
CHAPTER 6
Microinvasive carcinoma
S.E. Pinder |
|
I.O. Ellis |
|
S.J. Schnitt |
|
P.H. Tan |
|
E. Rutgers |
|
M. Morrow |
|
A lesion characterized by one or more clearly separate microscopic foci of infiltration of tumour cells into the mammary stroma, each less than or equal to 1 mm in size, and most commonly seen in the background of high-grade ductal carcinoma in situ (DCIS).
Historically, there has been wide variation in the definition of microinvasive carcinoma of the breast. Some authors have proposed that the definition of microinvasive carcinoma requires extension of the invasive cells beyond the specialized lobular stroma. However, it may be difficult to ascertain this, and there will be instances in which microinvasive carcinoma is diagnosed when convincing histological appearances are present, despite malignant cells or nests of cells not being clearly beyond the specialized lobular stroma.
Microinvasive carcinoma does not have an ICD-O code.
Microinvasive carcinoma is infrequent and is commonly over-diagnosed. It typically occurs in association with high-grade DCIS, but instances of microinvasive carcinoma accompanying lobular carcinoma in situ have been encountered and very rarely microinvasive cancer can be seen in the absence of an adjacent in situ component.
There are no specific clinical features associated with microinvasive carcinoma. In cases where microinvasive carcinoma arises in association with high-grade DCIS, the clinical presentation is mammographically detected microcalcifications, or less commonly a mass, asymmetry or architectural distortion. On ultrasonography, a solid hypoechoic mass has been reported in a small series {1515}.
The macroscopic appearance of microinvasive carcinoma, as with the clinical features, is that of the underlying in situ lesion. Most typically, ill-defined fibrous areas with comedo-type necrosis extruding from the surface are seen on close inspection of a sliced excision specimen, but in many cases no visible abnormality is evident.
Microinvasion is diagnosed most commonly in a background of extensive high-grade DCIS with prominent periductal chronic inflammation. Malignant cells are seen within the stroma, most often in small angulated clusters and less frequently as single cells. There is an absence of associated myoepithelial cells. The nature of the malignant cells is typically that of invasive carcinoma of no special type (NST). When arising in association with high-grade DCIS, the appearance of the infiltrating cells is also of high nuclear grade. Additional histological features commonly seen in association with micro-invasive foci are stromal oedema, desmoplasia, and chronic inflammatory cells.
Thorough sampling of large areas of high-grade DCIS should be undertaken so as not to miss foci of microinvasive (or frankly invasive) disease. Some reports have indicated that when microinvasion occurs, it is likely to be multifocal {1606}. It is therefore appropriate to search carefully for additional small foci when one such lesion has been identified and to confirm that the size of each focus does not exceed 1 mm in maximum dimension.
Care should be taken not to overdiagnose this lesion, particularly in uncertain cases. Indeed, subsequent histology review frequently “downgrades” a diagnosis of microinvasion or of lesions suspicious for microinvasion; in one series, only 21 of 109 cases (19.3%) were confirmed to be microinvasive lesions on review {1120}, highlighting the potential for over-diagnosis of this entity.
Although microinvasive carcinoma most commonly arises in large areas of high-grade DCIS, microinvasion can arise in association with all grades of DCIS and is very rarely seen with other precursor lesions of invasive breast cancer, such as lobular carcinoma in situ {975}.
The differential diagnosis of microinvasive carcinoma includes pure in situ disease and, conversely, frankly invasive breast carcinoma (i.e. > 1 mm in size). The size of the focus should be carefully measured with an ocular micrometer to exclude the latter. Care must also be taken to ensure that the changes do not represent DCIS involving either a terminal-duct lobular unit (TDLU) or a pre-existing benign process, such as sclerosing adenosis, radial scar or complex sclerosing lesion. Branching or distortion of ducts involved by DCIS also represents a diagnostic pitfall. Further haematoxylin and eosin (H&E) levels may be helpful in such cases.
Immunohistochemistry may also be of value in distinguishing microinvasion from its mimics. Immunostains for myoepithelial markers show that there is no myoepithelial layer surrounding microinvasive foci. Stains for keratins may be of particular value in highlighting the microinvasive foci and complement stains for myoepithelial cells. Immunostains for the basement membrane components laminin and collagen IV may be problematic since in situ lesions may show variable basement membrane loss and (micro)invasive foci may be at least partially surrounded by basement membrane. Particular difficulty in reaching a correct diagnosis may be seen when the patient has undergone previous needle biopsy (either needle-core or fine-needle aspiration) for pre-operative diagnosis, since displacement of benign epithelium (particularly from papillomas) or cells of carcinoma in situ may mimic microinvasion. The presence of granulation tissue and reparative fibrosis, adjacent fat necrosis and haemosiderin deposition, which are usually evident after needling procedures, should be sought.
When there is doubt about the diagnosis of microinvasive carcinoma or if the suspicious area is no longer seen on any further sections or immunostains, it is recommended that the case should be diagnosed as an in situ lesion with no definite evidence of established microinvasive or invasive carcinoma.
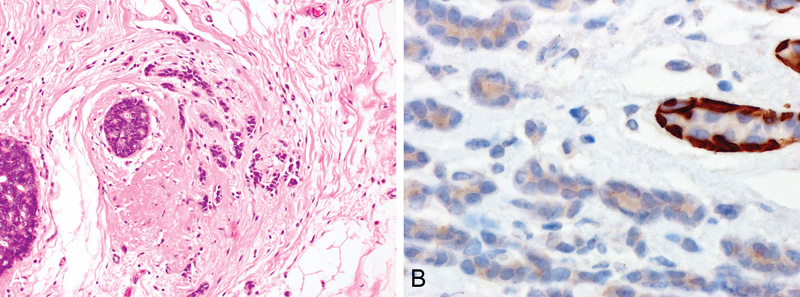
Fig. 6.01 Microinvasive carcinoma. A A small focus of invasive carcinoma of 0.8 mm in maximum extent is present adjacent to ducts with DCIS. The invasive carcinoma focally has a lobular morphology. B Immunostaining for CK14 shows no evidence of a myoepithelial cell (MEC) layer around the invasive tubules, in contrast to the distinct MEC layer around an adjacent normal tubule. Source: (A,B) Lakhani S.R.

Fig. 6.02 Microinvasive carcinoma. A Two ducts are filled by ductal carcinoma in situ, while small clusters of carcinoma cells invade the stroma (upper left quadrant of the field) admixed with a dense lymphocytic infiltrate. B Higher magnification shows small invasive cell clusters within stromal spaces distributed over a 0.7 mm area and surrounded by a dense lymphocytic infiltrate. C Immunostaining for actin highlights the vessel walls, while absence of myoepithelial cells around the tumour cell clusters confirms their invasive nature. Source: (A-C) Tan P.H.
There are no studies reporting the genetic profile of microinvasive carcinoma of the breast.
There are few data regarding prognostic or predictive significance of hormone receptor and HER2 status of microinvasive carcinoma of the breast. It seems logical to recommend assessment of these markers on this lesion; however, very commonly, the microinvasive foci are not present on the particular sections on which immunostaining is carried out, in which case the results for the associated DCIS should be reported.
The incidence of metastatic disease in axillary lymph nodes in microinvasive carcinoma of the breast is low. Review of the literature for accurate determination of the frequency of metastatic disease in sentinel lymph-node biopsy is impeded by the different definitions applied for the diagnosis of microinvasive carcinoma as well as pathological methods for handling and evaluating sentinel lymph nodes {136}. Between 0% and 20% of patients with microinvasive carcinoma are reported to have axillary metastasis (mean, 9.4%). However, caution is required in interpretation of these figures as most of these data are from very small series. For example, the highest reported frequency (20%) is reported from a series of 15 patients {291}. Nevertheless, in many centres sentinel lymph-node biopsy is undertaken in women with microinvasive carcinoma of the breast.
For the same historical reason of varying definitions of the diagnosis, robust data on the clinical behaviour of microinvasive lesions are not available. However, it appears that, if this restrictive definition is applied for diagnosis, patient prognosis is excellent and not clearly different from patients with pure DCIS of equivalent size and grade. In general, this condition is managed clinically in the same way as high-grade DCIS.
No predictive factors have been identified.
Microinvasive carcinoma is staged as T1mi in the TNM/AJCC classification.