CHAPTER 36
How do I write a grant?
Zachary D.W. Dezman and Jon Mark Hirshon
Department of Emergency Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
Introduction
Writing a successful grant combines art, skill, science, self-confidence and a touch of salesmanship. This chapter provides a brief introduction to grant writing in order to impart some key ideas and concepts. Creating a fundable proposal is a complex task requiring many steps and multiple components. A key early step of successful grant writing is finding an experienced mentor, both to help guide you through the funding process and to show the funding agency that you have knowledgeable expert support. There are many other components that go into successful grant writing: showing you have the appropriate collaborators (research is a team activity); institutional resources and environment (equipment, statistical support, appropriate space, etc.); appropriate financial structure and support; and appropriate ethical considerations (either human subjects or animals). Any application will contain instructions detailing the exact format of these supporting components, and a quick guide to writing NIH grants can be found through the NIH Examples of successful grants can often be found through your institution’s office of research development. However, this chapter will focus on how to write a compelling scientific argument for grants, primarily for the NIH.
General definitions
A grant is an amount of money given by a funding agency (e.g., government body, professional society, private donor, etc.) that provides financial support for a proposed project. It can provide primarily salary support (common in training grants), money for supplies (small start-up grants), or both (e.g., NIH R01 grants). A contract is similar, but designed to obtain financial support for specifically defined tasks or deliverables such as developing a specified output or program. Cooperative agreements obtain financial support for work that will be developed and specified in collaboration with the funding agency. It is important to understand the different funding structures, as they have implications about what you can and cannot do within a specific project. When in doubt, ask! You can discuss it with the grant/contract contact person included on the RFA, the departmental contact in the office of sponsored programs at your institution, or your mentor.
Getting started
Once you have identified a research question and a potential funding agency or source, speak with the program officer for the RFA or RFP (request for proposals). This can help inform you whether there is interest in your topic and whether there are other potential funding mechanisms available. Additionally, interpersonal relationships are important: getting to know the program officer can be very helpful, as he or she can inform you of what is currently of importance and interest within their institute or organization.
Overall grant tone
Throughout the remainder of this chapter, you will notice a common theme cropping up – “This project can be done;” “We have the support;” “This is an important project;” For those who have ever made a successful sale’s pitch, or obtained support from potential business investors, grant writing is much the same concept. In a formal and written fashion, you are trying to “sell” the reader on your idea and show them that you have every almost every resource lined up to complete the project, proving to them your project has a high chance of successful completion and that you are worth the additional investment.
As you write the grant, it may strike you “Why am I doing all of this work ahead of time? I can do much of this after I get funded.” Firstly, if you feel the hypothesis is strong and important enough for you to go through the effort of writing the grant, you should consider doing the project regardless of whether you get funding from the current RFA or not. Secondly, all of the thought and effort you put into project development ahead of time makes everything later – the poster showing the results, the final journal publication – much, much easier. Thirdly, your project will have greater chances of successful completion after you have gone through the effort of multiple revisions of your research plan. Finally, as general advice, a small focused project has greater likelihood of being funded than a large diffuse project. Great success is built through a series of small steps.
The importance of grant application instructions
A key early step to a successful funding application is to obtain the grant application instructions and to go through them carefully and to follow them precisely. This may seem intuitive, but grants have been returned without review because the margins were not right or the font too small. Any deviation from the instructions – especially page lengths – is an easy way for the institutional officers or an overstretched grant reviewer to reject your proposal without reading it. A second and equally critical point is to know your deadlines! Most granting agencies, and particularly the federal government, have a specific date for grant submission and there is no flexibility for a late application. These deadlines are for the final submission – not for when you need to route it through your institution before submitting it to the funding source. Know your institutional guidelines for submission – does it have to be sent to them one week in advance or two weeks? Your institution needs to review and approve the proposal prior to its submission to the grant agency.
Often you will be able to obtain a copy of a previous similar grant that was successfully funded. Having a model for success can definitely enhance the quality of your grant, but be aware that the writing requirements or proposal structure may have changed, so always confirm that your formatting, style, and length are correct. Each funding agency will have its own grant format, but many follow the NIH model in general.
Writing the research plan
The Research Plan is the core of your project, in which you clearly and explicitly define your hypothesis and how you will conduct your research. In the current NIH format, it will contain four main components: Specific Aims, Significance, Innovation, and Approach.
Specific aims
The first section, Specific Aims, should quickly focus the reader to your research topic and your specific hypothesis. It should be one page, and is often the only page that unassigned reviewers will read. Items to be covered, albeit briefly, include: general long-term goals of the research; specific objectives of the proposal and the hypothesis to be tested; expected outcomes; and importance of the research project and its potential impact. Often, the first paragraph is scripted as follows: what is known in the specific research field; what is not known/current knowledge gaps in the field; how the current research project will fill the identified gaps; and why this is important knowledge. It commonly ends with the specific, testable primary hypothesis of the proposed research project.
Significance
The Significance section will explain why your research topic and project are important. This section is usually 1–2 pages long (in a 12-page research plan). It may highlight critical barriers to progress in the field and how your research project will address them. You will also use this space to show how your project will improve the knowledge, technical capability, or clinical practice of the field. Within this section, you can give some thought as to how many people will be affected or how your project will change practice if the project is completed. A Significance section should answer the following questions: “Does this study address an important problem?” and “If our aims are achieved, what will be the result?” This section answers the “So what?” question – if you complete the research what difference would it make?
Innovation
The Innovation section tells how this project will challenge current practice or theories. This section is usually ½ –1 page in length. If your project uses a novel instrument, methodology, or approach, for example, “The ability of base deficit to predict mortality has never been examined in this population before,” then use this section to describe how your project is unique. Note that there is a fine balance between innovative and impractical. You may have a new approach to a problem, but if it is not realistic or feasible within the bounds of the support provided by the grant, (e.g., “Evaluating chest pain in astronauts on the lunar surface”), it will be perceived as too risky and will not be funded. A common mistake of new grant writers is to promise too much for the scope of the grant. It is much better to do small projects well than to promise the world.
Approach
If the research plan is the heart of your grant application, then the Approach section is the heart of the research plan. This section is the critical component of the grant that will specifically describe what you plan to do and how you plan to do it. It is usually 9–10 pages in length and has a more in-depth discussion of the general points previously raised in the Specific Aims page. Here you will go into a more detailed discussion of the information conveyed in your introductory paragraphs, such as the history of the problem, the risks to public, and so on. Not every reviewer will read your Approach, but it is important for the assigned reviewer who will be evaluating your grant to understand the entire context of your project and what you plan to do.
Your Approach section will include a more expanded discussion of your hypotheses as well as your long term goals. This is the section where the grant writer displays their thought process and method of evaluating their stated hypothesis. This is your opportunity to show how all of your resources – patient population, institution, study methodology, and so on. – will fit to evaluate your hypothesis. You also need to state what steps (specific aims) you plan to do in order to successfully complete your project, in other words your overall experimental design with a concrete sequence of action and a specific work plan.
As part of the Approach, preliminary data are usually included. It is not an absolute requirement for all grants, but most funded grants, especially at the NIH level, will have some. It is an easy way to elevate the quality of your proposal. What exactly do we mean by preliminary data? Some sort of information related to your hypothesis – perhaps you did a pilot study looking at the question (often done in translational studies). Perhaps you have looked at your patient database and you already know you have at least 10,000 cases and 20,000 controls (clinical and epidemiological studies). In the former, you are showing “proof of concept” and the purpose of the grant is to fund you to fully investigate your idea. In the latter, you are building towards your power calculations. In both cases, preliminary data show feasibility. Often, preliminary data form the core of an upcoming publication. As described above, it gives evidence in favor of your hypothesis, it allows you to do some initial calculations on power, and it shows achievability. The point of the grant is to show the funding agency how important your question is and that you have a high-quality method of testing your hypothesis that is achievable with all of the resources you have available.
Within the Approach, you need to discuss the precise procedures and methods needed to be able to accomplish your experiment or answer your hypothesis and specific aims. With clinical research, this may be a discussion of how you will enroll subjects, test them, record the data and then analyze the results. You need to describe the overall study structure – are you going to conduct a retrospective case–control study, a multicenter prospective cohort study, or a double-blind, placebo controlled clinical trial? Additional components of discussing the experimental activities include how you will collect the data, how they will be managed and how they will be analyzed. A Gant chart is a good way to convey a complex schedule within page limits (Appendix 36.1).
If you are writing a training grant, this is also a good way to show when you will be in different stages of training and when you will graduate. Reviewers expect a high level of detail – they need to understand exactly what you are planning and how you will accomplish your research. If you are relatively new to research, this is where additional team support is critical. Get the input from an epidemiologist or biostatistician if you are carrying out clinical or public health research, or from a senior laboratory scientist if you are carrying out basic science research. The key is to be clear and understandable so the reviewer knows exactly what you will do, how you will do it and when you will do it.
Also, within the Approach section it is important to discuss potential problems as well as possible alternative strategies. No researcher, especially a junior researcher, knows exactly what will happen with their project. The reviewers want to see that you have carefully thought through the project, identified potential pitfalls, and have come up with ways to address problems or alternate strategies for success. This is where you prove to the reviewer you have a robust study design, and that, while you cannot foresee all contingencies, you can anticipate some solutions.
The budget and budget justification
The budget and budget justification are key components, for you, for your institution, and for the funding source. The budget will show how the money will be spent, while the budget justification will explain why it is being spent in this manner. These components should clearly support the research – if the expenditures are not consistent with the research plan and the approach, reviewers will raise questions. Do not forget – academic institutions take a percentage of grants as indirects (to support the administrative infrastructure). This varies depending on the type of grant, the rules of the funding source, and the location at which the research will occur. However, it can be upwards of 50% of your grant, depending on your institution.
Ethical considerations
When conducting research on humans or animals, you will need to obtain the appropriate institutional review and approval. This may be required prior to grant submission, but is frequently necessary prior to fund disbursement. If carrying out multisite or international work, this may require multiple approvals. Again, it is important that the reviewers and the funding agency have confidence that you will conduct your research ethically and within the appropriate federal regulations.
Appendix 36.1 Gantt Chart
A Gantt chart is shown in Figure A36.1.1 describing the different stages of a research project: when each step is started, how long it takes, and when it should end. Note the key, and that the steps are listed chronologically, some steps occur concurrently. You should get information from your mentor on how long each of these steps is (e.g., IRB approval) or even run a small pilot to determine how long it will take you to enroll patients. You should also build into your timeline a factor of safety, to avoid delays.
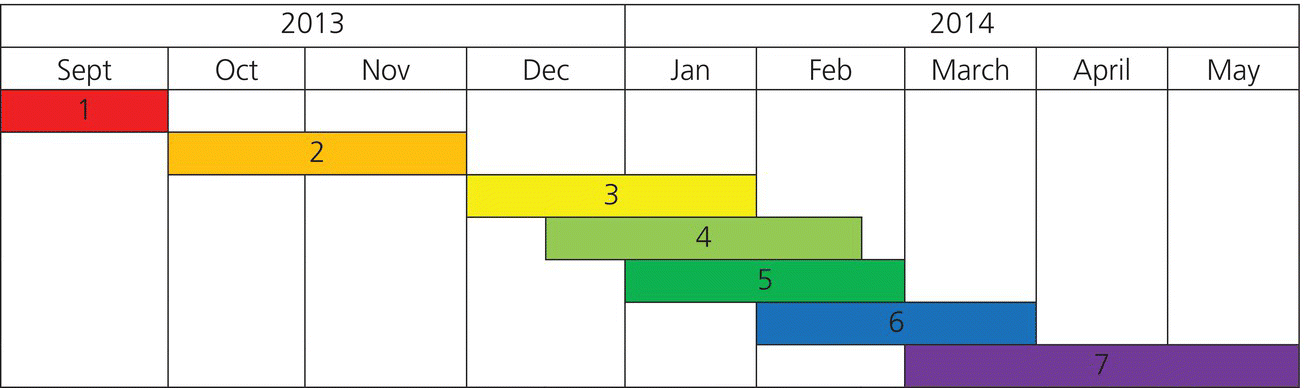
Figure A36.1.1 A representative Gantt chart.
Calendar/Mileposts
- Study design and project proposal development: One month.
- Institutional Review Board review and approval: Two months.
- Project Implementation with inservices for support staff: 1–2 months.
- Estimated data collection period: 1–2 months.
- Estimated sample testing period: 1–2 months.
- Data analysis: Two months.
- Journal article writing and submission: Three months.
Target Conference: ACEP, abstract submission date: Late April.
Appendix 36.2 Additional resources
- National Institutes of Health (2015) Grants & Funding. http://grants.nih.gov/grants/oer.htm (last accessed 16 May 2015).
- National Institutes of Health (2015) The K Kiosk – Information about NIH Career Development Awards. http://grants.nih.gov/training/careerdevelopmentawards.htm (last accessed 16 May 2015).
- The National Institute for Allergies and Infectious Diseases (2014) All About Grants: Tutorials and Samples. http://www.niaid.nih.gov/researchfunding/grant/Pages/aag.aspx (last accessed 16 May 2015).
- The National Institutes of Health Research Portfolio Online Reporting Tools: http://report.nih.gov/
- The National Science Foundation’s (nd) Find Funding. http://www.nsf.gov/funding/ (last accessed 16 May 2015).
- Grants.gov (nd) Find Open Grant Opportunities. http://www.grants.gov (last accessed 16 May 2015).