1. What is ecological stoichiometry?
2. Major patterns of nutrient content in organisms
3. Influence of stoichiometry on animal growth and community structure
4. Nutrient cycling in ecosystems
5. Influence of stoichiometry on species dynamics
6. Whole-lake food web experiments
7. Light:nutrient ratios and the ecology of Australia
Ecological stoichiometry examines how the nutrient content of organisms shapes their ecology. Although the chemistry of living things is constrained by their need to have a certain representation of major biomolecules such as DNA, RNA, proteins, lipids, etc., there is enough flexibility in these allocations that different species have nonidentical chemical contents. Thus, community structure is related to the portioning of elements in ecosystems. Stoichiometric considerations play a role in the rate of growth of animals, in the rates of recycling of elements by food webs, in the rate of mineralization of nutrients from organic matter, and in many other ecological phenomena. Stoichiometric models often have complex dynamics not seen in models lacking explicit treatment of stoichiometry, which suggests that stoichiometry is an important force shaping ecological dynamics.
autotroph. An organism that converts inorganic carbon to organic carbon and thus does not need to ingest or absorb other living things. Green plants (including certain algae and cyanobacteria) are photoautotrophs because they use light energy to make this conversion.
ecological stoichiometry. The balance of multiple chemical substances in ecological interactions and processes, or the study of this balance.
geophagy. The eating of dirt. This behavior may be used to balance mineral intake for animals living in low-food-quality environments.
growth rate hypothesis. Differences in organismal C:N:P ratios are caused by differential allocations to RNA necessary to meet the protein synthesis demands of rapid biomass growth and development.
heterotroph. An organism that relies on organic carbon for energy. Heterotrophs include herbivores, carnivores, and detritivores as well as omnivores that may feed on more than one trophic level.
homeostasis. Maintenance of constant internal conditions in the face of externally imposed variation. In ecological stoichiometry, homeostatic regulation of organism nutrient content causes some species to have narrower bounds to their chemical content than others.
nullcline. A set of points in an ecological model where the rate of change of one species is zero (it is at equilibrium). In community models, intersections of nullclines indicate points where more than one species is at equilibrium.
nutrient content. The quantity of an element in an organism’s biomass. May be measured as moles or grams per organism, as the percentage of mass made up by a given element, or as the X:C ratio, where X is a nutrient such as N or P.
threshold element ratio. The nutrient ratio of an organism’s food where that organism switches from limitation by one of those elements to limitation by another. For example, in the case of C:P, when food is above the TER, that organism will be limited by P, and when food is below the TER, that organism will be limited by C.
Some branches of ecology are oriented toward understanding the dynamics of individual species, and others focus on the fluxes of matter and energy among collections of species in ecosystems. Ecological stoichiometry fits between these two approaches because it deals with the patterns and processes associated with the chemical content of species. Numerous ecological phenomena from the success or failure of populations to the carbon storage of whole ecosystems have a stoichiometric component. The term ecological stoichiometry is relatively recent, but the field is based on some of the most classic of ecological studies.
Formally defined, ecological stoichiometry is “the balance of multiple chemical substances in ecological interactions and processes, or the study of this balance.” In addition, ecologists interested in stoichiometry often consider the availability of solar or chemical energy relative to the availability of one or more chemical substances.
Ecological stoichiometry is concerned with the contents of multiple elements in living and dead organic matter. There are approximately 90 naturally occurring elements, of which 11 predominate in living organisms. Only four of these (C, H, O, and N) make up about 99% of living biomass; the other seven (Na, K, Ca, Mg, P, S, and Cl) are essential to all living things. About 10 others, metals and nonmetals, are required by most but not necessarily all species. Finally, about eight other elements are required by more limited numbers of species. Some elements, especially C, H, O, and N, provide the atomic-level skeletons for biomolecules. Others are involved in materials providing structure at the organismal level, for example, the Ca and P in vertebrate bone. These elements all are generally required in high amounts. Other elements are used in energy transduction processes, where electrons are energized and deenergized. These elements, such as Fe and Mg, are just as necessary for life, but they are required in lower quantities. Although the theories and tools of ecological stoichiometry could be applied to any of these elements, most studies to date concern C, N, and P.
In the abiotic world (air, water, rocks, etc.), elements can be combined in almost limitless proportions. Living things, however, are based on a much more restricted chemistry utilizing carbon-containing organic molecules combined using more-or-less defined proportions of nucleic acids, lipids, proteins, and carbohydrates. But, and this is a crucial point in ecological stoichiometry, in spite of a commonality to the chemistry of living things, species throughout the tree of life do not have precisely identical chemistry. Nor do all species regulate their chemical content to the same degree. Ecological stoichiometry considers the many phenomena that emerge from the patterns of chemical content in organisms as well as the proximate and ultimate reasons they have the chemical composition they do. Some aspects of ecological stoichiometry arise because of a first-order commonality to the chemistry of life, whereas others arise as a result of the differences in chemistry among living things that one observes when one pays careful attention to the patterns of element content of different species.
Inorganic chemistry teaches us about the different characteristics of the chemical elements, their tendency to ionize, the number of covalent bonds they may form, etc. According to these physical principles, biochemistry makes use of the different elements in different ways. A special element is phosphorus. P makes up 1% of the soft tissues of most living things and a much higher percentage of some hard tissues such as bone. P has many biochemical functions. It is central to metabolism in the ATP/ADP energy capture and utilization system. It helps form the backbone of the information-carrying and protein-assembling nucleic acids (DNA and RNA; more on this role of P below). It combines with lipids to make cell membranes and therefore is involved in cell structure. Each of these different roles is crucial to the living cell. P also is a large component of the skeletal system of vertebrate animals. P is critical in these different ways, but not all cells or organisms combine these separate functions in the same ways; therefore, cellular or organismal P content can be species specific. Although necessary in all these ways and others, P is not always greatly abundant in nature relative to biological demand.
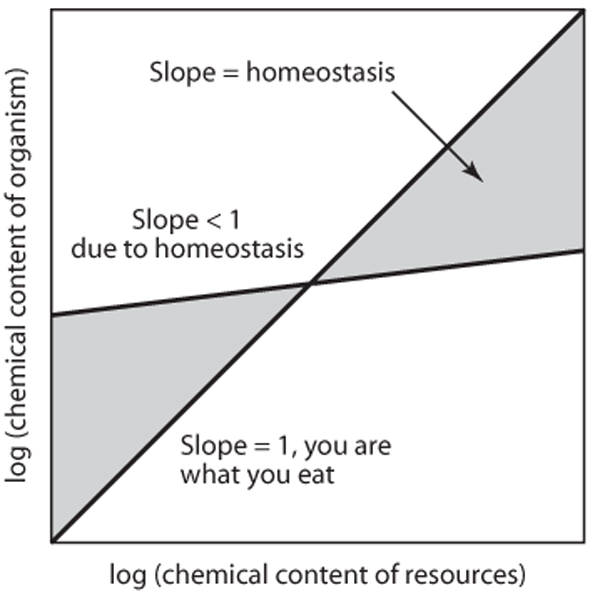
Living organisms concentrate certain elements while rejecting others. One of the hallmarks of life is its ability to maintain relatively constant internal conditions in the face of external variability. A wellknown example is the narrow range of temperature that a healthy endothermic vertebrate such as a human being maintains in spite of being exposed to wide environmental temperature fluctuations. The negative feedback associated with maintaining relatively constant internal conditions is called homeostasis. Homeostatic regulation of element content is a key aspect of ecological stoichiometry and requires a formal definition. The degree of stoichiometric homeostasis varies for different organisms and different elements. Because homeostasis is a resistance to change, we measure it by relating the elemental content of an organism to the elemental content of its food or its neighboring environment, as the case may be; homeostasis is indicated with the Greek letter eta (H) (figure 1).
Figure 1. Homeostasis in ecological stoichiometry. If one plots the logarithm of the element content of an organism versus the logarithm of the chemical content of the resources it consumes (both measured on a common scale), the slope is referred to as homeostasis. A slope of 1 indicates that the organism’s chemistry changes in lockstep with its food, or “it is what it eats.” Shallow slopes indicate a resistance to change. Homeostasis results from the tendency of living things to shape their own chemistry to their needs.
Homeostasis varies with the organism and with the chemical of interest. Photoautotrophic organisms (e.g., cyanobacteria, algae, and plants) often display a wide range of variation in C:N:P ratios according to conditions of light, nutrient, and growth rate as well as across different species and functional groups. Under severe nutrient limitation, autotrophs produce biomass with extremely low nutrient content (high C:nutrient ratio). This connection links ecological dynamics to stoichiometric patterns. In contrast, animals are more homeostatic and generally regulate their C:N:P ratios around stage- or species-specific values that are more nutrient rich than those for nutrient-limited autotrophs.
The wide range of autotroph C:N:P ratios in ecosystems reflects contrasts in the abiotic and biogeochemical conditions that supply CO2, light, and nutrients to photoautotrophs in different ecosystems. For example, in broad cross sections of both North American and Norwegian lakes, seston C:P ratio has been shown to be positively correlated with ecosystem light:nutrient ratio, which itself is determined by local conditions affecting light intensity (mixed layer depth, light attenuation) and external P supplies. In terrestrial ecosystems, local soil conditions, canopy development, and water supply interact to affect plant C:N:P stoichiometric ratios.
It is also increasingly recognized that various anthropogenic perturbations, such as atmospheric N deposition and increased CO2 concentrations can also affect autotroph C:N:P ratios in both aquatic and terrestrial ecosystems. For example, a doubling of CO2 concentration reduces plant N content by about 16%, on average. There are also broad-scale patterns in the N:P ratio of plant biomass in which N:P ratio decreases moving toward the poles. This pattern may reflect differences in edaphic conditions (e.g., differences in soil age that affect soil P supply) or effects of selection operating on plant growth rate (see growth rate hypothesis below). Despite the wide intraspecific variation in C:N:P ratios that can be produced by differences in growth conditions, there also are significant differences in plant stoichiometry that derive from phylogenetic affiliation. For example, legumes that harbor N-fixing symbionts generally have higher N:P ratios than other taxa.
Heterotrophs such as bacteria and metazoans also exhibit substantial variation in C:N:P ratios, but physiological variation caused by growth or dietary conditions is thought to be relatively minor compared to such effects in autotrophs. Heterotrophs are much more homeostatic in their element content than are autotrophs. Variation in element content in different heterotrophs reflects differences in organismal allocation to major biochemical and structural components. For microbes and small invertebrates (figure 2), C:N:P variation is tied to growth-related allocation to P-rich ribosomal RNA (the growth rate hypothesis), as the content of rRNA generally increases with growth rate, comprising a significant fraction of overall biomass and containing 8.6% P by mass. Indeed, in the bacteria, zooplankton, and insects shown in figure 2, on average about 50% (and sometimes over 90%) of total organismal P was contributed by the P contained in RNA. However, because growth rate decreases with increasing body size, the contribution of P in RNA to overall body C:N:P stoichiometry also declines with body size, becoming relatively insignificant (less than 10% of total P) for animals larger than about 0.1 g dry mass.
C:N:P ratios among larger vertebrate animals also vary considerably as a result of differential allocation to structural P in bones (the mineral apatite that makes up bone is 17.6% P by mass). In terrestrial vertebrates, the percentage of whole-organism mass devoted to skeleton increases with body size. In aquatic vertebrates, because of the suspension action of water, gravity exerts less of a selective pressure, and differences in skeletonization reflect other biological aspects; bony fish are well protected from predators, for example. These intra- and interspecific variations in vertebrate P requirements have important implications for their mineral nutrition and for their role in cycling of limiting nutrients in the ecosystem.
Figure 2. Intra- and interspecific relationships between total body P content (percentage of dry mass) and total body RNA P content. (RNA P content is the percentage of body mass contributed by the P contained in RNA; it is calculated by multiplying the RNA content by 0.086, the mass fraction of P in RNA.) The dashed 1:1 line indicates the condition of having all the cellular P made up by RNA-P. The figure shows P-limited Escherichia coli in chemostats (crosses), various freshwater crustacean zooplankton under different food conditions (dark gray circles and open triangles), larval Drosophila melanogaster during ontogenetic development (light gray circles), and field-collected mesquite-feeding weevils sampled during dry and wet years (black circles). Various shorter lines indicate significant intraspecific relationships. The longer solid line is a fit to the entire data set (R2 = 0.87); its slope is 0.97 (~ 1), indicating that, across the entire data set, variation in P content is directly and quantitatively attributable to variation in RNA content. In all cases shown, P- and RNA-rich organisms have higher growth rates than low-P and low-RNA organisms.
An animal’s niche is defined by numerous ecological factors, including climate, physical habitat structure, and presence or absence of predators. Some important niche dimensions are stoichiometric. These stoichiometric dimensions are likely most significant for detritivores, which consume nonliving food that may have very low nutrient content but also are often very important for herbivores because of the aforementioned differences in C:P and C:N ratios between plant and animal biomass.
In theory, there is only one particular composition containing elements in precisely the right proportions to optimize growth and maintenance of any given consumer at a particular moment in its life. Deviations from this optimum reduce growth rate and fitness, sometimes severely. Across terrestrial and aquatic habitats, there are numerous studies showing that one or more elements, including Na, N, P, Fe, or Ca, may be low enough in autotroph biomass to shape the foraging decisions or limit the growth of individual herbivore species. As a corollary, under these conditions, other resources including energy are in relative excess. Elements in surplus are not held with great efficiency by the metabolism of the consumer and instead are recycled to the environment, whereas dividends are paid on consumers holding that element most limiting their growth with high efficiency. Ecologists define a particular ratio in an organism’s food where it switches from limitation by carbon to limitation by another element as a “threshold element ratio” (TER). The actual value of the TER reflects the taxonomic identity and nutrient ratio of the consumer itself as well as its efficiency at processing carbon or nutrients.
When an animal cannot locate sufficient quantities of an element in its environment, that lack may limit the animal’s growth. C:N:P ratios in a variety of ecosystems indicate that animals, especially herbivores and detritivores, must often subsist on food with low element content. For example, in a large compilation of published values of C:N:P ratios for foliage in terrestrial plants, the average C:N and C:P ratios (moles: moles) were 36 and 968, respectively (N:P 28), in contrast to average C:N and C:P ratios of 6.5 and 116 for herbivorous insects (N:P 26.4). Similarly, freshwater seston (suspended organic matter containing phytoplankton and other microscopic biota on which filter-feeding zooplankton depend) also has high C:N and especially C:P ratios (10.2 and 307; N:P 30.2) compared to the average freshwater zooplankton species (6.3 and 124; N:P 22.3). Marine seston, however, generally has lower C:N and C:P ratios, much more in line with the elemental composition of marine zooplankton themselves. These contrasts suggest that, based on stoichiometric imbalance alone, marine food webs should operate more efficiently in processing organic matter than freshwater and especially terrestrial food webs. Indeed, existing data do indicate that a considerably greater fraction of the low-nutrient primary production of terrestrial food webs enters detrital food chains (that is, is not consumed by herbivores) than does so in freshwater and especially marine food chains.
A well-studied example is the waterflea Daphnia and the concentration of P in its algal food. This is a freshwater, herbivorous zooplankter and an important keystone species in aquatic food webs. Daphnia is considered a high-P zooplankter, consistent with its rapid-growth life history as explained by the growth rate hypothesis described above. Laboratory studies in which Daphnia have been raised on algal foods of varying C:P ratio have consistently shown that unless food quantity is very low, Daphnia growing on algal foods with C:P above about 300 or so show reduced rates of biomass gain, longer times to first reproduction, and reduced fecundity. Low-P zooplankton are much less susceptible to effects such as these. Similarly, studies examining stoichiometry across multiple lakes have shown that high-P Daphnia are less likely to be abundant in habitats where the potential food base has a high C:P ratio. Hence, the population-level dynamics that are well studied in the laboratory correctly predict certain aspects of community structure in the field.
Above, we focused on the single element most limiting for consumer growth. In an ecological interaction, mass must balance, and any matter that is ingested but not incorporated into consumer biomass must be returned to the environment in a solid, liquid, or gaseous state. The existence of stoichiometric mismatches between food and consumer, as well as differences in homeostatic regulatory ability of elements, therefore, has considerable bearing on the patterns of nutrient recycling in ecosystems.
One place this is observed is in litter decomposition. As described above, leaves of higher plants exhibit a wide range in C:N:P ratios. When leaves die, there is a range in C:N:P of the corresponding detritus that is as wide or wider than that in living leaves. Nutrient content in detritus also is lower than in leaves because of nutrient resorption before abscission. This potentially severe elemental imbalance between detritus and the organisms that consume it generates a strong stoichiometric component to litter decomposition. Litter of relatively high N or P content (C:N ≈ 10 or C:P ≈ 100) breaks down rapidly, as quickly as 1% loss of litter mass per day. In contrast, litter of low nutrient content (C:N ≈ 100 or C:P ≈ 1000) breaks down much more slowly, about 0.01% of mass per day. This contrast in litter breakdown rates and nutrient remineralization rates has a strong bearing on many aspects of terrestrial nutrient cycling, carbon storage, and other phenomena.
Stoichiometric constraints on nutrient cycling in ecosystems are well illustrated by consideration of the N:P ratio resupplied by foraging, homeostatic consumers. Theoretical analysis has predicted several patterns. First, the N:P recycled should generally increase with the N:P of the food consumed (but not in a linear way, see below). Consumers ingesting high-N:P food should tend to recycle high N:P ratios. Second, the N:P recycled should be a decreasing function of the N:P of the consumers themselves. High N:P consumers must retain N and lose P in order to meet their growth requirements. Finally, and perhaps most interestingly, the N:P recycled by homeostatic consumers should generally be a more extreme version of the N:P they eat. Consumers eating high-N:P food are best served by keeping P but losing N, and vice versa. This accentuation of nutrient ratios, if repeated over and over again in a relatively closed system while consumer biomass builds up, can cause N:P limitation patterns to diverge. This is an ecosystem instability generated by the homeostasis of the foraging consumer. We return to this subject below.
One example where such recycling effects have been studied is in lake communities where the zooplankton herbivores may be limited by high-N:P copepods or low-N:P Daphnia. Theory says that species shifts between these two groups should result in different nutrient limitation patterns, with recycling by copepods generating low-N:P conditions and recycling by Daphnia generating high-N:P conditions. Experimental studies of lake food webs generally have borne out those predictions; some of these results are described in an upcoming section.
The large stoichiometric imbalance between nutrientlimited autotrophs and the herbivorous animals consuming them suggests that food quality may play an important role in regulating herbivore dynamics in nature. However, nutrient recycling by the consumers themselves may ameliorate the low nutrient content of autotrophs via this important feedback. Thus, nutrient-autotroph-herbivore systems have the potential for interesting and complex feedbacks that may affect population dynamics and food-web structure. These interactions have been analyzed from a theoretical perspective in a number of mathematical models.
These models, “stoichiometrically explicit” versions of the famous Lotka-Volterra equations, generally contain several key components that distinguish them from nonstoichiometric models: variable and growthrate-dependent nutrient content of the autotrophic prey; strict homeostasis of nutrient content in the herbivorous consumer; and overall mass conservation of multiple elements in the system. We analyze these models by using nullclines. A grazer-autotroph model will have two nullclines, one for each trophic level. A nullcline is the set of all consumer and autotroph biomass values for which the rate of biomass change for a given population is zero. In most nonstoichiometric models, the shape of the nullclines is such that only one intersection is possible, meaning there is only one combination of autotroph and grazer densities where there is no change with time. Imposition of stoichio-metric food quality effects, which generally manifest when autotrophs have produced high biomass with low nutrient content, forces the consumer’s nullcline (where its rate of change is zero) to be hump shaped (figure 3). The hump means that the nullcline can intersect the autotroph’s nullcline to form more than one equilibrium point.
Figure 3. An example of nullcline analysis for a stoichiometrically explicit predator–prey model. In the figure, the gray box delineates the entire domain within which population fluctuations are confined because of the fixed amount of limiting nutrient (in this case, P) present in the system. The point on the autotroph axis labeled K represents the carrying capacity for the autotrophs. As discussed in the text, K reflects overall light intensity and is varied to examine potential dynamic impacts of light on the consumer–autotroph system. The interior of the gray box is delineated into two regions indicating situations where individual consumer growth is limited by C (low food quantity) or by P (low food quality). Intersections of the consumer (dark, solid) and autotroph (dashed) nullclines indicate potential equilibria. In this case, only one intersection is a stable equilibrium (solid circle) in which autotrophs have high biomass but consumer biomass is low and consumer growth is P-limited.
Stoichiometric models predict a much richer range of population dynamics than nonstoichiometric models. For example, increasing the parameter (K) for autotroph carrying capacity (to simulate the effects of increased light intensity) causes an intriguing series of bifurcations. As light intensity (∝K) increases, the dynamics shifts from a single stable point (with consumers limited by total food quantity) to a limit cycle to a second stable point (with consumers limited by poor food quality; situation shown in figure 3) to a stable point involving deterministic extinction of the consumer. Finally, a stoichiometric model containing one autotroph species together with two consumer species has also been analyzed to show that the two consumers can stably coexist with each other indefinitely under certain conditions of high light intensity and poor food quality. Under such coexistence, the single food type acts as two different resources, expanding the niche space.
The predictions of these stoichiometrically explicit models have been borne out in experimental studies. In an artificial ecosystem experiment involving a green alga and two species of Daphnia, increased light intensity led to very slow production of Daphnia biomass because of the poor food quality along with decreased trophic transfer efficiency from algae to Daphnia. As predicted by theory, near Daphnia extinction occurred in one mesocosm with highest light intensity, whereas high light also resulted in sustained coexistence of the two Daphnia species. In fact, the data showed that, under low light intensity (P-rich algae), there was normal density dependence in the herbivores: individual female fecundity was negatively correlated with Daphnia population size, indicating strong intra- and interspecific competition. However, under high light intensity (low-P algae), fecundity was positively correlated with Daphnia abundance, indicating intra- and interspecific facilitation. This latter relationship reflects the indirect effects of consumer-driven nutrient recycling: as the animal population built up, it cropped the low-P algae while excreting some P, thus increasing the P content of the remaining algae and improving its quality. A variety of additional studies have extended this work to field situations and have shown that modifications of light intensity and nutrient supply can significantly affect the C:N:P ratios of seston and thus alter the production and dynamics of zooplankton consumers under natural conditions.
Stoichiometric effects on trophic interactions and food web dynamics also occur in terrestrial ecosystems, as in the well-studied example of the role of low dietary N content (high C:N ratio) in limiting herbivore production. More recently, it has been shown that food P content can also play a role in limiting consumer performance in terrestrial settings. For example, experimental manipulation of the P content of the plant Datura wrightii significantly increased the growth rate of caterpillars of the moth Manduca sexta.
Previous sections explored the dual roles of stoichiometric mechanisms in affecting trophic dynamics: first, effects of food quantity and quality, and second, internal nutrient processing via consumer-driven nutrient recycling. In natural ecosystems, these mechanisms may come together in complex and interesting ways. We now consider a pair of whole-lake food web manipulations that were designed to evaluate stoichiometric dimensions to nutrient cycling related to food web structure. The experiments were based on predicted differential C:N:P stoichiometry of major herbivores that dominate under different food web conditions. Lakes with three dominant trophic levels (phytoplankton, zooplankton, zooplanktivorous fish) tend to have low zooplankton biomass dominated by copepods but lacking Daphnia because Daphnia are especially susceptible to predation by small fish. Such a zooplankton community will have high N:P ratio. In contrast, lakes with four trophic levels (the previous three plus piscivorous fish such as pike or bass at the top) tend to have high zooplankton biomass dominated by low-N:P Daphnia because the piscivores hold the zooplanktivores in check. The experiment thus relied on couplings of community structure and stoichiometry.
One experiment was performed in Lake 227 (L227), a lake that was experimentally eutrophied for more than two decades by addition of P-rich fertilizer, making this one of the longest-running whole-lake experiments in ecology. Its fish community lacked piscivores, and the lake supported dense populations of planktivorous minnows and a zooplankton community dominated by copepods and rotifers. Consistent with the low N:P ratios of the lake’s nutrient loading, the dense phytoplankton community was dominated by N-fixing cyanobacteria, as it had been for much of the period since low-N:P fertilization had begun in the mid-1970s. Into this configuration, 200 piscivorous pike were introduced to the lake over the course of 2 years, thus adding a fourth trophic level where it had not existed.
Figure 4. Food web dynamics after food web manipulation of experimentally eutrophied Lake 227 at the Experimental Lakes Area, Ontario, Canada. Two hundred piscivorous pike were introduced into the lake in 1992 and 1993, leading to elimination of planktivorous minnows (first panel). Consistent with a trophic cascade, zooplankton biomass increased dramatically (second panel), especially Daphnia (white portion of the zooplankton graph). Consistent with increased predominance of Daphnia in the zooplankton, the N:P ratio of total zooplankton biomass declined (third panel). Increased Daphnia and overall zooplankton biomass in 1996 was associated with a massive shift in phytoplankton community structure away from previous dominance by N-fixing cyanobacteria (fourth panel). This is consistent with an altered internal nutrientrecycling regime that disproportionately increased the availability of N relative to P (fifth panel), likely reflecting differential retention of P in the low-N:P Daphnia biomass. Error bars indicate +1 standard error.
Within a year of pike introduction, minnow densities had decreased dramatically, and during the third year following introduction of pike, a massive increase in zooplankton biomass (largely Daphnia) and a corresponding decrease in zooplankton N:P ratio were observed (figure 4). These changes were associated with large declines in algal biomass and overall increases in concentrations of dissolved nutrients, especially dissolved nitrogen, resulting in an increased N:P ratio of available nutrients. Most striking was the nearly complete absence of previously dominant cyanobacteria when Daphnia dominated the lake. These results provide support for a strong stoichiometric component of the trophic cascade, in which differential recycling of limiting nutrients by zooplankton taxa with different body N:P ratios affects phytoplankton community structure by altering the competitive arena.
A similar experiment was performed in Lake 110, a lake with a similar food web structure (dense minnow populations, no Daphnia in the zooplankton), but a lake still in its natural oligotrophic state. One crucial difference between L227 and L110 (and indeed other unfertilized lakes of the region) was that the C:P ratio of seston in L110 was considerably higher (>500 compared to <200 for L227). As for L227, piscivorous pike were introduced, and the response of the system was monitored over ensuing years along with dynamics of a similar oligotrophic lake (L240).
Figure 5. Food web dynamics after food web manipulation of naturally oligotrophic Lake 110 at the Experimental Lakes Area, Ontario, Canada. One hundred fifty-three piscivorous pike were introduced into the lake in 1992 and 1993, leading to elimination of planktivorous minnows (first panel). Inconsistent with a trophic cascade, zooplankton biomass decreased dramatically (second panel), including Daphnia (third panel), in both the manipulated lake (L110, light gray bars) and the reference lake (L239, dark gray bars). The zooplankton declines were associated with major increases in seston C:P ratio (fourth panel) in both lakes. These dynamics suggest that regional climatic effects altered phytoplankton growth conditions and worsened phytoplankton P limitation, raising seston C:P ratio and imposing a stoichiometric constraint on zooplankton and thereby truncating the expected trophic cascade. Error bars indicate ±1 standard error.
As in L227, pike introduction greatly reduced the activity and abundance of minnows (figure 5). However, instead of an increase of zooplankton biomass and especially of Daphnia, zooplankton biomass in L110 declined substantially, and Daphnia populations became nearly extinct. Interestingly, zooplankton in the reference lake underwent similar dynamics. These anomalous responses become understandable by noting that the year of Daphnia collapse corresponded to a year in which seston C:P ratios were unusually high in both L110 and L239. This suggested that regional climatic changes had altered nutrient supply to the lakes, accentuating phytoplankton P limitation and thus increasing seston C:P ratio and worsening food quality for zooplankton and especially for Daphnia. This stoichiometric constraint” on the trophic cascade was supported by later dietary P supplementation studies that showed that Daphnia in nearby, similar lakes do experience direct P limitation of their growth because of high seston C:P ratios.
Taken together, these two whole-lake experiments illustrate the ecosystem-scale operation of the two sides of the stoichiometric coin: food quality and nutrient recycling.
To further illustrate the scope and potential explanatory power of ecological stoichiometry, we close with a specific application that ties many observations together. Recently proposed, the “Nutrient Poverty/Intense Fire Theory” purports to explain a host of aspects of the ecology of Australia, the flattest, driest, and geologically oldest vegetated continent. Australia has a uniquely large proportion of highly nutrientdeplete soils. These high-light:nutrient conditions promote several notable features in the autotrophs. The surplus carbon (over nutrient availability) is used to produce foliage that is well defended from herbivores as well as large quantities of lignified tissues. These factors negatively impact foliovores. However, many of the plants also produce unusually high quantities of readily digestible exudates, and Australia is unusual in the number of vertebrates (mostly birds) that pollinate its flowers, attracted and supported, it would seem, by the high exudation rates.
In regard to the low-quality forage, note that large amounts of high-carbon plant biomass not consumed by herbivores build up and provide fuel for intense fire. Frequent fires characterize much of Australia, and these generate a positive feedback because fire volatilizes potentially limiting elements for animals; these elements include N, S, I, and Se, further depleting these potentially limiting elements.
The unusual plant biomass, caused by the positive feedback of high-light:nutrient conditions, presents challenges for the herbivores of the continent. The Nutrient Poverty/Intense Fire theory suggests that it is no coincidence that folivorous vertebrates of Australia are unusually small relative to other continents and have low metabolic rates. Another unusual aspect of the animals of the continent is the absence of geophagy (soil eating). Though geophagy is common on other continents, the extremely nutrient-poor soils of Australia may not provide adequate supplementation of trace elements to promote this style of foraging.
By examining the ratios of available energy to nutrients, particularly scarce nutrients, ecologists may identify processes not previously recognized as important for life forms or biotic adaptation on other continents.
Andersen, T., J. J. Elser, and D. O. Hesson. 2004. Stoichiometry and population dynamics. Ecology Letters 7: 884–900. This article provides an up-to-date review of the complex population dynamics that is characteristic of stoichiometrically explicit models.
Cebrián, J. 1999. Patterns in the fate of production in plant communities. American Naturalist 154: 449–468. This article summarizes a large amount of scientific literature on the processing of carbon in ecosystems, for example, how much of primary production is grazed versus enters into detrital food chains. The article shows how autotroph nutrient content (and growth rate) relates to carbon processing.
Elser, J. J., K. Acharya, M. Kyle, J. Cotner, W. Makino, T. Markow, T. Watts, S. Hobbie, W. Fagan, J. Schade, J. Hood, and R. W. Sterner. 2003. Growth rate–stoichiometry couplings in diverse biota. Ecology Letters 6: 936–943. This article examines the stoichiometric signatures of growth rate in diverse heterotrophs. P, RNA, and growth are closely coupled in small heterotrophs when P is limiting their growth.
Elser, J. J., W. F. Fagan, R. F. Denno, D. R. Dobberfuhl, A. Folarin, A. Huberty, S. Interlandi, S. S. Kilham, E. McCauley, K. L. Schulz, E. H. Siemann, and R. W. Sterner. 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408: 578–580. This article reviews the patterns of C:N:P ratios in autotrophs and herbivores from terrestrial and aquatic food webs. It shows that there often are large differences in the C:nutrient ratio across the plant-animal interface in terrestrial and freshwater systems.
Hessen, D. O. 2006. Determinants of seston C:P ratio in lakes. Freshwater Biology 51: 1560–1569. This article explores the reasons why organisms in lakes have such widely varying nutrient ratios. Lakes with high light but low nutrients have high seston C:P ratios and low numbers of P-rich herbivores.
Kay, A. D., I. W. Ashton, E. Gorokhova, A. J. Kerkhoff, A. Liess, and E. Litchman. 2005. Toward a stoichiometric framework for evolutionary biology. Oikos 109: 6–17. Like any phenotypic variation, stoichiometric differences among species are dependent on evolutionary history and subject to natural selection. This review considers a number of evolutionary facets to stoichiometric ecology.
Orians, G. H., and A. V. Milewski. 2007. Ecology of Australia: The effects of nutrient-poor soils and intense fires. Biological Review 82: 393–423. This thoughtprovoking recent article suggests that many of the unique aspects to the ecology of Australia are caused by high light and low nutrients, with a positive feedback of fire involved.
Reich, P. B., B. A. Hungate, and Y. Luo. 2006. Carbon–nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annual Reviews of Ecology and Systematics 37: 611–636. This article is a thorough examination of many relevant stoichiometric linkages in global change.
Sterner, R. W. 1995. Elemental stoichiometry of species in ecosystems. In C. Jones and J. Lawton, eds. Linking Species and Ecosystems. Boca Raton, FL: Chapman & Hall, 240–252. An early, concise examination of stoichiometric reasoning applied to ecological systems.
Sterner, R. W., and J. J. Elser. 2002. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press. This book is the most comprehensive examination of stoichiometry in ecology. Topics range from the molecular to the global.