Alternative Analgesics
Danielle Cosgrove didn’t think that the pain in her foot could get any worse. On holiday in Qatar in 2010, Cosgrove had rented an all-terrain vehicle (ATV) with a group of friends and ridden the sand dunes of the Arabian desert. It was a fun afternoon until disaster struck. The ATV rolled over, and Cosgrove’s left foot took the brunt of the impact: it was crushed, with skin shredded and bones sticking out. The ATV was not driveable, and the second-year university student was sprawled helpless in the baking desert sun for hours, waiting for help to arrive. When rescue crews finally brought her to the hospital eight hours later, she thought that she would finally get some relief from the unrelenting pain—but it just grew worse. Not even the strongest opiate medications on the market could take the edge off. In the weeks after her treatment, the pain spread beyond her foot and all over her body.
“It felt like my whole body had been dropped in a volcano,” she says. After her doctors had tried every possible therapy to no avail, Cosgrove was diagnosed with a rare, chronic neuro-autoimmune pain disease called complex regional pain syndrome (CRPS).
CRPS is described by those afflicted as the worst pain imaginable—more than three times the intensity of childbirth, as measured by the commonly used McGill Pain Questionnaire. A recent study found that nearly three-quarters of people with CRPS are at high risk for committing suicide from the intensity and chronicity of the pain1. Cosgrove herself experienced symptoms of depression, but she decided to try something different: the anesthetic-turned-party drug ketamine. Cosgrove says that it was the first medication that provided her with any kind of relief; pain that she had previously quantified as close to an ‘8’ or ‘9’ on a scale of 1–10 was now closer to a ‘4.’ In an experimental setting, ketamine has been used to treat everything from depression to migraines. Now, one of the most promising areas of low-dose ketamine treatment is for people such as Cosgrove, who have CRPS and have not responded to other forms of pain treatment.
CRPS is a rare condition that affects 26 out of every 100,000 people. By contrast, chronic neuropathy (pain that results from damage to peripheral nerves) affects up to 30 million people yearly in the US. The market for chronic-pain drugs in the US alone was estimated at more than $13 billion in 2014 and is only expected to rise. More than 240 million prescriptions for opioids were written in 2014, and yet a 2015 analysis revealed that little evidence existed to be able to determine whether these drugs were efficacious when used for longer than 12 weeks2. Moreover, opioids also carry a substantial risk of addiction and overdose: more than one-third of drug-overdose-related deaths reported in 2013 were a result of pharmaceutical opiates3.
Spurred by these statistics, researchers and pharmaceutical companies have renewed their interest in developing next-generation painkillers that move away from opiate pathways. Ketamine was one such drug that had shown early success at providing relief for patients with chronic pain. However, for CRPS, the drug must be delivered by intravenous (IV) infusion and comes with a litany of side effects, including hallucinations, agitation and nightmares. Cosgrove has gastrointestinal symptoms and profound mood shifts for a week after each treatment, which she receives roughly once every three months. These myriad side effects mean that ketamine, which has been approved by the US Food and Drug Administration (FDA) as an anesthetic, has yet to receive approval as a treatment for chronic pain or any other condition. Now, scientists in academia and industry are trying to develop new drugs that tap into ketamine’s pain-relieving benefits while avoiding its downsides.
Breaking the Cycle
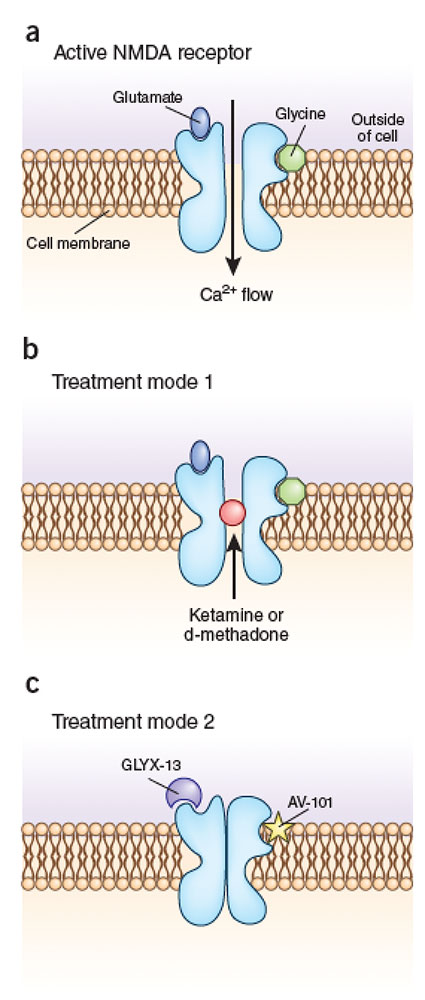
At the root of Cosgrove’s pain is an overactivated neural receptor called the N-methyl d-aspartate (NMDA) receptor. Ketamine works against the activity of this receptor, which is found in the membrane of neurons that affect learning, memory and pain in both the peripheral and central nervous systems. The receptor is activated when the amino acids glycine and glutamate bind to it simultaneously, which in turn opens an ion channel that enables the flow of calcium into the neuron. In pain-associated neurons, this calcium flow causes the nerve cells to send a barrage of pain signals to the brain.
Normally, glycine and glutamate dissociate themselves from NMDA receptors after the ion channel is opened, an action that inactivates the receptor. But this doesn’t seem to happen in people with chronic pain, and researchers still aren’t sure why. Furthermore, researchers have found that more NMDA receptors seem to be activated in those with chronic pain. In 1991, scientists at University College London showed in a rat model that the proliferation of activated NMDA receptors on peripheral nerves causes those motor neurons to fire even without a stimulus, and others have also since found this to be the case4. The result is a vicious cycle that is hard to turn off: more firing of neurons leads to greater proliferation of NMDA receptors.
Researchers hope that inhibiting the activation of NMDA receptor will interrupt the sequence of events. An early study testing Merck’s NMDA-receptor-blocking compound, called MK-801 or dizocilpine, found that the drug prevented the development of chronic pain in rats with a nerve injury5. The results of this early study showed that scientists were on the right track by setting their sights on NMDA-receptor antagonists, although this particular drug was never used clinically because subsequent animal trials showed that it could produce lesions in the brain. Another study found that injections of a different NMDA receptor antagonist, known as memantine, reduced chronic pain for up to two weeks in rats that had damage to the sciatic nerve6. By 2001, blocking NMDA receptors became a leading contender among approaches for the development of nonopioid painkillers.
“We keep getting hints that we’re almost there. We’ve really begun to tease out the role that the NMDA receptor plays in pain and other functions,” says Tony Yaksh, a researcher at the University of California, San Diego, who is studying ketamine’s pain-mitigating effects.
Ketamine works to switch off the receptor by sitting in the middle of the ion channel, similarly to a cork in a bottle. Ketamine also stays in the channel for a long time, which might explain why ketamine has so many side effects. Memantine, an NMDA receptor antagonist developed as an anti-Alzheimer’s drug by the German pharmaceutical company Merz, also sits in the receptor’s ion channel, but it dissociates rapidly and so its options for chronic-pain treatment are limited. Researchers needed to find a middle ground—a chemical that remained in the ion channel long enough to provide pain relief, but not so long that it induced major side effects.
“It was the classic Goldilocks dilemma—ketamine bound too long, memantine not enough. We needed something that was just right,” says Richard Mangano, chief scientific officer at Relmada Therapeutics, a company in New York City that is developing NMDA receptor antagonists for major depression and chronic pain. Their solution was d-methadone.
Breaking the chronic-pain cycle: (a) Individuals with chronic pain have increased numbers of the NMDA receptor in their pain-sensing neurons. In an active receptor, glycine and glutamate each bind to the receptor to open a central ion channel, which causes these neurons to fire and send a series of pain signals to the brain. (b) One mode of treatment, used by ketamine and d-methadone, works by corking the channel to prevent the flow of calcium and therefore the flow of pain signals. (c) Other drugs, such as GLYX-13 and AV-101, work by preventing the binding of glutamate or glycine, respectively, to the receptor to prevent the ion channel from opening.
Credit: Illustration by Marina Corral Spence/Springer Nature
Treatment Options
The version of methadone given currently to people with a heroin addiction is related to Relmada’s compound, but is structurally and functionally different. Whereas conventional methadone blocks the activity of the brain’s pain-sensing opioid receptors, d-methadone has essentially no opioid activity. Instead, d-methadone works by binding to the NMDA receptor. Similarly to ketamine, d-methadone sits in the middle of the ion channel, thus blocking the passage of calcium ions and decreasing the likelihood that pain-sensing neurons will fire. Unlike ketamine, however, d-methadone does not seem to stay bound for a long time, yet it surpasses the binding time of memantine. The company completed a multiple-ascending-dose trial a year ago, which identified a dosage range for the medication and determined that the drug was safe for healthy individuals.
The demand for fast-acting antidepressants, however, has caused some companies to re-evaluate their research on NMDA-receptor antagonists. Relmada told Nature Medicine that it has identified a greater market demand for drugs that can treat depression—another area in which researchers see a role for NMDA receptor antagonists. Last year, the company switched its focus from exploring d-methadone’s use for chronic pain to its use for major depression. Relmada intends to return to the pain trials after it has completed its depression studies, according to Mangano.
In a departure from Relmada’s strategy of blocking the ion channel in the NMDA receptor, VistaGen Therapeutics, a small biopharmaceutical company in San Francisco, has developed a drug called AV-101 that prevents glycine from binding to the NMDA receptor and so prevents the ion channel from opening. Unlike ketamine, AV-101 does not cause hallucinations or agitation, and it can be given in pill form. A phase 1 safety trial in healthy individuals, conducted in 2012, showed that the drug is safe. Participants also reported improved mood, which led VistaGen to begin investigating the drug as a potential antidepressant and to temporarily halt its exploration of the drug as a chronic-pain treatment.
VistaGen is not the first company to develop a compound that blocks glycine from attaching to the NMDA receptor. GlaxoSmithKline, for instance, had success with a similar therapeutic in rat models, but human trials for neuropathic pain failed to show any benefit from the drug. In one study, of 63 patients enrolled in the trial, neither the placebo group nor the group receiving the experimental drug GV196771 reported any difference in pain levels7.
Similarly to Relmada, VistaGen has shifted its NMDA research away from chronic pain, says Mark Smith, chief medical officer at VistaGen. “We’re all definitely still interested,” he adds, but the potential payouts from the development of a rapid antidepressant have caused the company to put the development of drugs for pain on the back burner for now.
Spinning Off
Not everyone has been lured away from the chronic-pain field, however. Northwestern University neurobiologist Joe Moskal founded Naurex, a company that developed an NMDA receptor modifier known as GLYX-13 for the treatment of depression. Naurex was bought in July 2015 by Allergan for $560 million to further develop the product, now called rapastinel, but Moskal is continuing to work on the drug. After founding another company called Aptinyx, Moskal is using GLYX-13 as a scaffold “to build fully synthetic molecules that are orally deliverable for use in chronic pain,” he says. The GLYX-13-based molecules that Aptinyx is building are currently under exploration in animal studies.
Jamie Sleigh, an anesthesiologist at the University of Auckland in New Zealand, says that although NMDA-receptor antagonists hold promise, pharma still has a long road ahead. Even though the drugs seem safe and show efficacy in animal models, the field is littered with similar drugs that ultimately failed in phase 2 and 3 trials. He also notes that chronic pain is a complex process, and so targeting the NMDA receptor alone might not be sufficient. “Chronic pain essentially rewires the brain and changes gene expression. It’s still not clear how to reverse that process, with ketamine or any other drug,” Sleigh says.
Cosgrove has been receiving ketamine infusions off and on for five years now, and is anxiously watching the field. “They’re coming out with new things all the time. Maybe the next drug will work even better than ketamine,” she says.
References
1. Lee, D.H. et al. Psychiatry Investig. 11, 32–38 (2014).
2. Chou, R. et al. Ann. Intern. Med. 162, 276–286 (2015).
3. Volkow N.D. & McLellan A.T. N. Engl. J. Med. 374, 1253–1263 (2016).
4. Woolf, C.J. & Thompson, S.W. Pain 44, 293–299 (1991).
5. Davar, G. et al. Brain Res. 553, 327–330 (1991).
6. Eisenberg, E., LaCross, S. & Strassman, A.M. Neurosci. Lett. 187, 17–20 (1995).
7. Wallace, M.S. et al. Neurology 59, 1694–1700 (2002).
--Originally published: Nature Medicine 23(1); 8-10 (January 2017). Reprinted with permission.