There would be no life on the Earth without the sun but a planet orbiting a star is actually extremely unlikely to have a biosphere, a thin sliver of space that harbors life and allows its evolution. The probes we sent to Mars did not uncover any evidence of life; Venus, our other neighbor, is too hot, and the remaining planets of the solar system are even less suitable for sustaining the only life we know of: carbon-based organisms that encode, in nucleic acids, complex programs for their reproduction and survival and run their metabolism with the aid of enzymes. Although we have been discovering many new extra-solar planets (planets orbiting another star) we have no indication that any of them support life (most are simply too big), and despite considerable resources invested in listening to space sounds we have not heard from anybody; all we hear are the radio waves emitted by the ionized interstellar gas that surrounds hot stars.
None of this is too surprising, considered in strictly energetic terms. What is needed is not simply a star (our galaxy alone has some hundred billion) with orbiting planets (again, they must be quite numerous) but a suitable “Goldilocks” star: not too big, not too small, not too cold, not too hot. Stars that are too massive do not last long enough to allow for the billions of years that, in the Earth’s case, were needed to produce complex life, and long-lived, dwarfish stars have insufficient luminosity to energize any planets that may orbit them. A Goldilocks star then has to capture a Goldilocks planet; one that is not too far away to have its water frozen all the time (like Mars), nor too close to have it vaporized (like Venus).
And that is merely the beginning of a long list of prerequisites that must be satisfied to achieve the conditions that make a planet habitable, or even the simplest life possible. The best way to demonstrate this is to imagine changing just one of the attributes that influence the delivery of solar energy to the Earth (playing the “everything-else-being-equal” game). What if gravity were twice as strong? What if its orbit were far more eccentric than its actual, nearly circular, course? What if its axis of rotation were not inclined? What if its rotation took 240 days instead of twenty-four hours? What if ninety (rather than thirty) percent of its surface were land? What if it had no water vapor and no carbon dioxide in its atmosphere? I will let you speculate about the unwelcome consequences of the first five ifs and give you just the answer for the last one: there would be no life on the Earth.

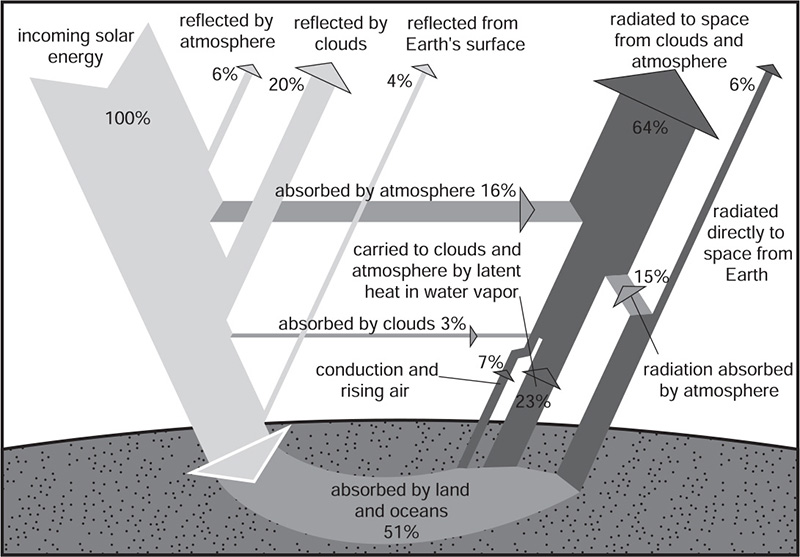
Figure 4 The Earth’s radiation balance (based on NASA image)
Our planet’s atmosphere lets incoming radiation (except for the shortest wavelengths) reach the surfaces and warm them (see Figure 4) but absorbs, temporarily, part of the outgoing longwave radiation. Without this absorption the Earth would be a “perfect black body radiator” and would re-radiate all the intercepted solar energy, leaving the planet at a temperature of 255 K (–18°C). At –18°C water would be permanently frozen, and there could be no life. Atmospheric gases, which selectively absorb part of the outgoing radiation (and then re-radiate it down and up) change a planet’s average radiative temperature (this is what makes Venus too hot and Mars too cold). On Earth, this “greenhouse effect”, caused mostly by water vapor and CO2, has been just right for the evolution and diversification of complex life, as it raises the mean surface temperature, by 33 K, to 288 K (15°C). This is about the temperature of a pleasant spring day in a temperate location and allows water to cover more than two-thirds of the planet’s surface, be copiously present in soils and air, and to account, on average, for about two-thirds of the fresh weight of living organisms (much more in some – up to ninety-five percent in green plant tissues and ninety-nine percent in phytoplankton).
After following the fate of the solar energy that reaches the Earth – that is, after examining the partitioning of incoming radiation, its return to space, and its atmospheric transformation, which determine the course and variability of the Earth’s climate – I will turn to the planet’s only important non-solar source of energy, its internal heat. This geothermal energy powers the global tectonics whose manifestations include not only a constant refashioning of the Earth’s continents and oceans but also some of the planet’s most violent natural phenomena: volcanic eruptions, earthquakes, and tsunami.
Despite stunning external biodiversity, living organisms share a relatively small number of fundamental metabolic pathways that use available energy to convert simple inputs into new living mass. Autotrophs (also called primary producers, which include all organisms able to synthesize new biomass from simple inorganic compounds) use two distinct ways to produce new biomass. Phototrophs (terrestrial plants, algae, phytoplankton, cyanobacteria, and green and purple sulfur bacteria) convert electromagnetic energy into high-energy phosphate bonds within ATP (adenosine triphosphate, the molecule chiefly responsible for the store and transport of energy within cells) and use this energy to produce new mass (phytomass) from atmospheric CO2 and the macronutrients (nitrogen, phosphorus, and potassium) and micronutrients (iron, calcium, silicon, and others) available in the soil. Chemotrophs (nitrifying bacteria (the bacteria which turn ammonia into nitrates), iron bacteria, non-pigmented sulfur bacteria, and methane-producing microbes) do not need light, only CO2, oxygen, and either an oxidizable element (hydrogen, iron) or a simple inorganic compound (hydrogen sulfide, ammonia). Their invisible metabolism is indispensable for the biosphere’s critical biological, geological, and chemical (biogeochemical) cycles.
Heterotrophs (also called chemo-organotrophs) are organisms that cannot synthesize new biomass from simple inorganic inputs and must get their building blocks from digested organic compounds: this includes most bacteria, fungi, and animals. Heterotrophs fall into four principal categories: primary consumers (herbivores), secondary and higher level consumers (carnivores), consumers of dead and decaying biomass (saprovores, detritivores), and species that resort to all the above strategies (omnivores). Modern energy studies have uncovered both great commonalities in plant and animal metabolism and many fascinating niche adaptations, and have also traced complex energy flows both on the large scale of individual ecosystems and the global scale of grand biogeochemical cycling, particularly the carbon and nitrogen cycles.
Sun and earth: solar radiation and its conversions
Astronomers like to point out that the sun belongs to one of the most common types of star (G2 dwarfs) unremarkable either for their size or their radiation. Most of their power comes from the proton-proton reaction, the fusion of hydrogen atoms into helium that proceeds at temperatures greater than 13 million K. The sun’s energy production (total luminosity) is immense, seen in terrestrial terms, as thermonuclear reactions in its core convert some 4.4 Mt of matter into energy every second: according to Einstein’s mass-energy equation, this works out to nearly 3.9 × 1026 W, a rate thirteen orders of magnitude (roughly 30 trillion times) greater than our use of all fuels (fossil and biomass) and primary (hydro and nuclear) electricity in 2015. Four and a half billion years ago, as the Earth was formed, the luminosity of the young sun was about thirty percent less than it is today. In that time, the sun has consumed just 0.03 percent of its huge mass but more than half of the hydrogen in its core. The rest of the solar story is of little concern to our civilization which will cease to exist long before the sun transforms itself, first into a red giant (one hundred times its present diameter) whose energy will melt the planet, and then into a much smaller, highly luminous white dwarf: the sun’s epochs are measured in billions of years, the history of complex civilizations has, so far, spanned only about 5,000.
We benefit from a perfect delivery: virtually nothing impedes the solar radiation as it streams through the cosmic void, when it reaches the topmost layer of the Earth’s atmosphere its flow amounts to about 1367 W/m2. This rate is called the solar constant, although monitoring from special satellites has revealed tiny, irregular, short-term fluctuations (up to 0.2 percent from the mean) that could not be previously observed because of atmospheric interference. There is also a more regular, tiny (about 0.1 percent), fluctuation connected to the sun’s eleven-year activity cycle.
SOLAR RADIATION
Overall, the sun’s spectrum corresponds very closely to a perfect black body, radiating at 6,000 K with the maximum emission close to 500 nm, in the lowest wavelengths of green light (491–575 nm). The visible part of the spectrum extends from 400 nm (deep violet) to 700 nm (dark red) (Figure 4): light’s diffraction in a rainbow or by a glass prism shows this beautiful color sequence. Human eyes have peak sensitivity for green and yellow (576–585 nm) light, with maximum visibility at 556 nm (near the end of green). Visible light carries about thirty-eight percent of the energy of incoming solar radiation, less than nine percent comes as ultraviolet (UV, less than 400 nm) radiation, which can neither be seen nor felt, and fifty-three percent is in infrared (IR, more than 700 nm) wavelengths, which include detectable heat (see Figure 5). The radiation that we measure at the top of the atmosphere and the radiation we receive on the ground (insolation) differ greatly, both in terms of overall quantity and in spectral composition. The most important quantitative adjustment is obvious: the solar constant measures radiation that streams through space and is perpendicular to a flat surface, but because this flow must be distributed over the planet’s almost perfect sphere, the average value, per unit area of the rotating Earth, is exactly one-quarter (about 342 W/m2) of the extra-terrestrial flow (the area of a sphere is four times larger than the area of a circle with the same radius). This incoming shortwave radiation is partitioned in three principal ways. Roughly twenty percent is absorbed as it passes through the Earth’s atmosphere. The absorption of UV radiation (mostly by stratospheric ozone) accounts for only about a tenth of the overall effect, but the elimination of frequencies below 300 nm was an essential precondition for the evolution of complex life. The remainder is absorbed by tropospheric clouds and aerosols (fine solid or liquid particles suspended in the atmosphere). Global albedo, the share of incoming radiation reflected by clouds and the Earth’s surface, without changing its wavelength, is almost exactly thirty percent. Fresh snow and the tops of tall cumulonimbus (thunder) clouds reflect more than ninety percent, dark soils and thick coniferous forests about five percent. About two-thirds of global albedo results from reflections from cloud tops; the remainder is split between reflections from surfaces and back-scattering in the atmosphere.
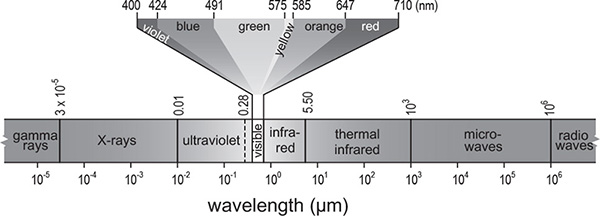
Figure 5 The electromagnetic spectrum
This means that average insolation amounts to almost exactly half of the solar constant, averaged per unit area of the rotating Earth, or approximately 170 W/m2. This insolation adds up to an annual global solar energy input of 2.7 x 1024J, or roughly 87 PW, more than five thousand times the worldwide consumption of fossil fuels and primary electricity in 2015. This makes it obvious that it is not a shortage of energy, but rather our ability to harness it and convert it into useful energy at an acceptable (both monetarily and environmentally) cost, that will determine the fate of our civilization. A tiny share of solar radiation could energize a civilization consuming a hundred times more energy than ours – but converting this abundant flow into affordable electricity is an enormous challenge.
On a cloud-free Earth, the average annual insolation would show a regular poleward decline, but tropical cloudiness causes a notable solar impoverishment of the equatorial zone, and monsoonal cloudiness does the same for the more northerly parts of Asia. Consequently, large parts of equatorial Amazonia, southern Nigeria (just 5° north of the equator), and provinces in the southern half of China (most notably the landlocked Sichuan, situated in a mountain basin at 30°N) receive less sunlight annually than New England, a region that extends from 40 to 45°N. And it is even less appreciated that the peaks of noon summer insolation are virtually identical in Jakarta (Indonesia’s capital, located at 6°S), and Edmonton (Alberta’s capital, nearly 55°N). These realities have major implications for any future large-scale attempts at direct (photovoltaic) conversion of insolation to electricity.
All the radiation absorbed by the Earth’s atmosphere and its solid and liquid surfaces is eventually re-radiated in IR wavelengths, and while the incoming radiation peaks at roughly 500 nm and ninety percent of it is shorter than four µm, the outgoing flux peaks at 9.66µm (a twenty-fold longer wavelength) and extends to just below three µm. This means that the incoming shortwave and the outgoing longwave streams of energy have a small overlap. Three major pathways maintain the Earth’s radiation balance: a small amount of energy is returned (through conduction and convection) as sensible heat, and about three times as much radiation leaves as the latent heat of evaporated water, which is released into the atmosphere after the moisture is condensed. Only a small share of the longwave emissions from surfaces (originating from the re-radiation of absorbed shortwave flux and the downward longwave emissions from the atmosphere) goes directly to space: some ninety-five percent is absorbed by the atmosphere’s greenhouse gases.
Atmospheric water vapor, the most important greenhouse gas, has several strong absorption bands; at wavelengths between 1 and 8µm, it adds about 20 K to the mean equilibrium surface temperature. Trace (but critically important) concentrations of CO2 (accounting for about a quarter of the current natural greenhouse effect), methane, nitrous oxide, and ozone increase the surface temperature by more than 10 K. The greenhouse effect has been responsible for maintaining a relatively narrow range of biospheric temperatures for the past 3.5 billion years but water vapor, the main contributor, could not have been the key regulator, because its changing atmospheric concentrations amplify, rather than counteract, temperature changes: water evaporation declines with cooling, and increases with warming. The best explanation involves gradual feedbacks between atmospheric CO2, temperature, and the weathering of silicate minerals: lower temperatures will bring decreased rates of silicate weathering and result in gradual accumulation of the released CO2 – and subsequent warming. The key regulatory role played by CO2 is the main reason for our concerns about relatively large anthropogenic increases of this gas caused mostly by combustion of fossil fuels and also by land use changes (above all by tropical deforestation).
Air and water: media in motion
Absorbed radiation provides three indispensable energy services: it heats continents and oceans (and the heat that these surfaces re-radiate does most of the heating of the atmosphere, keeping it in constant motion), it evaporates water and distributes it far from the sources of its origin, and it energizes photosynthesis. Given the relatively low specific mass of air (one cubic meter has a mass of just 1.2 kg near the Earth’s surface, a thousandth of that of water), only a very small fraction of insolation, perhaps no more than two percent, is needed to power the global atmospheric circulation which distributes heat, carries microbes, pollen, and seeds, and is responsible for the wind-driven weathering of continental surfaces. The global atmospheric circulation is energized by the continuous heating of the tropics, which creates a flow of cooler air from higher latitudes toward the equator (creating the so-called intertropical convergence zone) and sets in motion two vigorously moving loops of air commonly known as Hadley cells, after the English physicist George Hadley (1685–1768), who first described their existence.
Warm and humid tropical air first ascends (creating the equatorial low-pressure belt), moves poleward (in both a southerly and northerly direction), then cools, and descends (and is re-warmed) along a broad belt between 25° and 30° of latitude. This subtropical high-pressure belt creates desert zones, and the return flow of warm and dry air toward the equator generates persistent strong trade winds near the ocean’s surface. The existence of the trade winds was discovered in 1492, as they carried the three small ships commanded by Christopher Columbus (1451–1506) from the Canaries to the Bahamas in thirty-six days. A weaker circulation is also set off by the outflow of cold polar air that eventually warms up, rises, and returns at higher altitudes to close the loop. The mid-latitude (35°–50°) circulation (the Ferrell cell, named after a 19th-century American meteorologist) is driven by Hadley and polar cells, with air moving poleward near the surface and equatorward at higher altitudes. On a non-rotating Earth, ground winds in the mid-latitudes of the Northern hemisphere would be southerlies but the Earth’s rotation deflects them into the prevailing westerlies that bring plenty of precipitation to America and Europe’s western coasts.
The fastest near-ground winds are the product of the intensive summer heating that generates cyclonic (low-pressure) flows, ranging from innocuous localized thunderstorms to massive hurricanes. Even big thunderstorms, with a power of ten to a few hundred gigawatts, do not usually produce winds that strike objects with vertical power densities of more than 15 kW/m2, below the threshold for structural damage. North American hurricanes originate off Africa, first move westward, and then veer clockwise, frequently making landfall along the northern Gulf of Mexico, Florida, and the East Coast. Their Asian counterparts (typhoons) originate above very warm Pacific waters near the Marianas, move westward and repeatedly affect large parts of Southeast Asia, coastal China, the Korean peninsula, and Japan. Hurricanes or cyclones can have speeds up to 90 m/s (more than 300 km/h) and they strike vertical surfaces with power densities of up to 1 MW/m2, forces easily resisted by modern steel and concrete structures but not by wooden-framed houses.
While some hurricanes can endure for weeks and affect sequentially large areas along a path extending for several thousand kilometers, tornadoes are more restricted. The average path of an American tornado is only about 125 m wide (and often sharply delineated; a barely damaged house can stand across the street from a completely destroyed structure) and less than 10 km long, and they last less than three minutes. In contrast, tornadoes in the most violent (and relatively rare) category can generate winds in excess of 100 m/s and can strike vertical surfaces with more power than a typical hurricane.
WATER’S UNIQUE PROPERTIES
Water’s high specific heat capacity, 4.185 J/g°C, is several times that of soil and rock, and that is why the temperature of water rises and falls more slowly than that of solid surfaces and why it retains much more heat per unit of volume, making the ocean the world’s most massive temperature regulator. An Earth covered mostly by continents would repeatedly swing between high and low temperatures (similar to the oscillations experienced in large deserts). Moreover (as already noted), water has an extraordinarily high heat of vaporization, nearly 2.5 kJ/g at 20°C, which means that a large amount of latent heat can be moved over very long distances in water vapor and released tens, hundreds, or even thousands of kilometers away from its origin.
Evaporation draws water from bare soils and is intensified by transpiration (the movement of water from roots to leaves and then to the atmosphere) from vegetation but, obviously, the ocean dominates the Earth’s energy balance, not only because of its extent (just over seventy percent of the planet’s surface), but also because its low albedo (on average, six percent) means that it absorbs nearly four times more insolation than the continents. But because of water’s poor conductivity (less than one percent of that of even a poorly conducting metal) an inevitable consequence is the ocean’s strong thermal stratification. Sunlight penetrates only a thin sliver of the ocean’s average depth of 3.8 km, from less than 1m in highly turbid coastal waters that receive massive inputs of silt from large rivers, to about 200 m in the clearest tropical seas. Wind-generated waves mix the water within a similarly thin layer.
The surface temperature of this shallow mixed layer fluctuates daily and seasonally, and can rise to more than 25°C in the tropics. A more pronounced temporary warming takes place periodically in the Pacific Ocean where, normally, the strong trade winds off South America push the surface waters westward, creating cool surface water temperatures and causing the upwelling of nutrient-rich waters, which supports abundant marine life. But when the trade winds weaken, the surface waters off South America warm up, the upwelling is shut down (as is, largely, the fishing) and the westward expansion of warm surface waters extends along the equator to join warm water off Australasia. This recurrent warming phenomenon is known as El Niño and is associated with heavy rains and flooding in Peru and with drought in Australia and Indonesia. Its opposite is La Niña, which occurs when unusually strong trade winds create a larger than usual pool of cool water off the South American coast.
Below the thermocline (the layer of oceanic water where temperature declines rapidly with depth but nutrient concentrations and salinity increase), the water is always uniformly dark and close to 4°C, the point of its highest density. This is yet another property of this remarkable medium: while the density of other substances increases with decreasing temperature, water is at its densest at 3.98°C. This unusual temperature-density relationship makes it possible for fish to survive in northern waters, as ice forms at the surface rather than the bottom.
The cold waters of the deep ocean are brought to the surface only in restricted upwelling zones along the subtropical western coasts of the continents. This upwelling is compensated for by downward convection in giant oceanic cataracts that transfer surface waters to depths of several kilometers.
The planetary water cycle (evaporation-precipitation-runoff) moves, annually, nearly 580,000 km3. This equates globally to an average precipitation of about 3 mm per day, or 1.1 m a year, for every square meter of the Earth’s surface. Some 46 PW are needed to vaporize that mass of water, an energy that amounts to about fifty-two percent of total insolation. Latent heat thus greatly surpasses the kinetic energy of the cyclonic flows that bring summer rains: in thunderstorms the difference is commonly fifty- to one hundred-fold, in hurricanes the heat released during condensation is several thousand times the kinetic energy of the massive moving cyclone. But even the largest hurricane is an insignificant bearer of tropical heat, compared to Asia’s summer monsoons, which annually affect almost half of humanity and dump about 10,000 km3 of rain, from coastal Oman in the west to the Philippines in the east, releasing about five hundred times more latent heat than the most powerful hurricanes.
Only a small part of continental precipitation replenishes deep aquifers: about three-fifths are evaporated and less than a third is returned to the ocean by streams. Given the average continental elevation of 850 m, this stream flow has annually about 400 EJ (13 TW) of potential gravitational energy, an order of magnitude above the world’s total electricity use at the beginning of the twenty-first century. Only a small share of this enormous potential can be harnessed by building hydroelectricity generating stations; the degree of exploitation is limited by the availability of suitable sites to locate large dams, by competing needs for water (particularly for irrigation, cities, and industries), and by the necessity to maintain minimum streams flows to support aquatic life and flush away silt.
The earth’s heat: refashioning the planet
The other flow that energizes our planet is puny in comparison with solar radiation but its qualitative impact on the evolution of life and its effects on the fortunes of civilizations have been immeasurable, because the Earth’s internal heat is constantly recreating the ocean floor and re-assembling and splitting the continents. These grand geotectonic processes are accompanied by repeated, and often catastrophic, disasters. There are two sources of this internal energy: the basal heat from the slow cooling of the Earth’s molten metallic (largely iron) core and that from radioactive decay (particularly of uranium 235 and 238, thorium 232, and potassium 40). The latter flux is more important, and while the definite partitioning of the heat’s origins is still impossible, we have plenty of measurements to enable us to conclude that the aggregate global power of this geothermal energy amounts to some 44 TW.
Dividing this total by the Earth’s surface area gives a mean global flow of less than 90 mW/m2 compared to 170 W/m2 for average insolation, a difference of three orders of magnitude. The geothermal flux shows considerable spatial variation: the average for the ocean floor is more than seventy percent higher than that for the continents, where the areas of ancient crustal rocks (the Canadian Shield is a prime example) have the lowest rates. In contrast, the youngest sea floor oozes heat at a rate roughly three times as high as the oceanic average. The highest recorded large-scale averages are along the ocean ridges, where new floor is being created by upwelling of hot rocks (which is why the Pacific accounts for roughly half the Earth’s heat flow). Spectacularly high figures are reached at these hydrothermal vents, which spew water as hot as 360°C and reach power densities of many megawatts per square meter, rates equaled only by major volcanic eruptions.
About sixty percent of the Earth’s heat is converted into the formation of new sea floor along some 55,000 km of the ocean ridges, which divide the Earth’s crust (its thin, solid, topmost layer) into rigid and slowly moving geotectonic plates (Figure 6). The Pacific plate, the largest, is purely oceanic and in places is less than 10 km thick, while other plates have piggy-backing continents and crustal thicknesses of more than 100 km. Basaltic magma, rising from the underlying mantle along the ridges, creates about three square kilometers of new ocean floor at the mean global spreading rate of less than 5 cm/year. In a few places around the world – the Afar region in Eritrea and Ethiopia, the East African Rift Valley, and central Iceland – the rifting process can be seen on land. Diverging oceanic plates must eventually collide with more massive continental plates and the ocean floor must be recycled (subducted) back into the Earth’s mantle, a solid, nearly 3,000 km thick, layer between the crust and the liquid core. Deep ocean trenches are the most spectacular features of plate subduction.
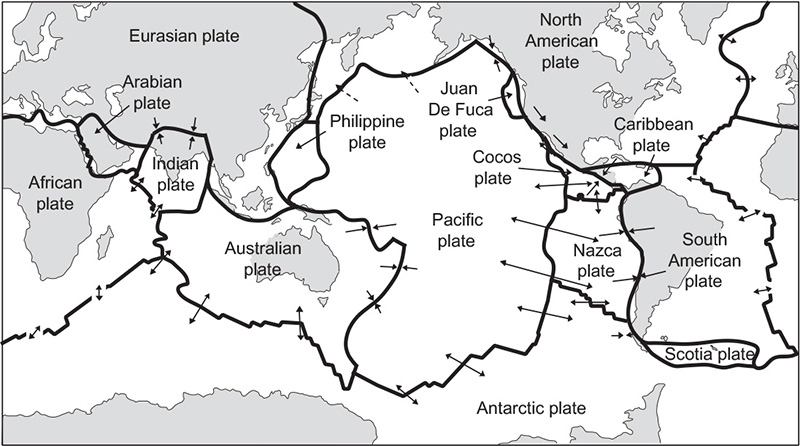
Figure 6 Geotectonic plates
This continuous recycling explains why there is no ocean floor older than about 200 million years (most of it is younger than 100 million years), and why the most violent earthquakes (often causing massive tsunami) and volcanic eruptions are concentrated along the subduction fronts. These zones make a huge semi-circle of deep ocean trenches around the Pacific, from the Aleutian Islands to Tonga (north of New Zealand), as the relatively rapidly moving Pacific plate is forced under the virtually immobile Australian and Eurasian plates. The other major type of collision between oceanic and continental plates results in the formation of prominent mountain ridges: the Himalaya is still growing slowly as the Indian plate collides with the Eurasian, and a largely spent collision of the African plate with the westernmost region of the Eurasian plate created the Alps.
Many details about the energetics and mechanics of the planet’s grand geotectonic process remain unclear, but there is no doubt that magma upwelling along the ridges and plate subduction along the trenches drive the Earth’s most massive cycle. New ocean floor, created by the convection of mantle magma is, on average, about 3 km above the abyssal plain, forming massive blocks of hot rocks with large gravitational potential energy, which furnish the push-power away from the ridges. Along the trenches, the sinking of the cold ocean floor produces the pull-power, as it applies torque to the viscous mantle. The importance of this force is attested by the fact that the average speed of plate movement correlates best with the length of subduction zones: the Pacific plate has short-term generation rates of up to 20 cm/year and long-term velocity of up to 90 km per million years. These speeds prove that mantle drag force (proportional to a plate’s area and its velocity) must be relatively small.
EARTHQUAKES AND TSUNAMI
As even the fastest moving plates travel at only about 0.5 mm a day, their continuous displacement cannot be perceived directly, but earthquakes and volcanic eruptions remind us repeatedly of the incessant energy flow from the Earth’s mantle. All but five percent of earthquakes are associated with subduction or collision zones, and all but ten percent take place in or near the Pacific’s coastal areas, in the appropriately named “Ring of Fire”. The energy released annually by earthquakes is equal to no more than one to two percent of the total geothermal flux but that is a process of continuous heat convection, while most earthquakes last only between a few seconds and half a minute, meaning that the larger ones have a great deal of destructive power. Consequently, in the twentieth century, earthquakes claimed more lives than volcanic eruptions, cyclones, and floods combined.
The easiest way to find an earthquake’s energy is through its relationship with the magnitude of tremors. A standard measure was introduced by Charles Richter (1900–1985) in 1935. Richter’s magnitude is the logarithm to the base 10 of the largest trace amplitude (measured in micrometers), recorded with a standard torsion seismometer 100 km from the tremor’s epicenter (the ground above the focus of the earthquake). The conversion to total energy, released as seismic waves, yields, as do other methods, only approximate values. The largest recorded earthquakes, with a Richter magnitude of 9.0, release nearly 1.5 EJ of energy, and if they take place in less than 30 seconds, the power is as high as 50 PW: no ephemeral energy discharge originating on Earth can be as powerful. At the same time, there is no strong correlation between an earthquake’s power and the overall death toll: residential density and, above all, the quality of housing construction are the key determinants of casualties. As a result, two of the twentieth century’s most famous earthquakes ended up with very different tolls: the 1906 San Francisco earthquake was roughly four times more powerful than the 1923 Tokyo quake, whose death toll (at nearly 143,000) was (mainly because of the collapse and burning of densely packed wooden houses) almost fifty times higher. The most deadly earthquake in recent history struck on July 28, 1976, in Tangshan, a large coalmining town in China’s Hebei province: its magnitude was 7.8 and it killed, officially, 242,219 people in the city and its surroundings but the real toll was much higher.
Some underwater earthquakes generate tsunami, massive seismic sea waves that can travel in the deep ocean at more than 600 km/h, while causing only a minimal disturbance at the surface. Once these waves hit shallow coastal waters they may rise to heights of up to several tens of meters and can strike shoreline vegetation and structures with vertical power densities of more than 1 MW/m2, rivalling, and often surpassing, the power of the fiercest tornadoes. The Pacific Ocean has the highest frequency of tsunami and Japan saw most of the tsunami-related casualties in modern history. The tsunami that struck the beaches of Honshū on June 15, 1896 was up to 30 m high and killed 27,000 people. The March 2011 earthquake off the coast of northern Japan had a magnitude of 9.0 and the tremor caused a huge tsunami that was responsible for most of the nearly 16,000 deaths and more than 2,500 missing people. But the most destructive recent tsunami was triggered by an undersea earthquake (magnitude 9.0), centered just off the northwestern tip of Sumatra’s Aceh province on December 26, 2004: it killed more than 200,000 people, mainly in Aceh but also along the eastern coasts of Sri Lanka and India, on western beaches of Thailand and (in much smaller numbers) right across the Indian Ocean, in Somalia. The overall energy release associated with this subduction-generated earthquake was estimated at 2 EJ.
The northwestern tip of Sumatra was also the site of one of the largest modern volcanic events: in 1883 a series of eruptions, culminating on August 26, destroyed most of Rakata, a small island in the Sunda Strait surmounted by the cone of Krakatoa, lifting about 20 km3 of ash and rocks into the atmosphere. Subsequent tsunami, rather than the eruption itself, caused most of the estimated 36,000 casualties.
Because they are intermittent and often of short duration, volcanic eruptions account for only a small share of the global release of geothermal energy: the best estimates put the share at around two percent of the total flux but there is enormous year-to-year variability, as decades may elapse between spectacular large-scale volcanic events, and some volcanoes erupt violently but briefly while others remain active for extended periods of time.
Heat nearly always dominates the overall release of volcanic energy (by one to three orders of magnitude) but its principal carriers are different. For many volcanoes, the heat is carried mostly by massive ash clouds that rise all the way to the stratosphere (most recently during the eruption of Mount Pinatubo in the Philippines on June 15, 1991), and cause lower ground temperatures worldwide for months. In contrast, Hawaiian volcanoes release their heat in the form of slow-moving lavas (smooth, rope-like, pahoehoe and crinkly aa); some of these flows can be closely approached to sample the hot magma. By far the most dangerous heat release are the pyroclastic flows that combine volcanic material ranging from fine ash to large rocks with hot (even above 500°C) gases. They can flow downhill at more than 100 km/h and smother everything in their path up to 100 km away. In 1902, such nuées ardentes (glowing clouds) killed 28,000 inhabitants of St. Pierre on Martinique after Mount Pelée erupted. In August 1997, pyroclastic flows destroyed a large part of the Caribbean island of Montserrat and they have been repeatedly observed at Unzen in Japan.
The eruption of Mount St. Helens on May 18, 1980 was the best monitored event of its kind. The total energy release over nine hours was about 1.7 EJ (52 TW). The best estimates for other large modern eruptions are: Krakatoa, 1.7 EJ, Bezymyannyi (Kamchatka), in 1956, 3.9 EJ, Sakurajima (Japan), in 1914, 4.6 EJ, and Tambora, in 1815, 8.4 EJ. But even Tambora’s energy was pitiful compared to the Yellowstone eruption, 2.2 million years ago, that released an estimated 2,500 km3 of ash. Even that was a minor event, compared to eruptions spread over some five million years (between 65 and 60 million years ago) that piled up about one million cubic kilometers of basalt lava to form the extensive (about 1.5 million km2) Deccan Traps in west central India.
Much like earthquakes, volcanoes are overwhelmingly associated with the margins of tectonic plates, but at a few locations powerful hot magma plumes have pierced through a plate, creating spectacular hot spots far away from any subduction or collision zones. The most famous example is the chain of Hawaiian Islands that extends, in the form of seamounts, all the way to Kamchatka. This volcanic chain is being created by a massive hot spot, that keeps piercing the Pacific plate on its northwestern-ward journey and is now situated underneath the western coast of Hawaii (where it manifests itself in continuing eruptions of Kilauea volcano) and just offshore from the island where Loihi, a large undersea volcano, will emerge (but not for tens of thousands of years) as the chain’s newest island. Another major hot spot pierces Africa’s plate in the center of the continent, creating the Virunga volcano chain on the borders of Uganda, Rwanda, and Zaire (the world’s last mountain gorillas live in the bamboo forest in the foothills of one of these volcanoes, the 4,500 m Mount Karisimbi).
Photosynthesis: reactions and rates
Photosynthesis is energized by the absorption of light by pigments in the thylakoid membranes inside bacterial and plant chloroplasts (the cellular organelles that give plants their green color). The energy efficiency of the conversion of simple inorganic inputs into new phytomass is surprisingly low. Introductory textbooks often outline the entire process in a simple equation in which the reaction of six molecules of CO2 and six molecules of water produces one molecule of glucose and six molecules of oxygen: 6CO2 + 6H2O = C6H12O6 + 6O2. The reality is vastly more complex. The key sequential steps were revealed for the first time in 1948 by Melvin Calvin (1911–1997) and his co-workers (Calvin received the 1961 Nobel Prize for Chemistry for this discovery). Most importantly, the process entails not only carbon fixation and oxygen evolution, but is also the complex exchange of oxygen and CO2 in two closely related cycles (the other being photorespiration).
Chlorophylls a and b, the two dominant plant pigments that can be excited by radiation, have rather narrow absorption maxima, the first being between 420 and 450 nm, the second between 630 and 690 nm. This means that photosynthesis is energized, overwhelmingly, by a combination of blue and red light, and because the pigments absorb virtually no light in the green and yellow parts of the visible spectrum those colors, reflected from the leaves, dominate in spring and summer and change only as the pigments begin decomposing in the fall. It also means that photosynthetically active radiation (PAR) amounts to only about forty-three percent of insolation. The energy absorbed by the pigments drives the electron transport (water is the electron donor and hence the source of oxygen) that involves three multi-enzyme complexes. This results in the production of NADP (nicotinamide-adenine dinucleotide phosphate, one of the two most important enzymes in cells) and ATP (adenosine triphosphate) which drive the incorporation of CO2-derived carbon into carbohydrates, a process that follows three distinct paths.
PHOTOSYNTHETIC PATHS
The proper name of this process is the reductive pentose phosphate (RPP) or Calvin-Benson cycle. In the first step, one of the biosphere’s most abundant enzymes, ribulose 1,5-bisphosphate oxygenase (commonly known as Rubisco, it accounts for about half of all soluble protein in leaves) catalyzes (increases the rate of) the addition of CO2 to a five-carbon ribulose 1,5-bisphosphate (RuBP) to form the three-carbon 3-phosphoglycerate (PGA). In the second step, NADPH (NADP with one hydrogen atom added) and ATP produce 1,3-bisphosphate (triose phosphate). Finally, the Rubisco is regenerated and the triose phosphate used either to form carbohydrates or fatty acids and amino acids.
Rubisco acts not only as a carboxylase (an enzyme which catalyzes the addition of CO2) but also as an oxygenase (an enzyme which catalyzes the addition of oxygen). In that role, it catalyzes the binding of oxygen to RuBP, leading to the production of PGA (which re-enters the RPP cycle) and glycolate (a two-carbon compound) whose breakdown releases CO2. Because the atmosphere contains so much more oxygen than carbon dioxide (20.95 percent versus 0.04 percent), the Rubisco-mediated photorespiration cycle (essentially an oxygen-consuming, carbon dioxide releasing counterpart of photosynthesis) can reconvert significant amounts of carbon (as much as half) and thus reduces the net efficiency of photosynthetic conversion. The numbers of carbon atoms in the final products of the two cycles give them their common name, the C3 (photosynthetic) and C2 (photorespiration) cycles (Figure 7). Unfortunately, most widely cultivated staples, as well as most vegetable and fruit species, use the C3/C2 cycle, giving the so-called C3 plants (which include rice, wheat, barley, rye, all tubers, all leguminous grains, and all oil crops) inherently low energy conversion efficiencies. Their cultivation also demands a great deal of water.
There is another photosynthetic path, which deviates in an important way from that dominant sequence. Its first step is the hydration of CO2 to bicarbonate (HCO3) in mesophyll cells (large cells that communicate, via stomata (holes), with the leaf’s surface); then, rather than using Rubisco, the bicarbonate is reduced, with a different enzyme, phosphoenolpyruvate carboxylase (PEP), to produce oxaloacetate, a four-carbon acid, which is subsequently transformed to malate (also with four carbons). This compound is moved into bundle sheath cells (buried deep inside the leaf, surrounding the vein), where the CO2 is removed. The released CO2 enters the RPP cycle, to be fixed by Rubisco. This C4 cycle is inherently more efficient because PEP carboxylase is a better catalyst than Rubisco and also because oxygen levels in bundle sheath cells (which exist only in C4 plants) are lower than in mesophyll cells, and hence Rubisco’s capacity to act as an oxygenase (as in the photorespiration process) is virtually eliminated while it catalyzes the C3 cycle.
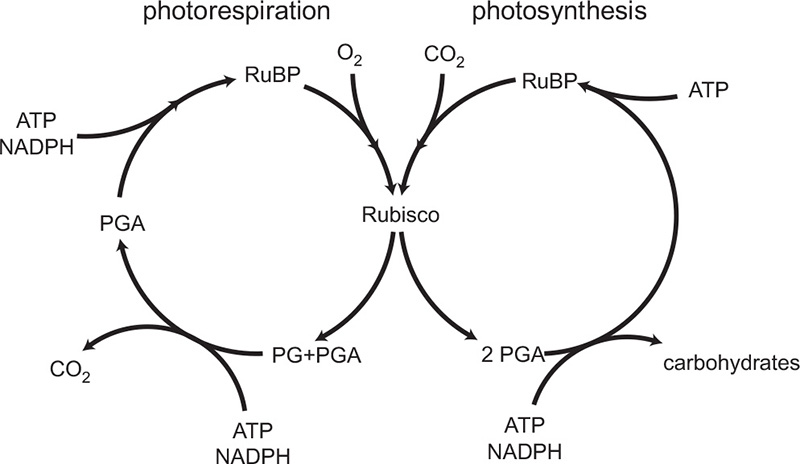
Figure 7 The C3/C2 cycle
C4 species have three more photosynthetic advantages: while C3 species are saturated by light inputs with power densities around 300 W/m2, there is no light saturation in C4 plants; while C3 plants perform best at temperatures between 15 and 25°C, C4 plants have their highest net photosynthetic rates between 30 and 45°C; and while C3 plants transpire, on average, around 1,000 moles of water (and as much as 4,000 moles) for every mole of CO2 they import, this ratio is just 400–500 for C4 plants. These three attributes mean that C4 plants thrive in sunny climates and are much more heat- and drought-tolerant (because inherently more water-efficient) than any C3 plant growing in the same conditions. Unfortunately, only three major crops – corn, sorghum, and sugarcane – follow the C4 photosynthetic path, which is also, unfortunately, shared by some of the world’s most invasive weeds (including crabgrass, a particularly obnoxious and ineradicable C4 species in gardens).
The final photosynthetic path, crassulacean acid metabolism (CAM), is restricted to succulents belonging to the Crassulaceae and Cactaceae families and to some orchids and bromeliads. This process is an adaptation to extreme aridity and high temperatures. These plants absorb CO2 during the night, when their water losses can be minimized, and convert it into malate using PEP produced from starch. During the day, NADPH and ATP are produced by light reactions, the CO2 is removed from the malate and high levels of cellular CO2 are converted by the RPP cycle. Commercially important CAM plants include pineapple, aloe (famous for its medicinal uses), opuntia (cactus pear), and agave (the sweet juice of agave is used to make Mexican tequila) and vanilla.
No matter which path a plant follows, it will consume a significant share of its fixed carbon in respiration, which produces energy by oxidizing sugars. This process takes place in special organelles (mitochondria) within plant cells, and the energy is used to maintain basic functions. These include the transport of sugars or starches (the photosynthate) from leaves to stems and roots, the uptake of macro- and micro-nutrients from soil, and their assimilation into organic compounds. The energy obtained through respiration is also used to make the complex organic compounds needed by organisms for their metabolism, supporting structures (roots, stems, trunks) and defense against heterotrophs, particularly insects. This last is accomplished by making the soft interior tissues inaccessible behind thick barks, waxy leaves, or thorns. Respiration may claim less than a fifth of all new photosynthate in crops (humans provide readily available nutrients through fertilization and defense with insecticides) but uses all of it in mature trees, which spend their energy completely on the maintenance of existing structures, as their newly synthesized phytomass is used to replace ageing parts, rather than form new tissues.
The maximum theoretical net efficiency of photosynthesis (after subtracting all respiration losses) is about four percent of insolation, but this rate can be approached only during brief periods and in the presence of adequate water and nutrients. Intensively tended (irrigated, fertilized) crops can average two percent efficiency during their growing season; the most productive temperate and tropical forests approach 1.5 percent. The global continental average is only 0.33 percent, and, because oceanic phytoplankton converts less than 0.1 percent of insolation into new aquatic phytomass, the average for the entire biosphere is less than 0.2 percent. To put it differently, the energy of only one in 500 photons which reach the planet’s ice-free surface gets converted into new phytomass. Given the immense flow of solar radiation, this inefficiency is largely irrelevant; the overall photosynthetic performance is impressive in quantitative terms and even more so in qualitative differentiation. The best global calculations show an annual net primary production (NPP) of about 120 billion tonnes of plant mass on the continents and 110 billion tonnes in the ocean.
Forests are generally the most productive ecosystems. The distribution of major biomes (complex large-scale communities of organisms) is limited not by insolation but primarily by temperature and precipitation: tropical rainforests need at least one meter of rain a year and an average annual temperature of more than 20°C; deciduous forests, the dominant natural vegetation of Western Europe and Eastern North America, occupy a broad niche, with annual average temperatures between 0 and 20°C and rainfall between less than half a meter and more than two meters a year. The annual rates of NPP (in absolutely dry matter) range widely for major ecosystems: from 1–3.5 kg/m2 (10–35t/ha, i.e. tonnes/hectare) in tropical rainforests, to 0.5–2.5 kg/m2 in temperate forests and 0.2–1.5 kg/m2 for most grasslands.
In qualitative terms, the richest tropical rainforests, in Amazonia, harbor more than six hundred different species per hectare, but most of this phytomass is stored in a relatively small number of large canopy and emergent (towering above the general canopy level) trees. Temperate and boreal forests are often dominated by just a few species, but some of these ecosystems, particularly the rainforests of the Pacific Northwest, can store much more wood per hectare (up to 3,500t) than their richest tropical counterparts. But the average phytomass in the above-ground vegetation of temperate forests (root phytomass is always difficult to assess, and so most phytomass totals are limited to above-ground growth) is similar to that stored in tropical formations, from 250 to 300t/ha. There is no universal conversion rate of these masses to energy: woods with higher lignin and extractives (resins, waxes) content will have higher heating values. The range for common North American species is from 17.8 MJ/kg for sweetgum to 21 MJ/kg for Douglas fir.
NPP rates are essentially equal to annual yields for crops that have not suffered any major damage from pests and diseases. These rates are, predictably, highest for C4 crops and for crops grown under optimum conditions. Many Iowa farmers harvest more than 12t/ha of corn, excellent harvests of English or Dutch wheat are around eight, and in Japan and China’s coastal provinces rice produces just over six. Leguminous crops (beans, lentils, peas) yield mostly less than two, but many vegetables can produce in excess of fifty. Because yields are reported at harvest moisture (which is less than fifteen percent for cereal and leguminous grains but ninety to ninety-five percent for many vegetables) the latter reduce to less than 5 t/ha of absolutely dry matter. Similarly, harvested stalks of sugarcane (excellent yields of this C4 plant are from 80 to 100 t/ha) contain only about twenty-five percent dry matter. Well-managed forests have (because of considerably higher respiration rates) annual increments of between 1 and 2 t/ha of dry matter but intensively managed plantations of fast-growing tree species (poplars, eucalyptus, pines) can produce more than three or four t/ha.
Heterotrophs: metabolism and locomotion
Heterotrophs have two basic ways of metabolizing complex organic compounds: anaerobically (without the presence of oxygen) and aerobically. The two groups of anaerobic fermenters that figure prominently in human affairs are the bacteria responsible for lactic fermentation and yeasts. Fermenting bacteria, which transform sugars into lactic acid, give us sour milk, yoghurt, and cheese, as well as sauerkraut, gherkins, and olives. Yeasts (fungi belonging to the phylum Ascomycota) produce alcohol from a variety of sweet or starchy substrates. The anaerobic path arose during the early history of the Earth, when its atmosphere had only a trace of oxygen; the aerobic became widespread after the oxygenating atmosphere was created during the past two billion years.
In the presence of oxygen, yeasts do not produce alcohol but carbon dioxide, to make dough that rises: leavened bread instead of pita or chapati. About half of the bacterial phyla are aerobic; they include commercially important species, such as the nitrogen-fixing bacteria (above all those of the genus Rhizobium) that provide leguminous species with this key nutrient (in the form of ammonia) in return for their supply of carbohydrate. All the species belonging to the kingdom Animalia are aerobic heterotrophs. The first task of heterotrophic metabolism is to break down carbohydrates into their constituent monosaccharides (glucose and fructose; sucrose, the world’s leading sweetener, is a disaccharide made by their combination), to hydrolyze (react in the presence of water) lipids into glycerol and fatty acids, and to decompose proteins into the constituent amino acids used to build new protein structures (muscles, organs, cells). ATP conserves the energy released by the degradation of nutrients; its transformation to adenosine diphosphate (ADP) makes energy available for the synthesis of new biomass and for locomotion.
Numerous enzymes catalyze these complex reactions. In fermentation, the final products are either lactic acid or ethanol and CO2 (we keep the gas in champagne but not in table wines) while aerobic metabolism produces, besides large amounts of ATP, CO2 (which we exhale) and water (which we lose through breathing and in sweat, urine, and feces). The maximum overall efficiency of anaerobic fermentation is around thirty percent, of aerobic metabolism about sixty percent, both for converting simple sugars or fatty acids. Every heterotroph has its own basal metabolic rate (BMR), the constant minimum power needed to energize its vital internal organs.
BASAL METABOLIC RATE
This rate is measured at complete rest, several hours after the last intake of any nutrients (digestion increases metabolism) and in a temperature-regulated setting. Oxygen consumption (or CO2 generation) served for decades as its best determinant. In 1932, Max Kleiber (1893–1976) noted that the basal metabolic rates of mammals, ranging from rats to steer, depended only on their body weight raised to the power 0.74. Later, he opted for the exponent of 0.75 (hence the so-called ¾ power law). When body mass (w) is expressed in kg and power in W then BMR = 3.52w0.75. When both variables are plotted on a logarithmic scale the rates are on, or very close to, a straight line (Figure 8). BMR measurements eventually extended the mammalian line from shrews to elephants and confirmed that its 3:4 slope also approximates the rates for some birds, some ectotherms (higher organisms that do not actively regulate their core body temperature) and many micro-organisms.
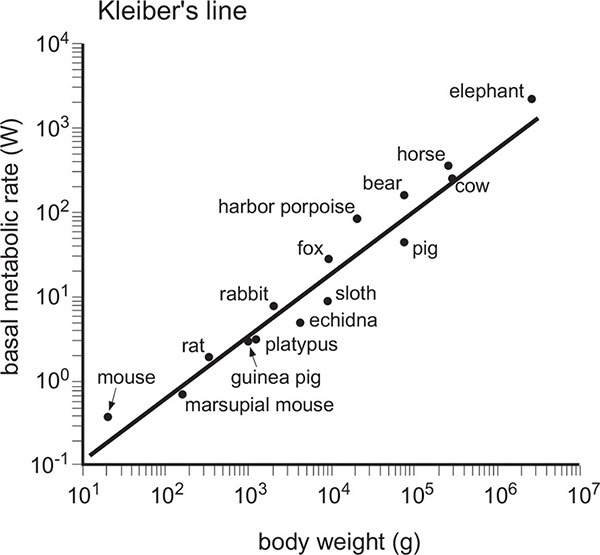
Figure 8 Kleiber’s line (plotted from data in Kleiber, M. 1961. The Fire of Life)
At close to 0.9, the exponent is considerably higher for carnivorous mammals, which means that their BMR is greater than that of similarly massive non-carnivores, and that it increases more quickly with greater body mass, making a rhino-sized tiger (1 t rather than 100 kg) impossible. On the other hand, the exponent is much lower (just below 0.5) for desert rodents, an adaptation that minimizes their energy needs (as well as water consumption) in a hostile environment. And while the BMR of many endotherms (vertebrates that actively maintain their core body temperature very close to a constant) and ectotherms may share the same exponent, endotherms must pay for their thermoregulation (37–38°C in mammals, and 38–42°C in birds) by having a BMR as much as 20–40 times higher than that of similarly massive ectotherms. Smaller, but significant, differences can be found among endotherms: for example, the large and almost constantly flying albatross (some circumnavigate the Earth every year) have a BMR almost twice as high as similarly-sized but occasional fliers.
The relationship revealed by Kleiber’s line also means that specific BMRs (W/g) decline exponentially with larger body mass (at a rate of roughly w−0.25): the smallest shrew needs about 100 mW/g, a nearly five orders of magnitude heavier steer requires only 1 mW/g, and a rufous hummingbird, the smallest (3.5 g) avian flier in Canada, has a BMR about twenty times higher than a hundred times heavier (3.5 kg) brown pelican (Figure 9). This limits the mass of the smallest endothermic organisms, because otherwise animals lighter than shrews and hummingbirds would have to feed constantly in order to compensate for high rates of heat loss, a consequence of having a relatively large body surface in relation to the body mass. At the same time, the low specific BMR of large mammals makes it possible for them to go for days without eating and, when some of them lower their BMR and stay at rest, to hibernate (live off the accumulated reserves of fat) for months (as do bears).
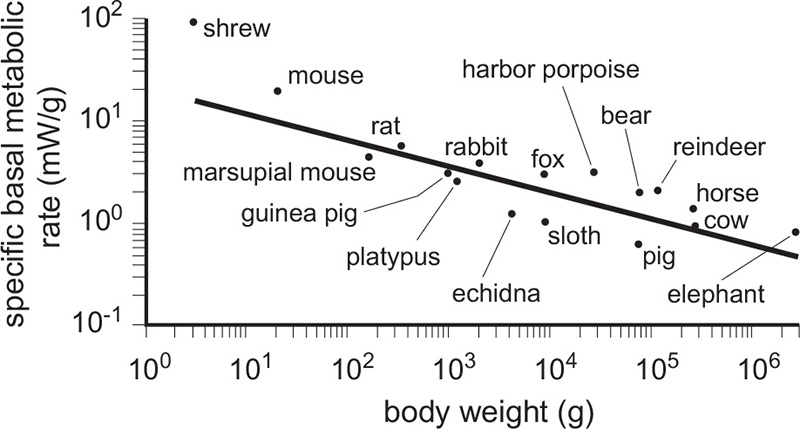
Figure 9 Specific BMR
The overall energy expenditure of heterotrophs with limited mobility (many invertebrates, and some reptiles and fish) is only a modest multiple of their BMR, while vigorous fliers and widely roaming mammals need most of their energy for their demanding activities. Observations over longer periods of time indicate that the total daily metabolism is about 2.5 times BMR for rodents and more than four times BMR for birds who forage on the wing. Running, flying, and swimming speeds, and endurance are limited by the metabolic scope (the ratio between short-term maximum energy expenditure and BMR) of heterotrophs. The average scope for mammals (independent of body mass) is about ten, for some fish and most birds about fifteen (but avian BMR is much higher than that of fish or mammals!), and commonly less than five for reptiles and amphibians. As a result, songbirds can develop up to 150 mW/g from their tiny bodies, while small rodents put out around fifty and an iguana less than five.
But running mammals give many false impressions: hares seem to be always running quickly, while canids (wolves, wild dogs, coyotes, jackals) often just amble. Whilst hares have a metabolic scope of around eight, canids have the highest known, surpassing thirty. They are the best sustained runners of the animal world: cheetahs may be record holders (105 km/h, greyhounds can do only fifty-eight) but their rushing distance while hunting is usually less than one hundred meters, and they often stop, panting with exhaustion, after running just for ten seconds. The slower wolves can pursue their prey non-stop for twenty minutes and cover, daily, more than thirty kilometers. And size matters, because the power available for running increases more quickly with larger body mass than does the cost of running. As a result, a massive animal may look too bulky and too muscle-bound to be a sprinter, but this could be a deadly miscalculation: you cannot out-run a bison, grizzly bear, or hippo!
If you are a good sprinter you may outrun an Asian elephant (although your chances of encountering one in the wild are now, unfortunately, negligible). These massive animals can do nearly 7 m/s (about 25 km/h), although it is still not fully clear if their rapid locomotion amounts to true running, so if you can run a hundred meters in less than fourteen seconds (the world record is 9.58s) you can elude them. Measurements show no major difference between the cost of running bipedally and quadrupedally, but besides their greater power, larger animal runners can also take advantage of the elastic strain that is stored, temporarily, in their long leg muscles and tendons and then released as elastic recoil: at high speeds this ability, which humans share with horses and cheetahs, can greatly reduce the overall energy needs of running.
Not surprisingly, comparisons of energy cost for the three main forms of locomotion show that swimming, thanks to water’s buoyancy, is the least costly way to move: it requires less than one-tenth the energy of running; while running is more energy-demanding than flying for body weights up to 1 kg. For heavier birds, the difference narrows, and the mass of the largest fliers is limited by the fact that the power needed for flight rises more quickly than the power that can be delivered by the pectoral muscles. Consequently, large birds have difficulty getting airborne (they often launch themselves into a strong wind from cliffs or steep hillsides: for example, American condors and royal albatrosses). There are very few fliers heavier than ten kilograms, the heaviest, the East African Kori bustard (13–19 kg), gets airborne only rarely, in a series of low-level hops.
The long-distance migrations accomplished by running, swimming, and flying are much easier when the animals can feed along the way, as do caribou, salmon, and Canada geese. But, counter-intuitively, songbirds migrating in cool weather may spend more energy in stopovers than during their interrupted flight. By far the most spectacular long-distance migration is the non-stop flight of tiny songbirds, as they cross forbidding expanses of ocean on their way from northern and temperate nesting habitats to subtropical and tropical winter sites. These migrations (guided by stars, sun, geomagnetic field, and polarized light) must be powered by fat, the most energy-dense fuel a body can store. Consequently, the maximum distance (discounting any helping winds) a small songbird will be able to fly non-stop will be limited by the amount of fat it can store before it embarks on a trip: many species add as much thirty percent to their normal body mass and have virtually no reserves left when they land (or literally drop exhausted on beaches) at their distant destination.
Energy in ecosystems: networks and flows
Thermodynamic imperatives rule the energy flows through ecosystems. Photosynthesis may be a relatively inefficient process, but autotrophs are at the top of the biosphere’s cascade of energy conversions, and hence their phytomass must always dominate the total biomass of an ecosystem. The more steps removed a living thing is from the primary energy of the Sun, the less energy is available at the successive feeding (trophic) levels (positions along what is often, though erroneously, referred to as a food chain – it’s almost always a web).
No scientific studies are needed to know that herbivores (primary consumers such as rodents and ungulates) are considerably more abundant than secondary consumers (carnivores, be they birds of prey that eat rodents, or ladybugs that eat aphids). These, in turn, are more numerous than tertiary consumers (secondary carnivores such as aquatic birds that feed on frogs that eat insects or on carnivorous fish). And, needless to say, access to food is greatly enhanced by being an omnivore and eating, indiscriminately, any available autotrophs, heterotrophs, or decomposing biomass – as long as it can be easily found, caught, and digested.
Omnivory is very common, and often is a matter of opportunity or need, as many normally herbivorous or carnivorous species can move up or down one trophic level, as some resources become temporarily abundant and easy to harvest or others become scarce. Humans, always no-holds-barred omnivores, have mastered this approach, as the foodstuffs we have consumed in times of natural plenty or desperate need range from whale blubber to willow bark.
Finally, decomposers play a critical role in every ecosystem, as they break down complex organic macromolecules and make essential macro- and micro-nutrients available, again and again, both to autotrophs and heterotrophs: they can, of course, feed on dead organisms at any trophic level.
In most terrestrial ecosystems, the feeding cascades are short. In the East African grasslands, paragons of large animal abundance, most transfer ends after just two steps with secondary consumers: ungulates (antelopes, gazelles, impalas, wildebeest) eat grass, felids (leopards, cheetahs, lions) and canids (wild dogs, hyenas) eat ungulates, and decomposers feed on their remains and anything else they can break down with their enzymes. No fierce carnivores can be found feeding at the sixth level from the sun. In tropical rainforests – with a greater, much more varied, standing phytomass and a greater variety of heterotrophs – three levels are common, and five are possible: fungi feed on plants, arboreal invertebrates feed on fungi, frogs feed on invertebrates, snakes feed on frogs, and birds feed on snakes.
Marine ecosystems are based on primary production by phytoplankton, a category of organisms that embraces an enormous diversity of tiny autotrophs, including bacteria, cyanobacteria, archaea (unicellular organisms that are outwardly indistinguishable from bacteria but have a distinct genetic make-up), and algae. Marine food webs are generally more complex than those of terrestrial biomes. They can extend to five and in kelp forests six trophic levels and, in an unmatched complexity the richest coral reefs may go seven.
A complete account of biomass within a unit area of any terrestrial ecosystem would show a pyramid-shaped distribution, with autotrophs forming a broad base, herbivores a smaller second tier, omnivores and first-order carnivores at the next level, and the rare top predators at the apex. The mass of the levels varies greatly among ecosystems, but phytomass is commonly twenty times larger than the zoomass of primary consumers and the zoomass of top carnivores may add up to less than 0.001 percent of phytomass.
In marine ecosystems the pyramid is inverted: the brief lives of phytoplankton (mostly between 12 and 48 hours) and the high consumption rates by zooplankton and larger herbivores mean that the total standing heterotrophic biomass could be between two and four times as large as the mass of the photosynthesizing phytoplankton. What is true collectively is also true individually, as most oceanic autotrophs are species of microscopic phytoplankton (their diameters average only about 10µm) while the organisms typical of higher trophic levels – zooplankton as primary consumers, small fish as secondary, larger fish and common squid as tertiary, and tuna as quaternary feeders – are progressively larger: before overfishing greatly reduced their mass and number mature Southern bluefin tuna could weigh more than 150 kg. There are notable exceptions: the largest marine mammals (blue whales, weighing up to 130 t) and fish (whale sharks, weighing up to 1.5 t) are filter feeders, consuming large quantities of tiny phyto- and zooplankton.
The declining numbers of heterotrophs in higher trophic levels are often associated with increasing body size: top predators commonly include the largest animals in their respective classes, be they golden eagles among the birds of prey, or tigers and lions among the felids. Herbivory has obvious energetic advantages, and in all modern ecosystems the animals with the largest body mass are megaherbivores (grazers with body mass greater than one tonne) such as elephants, hippos, and giraffes in the tropics, and moose and muskoxen in boreal and Arctic environments. This primacy was even more pronounced in the past, when the largest megaherbivores (be they the relatively recently extinct mammoth, or enormous dinosaurians, the heaviest one weighing perhaps more than 80 tonnes) were up to an order of magnitude more massive than today’s heaviest species.
Generalizations regarding the transfers within the trophic pyramid have been elusive. Pioneering studies done by Raymond Lindeman (1915–1942) on aquatic life in Wisconsin’s Lake Mendota found an efficiency of 0.4 percent for autotrophs, while the primary consumers retained less than nine percent, the secondary about five percent, and the tertiary feeders some thirteen percent of all available energy. These approximations were (erroneously) generalized into the “ten percent law of energy transfer,” with a corollary of progressively higher efficiencies at higher trophic levels. Subsequent studies proved that neither conclusion was correct, and showed that bacteria and herbivores can be much more efficient converters than carnivores. There are only two safe generalizations: first, no energy loss in any ecosystems is ever as high as that associated with photosynthesis, and second, energy losses during the subsequent transfers to higher trophic levels are never that large, but net transfers are commonly much lower than ten percent.
ENERGY EFFICIENCY IN ECOSYSTEMS
Final energy transfers in ecosystems are the products of exploitation, assimilation, and production efficiencies. The share of phytomass eaten by herbivores normally ranges from just one or two percent in temperate forests to as much as fifty to sixty percent in some tropical grasslands. This rate ignores occasional spikes caused by infestation of insects: gypsy moths can defoliate large areas of boreal trees and migratory locusts can strip more than ninety percent of available phytomass as they move through North African landscapes. Excluding soil fauna, the transfers are rarely above ten percent in any temperate ecosystem, and are mostly around one percent for vertebrates. It should be noted that the abundance of herbivores is not usually limited by the availability of phytomass, but rather by predation by carnivores, while the numbers of carnivores are generally limited by the abundance of prey they can capture.
Assimilation efficiencies (the share of ingested energy that is actually metabolized) clearly depend on feed quality: they are low (commonly less than thirty percent) among herbivores feeding on often digestion-resistant plant polymers, very high (in excess of ninety percent) for carnivores that consume high-lipid, high- protein zoomass. For many species the final rate, that of converting the digested energy into new biomass, negates the advantages of carnivory. This production efficiency is, regardless of their trophic level, much higher among ectotherms. Invertebrates can convert more than twenty percent, and some insects fifty percent, of assimilated energy into new biomass, while the mean for endotherms is around ten percent, for large mammals no more than three, and for small mammals and birds less than two.
Consequently, the share of energy available at one level that is actually converted to new biomass at the next level above – variously called trophic, ecological, or Lindeman’s efficiency – ranges from a small fraction of one percent for passerine birds to around thirty percent for insects. Moreover, the rates show few clear correlations based on taxonomic, ecosystemic, or spatial commonalities. In any case, trophic efficiency is not a predictor of evolutionary success, as both low-efficiency endotherms and high-efficiency ectotherms have done comparably well in similar ecosystems: for example, in African savannas elephants will harvest annually as much phytomass per unit area as termites.
In complex food webs, it is enough to reduce single energy flow by diminishing the abundance of a single species (be it through a climatic change or disease, or because of a human action) to get some unexpected results. A perfect example, which unfolded during the last quarter of the twentieth century, was the massive damage done to the kelp forests of the Pacific Northwest, and hence to the numerous species that depend on these giant marine plants, by sea urchins. The urchin stock was previously controlled by sea otters, but their numbers declined because of predation by orcas (killer whales). These large, sleek mammals have always preferred bigger prey (such as sea lions and seals) but once they became less available, mainly because of the combined effect of overfishing and climatic change, the orcas turned to otters.