2. The role of thermal stratification
3. Natural and cultural eutrophication
4. Ratios and sources of key nutrients
5. Whole-lake experiments and their role in eutrophication control policy
6. Nonpoint sources of nutrients
8. Internal recycling of phosphorus
9. Eutrophication in flowing waters
10. Eutrophication and the quality of drinking water
11. Eutrophication of estuaries
Increasing the inputs of the nutrients phosphorus and nitrogen to freshwater bodies and estuaries causes increased growth of nuisance algae, termed eutrophication. In lakes, eutrophication can be prevented by controlling inputs of phosphorus. In estuaries, there is still controversy over whether nitrogen, phosphorus, or both must be controlled.
epilimnion. The uniformly warm upper layer of a lake when it is thermally stratified in summer.
eutrophic. Eutrophic lakes are richly supplied with plant nutrients and support heavy plant growths.
eutrophication. The complex sequence of changes initiated by the enrichment of natural waters with plant nutrients.
hypolimnion. The uniformly cool and deep layer of a lake when it is thermally stratified in summer.
mesotrophic. Mesotrophic lakes are intermediate in characteristics between oligotrophic and eutrophic lakes. They are moderately well supplied with plant nutrients and support moderate plant growth.
oligotrophic. Oligotrophic lakes are poorly supplied with plant nutrients and support little plant growth.
thermocline. Thermal or temperature gradient in a thermally stratified lake in summer. Occupies the zone between the epilimnion and hypolimnion.
Eutrophication is the word used by scientists to describe the result of overfertilization of lakes with nutrients. The first symptom noticeable to casual observers is that the fertilized lakes turn green with plant growth. Paradoxically, we value the increased growth of plants that follows fertilization on land but abhor similar effects in our waters.
Eutrophication is derived from the German word eutrophe, which means “nutrient-rich.” The two nutrients that are responsible for increasing growth of algae and other aquatic plants are nitrogen and phosphorus. Eutrophic lakes typically have dense algal blooms. They can also have dense beds of rooted aquatic plants if the lakes have shallow areas with mud or sand bottoms.
The term eutrophication was coined by the German wetland scientist C. A. Weber in 1907 to refer to the rich wetlands in areas of Europe that received nutrient runoff from surrounding lands. The term was first applied to lakes by Einar Naumann roughly a decade later. The term oligotrophic (nutrient poor) was applied to nutrient-poor lakes, which generally have clear water and deep waters that contain high concentrations of oxygen. Lakes that are between these two extremes are generally termed mesotrophic. All three categories of lakes can undergo eutrophication if nutrient concentrations are increased. Recently, extremely eutrophic lakes have been termed hypereutrophic.
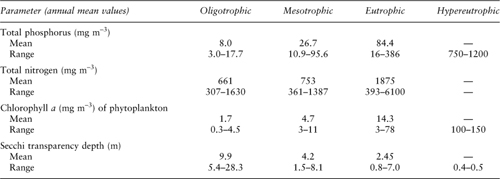
Table 1. General trophic classification of lakes and reservoirs in relation to phosphorus and nitrogen

Source: Modified from Wetzel, R. G. 2001. Limnology, 3rd ed. Amsterdam: Elsevier.
The early use of the terms was to refer to a lake’s appearance. Measurable indices of productivity, such as algal abundance, chlorophyll a, photosynthesis, or nutrient concentration were developed later and are usually used now to define trophic conditions in lakes (e.g., see table 1).
The term eutrophication became widely used by limnologists (scientists who study lakes and other fresh waters) to describe the complex sequence of changes in aquatic ecosystems caused by an increased rate of supply of plant nutrients to water.
The immediate response of an aquatic ecosystem to increased nutrients is an increase in photosynthesis and abundance of plants. This can give rise to increased productivity at all levels of the food chain, up to and including fish. But, as described in greater detail below, changes can also occur in the kinds of organisms inhabiting aquatic ecosystems during eutrophication to disrupt this transfer of energy up the aquatic food chain.
In order to understand all of the changes caused by eutrophication, a working knowledge of a lake’s thermal characteristics is necessary. Some eutrophic lakes are deep enough to have a thermocline, a sharp boundary separating the warm upper waters of a lake (known as the epilimnion) from cold deep layers (termed the hypolimnion). This occurs because cooler waters are more dense than warmer waters. Most swimmers have experienced the thermocline as they pass suddenly from warm water to cold during a deep dive. The depth of a thermocline is determined by the wind velocity and the size of a lake. It can be as shallow as a few meters in small lakes to 15 meters or more in large lakes. Typically, a thermocline in a north-temperate lake will form when a lake warms in May, and last until cooling of the overlying air in September or October causes convective mixing, slowly deepening the thermocline until eventually there is no difference in the density difference between upper and deeper layers of water, and the lake mixes totally.
The production of algae and other plants occurs chiefly in the epilimnion of a lake because that is where light for photosynthesis is greatest and where most nutrients enter the lake. But as the algae and other organisms in the epilimnion die, they sink slowly through the thermocline to decompose in the hypo-limnion. This decomposition consumes oxygen. If the rain of organic matter increases as it does when nutrient supplies are increased, oxygen in the hypolimnion can be depleted to very low concentrations. If oxygen concentrations become very low, it becomes impossible for air-breathing organisms to survive in the deeper layers of a lake. As a result, as a lake becomes more eutrophic, the species of fish and bottom-living invertebrates change from those that require high concentrations of oxygen to those that can tolerate low oxygen.
Eutrophic lakes can occur naturally in terrain with naturally rich soils and geologic sources of nutrients. But lakes can also become eutrophic very rapidly as the result of human influences. Cultural eutrophication is the term used to describe lakes that have rapidly increasing concentrations of nutrients and algal blooms as the result of human activity. Typical nutrient sources are sewage, manure, agricultural fertilizer, and in some countries, phosphorus-based detergents. Cultural eutrophication was first noticed in European lakes at about the turn of the twentieth century, as land was cleared and populations of humans and livestock increased. Similar observations were made soon after in North American waters. Also, by the early twentieth century, water was used as a vector to transport human wastes from populous areas to prevent diseases that were prevalent in earlier times. Typically, wastes were piped to the nearest lake or river where they were discharged. The eutrophying effect of nutrients was unknown at the time. More recently, studies of algal remains in lake muds have been used to deduce that eutrophication occurred in much earlier times. For example, G. E. Hutchinson, one of the pioneers in the study of eutrophication, deduced that Lago di Monterosi, a small lake on the road from Rome to Siena, Italy, underwent cultural eutrophication after the Romans built the Via Appia in 171 BC, which brought many more people to the lake. The modern road follows the same route.
Fish kills resulting from low oxygen in the hypo-limnion are frequently observed during cultural eutrophication. Several species of fish, including white-fish, cisco, and lake trout in North America, will suffocate rather than leave the cold hypolimnions that are their normal summer habitat. Some invertebrates, such as the opossum shrimp Mysis, disappear for similar reasons. The sight of hundreds or thousands of dead or dying fish on the surface of a eutrophic lake in midsummer has led those unfamiliar with the eutrophication problem to conclude that eutrophic lakes are dying. To the contrary, they are teeming with life, though not necessarily of the type that humans value.
Most plants require nutrients in rather set proportions in order to grow. Typically, algae contain roughly 40 g of carbon to every 7 g of nitrogen to every 1 g of phosphorus. This ratio is known as the Redfield ratio after the oceanographer Alfred Redfield who first discovered it. If any one of these three key elements is in short supply with respect to plant growth, it can limit plant production.
Phosphorus is the element that is usually the primary culprit in the eutrophication of lakes. In most lakes, phosphorus is very scarce with respect to the ratio in plants, compared to nitrogen or carbon. In many cases, precipitation falling on a lake’s surface or in its catchment is the only source of the element. But most of the phosphorus falling with precipitation on a lake’s catchment is typically taken up by terrestrial vegetation, so that only a few percent of what falls reaches the lake. Only in the case of lakes with catchments set in phosphorus-rich geologic substrates are lakes naturally eutrophic.
Nitrogen, too, enters largely with precipitation, but in most areas it is not as scarce as phosphorus, with respect to the nutrient demands of plants. It is usually in the form of nitrate (NO3) or ammonium (NH4), both of which are highly available to plants. But the atmosphere contains high concentrations of gaseous nitrogen. Some algae, most notably certain species of Cyanobacteria, are capable of fixing atmospheric nitrogen. (Cyanobacteria are commonly called bluegreen algae because of the color of certain diagnostic pigments. Although they contain chlorophyll, they are technically not algae because the pigments are not contained in a chloroplast as they are in true plants.)
Carbon is even more plentiful with respect to phosphorus. Most lakes have abundant supplies of carbon dioxide as a result of exchange with the atmosphere and the decomposition of organic material. In addition, the weathering of rocks and soils in a lake’s catchment supply bicarbonate (HCO3), which can be used by algae as well. In contrast to nitrogen, all species of algae can utilize CO2. Thus, of the three primary nutrients, phosphorus is the only one without a major source in the form of an atmospheric gas.
In a typical eutrophication scenario, human waste or animal manure is washed into a lake. These wastes contain high phosphorus with respect to nitrogen and carbon. Algae typically respond by increasing rapidly in abundance. Often, the new supply of phosphorus will allow them to outstrip the supply of nitrogen. This situation favors the nitrogen-fixing Cyanobacteria mentioned above. Many species of nitrogen fixers float on the surface of the water, where they form unsightly “blooms.” Most are too large to be eaten effectively by the typical invertebrates that occur in lakes, so they tend to accumulate, eventually falling to the bottom where their decay consumes oxygen, or washing ashore in unsightly windrows that rot and cause terrible odors (plate 22).
Early attempts to control eutrophication did not focus on nutrient control. Instead, attempts were made to poison the algal blooms by applying algal toxins such as copper sulfate or synthetic herbicides. These allowed only short-term control, and the algae were usually as abundant as ever in a few weeks. Little thought was given to the control of nutrient sources.
In the mid-twentieth century, a new source of nutrients caused eutrophication to accelerate: phosphate detergents. Before the advent of detergents, soaps were used to wash clothing. These did not work well in many waters, especially those that were high in calcium, magnesium, and bicarbonate, where scummy residues were often left on clothes. Detergents were developed by industrial chemists as more effective cleaning agents, and they quickly replaced soaps. Unfortunately, early detergents were very resistant to biodegradation, and they accumulated in lakes. As a result, it was not uncommon in cities such as Chicago or Milwaukee to be met by huge clouds of foam blowing down the streets when winds were onshore. Manufacturers responded by making detergents biodegradable, so that aquatic microorganisms could degrade them quickly. Unfortunately, many of these contained 20% phosphorus by weight. In the lower St. Lawrence and Great Lakes in the 1960s, roughly half of the phosphorus supplied by humans was as detergents, with most of the rest from human sewage. The industrial phosphorus effectively doubled the rate of cultural eutrophication.
Concern for the Great Lakes and many large European lakes led limnologists of the 1960s to search for ways to solve the eutrophication problem. There were literally thousands of studies of the nutrient requirements of algae, but until that time, researchers were not really focused on solving the problem in lakes. There were, however, two particularly important studies that helped to convince scientists and regulators that the key to controlling eutrophication was to control phosphorus.
The first of these was a long-term study of Lake Washington done by Tom Edmondson. He and his students had documented the increasing eutrophication of the lake during the early twentieth century. Edmondson convinced local regulators to divert sewage effluent from the lake. His analysis, published in Science, showed that the concentration of algae decreased in proportion to the decrease in the concentration of phosphorus.
The second influential study was a review of eutrophication done by Richard Vollenweider for the Organization for Economic Cooperation and Development. Vollenweider analyzed an extensive literature, deducing that phosphorus was the key to controlling eutrophication. He published the first models that related phosphorus input or “loading” to the state of eutrophication in lakes. He later revised these models to include the effects of flow through the lakes.
These two studies, and small lake experiments described later, were among those used by Jack Vallentyne, then cochair of the Scientific Advisory Board on the Great Lakes for the International Joint Commission (IJC), to convince the IJC to recommend to the Canadian and American governments that regulating the phosphate content of detergents and removing phosphorus from sewage were essential first steps to solving the eutrophication problem in the Great Lakes.
Controlling phosphorus was opposed by manufacturers of phosphate detergent and their allies. They mounted a Madison Avenue-style campaign to discount the evidence for phosphorus control, first arguing that carbon was far more likely the cause of eutrophication in the Great Lakes. This theory was tested at the Experimental Lakes Area (ELA) in a whole-lake fertilization experiment. Lake 227 had extremely low concentations of available carbon, much lower than in the Great Lakes. When fertilized with phosphorus and nitrogen, huge algal blooms were formed despite the scarcity of carbon. Although the algae showed symptoms of extreme carbon shortage, this was slowly made up by exchange from atmospheric supplies. The detergent people quickly changed their arguments to nitrogen as the cause, arguing that phosphorus recycled too quickly to be controlled effectively so that nitrogen control would be more effective. Of course, restricting nitrogen had no implications for the detergent industry. Its removal at the sewage treatment plant was also much more costly than removing phosphorus.
A second experiment at ELA proved the nitrogen control theory to be wrong as well. A double-basin lake, Lake 226, was separated into two basins by a watertight plastic curtain. Nitrogen and carbon were added to both basins but phosphorus only to one basin. The basin receiving phosphorus became very eutrophic; the basin receiving only nitrogen and carbon remained in a natural state. These simple experiments proved convincing to reluctant policy makers.
The experiments in these two lakes allowed another dimension of eutrophication to be investigated. As described above, floating blooms of nitrogen-fixing Cyanobacteria are often one of the most visible and objectionable effects of eutrophication. Lake 227 in the early years of fertilization did not have this group of algae. Although fertilization caused algae to increase, the species of algae did not change. We had added N:P at a ratio of 14:1 by weight in order to ensure that we were not confounding our investigation of carbon limitation by causing nitrogen to be limiting.
In contrast, the N:P ratio used in fertilizing Lake 226 was only 4:1 because it was designed to mimic the ratio in sewage. Nitrogen-fixing Cyanobacteria were the predominant algae to respond to fertilization, and the natural species of algae remained rare. To test whether this shift was a coincidence or whether the low N:P favored nitrogen-fixing Cyanobacteria, the N:P ratio in Lake 227 was decreased to 5:1 in the seventh year of fertilization. Within a few weeks, nitrogenfixing Cyanobacteria became as dominant in Lake 227 as they were in Lake 226. Measurements of nitrogen fixation confirmed that they were using gaseous N2 to overcome shortages of ionic forms. Obviously, although the amount of algal increase was caused by phosphorus addition, the species that became dominant were affected by the N:P ratio.
Although the arguments made by detergent manufacturers and their allies delayed legislation, science prevailed, and in 1973 phosphorus-control legislation was passed in Canada. In the United States, progress was slower, because phosphorus control was decided by individual states. But eventually, all states in the Great Lakes catchment regulated phosphorus. The resulting improvement in the state of the lower Great Lakes is a success story that should make limnologists proud.
In Europe, the debate over carbon did not occur, and there was minimal debate over the need to control nitrogen in fresh water. Instead, there was a rather fierce debate over whether to regulate the phosphorus content of detergents or to simply remove the phosphorus at the sewage treatment plant. Some countries decided to regulate detergents, others not, but all western European countries eventually regulated phosphorus loading to lakes.
In North America, the political focus on eutrophication diminished after phosphorus was controlled in detergents and sewage, even though clear evidence was emerging that nonpoint sources of phosphorus, such as fertilized land, feedlots, storm runoff from urban areas (which contains lawn fertilizer, pet excrement, and other high-nutrient materials), and leaky septic tanks were important. Fortunately, a few scientists kept studying these problems. Many lakes have become eutrophic as a result of excessive cottage development, land clearing, fertilizer applications, and urbanization in their catchments. These problems are much more difficult to control, and many of the necessary conditions are in the hands of local or municipal regulators who are not aware of the consequences of increased nutrient inputs for lakes.
Another cause of eutrophication was found to be the decline in predatory fish species. Steve Carpenter and Jim Kitchell and their colleagues at the University of Wisconsin deduced that declining piscivorous predators, as has happened in most lakes as the result of angling pressure, allowed increases in minnows and other small fish species because of reduced predation. The high populations of these small fish in turn depleted the populations of grazing zooplankton that under normal circumstances helped to control the abundance of algae through their grazing. The resulting trophic cascade caused lakes that were in a low algal state when all four levels of the food chain were intact to assume a high algal state when the top level of the food chain was removed. Carpenter and Kitchell proved their theories in a series of whole-lake experiments, showing that both the trophic cascade and phosphorus loading were important to controlling eutrophication.
Although control of nutrients and the integrity of biotic communities have allowed many lakes to recover, these efforts have not been universally successful. In some lakes, internal recycling of phosphorus from lake sediments keeps lakes from recovering. This phosphorus originated outside the lake, but in some lakes, anoxic conditions in deep water allow phosphorus to be remobilized into the lake during summer stratification or under winter ice. This recycling can go on for many years after external sources of phosphorus have decreased. The lakes with the greatest internal recycling appear to be those that have low concentrations of iron. The lack of iron is thought to lessen the precipitation of ferric phosphate, or coprecipitation of phosphates with ferric hydroxides, allowing phosphorus that would otherwise be immobilized in sediments to be released from sediments. Many mechanisms have been proposed to reduce this recycling, including bubbling of the hypolimnion with air or oxygen to prevent low oxygen, mixing of upper and lower strata in the lakes, or addition of iron, alum, or lime to attempt to keep the phosphorus in sediments from being released. None of these techniques has been totally successful in all systems, for reasons that are not well understood.
Streams and rivers can also suffer from cultural eutrophication. Slow-flowing streams show many of the same symptoms as lakes, with algal scums and low oxygen becoming major problems. In shallow clearwater rivers with rocky bottoms, huge mats of attached algae rather than plankton blooms are typical symptoms. As streams undergo eutrophication, typical groups of benthic invertebrates such as Trichoptera, Ephemeroptera, and Plecoptera typically decline and are replaced by chironomid (insect) and oligochaete species that are more tolerant of low oxygen.
Few states or provinces have guidelines for controlling the eutrophication of flowing waters.
In recent years, considerable attention has been paid to the role of eutrophication in degrading drinking water sources. Obviously, where sewage or manure is the source of nutrients, it is also the source of bacteria such as Escherichia coli and protozoans such as Cryptos-poridium and Giardia that cause gastrointestinal disease. But nutrients have indirect effects via their effect on aquatic organisms. Some species of bloom-forming Cyanobacteria also produce potent liver toxins, which occasionally cause the deaths of livestock or pets that drink water containing them. Several species of algae can also cause problems with taste and odor. As a result of these problems, high-quality drinking water becomes difficult and costly. In many cases, conventional filtration and chlorination of water will not suffice. In the worst cases, reverse osmosis must be used to eliminate chemicals of concern.
Most recently, much attention has been paid to the eutrophication of the coastal oceans, particularly in bays and estuaries. Although these are not generally used as drinking water (except in a few areas where shortage of fresh water has required desalinization of seawater), they support important fisheries.
The debate over what nutrient to control is alive and well in estuaries. Physiological studies show that algae are usually nitrogen limited. As was the case in fresh water, manyscientists have interpreted this as a signthat nitrogen control is necessary to reduce eutrophication.
Several studies suggest that this interpretation is incorrect, as it was in freshwater lakes. In the 1970s, phosphorus inputs were reduced to the Stockholm Archipelago, a part of the Baltic Sea just off the most populous part of Sweden. At the time, the main algal species in the Archipelago were nitrogen-fixing Cyanobacteria, and they showed signs of extreme nitrogen limitation. But a decline in phosphorus concentration was followed by a huge decline in algae. Unfortunately, the recovery was not complete because of phosphorus return from the sediments of the Archipelago, as described above for eutrophic lakes. As a result, whether or not to control nitrogen inputs in an attempt to cause further declines in algal blooms is still hotly debated.
A few studies suggest that if humans are determined to control nitrogen, phosphorus control is necessary as well. If the ratio of N:P decreases below the Redfield ratio, Cyanobacteria tend to become dominant, at least in estuaries where salinity is far below that of seawater, as in the Baltic Sea.
Carpenter, Stephen, James Kitchell, and J. Hodgson. 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Edmondson, W. Thomas. 1991. The Uses of Ecology: Lake Washington and Beyond. Seattle, WA: University of Washington Press.
Hutchinson, G. Evelyn. 1973. Eutrophication. American Scientist 61: 269–279.
Schindler, David W. 1974. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 184: 897–899.
Schindler, David W., and John R. Vallentyne. 2008. The Algal Bowl: Overfertilization of the World’s Freshwaters and Estuaries. Edmonton, AB: University of Alberta Press.