Major Events in the Evolution of Fungi
David S. Hibbett
OUTLINE
1. Fungi in the tree of life
2. Losses of flagella and diversity of the “basal fungal lineages”
3. Evolution of the dikaryon and multicellular fruiting bodies
4. Evolution of decayers and plant pathogens
5. Evolution of mycorrhizae, lichens, and endophytes
6. Evolution of animal pathogens and mutualists
7. The age of Fungi
Fungi represent one of the few clades of eukaryotes that evolved complex multicellular forms and diversified extensively on land, the others being plants and animals. In the process, Fungi have become integral to the functioning of ecosystems. They are the master decayers of plant biomass, playing a pivotal role in the global carbon cycle. As parasites and pathogens, they attack plants, animals, and each other, but they have also evolved intricate mutualistic symbioses, such as mycorrhizae and associations with the fungus gardening leaf-cutter ants. The number of extant species of Fungi is a matter of conjecture; one commonly cited estimate suggests 1.5 million fungal species, but only about 100,000 species have been described, and fungal molecular ecologists routinely detect species and even major clades that have no match in DNA sequence databases. Much progress has been made in reconstructing the phylogenetic relationships of the Fungi, but there are still unresolved branches in the fungal evolutionary tree. Adaptation in Fungi is largely biochemical in nature, involving the diversification of enzymes, effectors, and secondary metabolites that allow these anatomically simple organisms to exploit diverse substrates and hosts.
GLOSSARY
AFTOL. Assembling the Fungal Tree of Life, a multilaboratory collaboration supported by the US National Science Foundation that sought to reconstruct the phylogeny of Fungi and construct a higher-level classification for the group.
Biotroph. Fungus that obtains nutrition by association with a living host, whether as a pathogen, parasite, or mutualist.
Fruiting Body. Macroscopic multicellular structure that produces spores, including mushrooms.
Hyphae. Fungal filaments.
Mold. Asexual spore-bearing filamentous fungus (mostly Ascomycota). Also an informal term for certain fungus-like organisms (water molds, slime molds).
Mycelium. Network of hyphae; a fungal thallus.
Mycorrhiza. Symbiotic association of fungal hyphae and plant roots.
Saprotroph. Fungus that obtains nutrition by decaying dead organic matter. Necrotrophs (Fungi that kill and then decay a living host) blur the boundaries between saprotrophs and biotrophs (see above).
Yeast. Unicellular, nonflagellated fungus.
1. FUNGI IN THE TREE OF LIFE
Linnaeus considered Fungi to be members of the kingdom Plantae, and ever since mycology has been one of the traditional disciplines of botany; however, unlike plants and algae, Fungi have cell walls composed of chitin (as opposed to cellulose) and they are heterotrophic, with an absorptive mode of nutrition. The “five kingdom” system of the late 1960s was based largely on cellular, ultrastructural, and biochemical characters, and it classified Fungi as a separate kingdom of eukaryotes, distinct from the plants and animals, as well as the paraphyletic “protists.” Today, it is known from DNA sequence data that the Fungi form a clade in the eukaryotic supergroup Unikonta (“unikonts”), which also includes animals and several protist lineages.
Fungus-like taxa outside the unikonts include the Oomycetes, which have a filamentous habit and absorptive heterotrophy like true Fungi, but are actually members of the Heterokonts, a group that includes kelps and diatoms. Oomycetes include some of the most destructive plant pathogens, such as Phytophthora infestans (infamous as the cause of the Irish potato famine), as well as “water molds” that plague aquarium fish (some species also attack mammals, including humans). The suffix -mycetes and the term mold reflect the historical classification of these organisms as Fungi.
Another group of eukaryotes formerly classified as Fungi are the slime molds, which are in a clade within the unikonts termed the Mycetozoa. Slime molds include cellular forms (e.g., Dictyostelium discoideum), which forage as amoeboid unicells but can also aggregate to form a pseudomulticellular structure capable of gliding motility, and plasmodial forms (e.g., Physarum polycephalum), which alternate between amoeboid and uniflagellated swarm cells and eventually form a coenocytic, often brightly colored, multinucleate “supercell” that scavenges for bacteria along the forest floor. The closest relatives of Fungi within unikonts are the nucleariids, which are unicellular amoebae. Based on comparison with nucleariids and other clades of unikonts, such as choanoflagellates, it is likely that the common ancestor of the Fungi was a unicellular, possibly aquatic heterotroph, with a single posterior flagellum.
2. LOSSES OF FLAGELLA AND DIVERSITY OF THE “BASAL FUNGAL LINEAGES”
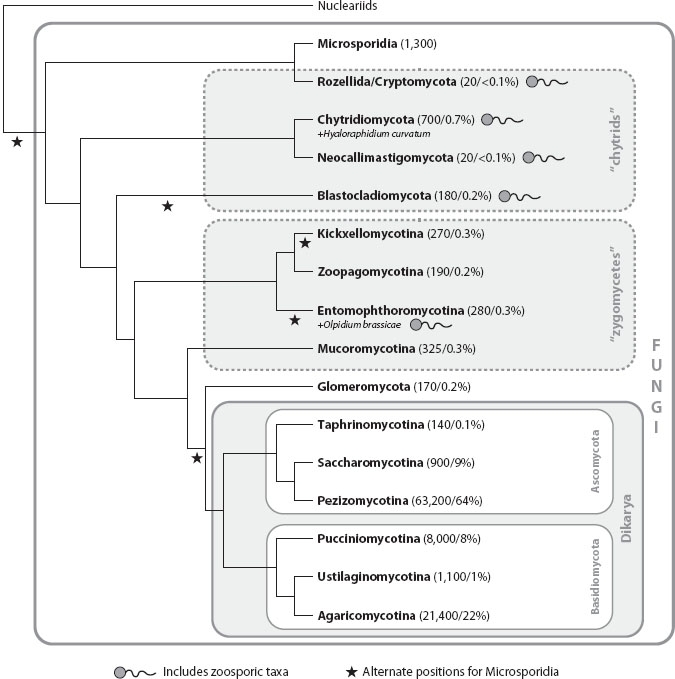
Reconstructing the deepest branching events in the fungal phylogeny is a work in progress. The topology presented here (figure 1) is based on a study that analyzed six genes in almost 200 species and that is reflected in the AFTOL (see glossary) classification of the Fungi. Other studies have resolved relationships differently, however, and this picture may change as more complete fungal genomes are included in phylogenetic analyses. Some of the most problematic aspects of the fungal phylogeny concern the “basal fungal lineages,” a paraphyletic assemblage containing diverse chytrids and zygomycetes (these are informal, purely descriptive terms, defined below).
Figure 1. Phylogenetic relationships of Fungi. Numbers of described species and proportion of all described Fungi in each group are indicated in parentheses following taxon names. Dashed lines delimit paraphyletic groups, not formal taxa. Branch lengths are not proportional to time. Relationships among chytrids and zygomycetes are particularly controversial.
Chytrids are Fungi that produce swimming zoospores, and in this regard they resemble the putative aquatic protist ancestors of the Fungi and other unikonts. Chytrids occur in both freshwater and marine environments, but they have also adapted to terrestrial habitats, including soil and the bodies of terrestrial organisms. Some chytrids have developed mycelial (see glossary) growth or produce rootlike extensions (rhizoids) that anchor the cells on the substrate and facilitate absorption of nutrients. Three independent clades of chytrids were included as formal taxa in the AFTOL classification: the Chytridiomycota, Neocallimastigomycota, and Blastocladiomycota. In addition, the recently recognized and informally named clade “Rozellida” or “Cryptomycota” is based largely on environmental sequences from aquatic habitats; the only formally named taxon in the group is the chytrid genus Rozella. Finally, Olpidium is a group of plant and algal parasites of uncertain placement that may represent another independent chytrid lineage.
Most chytrids are saprotrophs and can often be isolated by “baiting” with organic substrates containing cellulose or keratin (hemp seeds and snake skin are often used), but others have evolved biotrophic nutritional modes involving diverse hosts. For example, Rozella allomycis (Rozellida/Cryptomycota) is an intracellular parasite that inhabits another chytrid, the filamentous Allomyces (Blastocladiomycota). Other members of Rozellida/Cryptomycota appear to be phagotrophic or intracellular parasites, based on observations of cells harvested from water and sediments and visualized using fluorescently labeled DNA probes. Some chytrids have evolved plant parasitic lifestyles, such as Synchytrium endobioticum (Chytridiomycota), which causes black wart disease of potatoes, and Olpidium brassicae (which is of uncertain placement; see below), which causes club root diseases of cabbages and other Cruciferae. Animals are not immune to attack by chytrids; Catenaria anguillulae (Chytridiomycota) infects and kills nematodes, while Batrachochytrium dendrobatidis (also Chytridiomycota) is a devastating pathogen implicated in the ongoing global decline of frogs and other amphibians. More benign associations are manifested in the Neocallimastigomycota, which includes anaerobic rumen Fungi that benefit their herbivore hosts by digesting plant fibers.
Like chytrids, zygomycetes are united only by shared primitive characters (symplesiomorphies), specifically, the development of a mycelium lacking regular septa, and the absence of complex multicellular fruiting bodies. Beyond that, zygomycetes are wildly diverse in form and ecology. Zygomycetes are distributed across about five independent clades, the Mucoromycotina, Kickxellomycotina, Zoopagomycotina, Entomophthoromycotina (collectively, these four groups have often been classified as the Zygomycota, a name still in common use), and Glomeromycota.
Just about every imaginable fungal nutritional mode has evolved in the zygomycetes. The most familiar group, the Mucorales (Mucoromycotina), includes saprotrophic molds that are common agents of food spoilage, some of which are used to make fermented foods such as tempeh (Rhizopus oryzae), but members of this group also cause an extremely dangerous (and thankfully rare) fungal infection of humans called mucormycosis, while others parasitize mushrooms. Biotrophic associations with animals are widespread in other groups of zygomycetes as well. For example, members of the Entomophthoromycotina include insect associates, such as the fly pathogen Entomophthora muscae, which have potential applications in biocontrol (saprotrophs also occur in this group). Kickxellomycotina include a fascinating array of “Trichomycetes,” which live in the hindgut of aquatic arthropods, such as mosquito larvae and isopods, and Zoopagomycotina contains species that can attack and kill nematodes, rotifers, amoebae, and other Fungi.
Mycorrhizal associations have evolved in two groups of zygomycetes, the Endogonales (Mucoromycotina) and the Glomeromycota. Of these, the arbuscular-mycorrhizal (AM) associations of Glomeromycota are the most widely documented, and have been estimated to occur in 80 percent of plant species. Fossils of putative Glomeromycota spores have been found in the roots of early land plants from the Rhynie Chert, which has contributed to the view that the establishment of AM symbioses was a key event facilitating the colonization of land by plants. Members of the Glomeromycota and Endogonales form associations with extant non-vascular plants (bryophytes) as well as vascular plants, and both groups have been proposed to represent the oldest lineage of mycorrhizal Fungi. Both groups also contain simple fruiting bodies, including the “pea truffles” of the Endogonales and aggregations of spores termed sporocarps in certain Glomeromycota.
One of the most controversial issues in fungal evolution concerns the number of losses of the eukaryotic flagellum. In addition to the zygomycete groups discussed above, two other clades of nonflagellated Fungi are of relevance to this problem. One is an obscure planktonic fungus called Hyaloraphidium curvatum, which is nested within the Chytridiomycota. The other is a clade called Microsporidia, which are obligate intracellular parasites of diverse animals that are notable for their highly reduced genomes (some are fewer than 3 million base pairs, smaller than many bacteria), vestigial mitochondria lacking a genome (mitosomes), and a unique infection mechanism, the polar tube, through which the microsporidial cytoplasm is injected into the host cell.
The placement of Microsporidia within Fungi is not resolved; they could be the sister group to the rest of the Fungi, or they could occupy several positions closely related to different groups of chytrids or zygomycetes, with different implications for the number of losses of the flagellum. Finally, uncertainty in the placement of the chytrid Olpidium brassicae also makes it difficult to estimate the number of losses of the flagellum. In the six-gene study mentioned above, O. brassicae is closely related to certain zygomycetes (Entomophthoromycotina), and the Microsporidia are the sister group of the Rozellida/Cryptomycota clade (represented in that study by R. allomycis). This topology implies five independent losses of the flagellum within the Fungi, but alternative trees requiring fewer losses cannot be rejected.
3. EVOLUTION OF THE DIKARYON AND MULTICELLULAR FRUITING BODIES
About 98 percent of the described species of Fungi are in a strongly supported group called the Dikarya. The synapomorphy for which the group is named, the dikaryon, is formed through the cytoplasmic fusion of mating compatible haploid cells (monokaryons), but without the immediate fusion of the haploid nuclei, yielding a stable binucleate condition. Nuclear fusion typically occurs immediately prior to meiosis in specialized meiosporangia (cells producing haploid spores via meiosis), which in most Dikarya are the only truly diploid cells in the life cycle.
The Dikarya is composed of two sister clades, the Ascomycota and Basidiomycota, each of which is divided into three major groups, the Saccharomycotina, Taphrinomycotina, and Pezizomycotina (Ascomycota), and the Pucciniomycotina, Ustilaginomycotina, and Agaricomycotina (Basidiomycota). In Ascomycota, the meiosporangium is a saclike ascus, which produces spores internally, whereas in Basidiomycota the meiosporangium is a pedestal-shaped basidium, which produces spores externally on minute stalks. Both Ascomycota and Basidiomycota also contain species with asexual reproductive modes. Most common molds, including those used to make blue cheeses, and the fungus that produced the first antibiotic, penicillin, are asexual members of Ascomycota. In some cases, connections between asexual and sexual forms have been confirmed through cultural or molecular studies, but for many asexual forms the corresponding sexual stage, if it exists, has not been observed.
Many Basidiomycota produce hook-shaped projections called clamp connections at cell junctures in dikaryotic hyphae, which serve to apportion the individual haploid nuclei to daughter cells in mitosis. Ascomycota produce putatively homologous structures, termed croziers, but these are formed only at the bases of asci. Although many Ascomycota and Basidiomycota have filamentous growth, some have a yeast form (or alternate between the two forms), and two groups of Ascomycota, the Saccharomycotina and Taphrinomycotina, are composed almost entirely of yeasts. Clamp connections and croziers are features of hyphal forms, implying that the common ancestor of Dikarya was a mycelial fungus and that yeast forms in both Ascomycota and Basidiomycota have been derived by parallel simplification.
Multicellular fruiting bodies have evolved only within the Dikarya (excluding the minute sporocarps of certain Endogonales and Glomeromycota). Some fruiting bodies are inconspicuous and relatively simple crusts, but others are massive and may have complex, developmentally integrated structures. Examples include the gigantic bracket Fungi that grow on tree trunks, mushrooms with multiple layers of veil tissues (like the iconic “fly agaric,” Amanita muscaria), or the diverse gasteromycetes (Fungi with spores produced inside the closed fruiting body), such as stinkhorns, puffballs, and bird’s nest Fungi. There has been extensive convergent evolution of fungal fruiting bodies, with forms such as gilled mushrooms, coral Fungi, polypores, and puffballs all having been derived repeatedly. The majority of macroscopic fungal fruiting bodies, including all the preceding examples, are in the Agaricomycotina (Basidiomycota), but some Pezizomycotina (Ascomycota) have also evolved fruiting bodies, including various cup Fungi and the edible morels (Morchella spp.). One group of Taphrinomycotina, the genus Neolecta, produces simple club-shaped fruiting bodies; it is not clear whether these represent an independent origin of fruiting bodies or a retained plesiomorphic condition. Some Dikarya produce fruiting bodies underground, including the prized truffles in the genus Tuber (Pezizomycotina). Such hypogeous Fungi have fully adapted to the terrestrial habit, releasing their spores directly into soil or exploiting small mammals and other animal vectors for spore dispersal. At the same time, other lineages of Dikarya, both Ascomycota and Basidiomycota, have reverted to aquatic habitats. Lacking flagellated swimming cells, marine and freshwater Dikarya often have spores with elongate projections that aid in dispersal. Subsequent sections of this chapter highlight ecological innovations within the Dikarya, many of which are also echoed in the various chytrid and zygomycete lineages described previously.
4. EVOLUTION OF DECAYERS AND PLANT PATHOGENS
Dikarya play a key role in the global carbon cycle by decomposing plant cell walls, which are composed of the carbohydrates cellulose and hemicellulose, as well as lignin, a heterogeneous polymer highly resistant to microbial attack that gives woody tissues rigidity. Saprotrophic Dikarya attack diverse plant-derived substrates, including leaf litter, organic matter in soils, herbivore dung, and wood. The diversity and evolution of the enzymatic apparatus that enables Dikarya to cause decay is being revealed through biochemical and comparative genomic research. Many different kinds of carbohydrate-active enzymes involved in dismantling the cellulose and hemicellulose components of plant cell walls have diversified in both Ascomycota and Basidiomycota, and both groups include numerous taxa that decay nonwoody tissues. Selected economically important examples include the edible button mushroom Agaricus bisporus (Basidiomycota, Agaricomycotina), which has been adapted for cultivation on manure-straw compost mixture, and the mold Trichoderma reesei (Ascomycota, Pezizomycotina), a source of industrial cellulase enzymes. In contrast, efficient decay of massive woody substrates is limited to certain Agaricomycotina (although some Ascomycota do decay wood to an extent). Two principal forms of wood decay occur in Agaricomycotina: white rot, in which the carbohydrate and lignin portions of wood are degraded, and brown rot, in which the lignin fraction is chemically modified but not appreciably degraded. Lignin decay appears to involve the action of enzymes called class II fungal peroxidases, which are of interest for their potential application in bioremediation and other “green” technologies. The brown rot mode of decay has evolved independently in multiple lineages of Agaricomycotina with associated losses of genes encoding lignin-degrading peroxidases.
Plant pathogens have evolved in numerous lineages of Dikarya and have huge impacts on natural ecosystems and human agriculture. Selected examples in Ascomycota include the chestnut blight fungus (Cryphonectria parasitica), which devastated one of the dominant and most prized timber trees of North America, the American chestnut, and the ergot fungus (Claviceps purpurea), which infects rye and produces a mixture of alkaloids that cause a horrifying suite of symptoms in humans who ingest the grain, including convulsions, hallucinations, and impaired blood circulation leading to gangrene.
Plant pathogens also occur throughout Basidiomycota, with the largest concentrations among the “smuts” (Ustilaginomycotina), such as the corn smut fungus (Ustilago maydis), and the “rusts” (Pucciniomycotina), which include economically important pathogens of diverse crops that have complex life cycles involving multiple host plants. An example is the barberry-wheat rust (Puccinia graminis). The reliance of wheat rust on barberries (Berberis) has been recognized for centuries, leading to campaigns to control the pathogen by eliminating the alternate host in the area of grain fields.
Plant-pathogenic and saprotrophic Dikarya use many of the same families of carbohydrate-active enzymes to degrade plant cell walls. In addition, an active area of current research concerns the diversity and function of fungal effectors, small secreted proteins produced by plant-pathogenic Dikarya that suppress the immune response of host plants. Studies on fungal effectors are being conducted in Ascomycota, such as the rice blast fungus (Magnaporthe grisea), a widespread pathogen of one of the world’s most important cereal crops, and Basidiomycota such as Melampsora lini, a rust that infects flax. Comparisons of genes encoding fungal effectors often suggest strong diversifying selection, reflecting an evolutionary arms race between host and pathogen.
5. EVOLUTION OF MYCORRHIZAE, LICHENS, AND ENDOPHYTES
Mutualistic biotrophic associations of Dikarya and photosynthetic organisms include mycorrhizae, lichens, and endophytes. In each case, the fungus obtains its carbon nutrition in the form of sugars produced by the photosynthetic host. Mycorrhizal Dikarya aid their hosts by facilitating uptake of mineral nutrients, while some endophytes may confer enhanced drought tolerance, resistance to herbivores, or other benefits. Nonetheless, these associations may best be regarded as reciprocal parasitisms, with selection driving each partner to maximize its fitness at the expense of the other. An extreme outcome of such instability is manifested in mycoheterotrophic plants such as Indian pipe (Monotropa uniflora), a nonphotosynthetic angiosperm that actually draws sugars out of its fungal partners (mushrooms in the Agaricomycotina), thus reversing the normal flow of carbon in mycorrhizal symbiosis.
Most of the mycorrhizal associations of Dikarya are ectomycorrhizae (ECM), in which the hyphae of the fungus ensheathe and penetrate the roots of woody plants. ECM hosts include pines, oaks, hickories, birches, willows, dipterocarps, eucalypts, and certain legumes. These are often the dominant trees of extensive forests, so ECM have a large impact on terrestrial ecosystems, even if they do not involve as many plant species as the AM of Glomeromycota. ECM have evolved in certain Pezizomycotina (Ascomycota), but they are most diverse in the Agaricomycotina (Basidiomycota) (ECM also occur in the Endogonales). Within the Agaricomycotina, the ECM habit has clearly evolved repeatedly, but the precise number of origins is not well resolved. One lineage of mostly ECM mushrooms, the Boletales, also contains several mycoparasites and decayers, suggesting that reversals from the ECM habit to other nutritional modes are possible. ECM-forming Dikarya produce some of the most prized edibles, such as truffles, porcini mushrooms, and chanterelles, which are expensive, in part because they must be collected in forests containing their tree hosts.
Lichens and endophytes have also evolved in both Ascomycota and Basidiomycota, but they are much more numerous in Ascomycota (Pezizomycotina). Lichens, which involve associations with eukaryotic green algae as well as cyanobacteria, are able to colonize truly harsh environments, such as rock surfaces and tree trunks, and they play important ecological roles as primary colonizers. Endophytes are cryptic Fungi that produce no visible symptoms in the plants they inhabit. The diversity of endophytes has been revealed through direct culturing approaches as well as molecular surveys, which have found them in every plant species investigated so far. Phylogenetic studies in Ascomycota have revealed complex patterns of transitions between lichenized, endophytic, pathogenic, and saprotrophic lifestyles. Intriguingly, an “endolichenic” habit, in which Fungi inhabit lichen thalli, appears to have been an evolutionary precursor to the endophytic habit in some lineages of Ascomycota.
6. EVOLUTION OF ANIMAL PATHOGENS AND MUTUALISTS
Dikarya have evolved biotrophic associations with diverse animals, including humans, both as pathogens and as mutualists. Many human-associated Fungi occur as commensals or produce relatively mild infections, such as the yeast Candida albicans, which causes genital and oral yeast infections, as well as dermatophytes (Trichophyton and other genera), which are able to digest the keratin in skin, hair, and nails, causing annoying maladies known as ringworm and athelete’s foot. Other fungal pathogens are much more serious, such as Coccidioides immitis, the causal agent of coccidioidomycosis (San Joaquin Valley fever), which initiates as a lung infection but can become a deadly systemic infection. Another lung-borne pathogen is the unicellular Pneumocystis spp., which was thought to be a protist until it was shown with ribosomal RNA gene sequences to be a fungus. Like many fungal pathogens, Pneumocystis is common as a commensal in healthy individuals but can cause a serious opportunistic infection, particularly in immunocompromised individuals.
Diverse Dikarya attack insects, with perhaps the most dramatic being members of the genus Cordyceps, which produce elongate, stalked fruiting structures arising from the host’s body. Cordyceps species attack diverse arthropods, and one lineage has even switched from the underground larvae of cicadas to the underground fruiting bodies of certain truffles (an interkingdom host jump). Moreover, Cordyceps is closely related to the plant pathogen Claviceps purpurea, mentioned previously, further highlighting the evolutionary lability of fungal nutritional modes. Other nonhuman animals attacked by Fungi include nematodes, which are trapped by various “predatory” Fungi, whose mycelia are equipped with constricting or nonconstricting rings, and adhesive knobs, networks, and hyphal branches.
All of the preceding examples are in the Ascomycota, but Basidiomycota have also evolved diverse associations with animals, both benign and antagonistic. Examples include human pathogens, such as the yeast Filobasidiella (Cryptoccus) neoformans, which can cause fungal meningitis, or the “dandruff fungus” Malassezia globosa. One of the best-studied mutualistic associations of Fungi and animals is that of the neotropical leaf-cutter ants, which harvest large volumes of plant biomass to feed to several lineages of Agaricomycotina that are closely related to free-living saprotrophic mushrooms. In a striking case of convergent evolution, certain Old World termites, another group of social insects, cultivate a different group of saprotrophic Agaricomycotina in the genus Termitomyces.
7. THE AGE OF FUNGI
Fungi, with their ephemeral and often microscopic forms, are not well represented in the fossil record. Nonetheless, there are branching hyphae from the Silurian period (ca. 430 Ma), which may be the oldest fungal fossils, and forms attributed to Glomeromycota, Chytridiomycota, and Ascomycota occur alongside the oldest land plant fossils in the Devonian Rhynie Chert (ca. 410 Ma). Molecular clock studies have estimated the Fungi to be much older than the fossils alone would suggest, albeit with significant variance in age estimates. Various molecular clock analyses have suggested that the most recent common ancestor of the Fungi as a whole existed anywhere from 850 million to 1.5 billion years ago; the Dikarya are probably at least 500 million years old, and perhaps much older. While the exact timing and sequence of branching events deep in the fungal phylogeny are not resolved with confidence, it is clear that Fungi, through their activities as saprotrophs, pathogens, and mutualistic symbionts, have played a major role in shaping the evolution of terrestrial ecosystems.
FURTHER READING
Alexopoulos, C. J., C. W. Mims, and M. Blackwell. 1996. Introductory Mycology. 4th ed. New York: Wiley. This textbook contains a wealth of information on fungal forms, ecology, and impacts on humans.
Arnold, A. E., J. Miadlikowska, K. L. Higgins, S. D. Sarvate, P. Gugger, A. Way, V. Hofstetter, F. Kauff, and F. Lutzoni. 2009. A phylogenetic estimation of trophic transition networks for ascomycetous Fungi: Are lichens cradles of symbiotrophic fungal diversification? Systematic Biology 58: 283–297. Technical article reconstructing the historical pattern of switches between endophytic, endolichenic, and plant-pathogenic lifestyles.
Barron, G. L. 1977. The Nematode-Destroying Fungi. Topics in Mycobiology No. 1. Guelph, Ontario: Canadian Biological Publications. A classic text describing the surprisingly diverse Fungi and fungus-like organisms that trap and kill nematodes.
Berbee, M. L., and J. W. Taylor. 2010. Dating the molecular clock in fungi—how close are we? Fungal Biology Reviews 24: 1–16. Review article describing the opportunities and pitfalls of molecular clock dating in Fungi, including the integration of biogeography and fossils.
Floudas, D., M. Binder, R. Riley, K. Barry, R. A. Blanchette, B. Henrissat, A. T. Martinez, et al. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336: 1715–1719. Comparative genomic study describing the evolution of wood decay, and suggesting a fungal role in the decline of coal formation at the end of the Carboniferous.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, et al. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547. Current classification of Fungi with references to the phylogenetic analyses that form the basis of the taxonomy.
James, T. Y., F. Kauff, C. Schoch, P. B. Matheny, V. Hofstetter, C. Cox, G. Celio, et al. 2006. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443: 818–822. Comprehensive 6-gene, 200-species phylogenetic analysis of all major groups of Fungi.
Jones, M.D.M., I. Forn, C. Gadelha, M. J. Egan, D. Bass, R. Massana, and T. A. Richards. 2011. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474: 200–203. This technical article describes a major clade of Fungi known almost entirely from environmental DNA sequences.
Kirk, P. M., P. F. Cannon, D. W. Minter, and J. A. Stalpers. 2008. Ainsworth & Bisby’s Dictionary of the Fungi. 10th ed. Oxon, UK: CAB International. Essential desk reference for fungal biologists, with counts of described species for genera and more inclusive groups.
McLaughlin, D. J., D. S. Hibbett, F. Lutzoni, J. Spatafora, and R. Vilgalys. 2009. The search for the fungal tree of life. Trends in Microbiology doi:10:1016/j.tim.2009.08.001. Review article describing progress in fungal systematics from a historical perspective.
Rodriguez, R. J., J. F. White Jr., A. E. Arnold, and R. S. Redman. 2009. Fungal endophytes: Diversity and functional roles. New Phytologist 182: 314–330. Useful overview of the biology of endophytic Fungi.
Spatafora, J. W., K. W. Hughes, and M. Blackwell, eds. 2006. A phylogeny for kingdom Fungi. Mycologia 98: 829–1103. This special issue of Mycologia contains phylogenetic studies on all major groups of Fungi.
Staijch, J., M. L. Berbee, M. Blackwell, D. S. Hibbett, T. Y. James, J. Spatafora, and J. W. Taylor. 2009. The Fungi. Current Biology 19: R840–R845. Concise overview of fungal phylogeny emphasizing selected topics of interest to molecular biologists, and others.
Stergiopoulos, I., and P.J.G.M. de Wit. 2009. Fungal effector proteins. Annual Reviews of Phytopathology. 47: 233–263. Authoritative review of fungal effectors in selected model systems.
Taylor, T. N., E. L. Taylor, and M. Krings. 2009. Paleobotany. 2nd ed. Amsterdam: Elsevier. This is a lavishly illustrated compendium that presents the fossil record of Fungi in a broad botanical context.