Evolution of the Ecological Niche
Robert D. Holt
OUTLINE
1. Natural history, niches, and evolution
2. What is an ecological “niche”?
3. Complexities in the niche concept
4. The issue of genetic variation in niches
5. Demographic constraints on niche evolution
6. Niches evolving in communities
Every species and clade has a niche characterizing the range of environments (including abiotic as well as biotic factors) within which it persists, and outside of which it goes extinct. The niche describes how an organism with a particular phenotype performs in its demography (birth and death rates) as a function of environmental conditions. Given genetic variation in these traits, niches can evolve, sometimes quite rapidly, but niches also can show surprising conservatism. To understand niche evolution, one must draw on and integrate many areas of knowledge, ranging from detailed mechanistic understanding of individual performance to the mapping of genes to phenotypes, from life histories, mating systems, and population dynamics to population genetics, community ecology, and the broad spatial and historical perspectives of landscape ecology, biogeography, and paleobiology. Understanding niche evolution and conservatism is important to many basic questions in evolution, ecology, and biogeography, and it is also highly germane to many crucial applied issues.
GLOSSARY
Allee Effect. A positive effect of increasing population size on population growth rate.
Asexual Reproduction. Reproduction by cloning (i.e., making offspring that are genetically identical to the parent).
Dispersal. Movement of individuals across space; immigration is dispersal into a site, emigration is movement away from a site.
Evolutionary Rescue. A population that is declining in numbers toward extinction because the environment has changed, or because it has colonized outside its niche, yet nonetheless persists (because natural selection increases mean fitness sufficiently rapidly to allow the population to rebound from low numbers) is said to have experienced evolutionary rescue.
Extinction. The event marked by the death of the last individual of a population, species, or larger clade.
Hutchinsonian Niche. The range of environmental conditions (both abiotic, such as temperature, and biotic, such as density of a predator) for which the intrinsic growth rate r of a population is positive. If one plots r as a response surface undulating over an abstract space, where the axes are environmental variables, the niche is defined by that subset of this variable space where r > 0.
Intrinsic Growth Rate, r. The difference between birth and death rates (per individual, per unit time) when a population is sufficiently scarce that one can ignore competition for resources, interference, and other density-dependent effects.
Source-Sink Dynamics. A mechanism for sustaining some populations of a species outside its Hutchinsonian niche. In a heterogeneous landscape, a source habitat is one with conditions inside a species’ niche, where a population persists. This population can export individuals that end up in a habitat with conditions outside the niche, and so maintain a sink population.
1. NATURAL HISTORY, NICHES, AND EVOLUTION
From the air, many landscapes in northern climes such as Yorkshire, England, display lovely mosaics of land and water, tapestries of green vegetation, moors and woodland dotted with seemingly endless small ponds and lakes, reflecting glacial molding of the earth’s surface during the Pleistocene. A naturalist out for a Sunday stroll to scan for an elegant but rare butterfly, the small pearl-bordered fritillary (Boloria selene)—rumored to occur in a grassland sprinkled with violets, the butterfly’s required host plant—might from the corner of one eye see a glint of blue as a kingfisher dives to nab a fish in a pond, even as she hears the call of a swift soaring overhead to catch aerial insects. Each species seems to have its place, or way of life. What all naturalists know in their bones is that the world is intrinsically a highly heterogeneous place, and that to find a particular species, one must seek out habitats that match its conditions for life. These specifications for what a species needs to persist—which can often be quite subtle—constitute its niche.
So species have discernible niches. Each species across all these distinct habitats perused by our Yorkshire naturalist had a common ancestor, possibly a very long time ago, that also had its own niche, and so the current niche differences among these taxa must have emerged during evolution. Like any trait, given genetic variation, the niche requirements of a species can evolve, but sometimes species or clades can be surprisingly constant in their niches and key organismal traits related to niches—a phenomenon called niche conservatism (Wiens et al. 2010). In this chapter, I first present some necessary ecological background, including an exposition of the basic concept of the niche, and a brief discussion of some subtleties in the concept. I then turn to the crucial issue of the existence of genetic variation in the niche—which is necessary to fuel niche evolution—and sketch how the demographic context of selection can sometimes constrain niche evolution. I touch on how the community context often modulates niche evolution, and conclude by suggesting that the theme of niche evolution and conservatism is central to a range of vitally important applied questions.
2. WHAT IS AN ECOLOGICAL “NICHE”?
To understand what governs niche evolution, or its absence, it is important to have a crisp understanding of what is meant by the term niche. The word has many overlapping meanings in ecology (see Schoener 2009). In everyday English, a “niche” refers to a recess in a wall (e.g., a place that might hold a statue), and so statements about niches seem to be statements about the environment. In ecological usage, however, the word refers more subtly to how organisms relate to the environment. Our focus here is on the basic idea first formalized by the renowned ecologist G. E. Hutchinson (in his 1957 essay “Concluding Remarks,” discussed in Hutchinson 1978). Hutchinson suggested that the environment in which an organism lives could be graphically represented in terms of a set of axes, defining, for instance, the ranges of conditions impinging on organismal function (e.g., temperature, pH, toxin concentration), or resource availability (e.g., algal food supply for a zooplankter), or the intensity of different mortality sources (e.g., the abundance of a predatory fish species). The crucial idea is that one considers not just individual survival but more abstractly the dynamics of populations or lineages of reproducing individuals, reflecting the outcome of survival and births over many generations, and how these dynamics depend on environmental conditions. We imagine that a genetically homogeneous group of a few individuals of a given species is introduced into a habitat with a certain set of environmental conditions (including abiotic factors such as temperature, as well as biotic factors such as food availability, abundance of predators, etc.). For simplicity, we assume the species is asexual (i.e., a clone), or at least that mates have no trouble finding each other (for sexual species), and that the environment is constant, so that the genotype of these introduced individuals corresponds to a particular phenotype. We then watch what happens.
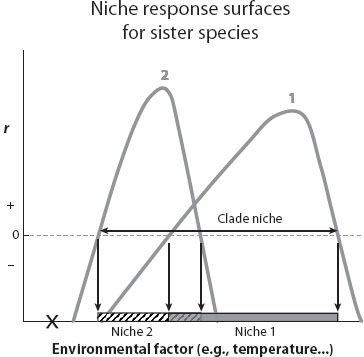
These individuals have an expected birth rate and death rate. The difference between birth and death rates is the net growth rate of the population, which for a population at low abundance is called its intrinsic rate of growth, denoted r. This concept is closely related to the population genetic concept of absolute fitness of a genotype. Because we are examining what is happening at low density, we assume that density-dependent effects such as crowding or competition for resources are negligible. If the intrinsic growth rate in a given habitat is positive, the population can grow in that environment; if negative, then without immigration or evolution, the population is doomed to extinction. If one now repeats this protocol across a large range of environmental conditions, and measures r for each, one builds up a profile, a kind of abstract landscape describing what is called the niche response surface for that particular genotype as a function of its environment (thereby making explicit the dependence of absolute fitness on the environment). Figure 1 shows a schematic example of niche response surfaces (which are curves in this case) for two related species, across a range of values of one environmental factor (e.g., temperature). The shape of the entire niche response surface is of ecological interest, and evolution can sculpt this shape. But a particularly important distinction is provided by the boundary in environment space separating zones of positive and negative population growth; this boundary defines the Hutchinsonian niche. This boundary cleaves the environmental states of the world into that set of conditions where a lineage goes extinct (r < 0, outside the niche), and another set where it potentially persists (r > 0, inside the niche). In figure 1, species 1 has a broader niche than does species 2, and the two species differ in the shape of their niche response and the environment in which growth is maximal. Some environments could potentially harbor both species (assuming they do not strongly compete), other environments just one. The niche of the clade spans more environmental space than any single member, but there are some environments (e.g., X in the figure) where neither species can persist.
Figure 1. Niche response surfaces along a single environmental niche axis, for two related species. A niche response surface is given by intrinsic growth rate, r, expressed as a function of an environmental variable (e.g., temperature). The Hutchinsonian niche of a species is defined by those environments where a species has a positive, rather than negative, growth rate. Species 1 is expected to go extinct if placed in environments outside the region shown in gray, and species 2 likewise perishes outside the hatched region. The niche of the clade is the union of these two niches. In a habitat with conditions X, neither species can persist.
The niche concept with minor modifications can pertain to individuals, or genotypes, or aggregates of individuals in populations, species, or broader phylogenetic clades. If conditions are outside the niche, individuals are not expected in the long run to have descendants, populations are not expected to persist, species will go extinct, and, finally, phylogenetic clades as a whole can disappear. Understanding niche limits can help explain why species’ borders occur where they do along gradients, or what determines the range of hosts that can sustain a parasite, or why phylogenetic clades disappear or proliferate in the fossil record.
To make this idea of the niche more concrete, let us return to our naturalist wandering over the Yorkshire landscape. Were she to dip a bucket in a pond and examine its contents under a microscope, the sample would teem with zooplankters, but with different types in different ponds. One small crustacean, the water flea (Daphnia magna) occupies some, but not all, water bodies (schematically depicted in figure 2A). English ecologists hypothesized that this distributional pattern could be explained by this species’ niche requirements and carried out lab experiments to test this idea. This species lives and breathes in water, so one niche axis—in versus out of the water—is so blatantly obvious, there is no need to quantify it. The ecologists surmised that more subtle aspects of water chemistry might explain why D. magna is absent from many lakes and ponds, even though it is present in others nearby. In particular, pH and calcium concentration should be key niche axes. Maintaining internal ionic balance is important for any organism, and pH influences that. Water fleas shed and replace their exoskeletons at each molt, and so require calcium. Conveniently, the daphnid grows asexually, so a clone was brought into the lab, and replicated into many copies. Small populations were then introduced into containers with different water chemistries and tracked, permitting the genotype’s intrinsic growth rate to be assessed across a wide range of combinations of pH and calcium availability (figure 2B).
Figure 2. This figure idealizes the study of the ecological niche of Daphnia magna reported by Hooper et al. (2008). (A) A map of ponds and lakes in the Yorkshire landscape, some with the zooplankter, and some without. (B) A laboratory study of intrinsic growth rates r of a clone of this zooplankter, grown with abundant food and no other species, as a function of pH and calcium concentration. The contours can be viewed as heights on a “mountain” emerging from the page, describing the growth rate of the daphnid, its niche response surface (to these variables). The Hutchinsonian niche consists of all those combinations of these abiotic variables where r > 0. (C) The water chemistries of each lake and pond in A, plotted in this same abstract environment space. In most cases, occupied ponds have conditions within the niche, and unoccupied ponds have conditions outside the niche; there are, however, a few intriguing exceptions (see text for more detail). (D) The long arrow describes a colonization event from an occupied pond with conditions inside the niche, to a pond with conditions well outside, where r ≪ 0. Evolution is unlikely, because extinction is rapid. If instead there can be a chain of colonization events, or spatially coupled sink populations, where at each shift a much smaller change in the niche is required for persistence (as indicated by the short arrow to just outside the niche, followed by short steps along a chain of habitats linked by thin lines), then gradually the lineage may evolve to include even radically different habitats in its niche. (Figures adapted from Holt and Barfield 2011.)
Almost without exception, water bodies where these abiotic factors predicted negative growth lacked the species (figure 2C); thus understanding abiotic niche requirements by using just two abiotic variables has strong explanatory power for interpreting this species’ distribution in Yorkshire. But the niche boundary in this two-dimensional space does not quite explain everything about the species’ distribution. Some sites seem suitable, yet lack the species. Maybe other unmeasured niche dimensions (e.g., the presence or absence of a voracious predator) explain these absences. Alternatively, r may be positive but low, making recovery from chance disturbances less likely. Finally, some ponds may simply be hard to reach or newly formed, and so not occupied because of the chance vicissitudes of colonization. Intriguingly, and conversely, a few sites have conditions a little outside the niche, but do have the species. One plausible explanation is that regular immigration from suitable sites (“sources”) can sustain populations in what is called a “sink” habitat, where conditions are outside the niche. Another possibility comes from the fact that the niche was quantified for just a single clone, yet Daphnia magna harbors considerable genetic variation. Maybe some genetic variants have niche requirements differing from the measured clone. Despite this possibility, it is clear that to an excellent approximation, the pH and calcium requirements describing niche limits of this clone also must describe the niches of a much wider array of genetic types, providing a plausible example of niche conservatism in a clade.
This experimental study helps define this species’ niche in the Yorkshire landscape but does not elucidate those aspects of organismal function that actually account for its niche response structure. A full understanding of the latter requires one to delve deeply into the rich mechanistic details of organismal biology, including physiology, morphology, behavior, and life history; each species’ story is likely to have some unique aspect that must be unraveled to really understand its niche. In effect, a full portrayal of niche evolution and conservatism requires a detailed understanding of the natural history and organismal biology (in its fullest sense) of each species. Covering this rich body of literature is beyond a short article—to do full justice to the theme would be like writing an advanced general biology text! Instead, the remainder of this chapter highlights general conceptual issues, illustrated by examples, which almost always arise when contemplating the evolution of species’ niches.
3. COMPLEXITIES IN THE NICHE CONCEPT
There are other dimensions of the niche concept that are important in ecology and evolution, which we barely touch on here. Direct measurements of a species’ niche are difficult, and in practice, ecologists often attempt to indirectly quantify the niche by characterizing patterns of resource use (see Schoener 2009). For instance, for an insectivorous lizard species, instead of plotting population growth rate against an environmental variable, as in figure 1, one might plot frequency of consumption of insects as a function of insect size, scaled against insect availability at each size.
The resource utilization niche concept has been particularly important in grappling with the problem of understanding the degree to which two species can be similar and still coexist. Species do not live alone, but instead are found in communities of interacting species. When species compete for resources or otherwise interfere, it can be a challenge to understand their coexistence. Indeed, in laboratory settings where pairs of related protozoa are put together, usually just one species dominates and persists, forcing the other to extinction. We will return briefly to this important issue at the end of this chapter.
Moreover, and crucially, understanding species coexistence requires analysis not just of what species need and can tolerate (as in the Hutchinsonian niche) but also of how they impact their environments via depletion of resources, or augmentation of natural enemies, or even alterations in physical or chemical conditions (what is called “niche construction”). This impact dimension of the niche (Chase and Leibold 2003) depends not just on the species but also on the feedback mechanisms present in the environment itself. As species evolve, these feedbacks may themselves change, altering conditions for persistence and coexistence. Even defining the “environment” can be quite tricky, since organisms can move to select their habitats and otherwise affect their living conditions. The environment in which organic evolution unfolds is itself in part determined by evolutionary processes.
Sometimes the growth rate of a species when rare can be boosted by an increase in its abundance, via what are called Allee effects; for instance, reproduction may be facilitated because mates can more easily find each other when the species is more common, or deaths may be reduced because there is protection in numbers against predation. Because of Allee effects, a species if sufficiently abundant may be able to persist in some environments where it cannot increase when initially rare (i.e., r < 0); the population “persistence niche” may exceed the population “establishment niche.”
4. THE ISSUE OF GENETIC VARIATION IN NICHES
Leaving aside such complexities, we return to the question of why the zooplankter does not inhabit a wider range of environmental conditions. The Hutchinsonian niche is a kind of abstract landscape (as in figures 1 and 2B), describing how absolute fitness (intrinsic growth rate) varies for a genotype (or species or lineage) over an abstract environmental space. To understand how niches evolve (and when they might not), it turns out one needs to think about two other kinds of landscapes (one abstract, one not), as well. Consider a thought experiment for Daphnia magna in Yorkshire. A waterspout sucks an aliquot of a daphnid from an established population and plops it into a pond, with conditions outside the niche of the source population, so the average growth rate of the colonizing population is negative. Without genetic variation, the colonizing clone simply goes extinct.
But given appropriate genetic variation in the source population, or if by chance a favorable mutation arises in the introduced population, evolution may occur that allows the population to persist and become established—and the niche of the clade will have expanded. Evolution by natural selection arises from variation in relative fitnesses among individuals (with a genetic basis) and can lead to evolutionary rescue of a population placed outside its niche (Gomulkiewicz and Holt 1995). A second landscape metaphor often usefully describes selection. The “adaptive landscape” portrays fitness as a function of genotype or a phenotypic measure (such as body size or temperature tolerance) in a given environment (including biotic interactions within and among species). In some (not all) cases, evolution can be described by a hill-climbing metaphor, where selection among alternative genotypes moves a population toward a local optimal fitness. This metaphor breaks down when individuals interact, such that fitness depends strongly on relative frequency; in this case, in effect the hill itself undulates as evolution occurs. But if a population is outside its niche, in general its numbers will be declining, and at low densities, so individuals may not encounter each other very often. This makes frequency dependence in selection less likely, and the adaptive landscape metaphor becomes a reasonably accurate characterization of the way selection occurs. As Charles Darwin noted in On the Origin of Species, reflecting on the struggle for existence, “a plant on the edge of a desert is said to struggle for life against the drought”; if fitness is determined largely by the ways in which individuals cope with physical and chemical conditions (i.e., the external environment), selection will straightforwardly favor whichever phenotype best tolerates these abiotic factors. The adaptive landscape describes how variation in phenotypes translates into variation in fitness in a given environment, and hence in the strength and direction of selection. One of the near-magical features of Darwinian evolution is that the effects on genetic composition of populations of even small differences in fitness cumulate and become amplified over time, leading to dramatic transformation within and among populations.
If niches are to evolve, there must be genetic variation among individuals in their phenotypes, leading to a heritable basis for variation in fitness as a function of the environment (i.e., in the niche). This issue requires much more empirical study and has not been addressed in detail in many species; nonetheless, there are some clear examples. At the level of entire species, there is considerable evidence for genetic variation among populations in climatic tolerances (Hoffmann and Sgrò 2011), implying intraspecific variation in ecological niches (see chapter IV.3). For instance, the Canadian tiger swallowtail (Papilio canadensis) ranges from Michigan to Alaska. Laboratory experiments suggest that Michigan caterpillars are so intolerant of many Alaskan summer temperatures that were one to move a population from Michigan to Alaska, it would go extinct. The ecological niche of the entire species is thus larger than that of local populations. In forestry, there are economic incentives to plant seedlings that will successfully mature into adult, log-worthy trees; thus many transplant studies have been carried out. The lodgepole pine, Pinus contorta, extends from Colorado to the Yukon. Seedlings often cannot grow and survive when planted at locations across the species’ range where thermal conditions are either much warmer or much cooler than their natal habitats. Though the physiological mechanism is not understood, this again suggests the existence of considerable geographic variation in the ecological niche of the lodgepole pine. Were a devastating blight to sweep across the range of the species and lead to mass local extinctions, leaving one remnant population behind, these experiments suggest one could not quickly restore the original range of the lodgepole, using individuals drawn from that sole surviving population.
The ultimate source of genetic variation that can permit niche evolution is of course mutation. Experiments probing the niche limits of clonal organisms have shown that when large populations are placed outside their niches (e.g., thermal tolerance zones for E. coli, salt concentrations for yeast), typically they go extinct, but very occasionally novel “Lazarus mutations” arise that can rescue these populations from extinction (see chapter III.6). Quantitative traits in sexual species can be under stabilizing selection, yet the species can maintain a pool of heritable variation in those traits because of recurrent mutation. This pool can provide the raw material to fuel niche evolution. Laboratory selection experiments on Drosophila (fruit flies) reveal that there can be substantial standing genetic variation permitting evolution of some niche traits; basically, conditions that are stressful for most individuals in the population may not be stressful for all.
Genetic variation in traits influencing the niche within species thus surely occurs, permitting species to be selected for increased fitness when absolute fitness is low (i.e., when conditions are outside the niche). But there is also increasing evidence that such variation may be lacking for crucial characters, leading to one explanation for niche conservatism for at least some species, along some niche axes (see chapter III.8). For instance, desiccation resistance and upper thermal limits can have little or no genetic variation in Drosophila populations. Plant species may be missing from soils with heavy concentrations of toxic metals, even though they reside in other habitats nearby, because they have no discernible genetic variation for resistance to those toxic conditions. Such examples are contrary to the conventional wisdom that genetic variation is ubiquitous for almost any trait and allows evolutionary responses to almost any selective pressure (Futuyma 2010). Even with genetic variation in single traits affecting the niche, genetic correlations among traits may constrain selective responses and hamper niche evolution (see chapter III.8). Leaving aside such genetic explanations for niche conservatism, ecological factors can also at times constrain niche evolution.
5. DEMOGRAPHIC CONSTRAINTS ON NICHE EVOLUTION
The third conceptual landscape pertinent to niche evolution is the “real” landscape, describing the ways in which environments as experienced by a lineage are structured over space and time. If lakes differing substantially in abiotic conditions are closely juxtaposed, our colonizing population of zooplankters is likely to end up in a lake with conditions well beyond its ancestral niche boundary (like the long arrow in figure 2D). Thus, its initial rate of decline will be large, rapidly reducing populations to low numbers and extinction. Theoretical studies suggest that the harsher the environment faced in colonization (as measured by the rate of decline in numbers), the less likely one will observe adaptation rather than extinction. If the geometry of the landscape is such that colonization is sporadic, and into habitats to which a species is so poorly adapted that the habitats lie well outside the niche, one expects evolutionary stasis even over long time horizons.
Reasons for Failed Adaptation in Colonization outside the Niche
Failed invasion outside the niche can reflect both the scarcity of appropriate genetic variation and demographic constraints operating outside the niche. If the colonizing population is initially genetically homogeneous, the potential for adaptation and persistence rests entirely on novel genetic variation, created by mutation—otherwise, the population is doomed. The likelihood of such mutations arising depends on the number of replication events that occur before a population goes extinct. If a population is plummeting rapidly to extinction, there will be scant opportunity for favorable mutations to arise; moreover, mutations of small positive effect on fitness (which arguably are more common than mutations of large effect) may not suffice. By assumption, outside the niche, r < 0. For a mutation to be favored by selection, it must have an effect δ > 0 on fitness (i.e., per capita growth rate) giving the mutant a higher relative fitness. But will the mutation be captured by evolution? Maybe not! The absolute growth rate of this mutant is r + δ. If r is negative, and δ is very small, then the net growth of the mutant type, r + δ, will still be negative (i.e., deaths of individuals carrying the mutation will exceed their births), and the lineage generated by the mutation will go extinct (along with the rest of the population). The harsher the environment (i.e., the lower r is), the larger the effect of mutation on fitness must be (i.e., the larger δ must be) for the new mutation to have any chance to persist. If most genetic variants that arise in the colonizing population have a small effect on the phenotype (and thus fitness), most will not lead to persistence. Mathematical models that take into account the inherent stochasticity of mutation and the chance vicissitudes of small population sizes have rigorously shown that the initial step of adaptation in a population suddenly exposed to an unfavorable environment (as can occur during colonization) requires mutations of large positive effect on fitness, and extinction may simply overwhelm the scope for adaptive evolution if such mutations rarely occur.
A comparable argument holds if adaptation depends not on novel variation but instead on variation sampled from a genetically variable source. For an introduction of an asexual species into a habitat to succeed, some individuals in the initial pulse of colonists must have a heritable positive growth rate, even though the average growth rate is negative. Figure 3 schematically shows what is needed. We imagine clonal genetic variation to be present among the colonists, expressed as variation in intrinsic growth rates among individuals in the colonized habitat. The left hump shows a population placed into a quite harsh environment; the right hump describes the same population in a less harsh environment. Both populations have equivalent levels of genetic variation in growth rates (the width of the curves is equivalent); however, in the harsh environment, note that no clones have a positive growth rate, so the population is doomed (without novel, highly favorable mutations). In the less harsh environment, a small number of individuals have a positive growth rate, so there is a chance the population will persist.
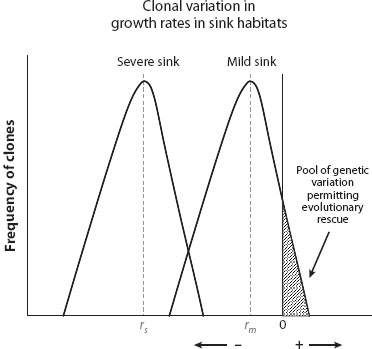
Figure 3. A demographic constraint on evolution during colonization outside the niche. We imagine that a group of colonists of an asexual (clonal) species has been taken from one habitat (a source), and placed in another (a sink). There is genetic variation among clones in their growth rates, so selection will occur. The humped curves depict heritable variation among individuals in their expected growth rates for two possible sinks; the curve on the left is for a severe sink, so the average growth rate is very negative, whereas the curve on the right is for a mild sink, where the average growth rate is only slightly negative. The amount of genetic variation in the growth rate of the colonizing population in each sink habitat is similar (as expressed by the width of these curves); however, for the population to persist without new mutations, some of these variants must have a positive growth rate, so what matters is not so much the mean growth rate but the tail of the distribution that exceeds zero growth. In the severe sink, no genetic variants have a positive growth rate, so extinction is assured. In the mild sink, some genetic variants have a positive growth rate, so there is at least a fighting chance of persistence.
The latter could describe colonization into a habitat only slightly outside the ancestral niche (as in the short arrow of figure 2D); adaptation and thus niche evolution would probably be more likely than they would be for colonization into a sharply different habitat (as in the long arrow of figure 2D). In the former case, the probability of some colonists having a positive growth rate is much higher. Also, with mutations arising in the sink, selection may be able to sort among a larger supply of mutations with rather modest effects on the phenotype and fitness, since only a small change in fitness might permit a positive growth rate in the novel habitat. If the structure of the environment experienced by an evolving lineage consists of gradual transitions between environmental states, rather than abrupt disjunctions, adaptation thus may be more likely to occur, and niches in a phylogenetic lineage will be evolutionarily labile, rather than conserved. Quite similar reasoning pertains to environments varying in time rather than space. Abrupt temporal changes in the environment that greatly lower fitness usually lead to extinction, rather than adaptation; however, if the same change occurs, but spread out in time rather than in a steep step, species may be able to adapt and persist, with an evolving niche tracking small environmental changes.
Extending these arguments, one can reason that the potential supply of favorable variation should increase with the initial number of colonists; larger numbers mean a more generous sample of preexisting variation found in the source, and they also provide a greater opportunity for novel mutations to arise in the sink as the population takes longer to decline to extinction from higher numbers. Experimental studies of adaptive evolution in harsh environments have shown exactly this predicted effect (see chapter III.6). For instance, in lab experiments, a sink habitat was created for an asexual species of yeast by adding salt to growth media. Most of the experimental introductions into the sink habitat went extinct, but some persisted and eventually grew as a result of adaptive evolution. The likelihood of persistence increased with the initial number of colonists, consistent with theoretical expectations. In the absence of absolute constraints on variation, niche evolution can certainly occur during colonization well outside the niche, but it may require a very large number of individuals in each colonization episode.
Reasons for Evolutionary Stasis in Sink Populations Maintained by Immigration
Returning to our spatial scenario, now imagine that some ponds in the Yorkshire landscape are connected by streams, permitting recurrent dispersal. Such recurrent immigration can sustain sink populations in environments outside their niches. This raises the issue of the interplay of gene flow and selection in determining local adaptation. The story is richly complex (Holt and Barfield 2011), but a few highlights are worth noting (see chapter IV.3).
First consider asexual species. The simplest effect is that immigration sustains the population in the first place, ensuring recurrent opportunities for adaptation to occur. If the spatial coupling of the habitats permits many separate attempts at colonization, one eventually might be successful. In general, the length of time required before recurrent immigration by a species succeeds in its adapting and persisting outside the niche increases with increasing harshness of the sink habitat, and with reduced numbers of individuals per colonizing episode. Even rare events, such as the appearance of a mutation with a large positive effect on fitness, are likely if one waits long enough. If density dependence is weak, an increase in immigration (as measured by the number of individuals arriving per colonization bout) can facilitate adaptation. Increased immigration enhances sampling of genetic variation from the source and also boosts local numbers, increasing the potential for local mutational inputs of variation. For a given rate of immigration, by contrast, increasing harshness in the sink (i.e., increasing difference in the environment between the source and sink) makes it harder for adaptation to occur; this reduces local population size, shrinking variation, and also makes mutations with small effect on fitness less likely to be captured by selection (as argued above for single colonization bouts). Lab experiments with the bacterium Pseudomonas have demonstrated these effects, using antibiotics to create sink habitats; a single antibiotic made a mild sink, and a cocktail of antibiotics generated a harsh sink. In both cases, increased immigration increased the rate at which the population adapted to the local environment. Moreover, the likelihood of adaptation was reduced when the sink environment was harsher (and adaptation was not seen at all over the timescale of the experiment with low immigration into the harsh sink). Both effects match theoretical expectations.
A quite different effect can arise when one considers sexual species. Given recurrent immigration, if immigrants mate with residents during each generation before selection occurs, a “migrational load” arises, diluting the effectiveness of selection. The reason is that immigrants tend to carry alleles that are maladaptive in the sink; when mixed with better-adapted alleles of residents, these lower the fitness of the offspring of resident individuals who mate with these immigrants. This is particularly likely to occur when immigration occurs into environments strongly differing from the source. In this case, resident numbers may be low, so most residents may mate with immigrants, rather than each other, and the genes flowing into the sink are likely quite maladapted there. The negative effect of gene flow on adaptation in marginal populations, leading to constraints on niche evolution within a species, is theoretically very plausible, but robust examples have been surprisingly hard to demonstrate. Douglas Futuyma (2010) has argued that speciation (defined by reproductive isolation between lineages) is crucial in diversification because it permits local adaptations to be captured by a lineage rather than washed away by gene flow. Speciation can potentially facilitate niche evolution and diversification in a clade, but note that the genes permitting persistence in a local environment must already be present—or the reproductively isolated population will simply disappear! This negative effect of immigration on niche evolution as a result of gene flow constraining local selection is also more likely for some life histories than others. If selection in the sink occurs immediately on immigrants, before they have a chance to mate with residents, then the migrational load imposed on local adaptation by immigration is weakened; the only migrants left will be those that by chance have higher fitnesses locally, and immigration, by boosting genetic variation, should facilitate niche evolution.
Another effect arises when reciprocal movement between source and sink habitats occurs, rather than one-way migration from the source to the sink (as assumed above). In this case, to understand evolution in the niche, one has to grasp that selection in effect averages over all the environments experienced by a lineage, but with differential weightings for different conditions. Because there may be few individuals in the sink, and they have low reproductive value there, selection tends to be automatically weighted toward conditions in the source (in effect “success breeds success”). If there is a trade-off between performance in the sink and that in the source, selection tends to favor the latter. Trade-offs are often simply assumed by evolutionary ecologists, but in practice they have been rather hard to definitively demonstrate. Trade-offs between fitness in habitats within and outside the initial niche tend to produce niche conservatism, particularly given large differences between source and sink. An alternative genetic mechanism for niche conservatism involves deleterious mutations. If a species for whatever reason is abundant in one habitat, and scarce in another, it may lose its ability to utilize the latter, not because of trade-offs in performance, but because selection is ineffective at weeding out deleterious mutations that degrade performance there (see chapter III.8; Holt 1996).
In some circumstances, however, these demographic and genetic constraints can be overcome, and niche evolution will occur. This can at times be dramatic, as in the adaptive radiations found on many oceanic islands. Analyses of the scenarios discussed above help identify circumstances for which niche evolution may be quite rapid. For instance, in source-sink environments, if dispersal is high into the sink, many individuals are forced to experience sink conditions, thus automatically increasing the “weighting” that selection provides such habitats, relative to sources. Transient periods when conditions are favorable in the sink (e.g., because competitors are absent) can also facilitate adaptation to it. Factors that go beyond these demographic models can make niche evolution more likely. For instance, individuals may have plastic responses, permitting them to shift their phenotypes so as to boost fitness in the sink environment. This dampens the rate of decline in the population and can permit adaptation to occur using even genetic variants of modest effect. Some species do seem to have abundant genetic variation that can respond to novel conditions (Hoffmann and Sgrò 2011), and in some cases, stress itself can pump up mutation rates or break down the developmental stabilization (canalization) of characters, which might provide variation for niche evolution.
6. NICHES EVOLVING IN COMMUNITIES
We have focused on how a single species evolves (or not) in a fixed environmental template. But as has been known since the time of Darwin, interactions among species crucially modulate the opportunity for niche evolution. The reason a habitat is a sink for a particular species may be that a superior competitor or voracious predator resides there, keeping r negative. Remove that other species, and the colonizing species may persist; then adaptation to local abiotic conditions can leisurely occur. This scenario helps explain the explosive evolution of adaptive radiations on islands, and can also account for rapid evolution in invasive species occupying novel environments. The spotted knapweed (Centaurea maculosa), for instance, was successfully introduced into North American environments at sites where the climate matches that found in its ancestral Eurasian range, but it then rapidly evolved the ability to live in different climatic regimes as it moved through the anthropogenically disturbed landscapes of the West, where disturbance had removed or weakened potential native competitors. Such bouts of rapid evolution ultimately slow, often as a result of intensifying interspecific interactions. Interspecific interactions may be highly significant in governing the likelihood of niche conservatism, versus rapid evolution. As a metaphor for this, consider dancers entering an empty dance floor. At first, some dancers (wallflowers) may stay put because of mysterious internal constraints, but other dancers wander widely and quickly across the entire floor. But as more and more dancers enter the room, it gets harder to move, because the space is preempted. Eventually, in a really crowded room, even though everyone continues to jostle and move locally in time to the music, no one really gets anywhere very fast.
One of the grand themes in the dance of life is a comparable patterning of movement in evolving and diversifying clades, measured against the spatially and temporally shifting template of environmental opportunities we call niche space. Understanding the determinants of the moves and halts in this dance—niche evolution and conservatism—is a crucial dimension of basic evolutionary biology, ranging from adaptive radiations, to biogeographical limits of species ranges, to understanding how ecological communities are structured. It is also increasingly a crucial dimension of applied evolutionary biology, for instance, in understanding species invasions and impacts of climate change (Hoffmann and Sgrò 2011), mitigating the risks of extinction of endangered species, or analyzing the conditions for disease emergence and the evolution of antibiotic resistance. There is the potential for creative application of many of these ideas to urgent applied questions. Consider conservation of an endangered species, which is declining because of environmental change. One hopeful message of models of niche conservatism and evolution is that in altered environments, anything that can be done that can improve the demographic performance of a population—even though the feasible measures that can be applied on their own cannot save the population—can indirectly make it more likely that evolution can help rescue it from extinction. Conversely, an understanding of these issues can help craft management strategies to prevent unwanted niche evolution, such as the evolution of resistance by microbes to antibiotics, or of agricultural pests to control measures. The central unifying theme of niche evolution and conservatism in ecology and evolutionary biology is one that cries out for a much deeper understanding, both empirically and theoretically.
Chase, J. M., and M. A. Leibold. 2003. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: University of Chicago Press. An accessible text treating ecological perspectives of both the Hutchinsonian niche and the impact dimension of the niche.
Futuyma, D. J. 2010. Evolutionary constraint and ecological consequences. Evolution 64: 1865–1884. An overview of how evolution can often be constrained, and how this sheds new light on a range of classical issues in ecology and evolutionary biology.
Gomulkiewicz, R., and R. D. Holt. 1995. When does evolution by natural selection prevent extinction? Evolution 49: 201–207. A characterization of the requirements for evolution by natural selection to act sufficiently strongly and rapidly to permit a species to persist in a novel, harsh environment.
Hoffmann, A. A., and C. M. Sgrò. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. A review of examples and theory related to rapid evolution in novel environments.
Holt, R. D. 1996. Demographic constraints in evolution: Towards unifying the evolutionary theories of senescence and niche conservatism. Evolutionary Ecology 10: 1–11. An exploration of alternative genetic causes for niche conservatism in source-sink environments, such as trade-offs and deleterious mutational loads.
Holt, R. D., and M. Barfield. 2011. Theoretical perspectives on the statics and dynamics of species’ borders in patchy environments. American Naturalist 178: S6–S25.
Hooper, H. L., R. Connon, A. Callaghan, G. Fryer, S. Yarwood-Buchanan, J. Biggs, S. J. Maund, T. H. Hutchinson, and R. M. Sibly. 2008. The ecological niche of Daphnia magna characterized using population growth rate. Ecology 89: 1015–1022. An excellent empirical study quantifying the ecological niche for a zooplankter.
Hutchinson, G. E. 1978. An Introduction to Population Ecology. New Haven, CT: Yale University Press. A classic text discussing the niche concept, with strong ties to community ecology.
Schoener, T. W. 2009. Ecological niche. In S. Levin, ed., The Princeton Guide to Ecology. Princeton, NJ: Princeton University Press.
Wiens, J. J., D. D. Ackerly, A. P. Allen, B. L. Anacker, L. B. Buckley, H. V. Cornell, E. I. Damschen, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13: 1310–1324. A synoptic review of niche conservatism, in terms of its existence, the mechanisms that explain it, and its implications.