CHAPTER 3
Reproduction: securing the future
Summary
In this chapter vegetative and sexual reproduction are compared. Next, the structures of cones, flowers, seeds and fruits are described. The compositions of floral diagrams and floral formulae are described. The diversity of flower shapes and forms is discussed in terms of coevolution with pollinators. The diversity of fruit structures is related to seed dispersal.
Introduction
Although some plants may live for years, or even for millennia, all must reproduce to establish further generations or to spread to new habitats. Most plants are able to reproduce sexually by forming flowers and, following pollination, setting seed (see Chapter 10 and below). Some hybrids, however, have lost this ability and reproduce vegetatively (see Chapter 11 and below). And some species are able to reproduce both sexually and vegetatively, thus increasing their reproductive flexibility.
Vegetative reproduction
Vegetative reproduction is a by-product of the modular and indefinite growth of plants that allows them to respond to their environment by producing adventitious shoots or roots from vegetative (i.e. non-sexual) tissues. Some plants, however, cannot reproduce vegetatively in this way, in particular many mature trees, for reasons that are not fully understood.
Examples of vegetative reproduction include the formation of clumps of individuals from a proliferating crown, as with many herbaceous perennials such as lupin (Lupinus spp.). Similarly, some bulb-forming plants such as bluebell (Hyacinthoides non-scripta) may produce new bulbs from buds located between the bulb scales of the parent. Alternatively, branches may arch over and root, giving rise to new plants, as with bramble (Rubus spp.), or adventitious roots may form from partially buried or broken branches, as in willow (Salix spp.). In some species modified stems called runners (stolons) grow along the ground, producing plantlets at the nodes, as in the case of strawberry (Fragaria × ananassa). In others, such as Kalanchoe daigremontiana, plantlets may form at the edges of the leaves, subsequently falling to the ground and taking root, whereas in some hybrid grasses, such as viviparous fescues (Festuca spp.), plantlets may replace the sexual structures of the flower. Some trees, for example, certain poplar (Populus) and elm (Ulmus) species, sprout adventitious shoots from their roots to produce a thicket of stems; others, such as Tasmanian holly (Lomatia tasmanica) produce adventitious roots from lower branches in contact with the ground, again resulting in a thicket of stems. The success of vegetative proliferation of this kind in trees is demonstrated to a startling extent by some very long-lived specimens. A European example is the 9550-year-old, and still living, Norway spruce (Picea abies) found in Sweden. An even older tree, however, has been found in Australia, this being a specimen of Tasmanian holly at least 43,600 years old and possibly as much as 135,000 years old (roughly the same age as ‘mitochondrial Eve’, the most recent common female ancestor of all humans alive today).
Although there is a great diversity of mechanisms for vegetative reproduction in the plant kingdom, the feature they have in common is that they result in progeny that are clones (see Chapters 5 and 11), with all the individuals in a clone usually being genetically identical to the parent plant. Vegetative reproduction is thus harnessed by the gardener, as in taking cuttings or layering, to produce large numbers of uniform individuals, a topic that is explored in detail in Chapter 11.
Sexual reproduction
Sexual reproduction, unlike vegetative reproduction, involves the mixing of the genetic material from two different individuals, the parents, and thus generates diversity (see Chapter 5). Genetic diversity among progeny is important, not least because it increases the possibility of survival of at least some individuals of a species where the environment is subject to change. It also creates the relatively rare possibility of new combinations of genetic information among progeny that may make a species more successful in an existing environment, perhaps, for example, because it can compete better with its neighbours or reproduce more effectively. Such a species is said to be more fit. The reproductive advantage so acquired is the basis of evolutionary change, the reproductively fittest individuals in each generation having a competitive advantage over the less fit individuals of the same species. Plant breeders harness and speed up the evolutionary process by performing artificial crosses with selected individual plants, followed by further selection for desirable traits. Most of our garden plants were produced from wild plants in this way, a subject discussed in detail in Chapter 5. A practical advantage of sexual reproduction in flowering plants for the gardener is that it results in the formation of seeds, which provide a convenient and long-term means of storing species and cultivars.
Cones and flowers
The process of sexual reproduction in seed plants involves the formation of male and female sexual structures either in cones (Fig. 3.1), as in the gymnosperms (conifers and their relatives), or in flowers, as in angiosperms. In dicotyledons (Fig. 3.2) the flower bud is surrounded by protective modified leaves, called sepals, which are sometimes shed or shrivel after the flower opens. Next come the petals, usually the most conspicuous part of the flower. In monocots, where sepals are absent (Fig. 3.3), the petals are called perianth segments. Petal and perianth segment numbers vary with family and may be separate, as in buttercups (Ranunculus spp.), or fused to one another, as in bellflowers (Campanula spp.). The male structures, which come next, are the anthers in which the microspores (which become the pollen grains; see Chapter 10) are produced. Numbers of anthers vary with family, as with petals. They are usually borne on stalks called filaments. Pollen grains have thick, fatty walls and are often richly ornamented. They are carried by wind, insects and other arthropods, or occasionally other groups such as bats or birds, to the female organs, where pollination and fertilisation occur. In wind-pollinated plants such as grasses and conifers the flowers usually lack both colour and scent, but are borne in such a way on the plant as to maximise the likelihood of pollen being picked up by the wind and carried to a receptive female. Where arthropods or other animals are responsible for pollination, coevolution of the plant species and pollinators usually involves the production of sweet nectar or scent in special glands at the base of the petals, or particular flower colours, to attract the appropriate pollinating species (see Chapter 13). White flowers, which appear to lack colour, reflect UV light in different ways and may therefore appear as different colours to the compound eyes of insects that can detect wavelengths invisible to the human eye (see Chapter 13). Scent is especially important as an attractant in the case of night-flying pollinators such as moths or bats. The shape of the flower – radially symmetrical (actinomorphic; Fig. 3.4) or bilaterally symmetrical (zygomorphic; Fig. 3.5), with separate or fused petals – is also fine-tuned to the pollinator with which the species coevolved. Finally, in some groups such as members of the Euphorbiaceae, the petals are insignificant and a ring of modified, coloured leaves (bracts) around the flowers serves to attract pollinators.

Figure 3.1 Newly formed cones of Dunkeld larch (Larix × eurolepis). Photograph by John Ford.

Figure 3.2 A half flower of evening primrose (Oenothera glazioviana). Drawing by Anne L.D. Bebbington.
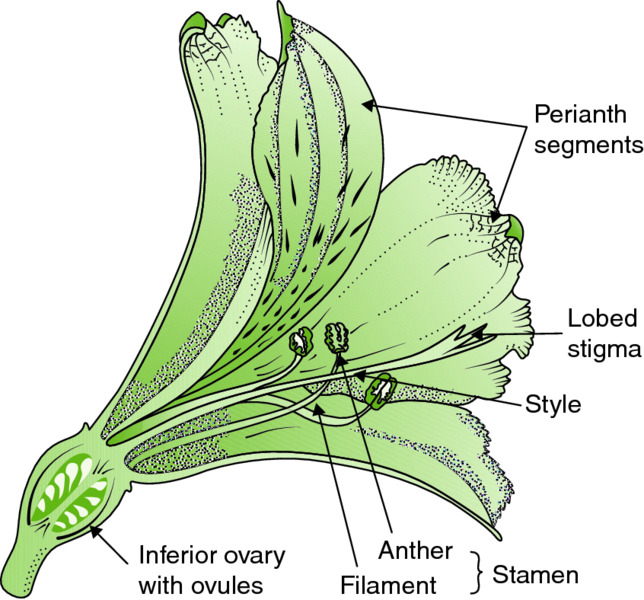
Figure 3.3 A half flower of a Peruvian lily (Alstroemeria sp.), a monocot. Note that in monocots there is only a single ring of petals (the perianth segments) with no outer ring of sepals, as there would be in a dicot flower. Note also that the ovary is inferior in flowers of the genus Alstroemeria (family Alstroemeriaceae). Drawing by Anne L.D. Bebbington.

Figure 3.4 Scan of a half flower of fuchsia (Fuchsia magellanica). This species has radially symmetrical (actinomorphic) flowers with four sepals (red), four petals (purple), two whorls of four stamens each, and an inferior ovary with a long style terminating in a four-lobed stigma. Scan by John Bebbington FRPS.

Figure 3.5 Scan of a half flower of snapdragon (Antirrhinum majus). This species has bilaterally symmetrical (zygomorphic) flowers with deeply five-lobed sepals and petals fused to form a corolla-tube. There are four anthers per flower, two pairs of different lengths, and the ovary is superior, terminating in a long, curved style with a pin-headed (capitate) stigma. Scan by John Bebbington FRPS.
The female structures (the ovules) of gymnosperms are unprotected by other tissues and the pollen reaches them direct, immediately followed by fertilisation. In the angiosperms the ovules are enclosed by an ovary, which may be above the ring of petals (superior, as in bellflowers, Campanula spp.; see also Fig. 3.6) or below it (inferior, as in courgette and marrow, Cucurbita pepo; see also Figs 3.2 and 3.4). The ovary possesses a stigma at one end, supported by a style, for reception of the pollen (Figs 3.2 and 3.3). The pollen grains germinate on the stigma surface to produce long pollen tubes, which grow through the surface and down through the tissues of the style, eventually reaching the ovaries, where fertilisation occurs, a process described in detail in Chapter 10.
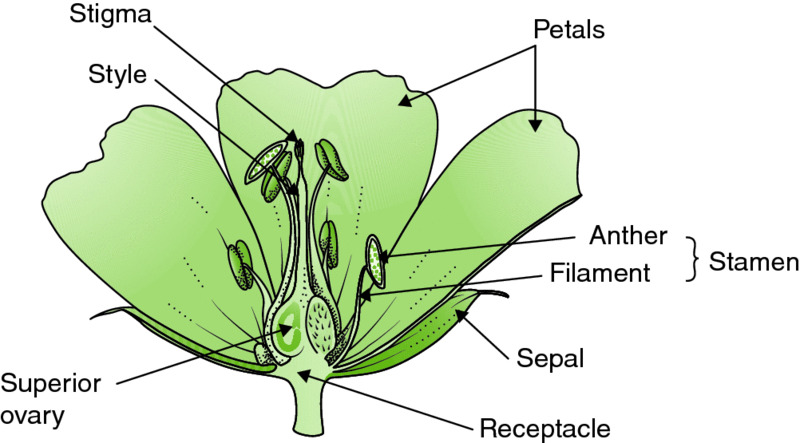
Figure 3.6 Half flower of Geranium ‘Rozanne’ to show the superior ovary. Drawing by Anne L.D. Bebbington.
Male and female organs in the gymnosperms are usually produced in separate, structurally different cones. It is easy to distinguish the soft, pollen-forming male ones from the much larger, woody female cones on pine trees (Pinus spp.). The male cones fall off the trees once the pollen has been released, whereas the female cones continue to grow and mature over a long period, sometimes several years. In the angiosperms the male and female organs may be formed in different flowers, as in hazel (Corylus spp.), or in the same flower, as in buttercup (Ranunculus spp.). However, most plants have inbuilt incompatibility mechanisms to prevent self-pollination, which would lead to inbreeding (see Chapter 5), or pollination by an unrelated species, which would produce an unfit hybrid. These mechanisms often rely on the recognition by the cells of the stigma of specific proteins on the surface of the pollen grains. Following recognition of alien pollen, fertilisation is aborted (see Chapter 5).
Plants bear their flowers in different ways, perhaps in part to facilitate pollination by wind or the insect and other pollinators with which they have coevolved. Often they are borne singly, as in poppies (Papaver spp.); sometimes they are borne all the way up the stem, as in foxglove (Digitalis spp.), to form a spike, or with each flower on the spike having a short stalk, forming a raceme, as in Delphinium spp.; or they may be borne on a branched structure to form a panicle, as in roses (Rosa spp.). In onions (Allium spp.) all the flower-bearing branches arise from a single apex, forming a dense head of flowers called an umbel, and in Hydrangea spp. these branches are subdivided to form a flat head of flowers called a corymb. In hazel (Corylus spp.) the male flowers form a catkin. In the daisy family (Asteraceae) all the flowers arise from a single platform, the receptacle, in such a dense mass as to form a composite structure resembling a single flower (Fig. 3.7) known as a capitulum. Very often, as in Fig. 3.7, the inner and outer flowers may be very different, the latter being expanded to give the appearance of petals and the former being very small and tube-like.

Figure 3.7 The compound flower of Echinacea pallida, a member of the Asteraceae. Note the outer ray florets and the inner mass of tube-like florets. Photograph by Chris Prior.
Alternation of generations
The genetic information needed to coordinate the development of a new plant is encoded in the genes within the DNA of the cells, packaged in elongate structures, the chromosomes, contained within the cell nucleus (see Chapter 5). The number of chromosomes per cell is fixed for each individual, but varies among, and sometimes within, species. Chromosomes normally occur as almost identical pairs. Indeed, they are identical for genes defining all the major characteristics of the plant, but may vary for minor characteristics like rate of growth, height or flower colour (see Chapter 5). Each cell of a plant therefore normally contains two almost, but not quite, identical sets of genetic information and is said to be diploid (but see Chapter 5 for discussion of the occurrence of other ploidy levels). When a cell divides in the shoot or root or apex to produce two daughter cells, the chromosomes also divide to produce identical pairs of daughter chromosomes. This division is termed mitosis. The process of sexual reproduction, in contrast, first involves a specialised form of nuclear division called meiosis, which results in a halving of the genetic material of the parent to produce specialised haploid cells, the microspores and ovules already described. Fertilisation of an ovule by a microspore restores the genetic complement and the resulting zygote is therefore diploid. This process is dealt with in detail in Chapter 5.
Following fertilisation a diploid seed develops from the zygote, and this is capable of germination to produce a new diploid plant that contains genetic materials from each of the parents. This alternation of haploid and diploid generations in seed plants has evolved over millions of years from a situation seen in more primitive plants, such as the ferns (see Chapter 1), in which there is an alternation between separate, free-living sexual (gametophyte) and spore-bearing (sporophyte) generations.
Because of its minute size and dependence on water for growth and fertilisation, the gametophyte generation of the fern is, as described in Chapter 1, very vulnerable and is thus the weak point of the life cycle. In seed plants, however, the risk of damage to the gametophyte generation has been reduced by its total incorporation into the sporophyte generation until after fertilisation has occurred. Thus, although there is an alternation of sporophyte and gametophyte generations, this is not apparent to the casual observer. The new sporophyte resulting from fertilisation can receive food from the parent sporophyte and can be dispersed in a complex unit called the seed (see Fig. 3.10). This contains the minute new sporophyte (the embryo), together with stored food materials to fuel germination and establishment of the seedling, a process described in Chapter 10. The whole structure is protected by a seed coat (see below and Chapter 10).
Floral diagrams and formulae
The disposition of the parts of the flower is usually displayed in formal descriptions (as in floras) using a floral diagram (Fig. 3.8). This provides a condensed and simplified view of the flower from above, with the individual parts displayed in one plane. The organs shown, starting from the centre, are: ovary; stamens; petals or perianth segments (the corolla); the modified leaves that enclose the bud (the calyx); and any reduced leaves that may be attached to the flower stalk (bracts and bracteoles). The ovary is usually shown in cross-section to reveal the arrangement of the carpels (see below). The anthers are drawn to show where they open to release the pollen. An absent organ important to the symmetry of the flower is indicated by a dot or asterisk. If one part of the flower is attached to another, the link is shown by a line. The separation of petals and sepals is also made clear.

Figure 3.8 Floral diagram of white dead-nettle (Lamium album): A = axis; Br = bract; Se = sepal; Pe = petal; St = stamen; Ms = missing stamen; O = ovary. Floral formula: K(5) C(5) A(4) G2. Artwork courtesy of the Royal Horticultural Society.
A floral formula (Fig. 3.8) may be used to supplement the floral diagram, in which K = calyx, C = corolla, A = androecium (male structures) and G = gynoecium (female structures). Figures are added to each letter showing the number of each part in a single flower. If the number exceeds 12, the symbol ∞ is used. If a flower part is absent, this is indicated by zero (0). Where whorls of sepals or petals are linked, this is indicated by square brackets. A bar above or below the number for the gynoecium indicates whether the ovary is superior or inferior. The floral formula usually starts with a symbol to indicate symmetry: ••• for zygomorphic (bilateral); + actinomorphic (radial); and for spiral. The floral formula does not indicate the overall shape and form of the flower. This is usually rectified by including in the formal description a half flower section, as in Fig. 3.2.
Seeds and fruits
The embryos of angiosperms may have single cotyledons, the seed leaves that enclose the embryo, as in the monocots (e.g. the Poaceae – grasses and their relatives) or two or more cotyledons, with the embryo between them, as in the dicots (broad-leaved flowering plants). The embryos and cotyledons are in turn surrounded by the integuments, which become the seed coat (testa) (see Chapter 10). Finally, the seeds are contained within carpels, the walls of which thicken to become the fruit. Carpels evolved from leaf-like structures folded over and fused along the edges.
Most of these structures may be seen very easily with the naked eye in the pea (Pisum sativum). If a mature pod, the fruit (Fig. 3.9a), is split open along its length the ancestral leaf-like form of the single carpel is obvious (Fig. 3.9b) The ‘beak’ end is the remains of the stigma with its short style. The remains of the flower, now shrivelled, may still be attached to the stalk end. Attached along one margin of the pod are the individual seeds, the ‘peas’, surrounded by a tough coat, the testa, derived originally from the integuments (Fig. 3.10a). This may be split to reveal the contents. Before doing so, however, the point where the pea was attached to the pod should be examined with a lens. The remains of the micropyle, the minute slot-like hole through which fertilisation by the pollen tube actually occurred, will be visible. If the testa is next removed, the two bulky cotyledons, the seed leaves that enclose the embryo, will be revealed. The embryo itself (Fig. 3.10b), close to the point of attachment to the pod, will be seen to have an embryonic shoot, the plumule, and an embryonic root, the radicle. Following germination these will grow to produce a new plant, initially using the food resources held in the cotyledons.

Figure 3.9 Drawing of (a) an entire pod of garden pea (Pisum sativum) and (b) a pod split open to reveal the internal structure of the fruit and seeds. Drawings by Anne L.D. Bebbington.
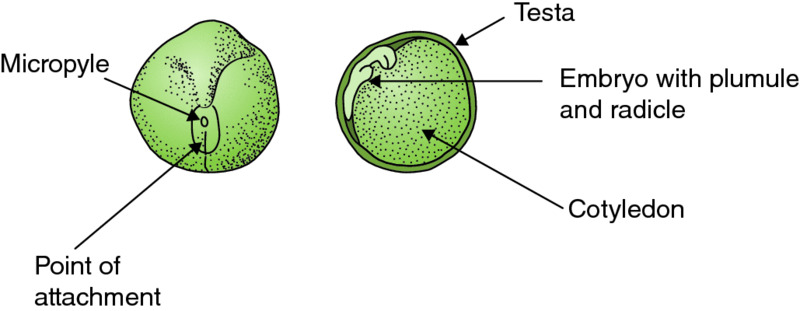
Figure 3.10 Two seeds of garden pea (Pisum sativum), one entire (a) and the other cut in half (b) to show the internal structure. Drawing by Anne L.D. Bebbington.
Other fruits
There are many other forms of seeds and fruits, some more and some less complex than the pea. All have evolved to provide protection for the embryo and to facilitate the dispersal of the seeds. In the weed species dandelion (Taraxacum officinale), for example, each carpel contains only one seed, and hardens to give protection; but it also develops a parachute of hairs at one end that enables it to be dispersed by wind. The paired carpels of Acer spp., each with a single seed, also become hard and develop membranous ‘wings’, again to aid wind dispersal. In wild progenitors of beans (Vicia and Phaseolus spp.) and in their relatives such as brooms (Cytisus spp.) the carpel walls dry and harden in such a way as to develop internal tensions. On a warm day, as the fruit ripens, these tensions cause the pods to split (Fig. 3.11) and the carpel walls to twist suddenly, often throwing the seeds considerable distances.

Figure 3.11 Dehiscing fruit of broom (Cytisus scoparius). Photograph by John Bebbington FRPS.
In other species the carpel develops into a fleshy structure, often brightly coloured and sweet, encouraging dispersal by animals and birds. These eat and digest the fruits, but the seeds, which are resistant to digestive enzymes, pass straight through the gut and may be deposited in the faeces many miles from the place of their formation. In plum (Prunus spp.) (Fig. 3.12), for example, a simple seed is surrounded by a three-layered pericarp or fruit wall, giving the stone, the flesh and skin. In tomato (Solanum lycopersicum) there may be two, three or more fleshy carpels comprising the fruit, each containing many seeds attached to a central thickened region called the placenta.

Figure 3.12 Plums are ‘true fruits’ with a three-layered fruit wall, the pericarp, consisting of skin, flesh and stone. The seed, which has two cotyledons and is surrounded by a brown, papery testa, is contained within the stone. Photograph by Chris Prior.
Finally in this brief list of examples, there are fruits in which the carpels harden to provide protection to the seed, but the structure on which the flower developed, the receptacle, swells to become fleshy and attractive to animals and birds. In strawberry (Fragaria × ananassa) (Fig. 3.13), for example, the receptacle grows to form the familiar red pyramid structure, a false fruit, with the tiny woody true fruits (achenes), each containing a single seed, dotted all over the surface. In apple (Malus spp.) the fleshy receptacle grows up and completely surrounds the fruit. If an apple is cut in half, the core, comprising the seeds, each with a brown testa, contained within about five horny carpels, is clearly visible. It is this core that is the true fruit, whereas the surrounding receptacle, with the remains of the flower emerging through the end opposite the stalk, is the false fruit. It functions in the same way as a fleshy true fruit, however, being attractive to animals and birds and thereby aiding seed dispersal.

Figure 3.13 Strawberries are ‘false fruits’ in which the red flesh is an expanded, fleshy receptacle, with the remains of the flower clearly visible around the base. The true fruits, which are small with a hard brown pericarp, are scattered over the surface of the receptacle. Photograph by Chris Prior.
Over the centuries gardeners and plant breeders have selected plants with particular seed or fruit characteristics that make the plants good to eat (such as tomatoes and apples) or attractive to look at, like the Chinese lantern (Physalis alkekengi). This selection has exaggerated particular parts of the seed and fruit, making it relatively easy to make out the component parts.
Conclusion
Flowers and fruits provide much of the colour and seasonal interest in a garden. They are also sophisticated structures vital for reproduction, variation and dispersal of species (see Chapters 5 and 10). They have evolved in response to environmental forces and have coevolved with pollinators and dispersal agents to produce a great natural diversity of structures, shapes, forms and colours. This diversity constitutes the raw material used by plant breeders who, by making crosses and selecting desirable hybrids (see Chapter 5), have produced the vast array of decorative and edible plants now available to the gardener. Knowledge of the many reproductive strategies used by plants allows the gardener to propagate desirable plants and insure against the loss of a single prized specimen.
Further reading
- Attenborough, D. (1995) The Private Life of Plants. BBC Books, London.
- Bebbington, A.L.D. (2014) Understanding the Flowering Plants. The Crowood Press, Marlborough.
- Bernhardt, P. (1999) The Rose’s Kiss: A Natural History of Flowers. Island Press/Shearwater Books, Washington, DC.
- Bowes, B.G. (1997) A Colour Atlas of Plant Structure. Manson Publishing Ltd, London.
- Capon, B. (1992) Botany for Gardeners. B.T. Batsford Ltd, London.
- Evert, R.F. & Eichhorn, S.E. (2012) Raven Biology of Plants, 8th edn. W.H. Freeman Publishers, New York.
- Glover, B. (2014). Understanding Flowers and Flowering – an Integrated Approach, 2nd edn. Oxford University Press, Oxford.
- Mabberley, D.J. (2008) Mabberley’s Plant Book, 3rd edn. Cambridge University Press, Cambridge.
- Mason, K. (2014) Flowers, fruit and seeds. In: The Fundamentals of Horticulture, Bird, C. (ed.), pp. 134–159. Cambridge University Press, Cambridge, and the Royal Horticultural Society, London.
- Pollock, M. & Griffiths, M. (2005) RHS Illustrated Dictionary of Gardening. Dorling Kindersley, London.
- Walters, S.M. (1993) Wild and Garden Plants (New Naturalist Series). HarperCollins Publishers, London.
Authors and affiliations
Written for the first edition (as part of Chapter 1) and revised for the second edition (as Chapter 3) by David S. Ingram, now Honorary Professor, Department of Science, Technology and Innovation Studies, University of Edinburgh, and Lancaster Environment Centre, University of Lancaster; updated for the third edition by Alastair Culham, Associate Professor and Curator of the Herbarium, University of Reading, and David S. Ingram.