Using the Human Genome to Improve Health
How are genes used in the field of health care?
Using the human genome sequence as a map, scientists are learning more every day about the relationship between our genome and human health. With this information, they are developing new ways to better diagnose, treat, and prevent human disease.
Even before the Human Genome Project, doctors frequently used genetic testing to identify carriers of certain genetic diseases. Doctors had some tools they could use to screen newborns for genetic disease and diagnose some types of cancer. But many diseases went unidentified until much later in life. Parents who might be carriers of genetic diseases passed on those genes to their babies without knowing the danger.
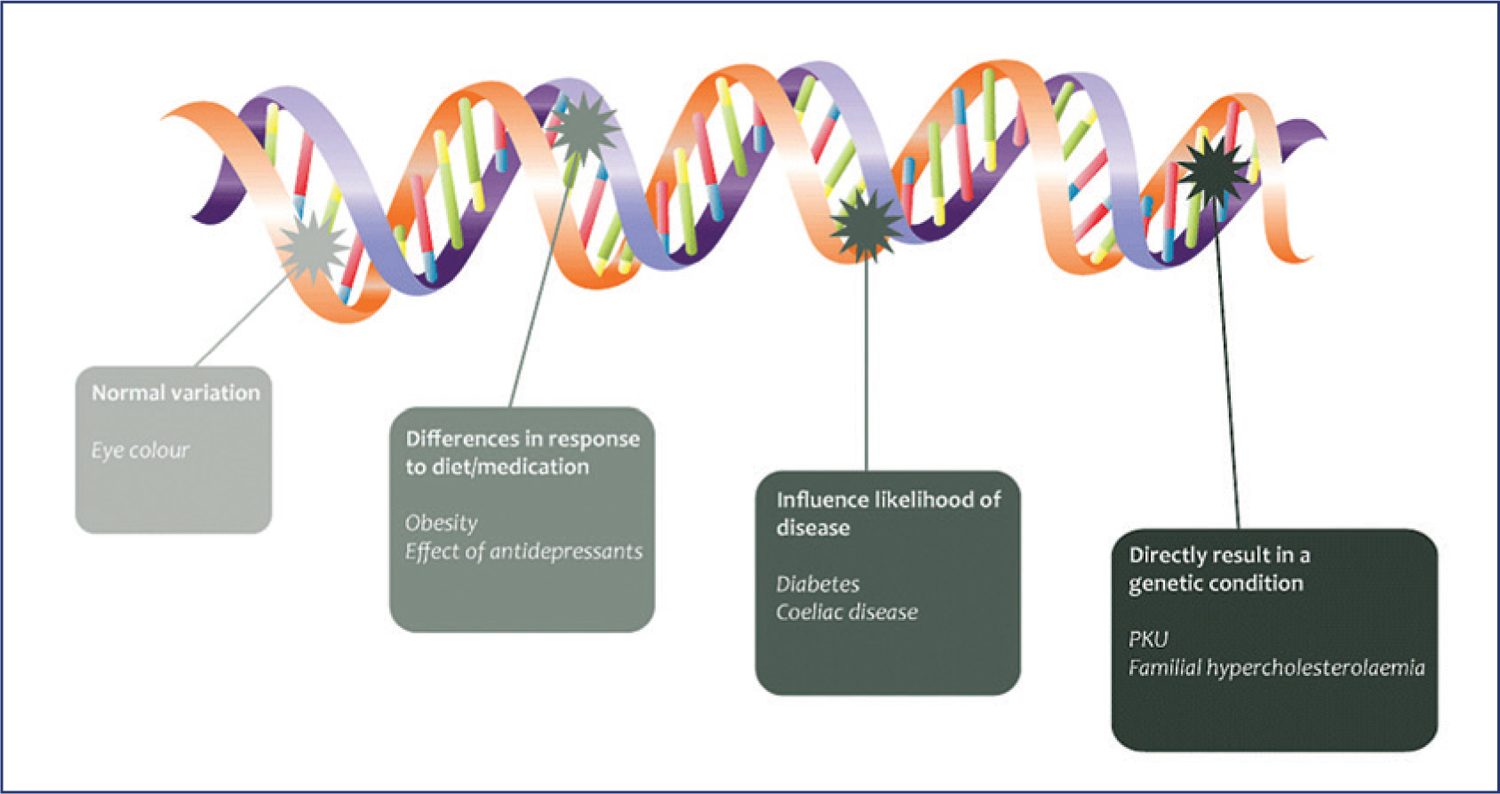
A genetic disease is an inherited condition caused by an abnormality in a person’s DNA—a mutation. As we discussed in the previous chapter, the abnormality can be as small as a change in a single base in one gene or it can involve the addition or subtraction of an entire chromosome.
Many of the diseases that doctors were able to diagnose and test for were single-gene diseases, caused by an abnormality in a single gene. These were simpler to find. With the completed human genome sequence, however, scientists have been able to greatly improve their ability to identify and diagnose many more conditions.
Since the complete sequencing of the human genome, scientists have discovered more than 1,800 disease genes! Plus, it now takes far less time to find a disease gene. Today’s scientists can find a gene suspected of causing a genetic disease in days, rather than the years it took before they had the human genome sequence as a tool.
As a result, there are now more than 2,000 genetic tests for human diseases. These tests allow patients to learn their own risk for genetic disease, while also helping doctors diagnose disease in patients.
Some diseases are so rare, you might not have heard of them before. Progeria is an extremely rare genetic disease of childhood that causes dramatic, premature aging. The disease affects approximately one in 4 million infants worldwide.
credit: Kemberly Groue
Children with progeria appear normal at birth. Within a year, though, their growth rate slows—they are shorter and weigh less than other children of the same age. Their appearance resembles that of an old person with baldness, wrinkled skin, pinched nose, and a small face and jaw relative to head size.
Children with progeria often suffer from symptoms of old age, such as joint stiffness, hip dislocations, and cardiovascular disease. To date, there is no cure for progeria. Children with it die on average at age 13, often from a heart attack or stroke.
In 2003, a team of researchers with the National Human Genome Research Institute, the Progeria Research Foundation, and other organizations discovered the gene that causes Hutchinson-Gilford progeria. The disease is caused by a tiny, point mutation in a single gene called lamin A (LMNA), located on chromosome 1.
A point mutation occurs when a single nucleotide base is changed, inserted, or deleted from a sequence of DNA. The LMNA gene consists of 25,000 base pairs and the substitution of a single pair causes the devastating disease.
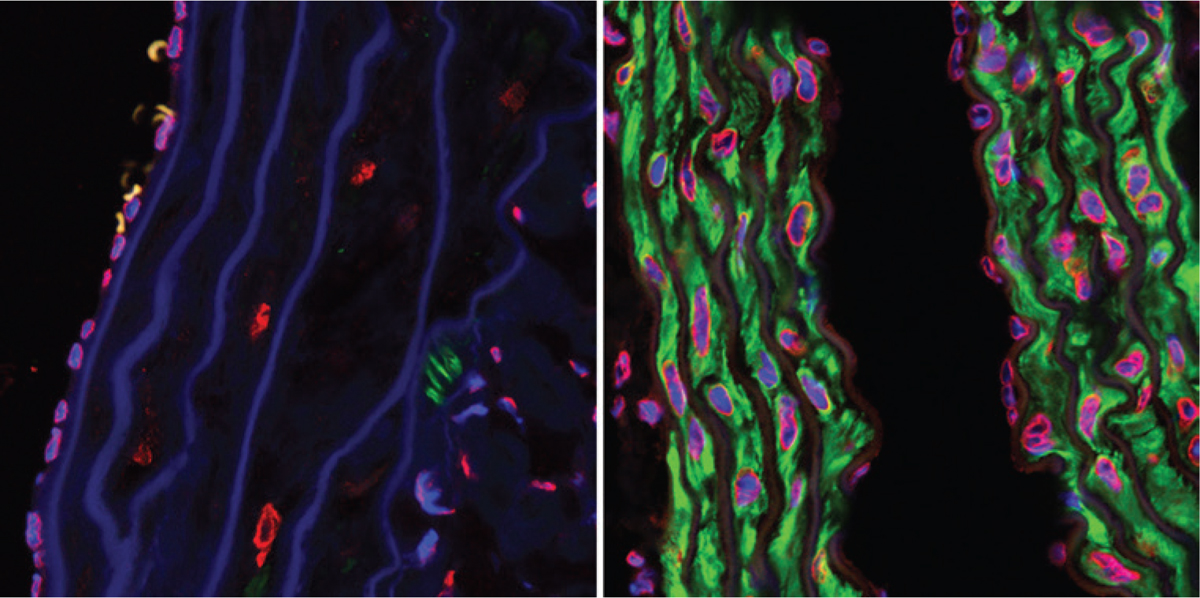
Progeria in mice—the left side are cells left untreated, while the right side are cells treated with a certain medication.
Why does this tiny change among thousands of bases matter? The LMNA gene codes for two proteins in humans, lamin A and lamin C. Both proteins have important roles in stabilizing the inner membrane of a cell’s nucleus. At first, such a small change would seem to have little effect on the gene and its production of lamin A protein. However, when testing cells from progeria patients, researchers found that the mutation in the LMNA gene causes the cell to produce an abnormal form of lamin A.
This abnormal protein changes the cell’s nuclear membrane, which can be especially harmful to cells and tissues in the cardiovascular and musculoskeletal systems. This finding could explain why the disease causes problems with organs and structures in these systems, such as the heart, muscles, and skeleton.
Researchers also noted that the parents and siblings of children with progeria were almost never affected by the disease. That led them to believe that the genetic abnormality was not hereditary. Instead, they found evidence that the genetic mutation occurs in the male sperm before the baby is even conceived.
With these breakthroughs, scientists developed a genetic test for Hutchinson-Gilford progeria syndrome. Before this genetic test, doctors used a patient’s physical symptoms to diagnose progeria. But these symptoms usually did not even appear until a child was a few years old. With a genetic test, doctors can diagnose the disease much earlier, which allows them to start treatment earlier.

Doctors at NIH working on cutting-edge genetic science.
While some diseases are caused by a single change in a gene, many more are linked to changes in multiple genes spread out across the human genome.
Some diseases are caused by a combination of genes acting together along with environmental factors. These diseases are called polygenic disorders. Using the map provided by the HGP, scientists are working to get a better understanding of how multiple genes interact with each other and the environment to cause human disease.
Do you or anyone you know have diabetes? Many people all around the world suffer from diabetes, a group of diseases that causes too much sugar to form in the blood. Diabetes is an example of a polygenic disorder.
One of the most common forms of the disease is Type 2 diabetes, a chronic condition that affects the way the body processes blood sugar. Blood sugar, also called blood glucose, is the body’s main source of energy. It comes from the food you eat. An organ in your digestive system called the pancreas produces insulin, a hormone that helps the body process glucose and turn it into energy for cells. In Type 2 diabetes, the body does not use insulin properly and too much glucose builds up in the blood.
Scientists have known that diabetes tends to run in families, which suggests there is a genetic factor that causes the disease. Scientists managed to identify more than 80 tiny DNA differences that appear to raise the risk of Type 2 diabetes in some people or protect others from the disease. Still, no single gene or combination of genes has been identified as causing Type 2 diabetes.
In 2017, a team of researchers made a potential breakthrough. They discovered that many of the changes across the genome in people with Type 2 diabetes affected the same DNA-reading molecule. The molecule, called Regulatory Factor X (RFX), is a regulator for many genes, which means that it controls when the gene turns on and off. Many of the DNA changes linked to Type 2 diabetes affected RFX’s ability to read certain sections of DNA in pancreas cells.
The researchers suspect that the DNA changes caused RFX to fail to read DNA’s genetic instructions properly, which affected the cell’s ability to use insulin effectively to regulate blood sugar. This discovery could explain how multiple changes in genes could cause the same disease.
This error is like having an oven temperature gauge that cannot measure the oven’s temperature correctly. The malfunction then affects the oven’s ability to cook food properly.
The vast majority of human diseases are like diabetes—polygenic. Diseases such as asthma, autism, cardiovascular disease, schizophrenia, inflammatory bowel disease, and certain autoimmune disorders are examples of polygenic disorders. Scientists hope that the insights learned by studying the human genome will lead to better ways to diagnose and treat these diseases.
Did you know that because of what we have learned about the human genome, doctors are developing ways to detect cancer with a blood test? Some cancers are even being treated not by where they are in the body, but by the changes in the genomes. Advances in genomic science and DNA sequencing are leading the way to a new understanding of cancer, how it develops, and how to treat it.
These changes involve DNA mutations that lead the cells to grow uncontrollably. As we discussed earlier, mutations regularly occur in the body, but typically our cells find and fix the mutations as the cells divide and replicate. In some rare cases, however, some mutations escape the cells’ repair. These mutations can develop into cancer.
Because of the HGP, scientists know what a healthy human genome looks like. They can use this to identify changes in the genome that lead to cancer. Some projects, such as the Cancer Genome Atlas, have sequenced the genomes of thousands of cancer samples. These projects show that some cancers have mutations in the same group of genes, even if they developed in different tissues.
Many of the mutations activate genes that either promote cell growth or turn off genes that prevent cell growth. This might explain the uncontrolled cell growth seen in cancer.
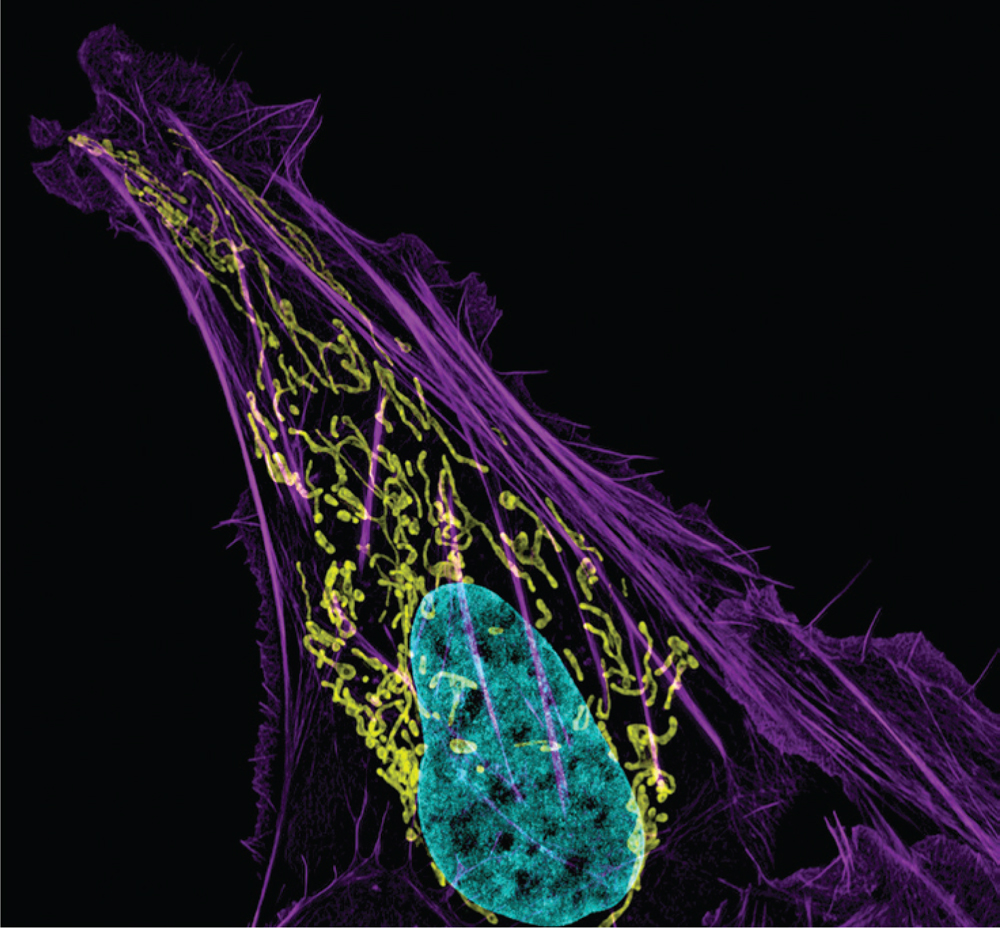
This image shows a cancer cell with DNA in blue, mitochondria in yellow, and actin filaments in purple.
credit: Dylan Burnette and Jennifer Lippincott-Schwartz, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health
As scientists learn more about the genome changes that cause cancers, doctors are exploring how to use this information to better treat patients. In 2003, Dr. Lukas Wartman was a fourth-year medical student when he was first diagnosed with a type of blood cancer called acute lymphoblastic leukemia (ALL). He immediately entered treatment with aggressive chemotherapy, which drove his leukemia into remission.
He graduated from medical school and started his career as a doctor and medical researcher. Wartman’s experience as a cancer patient led him to specialize in treating patients with leukemia and studying the disease in his laboratory at Washington University in St. Louis, Missouri.
Five years later, Wartman relapsed—his cancer had returned. At the time, researchers at his university were sequencing cancer genomes. They asked Wartman if they could study him and his cancer. The researchers sequenced the DNA in Wartman’s normal cells and compared it to the DNA sequence in his cancerous blood cells.
They found a mutation in the cancer’s genome in a gene called FLT3.
The researchers also identified a medication, already approved by the U.S. Food and Drug Administration (FDA) for treating other types of cancer, that had been used to treat patients with mutations in this same gene. Wartman started taking the medicine on a Friday. By Monday, his blood counts had improved.
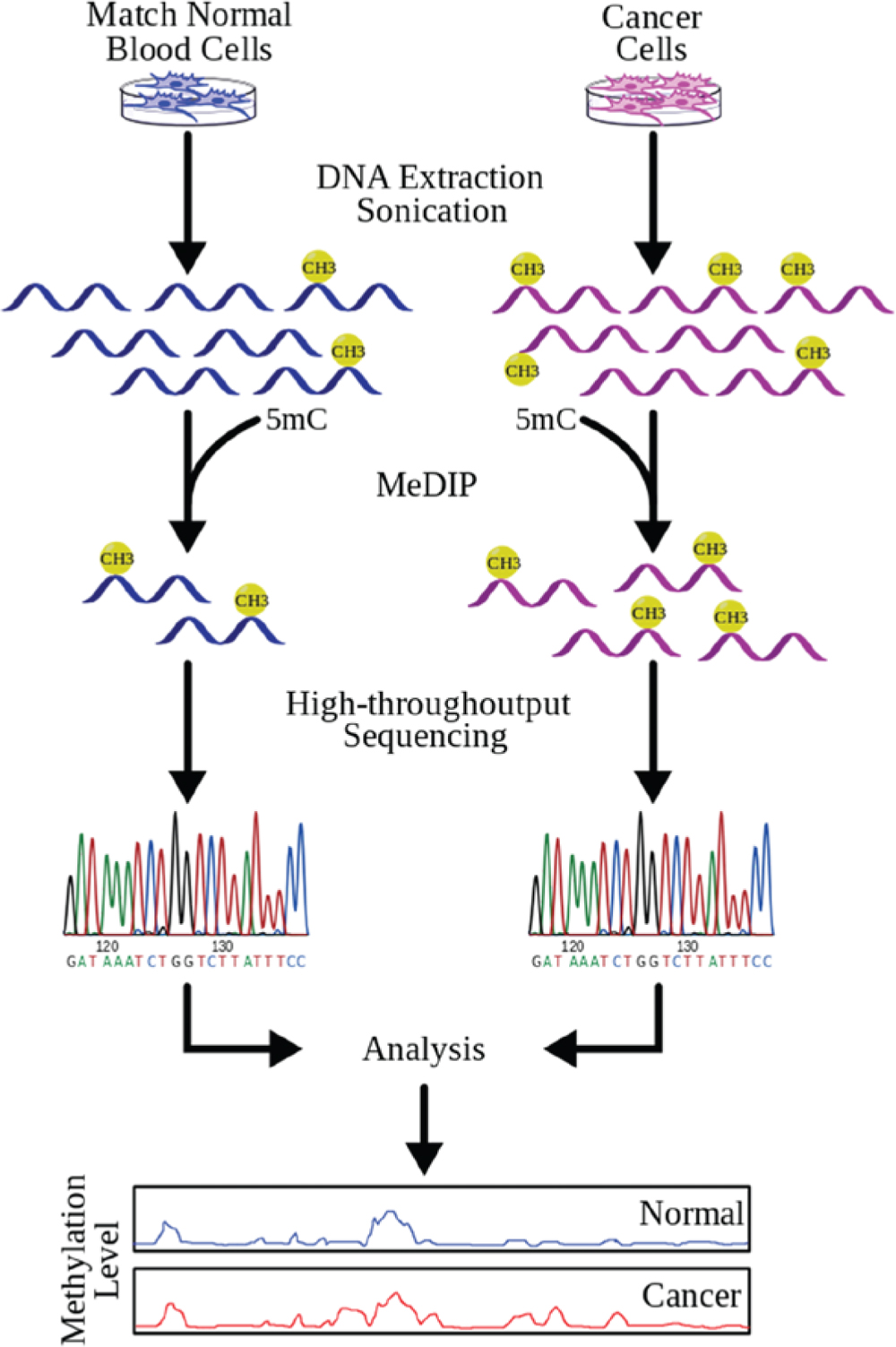
Researchers can pinpoint where cancer mutations occur and target those areas for treatment.
After several weeks, doctors could no longer detect leukemia cells in his blood. This meant that his leukemia had gone into remission. Although he would still need more treatment to make sure the cancer did not come back, Wartman believes that the insights into his cancer that genome sequencing provided saved his life.
Every person responds to diseases and treatments differently. What if there was a way to personalize your medical treatment to match your body’s unique genetic makeup? That’s the goal of genomic medicine.
Since the completion of the HGP in 2003, scientists have worked hard to figure out how each of our 20,000 to 25,000 genes functions and how it interacts with every other gene. With genomic medicine, doctors will be able to use a person’s DNA to determine the best possible treatment for them.
Small variations in specific genes may make one person more likely to develop a disease while a different variation may protect another person from that disease. Genetic variations may explain why one treatment works well for one person but not at all for another person.
As scientists learn more about how small gene differences affect health, they can develop better ways to prevent, diagnose, and treat many diseases and disorders. That’s what scientists hope genomic medicine can do.
In the United States, a public health program called newborn screening tests all babies at birth for up to 50 severe, inherited, and treatable genetic diseases. Through genomic medicine, doctors may one day be able to easily use whole genome sequencing at birth to screen all babies for a larger number of diseases and conditions.
Plus, rapid whole genome sequencing is being used for some very sick babies in hospitals. Because newborns are often difficult to diagnose, doctors can use rapid whole genome sequencing to get a genetic diagnosis within 50 hours. In many cases, the quick diagnosis helps doctors make time-sensitive treatment decisions that improve the newborn’s health outcomes.
Genomic medicine can also help doctors design more effective treatments for patients. Colorectal cancer is a type of cancer that forms in the colon (large intestine) or rectum. Low-dose aspirin therapy is often prescribed for patients with colorectal cancer to reduce inflammation. However, its effectiveness varies by patient.
Now, doctors may be able to use genomic medicine to determine which patients will benefit the most from aspirin therapy.
A study by researchers at the Dana-Farber Cancer Institute found that aspirin therapy can extend the life of colorectal cancer patients whose cancer has a particular mutation in the PIK3CA gene. In their study, 97 percent of patients who had the PIK3CA gene mutation and were treated with aspirin therapy were alive five years after diagnosis. That’s compared to 74 percent of those with the gene mutation who did not use aspirin. At the same time, aspirin therapy had no effect on the five-year survival rates for patients who did not have the PIK3CA mutation.
Genomic medicine may one day use genetic information to better treat people who are at risk for certain genetic diseases. This is already happening in some areas. For example, genetic tests can identify people with a high risk of certain cancers, such as women who carry the BRCA gene mutations and have a high risk of developing breast cancer. Knowing this genetic risk allows a woman and her doctor to decide on specific tests and treatments to reduce her risk of cancer.
Other genetic tests can identify breast cancer patients who are most likely to benefit from a drug called Herceptin. Genetic testing can also determine the best dose of a drug called mercaptopurine, which is used to treat leukemia and certain autoimmune diseases, so that the patient avoids severe side effects.
Have you ever experienced side effects from medication? Drugs affect different people in different ways. One person may find a drug very effective and easy to take, while another patient experiences severe side effects. Now, a new area of genomic medicine called pharmacogenomics is looking to use a person’s genome to improve drug treatment and reduce the negative side effects.

The field of pharmacogenomics studies how a person’s genes affect the way they respond to drugs. Researchers hope that, using genomics, they will be able to personalize what drugs and doses are prescribed to each patient for the best outcomes.
Imagine that you have high blood pressure. Before prescribing medication, your doctor sequences your genome. They might find a variation in your genome that means a commonly used drug to treat high blood pressure would have little effect on you and might increase your risk of heart attack. Your doctor would not prescribe that medication for you. Instead, they would prescribe another drug to reduce your blood pressure without negative side effects.
This type of scenario is already happening in real life. Abacavir is a commonly prescribed drug for HIV, the virus that causes AIDS. When some patients used the drug, they developed rashes, fatigue, and diarrhea. All of these complaints are symptoms of a potential immune system reaction. Scientists have identified a genome variation associated with the immune system that causes these reactions. Now, doctors test for this genome variation before they prescribe the drug. Patients have a better chance of staying well.
To learn more about disease, scientists compare the genomes of people with a disease to those without the illness. This comparison, called a genome-wide association study (GWAS), enables scientists to more easily identify the differences in genomes between sick and healthy people. With this information, researchers can develop new targets for therapies to treat and even prevent the disease.
Early on, the work of genomic association was slow and tedious. It was hard for researchers to identify which genes were involved in disease and find where they were located in the human genome. With the HapMap project and other technological advances in genomic science, researchers now have powerful tools to help them more quickly find the variations in DNA linked to a disease. With this information, they can predict whether a certain mutation is associated with a specific disease—eventually, they’ll be able to identify people at risk.
For example, researchers in the United Kingdom compared the genomes of 2,000 people who had one of seven common disorders. They compared those genomes to 3,000 healthy people. This comparison enabled researchers to identify new genetic markers associated with an increased risk for heart disease and diabetes. With this type of information, scientists can determine a person’s risk of developing a disease based on the markers in their individual genome.
We live in a very interesting present, and the future looks bright in terms of genomic health! But what about the past? Can the map of the human genome help us understand where we came from and how we developed? We’ll take a journey back in time in the next chapter.
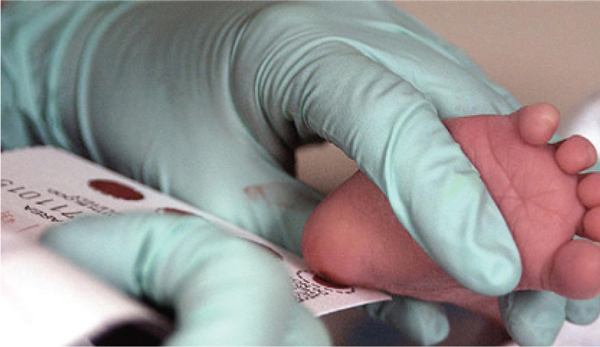
A blood test can show if an infant has inherited immune deficiency syndrome
credit: U.S. Air Force photo/Staff Sgt. Eric T. Sheler
KEY QUESTIONS
•Why might certain diseases get more attention from researchers studying genomics than other diseases?
•Personalized medicine used to be the stuff of science fiction! What other inventions that now appear in fiction might someday be fact?
Prescription drugs save lives around the world. They help prevent heart attacks and treat different types of cancer. However, not everyone has the same response to the same medication. Different responses to a medication are often caused by differences in the genome. A single DNA mutation in a person’s genome can be associated with an increased or decreased response to a particular medication.
When pharmaceutical companies develop a new drug, they test it on many people to see if any have different responses to the drug. They can then do a genetic screening to see if the people who had a different response have a mutation that the others do not have, or a single nucleotide polymorphism (SNP).
Once SNPs that are associated with an abnormal response to the drug are identified, doctors can screen patients for these SNPs so they will know how a patient will respond to the drug before they prescribe it. In this activity, you will choose a common drug and investigate how a genetic mutation is associated with a person’s response to this drug and how the mutation affects the way the body processes the drug.
•To start, choose a common drug to study. Some drugs have large amounts of data available.
•Azathioprine (Imuran) – treats rheumatoid arthritis
•Celecoxib (Celebrex) – treats arthritis
•Clopidogrel (Plavix) – prevents blood clots
•Mercaptopurine (Purinethol) – treats leukemia
•Warfarin (Coumadin) – prevents blood clots
•Learn about the medication. What conditions does it treat? What types of patients would use this drug? How does the drug affect the body? What pathways does it use?
•What changes in a person’s genome can affect how they react to this drug? How does this affect the treatment of their disease? How can doctors use this information to make treatment decisions?
To investigate more, consider that pharmacogenomic information is currently being used to prescribe medications for only a few conditions. What other diseases and disorders could benefit from using pharmacogenomic information to find better ways of using medications? Why?
Personalized medicine has great potential to improve human health. As genome research improves and the cost of genome analysis decreases, the opportunities to use information from a person’s genome to drive their health care decisions will increase. Here are some of the exciting possibilities.
•Using personal genome sequencing to diagnose patients with rare conditions
•Predicting a person’s risk of developing a medical condition
•Prescribing medications based on a patient’s genome
•Developing new drugs to treat diseases linked to specific genetic variants.
•In this activity, you will prepare a brochure that highlights the possibilities, breakthroughs, and limits of personalized medicine. The brochure will be read by the people who may benefit from personalized medicine but may be unaware of the advances in this field. The brochure should include an overview of personalized medicine. It should also present examples of success stories, along with discussing potential challenges. Choose from one of three subtopics to focus on in the brochure.
1.Genetic testing applied to rare disease to diagnose and/or develop a treatment plan
2.Using genetic testing to decide which drug and what dose to prescribe to a patient
3.Developing or prescribing drugs for diseases such as cancer that are targeted to the genetic makeup of specific patients
•Research the field of personalized medicine to gather information that you will use in the brochure. There are many news articles available on the internet about personalized medicine that you can read. The brochure should address the following questions.
•Why are doctors and patients optimistic about personalized medicine?
•How has personalized medicine helped patients already?
•How might personalized medicine help people with cystic fibrosis, cancer, or an undiagnosed disease?
•What are the challenges of personalizing medicine to individuals or small numbers of people?
•How will personalized medicine affect health care costs?
•What can we do to ensure access to personalized medicine is fair and equitable?
•Use markers and colored pencils or a computer to complete the brochure. Be creative!
To investigate more, choose one of the subtopics that you did not use and create a PowerPoint presentation to share information about the subject with others. Who is your audience? What information will be important to them and should be included in the presentation?
All cancers develop because of changes in the DNA sequence of our genome. These changes cause healthy cells to divide uncontrollably. This happens when the DNA change affects the activity of genes that normally stop cell growth or by turning on genes that control cell division. The result is a mass of cells—a tumor—that continues to grow. In this activity, you will compare DNA sequencing of a healthy cell and a tumor cell.
•Below are results of DNA sequencing for part of the DNA in a healthy cell and a tumor cell. Using this key, write the DNA sequence for each sample.
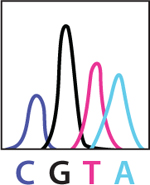
KEY
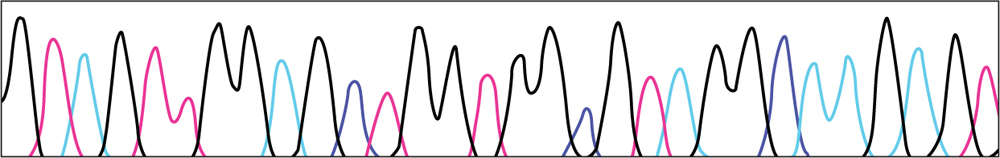
HEALTHY CELL DNA
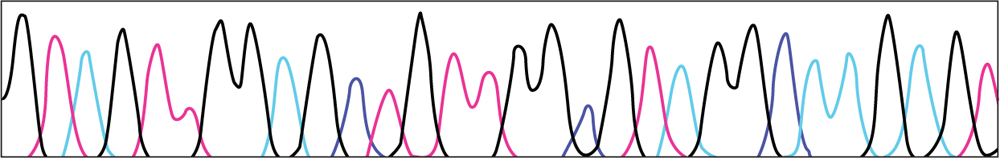
TUMOR CELL DNA
•Compare the healthy sequence to the tumor sequence. Is there a difference? If so, circle it.
•Does this mutation change the amino acid the DNA codes for? Use the Universal Genetic code to determine what amino acid the healthy sequence codes. (Remember that U – uracil represents T – thymine in the chart) What amino acid does the tumor sequence code for?
•Why does this mutation matter? How do you think it contributes to the development of the cancer tumor?
To investigate more, think about how understanding where this mutation occurs in a cancer tumor and what it does helps scientists develop more effective treatments for cancer.