CHAPTER 20
Garden ecology
Summary
In this chapter plants and gardens are considered from an ecological perspective. Garden function is dependent on food webs and element cycling on local and global scales. How a plant performs in the garden is strongly dependent on its natural habitat. Ecosystem diversity is strongly affected by past events such as ice ages. It also depends on grazing animals, pests and diseases, which prevent individual plant species from becoming overwhelmingly dominant. The balance of nature is dependent on plant diversity but many natural systems are unstable.
Ecology and ecosystems
Ecology: open and closed systems
Ecology is the scientific study of organisms in relation to their environment. The subject emerged in the nineteenth century mainly from observations on natural and semi-natural vegetation. In the twentieth century, ecology became more experimental and habitats created by human activities, such as metal-contaminated waste sites, proved to be just as interesting as natural ones. The vital, often dominant role of microbes was revealed. The idea that organisms and their environment can be viewed as interconnected systems was rapidly apparent to those who studied lakes (limnologists). This view gradually spread to terrestrial ecologists, so that by the early twenty-first century the word ecosystem was in common usage and recognized by all environmentally aware people.
All ecosystems are to some degree open – that is, they receive some inputs from outside. A lake receives water and nutrients from its catchment. Very likely it also receives colonizing plants and animals. Experiments with closed systems almost always result in eventual collapse, but such systems may persist for a surprisingly long time. In horticulture the most celebrated example is the Wardian case, a sealed, glazed container invented by Nathanial Ward in about 1829 and used during the heyday of botanical exploration to bring living plants from remote parts of the earth back to Europe.
The ecosystem concept gives us a useful framework for thinking about the world. The earth contains very large tracts of vegetation such as forest, grassland or tundra. When these are of sufficient extent, they function almost as if they were closed systems. They receive water and nutrients from the atmosphere and export them in rivers and as gases; but these tracts of land are so big that outside influences are relatively unimportant. In smaller tracts of vegetation such as arable fields, outside influences in the form of added water, nitrogen or phosphorus are necessary for the system to remain functional. Arable fields may nevertheless sustain a characteristic fauna and flora, which can be studied using methods that might equally be applied to extensive natural systems.
Whole gardens do not generally function as ecosystems, because their components are a heterogeneous mosaic; a shrubbery functions as a woodland ecosystem, a lawn functions as a grassland and a vegetable plot functions as an agroecosystem (cropland). Furthermore, many animals such as birds and butterflies forage over areas much larger than the individual garden.
Food webs
Green plants are the basis of all terrestrial life, using sunlight to fix carbon from the atmosphere and producing energy-rich organic matter in the process. The pathways by which ecosystems convert plant matter into other life forms are numerous and varied. Energy, which may roughly be equated with organic matter, is the key. Ecologists often describe plants and animals by what they eat, their trophic level (Table 20.1). This is an imprecise concept because many animals feed at more than one trophic level. In practice, many ecosystems and almost all soils have exceedingly complex food webs. Gardeners are well aware of animals feeding at two trophic levels; for example slugs and snails eating both decaying matter and green plants. Likewise foxes eat earthworms (detritivores), mice (omnivores) and fruit (plant material). Mice living in a Welsh spruce plantation ate seeds (plant material), fungi (decomposers) and beetles (mainly carnivores).
Table 20.1 Trophic levels in gardens, soil and ponds
| Trophic level | Garden | Soil | Pond |
| Primary producers | Green plants | Plant roots, mycorrhizal fungi | Water plants, including algae |
| Herbivores | Butterfly larvae, aphids | Tipulid larvae | Water flea (Daphnia) |
| Carnivores | Ladybirds | Centipedes, moles | Dragonfly larvae, adult frogs |
| Decomposers | Fungi, bacteria | Fungi, bacteria | Bacteria, archaea |
| Detritivores | Woodlice, wood-boring beetles | Earthworms | Caddis larvae, water shrimps |
| Herbivores/detritivores | Slugs, snails | Millipedes | Snails |
The immense complexity of food webs defies simple diagrammatic representation, but the concept of trophic levels is useful. In particular, the efficiency of tissue production when one organism eats another is low, typically 10%. This results in the pyramid of numbers, with carnivores having less than 1% of the biomass of plants. Decomposers are vastly more efficient at converting biomass than animals. In agricultural soils, both fungi and bacteria typically retain 40–70% of the carbon when they decompose plant litter.
Among detritivores, earthworms are truly remarkable. Their living biomass often exceeds 1 tonne ha−1. They are major ecosystem engineers in many parts of the temperate zone, affecting decomposition rates and soil texture, and providing a food resource for numerous vertebrates.
Nutrient and carbon cycling
At the scale of the garden, cycling of carbon, water and the major nutrients nitrogen (N), phosphorus (P) and potassium (K) are matters of practical importance, discussed in Chapter 22 under the heading ‘Conservation of resources in the garden’. Cycling of other nutrients is usually irrelevant because they are in adequate supply. In most gardens, water and carbon come from the atmosphere, whereas phosphorus and potassium either come from the soil or are added in fertilizers. Nitrogen is intermediate, with some entering from the atmosphere, but most coming from the soil or fertilizers (Table 20.2). Imports to the garden are in organic manures, mulches, chemical fertilizers and tapwater. Outputs are as produce and refuse, or through combustion, respiration and leaching.
Table 20.2 Sources (inputs) and sinks (outputs) of water and elements in gardens. Key: *** = major source or sink; ** = sometimes a major source or sink; * = minor source or sink
| Inputs | Outputs | |||||
| Atmosphere | Soil | Import | Atmosphere | Leaching | Export | |
| Carbon | *** | ** | *** | * | * | |
| Water | *** | * | ** | *** | *** | * |
| Nitrogen | ** | *** | ** | ** | *** | ** |
| Phosphorus | * | *** | ** | * | ** | |
| Potassium | * | *** | ** | * | ** | ** |
Phosphorus and potassium are mineral nutrients, whereas carbon, water and nitrogen are recycled through the atmosphere. Small amounts of mineral nutrients are available in rainwater and dust. These are sufficient to maintain vegetation on the surface of raised bogs and blanket bogs in northern Britain, but are totally inadequate for the growth of plants in gardens. Relative to the requirements of plants, most soils contain more potassium than phosphorus, so that phosphorus is more likely to be limiting. In temperate ecosystems plants are relatively profligate with potassium, returning it to the soil when leaves become leaky in autumn. Nitrogen and phosphorus are more strongly recycled within the plant.
In horticulture as in agriculture, nutrients are lost by removal in produce and may have to be replenished. As explained under ‘Conservation of resources in gardens’ in Chapter 22, the main loss through leaching is nitrogen. This is because decomposition in the soil normally converts organic nitrogen into nitrate. Nitrates are highly soluble and leach out. Phosphorus and potassium, on the other hand, are retained by most soils, especially those with a moderate or high clay content.
Carbon cycling in the garden is as complex as the food webs that drive it. Organic matter enters the soil both from above ground as litter, such as autumn leaves, and below ground through growth of plant roots. Fine roots of most plants have a short life span and turn over at a high rate. They produce exudates and in most species support mycorrhizal fungi (see Chapter 2). They are the main producers of soil organic matter in grazed grassland and lawns. If old grassland is cultivated, the organic matter then decomposes over a period of years, releasing organically bound nitrogen as it does so. In many non-grassy ecosystems, nutrient-poor conditions favour relatively high root production and high root/shoot ratios. Where nutrients and moisture are in ample supply, most production is above ground.
Plant parts produced above ground decompose at very different rates (Table 20.3). As a general rule, plants decompose faster in nutrient-rich environments than in nutrient-poor ones. Several other factors play a part. Low winter temperatures and waterlogging delay decomposition. Hard tissues decompose more slowly than soft tissues. Lignin is decomposed almost entirely by basidiomycete fungi (see Chapter 16), and does not decompose at all in the absence of oxygen.
Table 20.3 Decomposition rates and decomposer organisms for selected types of plant litter
| Organic matter | Speed | Organisms responsible | Comment |
| Apples | Fast | Bacteria, fungi | Process may be delayed if an anaerobic slush is formed |
| Ash leaves | Fast | Bacteria, earthworms | Forms mull humus |
| Oak leaves | Medium | Fungi, esp. on acid soil | Speeded up by soil animals |
| Pine needles | Medium | Fungi | Speeded up by soil animals |
| Straw | Fast | Bacteria, fungi | Takes up nitrogen from environment |
| Wood | Slow | Fungi | High lignin content; low surface area; takes up nitrogen |
| Coir | Slow | Fungi | Very high lignin content |
| Sphagnum moss | Very slow | Fungi | Pectin-like polysaccharides in cell walls |
Animals play a vital role in most ecosystems. In experiments where animals are excluded, microbial decomposition may start normally enough. Then it stops because microbes require relatively high amounts of nitrogen and phosphorus in their cells, which typically have a C/N ratio of 7:1 and a C/P ratio of 42:1. When animals are present, they digest the microbes and return N and P to the litter or soil, allowing decomposition to continue. Moreover, animals break up the litter, so there is a bigger surface area to be attacked by microbes.
The more recalcitrant (slower-decomposing) types of litter are recalcitrant because they contain chemically complex substances such as lignin (see Chapter 2). The extreme case is sphagnum moss (Sphagnum spp.), which does not contain lignin, but equally complex polysaccharides; its remains form most of the peat in bogs.
Cycling at the global scale
Whereas ecosystems are never completely closed, the earth’s crust, oceans and atmosphere are themselves nearly so. Very long-term processes such as subduction of rocks from the crust back into the earth’s mantle are of crucial importance on geological time scales but are irrelevant to the rapid environmental changes produced by human activity. Almost everybody is aware that atmospheric CO2 is increasing as a result of humans burning fossil fuels and clearing forests (see Chapter 14). Atmospheric CO2 rose from 280 ppm (parts per million by volume) in preindustrial times to about 320 ppm in 1960. The period since 1960 has seen a rapid increase, from 320 ppm to 400 ppm at the time of writing. Annual carbon emissions from fossil fuels are still increasing and further increases in atmospheric CO2 are inevitable.
From a gardening perspective, the carbon fertilization effect, that is the effect of elevated CO2 in increasing photosynthesis and crop yields, is of particular interest. Since the 1980s, numerous experiments have been made to test the magnitude of this effect. When CO2 was raised from the then ambient levels around 350 pm to 550 ppm, photosynthesis was found to increase by up to 30% in C-3 crops but by very little in C-4 crops (see Chapter 8), which were already carbon-saturated. Increased photosynthesis was not proportionally matched by increased yields, which rose by about 12% for C-3 crops in open-air experiments and zero for C-4 crops. Yield increases were greatest where crops were well watered and had a good nutrient supply.
The increasing concentration of atmospheric CO2 is reducing the rate at which heat radiates from the earth’s surface into outer space, warming the planet. The resulting climate change will vary from one part of the globe to another. Scenarios for southern Britain indicate milder, wetter winters and increased summer drought. Such conditions will favour evergreen trees and deep-rooted shrubs, but will make it harder for the gardener to maintain annual bedding, green lawns and late-flowering herbaceous borders. Summer crops such as maize, courgettes, runner beans and potatoes will need more irrigation.
The global element cycles that control climate are almost unimaginably large. The carbon cycle is fascinating, complex and not fully understood. If global values in gigatonnes (GT) are converted to kilograms per square metre, however, they can be related to the experience of gardeners and land managers (Table 20.4). Atmospheric CO2 is mostly taken up by the sea and then slowly released; the average residence time of a CO2 molecule in the atmosphere is about 3.5 years. This gives time for mixing, so that open-air values do not vary much except near particular sources. Reactive (fixed) nitrogen in the atmosphere lasts only a few weeks, so that there is much local variation. About half of global nitrogen fixation is due to human activity, amounting to 0.21 GT annually. If this amount were spread evenly over the 80 million km2 of habitable land, it would be equivalent to 26 kg of nitrogen per hectare. Atmospheric deposition of nitrogen in the United Kingdom is fairly stable at 336 kT annually, equivalent to 14 kg ha−1. The areas of maximum deposition, greater than 20 kg ha−1 annually, are in the mountains of Wales, northern England and southern Scotland. A healthy lawn requires much more nitrogen than this; for example, lawn grass clippings in a Colorado town are calculated to remove 120 kg ha−1 of N per year.
Table 20.4 Global carbon stocks and rates of emission, compared with production in lawns and grassland (habitable land excludes deserts and mountains, and is reckoned at 80 M km2)
| Stock or annual rate | GT Ca | kg m−2 | Explanation |
| Standing stocks | |||
| Atmosphere | 850 | 1.7 | Calculated for 400 ppm CO2 |
| Oceans | 38000 | 100 | |
| Total emissions to 2013 | 320 | 4 | Mean for habitable land |
| Living land carbon | 500 | 6.2 | Mean for habitable land |
| Soil | 1500 | 19 | Mean for habitable land |
| Soil microbes 0–30 cmb | 17 | 0.21 | Mean for habitable land |
| Soil organic matter 0–30 cmb | 2 | 10-year Colorado greenspace | |
| Soil organic matter 0–30 cmb | 6 | 50-year Colorado greenspace | |
| Annual rates | |||
| Fossil fuel emissions | 8.5 | 0.11 | Mean for habitable land |
| Deforestation | 1.5 | 0.02 | Mean for habitable land |
| Grass clippings | 0.15 | Lawns in Colorado | |
| Hay yield at Rothamsted | 0.10 | Unfertilized | |
| Hay yield at Rothamsted | 0.30 | Fertilized | |
aGT C = gigatonnes carbon.
bDepth of soil from the surface.
Phosphorus, unlike carbon and nitrogen, is effectively not distributed through the atmosphere and is often absorbed in the topsoil. Therefore continuous fertilizer applications can result in substantial long-term accumulation of P. A study in southern Britain found that 580 kg ha−1 P had accumulated in tilled soils over a 28-year period, and 430 kg ha−1 in fertilized grassland.
Habitats
Climate and soil tolerance
It is a commonplace of gardening that for plants to thrive the climate and soil must be right. The Royal Horticultural Society in the United Kingdom and the US Department of Agriculture rate plants according to their hardiness. Plants differ also in their ability to survive dry summers or tolerate wet winters. Likewise some plants thrive in an acid soil and others in alkaline soil. Over the past 150 years, gardeners have become increasingly interested in growing plants that are not merely suited to the site but that can thrive there with relatively little attention. Such planting was vigorously championed by William Robinson in the nineteenth century. He wrote that his idea of The Wild Garden ‘is applied essentially to the placing of perfectly hardy exotic plants under conditions where they will thrive without further care. It has nothing to do with the old idea of the “Wilderness”’. A more interventionist approach to ecological gardening has been championed in England by Beth Chatto and has many followers. In the United States it is a thriving industry.
The factors controlling where a plant can grow are various. Charles Darwin wrote that ‘when we reach the Arctic regions, or snow-capped summits, or absolute deserts, the struggle for life is almost exclusively with the elements’. In more clement regions ‘each species, even where it most abounds, is constantly suffering enormous destruction at some period of its life, from enemies or from competitors.’ This is of course not the whole story. Many alpine plants are hard to keep alive in a temperate climate. Some, such as glacier buttercup (Ranunculus glacialis), have to be frozen over the winter; most have to be kept dry.
Species can adapt to some extent to changing climate but all have their limits (Fig. 20.1), so that in response to large climate changes they have to move to where the environment is suitable. One way of visualizing climatic tolerance is as a climate limit or climatic response surface. These are analogues of species distributions, but plotted in climate space. Climate response surfaces have been widely used for predicting the effects of climate change, based on the assumption that species’ tolerances are fixed. The method has been successful in predicting where the great biomes (see Chapter 1) of the earth are found. It is less reliable when applied to individual species. For example, beech (Fagus sylvatica) reached Britain about 1000 BCE but is restricted as a native plant to southern England and Wales. It never reached its potential climatic limit in Scotland. An extreme example is Monterey pine (Pinus radiata), which is confined as a native to the coast of southern California but has escaped from cultivation in many parts of the Southern Hemisphere. Its natural climate limits are far narrower than those that apply in its introduced range.
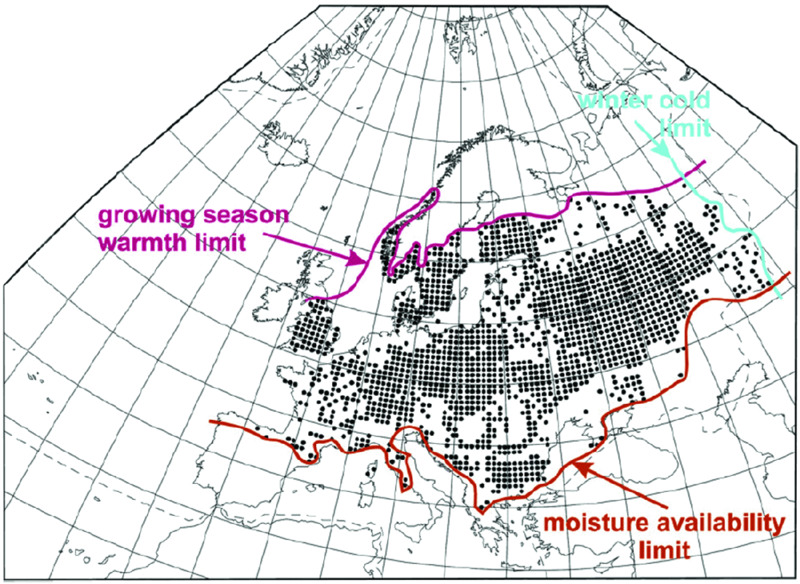
Figure 20.1 Limits to the natural distribution of small-leaved lime (Tilia cordata) in Europe. In the southern part of its range it is restricted to valleys with a humid microclimate. Its scarcity in western Europe is thought to be caused at least partly by woodland management. Data from Huntley, B. (2012) Reconstructing palaeoclimates from biological proxies: some often overlooked sources of uncertainty. Quaternary Science Reviews 31, 1–16.
Most plants in gardens grow well outside their natural climatic limits, because competition is reduced. In Britain, steppe grasses such as Stipa spp. can be grown alongside Arctic alpines such as mountain avens (Dryas octopetala) and Mediterranean plants such as lavender (Lavandula angustifolia). In natural broadleaved woodland, these light-demanding plants would be rapidly shaded out.
The soil tolerances of species cannot easily be plotted as a diagram. Just as many garden plants can be grown in climates to which they are not suited as wild plants, so also they can be grown in somewhat unsuitable soils. Broom (Cytisus scoparius) and foxglove (Digitalis purpurea) are confined as wild plants in Britain to acid soils, but cultivated strains can readily be grown in gardens at pH 8.0. Likewise stinking iris (Iris foetidissima) and shrubby cinquefoil (Potentilla fruticosa) are naturally confined to calcareous soils but have a much broader tolerance in cultivation. Plants are idiosyncratic in their tolerances; coping with these idiosyncrasies is one of the pleasures of gardening.
Natural processes and human land management
We have seen that plants can only thrive in the right physical environment. The effects of animals, including humans, are almost equally important. Grazing is the most important animal effect. Grasses are supremely adapted to be grazed by ungulates, hares and rabbits. When grass leaves are eaten, the base of the plant is left intact so leaves can sprout again. Most of the temperate zone is potentially either forest or grassland (steppe or prairie). Primeval forest, sometimes called wildwood, once occupied most of Europe and eastern North America. In Europe, almost none remains.
The difference between modern woodland and primeval forest is partly that large predators have been removed, along with beavers and wild boar. In the Middle Ages, deer were eliminated from most of southern Britain outside forests and parks. Now they are back in large numbers. The coppice woodland that William Robinson and Thomas Hardy knew so well has largely disappeared. This ancient form of woodland management is based on the principle that when young trees such as hazel, oak and ash are felled, they regrow from the base, yielding a supply of long, straight poles suitable for use in building and sustaining traditional woodland industries. If cutting was repeated on a roughly 10–20-year cycle, the tree bases (coppice stools) retained their juvenility and a continuous supply of poles was ensured. A bonus that came with traditional coppicing was that the regular felling of the trees allowed light into woodlands, thereby encouraging the profuse growth of spring flowers. With the move away from timber-framed buildings and the decline of traditional woodland crafts from the second half of the nineteenth century onwards, however, coppicing largely disappeared. There are still trees, but most have grown tall, with broad crowns that exclude sunlight. The result is that the wonderful displays of spring flowers that brightened woods as recently as the 1950s are seriously diminished, with the exception of a small number of woods where coppicing is still practised, such as that surrounding John Ruskin’s former home at Brantwood, near Coniston in Cumbria. The nuttery in Sissinghurst Castle Garden (Kent) derives its inspiration from these semi-natural coppice woods.
Outside woodland, grazing mammals maintain flower- rich grassland turfs, especially on chalk and limestone uplands. In the lowlands, hay-making is almost equally important in sustaining semi-natural grassland. It is hard for us nowadays to realise how nutrient-poor many grasslands were in the past. The turf was short and hard-bitten. The animals that fed on them grew thin in winter. Except on the most windswept coasts, grasslands are not natural in southern Britain. If grazing ceases, the land reverts to forest. There is extensive reversion to forest both in southern Europe and in the ‘old fields’ of eastern North America, but in the intensively farmed landscape of northwest Europe it is rarely seen.
Some methods of recreating flowery grassland and woodland are considered under ‘Habitat creation’, below. The basic principles are to keep coarse vegetation under control and make sure that plenty of light reaches the ground in spring. Soils with high fertility should be avoided, especially for grassland creation.
Slow-growing and fast-growing plants
If we ignore trees and shrubs and consider only herbaceous plants, there is still great variation. The biennial cotton thistle (Onopordum nervosum) will grow to nearly 3 m in just over a year (Fig. 20.2), whereas the perennial rock-rose (Helianthemum nummularium) will scarcely exceed 15 cm however long you grow it for. These two plants are intrinsically fast- and slow-growing. They exhibit their characteristic growth rates as seedlings and retain them up to the time they start flowering. Ecologists recognize these differences in terms of trade-offs. A large, fast-growing plant such as cotton thistle requires an ample supply of nutrients. If the nutrient supply is low, the plant is stunted and produces many fewer seeds. Both rock-rose and cotton thistle make excellent garden subjects but should not be mixed because they are rendered incompatible by their very different intrinsic growth rates.

Figure 20.2 Cotton thistle (Onopordum nervosum) 2.8 m tall is a fast-growing plant that requires a good supply of nutrients, but it roots so deeply that it is unaffected by summer drought. Photograph by Mark Hill.
One of the most obvious trade-offs is between rapid reproduction (r-selected plants, often annuals) and persistence in closed vegetation (K-selected plants). Plants that typically grow among ruins or in brownfield sites are called ruderals (from rudus, Latin for ‘rubble’). They have high reproductive output but do not persist. At the other extreme are plants such as rock-rose that persist indefinitely in closed, nutrient-poor vegetation. There also fast-growing plants such as nettle (Urtica dioica) and rosebay willowherb (Chamaenerion angustifolium) that persist indefinitely, forming dense, tall canopies. The plant ecologist J.P. Grime distinguished these two extreme persisting types as stress-tolerators (S) and competitors (C). He extended the meaning of ruderals (R) to include short-lived plants from a variety of non-rubble habitats such as arable fields. Grime’s terminology has attracted adverse criticism, mainly because S-type plants are not necessarily stressed by their environment, and C-type plants are herbaceous, excluding those most competitive of all plants, the trees. Grime’s types do, nevertheless, provide a convenient framework for consideration of plant behaviour in gardens.
Grime’s C, S and R types are examples of plant functional types, which are defined as groups of plants with consistent syndromes of traits. The syndromes corresponding to C, S and R include life history, growth rate, leaf toughness, palatability and even decomposition rate (Table 20.5). Broadly speaking, C-type plants maximize resource acquisition in consistently productive habitats; S-type plants maintain metabolic performance in unproductive habitats; and R-type plants complete their life cycle rapidly in habitats where events are frequently lethal to the individual.
Table 20.5 Syndromes of traits defining Grime’s plant functional types
| Plant trait | Grime type C | Grime type S | Grime type R |
| Life history | Long | Very long | Very short |
| Seed production | Delayed | Very delayed | Early |
| Life span of leaves and roots | Short | Long | Short |
| Potential growth rate | Rapid | Slow | Very rapid |
| Concentration of nutrients in leaves | High | Low | High |
| Concentration of carbon in leaves | Low | High | Low |
| Leaf toughness | Low | High | Low |
| Palatability | High | Low | High |
| Leaf decomposition rate | Rapid | Slow | Very rapid |
Data from Grime, J.P. & Pierce, S. (2012) The Evolutionary Strategies that Shape Ecosystems. John Wiley & Sons, Ltd, Chichester.
It is of interest to consider some garden plants in relation to their functional types (Table 20.6). Plants of type C and CR (CR is intermediate between C and R) are either species that should be placed at the back of the herbaceous border, or (as in the case of spearmint and horseradish) in a fertile part of the herb or vegetable plot where they will not invade and overrun other plants. The S plants are from infertile habitats, all except pasque flower coming from rocky ground. Bluebell, the SR plant, is difficult to classify in the Grime scheme; it and other bulbous plants do not fit Grime’s syndromes, being long-lived plants with delayed reproduction and leaves of low palatability (S characteristics), but having nutrient-rich, rapidly decomposing leaves and short-lived roots (R characteristics). Two of the R plants are signified as cornfield weeds; their natural habitat, like that of the cereals among which they grow, would probably have been semi-desert with abundant annuals. Cabbages in cultivation are CR plants, but wild cabbage on cliffs is a perennial of type CS. Persistent feral populations of parsley are found mainly on seashores in Britain; its natural habitat in Europe has been obscured by millennia of cultivation.
Table 20.6 Selected garden plants classified by Grime’s functional types; categories CR and SR are intermediate between the standard types
| Garden plant | Grime type | Habitat as a wild plant | Origin |
| Armenian cranesbill (Geranium psilostemum) | C | Tall herb-rich meadows | Turkey |
| Spearmint (Mentha spicata) | C | [Garden origin] | S Europe |
| Horseradish (Armoracia rusticana) | C | [Unknown] | Europe |
| Goldenrod (Solidago canadensis) | C | Dry ground near rivers | N America |
| Cotton thistle (Onopordum nervosum) | CR | Open stony ground | SW Europe |
| Lavender (Lavandula angustifolia) | S | Dry rocky scrub | S Europe |
| Thyme (Thymus vulgaris) | S | Dry rocky ground | S Europe |
| Chives (Allium schoenoprasum) | S | Rocky pasture | N Europe |
| Pasque flower (Pulsatilla vulgaris) | S | Calcareous grassland | N Europe |
| Edelweiss (Leontopodium alpinum) | S | Alpine rocky limestone turf | C Europe |
| Houseleek (Sempervivum tectorum) | S | Alpine sunny rocks | C Europe |
| Bluebell (Hyacinthoides non-scripta) | SR | Moist woodland | W Europe |
| Parsley (Petroselinum crispum) | R | [Unknown] | SE Europe |
| Dill (Anethum graveolens) | R | Cornfield weed | W Asia |
| Cabbage (Brassica oleracea) | CR | Chalk and limestone cliffs | S Europe |
| Larkspur (Consolida ajacis) | R | Cornfield weed | S Europe |
Grime’s types are relevant to how these plants should be cultivated. S-type plants should not be grown in fertile ground, where they are liable to be overrun by more vigorous neighbours or by rapidly maturing annual weeds, which are of R type. S plants in fertile soil may produce soft sappy growth that makes them susceptible to winter cold. Alpine plants are particularly badly affected, becoming susceptible to insect and fungal attack. R-type plants such as parsley and other annuals produce miserable stunted individuals when sown into poor soil. C species are more tolerant of poor soil because they can build up resources over several years. Goldenrod needs disturbance or open ground to get established, but once it has got going it can thrive on moderately fertile soil.
In addition to tall perennials, some annuals can potentially grow on poor soils if conditions are right. Rocky outcrops on hills often have small annuals growing among the rocks, for example hair-grasses (Aira spp.), thale cress (Arabidopsis thaliana), hairy bitter-cress (Cardamine hirsuta), and rue-leaved saxifrage (Saxifraga tridactylites). These species, of functional type SR, avoid the drought of summer by completing their life cycle in spring; they are small and of no horticultural merit. Some are garden weeds.
Competition and herbivory
The two processes that exclude or reduce both annuals and tall perennials from most grassland communities are competition and herbivory. Competition between plants in closed communities is inevitable, often because taller plants shade out shorter ones. Garden plants likewise compete with each other unless each has an allotted space separated by bare ground. Farmers and gardeners generally aim to reduce competition to the point where individual plants do not achieve maximum yield but are fairly close to it. Even where direct competition is minimal, plants can affect each other through shared pests and diseases. This is called apparent competition. For example, resistant plants may harbour a virus that devastates their neighbours, or a box hedge may harbour snails that devour nearby plants but leave the box untouched.
Herbivory can have an enormous effect even in annual communities. In the 1990s and 2000s many European arable fields were left unploughed for a period, under a scheme called Setaside. In the first year there were often many attractive broadleaved weeds. If the fields were unploughed for a second season, the broadleaved weeds almost vanished. Slug populations built up during the first year. Slugs generally avoid grasses, whose tissues are defended by silica bodies, so the slugs ate the broadleaved weeds, giving an overwhelming advantage to grasses.
Gardeners are well aware that slugs and snails can have a big influence on perennial communities. Many choice garden plants come from alpine regions and steppes, where slug populations are small or absent. These plants have no defences against mollusc grazing and suffer in gardens as a result. Fast-growing tall perennials may also be susceptible to grazing, especially by larger mammals such as rabbits, deer and domestic livestock. Experiments have repeatedly shown that when grazing mammals are removed from grassland, small slow-growing plants are replaced by tall ones. The effect can sometimes be reversed by increasing the grazing pressure again, but in the meantime many attractive wild flowers may have vanished.
Competition is therefore often mediated by herbivory. Where the soil is fertile, fast-growing plants can tolerate a higher level of grazing than on infertile soils. On infertile soils, loss of tissue to a herbivore is more serious. Thus we find that slow-growing plants (S-type species in Table 20.6) are often well defended against herbivores – by distinctive chemical flavours in lavender, thyme and chives; by toxic chemicals in the leaves of pasque flower; and by thick leathery leaves in houseleek. Edelweiss, on the other hand, is palatable to molluscs and is an example of a poorly defended alpine plant.
Tall, fast-growing plants have varying degrees of protection against herbivores. At one extreme, bracken (Pteridium aquilinum) is little eaten by insects and untouched by mammals and molluscs. Nettles (Urtica dioica) are well defended against mammals but relatively palatable to invertebrates such as the snail Arianta arbustorum and the larvae of lepidopterans. Tall grasses, on the other hand, are kept in check by grazing mammals but little eaten by molluscs. All of these tall plants have the potential to overtop and outcompete smaller ones, both in the wild and in the garden. In the garden, the gardener is the major agent of control, digging up and cutting back large plants so that small and medium-sized ones can thrive without excessive competition.
Habitat creation
Habitat creation in the wider countryside is closely linked to ecological restoration. This is now a thriving branch of knowledge, employing many consultants, nurserymen and contractors. There is an international Society for Ecological Restoration. There are journals such as Ecological Restoration, Ecological Management and Restoration, Journal of Applied Ecology and Restoration Ecology.
Many ecological restoration projects are essentially gardening on a large scale, with the proviso that long-term management is of a relatively low intensity. Wetland restoration has been particularly successful. The Royal Society for the Protection of Birds converted Lakenheath Fen from arable fields to reedbed. It is now a popular nature reserve with a visitor centre. Other restored habitats include wildflower meadows, flowery cornfields and mixed deciduous woodland. All of these can be created from arable fields, which often have the advantage of low soil organic matter and therefore low nitrogen. Other types of ecosystem, notably limestone grassland, heathland and peat bog, are much harder to restore and almost impossible to create within a human lifetime. Woodland is also slow to develop a natural character as many characteristic species of the ground flora are poor colonists.
After vegetation has been established, a sustained regime of management is essential unless the intention is to rewild with climax vegetation such as woodland. In Britain there are a few small areas that have been rewilded, notably the Broadbalk and Geescroft Wildernesses at Rothamsted in Hertfordshire, and the Hayley Wood Triangle and Monks Wood Wilderness in Cambridgeshire. These sites have developed a tree canopy but the vegetation does not have a natural structure. Both of the Cambridgeshire sites are adjacent to long-established woodland and therefore should be colonized by a full woodland flora over the next few centuries.
Only in the grandest gardens can gardeners wait for a century to achieve results. However, gardeners can intervene much more than would be acceptable in countryside habitat creation. It is no coincidence that the most popular habitats to create in gardens are those that are most successful in the countryside: ponds (Fig. 20.3), wetlands, grassland and woodland. Sowing cornfield annuals is also popular but does not create a habitat. Both in the country and in the private garden, the principles of habitat creation are the same: start with ground that is as clean as possible and make sure that the species you establish are those that you really want in the long run. The great English gardening writer Christopher Lloyd extolled the pleasure of wild gardening in grass. In The Well-tempered Garden, he claimed that ‘Where the grass has to be sown, the mixture is unimportant. Whatever one starts with, the end product will be the same, those grasses and other plants dominating which are best suited to their environment.’ Subsequent experience has shown this to be false in the wider countryside and equally false in gardens. A closed turf is not readily colonized by new species, so that coarse grasses such as cocksfoot (Dactylis glomerata) and tall fescue (Festuca arundinacea) may persist almost indefinitely. Likewise in the woodland garden, it pays to be careful about what is present at the beginning. Cow parsley (Anthriscus sylvestris) is pretty enough in a hedgerow but almost impossible to eliminate once established among bulbs. Over time it will overtop them.

Figure 20.3 A wildlife pond with a gently sloping ‘beach’ area allows birds and other animals easy access to the water. Photograph courtesy of Tim Sandal/Royal Horticultural Society.
Biodiversity
Why are there so many plants?
Broadly speaking, the number of plant species is determined by the rate at which they evolve and their dispersal dynamics. Mosses, which evolve slowly and can be dispersed by spores over a continent or hemisphere, have about 13,000 species whereas there are about 350,000 species of angiosperms (flowering plants). Garden plants are comparably diverse. The 2013 edition of the RHS Plant Finder listed 75,000 taxa for sale in Britain and Ireland.
The drivers for diversity of garden plants are various. We seek high yield, good flavour, attractive flowers, good windbreaks, ease of cultivation, suitability for differing sites, and so forth. There is also our love of novelty and diversity. Excellent plants may fail to please merely because they can be found in too many of the neighbours’ gardens. Many of these drivers apply equally to plants in the wild. High seed yield should increase numbers in the next generation. Good fruit flavour will attract birds that distribute seeds. Attractive flowers are better pollinated. All plants are to some extent habitat specialists. But how necessary are novelty and diversity in the wild?
Herbivorous animals are one major driver of diversity. Tall plants in a meadow may be grazed in preference to small ones. If a plant species is particularly abundant, then a specialist herbivore may increase in abundance and bring down the plant population. This is the basis of biological control. Plant defences are often effective against one category of herbivores but may attract others. Daffodils (Narcissus pseudonarcissus) are poisonous to mammals and are therefore avoided by sheep and cattle, but may be badly damaged by narcissus bulb fly (Merodon equestris).
Diseases are even more important than herbivores in promoting diversity (see Chapter 16?"?>). Elm disease has cut back almost all mature stands of suckering elm (Ulmus minor) in northern Europe. Ash dieback threatens to kill most individuals of a dominant tree (Fraxinus excelsior) in European woods. Ash woods may therefore become more diverse as other species replace the ash. Geneticists have demonstrated that there is a constant battle between plants and their diseases, with plants evolving resistance but at the cost of growing more slowly and therefore being more susceptible to competition. The outcome of this battle is most often to maintain high genetic diversity within species rather than for species to disappear completely.
In spite of the diversifying effects of herbivory and disease, natural ecosystems are often surprisingly species poor, especially in the northern coniferous forest (boreal) zone. Even herbaceous plants take a long time to evolve, so that the native British flora almost completely lacks endemic species (i.e. species that are restricted in the wild to a particular, clearly defined region). The most notable British endemic is Scottish primrose (Primula scotica), which is closely related to Scandinavian primrose (Primula scandinavica) and may be little more than an island race. Because evolution of species is slow, the diversity of plant species in the wild is mainly the result of events that took place thousands of years ago during or before the Pleistocene glaciations. It is an accident of history.
If the actual level of diversity is an accident rather than a necessary outcome of short-term evolution, then we would expect that different diversity patterns would be found elsewhere in the world. The richest plant communities in northwest Europe are grazed calcareous grasslands. High pH is not the direct cause. Indeed, one of the richest plant communities in the world is the fynbos of South Africa, which occurs on acid soil. The accident of history that produced this result is that Europe’s leached and acid soils were obliterated during recent glaciations, so that calcifuge (lime-intolerant) species such as heather (Calluna vulgaris) had to disperse over thousands of kilometres from distant outposts in the south. Limestone grassland plants did not have to travel far, because most of the continent had been covered with dry steppes.
The fact that species richness is to some extent an accident suggests that high biodiversity may in fact not be necessary for ecosystem function. Some of the issues are discussed below. They are explored in greater depth in Ken Thompson’s thoughtful and challenging book Do We Need Pandas? The Uncomfortable Truth about Biodiversity.
Diversity and stability
It is often stated that biological diversity promotes both ecosystem stability and productivity. From this it should follow that a well-stocked botanic garden would be one of the most stable and productive places on earth. Clearly this is not true, because a botanic garden is a highly artificial construct, its diversity dependent on the repeated introduction of interesting, but not necessarily complementary, specimens from elsewhere on the planet. Its stability, therefore, can only be maintained by horticultural intervention. Artificiality is not the only reason why a diverse ecosystem may be unstable. For example, some diverse natural systems such as savannah, chaparral and boreal forest may be inherently unstable because they regularly burn as a result of lightning strikes or other natural causes.
The theory that simplified artificial systems are inherently unstable and less productive was expounded by several eminent ecologists in the 1950s. From this it was argued that foresters should not plant monocultures of trees. Yet planting of conifer monocultures had been highly successful in central Europe and, indeed, some inherently stable natural conifer forests are in fact very species poor. Theoretical ecologists in the 1970s could find little justification for the theory. The argument continues, but it has become increasingly academic because so much depends on how stability is defined. In gardens, a species-poor lawn is not necessarily unstable, but it is highly likely to be invaded by broadleaved weeds.
Of much more relevance to horticulture is the question of how long a particular planting can be sustained. Crop rotations can reduce pest damage. Roses should not be planted where others have been grubbed out. Border perennials should at intervals be dug up and replanted, preferably in a new place. These practical requirements are symptoms of the fact that natural enemies build up, weakening old plants and facilitating their replacement with new species.
Ecological succession and the balance of nature
The balance of nature is an idea firmly embedded in the human psyche while at the same time being obviously impossible. Natural catastrophes have occurred in the past. An ice age would be seen as a catastrophe by many northern people: the last one ended only 12,000 years ago. About 6000 years ago, British elms suffered a major decline, thought to have been caused by a natural outbreak of elm disease. Small-scale disasters such as landslides are commonplace. Nevertheless, many natural and semi-natural ecosystems appear to be remarkably stable. When they are perturbed, they rapidly revert to the stable state.
That stable state is the climax, reached by succession. A classic succession is the replacement of heathland by forest. Heathland in much of Britain depends for its existence on a combination of burning, grazing and removal of produce in the form of sods or firewood. If these factors are removed, trees rapidly become established and the site reverts to woodland. This is an example of relay floristics whereby the plants that drive the succession come from outside the site, with the ground being prepared for them (so to speak) by the existing vegetation. In other successions, all the components of the succession are present from the start, but tall plants gradually overtop small ones. This is the initial floristic composition model.
A remarkable example of the importance of initial floristic composition is provided by the classic Park Grass experiment at Rothamsted in southern England. This experiment started in 1856 with a 2.8-ha area of flat hayfield that had been under grass for 100 years. Such traditional hay meadows, often emulated by gardeners, are known for their diverse and colourful flora. The experiment was designed to test the effects of inorganic and organic fertilizers on agricultural yield. Its complexity is almost mind-boggling, with more than 20 primary treatments, crossed with a secondary treatment to stabilize soil pH by adding lime. No modern researcher would be allowed to perform such an experiment because the treatments are not replicated. In spite of this, it has turned out to be one of the most successful ecological experiments of all time. The treatments were well chosen, including the addition of all the major nutrients N, P and K. Moreover, the effects of the treatments were overwhelming, reducing the need for replication.
The cutting regime, two cuts per year, is the same for all treatments, which differ in their effects on the soil. Regular addition of ammonium sulphate without lime lowered the soil pH from 5.5 to 3.6. Limed plots were brought up to pH 7. Plots that received no fertilizer or were fertilized with sodium nitrate have experienced little change in pH, which remains in the range 5.0–5.6. Except for rosebay willowherb (Chamaenerion angustifolium) on the most acid plots, almost no species invaded from outside. On the most acid plots, only three of the original species complement survived. Thus the mini-successions on each individual plot conformed almost exactly to the initial floristic composition model.
Another result, relevant to gardeners who seek for a species-rich and colourful wildflower meadow, is the overwhelming dominance of grass in plots that received both N and P. If P and K were added, the legumes thrived. In fact, all fertilizers reduced diversity.
Superimposed on the powerful major effects of the treatments were fluctuations in individual species. Goat’s beard (Tragopogon pratensis) increased from 1920 to 1950, was stable from 1950 to 1965, and then declined rapidly to 1980 when it had almost vanished. The authors speculate that it may have become more susceptible to infection by a rust fungus (see Chapter 16?"?>), but this is far from certain.
The mini-successions in the Park Grass plots are arrested successions, each responding to a stable management regime but not proceeding to the natural climax of woodland. Gardeners can achieve similar moderately stable results in lawns and long grass. Control of spring-flowering plants in woodland gardens is not so easy, as there is no mowing regime to control the vigour of the taller, more competitive species.
Unstable dynamics
Given that nature does not always achieve a balance, it is interesting to consider systems with fluctuations. Fire successions are found in many parts of the world, notably in heathland, conifer forests and chaparral. Combustible brushwood or timber builds up until the landscape becomes increasingly liable to burn as a result of a lightning strike or other natural event, or through human intervention. After a burn, the succession starts again, and the process is repeated. Shrubs from such ecosystems in the genera Ceanothus, Cistus, Erica, Juniperus, Lavandula, Rosmarinus, Spartium and Ulex tend to be relatively short-lived. In the garden they do not normally burn but become liable to frost or simply become ugly and therefore have to be replaced with young specimens every few years.
Another cause of instability is infestation by insects. Many insect outbreaks are completely natural. Some recur at regular intervals. The population of the larch bud moth (Zeiraphera diniana) has, for example, fluctuated regularly in the European Alps for at least 1200 years with the gap between population peaks averaging 9.3 years. Spruce budworms (Choristoneura spp.) devastate mature conifer stands over hundreds of square kilometres in North America, with no regular pattern and a return time of up to 60 years. Plagues of locusts are also natural, though fortunately they do not now occur in temperate Europe and North America.
Every gardener is aware that pest infestations vary from year to year. In this respect gardens are no different from natural ecosystems. Some pests such as Solomon’s seal sawfly (Phymatocera aterrima) recur every 2 years or so, whereas severe outbreaks of broom aphid (Aphis cytisorum) on laburnum are relatively unusual. The complexity of the factors that control these pests is formidable. Even the larch bud moth and spruce budworm cycles are not fully understood.
Individual species and their parasites may also fluctuate without there being any marked ecosystem consequences. The holly blue butterfly (Celastrina argiolus) is common in gardens throughout most of southern Britain, where its main food plants are holly (Ilex aquifolium) in the spring and ivy (Hedera helix) in the autumn. It fluctuates widely in numbers over a period of about 8 years, synchronously over much of southern Britain. Its caterpillars are parasitized by the wasp Listrodromus nycthemerus, whose only host is holly blue. In the year before a population crash, almost every holly blue caterpillar is parasitized. Then the butterfly disappears for a year or two, and it recolonizes, initially with very low levels of parasite attack, rebuilding its numbers rapidly. Then the parasite builds up again, and the process repeats itself. Such oscillations are called predator-prey cycles, and occur where there is a very powerful specialist predator. Where the predator is a generalist, its numbers will not necessarily fall when abundance of a particular prey species is low.
Conclusions
Ecological processes are as important in gardens as they are in nature. The difference is that in gardens, human activity such as clipping and digging takes the place of grazing, browsing and burrowing. Gardens are not in themselves ecosystems, but interact with the surrounding landscape on a variety of scales. An ecological approach to gardening is not only useful to achieve high yield and gratifying ornament, but enables the gardener to celebrate the astonishing richness of the processes that control plant performance.
Further reading
- Archer, D. (2010) The Global Carbon Cycle. Princeton University Press, Princeton, NJ.
- Crawley, M.J. (1997) Plant Ecology, 2nd edn, pp. xvii, 717. Blackwell Science, Oxford.
- Good, J.E.G. & Millward, D. (2007) Alpine Plants: Ecology for Gardeners. Batsford, London.
- Grime, J.P. (1979) Plant Strategies and Vegetation Processes. John Wiley & Sons, Ltd, Chichester.
- Grime, J.P. & Pierce, S. (2012) The Evolutionary Strategies that Shape Ecosystems. John Wiley & Sons, Ltd, Chichester.
- Thompson, K. (2010) Do We Need Pandas? The Uncomfortable Truth About Biodiversity. Green Books, Totnes.
Authors and affiliations
Written for the third edition by Mark Hill, independent consultant (to replace parts of Chapters 18 and 19 in the second edition).