Evolution of Life Histories
David Reznick
OUTLINE
1. What is the life history and why is it of interest?
2. The theory of life history evolution: A sampler
3. Other aspects of life history evolution
4. What have we learned?
5. Future research
The life history is a composite of all the variables that contribute to the way in which an organism propagates itself. The most important variables are how old it is when it begins to reproduce, how much it invests in reproduction, as opposed to other activities or structures, and how it allocates resources to offspring (many small versus few large). We are interested in life histories from a theoretical perspective because these variables are closely allied to an organism’s fitness, or its ability to contribute offspring to the next generation. We are interested in life histories from a natural history perspective because organisms in the real world display a vast diversity of life histories, so we would like to know why they evolved. Life history theory predicts the optimal allocation of resources to growth, maintenance, storage, and reproduction in response to external features of the environment, such as risk of mortality. The evolutionary optimum is defined as that allocation that results in the largest number of successful descendants. The empirical study of life history evolution has revealed that the different components of the life history evolve in a way that is consistent with assumptions made by theory. For example, there is support for the proposal that trade-offs are made between different components of the life history, such that investing heavily in reproduction early in life is associated with shorter life span. Empirical research also includes the experimental test of life history theory in natural populations and, as such, presents one of the best examples available of the experimental test of evolutionary theory in nature. Specifically, investigators have manipulated the risk of mortality in natural populations and have seen life histories evolve as predicted by theory. One future direction of life history research is to use it as a forum for developing a better understanding of the definition of fitness. Another is to use it as a forum for understanding how ecological and evolutionary processes interact with one another.
GLOSSARY
Adaptation. Any feature of an organism that has been shaped by the process of evolution by natural selection.
Antagonistic Pleiotropy. Pleiotropy means that a single gene influences more than one feature of the phenotype. The gene’s effects are described as antagonistic if the gene changes one feature of the phenotype in a way that increases fitness and another feature in a way that decreases fitness.
Fitness. In the context of life history evolution, fitness is the relative success of an individual at contributing offspring to the next generation. If we set the average success of an individual in a population as equal to 1, then we can score the relative fitness of individuals relative to this average as being either greater or less than 1.
Semelparity/Iteroparity. Parity refers to giving birth. Iteroparity means giving birth repeatedly during the course of an individual’s lifetime. Semelparity means giving birth only once, then dying.
Trade-off. In the context of life history evolution, a trade-off is a causal linkage between two features of the life history, such that increasing the resources devoted to one feature causes a decline in the amount devoted to the other. Trade-offs can also be dictated by other constraints, such as between the number and size of individual offspring, as shaped by the finite volume that a mother can devote to developing offspring.
1. WHAT IS LIFE HISTORY AND WHY IS IT OF INTEREST?
An individual’s life history is the composite of all the variables that contribute to the way it propagates itself. The two generic classes of variables that make up a life history pertain to timing and resource allocation. Timing variables include how old the individual is when it begins to reproduce and how often it reproduces. Allocation variables address how an organism divides up the resources with which it has to work. The categories of allocation include growth, maintenance, reproduction, and storage, such as in the form of fat. Reproductive allocation includes the ways in which these resources are in turn divided among offspring. An organism like an elephant, whale, or human produces only a handful of babies during its lifetime and devotes a very large amount of resources to each of them. An ocean sunfish (Mola mola) can produce many millions of eggs each time it reproduces, devotes little to each of them, and casts them to the fates of ocean currents. Allocation variables also include parental investment before or after the fertilization of gametes. For many organisms, all resources are invested in seeds, eggs, or live-born offspring who are on their own from birth. For others, the parents provide continued care, and hence make continued resource investment after birth.
We study life histories for two reasons. One is their theoretical importance. The other is their remarkable diversity.
Life History Theory
Life history theory is really a special application of the more general theory of evolution by natural selection. We use the word fitness to describe the differences among individuals in their ability to contribute offspring to the next generation. The key to understanding fitness is that it is first determined by the odds of surviving to maturity, then by the number of offspring that in turn survive to reproduce in the next generation. These features can evolve if they vary among individuals and if the variation is at least partly heritable. Life history characteristics therefore can evolve to maximize fitness and can be considered adaptations. Life histories represent a particular category of adaptations, as detailed in the next section. The reason they stand out as special is that they apply to the actual currency of fitness, which is the production of offspring. For that reason, a body of theoretical and empirical work has grown around explaining how and why life histories have evolved.
Life history theory occupies a special place in evolutionary biology because it contributed to a more generalized concept of fitness. When Darwin presented evolution by natural selection in The Origin, he defined fitness as survival. If individuals with a given phenotype are able to live longer, then they have more opportunities to reproduce. In the theory of life history evolution, fitness is defined instead as a composite measure of survival and age-specific reproduction, or all the different variables that make up the life history. One such composite variable is the intrinsic rate of increase (r), or the per capita rate at which a population increases in size. An alternative index of fitness that is sometimes used is R0, or the expected number of offspring produced during the lifetime of the individual. The rank order of the relative fitness of different phenotypes is equal to the rank order of each phenotype’s value for r or R0.
An important consequence of defining fitness in terms of such composite variables and in the context of life history evolution is that natural selection can cause the evolution of seemingly disadvantageous traits. For example, it can favor the evolution of reduced life span or reduced number of offspring produced during an individual’s lifetime, providing there are compensations, or trade-offs, in other components of the life history (see chapters III.1 and III.8).
Life History Diversity
Consider some of the extremes of life histories that we see in nature. The seeds of the desert annual plant Linanthus parryae lie dormant in the soils of the Mojave Desert for most of the year. They germinate in response to unpredictable winter rains. A single rain can cause some seeds to break dormancy, sprout, grow, and, if there is sufficient moisture, flower and set seed, all within a few months. All that remain at the end of the brief growing season are short, dry stalks and seed pods; otherwise, the species exists as a “seed bank” in the soil, accumulated in those years when there was sufficient rain to support a complete life cycle. Individual seeds remain dormant, possibly for decades, before responding to a promising winter rainstorm. Whole years can pass with no living plants being visible on the surface of the desert.
In contrast, bristlecone pines (genus Pinus), which inhabit equally arid, but high-elevation environments dispersed throughout the southwestern United States, replace the speed of the Linanthus life cycle with the ability to persist. These are the oldest-known living things, with the known ages of some individuals being close to 5000 years. It takes centuries for them to reach maturity. Their seed-producing cones take more than a year to mature. The large trunk of an old individual may contain only a narrow strip of living tissue connecting the crown of the tree to its roots; the remainder consists of decay-resistant dead wood.
The topic of life history evolution thus presents us with compelling questions and contrasts. If attributes like development time, frequency of reproduction, and number of propagules are so important in defining fitness, why are they also so variable? What features of the environment shape this remarkable diversity of life histories?
2. THE THEORY OF LIFE HISTORY EVOLUTION: A SAMPLER
Think of your life as a pie. The pie represents the available resources enabling you to survive and reproduce. The pie is divided into four slices, one each representing allocations devoted to maintenance, growth, storage, and reproduction.
Maintenance includes the cost of replacing the parts of your body that are constantly wearing out. Skin, blood cells, and the lining of your intestinal tract consist of cells that are in constant need of replacement, so they occupy a significant component of maintenance. Many other components of your body are also constantly being replaced or modified over time, at some cost in terms of consumed energy and resources. For adult humans, maintenance is the largest slice of the pie.
Growth is a major investment early in life, but many organisms either stop growing or dramatically reduce growth rate when they attain maturity, causing the size of this particular slice to decline with age. Mammals and birds, for example, virtually cease growth at maturity, so the investment in growth precedes the investment in reproduction. Fish, amphibians, reptiles, and most plants can grow throughout their lives, so there can be a temporal overlap between investment in growth and investment in reproduction.
Storage can be in the form of energy, primarily fat tissue, but also in the form of limiting nutrients or elements. In birds, calcium is a stored nutrient; it plays an important role in body fluids and bones, but it is also used periodically and in large amounts to produce eggshells. I will concentrate only on energy storage here. You may have difficulty envisioning fat as adaptive, but that is because we are all inclined to make more deposits than withdrawals. For many organisms, fat is a key feature of the life history, because it enables organisms to stockpile resources at a time when they are available, then save them for a later time when resources may be scarce, and it is ideal for producing offspring.
Reproduction is the piece of the pie devoted to making babies. This investment includes the gametes (eggs and sperm), and other forms of care provided by the parents. These other investments in reproduction can include finding, preparing, and defending a nest site, competing with others of the same sex to obtain a mate, or convincing members of the opposite sex to mate. You might be tempted to think of investment in reproduction as what is done with the surplus after all the critical needs for survival are met, but that is not necessarily the case. In some extremes it can displace maintenance. In most species of salmon, for example, reproduction happens just once, and it is like the flight of a ballistic missile. As salmon migrate from the sea into freshwater, they cease to feed. The entirety of the resources required to migrate upstream (sometimes hundreds of miles), prepare a nesting site, compete for mates, and produce gametes are derived from stored resources. Salmon cease investing in vital functions, like their immune systems, as they swim upstream. In their final days, they are depleted of fat reserves and can be covered with festering infections.
A consequence of thinking of life as a pie is realizing that the pie is finite in size. This means that increasing the size of any of these four slices necessarily reduces the size of other slices. This interdependence of the slices defines a central tenet of most life history theory, which is that all organisms must face trade-offs in allocating their resources to the various components of the life history, such that a gain in any one function comes at some cost to another function. For example, in a fish, reptile, or amphibian, increasing the current investment in growth might carry the benefit of producing more offspring in the future, if the individual survives, because larger individuals can produce more offspring, but increasing growth now can mean reproducing less now. The goal of life history theory is to predict the optimal allocations of this finite quantity of resources in a given set of circumstances. I offer an introduction to two types of life history theory that illustrate these principles.
r and K selection. This theory was first well articulated by Robert MacArthur and E. O. Wilson in 1967. It does not represent the inception of our interest in life history evolution, but it was the first big idea that captured the imaginations of evolutionary biologists and made the study of life history evolution an important empirical endeavor.
r and K are parameters from the equation used to describe logistic population growth, or population growth in conditions where resources can be limiting:
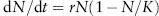
r = intrinsic rate of increase, or rate of exponential population growth when resources are unlimiting
N = population size
K = carrying capacity, or the maximum number of individuals the environment can sustain
The expression in parentheses can be thought of as a damping factor, or the degree to which the potential growth rate of the population is reduced because the environment is partially occupied. If N is very small, then the population growth rate is close to r. When N = K, the population ceases to grow.
In MacArthur and Wilson’s original formulation of the theory, they predicted that r-selection favors the evolution of “productivity,” or selects for traits that enable an individual to sustain rapid population growth. In addition, r-selected populations are those that are persistently far from their carrying capacity, perhaps because their abundance is frequently reduced by unpredictable events, like storms or droughts, or because they are kept scarce by predators. K-selection is selection in favor of the ability to persist in the face of limiting resources, such as when individuals are members of populations that are persistently close to their carrying capacity, perhaps because they occupy stable environments. MacArthur and Wilson envisioned how r and K selection would come into play when an organism colonizes an island. It will encounter abundant resources and experience an initial phase of r-selection, which would favor genotypes that “have a shorter developmental time, a longer reproductive life, and greater fecundity, in that order of probability” (MacArthur and Wilson 1967). After population expansion, island colonists will then experience K-selection, which “favors efficiency in the conversion of food into offspring” (p. 149).
Eric Pianka elaborated on MacArthur and Wilson’s predictions. He argued that r-selection would favor the evolution of early maturity, increased investment in reproduction, and the production of many offspring, each of which receive little investment from the parents, and short life span. All these changes in the life history, save the last, will increase the value of r. Pianka predicted that K-selection would instead favor the evolution of delayed maturity, decreased reproductive investment, the production of few offspring, each receiving high parental investment, and long life span. All these changes will reduce r, but are assumed to increase the ability of an organism to persist when resources are limiting. The concept of trade-offs is implicit in his predictions. Because r-selected organisms invest heavily in reproduction early in life, they invest less in growth and maintenance and have shorter lives. Conversely, because K-selected species invest more in their own growth and maintenance and less in reproduction, they tend to have longer lives.
Demographic Theory: How Mortality Risk Shapes Life History Evolution
There is a prominent feature of life histories that is not included in r- and K-selection. Many populations have age or stage structure, such as juveniles versus adults. Age classes can differ in the way they are affected by selection. Populations of the annual plant Linanthus parryae do not have age structure once seeds have germinated because all individuals germinate, mature, reproduce, and die in the same season. Populations of bristlecone pines do have age structure because they grow for centuries before they attain maturity, then live and continue to grow for millennia as mature, reproducing individuals. The probability of survival from one year to the next can change with the age, size, or stage of development of the individual. For example, if predators prefer large prey, then older, larger individuals may experience increased risk of mortality. On the other hand, older, larger individuals may be better at evading predators and defending themselves, so mortality risk may decline with age. The expected number of offspring is zero prior to maturity. The number of offspring produced can increase progressively after maturity if the individual grows, since fecundity is often directly proportional to size. In species with postnatal care, like birds and mammals, reproductive success often increases with age because the parents become more experienced and are better providers.
Since the populations of many organisms have such age structure, a body of theory was developed to address the consequences of differences among age groups in the risk of mortality and reproductive success. The theory deals with risks imposed from the outside, such as those of predators, disease, and competitors.
One common prediction from this body of theory is that when the risk of adult mortality is high, natural selection will favor the evolution of earlier maturity and increased allocation to reproduction. If we think of life as a pie, then this enlargement of the slice devoted to reproduction early in life means that some other slice must be smaller. There could be a reduction in growth, in maintenance, or in storage. Wherever the trade-off occurs, the expectation is that investing more in reproduction now will in some way detract from the future. Another prediction that emerges from some models is that a selective increase in the mortality rate of juveniles can also select for the evolution of delayed maturity and a decrease in the rate of investment in reproduction. The reason for the delay in the context of this model is also a product of the trade-offs associated with the pie of life. Deferring the investment in reproduction means investing more in growth and maintenance. In real life, this shift in investment can in turn mean more quickly outgrowing the size classes that are susceptible to predators, if predation on small individuals is the source of mortality. These alternative life histories resemble those predicted by r- and K-selection, but they can evolve independently of the population’s proximity to its carrying capacity. They evolve instead as a consequence of the probability of surviving as a function of age, the expected reproductive success of different age classes, and the sorts of trade-offs that exist between investments in different slices of the pie of life.
Alternative models have been developed that can yield different predictions. For example, Conrad Istock modeled the evolution of complex life cycles, which include larval and adult life stages separated by metamorphosis. In this context, higher mortality during the larval versus adult life stage is predicted to select for more rapid rather than slower larval development because metamorphosis can mean being able to make a transition to a less dangerous environment.
3. OTHER ASPECTS OF LIFE HISTORY EVOLUTION
Many features of life history evolution have emerged as specialized subdisciplines, or even disciplines in their own right. Here I offer a sampling of these spin-offs.
Iteroparity versus Semelparity
One prominent feature of the life histories of diverse organisms is whether they reproduce once, then die (semelparity) or are capable of reproducing multiple times (iteroparity). Pacific salmon (genus Oncorhynchus) are famous among fish for their single, suicidal investment in reproduction. Likewise, agaves are desert plants that may grow for decades before flowering once, then dying. The difference between the alternatives of single versus multiple reproductive events becomes more interesting when these alternatives are expressed in close relatives. Some populations of Atlantic salmon (Salmo salar) and American shad (Alosa sapidissima) are semelparous, while other populations have many individuals that will return to the sea after breeding, then come back to freshwater rivers to breed a second time. Some closely related species of plants, such as in the genus Echium, native to North Africa, Europe, Madeira, and the Canary Islands, can be either semelparous or iteroparous. The existence of such diversity among populations within a species or between closely related species tells us that semelparity is a life history that can evolve from iteroparous ancestors. It thus poses the question, “Why does semelparity evolve”? One simple answer, derived from theory, is that the evolution of semelparity is driven by the cost of producing the first offspring. Envision a salmon that must swim hundreds of miles upstream to reproduce. The cost of producing the first egg is swimming this great distance, facing barriers like waterfalls and rapids, surviving predators like bears, competing for a nesting territory, preparing the nest, then laying the first egg. All subsequent eggs are cheap by comparison and a far better investment, even to the point of death, than facing the risks associated with return to the sea, then once again facing the start-up costs of reproduction.
Offspring Number versus Size
Demographic life history theory generally considers how evolution shapes the size of the slice of the pie of life devoted to reproduction. It often does not address whether that slice is invested in many offspring, each receiving few resources, or few offspring, each receiving abundant resources. A separate body of theory has been developed that deals with the division of reproductive allocation among individual offspring. One benchmark paper in this literature was published by Christopher Smith and Stephen Fretwell. They predicted the optimal number and size of offspring for a parent to produce, given some simple assumptions. First, they assumed that the fitness of the parent increases with the number of offspring produced. Second, they assumed that the probability of survival of the offspring increases with its size at birth. It then becomes possible to define the fitness of the parent as the number of surviving offspring, which is in turn defined by some optimal combination of offspring number versus offspring size. Farrah Bashey found that guppies that live in rivers with abundant predators produce many small offspring, while those that live in headwater streams produce few but large offspring. In rivers with predators, guppies are scarce and food is abundant. In rivers without predators, guppies are abundant and food is scarce. She found that the fitness of small and large offspring is the same when food is abundant, but that large offspring have a substantial fitness advantage when food is scarce. This combination of results suggests that guppies gain by making more and smaller offspring in streams with predators because there is no sacrifice in being small at birth; however, when predators are absent and food is scarce, the advantage of making larger babies offsets the cost of making fewer.
Life Span and Senescence
A related body of theory deals with the evolution of life span and senescence. If there are trade-offs among various components of the life history, then it follows that maturing at an early age and investing heavily in reproduction can evolve only if there is some reduction in the investment in growth, storage, or maintenance. Any such reduction can be expected to take away from future reproduction or survival. George Williams captured this anticipated trade-off by proposing his antagonistic pleiotropy model for the evolution of senescence. He proposed that genes that increase reproductive investment early in life would at the same time cause the evolution of an earlier onset of senescence. Senescence is not defined as life span. It is instead defined as an age-specific deterioration in function, such as the ability to reproduce or the number of offspring produced. It is also defined as an acceleration in death rate with age. A consequence of earlier or more rapid senescence is a shorter life span.
It is this extension of life history theory to a consideration of senescence that shows how life history evolution can lead to seemingly counterintuitive responses. Darwin’s original concept of evolution by natural selection was that it acted primarily by selecting for the evolution of increased life span, yet here we argue that it can actually cause the evolution of decreased life span. This can happen because the benefit of early maturity and high reproductive investment early in life more than offsets the cost of reduced life span, at least in some circumstances. One of those circumstances is when an organism has a high risk of mortality imposed on it from the outside, such as a high risk of predation. If the chances of living long are small in spite of the amount invested in reproduction early in life, then the cost of high early investment is small, because most individuals will not survive long enough to realize that cost, regardless of what they invest in reproduction.
4. WHAT HAVE WE LEARNED?
Empirical research on the evolution of life histories has progressed in diverse directions. Here I will describe two of them. One productive line of research involves compiling descriptions of the life histories of many species of organisms into a single analysis that probes for statistical relationships among components of the life history, or between the life history and features of the environment. These analyses are capitalizing on the vast number of life history descriptions that have been generated over the past few decades. One pattern that has emerged in many studies is that the life histories of groups of organisms, like mammals or birds, array along what some refer to as a fast-slow continuum. At one end of the gradient are species that mature at an early age, devote abundant resources to reproduction, produce many offspring, and are short-lived. At the other end are organisms that are old at maturity, devote less to reproduction, often by producing few, well-provisioned offspring, and are longer lived. These studies show that the life history of any given species is not a random aggregation of life history components. Life histories instead evolve in an organized fashion, meaning that the way any one feature of the life history evolves is well correlated with the way the other components of the life history evolve. These correlations among different features of the life history are often consistent with the idea of trade-offs.
In one recent study, Peron et al. (2010) analyzed the association between senescence and the early life history. They compiled data from the published literature on 81 free-ranging populations of 72 species of birds and mammals. They evaluated the association between events early in the life history and the age at onset of senescence, defined as the age when an acceleration in mortality rate occurred. They found that the early life history predicted two-thirds of the variation in the age of onset of senescence and, specifically, that higher juvenile mortality, an earlier age at first reproduction, and the production of more offspring early in life combined to predict an earlier onset of senescence. This pattern of association was predicted by Williams and is consistent with antagonistic pleiotropy, or a more general trade-off between investment in reproduction early in life and the future potential to reproduce.
A second empirical approach has been the experimental study of life history evolution, either in the laboratory or in nature (see chapter III.6). This approach can bring us closer to the study of the cause and effect relationship between some feature of the environment that causes life histories to evolve and the way life histories evolve in response. It therefore offers a more direct way of testing and evaluating life history theory. A pioneering effort in experimental evolution was performed by Michael Rose and Brian Charlesworth on Drosophila melanogaster. They selected for reproductive success either early or late in life in replicate laboratory populations, by propagating successive generations of some populations from flies that had been the offspring only of young parents, and other populations from the offspring only of old parents. After 20 generations of selection, the early lines laid more eggs early in life, and the late lines laid more eggs late in life. The most interesting outcome was that the flies from the early lines had a higher rate of egg production early in their lives, but also had shorter life spans than flies from the late lines. These responses confirm the life history theory that predicts how life histories will evolve in response to differences in age-specific reproductive success. They show that selection on reproductive success can cause either a decrease or an increase in life span. Many other such experiments on the evolution of senescence in Drosophila have supported the predictions of life history theory.
I and my colleagues have pursued similar goals in our study of life history evolution in natural populations of guppies. The rivers in the northern range mountains of Trinidad offer a natural laboratory for studying life history evolution in action because they flow over steep gradients punctuated by waterfalls that separate fish communities. Species diversity decreases as waterfalls block the upstream dispersal of some species. The succession of communities is repeated in many parallel drainages, providing us with natural replicates. In the downstream localities, guppies live with a diversity of predators that prey on adult size classes of guppies (high-predation communities). Waterfalls often exclude predators but not guppies, so guppies found above waterfalls have greatly reduced risks of predation and increased life expectancy (low-predation communities). The only other fish found in these localities rarely preys on guppies, and when it does, preys on small, immature size classes.
We used mark-recapture methods to demonstrate that guppies from high-predation (HP) environments sustain much higher mortality rates than those from low-predation (LP) environments. We also found that, as predicted by life history theory, HP guppies mature at an earlier age, devote more resources to reproduction, produce more offspring per litter, and produce significantly smaller offspring than LP guppies. Laboratory studies confirm that all these life history differences between HP and LP guppies have a genetic basis. Genetic analyses imply that HP guppies invade guppy-free environments and evolve into LP phenotypes, and that some rivers represent independent replicates of this process.
Rivers can be treated like giant test tubes, since fish can be introduced into portions of stream bracketed by waterfalls, creating in situ experiments. We transplanted guppies from a high-predation environment below a barrier waterfall to a previously guppy-free environment above a waterfall, thus reducing their risk of mortality. As predicted by theory, the descendants of these fish evolved delayed maturation and reduced reproductive allocation, with some changes happening in four years or less. We also experimentally increased the risk of mortality by transplanting predators over a barrier waterfall that previously excluded predators but not guppies. These guppies evolved earlier ages at maturity within five years. Our results thus successfully test some predictions from life history theory in a natural setting. One by-product of this work is that it also reveals the potential rate of evolution by natural selection in nature. It can be remarkably fast.
5. FUTURE RESEARCH
Science moves like waves that gather energy, crest, then dissipate as they reach the shore. The cresting of a wave of science may mean that the important questions have been answered, but it may also mean that the easy questions have been answered, leaving behind the hard ones that few want to face, or perhaps that other topics have captured the imagination of the scientific community. The study of life history evolution seems to have crested, yet there remain important questions to answer. The results to date also suggest important new avenues of research. I will discuss one example of each of these venues for future inquiry.
The Elusiveness of Trade-Offs and Condition-Dependent Fitness
Trade-offs play a central role in life history theory and are supported by broad comparative studies and selection experiments; however, some studies appear to contradict the existence of trade-offs. One curious result that defies the expectations of antagonistic pleiotropy was reported for natural populations of water fleas (Daphnia pulex). Some genetic lineages grew faster and to larger body sizes, began to produce eggs at an earlier age, and produced eggs at a higher rate throughout their lives than other lineages in the same population. This seems like an impossible combination of attributes occurring as natural variation within a population. The rapidly developing lineages are superior in every way and should displace the other genotypes, yet the slowly developing genotypes persist. Later work suggested that the superiority of the rapidly developing lines is expressed only when food availability is abundant. When food is scarce, such genotypes lose out to those that are smaller, develop more slowly, and produce fewer eggs. These results tell us that our perception of trade-offs can depend on the environment we choose for assessing them. They also tell us that life history evolution represents adaptation to multiple dimensions of the environment. Risk of mortality is important, but so is resource availability.
Interactions between Ecology and Evolution
The rapidity with which life histories evolve raises another question about the factors that shape life history evolution and, more generally, about how ecological and evolutionary processes might relate to one another. The traditional perspective of the relationship between ecology and evolution is that ecological processes happen so much more quickly than evolution that we can treat organisms as constants (meaning that they do not evolve) when modeling ecological processes or studying ecological interactions in the real world. The traditional perspective is also that ecology shapes evolution. Field selection experiments with guppies tell us that evolution happens in a time frame comparable to that of ecology, so perhaps it is better to think of ecology and evolution as processes that interact in real time and reciprocally shape each other. In the case of guppies, the presence and absence of predators is associated with changes in guppy populations and features of the ecosystem that suggest that such interactions are important. When predators are absent, guppies become abundant and certain types of resources, like the invertebrates that guppies feed on, become rare. The individual growth rates of guppies are slower, and in other ways it appears that they have less to eat. Some features of guppy adaptations to low-predation environments suggest that they are also adapting to the way they have changed the environment in the absence of predators. This potential interaction between ecology and evolution represents our current, unfinished research.
There is good cause to think that what we are studying in guppies might be applicable to a diversity of organisms and in a diversity of climates. The study of ecology is replete with examples of organisms that have a major impact on the structure of their ecosystem. Every time we see such examples, we should think about how such impacts cause natural selection and evolution, both of the organism in question and of all others that occupy the ecosystem. A recent perspective by Tom Schoener (2011) offers a good summary of progress in the empirical study of such interactions. A second, by James Estes and others (2011), presents one context, the recent elimination of apex consumers in many ecosystems, that dramatically illustrates how individual species can restructure their ecosystems and hence potentially change the kind of selection that they, and many other members of the ecosystem, experience (see chapter VI.16).
Life history evolution has found practical applications. One is in fisheries biology. When humans harvest fish, they function as predators of the largest-size classes of prey. The field of fisheries management has focused on the population biology of exploited fish populations (meaning their abundance and age structure), but has not traditionally considered whether humans are causing these populations to evolve. A likely response to such predation is the evolution of earlier maturity and smaller body size. Smaller sizes in turn mean having less to harvest as food. We are now seeing such changes in some fish species used as sources of food. The mind-set had originally been that the resources of the ocean are too vast to deplete. As it became obvious that they could be depleted, the perspective of fisheries managers shifted to thinking that they could be actively managed. If they were overharvested, then harvesting could be reduced, with the expectation that exploited populations would rebound to their former state. If, on the other hand, exploited populations of fish have evolved and if their ecosystems have changed in response to their changed abundance and life histories, there may be no easy return to what they were in the past. Fisheries managers are only beginning to integrate evolution and its implications into their thinking about managing the exploitation of natural populations.
More generally, as the study of life history evolution has matured, it has become more integrated into other aspects of the study of ecology and evolution.
FURTHER READING
Estes, J. A., J. Terborgh, et al. 2011. Trophic downgrading of Planet Earth. Science 333(6040): 301–306.
MacArthur, R. H., and E. O. Wilson. 1967. The Theory of Island Biogeography. Princeton, NJ: Princeton University Press.
Pianka, E. R. 1970. On r- and K-selection. American Naturalist 104: 592–597.
Peron, G., O. Giminez, A. Charmantier, J. M. Gaillard, and P. A. Crochet. 2010. Age at the onset of senesence in birds and mammals is predicted by early-life performance. Proceedings of the Royal Society B 277: 2849–2856.
Reznick, D. 2011. Guppies and the empirical study of adaptation. In J. B. Losos, ed., In the Light of Evolution. Greenwood Village, CO: Roberts & Company, 205–232.
Roff, D. A. 1992. The Evolution of Life Histories. New York: Chapman & Hall.
Schoener, T. W. 2011. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science 331(6016): 426–429.
Stearns, S. C. 1992. The Evolution of Life Histories. Oxford: Oxford University Press.