6E | INTEGRATED PEST MANAGEMENT

FIGURE 6E.1 PESTS. CREATED BY VERNE J. ANDERSON.
THE GOALS OF this chapter are to:
- Introduce the concept of integrated pest management;
- Provide a deeper understanding of what pests are;
- Help staff of museums, historic houses, and archives develop sustainable strategies to manage museum pests; and
- Provide resources to further learn about integrated pest management.
This chapter provides a starting point for developing an integrated pest management plan and putting it into practice in your institution. There is much that is not covered here but there are resources listed at the end of this chapter that will help.
Pest Scenario
A collections technician opens a cabinet and watches in horror as a beetle strolls across a no-pest strip that had been placed in the drawer to protect the bird study skins inside. One of the study skins was shuddering. It was full of larvae, munching away. The pesticide strip had no effect on pests.
Sound familiar? In the past, the standard way to address any infestation was to use poison or call in an exterminator (who used poison). This would solve the immediate problem, but the infestation would eventually reoccur, so poison would be applied again, and so on, and so forth—often leaving a concentration of poisons. Yet the infestation would return. In some cases, such as the one described, the pest population would build up a tolerance to pesticide. This was obviously the wrong approach. It was at that time that new ways to approach the challenge of pests were explored.
INTEGRATED PEST MANAGEMENT
Integrated pest management (IPM) is a holistic approach to reduce pest activity using a combination of methods, including environmental management, cultural actions, and reduction of pesticides. This systematic approach is dependent on a variety of strategies to reduce the presence of pests, including pest identification, environmental control, building maintenance, and improved housekeeping. There is a reliance on regular and ongoing monitoring just to make sure that a new infestation has not appeared. If there is a new infestation, then it is caught before it becomes a disaster. These methods are more environmentally sustainable than previous, pesticide dependent methods.
Traditional pest control depends heavily on the use of pesticides. In museum collections, heavy metal pesticides, including arsenic, lead, and mercury, were historically used to protect objects sensitive to infestation. Taxidermists seem to have had their own formulas using arsenic soaps and other chemicals; these soaps were usually painted on the interior of the skins prior to mounting. During the first half of the twentieth century, neurotoxins and other organic compounds were developed, including DDT. These proved to be highly effective pesticides with increasingly worrisome side effects, both in terms of human health and the health of our planet.
IPM, as a term, was first used in the 1970s to describe less toxic methods being used to control agricultural pests, with an emphasis on integrating pest biology and cultural practices. In the early 1980s, an IPM approach began to be explored for nonagricultural needs. The National Park Service adopted IPM as the standard for pest management relating to both museum collections and to invasive species in the parks.1 IPM is now the accepted way to manage pests in museums, and elsewhere.
Why Use IPM?
Pest Scenario
1980. The collections are stored in a basement room that flooded over a long weekend. The water has been drained, and the space is dry again, all of the cabinets are opened to air out. A pest management company is sent in to fumigate as a preventive measure against the possibility of infestation. Once the treatment is completed, the curator sends a researcher to storage to examine the most sensitive textile collections. A clothing moth is seen fluttering about. The source—an Inuit child’s caribou parka—is discovered under a plastic bag. It is literally shimmering with adult clothes moths and larvae. The fumigation failed to solve the problem and the parka was damaged beyond saving.
Moral of the story: reliance on pesticides to deal with pest problems may not work. This is only one reason why collections care professionals use IPM methodology. To begin with, the history of pest control is the history of poisons. The chemicals used are effective in killing not only the targeted pest but are also detrimental to human health and to the health of the environment, as detailed in Rachel Carson’s 1962 book, Silent Spring. Many of the chemicals used in common pesticides remain as residues on the collection objects and in the spaces that were treated with them. A good example is mothballs. If you can smell that distinctive mothball odor you are inhaling toxic fumes. The active ingredient in most mothballs is either naphthalene or paradichlorobenzene (PDB). Technically, naphthalene is a repellent, meaning that it will discourage moths (or other pests) from eating anything around it, but it does not kill. PDB, on the other hand, is a pesticide—a neurotoxin—it kills. Naphthalene is flammable. Its effect on human health includes anemia through damage to red blood cells, dermatitis, sinus infections, fatigue, nausea, vomiting, and potentially several types of cancer. It has been banned in the European Union since 2008. PDB is less flammable and has mostly replaced naphthalene as the active ingredient in mothballs, air fresheners, disinfectants, and urinal cakes. It also is a suspected cancer causing chemical, with health effects similar to naphthalene. It is poorly soluble in water and does not break down in the ground, so it remains present in the environment. This smell is added to mothballs as a warning that there is a potentially dangerous chemical present, so that we detect it before it becomes hazardous. Not only are these chemicals bad for us, but they can be bad for the collection. They can add contaminants, cause fading, or other deterioration. PDB, for example, is a benzene ring combined with chlorine. In high enough concentrations it will cause fading.
BOX 6E.1 DEFINITIONS
- Biological methods—Understanding the biology and life cycle of the pest. This leads to an understanding of why it is present, what it is eating, and what environment it prefers.
- Cultural methods—What we can do to reduce (or eliminate) the threat to collections (and us) from the pest. Methods include repairing holes in the building fabric, improving housekeeping, reducing attractants such as food, water, and light, thus eliminating the reasons why the pest is there.
By employing IPM methods we concentrate our efforts on using biological and cultural means to reduce infestations (see BOX 6E.1, “Definitions”). We look for the source of the pests and address the reason as to why they are there, rather than simply applying pesticides again and again and waiting for a reoccur-rence. This multipronged approach is much more effective in reducing infestations and is better for the preservation of the collection.
Legalities
Many, if not most of the pesticides that were available to museums in the past are now listed as controlled substances and can no longer be purchased or used. Pesticides are highly regulated, and for good reasons; they are environmentally detrimental, causing serious damage to the biodiversity of the planet. Once used, many remain in the environment for a long time, causing significant collateral damage.2
There are state and federal laws governing the use and application of pesticides that include what chemicals can be used in public facilities such as museums or schools, who should apply them, and under what circumstances. For example, mothballs are no longer registered for use in museums. Mothballs can still be purchased over the counter at hardware and grocery stores, and they can be used in the home—just not in the museum. Other pesticides are registered but must be applied by a certified pest management professional (PMP). Pesticides can be used, and sometimes need to be used, but they should never be the first choice of a defense against an infestation. Always check to confirm that the pesticide used in the institution or on the grounds is registered and is properly applied.
How to Move Forward
The goal is to get rid of the pests. But how? Our toolbox is significantly reduced if we no longer can legally or ethically use pesticides. The Canadian Conservation Institute (CCI) has developed a tool to identify and reduce the risks of damage to collections, called the Framework for Preserving Heritage Collections.3 Ten factors or agents of deterioration have been widely acknowledged by the conservation field, one of which is pests (the others are physical damage, thieves and vandals, dissociation, fire, water, contaminants, light, incorrect temperature, and incorrect relative humidity).4 The agents are listed here because they interact with each other and can be used to help control infestations. This tool identifies three levels at which the risks can be addressed—the building level, the local level, and the procedures level. Finally, five strategies are used to address the risks—avoid, block, detect, mitigate, and recover or treat the damaged object. This framework provides a practical and systematic method for addressing risks. It is a logic model designed to develop control strategies that are adaptable to any given situation or institution. The tool will be referred to throughout this chapter and provides an excellent problem-solving method for dealing with pests.
Another important resource is the Museum Pest Network.5 This is a website, originally developed by the Society for the Preservation of Natural History Collections, designed to provide much-needed resources for pest problems. The site is meant to be a one-stop spot for information that a museum professional facing a pest challenge needs. Subjects covered include monitoring techniques, pest identifications, and treatment options. There are also additional resources and a link to join the Museum Pest Network List.
A successful integrated pest management program has six steps (see FIGURE 6E.2):
- Monitoring.
- Identification.
- Solutions.
- Prevention.
- Treatment.
- Evaluation and revision.
Monitoring will tell you what pests are present and leads to identification. Identifying the pests will inform the solutions that you choose to deal with them. Solutions will include a combination of exclusion (avoiding and blocking egress), improved housekeeping, and reduction of food and habitat. Treatment and recovery will reduce or remove the (hopefully) dead pests from the area and objects in the collection. Finally, you must evaluate the strategies to determine their effectiveness and revise procedures as necessary. If this last step is ignored, then it is likely that the problem will occur again. Documentation of processes, treatments, and findings is mandatory for evaluation. You do not want to repeat the same mistakes.
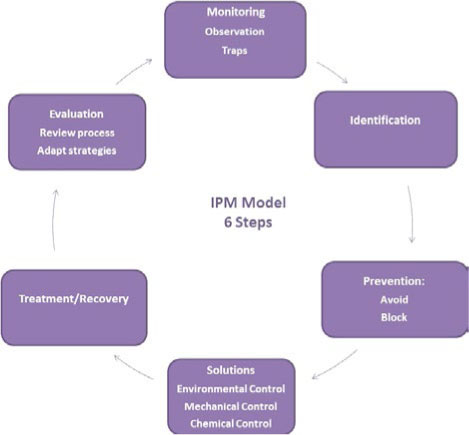
FIGURE 6E.2 THE SIX STAGES OF AN INTEGRATED PEST MANAGEMENT (IPM) PROGRAM ARE CYCLICAL. CREATED BY GRETCHEN ANDERSON.
Pest Identification and Risks
It is important to identify which pests specifically pose a threat to the collection. Once the pests are identified, you can make more effective decisions on how to deal with them by learning what the pests eat, how they live, what kind of environment they thrive in. With this information about their biology, we can determine why they are in the museum or historic house, and we can put our limited resources into the most cost-effective and sustainable way to meet the challenge. This is at the center of a successful IPM program.
What Is a Museum Pest?
Pests are animals and plants that come into conflict with humans. In the context of museums, archives, and historic houses, we usually mean insects, vertebrates (animals with backbones), and mold. Not all insects or vertebrates are pests. For example, there are more than a million species of insects and the clear majority are not classified as pests. The primary species we are most concerned with cause direct or indirect damage to our collections. This narrows the pool of suspicious (bad) actors even more. Some pest species are irritants; others cause serious damage, or human health issues. The focus of the following section is specifically on museum pests—what they are, why they are present, and what we, as collection care professionals, can do about them.
Pest damage to your collection will depend on many factors, beginning with what materials your collection is composed of. For example, if the collection is primarily paper-based, then any museum pest that eats or uses cellulose (paper) or sizing will be of primary concern, including silverfish, cockroaches, and mice (there are more; this list in not complete). Silverfish and cockroaches will graze on the surface, eating the sizing as well as the paper itself. Mice and rats will happily shred paper to make their nests. This is where understanding the basic biology and the habits of the pest species helps the collection care specialist determine why the pest is present and develop sustainable strategies to reduce the impact pests have on the collection.
Understanding Pests
Pest Scenario
Collection staff report small bugs in storage and panic ensues. The staff fear an infestation. A sample is taken to an entomologist and identified as ground beetles, which live outdoors in leaf litter. Ground beetles will not survive long inside the building, nor will they cause direct damage to the collection. The danger is that they will provide a food source for actual museum pests. We call these casual invaders. The solution: check for holes in the building fabric and block them. Clean the space to make sure that most of the beetles are gone. But do not panic!
Is the creature that is in the museum a pest or a casual visitor? Has it set up residence or is it just wandering through? The answers to these and similar questions will help to determine how to address the situation in the most efficient and cost-effective manner.
The first step is to identify the potential pest (e.g., insect, rodent, etc.), to name it. This will help determine if it is a casual invader or one that will cause damage. Once you know its name, knowledge of its life habits (e.g., what it likes to eat, how it likes to live, where it nests, how it breeds) can be determined. Only then can a strategy for dealing with it be built. IPM uses the biology and life habits to eliminate the risk.
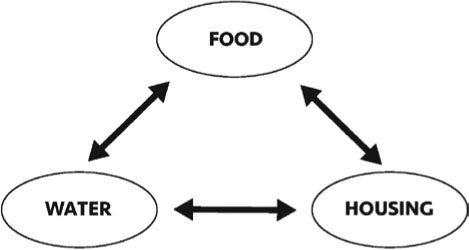
FIGURE 6E.3 ALL PESTS NEED THREE THINGS FOR SURVIVAL: FOOD, WATER, AND A HOME. CREATED BY GRETCHEN J. ANDERSON.
IPM does not have to be too complicated. One pest control professional put it this way. There are three things that all living organisms need: food, water, and a place to live (home). If you can eliminate access to just one of these attractants, it will be easier to eliminate or reduce pest presence (FIGURE 6E.3). It is important to learn a little bit about the biology of the pests that invade your institution because the more you know, the easier it will be to find the most efficient and cost-effective way to protect the collection.
What Is in a Name?
BOX 6E.2 BIOLOGICAL CLASSIFICATION
Biologists classify organisms by how similar they are to each other in a hierarchical system using Latinized names. The higher up the hierarchy of names, the broader the relationships; the lower down the hierarchy, the closer the relationships between species. When using integrated pest management (IPM) in the museum and other cultural settings, the focus is usually on the genus and species names, but often information can best be found using these in combination with the family name. For example, for purposes of identifying a museum pest, you might need to know that the American cockroach in the storage area is Periplaneta americana, but when looking for information about its habits, it is useful to know that it is in the family Blattidae. Here is how this looks in the hierarchy of biological classification:
Kingdom Animalia
Phylum Arthropoda
Class Insecta
Order Blattodea
Family Blattidae
Genus Periplaneta
Species americana
Names can be confusing. Common or regional names are often misleading. For example, the pocket gopher (a northern US name for a specific rodent) is known as a salamander in some places in the south ern United States. This is confusing because sala can cockroach (Periplaneta americana cies, removing the ambiguity in common names. Un derstanding the scientific name will help communi word, Mus (which is the Latin word for mouse) environment or food) not shared by other types of mice. If several species in a genus or an unknown species is referred to, the abbreviation “sp.” is used; thus, you could refer to any species of mouse in the genus Mus by writing the name as Mus sp.
What Are the Pests?
There are three basic categories of pest species:
- Arthropods (which includes insects, spiders, and centipedes);
- Vertebrates (e.g., mice, rats, pigeons, squirrels); and
- Mold (a fungus that grows on surfaces, often causing staining and structural damage).
Insects
Insects are the most pervasive and numerous museum pests. They are the hardest to find because they are small and often go unnoticed until the damage has been done. All insects are arthropods (which means “jointed foot”). All arthropods are invertebrates, meaning they have an external skeleton (exoskeleton), rather than an internal one like mammals or birds. There are many thousands of insect species, living under diverse environmental conditions, but all insects have six legs, and most have wings. Only a limited number of insects are classified as museum pests, and they are not the only arthropod commonly found in the museum. Spiders, centipedes, spring-tails, and others may be present. Pay attention to these because they may be pests or incidentals or an indicator of the presence of pest species. For example, spiders and centipedes hunt insects, so if you see them in a storage area, it is an indication that there are probably insect pests in the collection; a spider’s web can indicate where insect pests are living or gaining access to the collection.
There are two types of insect life cycles to be aware of—complete and incomplete metamorphosis. Species with complete metamorphosis deposit eggs, which hatch into larvae. The larvae undergo multiple molts as they grow and shed their exoskeletons, and then form pupae or cocoons from which they emerge as adult beetles, moths, flies, etc. In these species, the larval stage is the most voracious (hence, ent life cycle. Their eggs hatch into nymphs, which generally look like small versions of the adults. The nymphs go through several molts (the intermediate stages are called instars) until they become adults. These insects eat throughout their life, so all of the stages may cause damage to collections. Cockroaches and silverfish are examples of insects with incomplete metamorphosis.
Cultural heritage pests are often subdivided into categories based on their food preferences:
- Protein feeders such as dermestid beetles and clothes moths;
- Pantry pests such as grain moths and grain beetles;
- General feeders such as cockroaches, silverfish, and booklice; and
- Wood feeders such as powderpost beetles and termites.
Identify the Insect Pest
Insects can be identified at any life stage. You will most commonly see either the adults, the larvae, or nymphs, but you might also find tiny dried droppings or feces, called frass6 that can sometimes be used to identify pests. See BOX 6E.3 for a summary of the most common insect pests.
Protein Feeders
These are beetles and moths that exclusively feed on fur, feather, wool, leather, horn, and silk—anything that is made of protein. Collections made of these materials are at risk, including textiles, taxidermy mounts, many ethnographic objects, study skins, and horsehair furniture stuffing. The specific habits of protein feeders are species dependent. Most of the damage they do is caused by the larvae, who have voracious appetites, and the larvae may remain in the feeding mode for several years, depending on the species and environmental conditions. The adults of some protein feeders, such moths, do not feed at all. On the other hand, adult beetles (commonly called dermestids) will continue to cause damage, burrowing into cardboard or wood to lay eggs. Each species has its own preferred environmental conditions. Most dermestids like warm and humid conditions, but moths tend to prefer a drier environment. Most protein feeders like dark, still areas, although some are attracted to bright colors and the light when they are adults.
BOX 6E.3 INSECT PESTS
Protein feeders—Beetles and moths that go through complete metamorphosis. Among the common species to watch out for are:
Dermestids
- Carpet beetles (Anthrenus sp.)
- Fur beetles (Attagenus sp.)
- Hide beetles (Dermestes sp.)
- Odd beetle (Thylodria contractus)
Moths
- Webbing clothes moths (Tineola bisselliela)
- Case-making clothes moths (Tinea pellionella)
Pantry Pests—Insects with complete metamorphosis
- Drugstore beetles (Stegobium paniceum) eat starchy plant material, dried foods, plant specimens, and dried animal skins.
- Cigarette beetles (Lasioderma sericorne) feed on dried food, dried tobacco, plant specimens, and dried animal specimens.
- Grain beetles (Oryzaephilu surinamensis) feed on dried foods and grains. There are a number of related species (Family: Silvanidae) including the saw-toothed grain beetle.
General feeders—Insects with incomplete metamorphosis
- Cockroaches (Periplaneta sp.) feed on almost any food. There is a wide variety of roaches, from all over the world, but the most damaging species in museums are the oriental, German, and American cockroaches.
- Silverfish (Lepisma sp.) require high humidity and are often found in basements and under sinks. A related species, the firebrat (Thermophila furnorum) is more at home in hot dry climates. Both feed on starch and paper-based objects, often grazing on the surface and eating the finishes on the paper.
- Psocids (Liposcelis bostrychophila), commonly known as booklice, feed on mold. Their presence is an indication that humidity levels are too high (above 68 percent) and that microscopic mold is present. When feeding on the mold, they can damage paper.
Wood feeders—Most insects in this category go through complete metamorphosis, with the exception of termites.
- Furniture beetles (Anobium puntatum)
- Powderpost beetles (Lyctus sp.)
- Carpenter ants (Camponotus sp.)
- Carpenter bees (Xylocopa sp.)
- Deathwatch beetles (Xestobium rufovillosum)
- Termites (Isoptera sp.) often live in huge colonies that extend for miles. Complete destruction of the colony is not practical, so localized treatments are usually recommended.
Dermestids are easily identified. The larvae are generally torpedo-shaped with tufts of bristles in seg ments of the body. They are generally 0.5–10 mm long, depending on the species and the molt. The adults are round or oval, about 2–5 mm long, depending on species. They can fly, but are most often seen crawling. Colors vary in both the larvae and the adults, depending on the species. Some will burrow into wood or cardboard to pupate. The most interesting is the odd beetle (Thylodria contractus), which have larvae that are orange and curl into a C-shape if threatened. The adults are sexually dimorphic, meaning the male and female are physically different in appearance. The males look like small flies and fly well. The females look like the larvae, have vestigal wings, and do not fly.
Clothes moths are small and plain looking. The adults do not fly well, avoid light, and do not eat. The larvae of both common clothes moth species are white and soft-bodied, with dark heads. The case-making clothes moth larvae create cocoons out of the material they are feeding on and their frass, thus camouflaging it as part of whatever they are eating.
Pantry Pests
Pantry pests, also known as stored product pests, gen erally eat dried and processed food products. Food sources range from flour, corn meal, and spices to crackers, nuts, and dried fruits. Beetles, moths, and ants are common pantry pests. These pests may be found in unexpected places in storage, such as in ani mal skins. In one case, it was discovered that a tech nician was using corn meal to absorb fluids while skinning animals—a perfect food for grain beetles.
General Feeders
The general feeders eat protein, mold, starch, and paper. Archives, herbaria (botanical collections), any paper-based objects or documents, and dried foods are susceptible to these pests. They may graze on the surface of paper or feathers, or burrow into an object leaving holes and tunnels. The larvae may chew their way into wood or paper products to pupate (FIGURE 6E.4).
Wood Feeders
These insects will eat or nest in wood. Carpenter ants and carpenter bees prefer moist, soft, rotting wood; most termites and deathwatch beetles prefer dried dead wood and can digest food fiber. Some wood feeders make tunnels, but the presence of tunnels in a wooden object does not necessarily mean there is an infestation because most insects that live in live wood evacuate or die as soon as the wood is dead. Although we are not particularly concerned about species that live in live wood, it is important to keep in mind that they do leave tunnels, particularly between the bark and the wood.

FIGURE 6E.4 COCKROACH. CREATED BY VERNE J. ANDERSON.
Vertebrates
Vertebrates are generally easier to spot and to deal with than insects. Rodents (mice and rats) are the most ubiquitous, but birds, squirrels, and bats can also be a concern. Most are looking for a place to live, perhaps overwinter, or maybe just something to eat. Food is the main attractant for mice. The most practical way to deal with a vertebrate problem is to learn about the habits of the animal and block its access to the building or space.
Vertebrates will cause serious damage to the building and collections. Mice and rats chew constantly because their teeth continue to grow throughout their life. They urinate as they move along walls to mark their path and territory. Mice constantly forage for food and get most of their water from what they eat. In colder climates they often come inside buildings to get out of the cold. Once a house mouse is inside it will stay if there is food and a place to nest.
The house mouse is thought to have originated in Central Asia and has spread throughout the world by hitching rides with humans. They are territorial and rarely travel more than thirty feet from their nests. House mice are prolific; females reach sexual maturity in forty-two days and live for an average of one year. They produce an average litter of six and can have up to seven litters per year, producing up to forty-four offspring during this time.
Mice are smart, curious, and sociable. They can get through openings as small as 1/4 inch; if they can fit their head through a crack or crevice, they will squeeze their body through as well. Mice are attracted to human food and love chocolate (FIGURE 6E.5).
Rats are larger than mice and can be identified by their hairless tails. There are fifty-six species of rats. The black rat or house rat (Rattus rattus) and the Norwegian rat or brown rat (Rattus norvegicus) are the ones we are most familiar with and the ones known to spread diseases to humans. Like the house mouse, most rat species are invasive and have spread throughout the world, wherever humans live. They are attracted to human food and can cause a great deal of damage through chewing, defiling, and nesting.

FIGURE 6E.5 PEST INFESTATION. CREATED BY VERNE J. ANDERSON.
Birds
By nesting in attics and roosting on roofs, birds can cause a great deal of damage. Their droppings are acidic and will cause staining and aggravate corrosion on metal and can pose human health issues. Their nests are the natural habitat for clothes moths and dermestid beetles (which keep the birds’ nests clean by eating cast-off feathers and dried food).
Bats
Bats are generally looking for a place to live if they enter a building. Under the eaves or in the attic are ideal places for them to rear young and overwinter. Although bats may have a bad reputation through folklore, they are considered environmentally beneficial because of their main food sources (mosquitos and other insects) and many species are endangered through loss of habitat and contamination. However, bat guano is acidic, and like all mammals bats may carry rabies and must be dealt with carefully.7
Mold
We have all dealt with mold at one point or another. Mold is a fungus that spreads through spoors, which are found in the air almost everywhere. Under the right environmental conditions, mold will grow on almost any surface. Favorable conditions for mold growth usually mean relative humidity levels above 68 percent for a period of forty-eight hours or more. Mold can cause staining and structural damage to the object it is growing on, and several mold species are known to be toxic to humans. It is advisable to get the mold identified by an expert and wear personal protective equipment when dealing with it.8
Monitoring Strategies
To deal with a pest infestation, the first step is to identify the problem. You might see an insect flying, or a mouse scampering across the floor. You might notice that there are holes in a blanket or see tell-tale webbing on an object. A combination of observation and the use of traps is the best way to gather information about the pests that are present. Observation alone does not provide the complete picture—only what might be seen in a given instance. Traps provide a more complete picture of pest activity that includes around-the-clock information (what is caught or seen). The data (the pest species that are observed and trapped) should be carefully documented. These records provide information about what specific pests and occasional visitors are present, when they are likely to be seen, where they are concentrated, and where they are coming from. The documentation should include the date of the sighting (or date when the traps were checked), location of the observation or trap, the species seen, and the number of each type of pest. Systematic monitoring is the best way to gather the data needed to develop a strategy to reduce the risk to your collection.
The trap type will depend on your situation. Most of the traps discussed here are used for indoor situations, although there are traps designed for outdoor pests as well. Other types of traps may be more appropriate for your situation. Keep in mind that traps will not kill an entire population. They are used to monitor activity and show what species are present, but traps alone cannot prevent an infestation from occurring.
For most insect species, blunder traps are the most commonly used trap. These are also known as sticky traps or glue boards and consist of a piece of paper coated with a sticky substance. As they move about, the pests stumble on to the traps and stick to the surface. There are several inexpensive brands that are commercially available through pest management companies and hardware stores. The most common type of sticky trap is sold as a flat sheet that can be folded to form a tent or a box shape, which helps to keep dust off the adhesive. Homemade blunder traps can be made with double-sided tape placed on paper, with duct tape, or by an application of a material called Tanglefoot™. Sticky traps should be placed in out-of-the-way areas, along the junction of the floor with the wall, next to doors or windows, in cabinets, and next to (but not touching) objects. Blunder traps should be checked regularly, and the insects caught on them identified and counted. When a lot of in sects are stuck to a trap, it should be replaced; a heavily loaded trap is no longer effective as a trap and may become an attractant for pests that feed on the bodies of the insects stuck on the trap. When used inside of cabinets or drawers, care must be taken not to place sticky traps where objects other than pests can become stuck to them.
Pheromone traps are available for some insect species. These consist of a sticky trap with a pheromone lure—a chemical attractant that draws the males of a specific species to the trap. Pheromone traps are available for some of the more common species such as clothes moths and pantry pests but must be placed with care because they can attract males from the outdoors into the building. Pheromone traps are significantly more expensive than blunder traps.
Light Traps are useful for many insects, such as fruit flies, that are attracted to light, particularly ultraviolet light. Light traps are usually metal boxes that contain an ultraviolet lamp and have a sticky trap in the bottom. The insects fly into the light, die, and fall onto the sticky trap. Light traps are effective and are often used in kitchens and near garbage areas. It may be necessary to clean the area around light traps frequently as not all of the insects fall on the sticky trap, and ultraviolet light traps should not be placed where the light can shine on collection objects.
The best way to monitor mice and rats is with snap traps, which are the most effective and least cruel of all rodent traps. With a snap trap, the rodent is almost always immediately killed when it jostles the trigger.
There are several reasons why poison should not be used for rodents. Most rodent poisons (called rodenticides) are anticoagulants, combined with a food bait. The mouse or rat nibbles on the bait and goes back to its nest where it slowly bleeds to death, which is inhumane. The dead body, hidden away in the nest, then becomes a food source for dermestid beetles and other pests. The poisoned bait may also be infested with grain beetles or moths.
The use of glue boards for rodents is problematic because the rodent caught on the trap dies slowly of dehydration and may drag the trap away and die in hiding. When this happens, it creates the added problem of the dead mouse or rat becoming the attractant for dermestid beetles, starting a new pest problem. Snap traps should not be set in public areas where children can get into them. When using snap traps, set them perpendicularly against a wall, with the trigger facing the wall. Mice and rats run along walls for protection and will be more likely to trigger the trap if it is positioned properly.
Live animal traps are commonly used in public areas or loading docks where snap traps are likely to be set off by vibrations. Live traps are metal boxes with entrances that the rodent can get into, but cannot get out of. Live traps must be checked frequently because trapped mice can die of dehydration within a day. I once found that a female mouse had given birth to her babies in the trap, and all had died. Remember that mice and rats are territorial and if released outside the building, they are unlikely to survive but might come right back indoors. Live traps are useful for larger pests such as squirrels and racoons. Once trapped these animals must be relocated away from the museum.
Mitigation Strategies
A gallery staff member notices a small moth fluttering toward a painting and contacts the registrar. Panic ensues (FIGURE 6E.6).
This sort of situation is what the CCI Framework for Preserving Heritage Collections is designed for! Once the pest has been identified, the framework can be used to help develop mitigation strategies. Examine the three levels (e.g., building, local, and procedures) at which to act and the five strategies that you can use (e.g., avoid, block, detect, mitigate, and recover).
Avoid—Can you avoid the situation? How do you avoid it? This is where you begin to apply your knowledge of the pest because if you know what attracts the pest you might be able to avoid creating a habitat that attracts it. For example, this might mean creating a sanitary perimeter around the building because mice will be hesitant to cross a three-foot space with no clutter for hiding as it leaves them exposed to predators. For mice, a sanitary perimeter might mean a three-foot-wide and two-foot-deep span of cement or rock, which they will be hesitant to cross and unlikely to tunnel underneath to reach the building.
Other landscaping choices will significantly help to reduce flying insects from entering the building:
- Choose plants that do not attract unwanted pests (i.e., adult dermestid beetles are attracted to white flowers).
- Keep plantings away from doors and windows, trim trees so that they do not come close to the roof or exterior walls. When this simple step was taken at one museum, an ongoing carpenter ant problem was eliminated.
- Grade the ground around the building so that water flows away from the building. This will reduce mold growth from moisture moving through the foundation.
- Improve drainage by adding French drains to redirect surface water.

FIGURE 6E.6 DO NOT PANIC; THERE IS A SOLUTION. IT MAY TAKE WORK AND TIME, BUT THE SOLUTION CAN BE FOUND. CREATED BY VERNE J. ANDERSON.
Avoiding pests inside the building can be done as well. If the building has interior environmental control, can it be set to discourage pests from moving in? If this can be done and the desired environment would not damage the building, then it is worth exploring. Regulating the internal environment can be done at either the building level or the local level. Use dehumidifiers and fans to better manage humidity, being careful not to create additional problems. If a dehumidifier is used, make sure that it is regularly emptied or automatically empties into a drain. An improved and controlled environment will also enhance the condition of all collections along with helping to prevent pests.
Natural history museums usually have very large collections that are susceptible to moth and dermestid infestations, which can devastate organic materials and destroy both exhibition and research specimens. If the museum has the resources, these collections can be kept cool (60°F), with stable relative humidity (45 ± 5 percent). Pests will be unlikely to want to set up shop, no matter how tasty the objects are. This is a local solution specifically for storage because it is too cold for staff, researchers, or visitors.
Block—There is a lot that can be done to block pests from entering the building or gaining access to collections. At the building level, overall good building maintenance is mandatory. Remember that the building is the first line of defense to protect the collection and, in the case of a historic house or structure, it is the collection. Make it hard for the mice to get in; look for holes into the building, especially near the ground level, and block holes in the attic and roof. This will keep squirrels, bats, raccoons and birds out. Work with a PMP to determine when the best season is to seal these holes because you do not want to close the animals inside an attic. Remove bird nests from the eaves and attics to reduce clothes moths and dermestids. Other common strategies at the building level are common sense:
- Use screens on windows and doors.
- Maintain exterior surfaces in good repair (keep wood painted, replace deteriorating mortar for brick and stone). One museum was able to eliminate a mold infestation by repairing the mortar; the infestation was discovered before it became a major issue due to the identification of minute in sects, which ate mold and were discovered through monitoring.
- Seal holes and cracks in the exterior walls to prevent water from coming in.
- Maintain the roof in good condition.
Eliminate sources of standing water in places such as elevator shafts, along exterior walls, and in basement mechanical rooms. Make sure that the rooms adjacent to collection storage are dry and clean. Use storage cabinets and display cases that are well sealed, especially for objects that are sensitive to pest damage.
Housekeeping, as a standard operating procedure, is a vital strategy to be used in combination with other strategies. Go back to what the pests need—food, water, and a place to live. If the museum reduces clutter and is kept clean it will be easier to find the pests and will make it more pleasant for the staff and visitors. This means establishing responsibilities for who does the cleaning, what needs to be cleaned, and how often—in other words, a housekeeping plan. Do not forget the hidden, less-trafficked areas, which are often where the most pests will be found. The plan should be reasonable according to resources (FIGURE 6E.7).
The importance of managing food cannot be overstated. Food is one of the main attractants for pests that come into the building. Establish clear rules about where human food is allowed and not allowed, and whether food may be prepared on the premises (FIGURE 6E.8). Create a food management plan that works for the institution (see BOX 6E.4) but reduces the potential of infestations (the food management plan should be closely linked to the housekeeping plan). Isolate trash bins and dumpsters or move them away from the building. One large urban museum solved a rat problem by enclosing the trash dock.
A set of written procedures should be developed for live plants in the building. In an ideal world, live plants or cut flowers should not be allowed in the museum because they increase the likelihood of attracting insect pests. However, if plants are permitted, make sure that they are healthy and well cared for. Live plants should be closely monitored for pests

FIGURE 6E.7 HOUSEKEEPING. CREATED BY VERNE J. ANDERSON.

FIGURE 6E.8 FOOD IN THE MUSEUM. CREATED BY VERNE J. ANDERSON.
BOX 6E.4 FOOD MANAGEMENT
Important elements of a food management plan include:
- Make sure that kitchens and designated eating areas are kept clean.
- ∘ Check drains—keep them clean and keep the traps filled with water.
- ∘ Stove and preparation areas are notoriously hard to clean, but extra effort should be made.
- ∘ Do not allow food or garbage to stay in the building overnight.
- Use covered garbage cans and keep them clean. This will significantly reduce feeding and breeding grounds for fruit flies and other insects associated with garbage.
- Determine under what circumstances (if any) food and drink should be allowed in the galleries for special events and set strict standards for cleaning up immediately after the event. Although it is ideal that no food ever be allowed in the museum, it is understood that food at special events is often necessary.
- If your historic house or museum has a food-based educational program, develop procedures to make sure that the foodstuffs are maintained in such a way as to eliminate pantry pests. This might include defining the type of containers that dry goods (e.g., flour, sugar) are kept in or that dry goods be kept in a refrigerator or freezer.
- Determine where staff and volunteers should eat and where they can store their lunches.
(in one situation, live tropical plants were being used by a family of mice as their nesting spot!). Cut flowers may brighten a space but can also bring in pests and can cause water damage while being maintained. Take extra care to avoid damaging historic furniture if live plants are used; it is preferable to use silk flowers and plants instead because they will not attract pests. Avoid the use of wild flowers or flowers from the garden, if possible. If real flowers are mandated, use greenhouse flowers from a facility that uses systemic pesticides when growing the plants as these will be less likely to host unwanted insects.
Finally, keep monitoring, as it is an ongoing process. The only way to find out if the strategies you are engaging in are working is to be vigilant. If the methods are not as successful as you wish, then adapt. Go back to the beginning and tweak what is being done.
The best and least toxic method to deal with insects, mice, and mold is a combination of all these techniques:
- Monitor to determine where the pests are getting into the building;
- Block the access points in the building; and
- Improve housekeeping and clean up any dead animals, nesting spots, or debris left behind.
Use monitoring (with snap traps) and observation to track animal movements, and remember that they move three-dimensionally, climbing wires and pipes. Eliminate all access to food and improve housekeeping, and continue to trap; this is the one instance in which a monitoring method is also key to eliminating the pest.
Pest Scenario
An intern was doing an object check in a collections storage room in the basement of a museum. He noticed white fuzz growing on some African masks. Concerned, he called the conservator and described what he was seeing. The conservator dashed down to storage to confirm his fear. Indeed, all the wooden and hide objects in two rooms were covered in mold. The rooms were located on an outside wall that had no vapor barrier or insula tion. Everything was removed from the rooms and mechanically cleaned. The rooms were washed with a bleach mixture and the block walls painted with a fungicidal paint used on the interior of barns. Cabinets and shelving were thoroughly cleaned. A small heating, ventilation, and air-conditioning (HVAC) unit was installed for the entire storage area. Collections were returned to the space, reorganized, inventoried and a regular monitoring program initiated. Cabinets were moved away from the outer wall to allow for greater airflow, which was about 95 percent effective. A few years later, very small spots of mold were found in one space in the corner, which was a dead area with no airflow. Fans were added to improve the airflow and that did the trick.
Treatment Strategies
The steps in treatment are straightforward, and are as follows:
- Isolate and examine.
- Treat objects, storage furniture, and rooms.
- Clean objects and storage spaces.
- Clean and examine.
When a potential infestation is discovered, it is time to take action. Both the space where the infestation is found and the objects need to be treated. As discussed, the traditional approach of indiscriminately using general poisons is not advised and is often illegal, so what are the other options? For spaces, rooms, and cabinets, housekeeping is the answer. Clean out the cracks and crevices with a high efficiency particulate air (HEPA) vacuum. A HEPA vacuum removes minute (2.5 micron) particles and contains them in a bag. Follow vacuuming with soap and water treatment using a soft scrub brush; its old fashioned, but it works. If mold is a problem, seek the advice of a mold remediation specialist. Check the spaces for holes, cracks, and other egress and address those issues. Make sure that the space is thoroughly cleaned and dried prior to returning collections.
Isolation—When an infestation is discovered, the first thing to do is to carefully isolate and examine the infested objects. Determine if the infestation is active (alive) or not (old, dead). If it is not active, remove the frass, clean the object, and continue isolation to ensure that the pests do not return. Do not return the object to storage or exhibition until you are sure that the infestation is no longer active. If the pest infestation persists, decide how to treat it. Activity should be carried out away from the main collection, so that there is less possibility of spreading the infestation to other objects.
It is best to have an isolation room as far away from collections as possible. Use it for objects that are entering the building, either new donations, or new or returning loans. Examine all objects carefully before incorporating them in collection storage or exhibitions. Many infestations have been spread through the exchange or loan of objects among museums. In fact, it has been documented that the odd beetle, which originated in Asia, has been spread worldwide by the exchange of museum objects, particularly her-barium specimens.
Be creative with isolation methods. There is never enough space. At one museum with a serious dermestid infestation and absolutely no space for a permanent isolation room for examination and isolation, the registrar and IPM technician came up with the idea of using a tent, the sort that might be used to keep mosquitos away from a picnic. It was completely self-contained, with extremely small mesh screenings. The tent could be set up anywhere as needed, even in the middle of a collection storage space. This meant that objects of concern could be brought into the space and examined without fear of exposing other objects and the tent could be completely cleaned out, even dry cleaned if necessary, when the project was done.
Treatment choice will depend on several factors. The first is what materials are the objects made of, and whether they might be damaged by the treatment. Several commonly used treatments will be briefly described, but for the most up-to-date information and more detailed procedures, check the Museum Pest Network solutions tab.9
Thermal treatment can be one of two types, either low temperature (freezing) or high temperature. Both are well-researched and are the safest methods for killing insect infestations. Low temperature has been used for museum collections since the 1980s, and when done correctly, it has an excellent kill rate and will not damage most objects (see BOX 6E.5). The goal is to quickly freeze an object so that the pests in it will die. All life stages (adults, pupae, larvae, nymphs, and eggs) must be killed. The critical temperature is –22°F.10 The length of time needed for freezing to be effective depends on the object. For small objects or specimens (e.g., pinned insects), seventy-two hours is usually sufficient. For larger objects a longer time is required.
BOX 6E.5 PROCEDURES FOR FREEZING COLLECTIONS
- The infested object should be bagged in two layers of plastic, with as much air removed as possible. This prevents ice buildup on the object, eliminates the possibility of condensation damaging the object, and reduces fluctuation of equalibrium moisture content. Each layer of plastic should be well sealed.
- When the object is removed from the freezer it should be allowed to come up to room temperature before being unwrapped (allow at least twenty-four hours before opening the plastic bags).
- After treatment, the object should remain in isolation for at least two weeks to make sure the treatment was successful.
Of course, there are several types of objects that should not be frozen, including paintings on canvas, many audiovisual materials, wax, glass, and damp plant material. However, the method is so safe that some institutions use freezing as a preventive measure when moving large quantities of collections from one place to another.
Heat treatment is also successful and easily done. Research has shown that the critical temperature for killing insects (adults and eggs) is 130°F with a short exposure time. The equipment outlay can be simple and inexpensive. There are several strategies described on the Museum Pest Network website including using an oven, a heater, and fan, or a black bag in the sun. However, there are several caveats. Any material that is subject to damage at 130°F should not be treated with heat (e.g., waxes and resins). Care must be taken to not dry out the object, which will cause damage; the best way to prevent dehydration is to double-bag the object. There are some other methods described on the Museum Pest Network site that also work.
Controlled atmosphere treatments—The key feature of controlled atmosphere treatments (CAT) is the elimination of breathable oxygen. In essence, the insect dies through suffocation or dehydration. There are three primary methods for CAT in use in museums:
- Carbon dioxide (CO2),
- Inert gas (nitrogen or argon), and
- Oxygen scavenging.
The setup for all three methods is similar. An air-tight container is needed, as any leaks in the container will reduce the effectiveness of the treatment. With CO2 or inert gas, a vacuum is drawn to purge the container of breathable air. At the same time the CO2 or inert gas fills the container. The relative humidity of the gas needs to be regulated so that the objects inside do not desiccate.
CO2 treatment is considered safe for all objects. The initial expense is considerable because there are specialized systems required (called CO2 bubbles), which are commercially available, and special equipment to monitor CO2 levels. Local regulations need to be checked to determine whether a pesticide license is required. Treatment time is four weeks. Large mass treatments can be performed. Further information can be found at Museum Pest Network.11
Inert gas treatment is also considered safe for most objects, except for some mineral pigments, such as cinnabar and sienna, because these pigments can undergo a color transformation in the absence of oxygen. Like CO2 treatment, these require an outlay for materials and monitoring equipment. Unlike CO2 these gasses are not regulated as pesticides, so no license is required. More detailed description and product information is found at Museum Pest Network.12 Treatment time varies slightly for each of these methods but is generally two to six weeks.
The easiest CAT method to use is the oxygen scavenging treatment. An oxygen scavenger (a material that absorbs oxygen from the air) is inserted into a con tainer with the infested object. The container is sealed and left for twenty-one days. Inside the container the scavenger absorbs oxygen and the pests die. There is no need to adjust the relative humidity. The biggest challenge is to create an air-tight container. Instructions for this are on the Museum Pest Network website.13 This treatment can be used for almost anything with exception of the pigment Prussian Blue. Oxygen scavengers are not a registered pesticide, so no licensing is required. There are several special materials required, which are not absurdly expensive, but can add up, and large quantities of the scavenger are needed. The oxygen scavenger can only be used for one treatment and must be discarded after use.
Insect growth regulators (IGR) are synthetic pesticides that mimic certain insect hormones. They slow or halt an insect’s growth and break the cycle of breeding, hence knocking down the population. IGR, like other treatments must be used in partnership with other IPM actions. For example, a natural history institution discovered a serious infestation of drugstore beetles in its storage area. The beetles were drilling into study skins to pupate. The conservator and collection manager worked with a pest management company specializing in museums to use IGR technology as part of the overall holistic strategy to eliminate the beetle. The IGRs were used to slow insect breeding while the collection was processed through low temperature treatment. At the same time the facilities maintenance department found the egress point—an unused boiler on the roof, directly above storage, that was the beetles’ primary habitat. There were holes in the roof providing access to the room below. The boiler was removed, and the holes repaired. The cabinets were elevated above the floor on posts so that the floor was completely visible. The entire room and all cabinets were thoroughly cleaned, and housekeeping was improved. Ultraviolet light traps with sticky boards were placed in strategic places in storage. The monthly count of how many insects were caught on the traps went from a serious infestation to an occasional beetle being found.
Chemical Pesticide Treatments—There still is a role for pesticide treatments in museums, but they should never be used directly on collection objects but rather on the building. Localized applications of pesticides in cracks and crevices will help block insect pests from coming into the building or moving around once inside. These are residual treatments that normally are effective for about two weeks, unless the dust is washed away. If an occasional silver-fish is found, an IPM professional might use a gel or powder containing diatomaceous earth to deal with the problem—as the silverfish crawls over this abrasive material the insect’s exoskeleton is scratched causing it to die from dehydration.
Contracting with Pest Management Professionals
Many of the methods described in this chapter can be done in house, but there are times when it is necessary to bring in a PMP. For example, if termites threaten, then IGR or other chemical treatments will be required. Any time a registered pesticide is being used in a public institution a licensed PMP does the application. A trained PMP can be an important part of an IPM plan.
When contracting with a PMP it is important to understand what IPM is and how it relates to the institution. Define the goals and do the research. The PMP that you are seeking must have training and experience in IPM. Look at the PMP’s website and interview them for the position. Ask questions and get references. Make sure that the company is registered with the National Pest Management Association (NPMA) and any state organizations, and that they are properly licensed. A good PMP, who understands the need of the institution, is a major asset. Once the decision has been made, work with the PMP on a plan to move forward, both with the immediate infestation and with ongoing monitoring.
THE IPM PLAN
An IPM plan is a roadmap for how to proceed to achieve the desired result. It should be relevant to the needs of the institution and the collection. It should be practical and achievable. It is a process that will evolve. It is necessary to check the efficacy of the IPM plan over time, refining it as you become more familiar with the potential and actual pests and conditions at your specific institution. Although IPM procedures can be practiced without a plan, a good plan will put your actions into context, and help the staff and administration to see how everything fits together. This will help to communicate the priorities and justify expenses.

FIGURE 6E.9 IT IS IMPORTANT TO DEVELOP A PLAN AND TO SHARE IT. CREATED BY VERNE J. ANDERSON.
There are four key stages in developing an IPM plan:14
- Recognizing and identifying priorities for action.
- Identifying responsible staff.
- Acting on high priorities.
- Establishing procedures for future planning, financing, and review.
The procedures set the standards for how to go about the specific actions and treatments, describe best practices, and explain detailed actions that are to be followed to achieve the aim. They are training and reference tools, which provide consistency.
Sharing the Pain—Institutional Collaboration
Facing an infestation and building an IPM program can be overwhelming, so it is important to build collaborations to develop a team who can help. Look across the institution for roles that are natural fits for the team. Facilities and operations staff should be among your closest allies because they encounter pests, both dead and alive, on a regular basis, and so are vital to monitoring threats. Food services and special events staff deal with one of the major attractants—food—so they need to be involved. Education and frontline staff should also be included as well because they are the ones who are out in the public and see things that other staff may not. Collection staff who deal with pest risks on a regular basis are also important to include. Finally, administration and finance need to know what is going on.

FIGURE 6E.10 INTEGRATED PEST MANAGEMENT (IPM) WORKS BEST WITH A TEAM. BETTER IDEAS AND STRATEGIES ARE DEVELOPED THROUGH EDUCATION AND COMMUNICATION. CREATED BY VERNE J. ANDERSON.
Use the infestation and the IPM plan to educate your colleagues about the importance of IPM. People often find pests disgusting and gross. Use that to your advantage, empower the staff to help you with the solutions. Most often, the staff will be delighted to help get rid of the pests. Enlist the entire staff in helping to monitor by establishing a pest reporting system (paper or electronic, whichever works). Educate the staff as to what can be done without the use of pesticide and emphasize that IPM is more sustainable and environmentally friendly than the traditional methods of dealing with pest problems. Create an IPM team of key staff, making sure to include facilities staff. The more educated about pests and the building the team is, the better the chances that the solution will work.
CONCLUSION
An IPM program is a work in progress (BOX 6E.6). It is the way to gain control of the situation. It will take time, commitment, and a team effort. It will take accepting that you will never eliminate all the infestations that threaten your collection, but by working through this process, you will have a better idea of what risks your collection is facing and what to do about it.
BOX 6E.6 PESTS, CLIMATE CHANGE, AND SUSTAINABLE PRACTICES
Climate Change
- Changes in the species, distribution, and number of pests will occur as regional climates change.
Sustainable Practices
- Make sure that traveling exhibitions are pest free to avoid the introduction of invasive species.
- Reduce the use of chemical pest control by using an integrated pest management (IPM) approach with an emphasis on prevention and targeted responses to pests.
- Consider using sanitation; thermal control with cold (–20 to –30°C) or heat (55°C); or controlled atmosphere (CO2 or N2) before resorting to chemical control.
- Note that although reducing the use of air-conditioning saves energy, raising interior temperatures will increase the risk and rate of growth of insect infestations.
Source: Canadian Conservation Institute (CCI). Framework for Preserving Heritage Collections: Strategies for Avoiding or Reducing Damage, revised. Ontario, Canada: CCI, 1994.
By addressing the pest problem through holistic methods, many of the other risks to collections will also be reduced. All the actions taken to help avoid and block pests, better management of environmental conditions, and enhanced housekeeping, will improve conditions for the collections as well. Each institution is different and will require adaptations of the IPM principles. Solutions will depend on specific situations and must be determined by institutional mandate. A historic house museum, with a historic garden or natural landscape will find different challenges and solutions than an art museum in an urban setting. What is important is that IPM is a holistic system that can be creatively adapted to any cultural institution to reduce the presence of pests. •

FIGURE 6E.11 NO PESTS. CREATED BY VERNE J. ANDERSON.
RESOURCES
The following are books, articles, and web-based resources that are helpful when faced with an infestation. Note that a few older publications are listed which, although good for identification and basic information, include recommendations for pesticide treatments that are no longer considered appropriate. Always check the Museum Pest Network for the most up-to-date options.
Carrlee, E. 2003. “Does low temperature pest management cause damage? Literature review and observational study of ethnographic artifacts.” Journal of the American Institute for Conservation 42: 141–166.
Florian, M. L. 1988. “Ethylene oxide fumigation: A literature review of the problems and interactions with materials and substances in artefacts.” In A Guide to Museum Pest Control, edited by L. A. Zycherman, and J. R. Schrock. Washington, DC: Association of Systematic Collections.
Hawks, C. A., M. McCann, K. Makos, L. Goldberg, D. Hinkamp, D. Ertel, and P. Silence (eds.). 2012. Health and Safety for Museum Professionals. New York: Society for the Preservation of Natural History Collections.
Jacobs, J. F. 1995. “Pest monitoring case study.” In Storage of Natural History Collections: A Preventive Conservation Approach, edited by C. L. Rose, C. A. Hawks, and H. H. Genoways, 221–231. Iowa City: Society for the Preservation of Natural History Collections.
Jessup, W. C. 1995. “Pest management.” In Storage of Natural History Collections: A Preventive Conservation Approach, edited by C. L. Rose, C. A. Hawks, and H. H. Genoways, 211–220.
Iowa City: Society for the Preservation of Natural History Collections.
Nyberg, S. “Updated from Nov. 1987. Invasion of the Giant Spoor.” Soilinet Preservation Leaflets. Available at: cool.conservation-us.org/byauth/nyberg/spore.html.
Olkowski, W., S. Daar, and H. Olkowski. 2013. Common Sense Pest Control: Least Toxic Solutions for your home, garden, pets and community. Newtown: Taunton Press.
Rose, C. L., and C. A. Hawks. 1995. “A preventative conservation approach to the storage of collections.” In Storage of Natural History Collections: A Preventive Conservation Approach, edited by C. L. Rose, C. A. Hawks, and H. H. Genoways, 1–20. Iowa City: Society for the Preservation of Natural History Collections.
Story, K. O. 1985. Approaches to Pest Management in Museums. Suitland: Smithsonian Institution Conservation Analytical Laboratory.
Strang, T. 1996. “Detecting infestations: Facility inspection procedure and checklist.” CCI Note 3/2, Canadian Conservation Institute.
Strang, T. 1995. “The effects of thermal methods of pest control on museum collections.” In Preprints of the 3rd International Conference on Biodeterioration of Cultural Property, 199–212.
Strang, T., and R. Kigawa. “Agents of deterioration: Pests.” Available at: https://www.canada.ca/en/conservation-institute/services/agents-deterioration/pests.html (accessed August 25, 2019).
Zycherman, L., and J. R. Schrock (eds.).1988. A Guide to Museum Pest Control. Washington, DC: Association of Systematics Collections.
Useful IPM Web Resources
Bio-Integral Resource Center. Available at: www.birc.org/IPM.htm (accessed August 25, 2019).
Canadian Conservation Institute, Agents of Deterioration. Available at: https://www.canada.ca/en/conservation-institute/services/agents-deterioration.html (accessed August 25, 2019).
Museum Pest Network, https://museumpests.net/ (accessed August 25, 2019).
Museum Study LLC, provides courses on collections care subjects, including IPM. Available at: www.museumstudy.com (accessed August 25, 2019).
National Archives. Mold and Mildew Prevention of Micororganism Growth in Museum Collections. Available at: https://www.archives.gov/preservation/conservation/mold-prevention.html (accessed August 25, 2019).
National Pesticide Information Center. Available at: http://npic.orst.edu/ (accessed August 25, 2019).
National Park Service, Integrated Pest Management. Available at: https://www.nps.gov/orgs/1103/ipm.htm (accessed August 25, 2019).
National Park Service. Conserv-O-Grams, Leaflets 3/4, 3/6, 3/7, 3/8, 3/9, 3/10 are about IPM. Available at: https://www.nps.gov/museum/publications/conserveogram/cons_toc.html (accessed August 25, 2019).
Society for the Preservation of Natural History Collections, Food Management. Available at: https://spnhc.biowikifarm.net.wiki/Food_Management (accessed August 29, 2019).
NOTES
1. Museum Pest Network. Available at: https://museumpests.net/history-of-ipm (accessed August 22, 2019).
2. For example, the use of DDT in Borneo. Available at: https://www.youtube.com/watch?v=17BP9n6g1F0 (accessed August 15, 2019).
3. Available at: https://pub.cci-icc.gc.ca/resources-ressources/publications/category-categorie-eng.aspx?id=20&thispubid=382 (accessed August 25, 2019).
4. Available at: https://www.canada.ca/en/conservation-institute/services/agents-deterioration.html (accessed August 25, 2019).
5. Available at: https://museumpests.net/ (accessed August 25, 2019).
6. Frass can also include debris from what is being fed on, such as sawdust or fibers.
7. Available at: http://www.batcon.org/our-work/regions/usa-canada (accessed August 25, 2019).
8. Available at: https://www.si.edu/mci/english/learn_more/taking_care/mnm.html (accessed August 25, 2019) and https://www.nps.gov/museum/publications/conserveogram/03-04.pdf (accessed August 25, 2019).
9. Available at: https://museumpests.net/solutions/ (accessed August 25, 2019).
10. Strang, “Controlling insect pest with low temperature,” Canadian Conservation Institute Note 3/3, 1997, updated 2008.
11. Available at: https://museumpests.net/solutions-controlled-atmospherecarbon-dioxide-treatment (accessed August 25, 2019).
12. Available at: https://museumpests.net/solutions-nitrogenargon-gas-treatment/ (accessed August 25, 2019).
13. Available at: https://museumpests.net/solutions-oxygen-scavenger-treatment/ (accessed August 25, 2019).
14. Pinninger, Integrating Pest Management in Cultural Heritage (London: Archetype, 2015), 2.