and heat sensations. Because of this side effect, many patients refuse to stay on this medication despite its potential benefits. After about a year, my physician took me off niacin and I started on Lipitor, which was far more effective for me than niacin and which I tolerated very well.
So, why am I giving you this personal history? A study reported in the New England Journal of Medicine,18 along with an accompanying editorial, call into question the value of using niacin to treat CV disease. The AIM-HIGH trial, co-sponsored by the NIH and Abbott, looked at patients with established CV disease who were already on intensive statin therapy. The goal of this study was to see whether adding niacin therapy provided any extra benefit. The rationale for this was pretty sound. Unlike statins, niacin can significantly raise HDL and further lower LDL. Shouldn’t combining both modalities work better? Surprisingly, it didn’t. While the expected beneficial changes in terms of raising HDL did occur, adding niacin to intensive statin therapy was no different from adding a placebo in terms of preventing heart attacks, strokes, or other adverse CV events.
The NEJM editorial accompanying the AIM-HIGH study results entitled “Niacin at 56 Years of Age—Time for an Early Retirement?” basically questions further use of niacin given the copious data with statins showing the superiority of these drugs in CV disease therapy. This is causing some intense debate amongst cardiologists, who are unwilling to give up on niacin after this one study. The defenders of niacin correctly point out that there are other long-term studies with niacin currently underway that will provide a more definitive answer to the value of niacin for treating heart disease.
Niacin is a medicine that has been used by physicians for 56 years. Physicians take comfort in the fact that it has been around for so long and it has been taken by millions of people, so they know what the side effects are. Yet, niacin hasn’t been as intensively studied as newer classes of lipid modulating drugs. It is now being subjected to the same type of scrutiny demanded by the FDA of new drugs.
As was said earlier, decades of use does not ensure that a medicine is automatically safe and/or effective. Industry detractors seem to forget that pharmaceutical companies are full of people who also need medicine. I was my own case study in the effectiveness and risk–benefit profile of Lipitor versus niacin. For me, Lipitor was the answer. Whether or not that is also the case for others is a decision that a patient must make in consultation with his or her physician. However, one thing is for certain: Only long-term, well-controlled studies can provide assurance that a medicine is both safe and effective.
So, if your doctor recommends that you begin taking a statin, is he or she prescribing a drug for you that you don’t need? Based on recent clinical studies, this is not the case if diet and exercise have not completely solved your health concerns and you still have significant disease risk factors. However, pharmaceutical industry critics still believe that this type of medicine is “disease mongering.” This will be addressed next.
Drugs Target the Symptoms, Not the Cause
This was the fourth “secret that drug companies don’t want you to know.” First of all, it is untrue. Anyone who has needed an antibiotic knows that these medicines treat the cause of the illness by killing the bacteria that cause one’s fever, aches, and so on. The same can be said for people with AIDS, who take medicines discovered and developed by the pharmaceutical industry and which keep their disease under control. The same can be said for drugs that treat cancer or that enable one to quit smoking. Even Viagra can be said to treat a cause and not a symptom.
The fourth “secret,” however, has a different purpose than just attacking drugs that treat symptoms. After all, people take drugs to deal with symptoms routinely, such as ibuprofen for a headache or antacids for heartburn. But the intent of people who allege this is based on the premise that drug companies hype up conditions that really aren’t diseases in order to make money. Worse, they charge that the industry invents diseases.
The view that scientists in a pharmaceutical company sit around dreaming up new diseases and then convince people that their minor ailment urgently needs drug treatment is absurd. First of all, a company cannot simply declare a new disease and market a drug to treat it. A disease must be recognized by global regulatory agencies which set up criteria that a drug must meet in order to have even the most remote chance to be approved. Second, payers must believe that the condition is serious enough to warrant reimbursement of the cost of the drug to treat it. Third, physicians must believe the disease is serious enough to be willing to prescribe a drug to their patient to treat it. And finally, patients must be concerned enough about their pain or discomfort to be willing to seek treatment in the first place. Thus, in order for the “world disease mongering conspiracy” to be successful, patients, physicians, payers, and regulators must act in concert with the exploitative drug companies. Doesn’t this seem the least bit far-fetched?
Yet, what most people might consider preventative medicine, others regard as “disease mongering,” as exemplified by the following quote from the British Medical Journal.19
Some forms of “medicalisation” may now be better described as “disease mongering”—extending the boundaries of treatable illness to expand markets for new products.
—Ray Moynihan
Moynihan is a layman and one of the leading critics of the pharmaceutical industry. He has a strong belief that the industry effectively invents diseases by the medicalization of conditions in such a way that convinces healthy people that they are sick. One example used in this paper is in the area of osteoporosis:
Like high blood pressure or raised cholesterol levels, the medicalisation of reduced bone mass—which occurs as people age—is an example of a risk factor being conceptualized as a disease. . . . Slowing bone loss can reduce the risk of future fracture—just as lowering blood pressure can reduce a person’s chance of a future stroke or heart attack—but for most healthy people, the risks of serious fractures are low and/or distant, and in absolute terms, long-term preventive drug treatment offers small reductions in risk.
Is bone-thinning a disease? Of course not. But, if you are a petite female of Asian or northern European background, bone thinning is the first sign of osteoporosis. Unfortunately, when journalists like Moynihan make statements like this, it results in people ignoring health issues and not taking the steps necessary to forestall diseases like osteoporosis. Thus, it was refreshing to see Jane Brody’s view20 in a New York Times article entitled “A Reminder on Maintaining Bone Health.”
Brody believes that osteoporosis is underdiagnosed because of a reluctance to get bone density tests and is undertreated because people avoid drug therapy for fear of side effects. At age 60 she was found to have osteopenia, a condition characterized by low bone density but without frank osteoporosis. She likens osteopenia to prediabetes or prehypertension. At this stage, one doesn’t need to take drugs but lifestyle changes are recommended, such as regular weight-bearing and strength-training exercise, intake of calcium and vitamin D, smoking cessation, and limited alcohol consumption. However, people with osteopenia can benefit from drug therapy if they have already had a fracture.
But what about the safety of drugs for osteoporosis? Brody does an excellent job in discussing the risk–benefit profile of bisphosphonates, the major class of drugs prescribed for this disease.
On average, the bisphosphonates reduce the risk of a fragility fracture by 30–50%. By comparison, the risk of the most talked about serious side effect—an atypical fracture of the femur, or thigh bone—is minuscule.
What appealed to me about this article is the fact that an independent journalist from the New York Times has basically pointed out that bone loss is a real issue and needs to be treated long before symptoms arise. Brody’s messages are pretty clear: Monitor your bone health; at the first serious signs of bone loss, you should make lifestyle changes; if your bone loss evolves into early osteoporosis, you should work with your doctor to identify the medicine best suited for you. Unlike Moynihan’s minimization of a common disease of aging women and even some men, Brody has provided a thoughtful commentary on how best to approach bone loss. It is great advice.
Another term used by industry critics is “disease creep,” which was used in an essay by Jeanne Lenzer entitled “Disease Creep: How We’re Fooled into Using More Medicine than We Need.”21 Lenzer’s views can be summarized in her quote below:
Elevated cholesterol is not a disease. It doesn’t cause symptoms. It is a risk factor. People with high cholesterol levels are somewhat more likely to develop a heart attack or stroke, but they are at far less risk than individuals who already have cardiovascular disease. This is the definition of disease creep: when pre-conditions or risk-factors are treated as if they are the same as the actual disease state.
In Lenzer’s utopia, you wouldn’t get a statin until after you have already had a heart attack. The problem is that many first heart attacks are fatal—you don’t get a second chance to go on statin therapy then. She is correct in saying that just having high cholesterol alone does not justify taking a statin to prevent a heart attack or stroke. But CVD risk factors also include male sex, older age, family history of heart disease, post-menopause, smoking, obesity, high blood pressure, diabetes, and stress. If a patient presents to a physician with multiple risk factors and if diet and exercise have not been effective in lowering cholesterol levels to those recommended by the American Heart Association, that physician would be remiss if the patient wasn’t prescribed a statin. Waiting for a patient to first have a heart attack or stroke before providing such treatment would be irresponsible.
Lenzen implies in her article that the prophylactic use of statins may only prevent 1 in 50 heart attacks. I don’t necessarily agree with that number, but let’s say that is correct. There are 785,000 first heart attacks per year in the United States. Even employing Lenzen’s assumptions, the use of statins in the overall treatment paradigm of patients with multiple CVD risk factors would prevent thousands of heart attacks or strokes annually. Now that the most-studied statins like simvastatin and atorvastatin are generic, it would seem like the cost–benefit of statin use to prevent first heart attacks is noncontroversial. This isn’t “disease creep”—it is simply good medical practice.
What came as a big surprise to me in discussing “The Four Secrets That Drug Companies Don’t Want You to Know About” is the little respect that doctors are given in the health-care process.
I have a tremendous respect for doctors. It is incredibly difficult to get into medical school, and so the academic credentials of medical students are stellar. Then, they go through four years of medical school and another 3+ years of residency and then another year or more of study on a fellowship in their specialty. Only very talented and dedicated people get to be a part of this profession. And yet, to hear drug critics talk, you’d think that doctors have little knowledge of diseases and that they are at the mercy of a drug rep to teach them the latest medicine. The critics make it seem that doctors are only too eager to prescribe a new medicine especially when a drug rep offers them a free pen and pizza for lunch. Finally, another misconception is that doctors apparently turn weak-kneed when a patient comes in and asks for a new drug because they are unable to say no to such requests.
Well, my experience is a lot different. Doctors are very skeptical of new medicines. And why shouldn’t they be? They are smart individuals who have a great deal of experience in treating their patients. They attend scientific meetings in their specialty and read the latest medical journals. They know the strengths and weaknesses of the medicines they prescribe, and they know their patients’ conditions and needs. They also know that a major priority is “to do no harm.” Why would they switch to something new when they are already successfully treating their patients with effective medicines?
Doctors seek new medicines for conditions where no treatment exists. They will be interested in a new medicine when current treatments do not work for some of their patients. They will also be interested in a new drug if it has advantages over older ones. But they are the gatekeeper for the medicines that their patients get. It is not the drug company, it is not the drug rep, it is not really the FDA. Ultimately, doctors decide in consultation with their patients. It is this relationship that determines if new medicines have value. No amount of advertising or free drug samples should change this. To imply otherwise is to insult the entire medical profession.
Conclusion
My experience with The Dr. Oz show was very worrisome. To hear Dr. Abramson criticize the biopharmaceutical industry with partial facts was concerning enough. However, watching the audience completely buy in to the “four secrets” was alarming. It was clear that these people had no clue about the value that the biopharmaceutical industry brings to the world’s well-being.
President Obama signed the National Alzheimer’s Project Act into law in 2011. He has publicly stated that our national goal should be to cure this disease by 2025. Where do people think the experimental drugs to treat Alzheimer’s disease are coming from? Who does the public think will pay for the bulk of the clinical trials to prove or disprove whether the compounds designed to treat this disease actually work? Who will run the safety studies to show that such drugs will be safe for use in the general elderly population? Great research is done at the National Institutes of Health. Great fundamental research occurs daily at the universities and research institutes around the world. But the actual translation of this work into medicines occurs only in biopharmaceutical companies. The general public doesn’t have any appreciation of this.
At times, the industry doesn’t help itself. There is certainly a need for greater transparency in the R&D process, particularly with regard to clinical trial results. The industry hasn’t done a good job in educating the public on the contributions it has made in the battles against heart disease, AIDS, cancer, and so on. In addition, I personally wonder about the wisdom of direct-to-consumer advertising.
But this industry needs to be supported and appreciated by the public. All of us benefit from the R&D output of a healthy and robust biopharmaceutical industry. Attempts to belittle it with people talking about “The Four Secrets That Drug Companies Don’t Want You to Know” impact the long-term health of us all. Rather than fear these secrets, people should worry more about the industry’s productivity. Beyond the need for drugs to treat Alzheimer’s disease, patients are awaiting breakthrough medicines in diabetes, psychiatric disorders, cancer, antibiotic-resistant infections, and so on. For such breakthroughs to occur, biopharmaceutical R&D has to function optimally. Yet, industry productivity has sagged in the last decade. What is the cause of this drop? Can it be fixed? This will be discussed next.
References
1. Stipp, D. (2006) Take two possibly lethal pills and call me in the morning. Fortune, February 20.
2. Singer, N. (2011) Ingredients of shady origins, posing as supplements. New York Times, August 27.
3. De Kosky, S. T., Williamson, J. D., Fitzpatrick, A. L., Kronmal, R. A., Ives, D. G., Saxton, J. A., Lopez, O. L., Burke, G., Carlson, M. C., Fried, L. P., Kuller, L. H., Robbins, J. A., Tracy, R. P., Woolard, N. F., Dunn, L., Snitz, B. E., Nahin, R. L., Furberg, C. D. (2008) Ginkgo biloba for prevention of dementia: A randomized controlled trial. Journal of the American Medical Association, 300, 2253–2262.
4. Bent, S., Kane, C., Shinohara, K., Neuhaus, J., Hudes, E. S., Goldberg, D. O., Avins, A. L. (2006) Saw palmetto for benign prostatic hyperplasia. New England Journal of Medicine, 354, 557–566.
5. Barry, M. J., Meleth, S., Lee, J. Y., Kreder, K. J., Avins, A. L., Nickel, J. C., Roehrborn, C. G., Crawford, E. D., Foster, H. E., Kaplan, S. A., McCullough, A., Andriole, G. L., Naslund, M. J., Williams, O. D., Kusek, J. W., Myers, C. M., Betz, J. M., Cantor, A., McVary, K. T. (2011) Effects of increasing doses of saw palmetto extract on lower urinary tract symptoms; A randomized trial. Journal of the American Medical Association, 306, 1344–1351.
6. West, R., Zatonski, W., Cedeynska, M., Lewandoska, D., Pazik, J., Aveyard, P., Stapleton, J. (2011) Placebo-controlled trial of cytisine for smoking cessation. New England Journal of Medicine, 365, 1193–1200.
7. Jorenby, D. E., Hays, J. T., Rigotti, N. A., Azoulay, S., Watsky, E. J., Williams, K. E., Billing, C. B., Gong, J., Reeves, K. R. (2006) Efficacy of varenicline, an α4β2 nicotine acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation. A randomized controlled trial. Journal of the American Medical Association, 296, 56–63.
8. Etter, J. F. (2009) Cytisine for smoking cessation. A literature review and a meta-analysis. Archives of Internal Medicine, 166, 1553–1559.
9. Mann, J. J. (2005) The medical management of depression. New England Journal of Medicine, 353, 1819–1834.
10. Walsh, B. T., Seidman, S. N., Sysko, R., Gould, M. (2002) Placebo response in studies of major depression. Journal of the American Medical Association, 287, 1840–1847.
11. Wang, S. S. (2012) Why placebos work wonders. From weight loss to fertility, new legitimacy for “fake” treatments. Wall Street Journal, January 3.
12. Prayle, A. P., Hurley, M. N., Smith, A. R. (2012) Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: Cross sectional study. British Medical Journal, 344, d7373.
13. Ross., J. S., Tse, T., Zarin, D. A., Zhou, L., Kaumholz, H. M. (2012) Publication of NIH funded trials registered in ClinicalTrials.gov: Cross sectional analysis. British Medical Journal, 344, d7292.
14. Appel, L. J., Clark. J. M., Yeh, H-C., Wang, N. Y., Coughlin, J. W., Drum, T. G., Miller, E. R., Dalcin, A., Jerome, G. I., Geller, S., Noronha, G., Pozefsky, T., Charleston, J., Reynolds, J. B., Durkin, N., Rubin, R. R., Louis, T. A., Brancati, F. L. (2011) Comparative effectiveness of weight-loss interventions in clinical practice. New England Journal of Medicine, 365, 1959–1968.
15. Wadden, T. A., Volger, S., Sariner, D. B., Vetter, M. L., Tsai, A. G., Berkowitz, R. I., Kumanyika, S., Schmitz, K., Diewald, L. K., Barg, R., Chittams, J., Moore, R. H. (2011) A two-year randomized trial of obesity treatment in primary care practice. New England Journal of Medicine, 365, 1969–1979.
16. Berry, J. D., Dyer, A., Cai, X., Garside, D. B., Ning, H., Thomas, A., Greenland, P., VanHorn, L., Tracy, R. P., Lloyd-Jones, D. M. (2012) Lifetime risks of cardiovascular disease. New England Journal of Medicine 366, 321–330.
17. Heart Protection Study Collaborative Group (2001) Effects of 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: A randomized controlled trial. The Lancet, 378, 2013–2020.
18. The AIM-HIGH Investigators (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. New England Journal of Medicine, 365, 2255–2267.
19. Moynihan, R., Heath, I., Henry, D. (2002) Selling sickness: The pharmaceutical industry and disease mongering. British Medical Journal, 324, 886–891.
20. Brody, J. (2011) A reminder on maintaining bone health. New York Times, November 1.
21. Lenzer, R. (2011) Disease creep: How we’re fooled into using more medicine than we need. Available at www.newamerica.net/node/61818.