Chapter 4. Examples of Nanotechnology
4.1. Buckyballs: Their history and discovery*
Note
This module was developed as part of a Rice University Class called "Nanotechnology: Content and Context" initially funded by the National Science Foundation under Grant No. EEC-0407237. It was conceived, researched, written and edited by students in the Fall 2005 version of the class, and reviewed by participating professors.
““This year's Nobel Prize in Chemistry has implications for all the natural sciences. The seeds of the discovery were sowed by a desire to understand the behavior of carbon in red giant stars and interstellar gas clouds. The discovery of fullerenes has expanded our knowledge and changed our thinking in chemistry and physics. It has given us new hypotheses on the occurrence of carbon in the universe. It has also led us to discover small quantities of fullerenes in geological formations. Fullerenes are probably present in much larger amounts on earth than previously believed. It has been shown that most sooty flames contain small quantities of fullerenes. Think of this the next time you light a candle!””
-From the presentation speech for the Nobel Prize in Chemistry, 1996
Introduction
In 1996, the Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry, the most prestigious award in the world for chemists, to Richard Smalley, Robert Curl, and Harold Kroto for their discovery of fullerenes. They discovered fullerenes (also called buckyballs) in 1985, but the special properties of the buckyballs took a few years to prove and categorize. Although by 1996 no practical applications of buckyballs had been produced, scientists appreciated the direction this discovery based in organic chemistry had led scientific research, as well as its specific contributions to various other fields. The accidental discovery of fullerenes also emphasizes the benefits and unexpected results which can arise when scientists with different backgrounds and research aims collaborate in the laboratory.
What are Buckyballs?
Before going into detail about the actual buckyball, we should discuss the element that makes its structure possible, carbon. Carbon is the sixth element on the periodic table, and has been found to be at least a partial constituent in over 90 per cent of all chemicals known to man. Indeed, its electron-bonding properties grant it a versatility specific to carbon, allowing it to be so widely functionalized, and more importantly, the reason for life on Earth. Anything that is living is necessarily chemically based on Carbon atoms, and for this reason, substances containing carbon are called organic compounds, and the study of them is called organic chemistry.
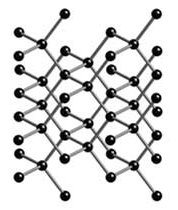
Though carbon is involved in chemistry with all sorts of other elements and compounds, it can also exist in pure carbon states such as graphite and diamond. Graphite and diamond are two different allotropes of carbon. An allotrope is a specific physical arrangement of atoms of an element. So although diamond and graphite are both pure carbon, because the crystalline structure of each is significantly different, their chemical and physical properties (as well as value) are very different.
Above: diamond Below: carbon. Notice how the structure of the two allotropes vary, even though they are both made of the same carbon atoms (black)
Figure 4.2.
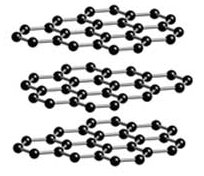
Diamond and graphite are not the only known allotropes pf carbon, chaoit and carbon(VI), discovered in 1968 and 1972, respectively, have also been found. Even more recently, the Buckminsterfullerenes, the subject of this module, were discovered at Rice by Smalley, Kroto,and Curl. Buckminsterfullerenes is actually a class of allotropes
Above: C540 Below: C60 Both of these are different allotropes of carbon. C60 is the most common and the most popularized of the Buckminsterfullerenes. Not shown is the second most common Buckyball, C70.
Figure 4.3.
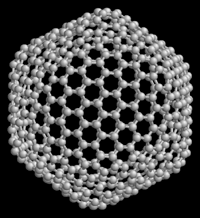
Figure 4.4.
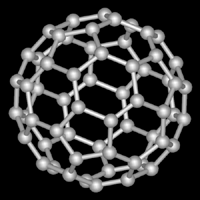
In fact, scientists have now discovered hundreds of buckyballs of different sizes, all with the trademark spherical-like shape. To differentiate them, each allotrope is denoted as C (for carbon) with the number of carbon atoms in the subscript (i.e. C80). Technically, the geometrical shapes that these buckyballs share are actually known as geodesics, or rather, polyhedrons that approximate spheres. Specifically, the commonly depicted C60 buckyball is a truncated icosahedron. A more satisfactory representation of it can be had in a soccer ball, with which it shares the exact same shape. It is made up of 12 pentagons, each surrounded by 5 hexagons (20 in all).
The Discovery
British chemist Harold W. Kroto at the University of Sussex was studying strange chains of carbon atoms found in space through microwave spectroscopy, a science that studies the absorption spectra of stellar particles billions of kilometers away to identify what compounds are found in space. This is possible because every element radiates a specific frequency of light that is unique to that element, which can observed using radiotelescopes. The elements can then be identified because a fundamental rule of matter stating that the intrinsic properties of elements apply throughout the universe, which means that the elements will emit the same frequency regardless of where they are found in the universe. Kroto took spectroscopic readings near carbon-rich red giants, or old stars with very large radii and relatively low surface temperatures, and compared them to spectrum lines of well-characterized substances. He identified the dust to be made of long alternating chains of carbon and nitrogen atoms known as cynopolyynes, which are also found in interstellar clouds. However Kroto believed that the chains were formed in the stellar atmospheres of red giants and not in interstellar clouds, but he had to study the particles more closely.
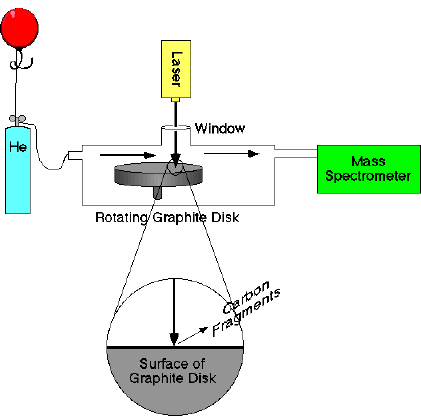
At the same time, Richard Smalley was doing research on cluster chemistry, at Rice University in Houston, Texas. “Clusters” are aggregates of atoms or molecules, between microscopic and macroscopic sizes, that exist briefly. Smalley had been studying clusters of metal atoms with the help of Robert Curl, using an apparatus Smalley had in his laboratory. This laser-supersonic cluster beam apparatus had the ability to vaporize nearly any known material into plasma using a laser, which is a highly concentrated beam of light with extremely high energy.
Through an acquaintance with Curl, Kroto contacted Smalley and discussed the possibility of using his apparatus to recreate the high-heat conditions of a red giant’s atmosphere in order to study the clusters of carbon produced, which might give Kroto insight as to the formation of the carbon chains. Smalley conceded and Kroto arrived in Smalley’s laboratory in Rice University on September 1, 1985 whom began working on the experiment along with graduate students J.R. Heath and S.C. O’Brien.
Smalley’s apparatus, shown above, fires a high energy laser beam at a rotating disk of graphite in a helium-filled vacuum chamber. Helium is used because it is an inert gas and therefore does not react with the gaseous carbon. The intense heating of the surface of the graphite breaks the C—C bonds because of the intense energy. Once vaporized, the carbon atoms cool and condense in the high-pressure helium gas, colliding and forming new bond arrangements. Immediately upon cooling several degrees above absolute zero in a chamber, the carbon leads to a mass spectrometer for further analysis.
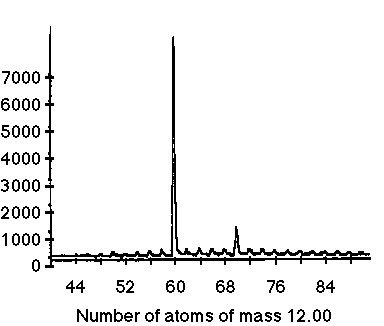
A mass spectrometer uses an atom or molecule’s weight and electric charge to separate it from other molecules. This is done by ionizing the molecules, which is done by bombarding the molecules with high energy electrons which then knocks off electrons. If an electron is removed from an otherwise neutral molecule, then the molecule becomes a positively charged ion or cation. The charged particles are then accelerated by passing through electric plates and then filtered through a slit. A stream of charged particles exits the slit and is then deflected by a magnetic field into a curved path. Because all the particles have a charge of +1, the magnetic field exerts the same amount of force on them, however, the more massive ions are deflected less, and thus a separation occurs. By adjusting the strength of the accelerating electric plates or the deflecting magnetic field, a specific mass can be selected to enter the receptor on the end. After adjusting the experiment, it became greatly evident that the most dominant molecule measured was 720 amu (atomic mass units). By dividing this number by the mass of a single carbon atom (12 amu), it was deduced that the molecule was comprised of 60 carbon atoms (720 / 12 = 60).
The next task was to develop a model for the structure of C60, this new allotrope of carbon. Because it was overwhelmingly dominant, Smalley reasoned the molecule had to be the very stable. The preferred geometry for stable molecule would reasonably be spherical, because this would mean that all bonding capabilities for carbon would be satisfied. If it were a chain or sheet like graphite, the carbon atoms could still bond at the ends, but if it were circular all ends would meet. Another hint as to the arrangement of the molecule was that there must be a high degree of symmetry for a molecule as stable as C60. Constructing a model that satisfied these requirements was fairly difficult and the group of scientists experimented with several models before coming to a conclusion. As a last resort, Smalley made a paper model by cutting out paper pentagons and hexagons in which he tried to stick them together so that the figure had 60 vertices. Smalley found that he create a sphere made out of 12 pentagons interlocking 20 hexagons to make a ball. The ball even bounced. To ensure that the shape fulfilled the bonding capabilities of carbon, Kroto and Curl added sticky labels to represent double bonds. The resulting shape is that of a truncated icosahedron, the same as that of a soccer ball. Smalley, Curl, and Kroto named the molecule buckminsterfullerene after the American architect and engineer Richard Buckminster Fuller who used hexagons and pentagons for the basic design of his geodesic domes.
Eleven days after they had begun, the scientist submitted their discovery to the prestigious journal Nature in a manuscript titled “C60 Buckminsterfullerene.” The journal received it on the 13th of September and published it on the 14th of November 1985. The controversial discovery sparked approval and criticism for a molecule that was remarkably symmetrical and stable.
How Buckyballs are made?
Experimentally, Smalley, Kroto, and Curl, first created the buckyballs using Smalley’s laser-supersonic cluster beam apparatus to knock carbons off of a plate and into a high pressure stream of helium atoms. They would be carried off and immediately be cooled to only a few degrees above absolute zero, where they would aggregate and form these buckyballs. This method however, resulted in low yields of buckyballs, and it took nearly five years until in 1990 newer methods developed by American and German scientists could manufacture buckyballs in large quantities.
The common method today involves transmitting a large current between two graphite electrodes in an inert atmosphere, such as Helium. This gives rise to a carbon plasma arc bridging the two electors, which cools instantaneously and leaves behind a sooty residue from which the buckyballs can be extracted.
These methods of producing buckyballs do deserve a great deal of applaud. However, humans cannot take all, or even most of, the credit for the production of fullerenes. As a matter of fact, buckyballs occur in nature, naturally, and in greater amounts than expected. Buckyballs are known to exist in interstellar dust and in geological formations on Earth. Even closer to home are the buckyballs that naturally form in the wax and soot from a burning candle, as the flame on the wick provides the sufficient conditions for such processes to occur. Buckyballs are the new sensation for us, but to Nature, they are old news.
Chemical and Physical Properties
Since buckyballs are still relatively new, there properties are still being heavily studied. Buckyballs’ unique shape and electron bonding give them interesting properties on the physical level, and on the chemical level.
Since spheres in nature are known to be the most stable configurations, one could expect the same from fullerenes. Indeed this is one of the reasons why Smalley, Curl, and Kroto initially considered its shape. Their tests showed that it was extremely stable, and thus, they reasoned, it could be a spherical-like geodesic. Also, fullerenes are resilient to impact and deformation. This means, that squeezing a buckyball and then releasing it would result in its popping back in shape. Or perhaps, if it was thrown against an object it would bounce back; ironically just like the very soccer ball it resembles.
Buckyballs are also extremely stable in the chemical sense. Since all the carbon-carbon bonds are optimized in their configuration, they become very inert, and are not as prone to reactions as other carbon molecules. What makes these bonds special is a property called aromaticity. Normally, electrons are fixed in whatever bond they constitute. Whereas in aromatic molecules, of which hexagonal carbon rings are a prime example, electrons are free to move (“delocalize”) among other bonds. Since all the fullerenes have the cyclo-hexanes in abundance, they are very aromatic, and thus have very stable, inert, carbon bonds. Buckyballs, though sparingly soluble in many solvents, are in fact the only known carbon allotropes to be soluble.
An interesting feature of Fullerenes is that their hollow structure allows them to hold other atoms inside them. The applications of this are abound, and are being studied to great extent.
Important to note about any new material is its health concerns. Although believed to be relatively inert, experiments by Eva Oberdörster at Southern Methodist University, presented some possible dangers of fullerenes. She introduced buckyballs into water at concentrations of 0.5 parts per million, and found that largemouth bass suffered a 17-fold increase in cellular damage in the brain tissue after 48 hours. The damage was of the type lipid peroxidation, which is known to impair the functioning of cell membranes. Their livers were also inflamed and genes responsible for producing repair enzymes were activated. As of 10/20/05, the SMU work had not been peer reviewed.
What have buckyballs contributed to science?
After the astrophysicists D.R. Huffmann and W. Kratschmer managed to produce larger quantities of fullerenes in 1990, scientists further investigated the structure and characteristics of buckyballs. Research on buckyballs has led to the synthesis of over 1000 new compounds with exciting properties, and over 100 patents related to buckyballs have been filed in the US. In addition, an important new material, nanotubes, has exploded onto the scientific scene in recent years. The discovery and manufacture of nanotubes resulted directly from research on buckyballs. Finally, although buckyballs have not yet been used in any practical applications, partly due to the high cost of material, researchers are using buckyballs to learn more about the history of our world, and companies are devising some interesting uses for buckyballs even today.
Nanotubes
Figure 4.7.
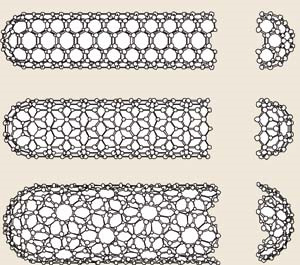
The discovery of nanotubes in 1991 by S. Iijima has been by far the buckyball’s most significant contribution to current research. Nanotubes, both single- and multi-walled, can be thought of as sheets of graphite rolled into cylinders and sometimes capped with half-fullerenes. Nanotubes, like fullerenes, possess some very unique properties, such as high electrical and thermal conductivity, high mechanical strength, and high surface area. In fact, carbon nanotubes provide a clear example of the special properties inherent at the quantum level because they can act as either semi-conductors or metals, unlike macroscopic quantities of carbon molecules. These properties make nanotubes extremely interesting to researchers and companies, who are already developing many potentially revolutionary uses for them.
What are buckyballs teaching us about our world?
A paper published on March 28, 2000 in the Proceedings of the National Academy of Sciences (PNAS) by Becker, Poreda, and Bunch uses buckyballs to provide new evidence for early periods in earth’s geological and biological history. By exploiting the unique properties of buckyballs, these three scientists were able to study geology in a new way. First of all, the unique ability to extract fullerenes (unlike graphite and diamond) from organic solvents allowed them to isolate carbon material in the meteorites, then the unique cage-like structure of fullerenes allowed them to investigate the noble gases enclosed within the ancient fullerenes. In their study, the researches found helium of extraterrestrial origin trapped inside buckyballs extracted from two meteorites and sedimentary clay layers from 2 billion and 65 million years ago respectively. The helium inside these buckyballs bears unusual ratios of 3He/4He coupled with non-atmospheric ratios of 40Ar/36Ar, which according to their research indicates extraterrestrial origin. In addition, they have shown that these fullerenes could not have been formed upon impact of the meteorite or during subsequent forest fires.iBecker, Poreda, and Bunch. 2982.
The discovery of the extraterrestrial origin of the enclosed helium has far-reaching implications for the history of the earth. For example, the existence of the carrier phase of fullerenes suggests that “fullerenes, volatiles, and perhaps other organic compounds were being exogenously delivered to the early Earth and other planets throughout time.”iiBecker, Poreda, and Bunch, 2982. With more research, it might even be possible to determine whether meteorite impacts on earth could have triggered global changes or even brought carbon and gases to earth that allowed for the development of life!
Uses
Why does it matter? Why should anyone care? These buckyballs are giving scientists information about allotropes of carbon never before conceived. More importantly, these buckyballs might allow engineers and doctors do what was never before possible. These are some of the applications for buckyballs currently in research.
Medical uses for buckyballs
Figure 4.8.
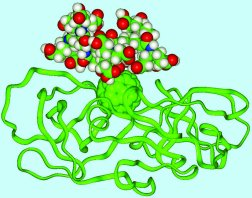
Buckyballs are now being considered for uses in the field of medicine, both as diagnostic tools and drug candidates. Simon Friedman, a researcher at the University of Kansas, began experimenting with buckyballs as possible drug treatments in 1991. Because buckyballs have a rigid structure (unlike benzene rings, often used for similar purposes), researchers are able to attach other molecules to it in specific configurations to create precise interactions with a target molecule. For example, Friedman has created a protease inhibitor that attaches to the active site of HIV 50 times better than other molecules. C Sixty, a Toronto based company that specializes in medical uses of fullerenes, plans to test on humans two new fullerene-based drugs for Lou Gehrig’s disease and HIV in the near future.
Another medical use for buckyballs is taking place in the field of diagnostics. Buckyballs unique cage-like structure might allow it to take the place of other molecules in shuttling toxic metal substances through the human body during MRI scans. Usually, the metal gadolinium is attached to another molecule and sent into the body to provide contrast on the MRI scans, but unfortunately these molecules are excreted from the system quickly to reduce the chance of toxic poisoning in the subject. Lon Wilson of Rice University and researchers at TDA Research have encased gadolinium inside buckyballs, where they cannot do harm to the patient, allowing them to remain inside the body longer, but still appear in MRI’s. So far this application has been successfully tested in one rat. Wilson and others have begun to develop even more applications for the tiny little cages that could one day help revolutionize medicine.
Engineering Uses
The Scanning Tunneling Microscope (STM) is one of the foremost tools in microscopy today; boasting the ability to to map out the topology of material surfaces at atomic resolution (i.e. on the order of 0.2 nanometers). The STM achieves this feat by bringing a needle point, functioning as a probe, within just several nanometers of a sample's surface. At these minute scales, even small disturbances can cause the tip to crach into the sample and deform itself. A possible solution to this problem would be the replacement of the standard needle point with a buckyball. As discussed previously, fullerenes bear amazing resilience due to their spherical geometry, and would resist distortions from such collisions.
European scientists are aiming to use buckyballs in circuit. So far, they have been able to attach a single fullerene to a copper surface, and then, through a process called shrink wrapping, fitted its center with a metal ion and made it smaller to increases electric conductivity by a hundred times.
Because of their shapes, they could be used equivalently to ball bearings, and thus allow surfaces to roll over each other, making the fullerenes equivalently lubricants
It has been shown that fitting a potassium ion in the buckyball causes it to become superconductive. Ways to exploit this are in the research stages.
Attaching metals onto the surface of fullerenes offers the possibility for buckyballs to become catalysts.
Conclusion
As we can see, we have come along way since that fateful year of 1985. Strides have been made. We have seen the rise of nanotubes and the new science of Nanotechnology. We are still studying the chemical and physical properties of buckyballs and continue to be amazed. They have already proved to us why they are important; their possible uses in medicine and in engineering are broad and profound, while the health risks they posed have yet to be fully analyzed. Only time will tell whether they will meet, or exceed our expectations as we unfold this brave new world.
Bibliography
Nobelprize.org: http://nobelprize.org/chemistry/laureates/1996/press.html
http://www.science.org.au/nova/024/024print.htm
http://blogcritics.org/archives/2004/04/10/084049.php
http://www.science.org.au/nova/024/024key.htm
http://www.sciencedaily.com//releases/2003/04/030418081522.htm
http://www.sciencenews.org/articles/20020713/bob10.asp
Gorman, Jessica. Buckymedicine: Coming soon to a pharmacy near you?. Science News Online: July 13, 2002, vol. 162, no. 2. http://www.sciencenews.org/articles/20020713/bob10.asp
Becker, Poreda, and Bunch. Extraterrestrial Helium Trapped in Fullerenes in the Sudbury Impact Structure. Science, Vol 272, Issue 5259, 249-252 , 12 April 1996.
Personal author: Aldersey-Williams, Hugh. Title: The most beautiful molecule : an adventure in chemistry / Hugh Aldersey-Williams. Publication info: London : Aurum Press, 1995. Personal author: Baggott, J. E. Title: Perfect symmetry : the accidental discovery of Buckminsterfullerene / Jim Baggott. Publication info: Oxford [England] ; New York : Oxford University Press, 1994.
4.2. Nanocars and the Development of Molecular Manufacturing*
Note
This module was developed as part of a Rice University Class called "Nanotechnology: Content and Context" initially funded by the National Science Foundation under Grant No. EEC-0407237. It was conceived, researched, written and edited by students in the Fall 2005 version of the class, and reviewed by participating professors.
In the late decades of the 20th century, the field of molecular manufacturing developed as materials and methods arose that facilitated development of new, useful designs. In this chapter, we take a look at the development of molecular manufacturing, where it stands today, and its some aspects of its future. Since the invention of the Scanning Tunneling Microscope in 1981, molecular manufacturing has reached various milestones that we will discuss in this section. In addition, we will take a look at a specific molecule that was synthesized by Rice University scientists that incorporated previously established molecular designs and mechanisms. This molecule takes molecular manufacturing further down the path of development.
In 2005, Rice University scientists Dr. James Tour, Kevin Kelly, and others built upon established milestones to reach new understandings of engineered, deliberate molecular motion. The team of scientists designed a molecular structure consisting of a chassis and axle system covalently bound to four separate Buckminsterfullerene (C60) molecules (figure 1) that facilitates rolling translational motion. The synthesis, structure, mobility, and observation of the nanocar will be discussed in subsequent sections of this chapter. But first, lets take a look at the developments in molecular manufacturing preceding the discovery of nanocar 1.
Figure 4.9.
A Brief Review of Early Advances in Nanoscale Design
To understand how the nanocar fits into the larger scheme of molecular manufacturing, we will review some instrumental developments in molecular manufacturing that jump-started the field. We will also introduce a couple of molecular components that facilitate the design of mobile molecules—bearings and axles.
The invention of the Scanning Tunneling Microscope (STM) by IBM’s Gerd Binning and Heinrich Rohrer in 1981 was vital to the development of molecular manufacturing and nanoscale design. The function of the STM is two-fold. First of all, STM imaging allowed scientists to visualize atomic surfaces. Secondly, STM tips are capable of directly manipulating individual atoms and molecules. Both of these functions have been instrumental in nanoscale design, and were both employed by the scientists involved in the design and observation of nanocar 1.
Early advances in nanoscale design came in the form of direct demonstration of the movement of single atoms and molecules with the use of an STM. The first of these demonstrations came in 1989 when IBM fellow Don Eigler spelled out the letters "I B M" on a nickel surface using 35 xenon atoms (figure 2, left). At first glance, the act seemed insignificant, criticized by many as merely a stunt. However, at the heart of the demonstration lies the fact that Eigler was able to move single atoms that could not even be observed less than a decade before. This demonstration was a step forward for the development of nanoscale design, and would be followed by subsequent developments. However, a complication of Eigler’s method was that it required experimental temperatures near absolute zero—an unpractical temperature for the design of useful products.
Figure 4.10.
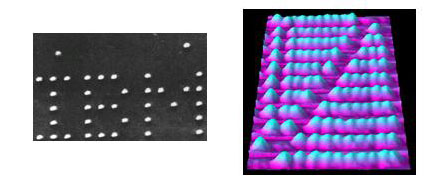
The second demonstration also came from IBM scientists and overcame the limitations of the previous experiment. In 1996, IBM’s Zurich laboratory produced a nanoscale abacus that consisted of individual C60 molecules that functioned as beads that could be pushed back and forth along eleven separate rails on a copper surface (figure 2, right). This time around, the components were manipulated at room temperature—a practical temperature for the design and application of nanoscale products. This demonstration represented another vital step in the advancement of nanoscale design. It indicated that molecules could be manipulated at room temperature and constructed into a functional design—also known as ‘bottom-up’ design.
Molecular Building Blocks
Since the invention of the STM, the field of molecular manufacturing has produced various molecules that serve as molecular building blocks for more complexly designed molecules that are emerging today. The set of building blocks necessary for the development of a design on any scale depends on the targeted function of that design. For example, in manufacturing an automobile, the necessary materials include bearings, axles, and various other components. The same idea applies to nanoscale design. Depending on the target function of a molecule, it is necessary to use various components. Here, we take a look at some of the molecular building blocks required to synthesize mobile molecules. Keep in mind that these components, and various others, will be used to describe the structure and function of nanocar 1.
The detailed structure and chemistry of the various systems used in the design and synthesis of molecular components is beyond the scope of this text. However, we will provide a general overview of a few molecular structures that are instrumental in the design of mobile, functional molecules. With the introduction of each molecular mechanical component, we will provide comparisons with its macroscopic counterpart in order to clarify the functionality of each system.
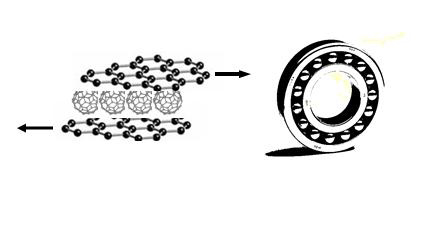
Bearings are structures that function to reduce energy loss to friction during various processes. Bearings are found in almost every rotating part of your car and facilitate smooth rotation of parts from the wheel up to the transmission. Researchers have investigated various systems to replicate this function on the molecular level. Here we take a look at the molecular bearing designed by scientists working in Japan. A monolayer of tightly packed C60 molecules was sandwiched between two single sheets of graphite to form a molecular bearing. The structure resulted in an ultra-lubricated system with zero frictional forces when the graphite sheets were moved along the rotating C60 molecules(figure 3).
Figure 4.11.
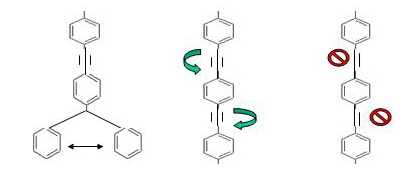
Axles function to transfer mechanical energy to turn a specific object such as a wheel. An effective axle is characterized by two functions: 1) it must be able to rotate freely and 2) must be in a fixed, linear position. The axle must be able to rotate freely because its function is dependent on its ability to transfer mechanical energy to rotate an attached structure. In the case of an automobile, the axle functions to transfer energy generated by the engine to rotate the wheels of the vehicle. An axle must be in a fixed, linear position because it must provide enough support to withstand forces placed on it, such as the weight of a chassis. On the molecular scale, the two functions of an effective rotor are encompassed in the structure of a triple bond, as opposed to single or double bonds (figure 4). A single bond is able to rotate freely, but is not in a fixed linear position. On the other hand, double bonds are in a fixed position, but are unable to rotate.
Figure 4.12.
As you have observed, the link between structure and function is vital to designs on both the macro and nanoscale. The structures described here do not, by any means, encompass the countless molecular structures that are required for the synthesis of functional molecules, but serve only to provide a general idea of the types of designs involved in molecular manufacturing. With this brief introduction to molecular manufacturing we are prepared to examine a specific example of molecular manufacturing: nanocar 1.
Design, Structure, and Function of Nanocar System
The structure of nanocars facilitates their function. Therefore, their structure and more importantly the precise, deliberate engineering of their structure through assembly and implementation represents vital progress in nanoscale design and molecular manufacturing. Studying the mechanics of rotors and motors from the bottom up, starting with the simplest molecules possible, the Tour group engineered nanocars as the first in a series of tools to test molecular mechanics and prove the viability of molecular design.
Central to understanding the implications of the Tour Group research is an understanding of the structure of the nanocars—their design and their function. To best address the nanocar structure we will analyze its design and assembly in three components: the wheel, the chassis, and the surface it operates on. As it turns out, each of these aspects is equally important in determining the functionality of the nanocar and is therefore the best way to analyze the structure of the nanocar system.
The driving characteristic of the wheel, if you will, is its ability to roll. Apart from this implicit necessity, the wheel must also be of a size that can be imaged with an STM. If a molecule is too big the STM cannot resolve it. Likewise, if a molecule is too small the STM cannot discern it from its neighbors. A wheel must also be large enough to have ‘ground clearance’ whereby the wheel is of sufficient height or radius to elevate the chassis high enough above the operating surface to avoid molecular interactions. Ease of synthesis must also be considered in the selection of a wheel. A molecular wheel must be reactive enough to bond with its chassis in order to synthesize the molecule.
Figure 4.13.

Clearly, there are many considerations that must be taken into account when choosing a wheel. The Tour Group at Rice University selected C60 as their wheel for their first nanocar. C60 is entirely composed of carbon with alternating pentagonal and hexagonal cyclical bond structures that form a spherical shell reminiscent of a soccer ball (figure 5). Ideally, this structure, under the right conditions, could roll with its compact surface and spherical shape. Furthermore, it is of a height that can be imaged with an STM and elevate the chassis to prevent interactions with the operating surface. In a subsequent section, we will learn more about how these concepts were tested and observed by the Tour Group.
Although C60 is an attractive molecule to test rolling versus sliding motion at the molecular level, there are some disadvantages to its structure. The properties of C60 limit the synthesis of the nanocar molecule. In particular, the pi bonds of the molecule react with the palladium catalyst to interfere with chassis synthesis. For this reason, the chassis was synthesized first and the C60 wheels were attached last.
The future of nanocar wheels will include the introduction of more controlled synthesis and variable size. In the short term, the Tour Group is investigating the use of carborane molecules, spheroid molecules comprised of carbon and boron, to have more control over the synthesis. They believe the carborane molecules will exhibit more compliant chemistry for their functional needs (figure 6). In the long term, the Tour Group is investigating large, complex organic molecules that are modeled after bicycle wheels with an outer rim and connecting spokes. This bicycle wheel would be made predominantly of carbon and allow for variable size depending on the length of the spokes and circumference of the rim.
Figure 4.14.
Continuing the analysis of the nanocar structure, we will investigate the chassis of the nanocar, including aspects of its design and functionality. When designing a viable chassis the Tour Group took into consideration three primary goals: one, how to physically connect the wheels, two, how to facilitate rotational motion of the wheels, and three how to develop a structure that allows the orientation of the molecule to be determined by STM. Molecular components of the chassis were also considered in order to maintain ease of synthesis. In addition to developing and understanding nanocar 1, the group also aims to add functionality to the chassis.
Figure 4.15.
The Tour Group addressed these goals in their design of the chassis in several ways (Figure 7). To physically attach the wheels to each other, the Tour Group designed a simple four-axle system reminiscent of a car. There are two axles in the front and two in the back connected to each other by a central shaft. Within the design of the axles, the Tour Group addressed the second goal of how to allow free spinning of the wheels. As discussed earlier, the axles are comprised of triple bonded carbon atoms, which allow for free rotation about the axle, while maintaining linearity with the adjacent axle (figure 4). The spinning of these triple bonds begins at 30 degrees Kelvin and has been shown to have virtually no frictional hindrance on the system. This is optimal for a preliminary research into rolling motion because it constrains free variables of the system. In other words, because the triple bonds do not add meaningful frictional forces to the system above 30 degrees Kelvin, all experimental results can be attributed to the chemistry of the wheel and its rolling or slipping interactions with the surface it operates on. Thirdly, the ease with which the nanocars can be resolved with an STM was in part dictated by the structure of the chassis. Specifically, the chassis was designed to have a central shaft longer than the length of the front or rear axles. The nanocar is wider than it is long. This is important in microscopy because it allows the observer to note the orientation and therefore the direction of translational motion of the nanocar. As far as the synthesis of the molecule, the Tour Group added functional branches to the phenyl groups on the axles and central shaft, which suspend the molecule in solution and allow for better mixing, better reaction, and better yields. These essential goals were addressed to produce a working nanocar; however, the goal of adding functionality was not described.
While the currently published nanocar is devoid of added functionality, the Tour Group is researching additions to the chassis to facilitate transport and motility. Nanotrucks are a popular idea and simply refers to a modified nanocar that can carry objects. A ‘bed’ could be synthesized into the chassis to carry substances ranging from metal ions to oxygen atoms. Of course, the object being carried would most likely be specific to the nanotruck synthesized and the particular chemistry of its ‘bed’. Furthermore, bonding of a metal ion to the chassis of a nanocar would allow for interactions with an electric field, providing a mechanism for controlled motion. Along this vein, the entire chassis of the nanocar could be designed to maintain a dipole moment creating a favored orientation in the presence of an electric field. Another form of motility that is being pursued is the addition of a light-driven single-directional molecular rotor. This type of rotor would ratchet forward through four isomeric states when stimulated by photons. The Tour group is looking to append such a motor to their chassis to create deliberate and controlled motion. Nanomachines that utilize this deliberate and controlled motion will be the next milestone in molecular manufacturing.
The last of the three components of the nanocar to explore is the surface it operates on. Surface chemistry plays an undeniable role in the functionality of the nanocar, and must be taken into account in designing the system. First, a surface must be found where rolling, not sliding, dynamics are likely. Secondly, a surface must be chosen on which observations of the molecule are clear and measurable.
Gold was chosen as the operation surface because it was theorized to be optimal for the aforementioned considerations. As it turns out, gold does accommodate these factors well. It allows for a special type of chemical interaction between itself and C60, known as charge transfer bonding. While we will not go into the details of charge transfer bonding, it will be sufficient for this textbook to consider the molecular interactions between the C60 and the gold surface as a type of van der walls forces or weak molecular interaction. This type of interaction is optimal for two reasons. One, if the bond strengths were any weaker the C60 molecule would simply slide like a bearing, as is the case with the C60 on graphite sheets. If the bond strengths were any stronger, the C60 molecule would not move at all. Therefore, the appropriate strength of molecular interaction between the wheel and the surface must be found to allow for rolling motion. Second, this interaction is temperature dependent and can be adjusted to control motion. Temperature controlled bonding interactions allow for the observers to cool the system down to where the bond strengths are more effective to give the STM time to resolve an image. Likewise, the system can be heated to allow for movement. Of course there is a range of possible temperatures that the system is constrained by, namely the temperature of decomposition of the nanocar. Therefore, the surface must be able to regulate motion while remaining within the available temperature range of the stable molecule. Each of these factors contributes to the ease with which a molecule’s motion can be observed. In the next section, we focus on this subject as we discuss how the Tour Group observed nanocar 1.
Observations: Rolling versus Sliding
Observations beyond the range of the naked eye require more deliberate and cautioned interpretation of what is observed. Specifically, factual and accurate information must be discerned out of various interferences that can lead to unsubstantiated conclusions. The Tour group had to surmount these uncertainties to conclusively show that the nanocars underwent rolling, rather than sliding, translational motion.
Figure 4.16.
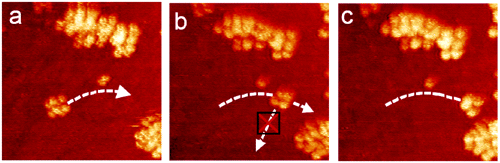
The choice of a gold surface enabled temperature dependent adhesion to the surface, and allowed targeted observation of specific molecules. In the picture to the left, you see several nanocars spread out along the Au(111) surface. As the researchers increased the temperature to 200°C, the cars began to move at such a rate that they noticeably displaced from their original position in a period of one minute (figure 8). This indicates that 200°C is a viable temperature for imaging nanocars on gold with STM. However, imaging of the cars at temperatures above 225°C is currently impossible due to the rapid motion of the molecules at that temperature. The nanocars are moving too quickly to be imaged by the one-minute capture rate of the STM. Once the system was heated to 300-350°C, the car began to decompose. These observations indicated that motion of the nanocars was dependent on temperature.
It was also observed that the cars did not exclusively undergo translational motion, but also rotated. In the image to the right, it can be observed that the cars pivot as they move across the frame, changing direction without moving forward. The Tour Group explained this rotation as an inability to synchronize the rotation of each wheel.
The Tour Group also observed three-wheeled molecules, or trimers, which they found useful in proving rotational motion of the C60 molecules. If the cars’ movement were in fact due to rotation, then the trimer would not be able to move translationally due to the fact that the three wheels cannot rotate in a coordinated manner, that is no two wheels can rotate the same parallel plane. This supports the idea that the C60 molecules are rotating, as opposed to sliding. To strengthen their assertion, the researchers heated a sample of the trimer molecules on the same gold surface to 225°C—a temperature at which the four-wheeled molecules rapidly moved out of the scanning range of the STM. Upon doing so, they observed that the trimers did not undergo significant translational motion, and remained within nanometers of their original position. This showed that both the wheels and axels of the trimer and nanocar allow for rotational motion, therefore substantiating the assertion that translational motion of the nanocar is due to rolling.
The researchers used an STM to pull the nanocar in order to see if there was preferential motion (ie. Due to rolling). When the molecules were pulled perpendicular to the axles (Figure 9; frame a) the molecule moved in the direction of the pull. But, pulling parallel to the axles resulted in no translational motion in the direction of the pull (frame b). Lastly, by pulling perpendicular to the axles, the nanocar resumed its forward path (frame c).
Figure 4.17.
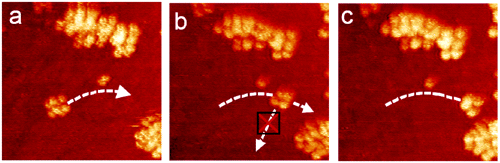
These experiments carried out by the Tour Group combined to substantiate the claim that the nanocars were rolling on the C60 wheels as opposed to sliding. This observation designates the molecule as the first nanocar capable of executing a predetermined, engineered motion. These experiments lay down a foundation of knowledge on the molecular mechanics of motion. Particularly, these experiments conclusively demonstrate rolling motion of the C60 on gold surfaces at a given temperature. This marks the first step in a greater understanding of molecular motion as it applies to molecular manufacturing.
The Future of the Development of Molecular Manipulation
Molecular manipulation as a science has developed in steps. Its early steps involved movement of atoms and molecules, along with the ability to observe those movements. Later came engineered molecular components that carried out predetermined functions, such as bearings and axles. At the present, nanocars are an example of the developments in motility and function of integrated components that serve a unified purpose. But more importantly, nanocars are an indicator of developments to come. They are ushering in an era of deliberate and controlled motion at the molecular level.