
It was bitterly cold that night. As the temperature slid silently towards zero, a caged thylacine paced up and down under the starry Tasmanian sky. Numbed by the cold, sinking slowly into hypothermia, the stripy, wolf-like beast cried out. But there was no one around to listen, no one to care. The keepers had long since locked up and gone home.
It was 7 September 1936, and the height of the Great Depression. At the Beaumaris Zoo in Hobart, times were hard. To keep costs down the zoo employed disinterested cheap labour – ‘sussos’, as they were known – to look after the animals. They worked for minimal wages with minimal supervision. As a result, cages were left uncleaned and animals were left to pick over the rotting remains of previous meals. Their most basic needs neglected, many of them became sick, but no one bothered to call the vet. Vets cost money.
The thylacine, who was known as Benjamin, lived in a sparse, rectangular enclosure at the back of the zoo. Although it was the start of spring, the daytime had been unseasonably hot. The single tree that covered Benjamin’s enclosure had yet to sprout leaves, and the thylacine had been left without shade. Visitors came. Visitors went, but Benjamin was too dehydrated to take much notice. At dusk, the keepers were meant to open a sliding wooden door and shoo the animal into its covered nighttime pen, but they forgot … again. The exhausted thylacine was left alone to face the elements. While his keepers slept in their beds, Benjamin was unable to get to his. And when they turned up for work the next day, Benjamin was dead. In a final act of indifference, they threw his cold, lifeless body out with the trash.
It was a sad, pointless death that could so easily have been avoided, but made all the more poignant by the fact that Benjamin was irreplaceable. He wasn’t just the last thylacine in the zoo. He was the last known thylacine on Earth.
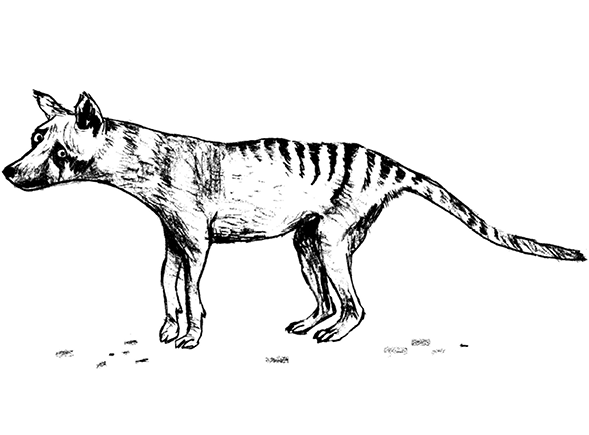
Thylacines, known also as Tasmanian tigers and Tasmanian wolves, were enigmatic and unusual animals. Think of a large dog with pointy ears, wearing black eyeliner and a tiger onesie. It had the head of a wolf, the stripes of a tiger and the stiff tail and pouch of a kangaroo. People said it walked like a ‘dog with a broken back’, yet it could turn on the speed and disappear into the bush in the blink of an eye. A fierce predator, it fed on wombats, rodents and birds. Notoriously vocal, it made a variety of bizarre noises: hisses, snuffles and a particular wheezy rattle that made it sound like it needed an inhaler. It looked like a placental mammal, yet was a marsupial. But where most marsupials have pouches that open upwards, the thylacine had one that pointed down.1 Unusually, both sexes had a pouch. Females used theirs to nurture their babies, while males used theirs to protect their pendulous, dangling scrotums from the prickly Tasmanian scrub2 .
They first appeared around 25 million years ago. Thylacine fossils have been found at the Riversleigh World Heritage Site in Queensland, Australia, alongside the remains of other fantastical beasts: carnivorous kangaroos; tree-climbing crocodiles; and one of the largest birds ever found, a flesh-eater dubbed the ‘demon duck of doom’ (Bullockornis planei). Back then there were at least six different types of thylacine, some as big as Labradors, others as small as Chihuahuas. ‘Paris Hilton would have been able to carry one of these things around in a little handbag,’ palaeontologist Michael Archer from Australia’s University of New South Wales told the 2013 TEDx De-extinction event, ‘until a drop-croc landed on her.’ Then gradually, the world began to change. It became cooler and drier, and the different types of thylacines struggled to adapt. By four million years ago, they had been whittled down to just one, the modern thylacine, Thylacinus cynocephalus, ‘the pouched dog with a wolf-like head’. Then it, too, began to disappear.
Sixty thousand years ago, Australia, New Guinea and Tasmania were part of a single landmass, still home to the thylacine. But then humans arrived from Asia. Where the thylacine had been an apex predator for millions of years it now found it had competition. By hunting the same animals as the thylacine, humans began to nudge the delicate balance of the Australasian ecosystem out of kilter. The landmass split. By 10,000 years ago, thylacines were extinct in New Guinea. Then, 3,500 years ago, new colonists arrived in Australia and brought dingoes with them. Used as a hunting companion, the pack animals helped humans become even better at killing things. Out-competed and out-manoeuvred, thylacine numbers declined until, one day, the Tasmanian tiger was gone from mainland Australia, too.
Tasmania was the thylacine’s last stronghold, but even that was to fall. In the early nineteenth century, settlers arrived from Europe, determined to turn Tasmania into a little slice of home. They built churches, houses and sheep farms, and replaced native animals with European alternatives. Rumours began to circulate that the thylacine was a sheep killer, and from a small Chinese whisper a larger wave of paranoia swept the island. If the gossip was to be believed, thylacines not only killed sheep, they sometimes took small children too. In the end, people were so frightened that the Tasmanian government placed a bounty on the thylacine’s head. Every man with a gun turned against them. Thylacines were slaughtered in their thousands.
The tragedy is that the rumours were unfounded. There’s no concrete evidence to suggest that thylacines ever took children. The stories were folklore, most likely made up to ensure that curious kids kept their distance. Nor is there any evidence to suggest that thylacines killed more than just the odd sheep. They certainly weren’t the blood-thirsty murderers they were made out to be. The truth, according to biologist Robert Paddle from the Australian Catholic University (author of The Last Tasmanian Tiger: The History and Extinction of the Thylacine), is that thylacines were used as scapegoats. Sheep farms failed, not because thylacines killed sheep, but because of bad weather and poor management. Rather than admit their failings, it was easier for the middlemen of Tasmania’s sheep industry to blame it all on someone, or something, else. Thylacines became the fall guy and then paid the price.
By the early 1900s, thylacines were incredibly rare in Tasmania, and by 1936, there was just one left – Benjamin. Three years before he died, Benjamin was filmed in his outdoor enclosure by Australian naturalist David Fleay. It’s one of the most haunting pieces of footage you’ll ever see. Google it, I urge you. The film is washed out and grainy. There’s no sound. A black and white Benjamin paces around his small, sparse enclosure. He eyes the camera and seems to yawn. He looks like he’s given up already. The film loops and repeats. But it’s what happened after the camera stopped rolling that, for me, brings Benjamin to life. Thylacines, you see, ‘yawn’ when they feel threatened. That staggering 120-degree gape was a sign that they were not happy, that if pushed, they were likely to attack. A black box on a tripod with a curtain and a pair of legs sticking out was definitely threatening, and Fleay either ignored or was unaware of the warning sign. When the camera stopped rolling, the last living thylacine on planet Earth bit the cameraman on the arse – not once, but twice! Thylacine 2 – Homo sapiens 0! It’s a wonderfully defiant glint of life at the conclusion of an otherwise tragic and sorry story.
Ironically, the thylacine finally received full legal protection in the year that Benjamin died, but it was much too little, much too late. Since then it has become a much-missed Tasmanian icon and many people refuse to believe it has gone. Since Benjamin’s death, there have been over 4,000 reported sightings of possible thylacines. Roberta Westbrook, the landlady of the Mole Creek Hotel in Northern Tasmania, claims she saw one in 1997 when she was driving along the road from Mole Creek to Paradise. Its eyes, she says, were dark, like the animal wore eyeliner. Then in 2010 a French backpacker spotted a similar animal on the same stretch of road. A kohl-pencilled fox or genuine thylacine? On the remote western side of New Guinea, locals talk of a thylacine-like creature they call the dobsegna, which has been spotted as recently as 1997. And from Australia, there are grainy photos and blurred videos. One piece of film, shot from a car in 1973, shows an apparently striped, dog-sized animal run out of some trees and cross a road. Its gait is part dog, part kangaroo and its tail is held out stiffly. But in the blink of an eye, the animal is gone.
The problem, however, is that the ‘evidence’, if one can call it that, is either equivocal or downright damning. Some photos and films are proven fakes, others too fuzzy to make an unambiguous ID. Stories are just that, while eyewitness testimonies come without hard proof. Samples of suspected thylacine hair and droppings have all turned out to come from other native wildlife. One study by the Queensland Museum’s Ralph Molnar found that patterns of thylacine sightings fail to match those of other Australian wildlife, as would be expected, but do mirror sightings of UFOs! Just like flying saucers, sightings of thylacines tend to be made by single individuals, last just a few minutes and occur late at night, often after the pub has shut. These days we have camera phones glued to our fingertips, yet nobody has been able to take an image good enough to convince the doubters. In the absence of a body or DNA proof, the thylacine, in my eyes at least, remains resolutely and sadly dead. Why chase rainbows when something practical can be done?
In a Pickle
An Australian by birth, Michael Archer grew up in the Appalachian Mountains in the eastern United States. As a child, he was more interested in the local wildlife than he was his fellow classmates. ‘I was a bit of a sociopath,’ he says. ‘I didn’t enjoy the company of people that much; snakes and turtles seemed much more reasonable to me.’ Then he discovered fossils in the rocks and boulders around his home, and a lifelong interest in the lives of animals long gone was born. He collected and stored all that he found in a special room in his house. Then one day, on a visit to New York, he heaved two fossil-filled suitcases up to the front desk of the American Museum of Natural History and asked someone to take a look. The late curator of invertebrates, Norman D. Newell, graciously obliged and identified his specimens for him. It was an act of kindness that inspired Archer to follow a career in science. He studied Geology and Biology at Princeton then, while a Fulbright Scholar at the Western Australian Museum in Perth, became intrigued by the museum’s collection of pickled Australian animals. Many of the specimens, he realised, had never been properly identified. ‘They were unknown to science,’ he says. It made Archer realise just how little was known about this cache of Australian biodiversity. For his PhD at the University of Western Australia, he studied carnivorous marsupials, including the thylacine, and went on, through much of his career, to catalogue many of the amazing finds from Riversleigh. But it was a visit to the Australian Museum in Sydney in 1976 that would change everything.
‘Staring down at me from a shelf full of skulls and skeletal tissue was a thylacine pup in a glass jar,’ he says. The pup had been acquired over a hundred years before by museum curator and collector George Masters. A Victorian ‘Crocodile Dundee’, Masters travelled widely through Australia and Tasmania, wrestling venomous snakes and shooting all manner of fauna to bring back for the collections at the Australian Museum. At one stage he had collected more than half of the museum’s natural history exhibits, and the thylacine was among them. Exposure to daylight and the alcohol it had been preserved in had long since bleached most of the colour from the tiny thylacine’s body, so the pup was white as a sheet. Only the faintest of stripes could be seen on its back. It lay curled up with its tail tucked under its bottom, its forepaws curled up as if begging. Wrinkly folds of skin hung loose over its podgy belly and its eyes were squeezed shut. Archer was mesmerised.
In the years that followed, he went to see the pup several more times. Because of his research interests, Archer realised better than most the pivotal role played by humans in the thylacine’s extinction. ‘We killed these things,’ he tells me. ‘We slaughtered them. What I think is important is that, if it’s clear that we exterminated these species, then I think we not only have a moral obligation to see what we can do about it, but I think we’ve got a moral imperative to try to do something if we can.’ The ghostly thylacine pup haunted him. Then, during one visit in 1990, a thought popped into his head. Archer knew enough about preservation to realise that alcohol preserves not just cells, but the DNA inside them too. If the pup had been pickled promptly after its death, perhaps its DNA was still in good shape and could be used for cloning. Perhaps mankind could undo some of the wrongs it had done and de-extinct the thylacine.
It was an audacious idea. In 1990, claims that DNA had been retrieved from ancient tissue samples were largely met with scepticism, and mammals had yet to be cloned. Archer asked around his geneticist friends to see what they thought. ‘There was uproarious laughter,’ says Archer. ‘Colleagues thought the idea was completely ridiculous.’ But he wasn’t put off. He decided that if he ever got the chance, he would give it a go.
The opportunity came around a decade later when Archer was appointed Director of the Australian Museum, home of the pickled thylacine. On his orders, the pickled pup was removed from its ethanol bath and a tiny piece of tissue removed. Geneticists Don Colgan and Karen Firestone then ran tests to see if the tissue still contained DNA. Much to their delight, they found that it did.
The plan was to create a ‘living library’ of thylacine DNA, where recovered genetic material would be stored in living bacteria that could be kept in the lab. From this Archer hoped to amass material for cloning. The thylacine genome would be reconstructed inside a cell belonging to the animal’s closest living relative, the Tasmanian devil (Sarcophilus harrisii), and if a cloned embryo could be made, it would be nurtured using the surrogacy skills of the same species. Beyond that, Archer envisaged a scenario where cloned thylacines would be raised in captivity, allowed to breed naturally, then released back into the wild. ‘There’s plenty of places in Tasmania where they could still live,’ he says. On one visit, Archer went to the beech forests on the island’s south side where a local man, Peter Carter, who remembered the animals from his childhood, showed Archer around. Carter told him how the thylacines used to circle his old hunting shack at night, and how when he was a boy, he was allowed to keep one on a lead. Back in the day, despite all the erroneous rumours of sheep killing and child snatching, thylacines, it seems, were kept as pets. Some museum specimens bear rough rings of fur round the neck where their collars rubbed. And historical records reveal how in 1831, Tasmania’s first ever shop, a livery stable in Hobart, had a live pet thylacine for sale. Archer sees no reason why de-extincted thylacines couldn’t be kept as pets today. ‘We’re at a situation where, increasingly, wildlife isn’t safe in the wild anymore,’ he says. ‘We need additional strategies to help protect what we’ve got.’ It’s a controversial idea. Many people won’t be happy with the idea of de-extincting a wild animal only to have it live as a lapdog, but Archer’s idea would be to have pet thylacines and captive and wild populations. It’s a natural leap for him to make. Over the years, he’s kept all manner of Australian wildlife as pets in his home, including wallabies, quolls, possums and fruit bats. ‘Marsupials,’ he says passionately, ‘make excellent pets.’ But the purpose wouldn’t be companionship. It would be to raise the thylacine’s profile, to make people care more about wildlife and the rate of its disappearance. ‘No animal we’ve ever put our arm around has ever gone extinct,’ he says. But one step at a time …
Shortly after his team extracted snippets of DNA from the thylacine pup, Archer fronted a press conference where he told the world’s media ‘Ladies and gentlemen, we are here to announce what is probably the biological equivalent of human beings taking their first step on the moon.’ It was a bold, headline-grabbing statement, and one that I think, with the benefit of hindsight, Archer has come to regret. From that moment on, the world’s media was captivated. Journalists badgered Archer and his team for constant updates and the Discovery channel filmed their every move. It created a lot of pressure, but the media didn’t grasp the subtleties of what was going on. Where before the team had isolated bitty, fragmented strands of DNA, they now found themselves able to retrieve whole genes, not just from the pickled pup but from other dried-up specimens. It was a significant step but all the media saw was a big, fat absence of a living, breathing thylacine. ‘That was the biggest problem with the thylacine project,’ says Archer. ‘From day one, it was conducted in the full glare of the media spotlight.’ Then, in 2003, Archer left the Australian Museum to become Dean of Science at the University of New South Wales, where he can still be found today. At the Australian Museum, the thylacine project fell by the wayside. ‘I never expected that the thylacine project wouldn’t continue without me,’ says Archer. ‘It was a huge disappointment.’
Since then, no one has picked up where Archer left off. There is, at present, no coherent plan to de-extinct the thylacine. But relevant research is continuing to build. The thylacine’s mitochondrial genome has been published, and there’s evidence to suggest that DNA sequences from preserved specimens might still actually work. In 2008, Andrew Pask from the University of Melbourne and colleagues extracted a tiny snippet of DNA from some century-old pickled thylacines. The fragment wasn’t a gene but a stretch of DNA that switches on the gene that codes for collagen, the structural protein found in bone and cartilage. They joined the fragment to another gene that produces a blue pigment, and injected the hybrid DNA into developing mouse embryos. Fourteen days later, when the embryonic mice were processed, they were very blue indeed, hinting that the thylacine DNA had managed to switch on the rodent collagen gene. Pask may not have resurrected the thylacine, but he did bring its DNA back to life. That has to be a step in the right direction. For now, it’s as close to a living thylacine as anyone has got, but Archer is not disheartened. He has another de-extinction project on the go.
A Frog in the Throat
The Lazarus Project seeks to bring back one of the most bizarre animals in the world. It’s everything the thylacine isn’t. This creature wasn’t furry or pouched, big or stripy. It didn’t have sharp pointy teeth or a long tail. It never inspired fear or fable. The truth is, few people outside of its native homeland have ever heard of it. It never bothered sheep, people or anything very much, but it might have hurt a fly. The animal was a humble, slimy frog that lived, until very recently, in the bubbling creeks of Queensland, Australia. But it had one hell of a party trick. Wait for it … drum roll please … female frogs burped up fully formed froglets. I kid you not, they really did.
Think for a moment how peculiar that is. Female frogs, we are told at school, ditch their blobby frogspawn in a ditch or pond. After the eggs have been fertilised by a male, they hatch into tadpoles, which then turn into frogs. It’s a process that plays out in jam jars and buckets all over the world, much to the wonder of transfixed children. That’s how frogs have their young. Mother frogs do not, instinct tells us, belch up froglets. Burping releases gas, not babies. If it were any other way I’d pop out quadruplets every time I had a gin and tonic. Thankfully this is not the case, yet here is an animal that somehow ejects fully formed froglets from its mouth. It gives birth by burping.
Now, I’ve experienced giving birth via the conventional route … several times. On one occasion I even had to push out two whoppers in the space of an hour. But it’s not pretty. In that short time, I experienced pain that was off the Richter scale, communicated only in words of four letters, and nearly punched the midwife. Repeatedly. The babies came out beautifully but my vestibule3 was left in tatters and any dignity I’d once had was washed away when the tsunami that was my waters broke. Burping up a baby, in contrast, seems like an excellent idea. Who hasn’t, at some point, enjoyed the satisfaction of a loud, resonating belch? Be honest. The freedom! The release! The production of a sound so at odds with your normal voice it shouldn’t be possible. But it is. A frog that belches and gives birth at the same time is nothing short of an evolutionary masterstroke.
The southern gastric-brooding frog (Rheobatrachus silus) was discovered in 1973, the same year that Elvis Presley’s ‘Aloha from Hawaii Via Satellite’ concert attracted more viewers than the Apollo moon landings. It was chanced upon by biologist David Liem during a routine field survey of the rocky streams of south-east Queensland. As frogs go, it wasn’t much of a looker. Unremarkable. Drab. Not particularly big. Not particularly small. But Liem realised it was different to other Aussie frogs. It had large, bulging eyes, a short, blunt snout and an excessively slimy body that made the animal difficult to handle. To Liem it looked more like a frog found in Africa, Xenopus laevis, than it did any native amphibian. Yet here it was, hiding among the stones of a fast-flowing Australian creek. Unaware of its unusual talent, Liem’s colleagues, biologists Chris Corpen and Greg Roberts, captured a few of the animals and because it was getting late, decided to take them back to their house for an overnight stay. They would take them to the lab later.
As house guests go, they made quite an impression. The amphibians were in a tank in the living room when one of the housemates noticed that a big frog seemed to be eating a smaller one. But when the researchers looked more closely, they realised that the little amphibian was coming out of, not going into, the bigger frog’s mouth. It was all a bit odd. The scientists were impressed but not totally flabbergasted. After all, male Darwin’s frogs (Rhinoderma darwinii) are known to rear and transport their tadpoles in enlarged vocal sacs. Perhaps, the researchers reasoned, this was a male frog that was doing something similar.
Then it happened again. The frogs had been transported to the lab and one of them was to be moved to a separate tank. But when the researcher plunged his hand into the water, the frog slipped through his fingers, rose to the surface and much to everyone’s surprise, burped up not one, but six little froglets. Over the next few weeks, three more tiny frogs appeared, so the researchers decided to examine the parent frog more closely. But when they tried to pick it up, the frog raised its head and belched up octuplets. Then, without warning or time to call the midwife, five more froglets followed. When team leader Mike Tyler at the University of Adelaide and colleagues were finally able to dissect the frog, they found the animal they had presumed was male was in fact female. The mother hadn’t burped up her offspring from a vocal sac, but instead had nurtured them inside her very big, very stretchy stomach.
The gastric-brooding frog was quite unique. A female frog, we now know, would lay her eggs as normal, then swallow them once they had been fertilised. The eggs would then slip down into her stomach, where they would hatch into tadpoles and spend the next six weeks metamorphosing into frogs. A pregnant mother could fit a staggering 20 froglets inside her TARDIS-like body, an impressive feat given that she was just 7cm (2.8in) long, and each full-term froglet measured 1cm (0.8in) in length. As her babies grew, her stomach became stretched as thin as a plastic sandwich bag, filling her body cavity until her lungs became so squished she had to breathe through her skin. With her buoyancy and centre of gravity altered, the bloated female could no longer float horizontally and instead was forced to dangle vertically in the water with her head sticking out. Then when she was ready, she’d simply burp up her babies, one or more at a time, as and when they were ready. And after that, her stomach would return to normal as if nothing out of the ordinary had ever happened.
Mike Tyler and colleagues described this ‘unique form of parental care’ in the journal Science in 1974, but it didn’t receive the fanfare that was expected. Instead of awe and wonder, the paper was met with scepticism and disbelief. Gastric-brooding, it seemed, was an outlandish, impossible idea. A stomach can’t turn into a womb any more than a womb can turn into a stomach. Tyler, critics proclaimed, must have got it all wrong.
So Tyler set about studying and photographing the frog further until he had amassed enough data for a second, more detailed research paper that described both the animal and its behaviour. This time he included his photographic evidence. The most iconic image is of a female held snuggly between her captor’s thumb and forefinger, her mouth wide open with a mini frog climbing out. It looks like the ugliest Russian doll you’ve ever seen, but it was irrefutable. When Tyler published the second paper in 1981, the response was completely different. The baby-burping gastric-brooding frog was featured in newspapers, magazines and journals around the world. Several groups began to study the frog.
But just as interest in the gastric-brooding frog was mounting, its numbers in the wild were waning dramatically. Tyler and his team visited the creeks of south-east Queensland every month between 1976 and 1980, but the little frog became harder and harder to find. The last wild gastric-brooding frog was spotted in 1981, and although people continued to look for it, it has never been found since. After that, the last two adult frogs that Tyler had kept in his laboratory died in 1983 and the species became officially extinct. Just 10 years after it had first been spotted, the southern gastric-brooding frog was no more. What a loss.
Then, on New Year’s Eve 1984, some good news! A man who would later become known as ‘The Frog Whisperer’ for his ability to find and catch wild frogs, was taking a dip in a waterfall, high up in the mountains of Queensland’s Eungella National Park. Biologist Michael Mahony, then at Sydney’s Macquarie University, was taking a well-earned break after a hot, humid day spent collecting frogs for his PhD project when one of his colleagues spotted an orangey-brown frog disappear under a stone. From his knowledge of the local fauna, Mahony knew this had to be something unusual – the only orangey-brown amphibian around those parts was the cane toad, but that wasn’t found in mountain streams. He felt around under the rocks and clasped his hands around it, but the creature was unusually slimy, a trademark of the slippery gastric-brooding frog. ‘So I knew what it was before I saw it,’ he says. Mahony had discovered a second species of gastric-brooding frog, the northern variety (Rheobatrachus vitellinus).
These frogs were not difficult to find. Mahony and colleagues were able to collect dozens of them in just one night. Hopes were high that the animal could be studied and the secrets of its gastric-brooding unravelled. ‘Finding the new species of gastric-brooding frog was like a second chance,’ says Mahony, ‘but it was bittersweet.’ Almost as soon as it was discovered, the northern gastric-brooding frog disappeared too. Queensland scientists, charged with monitoring the species, watched as local populations crashed. ‘There were no proactive efforts to save the frog,’ says Mahony, ‘the scientists effectively monitored it into extinction.’ Less than a year after Mahony identified the slippery first specimen, the northern gastric-brooding frog disappeared from the streams of Eungella. It too had become extinct.
The truth of it is that we humans have inadvertently been spreading mass ‘frogicide’ around the globe in the shape of a nasty fungus that infects and kills frogs and other amphibians. Disseminated around the world via the commercial trade in amphibians for the pet and food industries, the chytrid fungus (Batrachochytrium dendrobatidis) is now found on every continent where there are amphibians (that’s everywhere except Antarctica). It enters the animals’ bodies through their skin, upsets their fluid balance and kills by causing heart failure. Chytrid has caused mass mortalities, population declines and extinctions on multiple continents, and in terms of hammering biodiversity is the most significant disease of vertebrates in recorded history. Over the past 30 years it has caused the catastrophic decline or extinction of at least 200 species of frogs. Although the fungus wasn’t formerly recognised until 1999, analysis of a preserved museum specimen suggests that amphibians living in the gastric-brooding frog’s backyard – the mountain ranges of south-east Queensland – were infected with the fungus as early as 1978. The last wild southern gastric-brooding frog was spotted just three years later. ‘We think gastric-brooding frogs were highly susceptible to chytrid,’ says Mahony. Once again, it seems we humans are responsible for the disappearance of yet another species.
But Michael Archer hopes to change all that. Under the auspices of the Lazarus Project, he has amassed a formidable team of experts, including Michael Mahony and Mike Tyler, to de-extinct the gastric-brooding frog. As with the thylacine project, Archer sees the Lazarus Project as an opportunity for mankind to atone for its crimes against biodiversity, but it’s also more than that. Like the thylacine, the gastric-brooding frog represents something quite unique in evolutionary terms. There’s an argument to be had for bringing it back purely because it is so unusual. It’s physically and genetically distinct. The female’s ability to turn its stomach into a makeshift womb sets it apart from anything else that is alive on the planet today. If we could de-extinct the gastric-brooding frog, then we’d have an opportunity to understand how these changes occur, to explain how it is that female frogs don’t digest the eggs that they swallow. Preliminary studies from when the frogs were still alive suggest that the eggs’ jelly coats contained a substance, prostaglandin E2, which dampened the production of stomach acid, and that when the tadpoles hatched they produced the same hormone-like substance. Archer argues that if we de-extincted the gastric-brooding frog and worked out how it managed its gastric acid secretions, then it may lead to the development of new therapies for stomach ulcers or for people recovering from stomach surgery. But first Archer has to clone it.
Early Days
Luckily for the Lazarus Project, other types of frogs have already been cloned. Back in the fifties, people weren’t interested in cloning frogs per se, but in a question that had been nagging cell biologists for over half a century: what happens to the genome of a fertilised egg as it starts to develop and turns, ultimately, into an adult animal? In the very early days of embryonic life, the cells inside an embryo are totipotent, meaning they can turn into any of the many hundreds of different cell types found in the adult animal – heart, muscle and nerve cells, for instance. But as the embryo develops from a seemingly unimpressive blob of cells into something more recognisable, with limbs, organs, tissues and the like, the cells start to lose their totipotency. They become less able to generate different cell types. So the question that kept cell biologists up at night was, as an embryo develops to adulthood, do its genes stay the same but are somehow switched on and off differently, or are the parts of the genome that are no longer needed somehow lost? Nuclear transfer experiments held the answer. If the DNA-containing nucleus of an adult cell could be transferred into an egg stripped of its own genetic material, what would happen? If development proceeded as normal, then the requisite genes must have been reactivated. But if the cloned egg failed to divide, perhaps the DNA needed for development had somehow been lost forever …
Scientists had been trying and failing to take a nucleus from one cell and put it into another, physically separate cell, but the cells broke so easily that most people thought the task was simply impossible. So when one man, Robert Briggs, applied for funding from the National Cancer Institute (NCI) for his nuclear transfer experiments, the ‘hare-brained scheme’ was roundly dismissed. But Briggs, a banjo-playing ex-shoe factory worker, didn’t give up. The NCI relented and Briggs acquired the assistance of a talented researcher called Thomas King. For their work, they chose to focus on a beauty of a frog called Rana pipiens, the northern leopard frog. With its green body and striking black spots, it looks like it has hopped straight out of a colouring book. Briggs and King took a single cell from an embryonic frog, sucked out its nucleus with a fine glass pipette, then injected it into an egg that had had its own nucleus removed. It was frustratingly slow work and the duo spent two years honing their methods and gathering data. But what they found was that 40 per cent of the DNA-infused eggs developed into embryos and then tadpoles. It was the first de facto nuclear transfer experiment, and a huge technical breakthrough. They published their research in 1952 to much scientific acclaim. But while it sort of answered the question they were interested in, it didn’t quite nail it. Briggs and King had shown that DNA from embryonic frog cells could direct development, but what of DNA from older, more specialised cells? It would take a man who would later become known as the ‘Godfather of Cloning’ to sort that one out.
At school, British-born John Gurdon was advised against becoming a scientist. In his end-of-term report his teacher wrote, ‘I believe Gurdon has ideas about becoming a scientist. On present showing, this is quite ridiculous. If he can’t learn simple biological facts he would have no chance of doing the work of a specialist, and it would be a sheer waste of time both on his part and of those who would have to teach him.’ Gurdon can recall the words, written over 60 years ago, as if it were yesterday. He even keeps the old report pinned above the desk in his office because it amuses him. And he has every right to be amused. He has done what every schoolchild longs to do: prove his teachers spectacularly wrong.
For his experiments, Gurdon chose a different frog to study. If Rana pipiens is a frog supermodel, then Gurdon’s frog, the African clawed frog (Xenopus laevis) would struggle to get a photo-shoot for a knitting catalogue. Imagine a ball of dirty brown modelling clay that’s been catapulted at a wall, then had frog arms and legs stuck on. That’s Xenopus laevis. As frogs go, this one more than deserves a kiss from a princess. But as we all know, it’s not what you look like, but what you do with your life that matters, and this frog was part of arguably one of the most important pieces of biological research of all time. Gurdon chose to isolate nuclei, not from embryonic frogs as Briggs and King had done, but from tadpoles, specifically from the cells that line the tadpole’s gut. The rationale was that these ‘intestinal epithelial’ cells lacked totipotency. These were cells that could not generate other cell types or change their own identity. Once an intestinal epithelial cell, always an intestinal epithelial cell. Researchers talk about cells such as these being ‘committed’, a nice analogy that somehow implies the cell has taken a vow of ‘intestinal epithelial-ness’. It will never covet other cell types again. If the nucleus from a committed cell could direct development after being plonked into an enucleated egg,4 it meant that the genes for directing development were all still there. Gurdon transferred the tadpole nucleus into enucleated frog eggs and managed to produce not one, but 10 cloned tadpoles. He proved that genetic material from mature, committed cells still contains all the information needed to make an entire organism. It was a conceptual leap forwards, and one that has spurred the fields of stem cell biology and regenerative medicine ever since. It paved the way for mammals to be cloned, for Dolly and forays into de-extinction. But it also set the stage for therapeutic cloning. Gurdon realised that if you could take DNA from someone who was ill, with Alzheimer’s, say, and reprogramme it to an embryonic state through nuclear transfer, you could use the resulting embryo to harvest ‘spare part’ cells that could be used to treat the disease. The same cells could also be used in culture to test new medicines, speeding up the process of drug development. In 2012, Gurdon was awarded the Nobel Prize for Physiology or Medicine for his research into cellular reprogramming, and he remains an inspiration to anyone out there who, like me, suffered from crushingly low teacher expectations and perennially poor school reports. You don’t have to be top of the class to do well in life.
But what of the gastric-brooding frog? Does John Gurdon’s work bring the dream of resurrecting the bizarre baby-burper back to life any closer? You’d be forgiven, thanks to Gurdon’s seminal frog-cloning work, for thinking that it’s easy to clone frogs. If they could do it in the fifties, surely it’d be a doddle to do decades later …
Almost Tadpoles
The first step for Michael Archer and the Lazarus Project was to find a source of gastric-brooding frog DNA. So Archer called up his friend, Michael Tyler, who had kept the amphibians in his laboratory in the seventies, to ask if he had saved any of the frogs after they died. Much to his delight, he found that he had. Decades before, Tyler had had the foresight to realise that one day, material from the gastric-brooding frog might be of interest to others. The remains of a couple of dozen individuals lay almost forgotten at the bottom of his laboratory freezer. There were whole frogs, bits of frogs, adults and juveniles; a veritable treasure trove of cells and hopefully DNA for Archer’s cloning experiments.
However, the body parts were now over 30 years old and had been frozen without cryopreservatives. So when the scientists thawed a bit of tissue and tried to grow cells from it, the cells obstinately refused to play ball. They just sat there in the petri dish, floating around, long dead. When biologists cloned frogs during the fifties, their starting material was in good shape. The gastric-brooding frog tissue, in contrast, seemed of such poor quality that the Lazarus team fully expected their cloning experiments to croak. And where Briggs and King and then Gurdon had used DNA from embryonic frogs and tadpoles respectively, Archer’s team was having to use DNA from much older specimens. Their cells were more mature, more specialised and arguably less able to have their biological clock ‘reset’ to drive embryonic development. ‘It is relatively easy to obtain cloned animals starting with nuclei from embryonic cells,’ says Gurdon. ‘It is much more difficult to obtain normal adult animals from the nuclei of specialised cells.’
This was the plan. Drawing on the experiences of Gurdon, Briggs and King and many others, the Lazarus team planned to take the nucleus from one of their hopeless-looking cells and inject it into a live frog egg that had had its nucleus removed or inactivated. But because there were no gastric-brooding frog eggs in the freezer, the team would have to use the eggs of a living, related species for their experiments. It’s another layer of complication that only lengthens the odds of success. ‘No one has ever cloned a frog using the nucleus of one species in the enucleated egg of another species,’ says Gurdon. On Michael Mahony’s advice, they decided to plump for the Great Barred Frog (Mixophyes fasciolatus), a common-enough frog with a big, yolk-rich egg, which could be collected from the wild by driving around the rainforests of New South Wales at night (where Mahony is based and the cloning experiments were to be done) and trying not to run them over as they hopped across the road.
With the frogs and their eggs safely collected, they began their experiments. Very carefully, they transferred hundreds of nuclei into hundreds of recipient eggs and waited. Freshly fertilised ‘regular’ frog eggs take a couple of hours to start dividing, so if the cloned eggs were to do the same, the team reckoned it would probably happen on a similar timescale. But the clock ticked, hours passed and the reconstituted eggs, disappointingly, did nothing.
But then, unexpectedly, one of the reconfigured eggs did start to divide. The single cell split into two cells, which split into four cells and kept on going. The team high-fived around the laboratory. The nurturing environment of the Great Barred Frog’s egg had somehow reactivated the dead nucleus and a tiny gastric-brooding frog embryo was beginning to form in front of their very eyes. ‘When we saw the first, then the second, then the third cell division, we knew we were on to something,’ says Mahony. The Lazarus nucleus had come back to life. But where biblical comeback king Lazarus of Bethany endured an alleged four days of death before being brought back to life by a famous bloke with a beard,5 the DNA of the dead gastric-brooding frog had somehow survived 30 years in an Australian chest freezer. It was nothing short of a miracle. The team had successfully de-extincted the southern gastric-brooding frog.
Back in the petri dish, the plucky cell continued to divide until it had formed a tiny, featureless ball of a few hundred cells. That may not sound like much, but it’s from such humble beginnings that all multicellular life begins. But then, just as suddenly as it had started, cell division stopped. The embryo never turned into a tadpole, much less a frog. The gastric-brooding frog was gone all over again.
Genetic tests confirmed that the early embryo had indeed contained gastric-brooding frog DNA, and that the DNA was being replicated as the cells divided. It was a genuine clone, but a clone that unfortunately never made it past the first few days of life. Still, Archer announced the team’s progress at the 2013 TEDx De-extinction event to a heart-felt round of applause and a flurry of media reports. Later that year, the team bagged a place in Time magazine’s top 25 inventions of 2013, alongside an artificial pancreas, a new atomic clock and the ‘cronut’, the bastard pastry child of a croissant and a doughnut.
The Lazarus frog had failed to make it past an important milestone in embryonic development known as gastrulation. A crucial period in early life, gastrulation occurs when the hollow ball of early embryonic cells starts to fold in on itself to form a layered structure known as the gastrula. These layers then go on to generate various defined tissue types such as blood vessels, bone, skin and brain. Organs and body parts can’t develop until gastrulation has occurred, and the whole process is underscored by a complicated flurry of genetic and molecular activity. For some as-yet unknown reason, the Lazarus embryos stopped dividing just as they were on the brink of gastrulation.
But the team was unfazed. With every cloning attempt, they ran a detailed series of control experiments. In one, they kept everything the same, but instead of transferring a gastric-brooding frog nucleus into an enucleated egg, they injected a nucleus taken from a living Great Barred Frog. The modified egg divided for a few days, just as the cloned gastric frog embryos had done, then stopped at exactly the same point, just before gastrulation. It sounds like bad news, except that it isn’t. Because embryos from both living and dead donor nuclei fail at the same point, the control experiment suggests that it’s probably not the dead gastric-brooding frog nuclei that are the problem. More likely, it’s the cloning process itself. So if the cloning process can be improved, perhaps the Lazarus embryos can be nurtured beyond gastrulation, into tadpoles and then into frogs.
The Lazarus scientists believe that the gastric-brooding frog’s DNA still contains the information needed for making a baby frog, it’s just a matter of getting the egg to reactivate these genetic signals. ‘I think it’s the egg that is the brick wall,’ says Mahony. Just as egg and sperm are vital for normal fertilisation, so too the egg plays a vital role when life is created through cloning. Its cytoplasm (that’s pretty much everything inside the egg minus the nucleus) contains an as-yet uncharacterised cocktail of molecules that can physically reconfigure and alter the activity of DNA. These molecules can reorganise, repackage and reactivate DNA, somehow switching on the signals that guide embryonic development. But if the egg becomes damaged, the signals can fail. Embryonic development may not happen at all, or if it does, it may not last that long. Although the eggs of the Great Barred Frog ‘look’ good, perhaps they are the reason that Lazarus embryos have yet to turn into anything resembling a tadpole. It’s also hard to remove every last scrap of Great Barred Frog nuclear DNA from the egg, another hurdle the team is working hard to overcome.
They’re trying to turn things around by using only the best eggs for their cloning experiments. And it’s here, speaking with another member of the team, Andrew French, that I realise the level of detail, dedication and doggedness required to take the Lazarus embryo to the next level. Let me explain. It’ll give you an idea of just how hard these guys are working. To get the best eggs requires the best frogs, but the Great Barred Frog is a seasonal breeder that only lays in the summer months between September and February. It’s on these warm, wet nights that one of the team hops into a four-wheel drive and cruises around the forests of New South Wales, collecting females as they crawl out of the leaf litter and cross the road to hop down to the creek to breed. It takes a few weeks of sleepless nights to collect the few dozen females needed for a round of cloning experiments, and the ladies are then chauffeur-driven back to the lab where they are treated like frog royalty. ‘We need to get them in tip-top shape,’ says French. So they are fed titbits of chicken and live free-range crickets. Just as with human IVF, the frogs then receive hormone treatment to stimulate ovulation. But you can’t buy frog hormones over the counter, so instead the team has to ‘make’ and purify the hormones themselves, from the pituitary glands of cane toads. The hormones are then injected into the females at roughly the same time so that they all, in theory at least, ovulate their 500 or so eggs on the same day – the designated day for the cloning experiments … except that it never really pans out that way. For every five or so hormone-injected females, only one or two ovulate when they are meant to. French can tell when this is happening because ovulating females frantically wiggle their back legs to and fro in an almost contraction-like fashion. The eggs need to be used when they are as fresh as possible, so if the female doesn’t lay them straight away, French will delicately massage her abdomen until she does. But the freshly laid frog eggs are still not ready for cloning experiments. Ask any five-year-old and they will tell you that frog eggs sit inside a protective layer of jelly. It’s this gelatinous mass that is the nemesis of frog-cloners. There is, in fact, not one but multiple layers of jelly surrounding the egg, and in texture it’s like the silicone putty used to seal windows. Peel one layer away and another seems to miraculously spring up in its place. Sperm can somehow manage to get through these multiple membrane layers, but the fine glass needles of cloners cannot. The needle either bounces off or shatters. These are slimy, difficult eggs to work with. It’s incredibly frustrating, so the team is refining methods to de-jelly their frog eggs. It’s a complex procedure that involves chemicals, UV light and physically teasing the membranes off with fine forceps. ‘We are having to re-invent Gurdon’s protocols from the 1950s,’ says French. The hope is that by collecting and treating the eggs with extreme care and respect, by paying attention to every single component of the protocol leading up to nuclear transfer, the eggs will be in top shape, and able to reactivate the dead, DNA-containing nuclei from gastric-brooding frogs. And it looks as if their hard work is finally paying off.
‘Every time we re-run the experiments we get better results,’ says French. For every 100 or so nuclear transfer experiments done – where the nucleus from a dead gastric-brooding frog cell is injected into a de-jellied, enucleated live frog egg – around seven or eight live Lazarus embryos are created. Most fail before gastrulation but every now and then, once in a while, when there’s a blue moon in the sky and the planets align, the odd cloned embryo keeps on dividing. The team has watched with great excitement as a small number of amorphous cloned Lazarus embryos begin to develop recognisable tadpole-like features, in the shape of a small bumpy ridge destined to become the animal’s backbone. ‘They’re almost tadpoles,’ says French. Little by little, as the experimental protocols creep closer to the optimum, the embryonic frogs are starting to live a little bit longer, to develop a little bit further and to become more tadpole-like. The frustration for now, however, is that the ‘almost tadpoles’ remain just that: ‘almost’. They don’t turn into tadpoles, much less froglets or frogs. Embryonic development grinds to a halt after the primitive backbone is made, and the embryos all die.
But I’m not disheartened. I’ve spoken to these scientists and I know they’re not going to give up easily. They have optimism, team spirit and a never-ending list of experimental variables they will continue to tweak until they get their procedures ‘just so’. Next step, to take a nucleus from a living cloned embryonic gastric-brooding embryo (before it stops dividing) and use that for cloning. ‘We think that re-cloning might be the best option,’ says French. By cloning a clone they hope to push subsequent embryonic development all the way and bring the gastric-brooding frog back from the dead.
When British scientists Patrick Steptoe and Robert Edwards first tried to create human embryos in a dish back in the late sixties, they found it immensely difficult. Their attempts to artificially meld human sperm and human egg met with failure time and time again. Indeed, it took the pioneers of in vitro fertilisation (IVF) eight years from when they first witnessed an IVF egg dividing in a dish to the birth of the world’s first test-tube baby, Louise Brown, on 25 July 1978. It’s hugely unrealistic to expect experiments of this nature to work anything like first time. ‘Ultimately you need all the conditions to be right on the day … and then it’s a numbers game,’ says French. Transfer enough donor nuclei into enough eggs, continue to learn from your experiments and refine your protocols, and eventually the gastric-brooding frog will rise Lazarus-like, not from a stony grave, but from the bottom of a chest freezer in a laboratory in Adelaide. ‘Watch this space,’ says Michael Archer. ‘I think we’re going to have this frog hopping glad to be back in the world again.’ If I were to choose one animal from the whole of the history of time to bring back to life again, it might well be the gastric-brooding frog.
NOTES
1 Water opossums (Chironectes minimus) also have downward-facing pouches. Alive and well today in the freshwater streams and lakes of Central and South America, the water opossum’s pouch can be closed and made watertight by a strong ring of muscle. Babies get to stay dry, while the males’ genitalia, hidden therein, can’t get tangled up in underwater plants. Phew!
2 The first scientific description of the thylacine, recorded in 1805 by Tasmania’s Lieutenant-Governor Paterson, described the amply endowed thylacine thus: ‘Scrotum pendulous, but partly concealed in a small cavity or pouch in the abdomen … Eyes large and black … which gives the animal a savage and malicious appearance.’
3 See Chapter 3 for an explanation of terminology.
4 One that’s had its nucleus removed.
5 Not Harvard biologist George Church, the other one.