The Origin of Life
David Deamer
Nothing in biology makes sense except in the light of evolution. —Theodosius Dobzhansky
OUTLINE
1. Defining life in evolutionary terms
2. Plausible sites for the origin of life
3. Conditions required for life’s origin
4. Self-assembly of boundary membranes and compartments
5. Prebiotic polymerization reactions
6. How could evolution begin?
7. Evolution in the laboratory
The prebiotic environment had a variety of simple carbon compounds and energy sources that could drive chemical reactions. These reactions produced ever more complex carbon compounds, some of which could assemble into membranous compartments, while others could link up to make polymeric chains. The polymers became encapsulated in the compartments, producing vast numbers of protocells. The variable protocellular compartments are a microscopic version of what is now called combinatorial chemistry; that is, each protocell contained a different mix of polymers and monomers, and represented a natural experiment. Some of the polymers happened to have potential catalytic abilities, while others could in some way replicate. A rare few of the cellular compartments contained both catalysts and replicating molecules in which the catalysts could speed up replication, and the replicating polymers could carry a kind of genetic information that coded for the monomer sequence in the catalysts. Biological evolution began with compartmented systems of molecules that could grow and reproduce. Within the populations of primitive microorganisms there would be variations in their abilities to compete for resources, and to withstand stress. These variations became selective factors that initiated subsequent stages of biological evolution.
GLOSSARY
Amphiphile. A molecule having both hydrophilic and hydrophobic components. Typical hydrophilic groups are carboxylate, phosphate, and sulfate, and the hydrophobic portion is a hydrocarbon. Examples of amphiphiles include most lipids, fatty acids, sterols, and detergents.
Combinatorial Chemistry. A way to perform multiple chemical reactions in parallel. Each combination can be different, so information about reaction conditions is obtained much more rapidly than by performing a series of experiments with different conditions one after another.
Lipid Bilayer. All cell membranes use a bimolecular lipid layer as a barrier to free diffusion of ionic and polar solutes.
Liquid Crystal. An organized structure formed by amphiphilic molecules, often in the form of semicrystalline layers (smectic phase) or rods (nematic phase), in which the molecules are not fixed in place but instead move by diffusion within the layer or rod.
Multilamellar Matrix. The nanoscale organization of many phospholipids and soaps that associate as layers of molecules.
Protocell. A microscopic membrane-bounded system of interacting polymers that represents an evolutionary stage on the pathway to the origin of life.
RNA World Hypothesis. The conjecture that life passed through a phase in which RNA functioned both as a catalyst and to store genetic information. The RNA life-forms were later replaced by living systems using RNA, DNA, and proteins.
Vesicle. Spherical structures bounded by a lipid bilayer, sometimes referred to as liposomes.
Dobzhansky was among the great pioneering geneticists of the last century, and the quote at the beginning of the chapter is the title of an essay he wrote in 1973, late in his career. The quote is often used to challenge biology teachers and professional biologists to think more deeply about evolution, and it will be used here in a slightly different context: Does the origin of life make sense in the light of evolution?
Although Charles Darwin knew virtually nothing about biochemistry and molecular biology, he would have agreed that evolution began with the origin of life. In On the Origin of Species, he asks the question we are addressing here: “Looking at the first dawn of life, when all organic beings, as we may believe, presented the simplest structure, how, it has been asked, could the first steps in the advancement or differentiation of parts have arisen?”
What would Darwin have written if he understood that life began 4 billion years ago, when the systems of replicating molecules were much simpler than bacteria today? He would likely propose that evolution by natural selection could not begin with a single molecule, but there must have been a way to generate large numbers of primitive systems of molecules in the prebiotic environment. Furthermore, there would be considerable variation in the properties of such systems. The requirement for variation within a population means that the first life-forms capable of evolution were not simply a mixture of reproducing molecules, but instead consisted of microscopic systems of interacting molecules encapsulated in some sort of boundary structure, referred to here as protocells. Each protocell was a kind of natural experiment, and the primary hurdle they needed to overcome was to capture available sources of energy in order to grow by polymerization of nutrient monomers, then to reproduce. Heterotrophic life today does this by accumulating simple molecules from the environment. Chemical energy is then used during metabolism to activate them so they can be linked into polymers such as proteins and nucleic acids. The earliest cells also needed to store genetic information and replicate it when they reproduced, so their properties were passed along to the next generation. A certain amount of error in this process was inevitable, what we now call mutations. The imperfections in replication were important, because they meant that life could explore different niches and begin the long trek to cellular life as we know it today.
The next question concerns a process by which large numbers of natural experiments could be generated by organic carbon compounds in the prebiotic environment. Three things are necessary: a way to produce the microscopic equivalent of test tubes, a suitable source of energy, and a way to synthesize polymeric molecules. If the polymers can be encapsulated as protocells and provided with an energy source, the system has the potential to become more complex. Because life somehow emerged from the complex environment of the prebiotic earth, and did so soon after liquid water first condensed on the planet, it seems possible that its origin can be reproduced in the laboratory under just the right set of conditions and components.
1. DEFINING LIFE IN EVOLUTIONARY TERMS
At some point in the near future, a claim will likely be made that artificial life has been fabricated in the laboratory. For this claim to be convincing, it will be necessary to show that the system has properties that fall within an accepted definition of life. The problem is that no definition is generally accepted by biologists. Even the simplest microorganisms are extraordinarily complex, and dictionary-style definitions don’t easily encompass such complexity. Because life is a complex phenomenon, one approach to a definition is to describe a minimal set of properties associated with the living state. What follows is a single paragraph that incorporates properties of terrestrial life as we know it; taken together, the properties exclude anything that is not alive:
The machinery of life is composed of polymers, very long molecules composed of subunits called monomers. The primary polymers of life are nucleic acids and proteins, often called biopolymers by definition. The polymers interact within a membranous boundary that has three primary functions: containment, transport of nutrients, and energy transduction. Biopolymers are synthesized in the container by linking together monomers—amino acids and nucleotides—using energy available in the environment. Polymer synthesis is the fundamental process leading to growth of a living system. Nucleic acids have a unique ability to store and transmit genetic information, and proteins called enzymes have a unique ability to act as catalysts that increase the rates of metabolic reactions. The genetic and catalytic polymers are incorporated into a cyclic feedback-controlled system in which information in the genetic polymers is used to direct the synthesis of the catalytic polymers, and the catalytic polymers take part in the synthesis of the genetic polymers. During growth, the cyclic system of polymers reproduces itself, and the cellular compartment divides. Reproduction is not perfect, so that variations arise, resulting in differences between cells in a population. Because different cells have varying capacities to grow and survive in a given environment, individual cells undergo selection according to their ability to compete for nutrients and energy. As a result, populations of cells have the capacity for evolution.
There is no doubt that a claim of synthetic artificial life would be convincing if the system incorporated all the above properties; however, if the properties are deleted one by one, the definition becomes blurred and the claim weaker. Suppose the system reproduced perfectly so that evolution could not occur. Would it still be considered alive? Most would say yes, so the ability to evolve might not be a fundamental property of life. But consider another system in which all the nutrients required for growth were present in the medium so that no metabolism was required. This system would resemble a virus that requires the cytoplasm of living cells to reproduce; viruses, however, can evolve, so they seem to exist in the border between life and nonlife.
2. PLAUSIBLE SITES FOR THE ORIGIN OF LIFE
We can now briefly describe a few examples of sites and conditions proposed as being conducive to the origin of life. Each of these is characterized by one or more properties believed to have promoted certain chemical or physical processes conceivably related to steps involved in the pathway to life. For instance, mineral surfaces such as clay were suggested many years ago by Desmond Bernal and promoted by Graham Cairns-Smith, who thought a genetic takeover might have occurred as organic compounds were adsorbed to and organized by the clay: “We have, as it were, identified the organization responsible for the ‘crime against common sense,’ the origin of life. And it is true that the proposition that our ultimate ancestors were mineral crystals was not widely anticipated.” There is some experimental support for the idea that clays were involved in life’s origins. James Ferris and coworkers have made an extensive study of montmorillonite clay and demonstrated that chemically activated mononucleotides in the form of imidazole esters do in fact adsorb to the mineral surface. When concentrated as near neighbors, polymerization into oligomers up to 15 or more nucleotides in length can occur.
Another surface reaction has been suggested by Gunther Wächtershäuser, who proposed that life could begin as two-dimensional synthetic chemistry on a special mineral surface called pyrite, a crystalline mineral composed of iron sulfide. According to Wächtershäuser’s idea, pyrite has two special properties. The first is that it has a positive surface charge; it therefore adsorbs negatively charged solutes such as carbonate and phosphate. Furthermore, when hydrogen sulfide reacts with iron in solution to produce iron pyrite, the reaction can potentially donate electrons to the bound compounds and drive a series of energetically uphill chemical reactions that otherwise could not occur in solution. Wächtershäuser sees these reactions as the beginning of metabolism, occurring on a flat mineral surface rather than in the volume of a cell. He refers to this stage of life’s history as the “Iron-Sulfur World.” After metabolic processes were initiated in this way, the reaction pathways would become encapsulated in membranes to produce the more familiar forms of cellular life.
Yet another site was proposed by Jeffrey Bada and Stanley Miller, who suggested that the early earth may have been covered by a global ice sheet. Under these conditions, organic compounds are preserved for much longer time intervals, and they speculated that occasional melting produced by impact events would release the organics and initiate chemical reactions necessary for life to begin.
All these proposals involve reactions of relatively simple compounds; however, for cellular evolution to begin, there must have been a point at which complex interacting systems of polymeric molecules were encapsulated within boundary membranes, and none of these proposals addresses this requirement. The rest of this article will describe a process by which the first protocells could emerge and be exposed to selective processes.
3. CONDITIONS REQUIRED FOR LIFE’S ORIGIN
We can start by describing conditions likely necessary for life to begin on the early earth 4 billion years ago, and then see whether a plausible site exists encompassing all the conditions. Certainly liquid water was required, a dilute solution of potential monomers. The primary monomers of life today are amino acids, nucleobases, pentose sugars (ribose and deoxyribose), and phosphate, all of which have been synthesized in simulated prebiotic conditions or demonstrated to be present in carbonaceous meteorites. There must also have been an energy source capable of driving polymerization reactions, involving a chemical reaction in which monomers could be linked into random polymers, some of which would have weak catalytic activity. A process would be required to concentrate dilute solutions of monomers to the point they could react; furthermore, the polymeric products of the reaction must be confined in some sort of compartment so they can interact with one another rather than being dispersed. The result will be large numbers of compartmented systems of polymers that are exposed to selective conditions so that evolution can begin. The next section will describe a plausible site and experimental systems in which membranous boundary structures self-assemble from amphiphilic molecules. Within the structure, polymers can be synthesized from monomers by an energy source that was ubiquitous in the prebiotic environment, followed by encapsulation of the polymers to produce protocells.
4. SELF-ASSEMBLY OF BOUNDARY MEMBRANES AND COMPARTMENTS
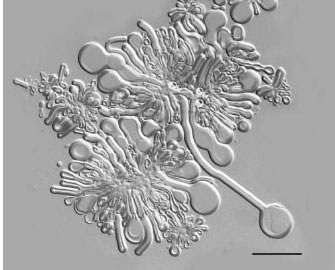
It has long been known that a phospholipid called lecithin can be extracted from egg yolks. Experiments conducted in the 1960s showed that if lecithin is dried on a microscope slide and then exposed to water, long wormlike structures called myelin figures grow (figure 1). Alec Bangham and his coworkers at the Animal Physiology Institute at Babraham, Cambridge, UK, added a dilute salt solution to egg lecithin in a test tube and found a milky suspension was produced consisting of immense numbers of cell-sized spherical globules. Using an early version of electron microscopy, they observed that the globules were multilamellar structures composed of many lipid bilayers. Furthermore, the globules could be dispersed into vesicles now called liposomes, and the membranous lipid bilayer is now understood to be the primary structural component of all cell membranes.

Figure 1. When phospholipid on a microscope slide is exposed to a dilute salt solution, the dry material begins to absorb water and grow out into tubular structures called myelin figures. These are unstable and ultimately break up into vesicles called liposomes. The tubules and vesicle boundaries are composed of lipid molecules that self-assemble into multilamellar bilayers in aqueous phases. A reasonable assumption is that the earliest forms of cellular life also used lipid bilayer membranes as boundary structures, but composed of lipid-like molecules such as fatty acids and alcohols available in the prebiotic environment. Bar shows 25 micrometers.
Could similar self-assembled compartments have been present on the prebiotic earth? This question has been addressed by investigating organic compounds in carbonaceous meteorites. One such meteorite fell near Murchison, Australia, in September 1969, and more than 100 kg of scattered fragments were collected and distributed to interested scientists. In 1970, Keith Kvenvolden and a group of researchers at NASA Ames analyzed a sample of the meteorite and convincingly demonstrated that amino acids, one of the essential organic compounds composing all life on earth, were present in the meteorite. This study established that amino acids, the fundamental building blocks of proteins, can be synthesized by a nonbiological process that occurred in the asteroid parent body of the meteorite. It seems reasonable to think that amino acids would have been synthesized on the prebiotic earth by similar reactions.
But what about membrane-forming compounds? In 1985, samples of the Murchison meteorite were extracted with an organic solvent, and a drop of the solution was dried on a microscope slide. When a dilute salt solution was added, amphiphilic compounds in the extract assembled into cell-sized membranous vesicles (figure 2), suggesting that similar cellular compartments were likely to be present when the first liquid water appeared on the earth more than 4 billion years ago.
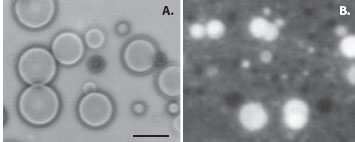
Figure 2. Carbonaceous meteorites like the Murchison chondrite contain long-chain monocarboxylic acids that can assemble into microscopic vesicles (A). These are true membranes capable of encapsulating a fluorescent dye such as pyranine (B). Bar shows 25 micrometers.
The next question concerns how polymers can be encapsulated in the empty membranous compartments to produce protocells. It is known that lipid membranes fuse into multilamellar structures when dried, so one possibility is that wet-dry cycles in hydrothermal sites associated with volcanic activity on the early earth could carry out such a process. In early studies it was found that liposomes dried in the presence of nucleic acids or proteins trapped the polymers between the layers. When water was added back to the dry film, the lipid layers formed vesicles again, but now with up to half the large molecules trapped inside (figure 3). This seems a very plausible process by which primitive protocellular systems of polymeric molecules could be produced on the early earth.
Figure 3. Phospholipid vesicles can be dried in the presence of a solute, in this case short strands of duplex DNA. When water is added to the dry material, multilamellar vesicles assemble (A), trapping the DNA between lipid bilayers. Here the DNA has been labeled with a fluorescent dye so that it can be visualized in association with the vesicles (B). After a few minutes, the multilamellar structures begin to form myelin figures that then break up into smaller vesicles containing the DNA (C). Cycles of wetting and drying would be common in the prebiotic environment, and represent a simple process by which cellular compartments could form containing encapsulated reactants and polymers. Bar shows 50 micrometers.
5. PREBIOTIC POLYMERIZATION REACTIONS
Given cellular compartments and a way to encapsulate biopolymers, how could prebiotic polymers have been synthesized? This question has not yet been answered, but several possibilities have been experimentally tested. In one approach, dispersions of lipid vesicles were prepared and mononucleotides, the monomers of RNA, were added to make a ratio of about one nucleotide per lipid in the solution. The mixture was warmed to 85 °C while being dried with a gentle stream of carbon dioxide to simulate the prebiotic atmosphere. A small amount of water was then added, the mixture was stirred for a few seconds to disperse the lipid vesicles, and the wet-dry cycle was repeated up to seven times. The idea was to simulate the conditions of a volcanic hydrothermal area on the early earth in which a continuous drying and wetting process occurred at the edges of pools. The water would be fairly hot (80–90 °C) and weakly acidic.
When the solution was analyzed for the presence of polymers, it was found that RNA-like molecules had been synthesized, ranging from 20 to 100 nucleotides in length. The yields were low by the standards of organic synthesis: fewer than 0.1 percent of the nucleotides had been linked into longer polymers, representing a few micrograms of product from the milligram quantities of nucleotides present in the mixture; however, there is no reason to think that high-yield polymerization reactions occurred in the prebiotic environment, in which mixtures of hundreds of different organic compounds would be present. The reactions leading to early biopolymers were almost certainly very low yield.
An important outcome of these experiments was that when the last cycle of hydration was completed by adding water, the lipid captured the RNA in vesicles. Such protocells represent a first step toward cellular life in an RNA world, that is, microscopic membrane-bounded compartments containing complex mixtures of polymers with the potential to be both catalysts (ribozymes) and carriers of information.
6. HOW COULD EVOLUTION BEGIN?
To summarize what has been said so far, the origin of life can be understood as an emergent phenomenon that occurs when water, mineral surfaces, and atmospheric gases interact with organic compounds and a source of energy. Hydrothermal conditions and processes act in concert to “pump” a random assemblage of simple organic compounds away from equilibrium toward increased complexity. The primary conditions are cyclic processes driven by a suitable input of energy, and capture of small amounts of the mixture in compartments that permit a natural version of combinatorial chemistry, resulting in vast numbers of microscopic molecular systems, each a kind of natural experiment.
We can now consider the physical and chemical conditions prevailing on the prebiotic earth that could drive the first steps of evolution. The early earth had oceans, volcanic landmasses, and an atmosphere of carbon dioxide and nitrogen gas. The most plausible site for the origin of life was not the open ocean or dry land; instead, there is reason to think that the most conducive conditions for life to begin existed in places where liquid water and the early atmosphere formed an interface with mineral surfaces such as volcanic rocks. Interfaces have special properties, because they allow three essential processes to occur that happen nowhere else: wet-dry cycles, concentration and dilution, formation of compartments, and combinatorial chemistry.
Cycles: The fluctuating environment required to provide cycles most likely took the form of pools in volcanic sites where hot water constantly underwent wetting and drying. The pools contained complex mixtures of dilute organic compounds from a variety of sources, including extraterrestrial material delivered during the last stages of the earth’s formation, and other compounds produced by chemical reactions associated with volcanoes and atmospheric reactions. Because of the fluctuating environment, the compounds underwent cycles in which they were dried and concentrated, then diluted on rewetting.
Compartments: During the drying cycle, the dilute mixtures would form very thin films on mineral surfaces, a process necessary for chemical reactions to occur. Not only would the compounds react with one another under these conditions but the products of the reactions would also become encapsulated in microscopic compartments by membranes that self-assembled from soap-like compounds. This process produced vast numbers of protocells that appeared all over the early earth, wherever water solutions were undergoing wet-dry cycles in volcanic environments similar to today’s Hawaii or Iceland.
Combinatorial chemistry: The protocells represented compartmented systems of molecules, each different in composition from the next, and each representing a kind of microscopic natural experiment. Most of the protocells remained inert, but a few happened to have the capacity to capture energy and smaller molecules from outside the encapsulated volume. As smaller molecules were transported into the internal compartment, energy was used to link them into long polymeric chains. Polymers have emergent properties that far exceed what monomers can do; for instance, both the primary biopolymers of today’s life—proteins and nucleic acids—can act as catalysts, and nucleic acids carry and transmit genetic information, yet individual amino acids and nucleotides lack these properties.
Life began when a few of the immense numbers of protocells found a way not just to grow but also to incorporate a cycle involving catalytic functions and genetic information. According to this hypothesis, cellular systems of molecules, not individual molecules, were the first forms of life.
7. EVOLUTION IN THE LABORATORY
We can now address a simple question central to our understanding of the origin of life: Can nonliving molecular systems evolve? Can genetic information really appear out of nowhere? An answer to that question was provided by Andrew Ellington and Jack Szostak in 1990, then elaborated by David Bartel and Szostak in 1993. Their goal was to determine whether a completely random system of molecules could undergo selection in such a way that defined species of molecules emerged with specific properties. Bartel and Szostak began by synthesizing many trillions of different RNA molecules about 300 nucleotides long, all present as random sequences of nucleotides. They reasoned that buried in those trillions were a few catalytic RNA molecules called ribozymes that happened to weakly catalyze a ligation reaction, in which one strand of RNA is linked to a second strand. The RNA strands to be ligated were attached to small beads on a column, then exposed to the trillions of random sequences simply by flushing them through the column. This process could fish out any RNA molecules with even a weak ability to catalyze the reaction. They then amplified those molecules in an enzyme-catalyzed process and put them through for a second cycle, repeating the process for 10 rounds.
The results were astonishing. After only four rounds of selection and amplification, an increase in catalytic activity was seen, and after 10 rounds, the ligation rate was 7 million times faster than the uncatalyzed rate. It was even possible to watch the RNA evolve. Nucleic acids can be separated and visualized by a technique called gel electrophoresis. At the start of the reaction, nothing could be seen, but with each cycle new bands appeared. Some came to dominate the reaction, while others went extinct.
Bartel and Szostak’s results demonstrate fundamental principles of evolution at the molecular level. At the start of the experiment, each molecule of RNA was different from all the rest. There were no species, just a mixture of trillions of different molecules, but then a selective hurdle was imposed in the form of a ligation reaction that allowed only certain molecules to survive and be reproduced enzymatically. After a few generations, groups of molecules began to appear that displayed ever-increasing catalytic function. In other words, in a mixture that initially contained completely random RNA molecules, species of molecules appeared in an evolutionary process closely reflecting the natural selection outlined by Darwin for populations of living organisms. These RNA molecules were defined by the sequences of bases in their structures, which caused them to fold into specific conformations that had catalytic properties. The sequences are analogous to genes, because the information they contained was passed between generations during the amplification process.
The inescapable conclusion is that genetic information can emerge in random mixtures, as long as there are populations containing large numbers of polymeric molecules with variable sequences of monomers, and a way to select and amplify a specific property. A similar process must have occurred on the prebiotic earth to bring the first forms of life into existence. The origin of life is best understood as a metaphor of combinatorial chemistry, but at a level far beyond what is possible in the laboratory. Will we ever discover the combination of ingredients that gave rise to life? There is reason to be optimistic. We need to apply what we know about the chemistry and physics of living systems to develop plausible hypotheses, then be brave enough to test them experimentally.
FURTHER READING
This article was adapted in part from First Life (Berkeley: University of California Press, 2011), which is a more detailed account of the research briefly described here.
Bartel, D. P., and J. W. Szostak. 1993. Isolation of new ribozymes from a large pool of random sequences. Science 261: 1411–1418. The authors convincingly demonstrate how a catalytic function can emerge from a completely random system of RNA molecules undergoing selection and amplification.
Deamer, D. W., and J. Szostak, eds. 2010. Origins of Life. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Each chapter of this multiauthored book provides an expert analysis of processes leading to the origin of life.
Hazen, R. M. 2007. Genesis: The Scientific Quest for Life’s Origin. Washington, DC: National Academies Press. Robert Hazen, a research scientist at the Carnegie Institution of Washington, has written an engaging first-hand account of what it is like to investigate the origin of life.
Sullivan, W. T., and J. Baross, eds. 2007. Planets and Life: The Emerging Science of Astrobiology. Cambridge: Cambridge University Press. The editors of this book invited experts to write chapters about their specialty, using language that would be appropriate for undergraduate courses.
ONLINE RESOURCES
Astrobiology: Life in the Universe. National Aeronautics and Space Administration.
astrobiology.nasa.gov/
NASA’s Astrobiology website introduces concepts and news related to the origin and distribution of life in the universe.
Exploring Life’s Origins: A Virtual Exhibit.
exploringOrigins.org/
This website, featuring molecular animations by Janet Izawa, visualizes the results of origins of life research for broad audiences.