CHAPTER 6
Soils and roots
Summary
This chapter considers the requirements of plants from soils. It goes on to discuss how soils are formed, how to recognise their key features, and the physical, chemical and biological properties of different soil types. Finally, the structure and growth of roots is described, with special emphasis on their role as absorbing organs.
Introduction
Why do plants need soil?
Most plants of interest to gardeners are grown in soil of some kind. However, plants can and do grow perfectly well in the absence of soil; examples include floating aquatics and plants raised in nutrient solutions in commercial hydroponic systems. These examples are, however, quite specialised. Submerged aquatics rely on an internal anatomy that allows the rapid movement of oxygen through the plant (see Chapter 8), whereas commercial hydroponic systems rely on sophisticated techniques for the analysis and maintenance of the growing solutions. Soils, in contrast, provide the essential requirements of plants with relatively little attention, provided that they are properly managed. Because the responses of shoots and roots are closely linked (see Chapter 12), gardeners should know something of the root environment so that they can manage it effectively to produce the plants they want.
What do plants want from the soil?
Soils supply several of the requirements needed for healthy plant growth, including anchorage for roots to stabilise the plant, water and nutrients for growth, oxygen to allow roots to respire, and a buffer against changes of temperature and pH. The importance of soils for anchorage is readily appreciated after a storm, when trees growing on shallow soils may be blown over.
As a general rule, it is rare for roots to occupy more than 5% of the total soil volume, even in the upper 10–15 cm where they are generally most abundant. The volume occupied decreases very rapidly with depth and is often no more than 0.01% at 50 cm. This means that only a small fraction of the soil within the rooting zone is in direct contact with roots. The implication of this for gardeners is that managing soil fertility is essentially about providing a medium in which roots can grow and proliferate to form a network capable of harvesting water and nutrients located at some distance from the root surface.
How soils are formed
Soil is a dynamic medium and changes with time. Some changes may occur in a matter of minutes, such as the production of a hole by an earthworm; others, such as the formation of humus from plant remains, take a few years; whereas yet others, such as the weathering of rocks and the formation of clay minerals, may take several centuries. Temperature and rainfall affect the rates at which rocks and minerals weather to form the basic solid materials that constitute a soil. These are essentially long-term processes, but temperature and rainfall also have short-term effects, such as on the leaching of nutrients and the rate of biological processes, particularly the decomposition of organic materials.
Differences in the types of rocks and sediments that constitute the parent materials of a soil affect the end products, especially the types and sizes of mineral particles. In the United Kingdom, the variety of rocks exposed to weathering covers almost the whole geological time course, but the soils at a particular place are rarely dominated by the underlying geology. This is because glaciers in past ice ages have moved materials around, and wind erosion from continental Europe and North Africa has deposited materials over the original rock deposits. Thus in many places drift and soil materials eroded from elsewhere are the dominant precursors of the soil mineral matter. Most soils in the United Kingdom are less than 10,000 years old, and many in southeastern England are of even more recent origin. The topographic location also influences the type of soil that forms, mainly because of the drainage conditions. Generally, flat areas allow more rain to permeate the soil than sloping areas, where lateral movement is possible. At the base of slopes water may accumulate, either at depth or at the surface, to form waterlogged soils, known as gleys. If surface water persists for prolonged periods and vegetation is plentiful, peat bogs are formed.
Soils also contain a vast number of living organisms, most of which are not visible to the naked eye (see below). The interaction of these living organisms with the dead organic materials produces a vast array of chemicals, some of which promote weathering of soil mineral particles, whereas others join particles together, or form a reserve of nutrients that biological activity can make available to plants. Roots and their associated organisms and exudates can also promote the weathering of minerals.
A feature of soils is that they usually show some horizontal banding, most obviously of colour, but also of other properties. Usually the upper 20 cm or so has the darkest colour because of the presence of organic matter. The lower parts of the soil frequently accumulate smaller particles and other materials washed downwards. These horizons of the soil indicate the dominant soil-forming processes. Most gardeners only look at the top horizon, the topsoil, but it is the second and subsequent horizons, the subsoils, that often make a difference to the growth of long-lived plants because of their contribution to root growth and the supply of nutrients and water.
The end result of all these interacting factors is to produce soils of almost infinite variety. Even in gardens of fairly modest size there are likely to be areas noticeably different from one another that need to be managed individually if the best results are to be obtained. In addition to the soil-forming factors is the special influence of one particular organism, humankind. Gardeners contribute in many ways to the formation of new soils by altering the drainage, moving soil materials to create deeper soils, adding waste products such as manure to promote biological activity, and adding fertilisers and manures to improve the nutrient status. Less well appreciated are the off-site effects of human activities that also have an effect on the development of soils. For example, industry and the burning of fossil fuels result in the soils of most of Europe and North America receiving inputs of nitrogen (N) and heavy metals from the air. In southern England, the average input of N is about 25 kg per hectare per year; this is equivalent to applying 36 g (a large spoonful) of Growmore (an inorganic compound fertiliser formulated during World War II) to a square metre. Similarly, the cadmium content of the topsoil at the research institute at Rothamsted, Harpenden, Hertfordshire has increased by 40% since 1850. These and other industrial inputs to soils will have significant consequences if they continue unabated.
In simple terms, soils are made up of mineral matter and organic matter, with the spaces between these solid materials filled with either water or air. The organisms live on the surfaces of the solid material and in the spaces. Soils, then, are complex media that have physical, chemical and biological properties. The aim of gardeners must be to manage these to the best advantage of plants. To achieve this effectively demands some understanding of the properties of the individual soil components and how they interact with each other.
Physical properties of soils
Soils are frequently defined in terms of their texture. This is a summary term describing the relative quantities of mineral particles of less than 2 mm diameter. Particles larger than 2 mm in diameter are usually referred to as stones, gravel or pebbles, and have no part in the description. The distribution of particles less than 2 mm in diameter cannot easily be altered, and influences many other important soil properties such as aeration, water-holding characteristics and ease of cultivation. This explains why this method of definition is so widely used; it is a stable, shorthand description of a range of properties.
For convenience, soils are allocated to a textural class depending on their content of sand, silt and clay-sized particles. Sand-sized particles are typically 0.05–2 mm diameter, silt particles are 0.002–0.05 mm, and clay particles are less than 0.002 mm in diameter. With a little practice it is possible to determine the textural class by rubbing a small ball of moist soil between the fingers and thumb (see Fig. 6.1). It is, however, important to have removed roots, stones, insects, earthworms and slugs before doing this; substantial amounts of organic matter are especially misleading, because silt particles and finely divided organic matter both feel silky to the touch.
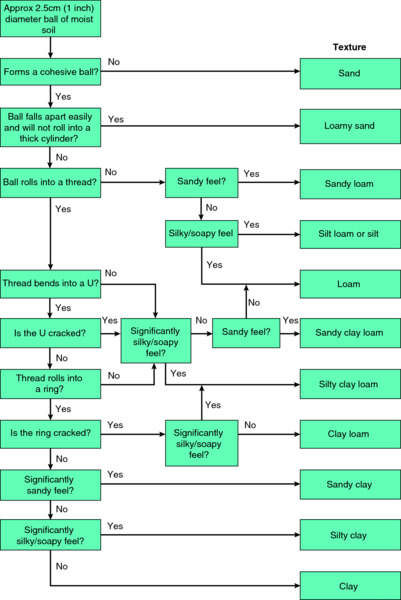
Figure 6.1 A simple guide for assessing the texture of a soil using properties measured by rubbing a small ball of soil between fingers and thumb. Image by Peter Gregory.
Sand particles usually consist of quartz, are rounded or irregular in shape and are not sticky when wet. Sands possess good drainage and aeration, but may be prone to drought. Silt particles are intermediate in size between those of sand and clay and they too are dominated by quartz. They give the soil some cohesion and plasticity but may cause the soil surface to become compacted and form a crust when dry. The clay-sized particles are dominated by clay minerals rather than quartz. They have a very large surface area in relation to their unit mass, about 10,000 times more than that of sand. This allows the adsorption (binding) of water and nutrients, which results in wet clays being sticky and cohesive.
Most soils are a kind of loam, with an ideal composition of sand, silt and clay particles in about equal proportions. The term loam implies nothing about the quantity of organic matter present, although in everyday language gardeners often erroneously refer to soils with moderate amounts of organic matter as ‘loams’. Another factor that can lead to misunderstanding is that the word ‘clay’ is used here to describe the size of particles; however, it is also used (see below) to describe a wide range of different minerals, with different chemical properties. The terms ‘light’, ‘medium’ and ‘heavy’ are also commonly used to describe texture, and relate to sandy soils, loamy soils and clayey soils, respectively. These descriptions reflect the water content of the soils when drainage has ceased.
The texture of a soil is not easily modified and, apart from the incorporation of large amounts of sand to improve the physical properties of clay soils for horticultural purposes, is not changed by cultural management. However, other physical properties of a soil can be substantially altered by management practices such as cultivation, drainage and manuring, which all affect soil structure, especially in the topsoil. The term structure describes the way in which the sand, silt and clay particles are grouped or arranged together into units known as aggregates. Essentially, it is only these aggregates that are altered by most gardeners.
Within a soil, different structures are often present in different horizons. Spheroidal structures (granules and crumbs) are typical of many surface soils, particularly those under grass, and soils high in organic matter. In the subsoil, plate-like, columnar, prismatic and blocky structures usually dominate. Between the structures are spaces called pores, which are normally filled with air and water. The exact process of how the structures are formed is complex and variable, but living organisms and plant roots play key roles. Plant roots tend to compress soil particles into small aggregates as they elongate through soils, and similar compression and contraction occurs as plant roots take up water, and soils are wetted and dried. Plant roots and microorganisms also exude sticky organic compounds, mainly long-chain sugars known as polysaccharides, that can bind particles together. The decomposition of organic materials by microbes also produces other organic compounds that interact with clay-sized particles to cement them together. Thus, clay particles and fine organic materials are important components of aggregates (see Fig. 6.2),especially in topsoil, where living organisms and organic matter are most frequently found. In the subsoil, downwardly moving organic materials, clay minerals, oxides of iron and aluminium, and salts such as calcium carbonate can all act as cements.
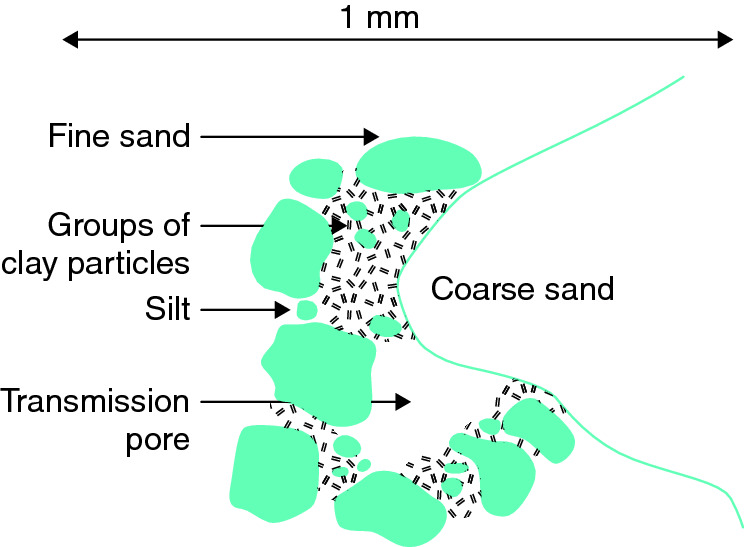
Figure 6.2 Diagrammatic representation of soil microstructure examined with a light microscope, showing sand and silt particles joined together by groups of clay particles to form a structure containing pores. Spaces between the groups of clay particles and the surfaces of the solid particles contain organic matter, but these cannot be seen clearly with a light microscope. Image by Peter Grgory.
The pores are an integral part of a soil and are filled with water and air. The proportions of water and air change as rain falls, as plants take up water, and as evaporation occurs from the soil surface. The size, shape and continuity of the pores determine how much of the rain falling on a soil drains away, is held for plant use, or is held so firmly that plants cannot use it. Table 6.1 summarises a useful classification of pores in terms of size and function. In crude terms, if a pore can be seen with the naked eye it will not hold water in a freely draining soil.
Table 6.1 Classification of pores by size and function
| Pore class | Pore diameter (μm) | Pore function |
| Transmission (macropore) | > 50 | Drainage after saturation |
| Aeration when soil is not saturated. Both aeration and drainage require interconnection of pores | ||
| Root penetration. Root diameter is typically < 200 μm | ||
| Storage (mesopore) | 50–0.2 | Store water available for plant use |
| Residual (micropore) | < 0.2 | Hold water so strongly that it is not available to plants |
| This water controls to a large extent the mechanical strength of the soil |
From Rowell (1994).
In general, sandy soils have about 35–40% pore space, loams 45–55%, clays 50–70% and peats 80%. However, it is the sizes of the pores making up the pore space that differ significantly among soils of different textural classes. In sandy soils, most of the pores are typically the larger transmission pores, with only a few of the very small residual pores and some storage pores. This contrasts with clayey soils, which have fewer transmission pores, but three to four times the volume of residual pores. Sandy soils drain easily, hold only modest amounts of water available to plants, and retain little water after drying out by plants. In contrast, clays do not drain readily, hold more water available for use by plants, and contain water even after prolonged dry periods. The critical limits for the three categories of pores are as follows. If transmission pores form less than 10% of the soil volume, drainage may be a problem; if storage pores form less than 15% of the volume, water availability is likely to be restricted; and if residual pores form more than 20% of the volume, mechanical difficulties will be experienced because the soil will be sticky when wet.
Water is held in soils against the force of gravity because of forces acting between the water and the surfaces of the soil particles. These forces result in water being held inside small pores, at the necks of larger air-filled pores, and as thin films on particles enclosing air-filled pores (see Fig. 6.3).
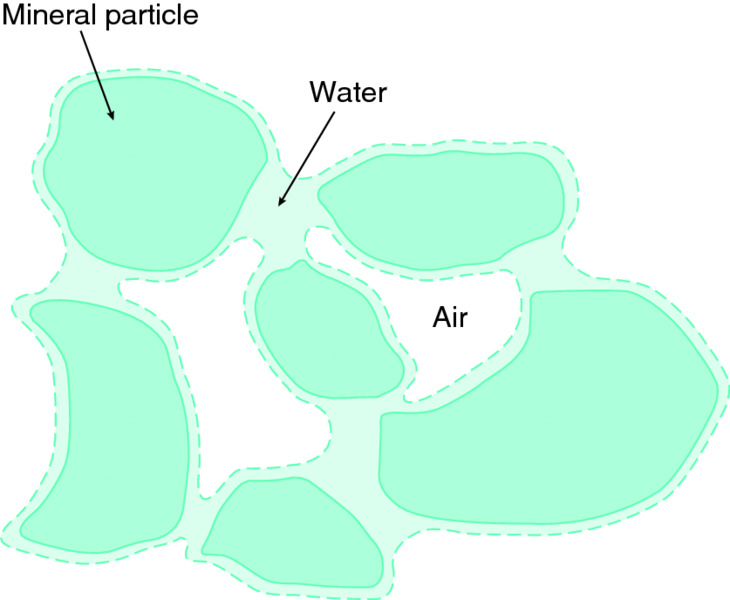
Figure 6.3 Water, air and mineral particles in a freely drained soil. When drained, i.e. not saturated, air occupies the larger transmission pores and water is present in the smaller storage pores and as films on the surface of soil particles. Image by Peter Gregory.
Chemical properties of soils
The sand- and silt-sized components of the soil consist mainly of quartz and are therefore largely chemically inert, apart from the finer materials that are smeared across their surfaces. It is the clay-sized fraction, less than 2 μm in diameter, together with some components of the organic matter, that is chemically active. As a result of weathering processes, the clay-sized particles are chemically altered and become electrically charged, enabling them: to adsorb ions (charged atoms and molecules), including some that are plant nutrients; to adsorb water, with consequent swelling and plasticity; and to disperse, as a consequence of repulsion between charged surfaces. Together, the various processes producing clay minerals result in a suite of materials with different properties, and for this reason not all clay soils behave in the same way.
The clay-sized fraction consists of very fine colloidal material. There are three types of colloidal material in the clay-sized fraction: clay minerals, alumino-silicates and sesquioxides of iron and aluminium. For clay minerals, each clay particle is made up of a series of plate-like layers stacked one on top of the other, like the pages in a book. Each layer comprises horizontal sheets of silicon, aluminium, magnesium and other positively charged ions, surrounded and held together by oxygen (O2) and hydroxyl (OH) groups. The sheets are of two types: tetrahedral sheets composed of silicon atoms bound to oxygen atoms; and octahedral sheets composed of either aluminium or magnesium atoms, each bound to either six oxygen atoms or six hydroxyl ions. These sheets are combined together into layers of clay minerals of three main types, as illustrated in Fig. 6.4.
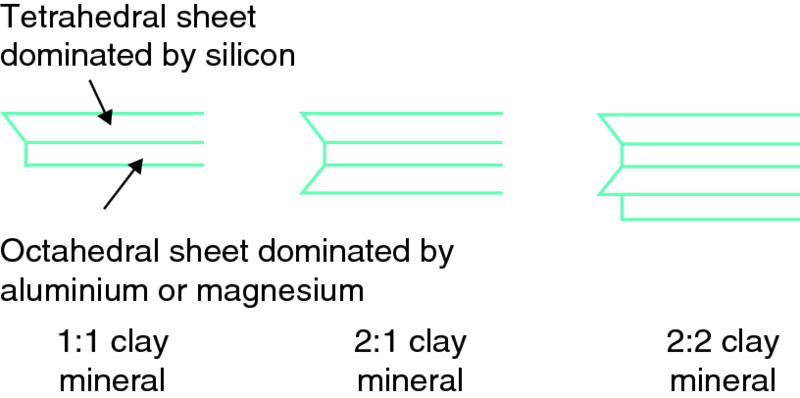
Figure 6.4 Diagrammatic representation of the chemical structure of various types of clay minerals making up the clay fraction of soils. Three basic types of clay mineral are produced depending on the arrangement of the tetrahedral and octahedral layers. Image by Peter Gregory.
All clay minerals are small, and this results in a large surface area that can adsorb ions, water and gases. In addition, another important property distinguishes clay minerals, and is of vital importance to plants. Due to the weathering processes that led to their formation, impurities are often found in both the tetrahedral and octahedral layers, and this produces clay minerals with a permanent negative charge. These charges are always balanced, however, by positively charged ions, called cations, that are adsorbed onto the surfaces of the minerals. In general, calcium, magnesium, potassium and hydrogen balance the negative charge, but they are interchangeable, depending on their availability in particular soils. Such ions are known as exchangeable cations, the total charge as the cation exchange capacity (CEC), and the process as cation exchange. The CEC and the balance of ions are important to gardeners because they determine such important features as the pH (acidity or alkalinity; see Chapter 7) of the soil, and the ability of a soil to replenish some nutrients such as potassium to the soil solution.
Unlike the clay minerals, some of the alumino-silicates and sesquioxides of iron and aluminium do not swell or shrink. They do not have a permanent negative charge, although it is possible for a charge to develop, associated with the hydroxyl groups at the edge of the particles. This charge is variable, depending on the pH of the surrounding soil solution. In alkaline soils the charge is negative, but in acid soils the charge is positive and the soil has an anion exchange capacity (AEC), the opposite of a CEC, satisfied by negatively charged ions such as chloride, sulphate and nitrate. In temperate soils the AEC is rarely evident, but soils in tropical gardens may have this property.
Humus is a mixture of complex compounds that have been synthesised by microorganisms in the soil from the breakdown products of plant and animal remains or by alteration of such materials. It is typically brown or dark brown in colour, and gives many temperate soils their characteristic brown to olive appearance. Humus colloids are not crystalline and are composed essentially of carbon, hydrogen and oxygen. They have a pH-dependent negative charge, never a positive charge, so that humus, like clay minerals, adsorbs cations.
Biological properties of soils
A teaspoon of moist garden topsoil will contain several million bacteria, a million or so fungi, and ten thousand or so protozoa. These microorganisms live mainly on each other and on plant and animal residues, which they convert into many chemical forms. The decayed and decaying organic matter in soils results from the combined activity of the soil fauna and flora, and this, together with the burrowing and mixing activities of live animals, means that the soil is a continually changing, living medium.
These living organisms are an essential part of the soil-forming process, and the carbon compounds produced by the photosynthesis of plants are broken down by them. In soils that have been formed for some time, a vast array of organisms is present, living on each other and on dead organic matter returned to the soil from plants. Microorganisms convert organic residues into a resistant residue, humus. This combines with living organisms, dead cells and mineral particles to form the solid component of a soil. The term soil organic matter is used to refer to all the organic material in soil, including humus. Soil organic matter comprises living material (soil animals, plant roots and microorganisms) and dead material, including shoot and root residues and humus.
The amount of organic matter present in a soil depends on the amount and type of vegetation, the climate and the physico-chemical properties of the soil. Thus woodlands and grasslands generally have more organic matter than cultivated soils; temperate regions generally have greater amounts than Mediterranean regions; and clayey soils generally contain greater amounts than sandy soils because the organic material is ‘protected’ by binding to clay particles .
In the United Kingdom and much of northern Europe only about 2–5% of a cultivated topsoil is organic matter, and of this only a small proportion is living. For example, a cultivated topsoil may contain 4.5 kg of organic matter per square metre, of which only about 2.5% (112 g) will be living microorganisms, called the microbial biomass. Although small, this component is vital, because almost all the plant and animal residues must pass through it if nutrients are to be released for plant use.
Some of the more important groups of organisms present in soils are shown in Fig. 6.5,and an indication of numbers and biomass is given in Table 6.2. The microorganisms are the most numerous and have the highest biomass. Because activity is generally related to biomass, the microorganisms, together with earthworms, dominate biological activity in the soil, with 60–80% of soil metabolism attributable to the microflora. In some areas ants and termites are important, and in some tropical soils the activities of termites and their associated fungi play a major part in soil regeneration.
Table 6.2 Numbers and liveweight biomass of the organisms commonly found in surface soils
| Organism | Number per m2 | Biomass (g m−2) |
| Bacteria | 1013–1014 | 45–450 |
| Actinomycetes | 1012–1013 | 45–450 |
| Fungi | 1010–1011 | 110–1100 |
| Algae | 109–1010 | 5.6–56 |
| Protozoa | 109–1010 | 1.7–17 |
| Nematoda | 106–107 | 1.1–11 |
| Other fauna | 103–105 | 1.7–17 |
| Earthworms | 30–300 | 11–110 |
After Brady & Weil (2008).
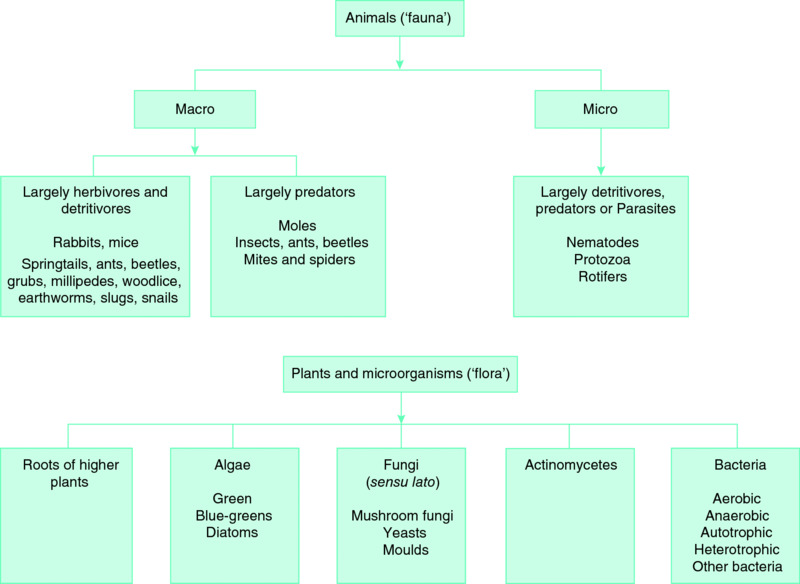
Figure 6.5 A general classification of some of the more important groups of organisms that are commonly present in soils.
Bacteria are single-celled organisms. They rarely exceed 4–5 μm in size, and the smaller ones are about the same size as a clay particle (2 μm). They exist in clumps or colonies, and different types use different food sources. Most soil bacteria are heterotrophic, meaning that their energy and carbon source come directly from organic matter; together with fungi they effect the general breakdown of organic matter in soils. A smaller group of bacteria obtain their energy from inorganic substances such as ammonium, sulphur and iron, and most of their carbon from carbon dioxide. This group plays a vital role in controlling the availability of nutrients, especially nitrogen, to garden plants (see later).
Actinomycetes are a specific group of bacteria that are unicellular but resemble fungi because their elongated cells are filamentous and frequently branched to form an extensive colony. Their main food source is soil organic matter, which they break down. They appear to be more capable than many other bacteria of breaking down some of the more resistant compounds produced by plants. However, whereas other bacteria can survive across a wide range of pH levels, the optimum for actinomycetes is between 6.0 and 7.5.
Most soil fungi are multicellular organisms forming long hyphae normally 5–20 μm in diameter, although a few species of unicellular yeasts also occur. The soil fungi can use a wide variety of carbon substrates as a source of energy. Some can utilise only simple carbohydrates, alcohols and organic acids, whereas others can decompose polymers such as cellulose and lignin (see Chapter 2). Some are parasites of plants (see Chapter 17) whereas others, such as mycorrhizas, have a symbiotic relationship with their host (see Chapter 2). Overall, fungi are the most versatile group of microorganisms and, because of their role in humus formation and aggregate stabilisation, play a significant role in all soils.
Much has been written on the importance of earthworms. They ingest leaf and root material along with mineral particles, which are subjected to digestive enzymes and grinding processes within the animal. The mass of material passing through the body of a worm daily may equal its own body mass, and the casts produced have significantly greater nutrient content and structural stability than the bulk soil. Earthworms must have organic matter as a food source, and prefer a well-aerated but moist habitat. Most thrive when the pH of the soil is between 6 and 7, but a few species tolerate lower levels of pH. Many native species of earthworm are killed by the disruption of their food sources and burrows that results from cultivation. Where earthworms are not present, for example in acidic, dry or very wet conditions, the accumulated litter is broken down only very slowly.
From the description above it is clear that inputs of new organic materials are essential to maintaining an active population of living soil organisms. It should also be clear that adding organic materials will not necessarily increase the organic matter content of a soil. Much may simply decompose to carbon dioxide, which is lost to the atmosphere, and inorganic ions, which may be lost by drainage.
Roots and soils
Roots, which were introduced in Chapter 2, have several functions. They absorb water and nutrients from the soil and transport these from the site of absorption to the shoot; they form the site of synthesis for several plant hormones (see Chapters 2 and 12), which may act in either the root or the shoot or both; they may act as storage organs as, for example, in root vegetables such as carrot; and they serve to anchor the plant.
A typical young root is shown in Fig. 6.6. It comprises a root cap, behind which are the dividing cells of the meristem, which later differentiate into cells forming the epidermis, the cortex and the stele. During this differentiation the cells increase in volume and elongate to push the root through the soil. Behind this zone of elongation, the cells of the stele themselves differentiate to form the endodermis, phloem and xylem. The xylem is essentially a mass of non-living pipes that transports water and nutrients upwards to the shoot, while the phloem consists of living cells that allow bidirectional movement of assimilated materials. The endodermis gradually becomes thickened with deposited corky material, suberin, as the plant ages. The suberisation of the roots strengthens them, but also prevents the movement of some nutrients to the xylem and thence to the shoot.
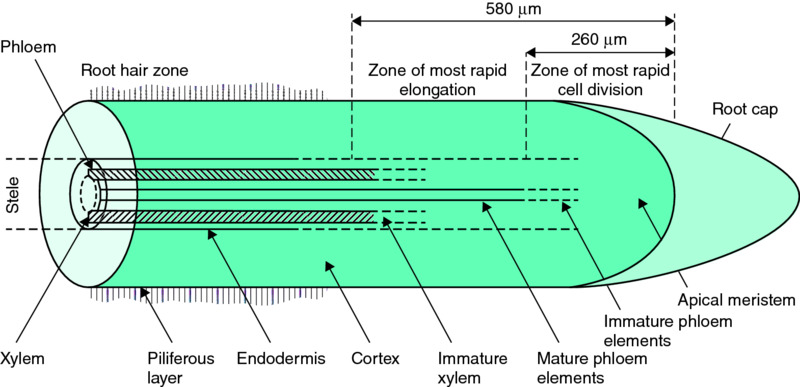
Figure 6.6 Diagrammatic representation of a longitudinal section through a young root showing the spatial arrangement of the root tissues. Image by Peter Gregory.
The outer cells of the root cap may exude materials that coat the root tip with mucilage (see Fig. 6.7, left). This mucilage is very noticeable in some plant species, for example grasses (Poaceae); it ensures good contact between the root and the soil, and aids the transfer of water and nutrients to the plant. So effective is this material that it is impossible to detach soil particles from some roots, and they exist within a rhizosheath (Fig. 6.7, right). The root hairs also act to increase contact between the root and the soil particles. In addition, because they effectively extend the surface of the root outwards, they are able to mine a zone of soil quickly that would otherwise take some time to deplete. This process is particularly important in the acquisition of nutrients, such as phosphate, that otherwise move slowly through the soil. Mycorrhizal fungi may serve a similar purpose (see Chapter 2).
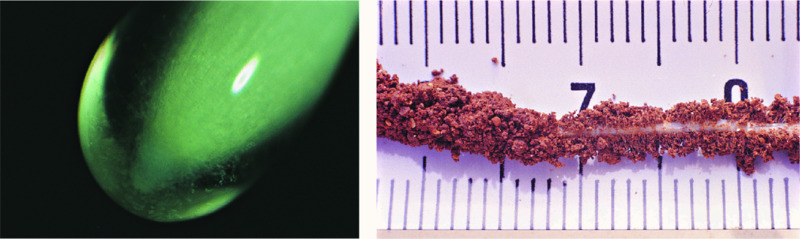
Figure 6.7 (left) Mucilage production at the root tip of a maize (Zea mays) root; and (right) the binding of soil particles to a root by mucilage and root hairs to produce a rhizosheath that is very difficult to remove. The production of rhizosheaths is common in many species, especially as the soil dries. Image by Peter Gregory.
However, most roots do not look like or function like young roots. Root hairs are ephemeral and generally last for only a few days. As roots age, the epidermis and cortex are attacked by other organisms, become desiccated during dry periods, and eventually disappear. Older roots of annual species may have the endodermis as the outer layer for a significant part of the growing period. Roots of most dicotyledonous plants undergo secondary thickening (see Chapter 2) in which a corky periderm is formed and the endodermis, cortex and epidermis are sloughed off to leave a woody root; this is most noticeable in trees and shrubs.
Water can be taken up along the whole length of the root system if it is available in the soil and if contact between the root and soil is good. The water directly surrounding a root system is only a small fraction of the total needed to supply the amount that transpires during the course of a sunny day. To make up the deficit, liquid water must flow from some distance away, often up to10 mm, to reach the root surface. This cannot happen if there is an air gap between the soil and the root.
Two different situations have to be managed by gardeners. The first is on sandy/silty soils with high organic matter content where ‘puffiness’, the presence of large amounts of air, may lead to poor soil-root contact. This can be overcome by firming the soil, manually or with a roller. The second situation is more difficult to manage because it occurs where there are only a few cracks in the subsoil and the roots are limited to following them. As the soil dries, the roots may lose contact with the soil and hang in the air between the cracks.
The activity of roots in taking up nutrients is rather more complex than that of taking up water, because of the role played by the endodermis. Older books on this subject often suggest that nutrient uptake is restricted to an absorbing zone close to the root tip. This is now known to be incorrect. Ions such as nitrate, ammonium, phosphate and potassium can be taken up along the whole length of a root and transported to the xylem for onward transportation to the shoot. For calcium and iron, however, although limited uptake can occur along the length of the root, uptake is greatest nearest the root tip and translocation to shoots via the xylem is confined to this zone. The reason for this differing behaviour by different nutrients lies in the pathways they use to move from cell to cell within the root. Nitrate, ammonium, phosphate and potassium move mainly inside the living parts of cells (i.e. the protoplasts, which are linked to one another across the cell walls by plasmodesmata), whereas calcium and iron move initially in the non-living cell walls, a route that may be blocked by the suberised endodermis (see Chapter 2).
Little research has been done on the roots of garden plants and there are only few data available on the roots of vegetables, mostly in soils with a sandy texture. Pulling up a plant to examine the roots often gives a false impression of the depth and size of the root system because so many of the fine roots become detached. The roots of most garden vegetables normally grow to at least 50 cm (see Table 6.3),and the roots beneath a lawn often penetrate to 1.5 m if the soil is deep enough. Beneath each square metre of ground there will be several kilometres of roots, with about 40 km m−2 in the case of grass. In fact, the values shown in Table 6.3 are something of an underestimate because the methods used to obtain them ignore the very fine roots and root hairs.
Table 6.3 The maximum depth of rooting and length of root systems of some vegetable crops grown on a silt loam
| Crop | Maximum rooting depth (m) | Total length (km m−2) |
| Broad bean | 0.8 | 1.76 |
| Cauliflower | 0.8 | 11.9 |
| Lettuce | 0.6 | 2.37 |
| Onion | 0.6 | 1.83 |
| Parsnip | 0.8 | 5.65 |
| Pea | 0.7 | 5.46 |
| Turnip | > 0.8 | 15.42 |
| Grass | 1.5 | > 40 |
From publications of the former Horticulture Research International, Wellesbourne, UK.
The weight of a root system is also a substantial component of the total plant weight, although its relative size generally decreases with time. For example, in many crops of peas (Pisum sativum) and beans (Vicia faba) up to 40% of the total plant weight prior to flowering may consist of roots. After flowering, as the pods start to grow, root growth occurs at a much slower rate, and the roots typically form only 15–20% of the total plant weight. In the final stages of pod filling, the plant allocates most of its resources to the pod and only a small amount to the roots, so the root system begins to degenerate.
In general, most of the root length is located in the top 15 cm of soil, and the quantity decreases very rapidly with depth. The zone of maximum root length coincides with the most chemically and biologically fertile zone of the soil. Water, however, is more evenly distributed in the soil profile, so the depth of rooting and the roots growing in the subsoil are important for the acquisition of water. A deep root system is one mechanism that plants have to tolerate drought (see Chapter 8).
Among the many soil conditions that can impede root growth, the most important that can be managed by gardeners include compaction, a shortage of oxygen due to waterlogging or poor drainage, dryness, low nutrient supply, and a pH that is too low.
Conclusion
Soils may be very diverse, even within the space of a very small garden, having been derived from a wide range of rock types, drift materials and organic matter. Different mixes of sand, silt and clay particles give rise to soils of different textures. Soils are living materials that are populated by large numbers of micro- and macroorganisms, which play a central role in the breakdown of organic matter and the release of nutrients, and in maintaining and enhancing soil structure and fertility. Understanding the formation and properties of different soil types, the nutrient requirements of plants, and the structure and growth of roots, is an essential prerequisite for the development of appropriate soil management strategies.
Further reading
- Brady, N.C. & Weil, R.R. (2008) The Nature and Properties of Soils, 14th edn. Pearson-Prentice Hall, New York.
- Gregory, P.J. (2006) Plant Roots: Growth, Activity and Interaction with Soils. Blackwell, Oxford.
- Gregory, P.J. & Nortcliff, S. (2013) Soil Conditions and Plant Growth. Wiley-Blackwell, Oxford.
- Rowell, D.L. (1994) Soil Science: Methods and Applications. Longman Scientific & Technical, Harlow.
- White, R.E. (2006) Principles and Practice of Soil Science: The Soil as a Natural Resource, 4th edn. Blackwell, Oxford.
See also Chapter 7, ‘Further reading’
Authors and affiliations
Written for the first edition as part of Chapter 4 by Peter J. Gregory, previously Chief Executive (now Director of External Affairs), East Malling Research, and Professor of Global Food Security, University of Reading, and updated for the second and third editions (as Chapters 6 & 7) by Peter J. Gregory and by Paul Alexander, Head of Horticultural and Environmental Science, Royal Horticultural Society.