CHAPTER 19
Maturation, ripening and storage
Summary
The optimal point of consumption of fruit, vegetable, flower and seed crops is usually at a well-defined point in the development of the product on the plant; however, consumers prefer fresh produce to have a shelf life or vase life of days, weeks or even months. Much scientific and agronomic effort has been put into discovering the biology behind the maturation and ripening of different crops, and how the process that leads to rotting in the absence of intervention can be slowed down or halted so that the product is held in suspended animation at the optimum stage in its life cycle. This chapter explores the fundamental processes that affect the quality of fresh produce and how shelf life and vase life can be extended in commercial and domestic settings.
Introduction
Gardeners generally harvest their produce for immediate consumption when it has reached the right stage of maturity or ripeness. By choosing varieties that mature at different times, or by delayed planting or repeat sowing, the seasonal nature of production from the garden can be maintained. However, in some circumstances people may also wish to store the harvested crop for an extended period. If seeds are to be saved for sowing the next year they must be kept in a viable condition over the winter. Late-ripening cultivars of apples can be maintained in good condition for some time if stored in the right way. Even cut flowers may be kept for longer than normal if treated appropriately.
In commercial horticulture, post-harvest handling and storage of produce is required to provide consumers with fresh products irrespective of the distance from the growing area, and time of year. The need to develop better techniques for maintaining product quality and extending storage life has led to continuing advances in understanding the changes that take place after harvest.
This chapter is written to provide the gardener with some understanding of the physiological changes that occur in storage structures, seeds, fruit and flowers during maturation and following harvest, especially those that affect their longevity in store. Although gardeners do not normally have access to commercial storage facilities, some knowledge of the theoretical basis for the most important aspects of commercial practice contributes to our understanding of the processes that occur during ripening and after harvest, and is therefore included in this chapter.
Changes in metabolism and ethylene synthesis
Respiration and water loss
When a plant tissue has reached maturity, its respiration rate will either remain roughly constant or slowly decrease with age. An exception to this behaviour is the marked rise in respiration, known as the climacteric, that accompanies the onset of ripening in many fruits and senescence in flowers. Both ripening and the climacteric are triggered by the endogenous production of ethylene, and both processes may be stimulated to occur prematurely by the application of this gaseous hormone (see below).
Although synthetic (anabolic) processes occur during maturation, the balance of metabolism is destructive, or catabolic, and ultimately produce becomes senescent. The respiration rate of a product is an indicator of the rate of metabolism, and in general higher respiratory activity is associated with a shorter storage life. Moreover, fruit and vegetables with a higher content of stored materials that can be utilised in respiration will, on the whole, remain in good condition for longer than those with only small amounts.
Once harvested, fruits and vegetables can no longer take up water and gradually become dehydrated and shrivel. Thus, preventing water loss as much as possible can contribute significantly to their post-harvest life. Similarly, although cut flowers may still absorb water through their stems, its uptake can be severely reduced if the water-conducting vessels become blocked. Seeds are different in that they lose water during ripening, and here storage life depends on keeping them in a dry, dormant condition (see Chapter 10).
The objective of commercial storage is to delay senescence, preserving the desired characteristics of fresh horticultural produce and providing the market with a continuous supply of products with high nutritional value and acceptable sensory quality. To do this, technologies are used to reduce respiration and conserve moisture within the product. Many of these techniques are not available to gardeners, but an understanding of the underlying physiology, especially the importance of preventing water loss, the role of ethylene and the need to reduce the rate of respiration, can be used by them to increase the life of harvested produce.
Ethylene
During the nineteenth century, when coal gas was used for street lighting, it was observed that trees near the lamps shed their leaves earlier than other trees. Later, ethylene was identified as the active compound. Natural gas does not contain ethylene, so gas-induced leaf loss would not be observed today. Ethylene (CH2=CH2) is a colourless gas that has particular significance in the ripening of climacteric fruits, and in the senescence of leaves and flowers. The dramatic increase in ethylene concentration in climacteric fruits such as apple (Malus domestica), pear (Pyrus communis), plum (Prunus domestica) and tomato (Solanum lycopersicum) is required to trigger and synchronise the ripening processes, and does not occur until they approach full development. From an evolutionary viewpoint, this could safeguard against premature ripening at a stage when the seeds are immature and the attractiveness of the fruit for consumption by animals to facilitate seed dispersal is less than optimal. When unripe climacteric fruits are treated with ethylene the onset of ripening is hastened. In contrast, fruits such as Citrus species and cultivars and grapes (Vitis vinifera) do not exhibit a dramatic rise in ethylene production and are called ‘non-climacteric’. In these cases the application of ethylene does not trigger ripening. One challenge for scientists is to find out whether manipulation of ethylene production and response by the plant to external ethylene can prolong the life of fruit, flowers and leaves beyond that which they would normally have if left undisturbed.
Clearly, ethylene has important commercial implications for the storage and marketing of fruit, vegetables and cut flowers. The gas is both friend and foe within the trade – on one hand it can be used to induce ripening-on-demand but, because the progression from ripening to rotting is a continuum, it can also be a key inducer of flower, leaf and fruit spoilage and loss of shelf life. The ability of ethylene to initiate ripening is exploited in the marketing of several types of fruit such as avocado (Persea americana), banana (Musa spp.) and tomato. The commercial ripening of bananas is a routine operation carried out in importing countries to provide fruit to consumers at a specified colour stage. Harvesting bananas in an unripe (green) condition and maintaining cool temperatures (13–14°C) during transport facilitates their supply to distant markets; subsequent ripening with ethylene ensures uniformity in ripening and product quality. There are some instances where ethylene treatment is useful for non-climacteric fruit, for example in the de-greening of citrus fruits where certain cultivars become edible before the green colour of the peel has disappeared. In such cases ethylene gas is used to encourage the degradation of the chlorophyll in the peel.
The gardener may also make use of the knowledge that the ripening of climacteric fruits harvested in an immature condition is hastened by treatment with ethylene. For example, it may be useful to stimulate ripening of immature tomatoes at the end of the growing season by enclosing the green fruits with red, ripening fruits or other ripe climacteric fruits such as apples or bananas, all of which will be emitting ethylene gas as part of their own ripening process. The greatest stimulation of ripening is achieved in warm temperatures.
Although ethylene gas may be used to improve the quality of certain types of fruit, its presence in the atmosphere may also have detrimental effects. The build-up of ethylene generally reduces the quality of vegetables, although there is a range in sensitivities to the hormone. Vegetables that display high sensitivity include broccoli (Brassica oleracea), Brussels sprouts (B. oleracea), Chinese cabbage (B. chinensis), cauliflower (B. oleracea), cabbage (B. oleracea), cucumber (Cucumis sativus), endive (Cichorium endivia), sweet corn (Zea mays), lettuce (Lactuca sativa) and spinach (Spinacia oleracea). Adverse effects include loss of visual quality due to accelerated de-greening, senescence and impaired eating quality, such as toughness in asparagus (Asparagus officinalis) or bitterness in carrots (Daucus carota).
Vegetables may be exposed to high concentrations of ethylene during distribution and retailing, chiefly because of the presence of ripening fruit. Care is therefore taken to avoid unnecessary mixing of vegetables with fruit. Similarly, gardeners are advised not to store pome and stone fruits, which produce large amounts of ethylene, in the same refrigerator as ethylene-sensitive vegetables. However, the cool temperatures in a domestic refrigerator do reduce ethylene production and slow the overall rates of deterioration of sensitive vegetables.
Another effect of exposure to ethylene is the accelerated senescence and fading of cut flowers. Sometimes this is a response by the flower to being disturbed in the vase and receiving a false-pollination signal, as is the case with orchid flowers, which can last for months if undisturbed but senesce and drop off within two to three days if the flower is knocked. Since the purpose of the flower is to encourage pollinators to visit, once pollination has occurred it is no longer needed by the plant, which then removes it as quickly as possible. Gardeners should ensure that ethylene-sensitive flowers are not stored with climacteric fruits such as apples and pears, because the presence of ethylene in greenhouses, storage rooms or anywhere in the distribution chain can have serious detrimental effects on the keeping quality of flowers. These include the downward bending of leaves (epinasty), withering, ageing, yellowing and the abscission of flower parts and leaves (Fig. 19.1).

Figure 19.1 The effect of ethylene gas on torenia (Torenia fournieri) has caused them to lose many of their flowers (right). This flower drop has been effectively prevented by treatment with the anti-ethylene agent MCP (centre). Photograph courtesy of Michelle Jones, Department of Horticulture and Crop Science, Ohio State University.
Various types of technology exist to reduce ethylene concentrations around stored fruit and vegetables: these include its removal by ventilation with fresh air, oxidation using chemical agents such as potassium permanganate and ozone, or physical/chemical methods such as with heated catalyst systems. Although important benefits of ethylene removal have been established for apples, it is not at present commercially practical in the United Kingdom. Gardeners who store apples should ensure that the fruit is as well ventilated as possible.
Both ethylene production and ethylene action may be blocked by specific inhibitors. The gas molecules bind to specific receptors on the cell membranes and set up a cascade of reactions leading to the production of more ethylene and the induction of ripening or senescence responses. One way of preventing these physiological responses is to use a chemical that is structurally very similar to ethylene, and recognises and binds to the receptor in the plant, but then blocks it, preventing the downstream cascade of events from occurring. Past practice focussed on the use of silver ions applied as silver thiosulphate (STS), but use of this metal caused problems linked to its safe disposal in the environment. The most common treatment at present is 1-methylcyclopropane (1-MCP), which is applied as a gas, often during the normal transport or storage phases of the supply chain, and which has no problems associated with disposal. 1-MCP is now routinely used on a range of ethylene-sensitive cut flowers and on many varieties of apple found in major supermarkets. Its use on leafy vegetables is more varied as occasionally its application can cause the generation of off-flavours, and if the cold chain is broken, allowing the synthesis of new ethylene receptors, the crop can still senesce and fail very rapidly.
Although less efficient than 1-MCP, carbon dioxide at high concentrations, in the range of 5–10%, inhibits many responses to ethylene, such as the induction of fruit ripening. This is discussed later in relation to the controlled atmosphere storage of fruits.
Maturation and ripening
Seeds
The embryos of all seeds develop to a particular stage that is characteristic of the species. When the embryo reaches full size, the deposition of food reserves is completed (see Chapter 9), physiological maturity is attained, and ripening or dehydration begins. In hot summers the moisture content of the seed may be reduced to 10%, although a water content of 15% is usual in an average summer. Once this stage of desiccation is reached, the seed is usually shed from the parent plant. By this time it is physiologically inert and will remain in a dormant state (see Chapter 10) until the conditions are right for germination.
Fruits
The botanical definition of a fruit is a ‘seed receptacle developed from an ovary’ (see also Chapters 2 and 10). A consumer definition is more likely to concentrate on sensory qualities, the aromatic flavours and sweetness that distinguish a fruit from a vegetable. In nature, the aromas and palatable nature of most fruits are important to ensure that seeds are consumed and dispersed by animals. The tissues that form various fruits are derived from different parts of the flower. Some examples from temperate fruits that are common in gardens include the receptacle (strawberries, Fragaria × ananassa), accessory tissues (apples) and the placental tissue septum (tomato). Ripening is a term reserved for fruits and relates to changes in colour, texture, flavour and aroma. The state of ripeness at the point of harvest has particular implications for producers and consumers alike. Generally, fruits that are allowed to ripen on the plant achieve a higher sensory quality, but are more susceptible to damage during post-harvest handling and may have a reduced storage life. The biochemical changes associated with fruit ripening and the controlling mechanisms continue to attract much research attention. A better understanding of the ripening processes may eventually provide strategies for the more effective control of ripening.
Although fruit is important in a healthy diet, most people also eat fruit for enjoyment. The perception of flavour is an important part of sensory quality (see Chapter 13), and it is vital that procedures to extend the period of availability of fruits do not result in an unacceptable loss of taste and flavour. The taste and flavour of fruits are derived from a complex mix of sugars, acids, phenolic compounds and a wide range of volatile chemicals. The sugars and acids derive from photosynthesis, and in some fruits such as apples and pears the sugars are converted to starch during fruit development. Fruits that accumulate carbohydrates as starch are often picked at an immature stage and only achieve an acceptable taste and flavour during subsequent ripening when the starch is broken down again to form the sugars glucose, fructose or sucrose. Fruits continue to accumulate sugars from the plant during maturation and ripening, and for some, such as strawberry, this contributes significantly to flavour at harvest. Strawberries that are harvested too early will never develop sufficient sweetness and flavour for commercial acceptability.
Malic and citric acids are the main organic acids of fruits, and these are significant components of taste. It is therefore important to achieve the correct balance between the acid and sugar concentrations in the fruit. Excessive acidity is often associated with harvesting fruits in an immature or unripe state. The concentrations of acids decline during ripening and are complemented by corresponding increases in sugar concentration. Phenolic substances such as tannins are responsible for astringency and generally result in an adverse reaction from consumers. Although most fruits lose their astringency during ripening, this remains a major quality problem in fruits such as persimmon (Diospyros kaki) because of the presence of water-soluble tannins.
Specialised volatile flavour compounds are produced in ripening fruit and these provide the unique sensory character associated with the different types of fruit. In apples and pears, the major volatiles are aliphatic esters, although terpenoids and aldehydes may also contribute to flavour. In strawberries esters, alcohols, carbonyls and sulphur-containing compounds are important. In stone fruit, mostly of the genus Prunus, lactones have a role in flavour development, particularly in peaches (Prunus persica) and nectarines (P. persica var. nectarina).
The visual, structural and chemical changes that occur during ripening in soft fruits such as strawberry and raspberry (Rubus idaeus), stone fruits such as cherry (Prunus avium) and plum, and pome fruits such as apple and pear are associated with an orchestrated sequence of biochemical changes that are under genetic control. The extent to which these underlying changes progress determines the visual and eating quality of the product at the point of sale and consumption.
Vegetables
Vegetables are derived from virtually all types of plant tissue, and include underground organs such as swollen roots (e.g. carrots) and swollen stem tubers (e.g. potatoes, Solanum tuberosum), and above-ground parts such as leaf blades (e.g. spinach), axillary buds (e.g. Brussels sprouts), swollen inflorescences (e.g. broccoli) and seeds (e.g. sweet corn). From the consumers’ point of view, vegetables also include some fleshy fruits such as tomatoes and cucumbers, and immature fruits such as peas (Pisum sativum) and bean pulses (Phaseolus spp.). The part of the plant that the vegetable develops from has important implications for its behaviour during storage. Young tissues that are respiring and transpiring actively, such as the leaves of lettuce and spinach, are likely to lose quality particularly rapidly, and in commercial practice immediate steps are taken to reduce their rate of metabolism and transpiration by reducing the temperature.
The stage of development is also critical in the maintenance of quality, particularly in biennial vegetables where some kind of storage organ is formed in the first season, followed by flowering and seed formation in the second year. Although biennial vegetables are extremely varied morphologically, they all cease growth in the autumn. The rate of metabolism of their storage organs declines naturally, which makes them well adapted for long-term storage.
Cut flowers
There is a large variation in the structure of flowers grown commercially for cutting. Examples include corymbs (spray carnation), umbels (belladonna lily, Hippeastrum spp.) and spadix plus spathe (anthurium, Anthurium andraeanum). The functional life span of the flowers of different species varies from a few hours to several months, although for most commercial species the approximate storage period is a few days to a few weeks. Generally inflorescences have a low carbohydrate reserve and in this regard are similar to many leafy vegetables. They also have a large surface area in relation to their mass and this makes them very vulnerable to water stress. Flowers that have lost 10–15% of their original fresh weight are normally wilted.
Ethylene has a major role in the senescence of some cut flowers such as carnation, but some other species are insensitive to this hormone. These include Chrysanthemum cultivars, aster (Michaelmas daisy, Aster novi-belgii), sunflower (Helianthus annuus), nerine (Nerine bowdenii), liatris (Liatris spicata), zinnia (Zinnia elegans) and rudbeckia (Rudbeckia hirta).
Senescence in cut carnation flowers is characterised by a rise in respiration rate and ethylene production, and in this respect the carnation behaves much like a climacteric fruit. In carnation and some other species of flowers, pollination stimulates ethylene production and causes senescence of the petals. The functional significance of pollination-induced senescence may be to save energy and sugar reserves that would otherwise be used in maintaining elaborate flower structures, and to prevent further visits by pollinators such as bees.
The physiological processes that cause the deterioration of cut flowers have many similarities with those that operate in fruits and vegetables. This is not surprising, for some vegetables are derived from flower buds (e.g. artichokes, Cynara scolymus) or swollen inflorescences (e.g. broccoli). Decorative foliage plants used by florists (florist greens) are likely to behave in a similar way to leafy vegetables.
There are many causes of deterioration in cut flowers. The effects of disturbance in the vase have already been mentioned (see above). Depletion of food reserves reduces the amount of energy available to maintain cell structure and function and, as with all fresh horticultural crops, there is a risk of attack by bacteria and fungi. Excessive loss of moisture by transpiration causes wilting of both flowers and foliage. Bruising and crushing during handling may stimulate respiration and hasten the senescence process. Exposure of flowers to warm temperatures is likely to increase respiration and accelerate senescence, whereas in some flowers low temperatures may result in chilling injury (see below). Colour changes or fading may affect flower quality and acceptability, and in sensitive varieties the accumulation of ethylene in the storage environment may have serious consequences by accelerating the ageing process (Fig. 19.1).
Pre-harvest influences on storage quality
Diseases and disorders
Fresh fruits and vegetables are highly perishable, and spoilage during storage may occur as a result of attack by fungi, bacteria and viruses or by the development of functional or physiological disorders (see Chapters 17 and 18). Soft rots caused by bacteria such as Erwinia and Pseudomonas spp. affect practically all vegetables and especially carrot, celery (Apium graveolens var. dulce) and potato, but fungi cause most of the spoilage that occurs in fruit and vegetables during storage. Genera such as Penicillium, Botrytis and Sclerotinia commonly affect most fruit and vegetables, and are likely to affect produce stored by the gardener. Some of the physiological disorders of apple such as superficial scald, a skin browning disorder, and bitter pit (Fig. 19.2), an internal calcium-deficiency disorder characterised by brown ‘corky’ lesions, are also likely to occur.
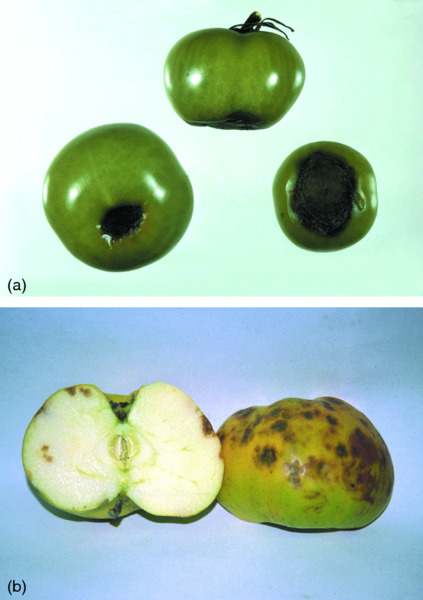
Figure 19.2 Blossom-end rot of tomatoes (Solanum lycopersicum) and bitter pit of apples (Malus spp.). Photographs courtesy of the Royal Horticultural Society.
Stems and leaves of cut flowers and florist greens are usually contaminated with bacteria and quickly contaminate clean vase water. Bacteria isolated from cut-flower containers include species of Achromobacter, Bacillus, Micrococcus and Pseudomonas. There is little information on the loss of quality or level of wastage in cut flowers that can be attributed to pathogens, but good hygiene combined with the use of biocides is usually sufficient to prevent the significant decay of most cut flowers.
Climatic factors
The potential of fruits and vegetables to develop particular diseases or disorders is influenced by climatic and field or orchard factors. The extent to which disease can develop in stored crops is determined by the number of viable pathogenic microorganisms present and their ability to infect the plants in question. Because the propagules of many of the important fungal diseases of apple are dispersed by rain, it is not surprising that wet growing seasons are associated with a higher incidence of storage rots in this crop. High rainfall in August and September is associated with greater rotting in stored ‘Cox’s Orange Pippin’ apples due to Gloeosporium spp. Infection of apples by Phytophthora syringae occurs during periods of heavy rain just prior to or during harvest, spores being spread from the soil to the fruit either by direct contact or by rain-splash.
Weather conditions during the development and harvesting of vegetable crops also affect their susceptibility to disease after harvest. For example, Phytophthora rot of winter white cabbage (Phytophthora porri) is most likely to develop when cabbages are harvested in very wet field conditions. Similarly, harvesting root crops in wet weather may result in considerable amounts of soil and disease inoculum being taken into store with the produce.
Climatic conditions during the development of the fruit affect the susceptibility of stored apples to physiological disorders. Cool wet summers are known to reduce the tolerance of apples to low storage temperatures, and in some years it may be necessary to raise the storage temperature slightly to avoid a deterioration of the fruit flesh referred to as low temperature breakdown. Dull sunless summers may increase the risk of core flush, which as the name implies is a physiological disorder that results in a pink discoloration in the core of the fruit. Warm dry summers increase the risk of deleterious conditions such as superficial scald, bitter pit and water core. In such years it is particularly important to apply chemical antioxidants to the harvested fruit to prevent scald development later in the storage period. Mathematical models based on temperature and rainfall measurements made in the orchard during fruit development have now been developed in order to predict the risk of physiological disorders in different consignments of ‘Cox’s Orange Pippin’ apples.
In many cases, control of post-harvest diseases is only possible by eradicating the causal organisms, or protecting the crop in the field or orchard before harvest. Examples of this include such fungal diseases as grey mould of strawberry (Botrytis cinerea), ‘bull’s-eye-rot’ of apple (Gloeosporium spp.), and ‘neck rot’ of dry bulb onions (Botrytis allii).
As most cut flowers are produced as protected crops under glass, it may be less relevant to consider pre-harvest climatic influences because these are largely under the control of the grower. If flowers are exposed to high light conditions before cutting, sugars accumulate and storage life is improved. Any subsequent exposure to light may also be beneficial, because cut flowers retain their capacity to photosynthesise and produce carbohydrates.
Seed vigour (see Chapter 10) and seed longevity are closely related, and other things being equal, high-vigour seeds will retain their viability longer than low-vigour seeds. Weather conditions during ripening may sometimes adversely affect seed vigour and storage potential. Excessive rainfall may lead to an increase in fungal infection, whereas cycles of wet and dry periods may damage the seeds as they swell and contract under the changing moisture conditions.
Field factors
Nutrition during the pre-harvest phase of fruits, storage organs and flowers can have a major impact on post-harvest quality. A great deal of research has been carried out on the effects of orchard factors on the storage quality of apples and pears. There have been numerous investigations of the effects of tree factors such as rootstock, age, cropping level and pruning, and of soil and nutritional factors and the use of orchard sprays such as fungicides, insecticides and growth regulators, and much of the wisdom gained from these findings can be applied to other fruit and vegetable crops that may be grown in open field or under protection. The importance of achieving adequate calcium nutrition in fruit and vegetables is universally recognised. Calcium deficiency impairs membrane permeability in plant cells, and membrane leakage is followed by a major disintegration of membrane structure and reduction in growth of meristematic tissues. An under-supply of calcium to apple crops, possibly in combination with adverse climatic conditions (see above), results in bitter pit, for which the most effective remedy is the repeated application of orchard sprays containing calcium chloride or calcium nitrate. The nutritional state of apples has such a major effect on their storage quality that mineral analysis standards are available for growers, to help them select the most suitable storage and marketing strategy for any particular consignment of fruit.
Calcium deficiency is also a major cause of losses in vegetable crops, causing blossom-end rot in tomato (Fig. 19.2), tip burn in lettuce, and blackheart of celery. Blossom-end rot can be controlled by ensuring sufficient application of calcium during the growing season, but tip burn in lettuce is more difficult to avoid, because calcium is a very immobile element in the plant, becoming integrated within the membrane structures and unable to be translocated from one part of the plant to another. Tip burn in lettuce is, therefore, very hard to control, as it tends to appear on new leaves produced once the deficiency threshold has passed, and these are often within the heart of a lettuce and not visible at harvest. Floral crops and some herbs may be exposed to high levels of phosphate prior to harvest, which has the effect of ‘hardening’ the crop and making it more robust when subjected to post-harvest handling practices that would otherwise induce excessive bruising and damage. Potassium has more recently been highlighted as an important element for increasing post-harvest quality and longevity, and many protected crops (i.e. those grown under glass or in plastic tunnels) are supplied with high concentrations of potassium during production. Alternatives to the application of inorganic ions in chemical fertilizers are being explored, and seaweed extracts have been found by commercial producers and gardeners alike to be highly effective nutritional supplements for fruit, vegetable and flower crops.
For gardeners who rely on self-saved seed for the next crop, the best way to produce high-vigour seeds that store well is to ensure that the mother plant has adequate nutrition, so that the seeds mature fully on the parent plant. It is also important to ensure that the mother plant has completed senescence before the seeds are collected, as this process of remobilizing nutrients from leaves and stems into the developing seeds is important, ensuring that the next generation will have a high germination rate and produce healthy seedlings.
Varietal factors
The strong genetic basis for the storage potential of different apple cultivars is well recognised, and despite the application of modern storage techniques maximum storage varies from a few weeks for cultivars such as ‘Discovery’ to up to 10 months for cultivars such as ‘Bramley’s Seedling’. There are also marked differences in the storage potential of pear cultivars and these are taken into account in storage recommendations provided to the UK fruit industry. Regardless of variety, however, the storage of pears is difficult for gardeners, as the fruits bruise easily and are hard to ripen to a satisfactory quality.
Harvesting, handling and preparation for storage
Time of harvest
The physiological maturity of a vegetable does not always coincide with its commercial maturity. With the exception of biennial types, most vegetables are picked at a stage of maturity determined by the demands of consumers or processors rather than at a stage that might provide a more sustained storage or shelf life. Thus there is often a distinction between physiological maturity and commercial maturity. Physiological maturity refers to progress through a series of developmental stages that typically include the growth, maturation, ripening and senescence of an organ or organism. Commercial maturity reflects the stage at which the market requires the plant organ. For example artichoke, broccoli and cauliflower are harvested when the inflorescence is sufficiently well developed. Cucumbers, green beans (Phaseolus vulgaris), okra (Abelmoschus esculentus) and sweet corn are marketed as partially developed fruits. The time of harvesting of biennial types is determined by their progress to the dormant state, and harvesting is undertaken when growth ceases in the autumn. At this time the diminished rate of metabolism (respiration) makes them suited for long-term storage.
There may be other factors that determine the time of harvest, including the development of pest and disease problems in vegetables left in the ground, and the likelihood of adverse weather conditions that may affect the ability to harvest. Time of harvest may be critical in order to prevent loss of quality in the harvested product. For example, late harvesting of onions (Allium cepa) leads to a greater proportion of split and shed skins and to the staining of bulbs left in the field. In commercial horticulture onions are therefore harvested in the green. This is made possible, however, by facilities for fast and efficient drying, and unfortunately such facilities are not usually available to gardeners. There are, however, a few exceptions: immature seeds of Ranunculaceae produce seedlings more readily than mature seeds, whereas some capsules, such as those of hellebores (Helleborus spp.), release their seed before they are completely dry, so must be collected early.
Most of the ripening changes in non-climacteric fruits such as strawberries and cherries take place while the fruits are still attached to the plant, so these are normally harvested when they are fully ripe, at optimum consumer quality. Although climacteric fruits such as plums, apples and pears will also ripen on the tree, they are commonly harvested in an under-ripe condition but at a stage where optimum consumer quality will be achieved after storage. Avocados are an exception and only ripen when detached from the plant.
For the long-term storage of apples, the fruit must be picked before the onset of the climacteric rise in respiration rate. This applies to storage by both gardeners and commercial growers. Commercial judgements of the correct time to pick apples for long-term storage are based primarily on starch content, firmness and sugar concentration. Where there is no requirement for storage, apples are left on the tree to ripen, leading to superior eating quality.
The main objective with cut flowers is the longest possible vase life, with flowers reaching their full floral development and being fully open in the vase. Flowers are harvested at various stages of maturity in different parts of the world; US purchasers prefer to buy more open flowers that are showing their final shapes and colours, whereas most European purchasers prefer to buy flowers in the bud so that they open some time later in the home. Many types such as carnations, lilies such as Alstroemeria and Lilium longiflorium, and daffodils (Narcissus pseudonarcissus) may be picked at the bud stage, but others such as orchids (Orchidaceae) should be fully developed before they are cut.
Handling
Fruits and vegetables are susceptible to decay caused by a wide range of bacteria and fungi. Consequently great care is needed in both harvesting and post-harvest handling to avoid damage that might allow infection by pathogens. Commercial fruit crops destined for the fresh market are picked by hand to avoid damage, but those for processing, such as blackcurrants (Ribes nigrum), may be harvested mechanically. Harvesting field vegetables by hand may not be feasible, particularly with root crops, and particular care is required with mechanical harvesting as damage may result in direct loss of visual quality, internal damage such as bruising in potato, and access to pathogens.
Cut flowers and herbs are highly perishable and should be handled promptly and carefully. Any physical impacts will damage and bruise the blooms, thereby affecting their visual quality and reducing vase life by stimulating ethylene production. Hygiene is particularly important: dead or decaying plant material remaining in containers used for fresh cut flowers is likely to be a source of ethylene and of microorganisms that may cause decay. Some of these, particularly bacteria, are implicated as a cause of stem blockage in freshly harvested flowers, reducing water uptake and hastening the onset of wilting. Additionally, knives used for cutting bunched herbs to a consistent stem length need to be kept sharp and sterilised frequently to avoid introducing bacteria into the cut stem ends.
Seeds need to be handled carefully as they do not store well if damaged. After collection they should be dried quickly at a relatively cool temperature before being transferred to storage conditions.
Post-harvest treatments
Various treatments may be applied to harvested horticultural crops before they go into storage; all are aimed at maintaining quality or minimising decay. Some crops, such as dry bulb onions, require fast and efficient drying until the necks of the bulbs are tight and dry. This may be achieved by blowing heated air through the store until the onions have lost about 3–5% of their original weight. In countries where fine weather after harvest is normally experienced, adequate curing of onions may be achieved in the field over a period of 2–4 weeks. In subsequent storage a comparatively low relative humidity (65–70%) is required in order to prevent re-rooting and growth of shoots, and to minimise the development of ‘neck rot’.
Potato tubers require ‘curing’ after harvesting, to stimulate the production of suberised corky periderm tissue and thereby reduce moisture loss during storage, and to increase resistance to infection by Fusarium spp. and other rot-forming organisms. The periderm, comprised of corky cells, that forms around wounds inflicted during harvesting effectively heals cuts and bruises and thereby forms a physical barrier to infection. Potatoes are cured at temperatures of 10–15.5°C and 95% relative humidity for up to 2 weeks. Washing may be important to remove surface deposits of debris, dirt and sap from certain crops such as carrots, but in others contact with water may increase the spread of disease. Where washing is required, clean water is essential and disinfectant may be added to the rinse water to kill bacteria and fungal spores.
Other post-harvest treatments include waxing, to enhance appearance and reduce water loss in commodities such as apples, citrus and sweet potatoes (Ipomoea batatas), and the application of chemicals to suppress sprouting, maleic hydrazide and chlorpropham (CIPC) being the compounds most commonly used. Maleic hydrazide is applied to the potato crop prior to harvest and is translocated to the developing tubers, where it arrests cell division but does not limit cell expansion. It is important to time the application correctly in order to prevent tuber yield and size being limited. Chlorpropham is applied to potatoes 2–3 weeks after they have been placed in storage, to prevent cell division and hence sprout formation.
Fungicide treatments to prevent post-harvest infection in potato include fludioxonil, azoxystrobin and difenoconazole. Few treatments are available to prevent post-harvest losses in apples and pears, as these are usually consumed raw and consumers are very wary of pesticide residues. Superficial scald, a physiological condition that affects many apple cultivars grown in hot dry conditions, is usually only a serious problem in the United Kingdom on the culinary cultivar Bramley’s Seedling. It occurs following the death of cells in the epidermal and hypodermal layers of the fruit as a result of the oxidation of alpha-farnesene, which occurs naturally in the cuticle, into harmful conjugated triene compounds. Superficial scald in Bramley’s Seedling fruits can be prevented by the application of full-strength (2000 ppm) diphenylamine (DPA), an antioxidant, where fruit is to be stored beyond November in ventilated controlled atmosphere (CA) conditions, with 8–10% CO2. Where possible the regulation of storage atmosphere is used in place of chemical treatment, so fruit stored in scrubbed, low-oxygen stores at 5% CO2 + 1% O2 need not be DPA treated where fruit is to be marketed by the end of March. Fruit stored in this atmosphere for longer periods should be treated with half-strength DPA solution.
Post-harvest treatment with calcium is mainly restricted to apples, and in particular to cultivars such as ‘Cox’s Orange Pippin’ and ‘Egremont Russet’, which are susceptible to a range of physiological disorders caused by insufficient accumulation of calcium in the fruit during development, notably bitter pit. Although affected fruits are edible, the pitted areas are bitter in taste and severely affected fruits are unpleasant to eat.
The use of preservative solutions throughout the marketing chain is particularly important to maintain the quality and vase life of cut flowers. Several commercial preservative materials are available. These usually contain sucrose to provide the energy that the flowers require in order to maintain normal cell functions and to complete development, if this is not already completed at the time of harvest. Biocides such as 8-hydroxyquinoline citrate and aluminium sulphate, are included to kill microorganisms. An acidifying agent such as citric acid is also included to improve water uptake by lowering the pH. Low-pH water (pH = 3.5) travels faster in the water-conducting vessels of the plant (xylem), thereby preventing or reducing the wilting that frequently occurs in field-grown flowers. The use of ethylene inhibitors as a pulse treatment after harvest has already been described (see above).
The storage environment
Fresh produce
The deterioration of fruits, vegetables and cut flowers after harvest may be expected to occur under conditions that accelerate the rate of respiration and promote water loss, notably where temperatures are high and humidity is low. The consequences of such conditions will vary according to the extent to which a particular type of produce is adapted to withstand stressful conditions.
Temperature is one of the most important factors affecting the keeping quality of horticultural produce. Lowering the temperature reduces the rate of respiration and other metabolic processes, and thereby reduces the rate of senescence and of ripening in fruits. The highest freezing point for fruits varies between −3.0°C (pomegranates, Punica granatum) and −0.3°C (avocados), and for vegetables between −2.2°C (Jerusalem artichokes, Helianthus tuberosus) and −0.1°C (endive and escarole, Cichorium endivia). The highest recorded freezing point for blooms varies from −0.5°C for Easter lilies (Lilium longiflorum) and roses (Rosa spp.) to −0.7°C for carnations.
It might be assumed that the longest storage or shelf life of fruits, vegetables and cut flowers is likely to be achieved by maintaining temperatures at slightly above their freezing points, and the recommended temperature for the storage of most types of vegetables of temperate origin is 0°C. This applies to many of the field vegetables grown in the United Kingdom such as roots and onions, brassicas and legumes. Even at 0°C the storage life of vegetables varies from 5–8 days (sweet corn) up to 8 months (onion).
There are a number of plants, however, particularly those originating in tropical or subtropical regions, that are injured when stored at temperatures well above freezing. For such plants the full potential benefit of refrigeration cannot be realised. Chilling stress leads to an altered metabolism and the development of a range of injury symptoms. Among vegetables affected by chilling injury, probably the most economically significant are squash (Cucurbita spp.), cucumber, aubergine (Solanum melongena), tomato, snap beans (Phaseolus vulgaris), sweet pepper (Capsicum annuum) and potato. The storage of potatoes at temperatures below about 6°C results in the hydrolysis of starch to form sugars. This ‘low-temperature sweetening’ imparts an unacceptable dark colour to potato crisps and chips.
Chilling-sensitive fruits that are most familiar to UK consumers include avocados, bananas (Musa spp.), grapefruits (Citrus × paradisi), lemons (Citrus limon), mangoes (Mangifera indica), melons (Cucumis melo) and pineapples (Ananas comosus), all of which are tropical in origin. Of the fruit types important in UK production (apples, pears, plums, cherries, strawberries, raspberries and blackcurrants) only apple is regarded as susceptible to chilling injury and this is highly dependent on the cultivar. Cut flowers are commonly kept at about 4°C at the wholesale level and during transport, but there are some types that require much higher temperatures in order to avoid chilling injury. These include some orchids such as Cattleya (7–10°C) and Anthurium (13°C). Chilling injury may result in failure of flowers to open properly or discoloration of sepals and petals. Many herbs are native to hot climates and can be susceptible to chilling. The primary example is basil, which rapidly shows blackening and breakdown of the leaves if it is exposed to temperatures below 12°C for any length of time.
Low-temperature storage methods
Various types of mechanical refrigeration systems are used for the commercial storage of fruits and vegetables. The construction and refrigeration systems required for any particular application are dictated by the characteristics of the produce, and in particular by the rate of cooling required to maintain quality after harvest. Suggested cooling times vary from 3 hours for highly perishable products such as soft fruit, sweet corn, asparagus, calabrese (Brassica oleracea) and spinach, to up to 6 weeks for potatoes and onions. Quick methods of cooling such as forced-air cooling, vacuum cooling and hydro-cooling are required to remove the field heat rapidly from perishable crops. Forced-air cooling has been increasingly used to pre-cool cut flowers that are already packed into ventilated crates prior to shipment under continuous refrigeration. Forced-air systems are available that maintain a high humidity (95–98% relative humidity) while cooling to the required temperature within 1 hour.
Precise control of the temperature is a major factor limiting the ability of the gardener to store fruits and vegetables effectively. However, commercially, refrigeration is of prime importance in extending the storage and shelf life of fresh horticultural crops. It is particularly important in the distribution of highly perishable vegetables such as asparagus and lettuce and soft fruits such as strawberries and raspberries, although the use of refrigeration for these types of crops cannot extend the season significantly. This can only be achieved by the choice of cultivar, repeated sowing and cultural techniques. The use of refrigeration as a means of extending the marketing period is most appropriate for perennial fruit trees that produce one crop a year.
Root crops such as carrots and parsnips (Pastinaca sativa) are generally kept in the ground during the winter, when lower soil temperatures reduce respiration rate and developmental changes, and high soil moisture prevents desiccation. Gardeners can extend ground storage time of carrots by covering the soil with straw and pinning it down with horticultural fleece, or by making a smaller version of an agricultural ‘clamp’, which farmers use to store root crops such as potato, turnip and sugar beet (see section on vegetables under ‘Non-commercial storage’, below).
Refrigeration is the major method of slowing deterioration and extending the life of cut flowers, and is used extensively during transport and distribution. It is perhaps more appropriate to talk about refrigerated transport than storage of cut flowers. For most species maximum storage life may be achieved without water in moisture retentive containers at −0.5 to 0.5°C. This dry-pack method of storage has extended the storage life of many cut flowers such as carnations and roses. For some products, for example potted miniature roses, a conditioning period during the pre-shipment phase, where the plants are exposed to a cool, dark, dry environment, can help them adapt and survive the more extensive period of the same conditions when they are subjected to road freight or shipping.
Controlled atmosphere (CA) storage
The effectiveness of refrigeration in extending the storage life of produce is limited by the susceptibility to chilling injury of each type of fruit or vegetable. An important advance was the discovery that modifying the storage atmosphere, by increasing the concentration of carbon dioxide and decreasing the concentration of oxygen, could extend the life of fruit and vegetables beyond that obtainable by cold temperatures alone.
Lowering the oxygen concentration depresses the rate of aerobic respiration by decreasing the amount of oxygen available for oxidative reactions. The oxygen concentration recommended for CA storage of apples is typically 1–2% (air is normally 21%). However, if the oxygen concentration is too low, anaerobic respiration may occur, leading to the production of fermentation products such as ethyl alcohol and ethyl aldehyde that would impart off-flavours to the fruit. Consequently, precise control of the atmosphere within the storage chamber is extremely important.
The carbon dioxide (CO2) concentration for stored apples may be as high as 10% (air is approximately 0.04% CO2). Because CO2 is the end product of aerobic respiration, increasing the ambient concentration may lead to a reduction in respiration rate due to feedback inhibition. However, even at a concentration of 5% there is little effect on respiration rate, and it is now known that CO2 increases storage life by inhibiting the production of ethylene, as discussed above.
CA storage has major advantages over cold storage in air for the preservation of many types of fruits and vegetables, and the technique is widely practised in many of the world’s production areas for horticultural crops. Recommended concentrations of oxygen and carbon dioxide are available for apples, pears, Nashi (Asian pear, Pyrus pyrifolia), fruits other than pome fruits, and vegetables. Several thousand tonnes of UK-grown onions are stored in CA in order to extend the period of availability, and winter cabbage is kept for long periods in CA storage to provide continuity of supply to the fresh market and for processing into coleslaw. Although CA was traditionally practised in purpose-built stores the technology has progressed to include shipping containers and the packaging of produce in semi-permeable films to generate CA conditions within the package. This latter form of regulating the atmosphere around produce has been taken further in the development of modified atmosphere packaging (MAP). A substantial industry manufactures polymer films of known (usually around 40 μm) thickness and with laser-induced microperforations to allow gas exchange with the external environment. It is becoming increasingly common to induce a modified atmosphere within the bag at the time of packing, particularly in cut fresh lettuce and fruit crops. This prevents the browning caused by oxidation of phenolic compounds, which would otherwise render the product unsaleable. Consumers may notice that some of these products will last well in the refrigerator if they are unopened, but deteriorate rapidly if the pack is opened and only partially consumed. This is because the modified atmosphere inside the pack is lost as soon as the bag is opened, so oxidation then proceeds rapidly.
CA technologies are generally unavailable to the gardener, although storing apples and pears in polythene bags has been recommended as a means of reducing water loss and preventing shrivelled or leathery fruit. The bags should not be tightly sealed, otherwise oxygen will be depleted and carbon dioxide will accumulate to damaging concentrations. Some outlets now provide storage bags for increasing the shelf life of fruit and vegetables to be kept in the refrigerator, and these are essentially microperforated bags similar to those used for bagged salads.
CA storage is not generally recommended for cut flowers because of the small margin of safety between effectiveness and phytotoxicity, the small volume of any one cultivar and the high cost of treatment.
Seeds
Seeds are stored for a variety of reasons: to maintain seed stocks for growing from one season to the next; to keep stocks for sale; to maintain breeding lines; and to conserve genetic material. Food and grain seeds are stored before processing and consumption. The basic difference between storing fresh produce and seeds is that the latter are already dry, and storage methods aim to restrict water uptake rather than to reduce water loss.
Seed vigour and seed longevity are closely related and, other things being equal, high-vigour seeds will retain their viability for longer than low-vigour seeds. Thus the factors that increase vigour (see Chapter 10) will also normally increase longevity. Similarly, the genetic constitution affects both vigour and storage potential. Seeds of onions, parsnip and lettuce have low vigour and store less well than, for example, the high-vigour, starchy seeds of round-seeded peas. However, the conditions under which the seeds are dried and stored are also extremely important in determining longevity.
Because all seeds lose vigour and viability during storage, it is important to establish the optimum storage conditions for different types of seeds. In 1973 Roberts divided seeds into two broad categories, depending on their storage behaviour. Seeds that can be dried to low moisture contents and stored at −18°C for long periods are termed orthodox seeds, whereas those that are killed if their moisture content is reduced below some relatively high level (12–31%) are termed recalcitrant seeds. The latter include many tropical species, especially tropical fruits such as mangoes (Mangifera indica), coconuts (Cocos nucifera) and jackfruit (Artocarpus heterophyllus), as well as several large-seeded temperate species such as oak (Quercus robur), horse chestnut (Aesculus hippocastanum) and sweet chestnut (Castanea sativa). More recently an intermediate group has been identified, where drying below 10–12% moisture results in early loss of viability; coffee (Coffea arabica) belongs in this group.
Damaged seeds do not store well, and it is important to minimise damage during and after harvesting. A common cause of post-harvest damage with consequent loss of viability is drying the seeds at too high a temperature. There is no real ‘safe’ temperature at which to dry seeds, but seed banks minimise deterioration and thereby safeguard longevity by drying at 15°C and 15% moisture content of the air. Recent research has enabled scientists to preserve seeds at ultra-low temperatures using cryopreservation. This approach is being used to safeguard the longevity and diversity of rare plants that might be lost if seeds were stored conventionally.
Once the seeds have been dried, their longevity is strongly influenced by their moisture content and by the storage temperature. Seed longevity is related directly to the interaction of a number of environmental factors, notably temperature, moisture content of the seed and oxygen levels. The relationship between temperature and seed moisture content is an interesting one. Generally, the lower the moisture content and the lower the temperature the greater the seed longevity, as under these conditions the seeds are maintained in a state of suspended animation. Thus the wetter and warmer the seeds are in storage, the faster they lose viability and vigour. Therefore orthodox seeds should be stored under dry and cool conditions. To maintain the viability of orthodox seeds for as long as possible, the ideal conditions are hermetic storage at a temperature below −18°C (the lowest limit of a domestic freezer) and a moisture content below 5%. In these conditions metabolic processes are reduced to a minimum level. Predictions of storage longevity under these conditions are given in Table 19.1.
Table 19.1 Estimate of probable regeneration intervalsa for seeds stored at −20°C and 5% moisture content
| Plant | Cultivar | Probable regeneration interval (years) |
| Barley | ‘Proctor’ | 70 |
| Rice | ‘Norin’ | 300 |
| Wheat | ‘Atle’ | 78 |
| Broad bean | ‘Claudia Superaquadulce’ | 270 |
| Pea | ‘Meteor’ | 1090 |
| Onion | ‘White Portugal’ | 28 |
| Lettuce | ‘Grand Rapids’ | 11 |
aThe regeneration interval is the predicted time for viability to fall to 95% of its initial value. Thus, it will take the barley cultivar ‘Proctor’ 70 years to fall from 99% viability to 94% viability, if stored under optimum conditions.
Based on data in Roberts, E.H. & Ellis, R.H. (1977) Prediction of seed longevity at sub-zero temperatures and genetic resources conservation. Nature, 368, 431–3.
Trends in commercial storage
Over the past decades there have been major changes in the storage requirements for horticultural crops. Storage initially provided consumers with fresh produce during the winter months, and the major objective was to provide producers with the means to store various crops without undue wastage. In due course, advances in storage technology for permanent structures and for transportation contributed to international trade, and eventually the seasonal aspect of fruit and vegetable production was eroded. In a highly competitive global market it is now increasingly important to provide fresh produce of the highest visual and eating quality, while the diversity of fruit and vegetables that consumers expect throughout the year has increased exponentially; consequently very few people eat truly seasonally, and most supply chains have a global dimension for at least part of the year. The development of storage recommendations has reflected the increasing demand for high standards of produce quality, in addition to the control of diseases and disorders, against a background of decreasing permissions for chemical usage.
As consumer demands for quality change in the more health-conscious society of today, storage techniques are being developed that maintain or improve components of food that contribute to a healthy diet. Research is being directed towards maximising sensory attributes such as taste, aroma and texture, essential nutritive compounds such as carbohydrates, proteins, vitamins and minerals, and bioactive substances such as polyphenols, carotenoids, phyto-oestrogens and dietary fibre. There is also increased consumer awareness of undesirable attributes such as mycotoxins and pesticide residues, and stringent safety standards are already in place across all developed countries.
Changes in consumer awareness of the health, nutritional and ecological aspects of food quality are leading to continual changes in the market place; future developments in pre- and post-harvest management practices will reflect these changing needs. To provide fruit and vegetables in the freshest condition possible there will be increased use of cool-chain marketing, chilled display cabinets in retail shops and perhaps an increased use of polymeric film packaging. The demand for ready-to-use fruit and vegetables is likely to grow, with consumers willing to pay for both quality and convenience.
Non-commercial storage
Although this chapter is not intended primarily to provide practical guidance on the storage of garden products, it may be useful to summarise key aspects of post-harvest biology and storage, to help gardeners manage their own produce effectively after harvest.
Seeds
These should be collected as soon as they are ripe and laid out to dry in seed trays lined with paper. Where seeds are thrown from the ripening pods as with Erodium spp., or shaken out as with Papaver spp., the seed heads should be collected as they approach maturity and ripened in paper bags.
The best way to store seeds is to keep them cool and dry. A popular and effective method is to keep them in an airtight container, preferably with a desiccant, in a refrigerator. However, it is important that the container is hermetically sealed, otherwise the seeds will equilibrate with the moisture content of the refrigerator. This will hasten their deterioration, even though they are being stored at a low temperature.
When the seed is removed from the refrigerator, it should be allowed to equilibrate with the air temperature before the container is opened. This ensures that the warm ambient air, which has a higher humidity than the air in the container, does not rapidly enter the seeds, possibly causing membrane or cell damage.
For long-term storage the best practice is to dry seeds thoroughly, place them in an airtight box over a desiccant such as silica gel and keep them in a freezer at a temperature of −13 to −15°C. If it is necessary to after-ripen the seeds (see Chapter 10) they should be kept for 2–3 months before being frozen. For short-term storage, when seeds are to be sown the following year, it is sufficient to put completely dry seeds into sealed paper bags and store them in a cool dry place.
Soft fruits
Soft fruits such as strawberries and raspberries are highly perishable and should be picked when mature but not over-mature. Eating quality will not improve after harvest. Fruits should be picked during the coolest part of the day and stored in a refrigerator until required. It is important not to overload the refrigerator, because the heat produced by the metabolising fruit may raise the temperature to a level that is not only inappropriate for fruit storage but also exceeds that recommended for other stored foods.
Stone fruits
Stone fruits also benefit from refrigerated storage, but it is unlikely that a domestic refrigerator has sufficient capacity to store significant quantities. Cherries should be picked when they have achieved the desired eating quality, but plums should be harvested slightly under-ripe, when the background colour is green/yellow as opposed to yellow, if they are to be stored. Only perfect fruits should be kept, as any physical damage or lesions caused by insects or disease are potential sites for infection by rot-forming organisms and can cause healthy fruit in the same batch to deteriorate.
Pome fruits
Of these, apples are most amenable to storage, particularly cultivars that mature late in the season, from the end of September onwards. Apple cultivars differ markedly in their rate of ripening and senescence and consequently in their storage potential; they also differ in the ways that they interact with the storage environment. Gardeners should not attempt to store early varieties of apple, and it is not worth taking much trouble with mid-season varieties, but varieties picked from the middle of September onwards are likely to keep for periods varying from several weeks to several months. Of the major commercial cultivars currently in production in the United Kingdom, the most suitable for storage by gardeners include ‘Cox’s Orange Pippin’, ‘Red Pippin’ (‘Fiesta’), ‘Bramley’s Seedling’, ‘Gala’ and ‘Jonagold’.
Gardeners do not have access to the sophisticated methods of determining harvest maturity that are used commercially. The general recommendation is to pick apples for storage when the fruits can easily be detached from the tree by gentle twisting, and before the best eating quality in terms of sugar content and aromatic properties has been achieved.
The need to select only perfect apples for storage cannot be over-emphasised. They should be kept in a well-ventilated, cool (4°C) frost-free cellar or outbuilding. Storage in perforated polythene bags is preferred to prevent the shrivelling that some cultivars are especially prone to. The bags must not be sealed completely, otherwise the oxygen in the bag will be depleted and alcoholic off-flavours develop. Because the apples are in contact, there is the potential for the spread of disease within the bags, so regular inspection is necessary to remove any rotted fruits. A more traditional method is to wrap the fruits in newspaper and store them in single layers in wooden, slatted trays.
Gardeners may improve the storage potential of apples by spraying their trees with 0.8% (w/v) flake calcium chloride at intervals of 10–14 days from late June to harvest. Damage to the foliage may be avoided by spraying at temperatures below 21°C, preferably in the evening. Post-harvest application of calcium compounds such as calcium chloride provides an additional means of supplementing calcium in the fruit.
The storage of pears presents difficulties for the gardener, particularly in handling and ripening to a satisfactory quality, regardless of cultivar. Pears do not ripen properly when left on the tree and are difficult to store because they ripen unless kept below 0°C. Sound pears should be kept as cool as possible and inspected regularly. The extent of softening may be gauged by gentle squeezing. Ripening should be completed by transferring the fruits to room temperature. Although polythene bags may be used for pears, it is particularly important to provide sufficient ventilation, otherwise the build-up of carbon dioxide and the depletion of oxygen may damage the fruit.
Vegetables
The traditional method of storing vegetable root and tuber crops is to make a clamp or ‘pie’. This was the original method of storing potatoes following their introduction into the British Isles during the sixteenth century. Garden clamps are used mostly for potatoes, but also for other vegetables such as beets, carrots, swedes (Brassica napus) and turnips (B. campestris). Clamps are piles of roots or tubers on a straw base, often triangular in vertical cross-section, that are covered with a layer of straw and a layer of soil to provide protection from frost and rain. Clamps should be constructed on well-drained land and have a trench dug out around the structure to provide drainage for rain running off the sides. Ventilation holes plugged with loose straw are required in the ridge of the clamp to prevent a build-up of carbon dioxide and the depletion of oxygen through respiration. Only healthy roots or tubers should be placed in the clamp. When storing potatoes, it is particularly important to exclude light to prevent greening and the concomitant build up of toxic alkaloids in the tubers. Exposure to below-freezing temperatures leads to the breakdown of starch and the development of ‘sweetening’.
Onions are particularly suitable for storage by the gardener. They should be harvested when the foliage is brown and brittle, pulled or dug from the ground on a fine sunny day and left on the surface of the soil to dry. When they are dry, remove any soil, dead roots and loose, dry skins. Store the onions in trays in a cool dry place, or rope them if preferred. Check the condition of the bulbs at intervals and use before sprouting signals the break of dormancy.
Cut flowers
Although the gardener is not likely to need to store cut flowers, general methods of extending the vase life of flowers from the garden or from retail stores may be relevant. Cutting garden flowers at the bud stage may be appropriate for roses, lilies and gladioli. Flowers should be handled carefully and placed in fresh clean water, with leaves removed from stem sections below the water level. Microorganisms sometimes block the water-transporting tissues and lead to premature wilting, and the practice of cutting the base of stems every few days helps to ensure that stems continue to take up water. Proprietary preservative products should be used where available, and excessively warm, dry atmospheres avoided. A homemade preservative solution can be achieved by combining two teaspoons of sugar, a tablespoon of lemon or lime juice and a drop of bleach in 1 litre of water.
Conclusion
Wherever possible, practical advice has been provided on the storage of fruits and vegetables that might be grown by gardeners. In addition, many procedures used commercially are also described, and may provide the more adventurous gardener with a basis for experimentation. One of the joys of gardening is to keep experimenting, learning from one’s failures and rejoicing in one’s successes.
Further reading
- Copeland, L.O. & McDonald, M.B. (2001) Principles of Seed Science and Technology, 4th edn. Springer, Dordrecht, The Netherlands.
- Jackson, J.E. (2005) The Biology of Apples and Pears. Cambridge University Press, Cambridge.
- Kader, A.A. (2002) Postharvest Technology of Horticultural Crops, 3rd edn. University of California, Agriculture and Natural Resources, Publication 3311, 535 pp.
- Kader, A.A. (2005) Postharvest handling. In: The Biology of Horticulture, 2nd edn, Preece, J.E. & Reed, P.E. (eds), pp. 379–407. John Wiley & Sons, Inc., Hoboken, NJ.
- Kays, S.J. (1991) Postharvest Physiology of Perishable Plant Products. Chapman & Hall, London.
- Paliyath, G., Murr, D.P., Handa, A.K. & Lurie, S. (2008) Postharvest Biology and Technology of Fruits, Vegetables, and Flowers, Wiley-Blackwell, Oxford.
- Prusky, D. & Gullino, M.L. (eds) (2014) Postharvest Pathology: Plant Pathology in the 21st Century. Proceedings of ICPP 2013. Springer International Publishing AG, The Netherlands.
- Roberts, J.A. & Tucker, G.A. (eds) (1985) Ethylene and Plant Development. Butterworths, London.
- Seymour, G.B., Taylor, J.E. & Tucker, G.A. (eds) (2012) Biochemistry of Fruit Ripening. Springer, The Netherlands.
- Shewfelt, R.L. & Bruckner, B. (eds) (2000) Fruit and Vegetable Quality: An Integrated View. Technomic Publishing Company, Inc.
- Thompson, A.K. (2010) Controlled Atmosphere Storage of Fruits and Vegetables, 2nd edn. CAB International, Wallingford, Oxon.
- Thompson, P. (2005) Creative Propagation, 2nd edn. Timber Press, Cambridge.
- Wills, R., McGlasson, B., Graham, D. & Joyce, D. (1998) Postharvest: An Introduction to the Physiology & Handling of Fruit, Vegetables & Ornamentals, 4th edn. CAB International, Wallingford, Oxon.
Authors and affiliations
Rewritten and extended for the third edition by Carol Wagstaff, Associate Professor in Crop Quality for Health in the Department of Food and Nutritional Sciences, University of Reading (to replace Chapter 12 in the first edition/Chapter 17 in the second edition, written by David S. Johnson, then a Team Leader at Horticulture Research International, East Malling).