CHAPTER 7
Respiratory Anatomy and Physiology1
1The authors have retained several portions of the first edition’s chapter, written by Mark Burno, Casey A. Harper, Matthew Chao-Ben Chia, and M. Christine Stock with permission of the authors we were able to contact.
Introduction
The respiratory system consists of the upper and lower airways, lungs, and respiratory muscles whose main function is gas exchange, which provides oxygen to the blood for delivery to cells and removes carbon dioxide that is generated by cell metabolism. Other functions of the lung include acid-base balance (covered in Chapter 10); synthesis of the surfactant, which lubricates the inside of the lung; and immune defense against any airborne diseases.
Anesthesia and surgery may affect all these functions, particularly gas exchange. With a thorough understanding of respiratory anatomy and physiology, along with knowledge of the physiologic changes that occur during surgery, the anesthesia technician will be better suited to assist the anesthesia provider in caring for the physiologic perturbations that may occur while a patient is under anesthesia.
Anatomy
Atmospheric gases, most importantly oxygen, enter the body through the nose or mouth during inspiration. They flow into the lungs and deliver oxygen to the blood. At the same time, carbon dioxide (a by-product of cellular metabolism) moves from blood into the lungs. During expiration, carbon dioxide is expelled into the atmosphere or anesthesia circuit, if the patient is under general anesthesia. This section presents the anatomy and structures of the upper airway, lower airway, lungs, and muscles involved in breathing.
Upper Airway
The upper airway consists of the nose, mouth, pharynx, and larynx (Fig. 7.1). The nose consists of the nasal septum, cribriform plate, lateral walls, and curved structures called conchae, which are also called turbinates. The conchae in the nasal cavity help to filter large inhaled particles and to warm and humidify inspired gases. Olfactory and respiratory epithelia line the nose, forming a mucus blanket to further aid in filtering particles. The abundant blood supply to the nose comes from the internal and external carotid arteries, which branch into the ethmoid arteries, sphenopalatine artery, and septal branch of the superior labial artery. Venous drainage occurs through the facial, ophthalmic, and sphenopalatine veins. Given the high vascularity of the nose, bleeding during nasotracheal intubation is not uncommon. To decrease this bleeding risk, the anesthesia provider usually administers an intranasal medication, such as phenylephrine, to cause constriction of blood vessels in the nose.
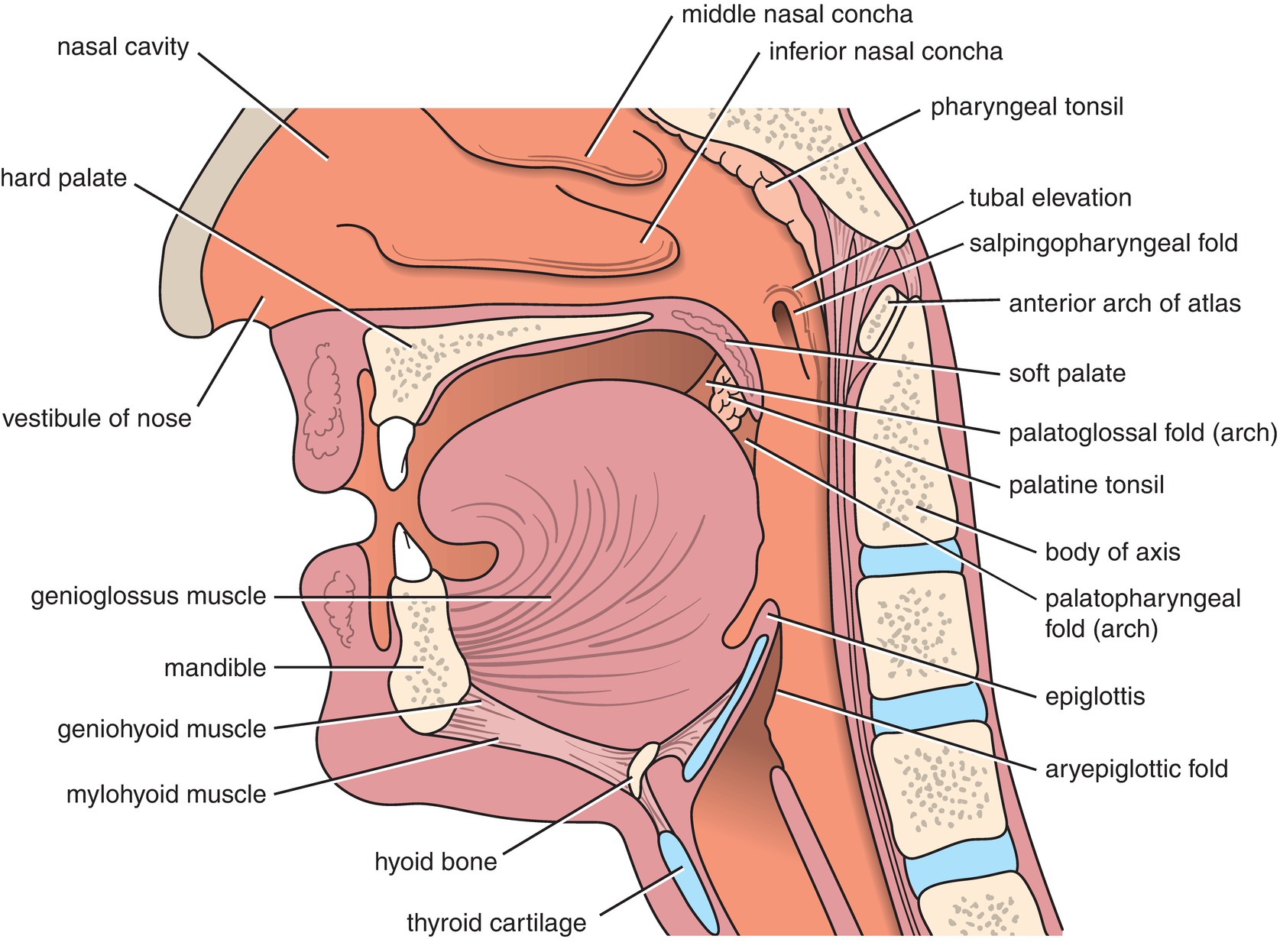
FIGURE 7.1. Sagittal section of the head and neck, showing the relationships of the nasal cavity, mouth, pharynx, and larynx. (From Snell RS. Clinical Anatomy by Regions. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008, with permission.)
The mouth consists of the upper and lower teeth, the tongue, and the hard and soft palates. A layer of squamous epithelium helps protect the mouth from injury during chewing. Preventing the mouth from getting dry, salivary glands empty into the mouth and help to humidify inspired gases. Gases moving through the mouth are heated, humidified, and filtered, similar to gases that pass through the nose. However, due to the lower surface area, this occurs to a lesser degree. The risk of significant bleeding is less in the mouth compared to the nasal cavity. This is due to the fact that the vascular supply of the oral cavity is deeper and the salivary glands lubricate the oral mucosa. This lubrication by saliva decreases injury and bleeding upon instrumentation.
The pharynx is a tube composed of muscle and connective tissue that helps to stent open the airway during inhalation and exhalation. It runs from the posterior nasal cavity passing posteriorly to the oral cavity to the superior portion of the esophagus and is conventionally divided into three segments: the nasopharynx, oropharynx, and laryngopharynx. The nasopharynx is the upper portion of the pharynx. It lies posterior to the nasal cavity, extending from the base of the skull to the posterior portion of the soft palate. Gases move through the nasopharynx to enter the oropharynx. The oropharynx begins at the posterior section of the mouth and consists of the posterior tongue, salivary glands, tonsillar pillars, and soft palate. Gases from the oropharynx pass into the laryngopharynx, which begins inferior to the oropharynx at the superior border of the epiglottis and extends inferiorly to the superior junction of the esophagus. It is posterior to the larynx and is in continuum with the esophagus.
The oropharynx and laryngopharynx contain muscles that aid in stenting open the pharyngeal passage during inspiration and expiration. These muscles relax during sedation. They relax further after induction of general anesthesia and still further after patients receive neuromuscular blocking drugs. Consequent to this relaxation, the patient is at increased risk of airway obstruction and difficulty with mask ventilation. For this reason, anesthesia providers may utilize an oral or nasal airway or a two-handed bag-mask ventilation technique in an attempt to overcome any obstruction. The tongue also relaxes and may contribute significantly to airway obstruction, particularly in the supine position. Extrinsic muscles of the tongue anchor it to different bones, allowing movement of the tongue in multiple directions. Relaxation of these muscles can result in posterior movement of the tongue leading to obstruction of the pharynx.
The larynx is composed of muscle and cartilage and contains the epiglottis; the arytenoid cartilage, which borders the posterior entrance to the trachea; vocal cords; cricoid cartilage; hyoid bone; thyroid cartilage; and cricoid membrane (Fig. 7.2). It is located anterior to the laryngopharynx and superior to the trachea and may be divided into three sections called the supraglottic, glottic, and subglottic larynx. The vocal cords form the glottic opening and the entrance to the subglottic larynx and the trachea. The muscles and cartilage of the larynx are intricately associated in order to accomplish its functions. With elevation of the larynx, as during swallowing, the epiglottis folds over the vocal cords to prevent the aspiration of food or other material that enters the pharynx from the mouth or from the stomach from entering the trachea and lungs.
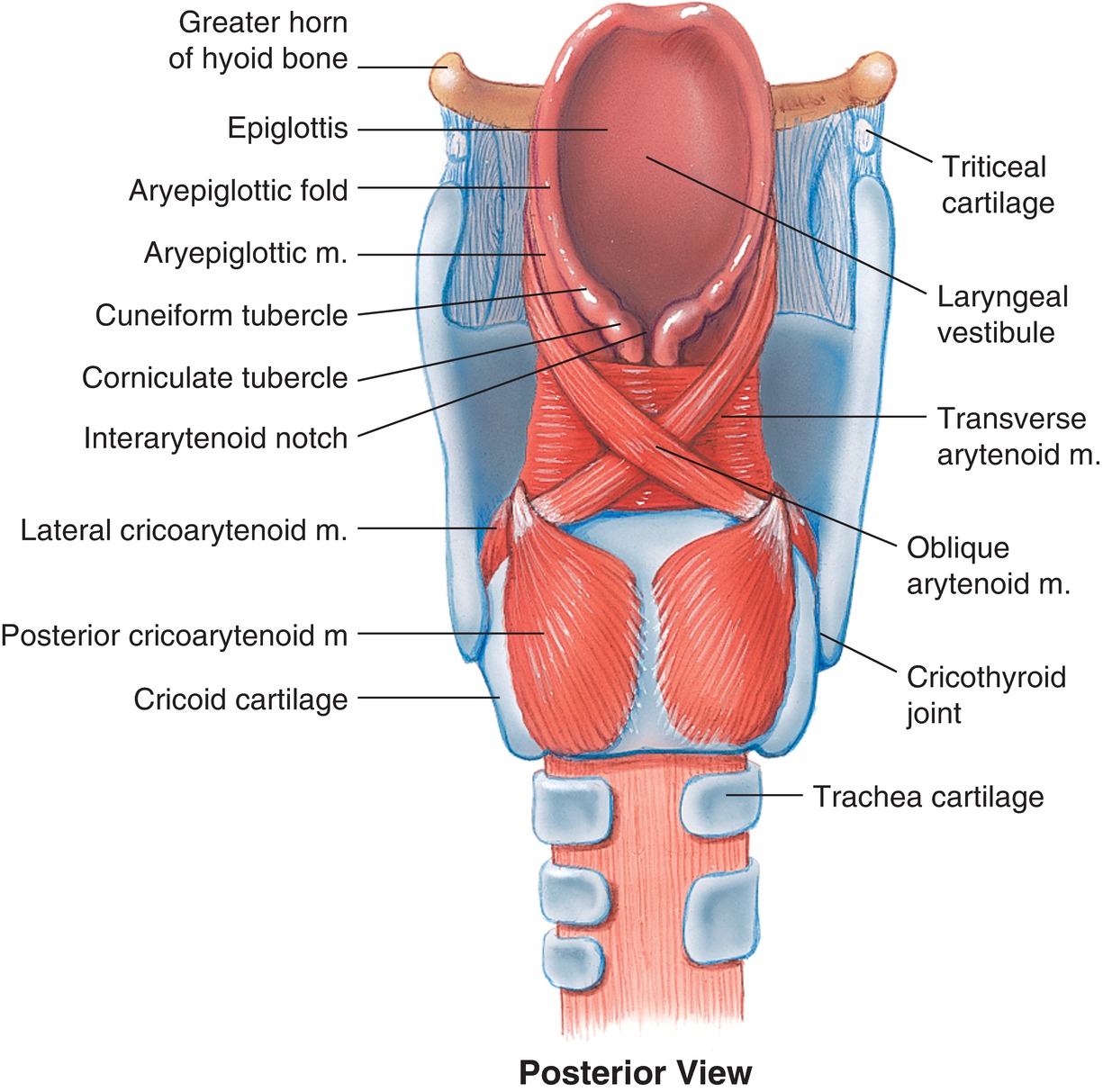
FIGURE 7.2. Larynx posterior view. (With permission from Lippincott Williams & Wilkins.)
The thyroid cartilage is easily palpable in most people. It commonly identified as an anterior protrusion in the neck referred to as the Adam’s apple. This is more easily visible in men but also identifiable in women, highlights that the trachea and larynx are larger in men, and has a slightly different position in the neck. The cricoid cartilage is a ring of cartilage just below the thyroid cartilage. It circles the larynx and is separated from the thyroid cartilage by the cricothyroid membrane (Fig. 7.3). The cricoid cartilage is an important landmark to the anesthesia provider during difficult airway situations or during placement of a transtracheal nerve block. When unable to ventilate or intubate the patient, either orally or nasally, it may be necessary to access the airway by making an incision in the cricothyroid membrane (cricothyroidotomy). This incision then allows a conduit such as an endotracheal tube to be placed in the airway and facilitate ventilation (see Chapter 57, Airway Emergencies). The cricoid cartilage may also be important during induction of general anesthesia when the risk of aspiration of stomach contents is increased. Since the esophagus travels posterior to the cricoid cartilage, direct pressure to the cricoid cartilage, also known by the eponym Sellick maneuver, can occlude the esophagus. Although somewhat controversial, this action is thought to decrease the chance of stomach contents passing passively through the esophagus and into the laryngopharynx to descend through the trachea and into the lungs. This cricoid pressure should not be confused with the “BURP” maneuver, which involves applying pressure on the larynx itself “backward, upward, and rightward” to improve visualization of the vocal cords during direct laryngoscopy. The anesthesia provider may guide you in doing this. You, as the anesthesia technician, should learn the location of the cricoid cartilage (used for one airway assistance technique) and the larynx (used for another) as you may be called on for either.
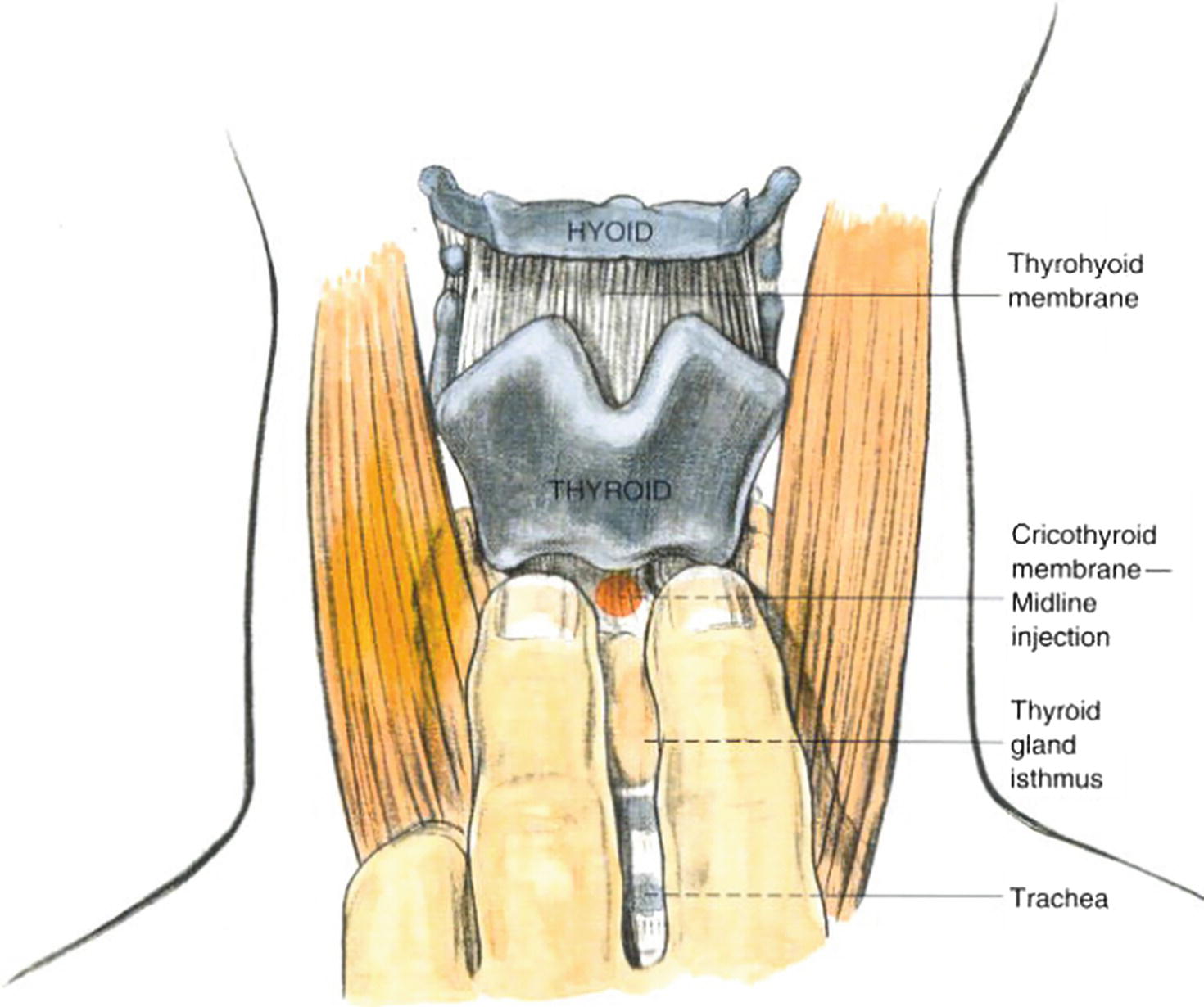
FIGURE 7.3. Translaryngeal block. (From Brown D, ed. Atlas of Regional Anesthesia. 2nd ed. Philadelphia, PA: Saunders; 1999:215-221, with permission.)
During direct laryngoscopy, a laryngoscope is inserted into the mouth sweeping the tongue to the and into the posterior pharynx (Fig. 7.4). The epiglottis is identified and lifted to expose the arytenoids and vocal cords. An endotracheal tube can then be passed through the mouth and vocal cords into the trachea. The anesthesia provider uses one hand on the blade and one on the tube: if cricoid pressure is to be held, or if the larynx is to be manipulated to improve view, this must be done by an assistant.
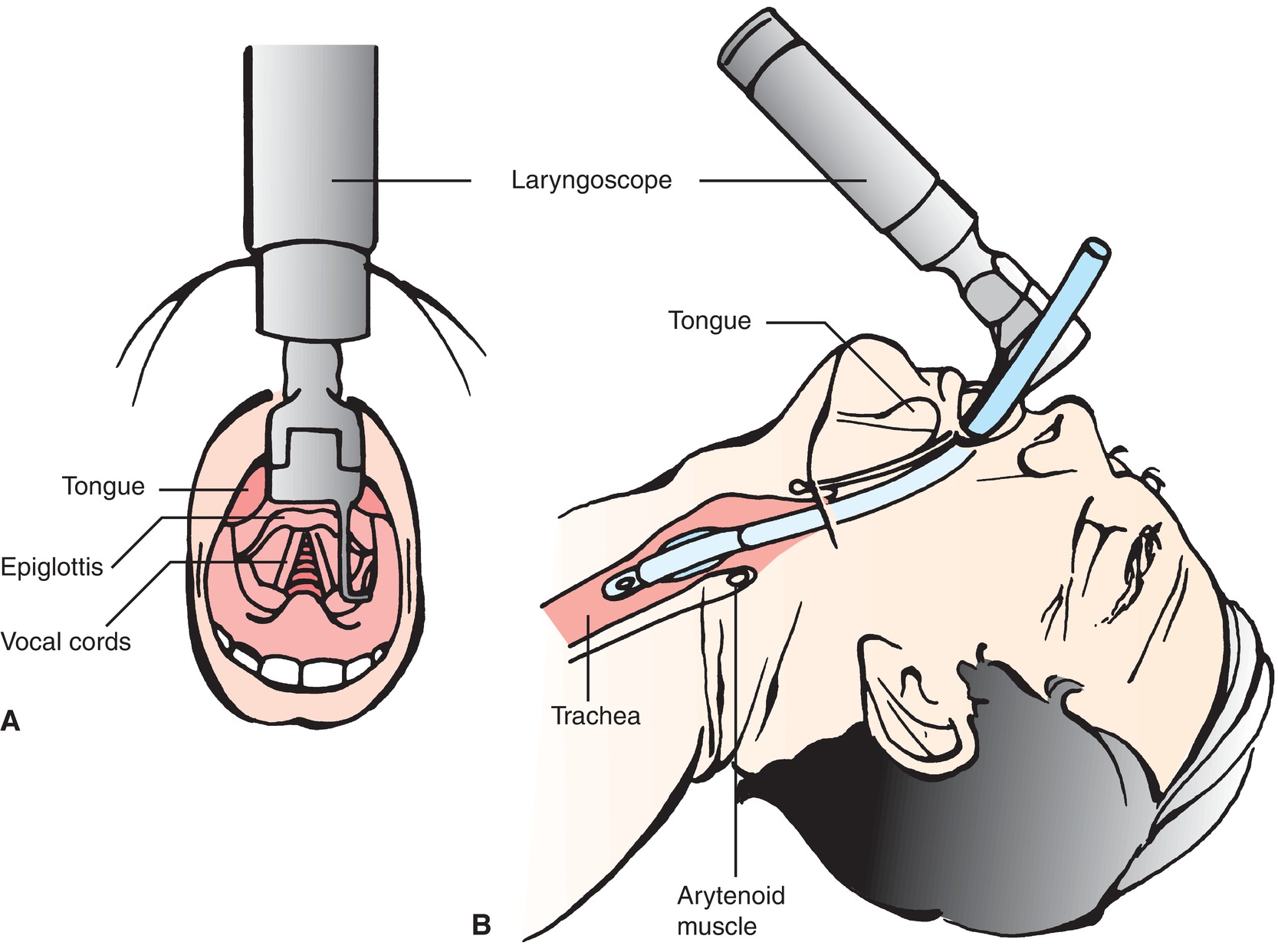
FIGURE 7.4. Direct laryngoscopy and endotracheal intubation. A: Direct view of upper airway. B: Side view of upper airway. (From Nettina SM. The Lippincott Manual of Nursing Practice. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001, with permission.)
Familiarity with the sensory nerves of the nose, mouth, nasopharynx, oropharynx, laryngopharynx, and larynx is useful when a patient requires placement of an endotracheal tube through the nose or mouth while awake (Fig. 7.5). This knowledge allows the anesthesia provider to adequately anesthetize the nerves to make the experience as comfortable as possible for the patient and facilitate ease of placement for the provider.
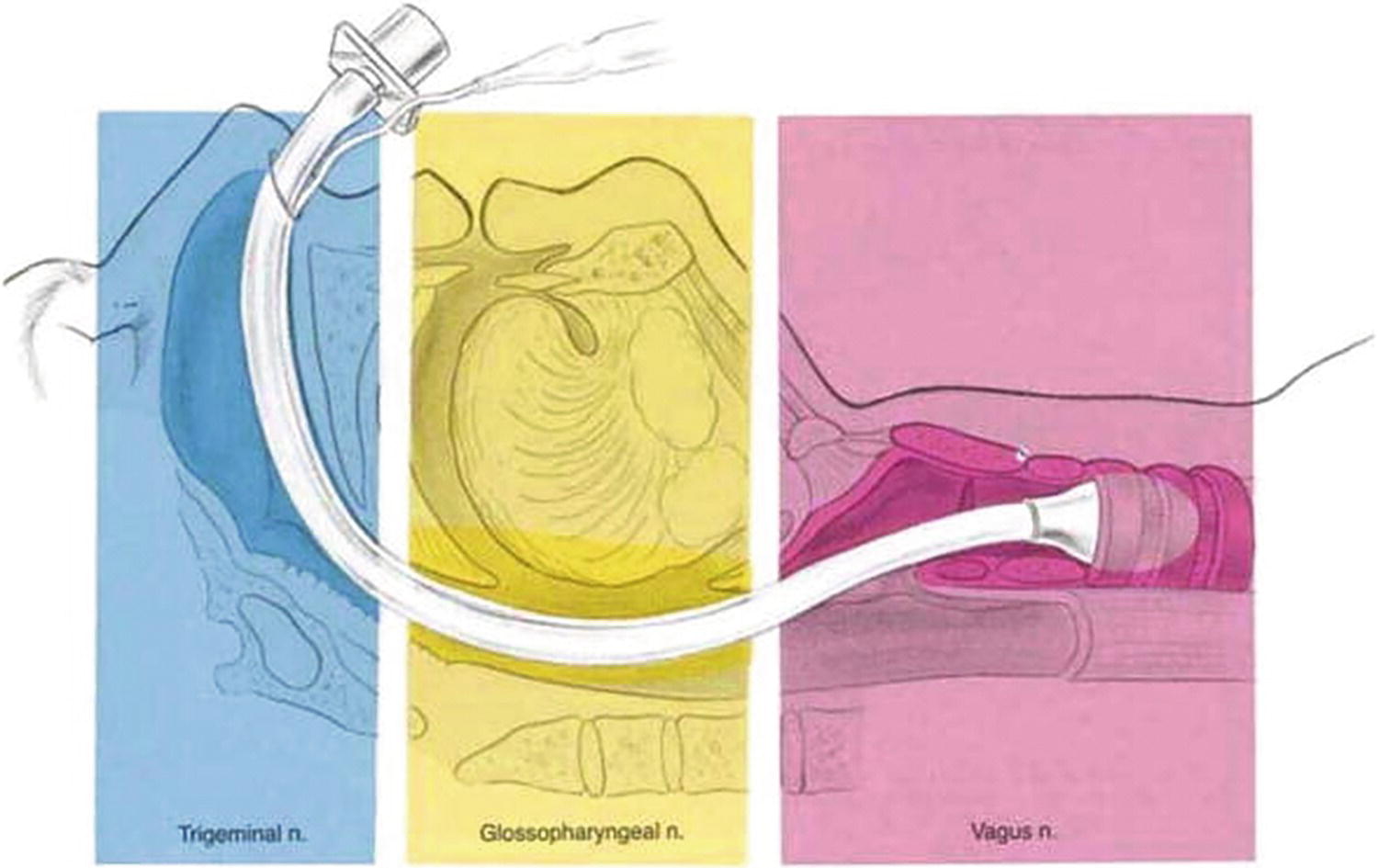
FIGURE 7.5. The innervation of the upper airway. (From Brown D, ed. Atlas of Regional Anesthesia. 2nd ed. Philadelphia, PA: Saunders; 1999:215-221, with permission.)
The internal nasal cavity is innervated by branches of cranial nerve V, the trigeminal nerve, which is named for its branches V1 (ophthalmic nerve), V2 (maxillary nerve), and V3 (mandibular nerve). The internal nasal cavity is innervated by the ophthalmic and maxillary branches of the trigeminal nerve. The application of topical anesthetics to the nasal cavity is one of the most common methods of anesthetizing these nerves. This allows an endotracheal tube to be passed painlessly through the nasal cavity and into the nasopharynx.
Sensory nerves of the mouth include the maxillary nerve but mainly the glossopharyngeal nerve (CN IX). These provide sensory innervation to the oropharynx and require anesthetizing prior to placement of an orotracheal tube. There are several techniques for anesthetizing these nerves with aerosolized local anesthetics being the most common. Once either the nasopharynx or oropharynx has been adequately anesthetized, the laryngopharynx also requires anesthesia in order to pass the endotracheal tube.
Innervation of the larynx is provided by the superior laryngeal nerve and recurrent laryngeal nerve, both branches of the vagus nerve (CN X). Sensory innervation of the laryngopharynx above the vocal cords (supraglottic and glottic) is via the superior laryngeal nerve. The recurrent laryngeal nerve is responsible for sensory innervation of the subglottic region. The laryngopharynx may be anesthetized by applying topical anesthetic to the mucosa of the laryngopharynx. Alternatively, the superior laryngeal nerve passes fairly superficially near the hyoid bone before it supplies the lining of the laryngopharynx. This nerve can be easily anesthetized at this location with a small injection of local anesthetic (superior laryngeal nerve block). The recurrent laryngeal nerve (subglottic area) may be anesthetized by application of topical anesthetics usually through the fiberoptic scope or via transtracheal block performed by injection of local anesthetic through the cricothyroid membrane directly into the trachea (Fig. 7.3). After anesthetizing the nerves by application of topical anesthesia or by nerve block, an endotracheal tube may be passed more comfortably through the upper airway, including the laryngopharynx and larynx, and into the trachea.
Lower Airway
Gases pass through the larynx into the trachea en route to the lungs. The lower airway begins at the level of the trachea, just below the glottic opening, and includes the trachea, bronchi, bronchioles, respiratory bronchioles, and alveoli. The lungs consist of five lobes, which are distributed two on the left and three on the right. The left is divided into the upper and lower lobes. Having only two left lobes accommodates the heart’s location in the left side of the chest. The right lung is divided into the upper, middle, and lower lobes. The lower airway is composed of tubes for airflow (conducting airways) and structures to allow gas exchange between the lungs and blood (respiratory airways). The airways of the conducting zone do not participate in gas exchange; their function is to allow the transfer of air between the atmosphere and the respiratory zone where all gas exchange occurs.
The trachea branches into two mainstem bronchi. The branch point between the trachea and mainstem bronchi is called the carina. Visual identification of the carina during intubation with a fiberoptic bronchoscope is helpful because the ideal location of the endotracheal tube is a few centimeters above the carina, allowing both lungs to receive gas from the tracheal tube. If the endotracheal tube is placed into one of the mainstem bronchi, only that lung will be ventilated. This is referred to as a mainstem intubation and usually results in decreased blood oxygen levels and increased peak airway pressures.
The trachea is a muscular tube reinforced by anteriorly placed C-shaped rings of cartilage and is a conducting airway. The trachea terminates at the carina where it divides into two bronchi called the left and right mainstem bronchi. The right mainstem bronchus branches from the trachea at a less acute angle than the left mainstem bronchus (Fig. 7.6). This difference in branching angles explains why liquid entering the trachea is more likely to enter the right lung. Clinically speaking, if a patient has stomach contents enter the trachea and descend to the level of the bronchi, those contents are more likely to flow into the right mainstem bronchus. It is more difficult for stomach contents to enter the left mainstem bronchus because the left bronchus diverges at a more acute angle from the trachea. This is also why airway devices that pass the carina (mainstem intubations, bronchial blockers, double-lumen tubes) have a tendency to go down the right side.
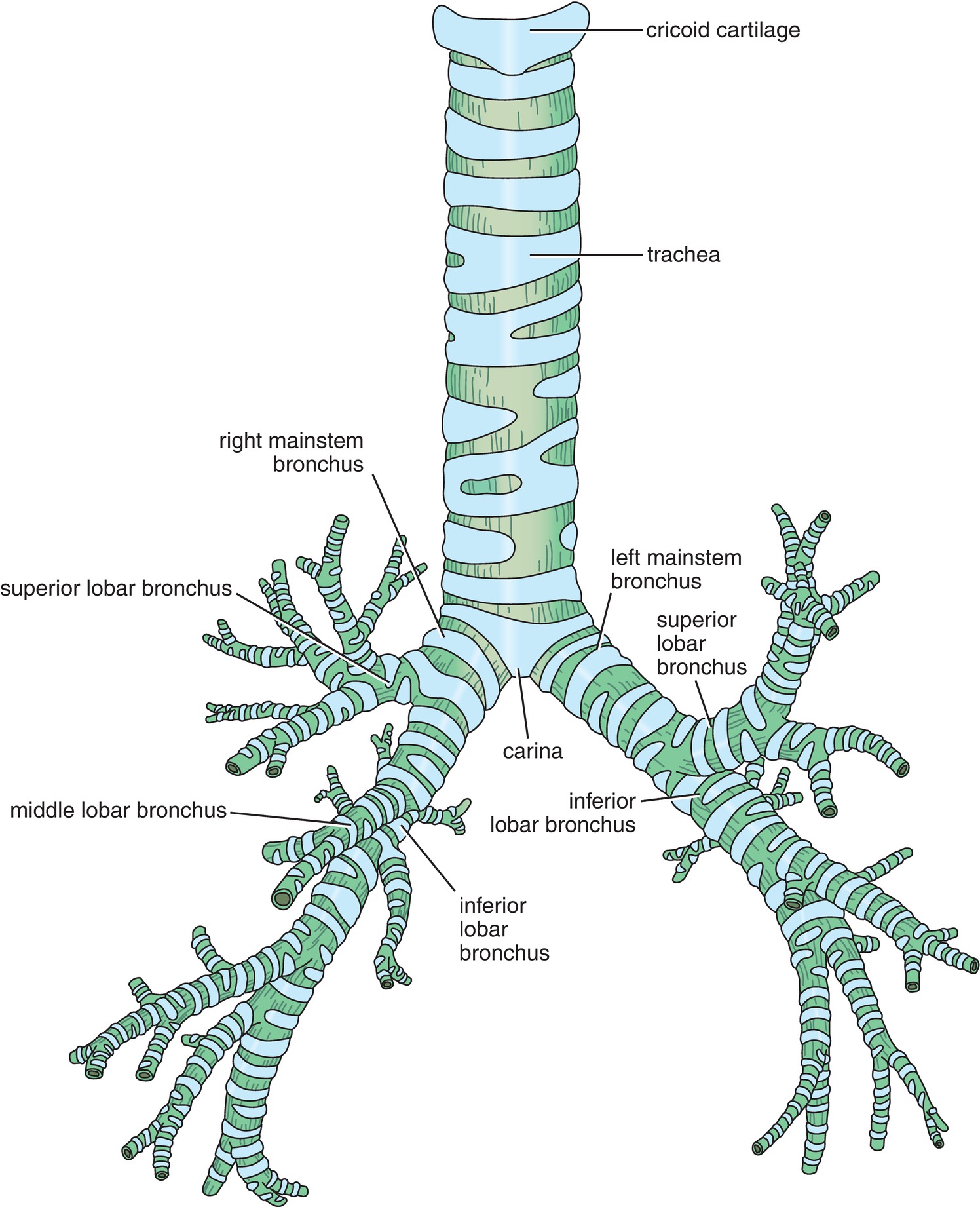
FIGURE 7.6. The trachea, carina, and bronchi. (From Snell RS. Clinical Anatomy by Regions. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008, with permission.)
The first branch of the right mainstem bronchus is called the right upper lobe bronchus. This branch is usually seen very shortly after entering the right mainstem bronchus. The right upper lobe bronchus is directed posteriorly, and identifying this structure during fiberoptic bronchoscopy helps to confirm that the scope is in the right mainstem and is a useful landmark in helping the anesthesia provider orient themselves.
The trachea, bronchi, and bronchioles are conducting airways and do not participate in gas exchange between the airway and blood. They are lined with respiratory epithelium and goblet cells that produce mucus and help to filter particles. The respiratory epithelium has attached cilia, which are tiny hair-like structures that beat in a coordinated fashion to help move mucus toward the larynx and into the laryngopharynx so that it may be cleared. Ciliary function is impacted by many factors. Relevant to the anesthesia provider, an endotracheal tube hinders the removal of mucus, resulting in buildup that may need to be suctioned before removal of the endotracheal tube. In addition, inflammatory conditions of the bronchi not only increase mucus production but impair ciliary function (which literally sweeps out microscopic particles from the lung) and thus clearance of mucus. This kind of inflammation can be caused by many things: diseases like asthma or chronic bronchitis, infections, chemical irritants like air pollution or cigarette smoke, or physical irritants like airway devices and drying gases.
Respiratory bronchioles and alveoli comprise the respiratory zone, the area distal to the conducting zone where all gas exchange occurs. In this zone, the exchange of oxygen and carbon dioxide occurs because the alveoli bring the inspired gas into close proximity to blood flowing through the pulmonary capillaries. The alveoli are sacs that have very thin membranes to facilitate gas exchange with the capillaries (Fig. 7.7). Alveoli are the main sites where exchange of oxygen and carbon dioxide between the blood and lungs occurs. The respiratory bronchiole is responsible for 10% of gas exchange, while the alveoli are responsible for the other 90%. Groups of alveoli are normally clustered together like a bunch of grapes with a single grape termed an alveolus. The wall of the alveolus is extremely thin and contains three cell types: type 1 pneumocytes, type 2 pneumocytes, and alveolar macrophages. Type 1 pneumocytes are the most abundant and are very thin cells that account for gas exchange. Type 2 pneumocytes are specialized cells that secrete surfactant (the lubricant, which allows the alveolus not to get stuck to itself), while alveolar macrophages engulf any particles that escape the conducting zone’s mucociliary clearance.

FIGURE 7.7. Pulmonary alveoli and capillaries. A: External lobar anatomy of the lungs. B: Depiction of visceral and parietal pleura. C: Functional anatomy of the lungs. (A and B from Sadler TW. Langman’s Medical Embryology. 9th ed. Image Bank. Baltimore, MD: Lippincott Williams & Wilkins; 2004; C from McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000.)
The lungs and interior chest wall are both lined by a thin layer of tissue called the visceral and parietal pleura, respectively. Under normal circumstances, the parietal and visceral pleura meet and are only separated by a thin layer of fluid in the area referred to as the pleural space.
The natural tendency of the lungs is to move toward a state of collapse due to the fact that the tissue that composes the alveoli and surrounding supportive tissue has an elastic component. However, the lungs do not collapse under normal circumstances due to the thin layer of fluid in the pleural space in effect adhering the lung to the chest wall. The pressure in the pleural space is subatmospheric (approximately −5 cm H2O at rest). This tendency for the lungs to remain adherent to the chest wall is similar to how two pieces of glass stick together when a thin layer of water is placed between the glass pieces. The lungs slide along the chest wall during inspiration and expiration, but the lungs do not pull away from the chest wall or collapse under normal circumstances.
If air enters the thorax through a defect in the chest wall or the lung, air may enter the pleural space, raising the pressure in the space and allowing the lungs to pull away from the chest wall, causing lung collapse. Air in the pleural space is referred to as a pneumothorax. In addition to air, many other substances may enter this pleural space causing compression of the lung and alveoli, referred to as atelectasis. Blood in the pleural space is referred to as a hemothorax. Fluid may also accumulate in the pleural space, due to various causes, and this is referred to as a pleural effusion. Occasionally, this fluid is pus, and in this situation, it is referred to as an empyema.
As previously mentioned, elastic tissue in the lungs creates a force that has a tendency to collapse alveoli. This counteracts the force exerted on the lungs by the chest wall and negative pressure in the pleural space that typically holds the lungs open. Furthermore, surface tension created by liquid lining the inner surface of alveoli adds to the forces collapsing alveoli. Surface tension occurs because the molecules in the liquid are attracted more strongly to themselves than they are to the gases in the alveoli and occurs where liquids meet gas. The entire interior surface of the sphere-shaped alveoli is coated in liquid; thus, the surface tension within the alveoli is significant. This force is disadvantageous to the alveoli because it could cause collapse of the alveoli. Surface tension would be a greater force causing alveoli to collapse if it were not for surfactant that lines the alveoli. Surfactant decreases surface tension by decreasing the attractive forces between the water molecules. Decreasing the surface tension reduces the tendency for alveoli to collapse.
While the lungs have a tendency to collapse, the chest wall has the opposite tendency to expand and thereby increase the volume inside the chest. An equilibrium is established where the tendency of the lungs to collapse is balanced by the tendency of the chest wall to expand. The equilibrium normally occurs after exhaling a normal volume breath. If a patient has lung disease or an abnormality of the chest wall, this equilibrium may be altered. A lung or chest wall abnormality may cause this by changes in lung compliance or elasticity.
Compliance of the chest wall and lungs determines at what lung volume the forces will be in balance. Compliance is how easily something changes volume when subjected to a pressure change. For instance, some balloons inflate quite easily when air is injected. They have a high compliance and low elasticity. Other balloons, due to the thickness of their walls or composition of their material, require much more pressure to inflate and have low compliance and high elasticity.
Anatomy of the Thorax
The thorax is a cone-shaped cavity supported by vertebrae and protected by ribs. It houses the lungs, heart, and many major blood vessels, including the ascending aorta and proximal descending aorta. The volume of the thorax may be increased in three directions: anterior-posterior (front-back), superior-inferior (up-down), and lateral-medial (side-side). Elevation of the ribs is responsible for increasing thoracic volume in the anterior-posterior and medial-lateral axes because the ribs are set at a downward angle, similar to the handle of a bucket. As you elevate the ribs, the diameter of the thorax increases and, thus, the thoracic volume. The diaphragm creates the floor of the thorax, and when it contracts, it moves in the superior-inferior axis and thus increases thoracic volume while decreasing intrathoracic pressure (Fig. 7.8). When a patient is face down, or prone, thoracic excursion is limited due to the weight of the patient on the thorax. Thus, anesthesia providers allow space for the abdomen to move during inspiration to allow lung volume expansion.

FIGURE 7.8. Action of the diaphragm and the rib cage during inhalation (A) and exhalation (B). (From Cohen BJ, Taylor JJ. Memmler’s the Human Body in Health and Disease. 11th ed. Baltimore, MD: Wolters Kluwer Health; 2009, with permission.)
Lung Volumes
The volume of gas a person breathes in and out varies depending on whether he or she is at rest or engaged in vigorous physical activity. The total volume of gases inspired in 1 minute is called the minute volume or minute ventilation. Different conditions and diseases may change the typical minute volume. A volume is a quantity of gas that can be inspired or expired and is not divided further into smaller quantities. A capacity is the combination of two or more volumes. Total lung capacity, the volume of gas the lungs can hold after the maximal inspiratory effort, can be divided into various volumes and capacities (Fig. 7.9). If a person were then to expel as much gas as possible, there would still be some gas left in the lungs that he could not empty, no matter how hard he tried. The remaining gas volume is called the residual volume. When the person is breathing normally, the volume of gas inspired and expired is called the tidal volume. At end expiration, while breathing quietly, the tendency of the chest wall to expand is matched with the tendency of the lungs to collapse and equilibrium exists. This is what makes it quite comfortable to cease expiration at the end of expelling a tidal volume. The functional residual capacity (FRC) is the volume of gas in the lungs at the end of a normal exhalation, when all the forces in the chest are in balance. If the person then continues to exhale, the process becomes active and muscle contraction is necessary.
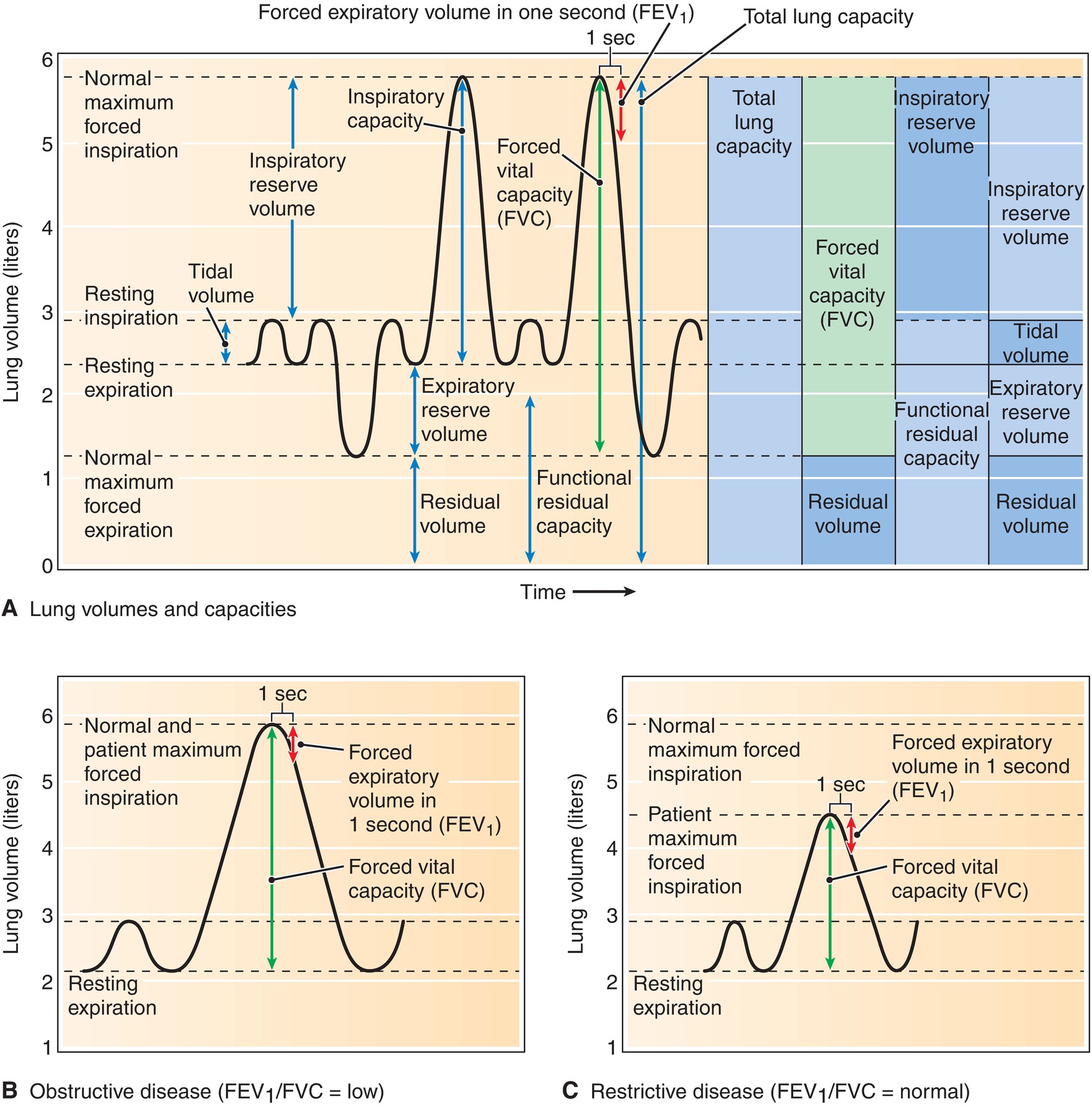
FIGURE 7.9. Spirogram, showing lung volumes and capacities (A), obstructive disease (FEV1/FVC = low) (B), and restrictive disease (FEV1/FVC = normal) (C). FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
FRC, serving as a reserve source of oxygen and a patient’s ability to tolerate periods of apnea, is a very important concept to anesthesia providers. The forces in the patient are in balance at the moment the patient goes to sleep: she is not doing any active breathing; thus, the total amount of gas in the lungs is her FRC. This FRC contains oxygen that can be absorbed into the blood during a period of apnea. A lower FRC means that the patient does not have as much oxygen reserve; thus, there is less time between when the patient becomes apneic and when the oxygen saturation of hemoglobin in the blood begins to fall. The FRC can be affected by various factors including body habitus, patient position, and gender. Preoxygenation of the lungs prior to the induction of anesthesia increases the “functional” FRC by the process of replacing the gases, mainly nitrogen, in the lung with oxygen, thereby increasing the allowable apnea time. In patients with a decreased FRC undergoing general anesthesia, the airway needs to be secured in a more efficient fashion, so as to avoid rapid desaturation.
The thoracic volume at which equilibrium exists between the chest wall’s tendency to expand and the lung’s tendency to collapse may vary with certain patient conditions or diseases. For instance, in chronic obstructive pulmonary disease (COPD), the elastic component of the lungs is degraded, so lung compliance is increased. This means the lung volume at end expiration is larger than the lung volume in a person with healthy lungs. Thereby, FRC is normal to slightly increased. Alternatively, in a patient with restrictive lung disease, where the lungs are stiffer, less compliant, and more elastic, a smaller lung volume at end-expiration exists. The smaller end-expiratory lung volume is due to the lungs having a greater force shrinking the size of the lungs that resists the chest wall’s tendency to want to expand and increase the lung volume, reducing FRC.
Blood Supply to the Lungs
Lung parenchyma has a dual blood supply. The first is the pulmonary circulation consisting of pulmonary arteries and veins and carrying the full output of the right side of the heart. The pulmonary arterial supply carries deoxygenated blood from the right side of the heart to the alveoli to be oxygenated and to unload carbon dioxide. Blood flows from the right ventricle through the pulmonic valve and into the main pulmonary artery where it branches into the right and left pulmonary arteries and further branches into smaller and smaller arteries. The right pulmonary artery divides into upper, middle, and lower branches to provide blood supply to the respective lobes of the right lung. Similarly, the left pulmonary artery divides into upper and lower branches to supply their respective left lobes. The small arteries branch into even smaller arterioles and finally into capillaries. Capillaries form networks around the alveoli, which facilitates gas exchange between the two so that oxygen may more easily diffuse into the blood and carbon dioxide may more easily diffuse into the alveoli (Fig. 7.7C). The capillary blood is collected by venules, which empty into veins that drain into the four main pulmonary veins. These veins empty into the left atrium of the heart.
The second source of blood to the lungs is a small bronchial circulation that arises from the aorta. These bronchial arteries carry oxygenated blood to the bronchi, which are not oxygenated directly from the alveoli.
Physiology
Nose, Mouth, Pharynx, and Larynx
In this section, we discuss the physiologic functions of the respiratory system as they relate to respiration and maintenance of homeostasis in the blood. The lungs are exposed to an enormous quantity of gases each day and contain delicate tissue in the alveoli that need to be protected. The conducting airways contain protection mechanisms that filter, humidify, and heat the inspired gases to decrease the risk of damaging the airways and the alveoli. The main function of the lungs is to deliver oxygen to the blood and remove carbon dioxide. This is accomplished by the body’s attempt to match the delivery of inspired gas to the alveoli (ventilation) to the delivery of blood flow to the lungs (perfusion). This allows blood to absorb oxygen and release carbon dioxide. This is termed ventilation-perfusion matching. For optimum gas exchange to occur, ventilation and perfusion must be matched at the alveolar level.
Another protection mechanism is the body’s attempt to provide inspired gases of consistent humidity and temperature to the alveoli. As ambient temperature and humidity vary markedly, both the nose and mouth are able to heat, humidify, and filter inspired gases. The nose is more effective than is the mouth at these functions because the nose has a larger surface area over which the gases may flow. The hairs near the nares filter the gases and trap particles. The abrupt change in direction of airflow in the nasopharynx also traps particles. Due to their momentum, the moving particles do not abruptly change direction 90 degrees to descend into the oropharynx and instead impact in the nasopharynx. Gas flow through the mouth also abruptly changes direction in the oropharynx, which allows some particles to be filtered.
The pharynx also warms, humidifies, and filters gases, albeit to a lesser degree. One of its principal roles is protecting the airway from aspirating solids or liquids during eating and drinking. This is accomplished by the swallowing reflex, in which a coordinated muscular contraction passes a bolus of food or liquid smoothly over the tongue, into the hypopharynx and esophagus, directly over the larynx, which is fully covered by the epiglottis and the closed vocal cords. In other words, the larynx senses foreign material, liquid or solid, and causes the vocal cords to close and the epiglottis to cover the vocal cords and trachea to prevent the solid or liquid from entering the trachea.
The trachea, bronchi, and bronchioles (conducting airways) also heat and filter the gases before they reach the respiratory bronchioles and alveoli. Cells lining the conducting airways secrete mucus to catch and filter particles. This mucus is then expelled from the airways via cells that have hair-like protrusions called cilia that beat in a coordinated fashion moving the mucus into the pharynx to be cleared into the esophagus and stomach.
The functions of the pharynx and the larynx have important implications during anesthesia. The placement of a nasotracheal or orotracheal tube bypasses the normal warming, humidifying, and filtering functions of the nose and mouth. These functions must then be replaced by functions of the anesthesia circuit or ventilator. General anesthesia abolishes the reflex-mediated coordinated muscle contraction that prevents solids or liquids entering the trachea. A patient with a stomach full of food or gastric secretions can aspirate stomach contents during anesthesia. This is most likely during induction of general anesthesia prior to placement of an endotracheal tube. However, placement of an endotracheal tube does not guarantee that aspiration will not occur.
Patient factors that slow gastric emptying (e.g., diabetes, obesity) or in whom the muscular valve between the stomach and esophagus is dysfunctional (e.g., gastroesophageal reflux) are at increased risk for aspiration during anesthesia. This risk of aspiration must be taken into account by the anesthesia provider prior to induction of general anesthesia and is an important factor in determining how to manage the airway during anesthesia. The anesthesia provider must determine if the case can be delayed to allow the stomach to empty, or to proceed, either because the case is an emergency or waiting will not change the risk. If the case proceeds, the anesthesia provider will determine if the case can be performed under minimal sedation or regional anesthesia, in which case the patient’s airway reflexes may be preserved. If general anesthesia is necessary, the anesthesia provider will take special precautions to minimize the risk of aspiration including placing an endotracheal tube as quickly as possible once anesthesia is induced to minimize the time the airway is unprotected. This is termed rapid sequence induction. In addition, the use of cricoid pressure may be utilized to prevent the aspiration of stomach contents. Aspiration can also occur on emergence, and the swallowing reflex must recover after general anesthesia to protect the airway again.
The protective reflexes of the larynx can also be detrimental. In partially anesthetized, or even awake, patients, stimuli to the larynx (e.g., mucus, blood, or saliva) can cause the vocal cords to spasm and close termed laryngospasm, thereby making ventilation difficult. This is an emergency situation and the anesthesia technician might often be called upon for assistance by the anesthesia provider.
Diffusion
The word “respiration” can be confusing, as it has two definitions. One definition refers to the acts of inhalation and exhalation of gases into and out of the lungs. In this chapter, we will call that process of inhalation and exhalation by the lungs “ventilation”—this will avoid confusion and is also common use in anesthesia practice. The second definition of respiration is a cellular activity, the use of oxygen and nutrients to produce energy for all cellular processes. Chapters 4 and 5, Cardiovascular Anatomy and Physiology and Cardiovascular Monitoring, discussed the ultimate goal of the cardiovascular system, delivering oxygen and nutrients for this cellular respiration.
As previously stated, oxygen diffuses from the alveoli into pulmonary capillaries, while carbon dioxide diffuses from the blood into the alveoli. Diffusion refers to molecules moving from an area of higher concentration to an area of lower concentration. Because the concentration of oxygen in the alveoli is usually higher than the concentration of oxygen in the blood, oxygen moves from the alveoli into the blood. The opposite is true of carbon dioxide. The concentration of carbon dioxide is usually higher in the blood than it is in the alveoli, so carbon dioxide diffuses from the blood into the alveoli. Diffusion stops when the concentration of oxygen, or any other gas or liquid, in both places is equal (Fig. 7.10).

FIGURE 7.10. General view of the ventilatory system showing the respiratory passages, alveoli, and gas exchange function in an alveolus. (From McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007, with permission.)
Resistance to Airflow in the Airways
Gas must flow through the airways to reach the alveoli. Any resistance to gas flow can diminish the amount of gas that reaches the alveoli. Total resistance depends on the amount of gas flowing at one time and on the cross-sectional area of the airway. For example, the nasal passage accounts for a significant amount of resistance because of its relatively small cross-sectional area. The trachea, however, has a lower resistance to gas flow when compared to the nasal passage, because its cross-sectional area is larger. The trachea and mainstem bronchi each create a similar amount of resistance to gas flow. Although the main bronchi have smaller diameters compared to the trachea, adding the cross-sectional areas of each bronchus together creates a cross section similar to the cross section of the trachea. Certain medical conditions, such as asthma and COPD, reduce the cross-sectional area in the bronchi and bronchioles, thus increasing the resistance to gas flow (Fig. 7.11). This becomes important to the anesthesia provider as these patients may require longer inspiratory and expiratory times in order to achieve the same amount of gas flow and complete exhalation of inspired gases.
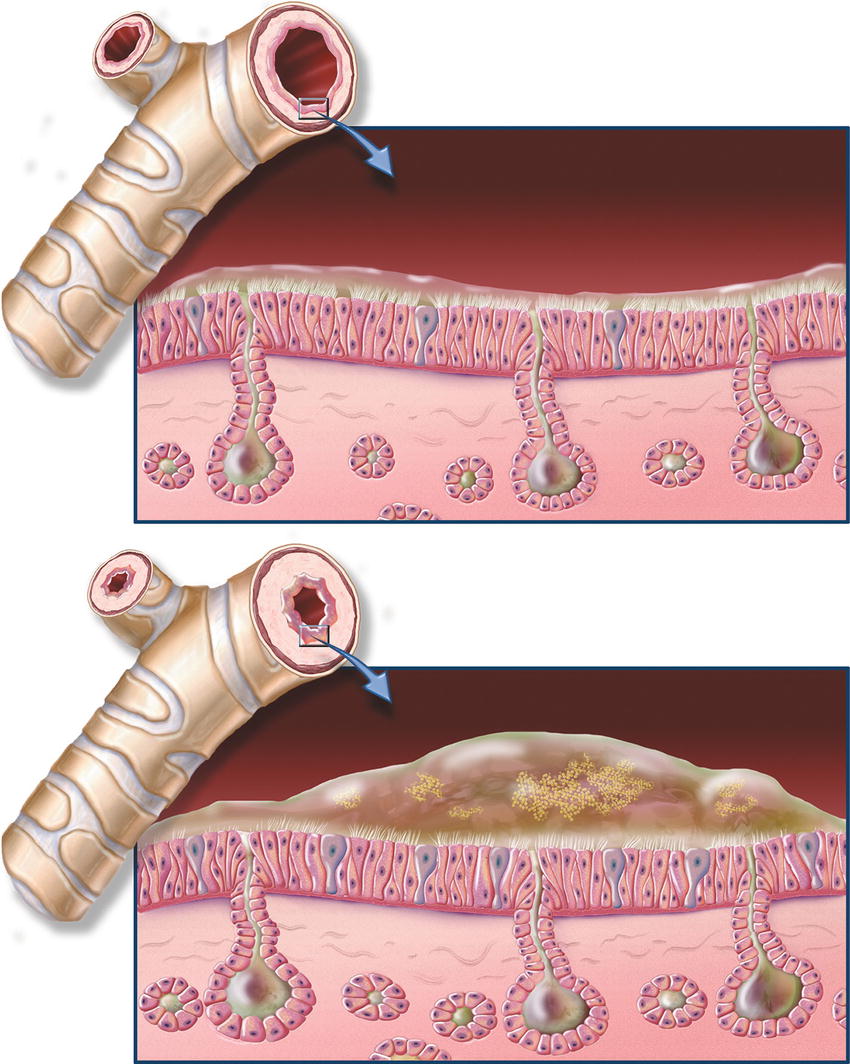
FIGURE 7.11. Chronic bronchitis and normal bronchial anatomy. Chronic obstructive pulmonary disease (COPD), close-up of cross section of healthy bronchi next to normal bronchial tube; close-up of cross section of bronchi with chronic bronchitis next to narrowed bronchial tube. (Image © ACC used with permission.)
Gas Exchange in the Alveolar- Capillary Unit
For gas exchange to occur efficiently, there must be ventilation-perfusion (so-called V/Q) matching. Abnormalities in V/Q matching can lead to either “dead space ventilation” or “shunting.” Dead space ventilation occurs wherever there is ventilation without perfusion. That is, alveoli receive fresh gas, but little or no blood flow arrives at the capillaries surrounding those alveoli. As a result, no gas exchange happens. Dead space ventilation results in more of a problem with carbon dioxide elimination than oxygen uptake. It wastes patient breathing efforts and increases the work of breathing.
Dead space ventilation may occur anytime blood is obstructed from passing through vessels (e.g., a blood clot, fat embolus, amniotic embolus, etc. in a blood vessel), when the pulmonary blood vessels are constricted, or when total cardiac output is so low that there is significantly reduced pulmonary blood flow to all or parts of the lung. An inevitable kind of dead space ventilation also happens during every breath; a portion of each breath is “wasted” going through the large airways (and the endotracheal tube if present), which are not gas exchange surfaces. This is about 150 mL per breath in an adult. In Chapter 27, the Breathing Circuit, you will read further about this mechanical dead space as a part of the breathing circuit. As an anesthesia technician, your setup of the breathing circuit outside the patient will play a critical role in the clearance of carbon dioxide.
Shunting occurs wherever there is perfusion but no ventilation. That is, blood flows through capillaries surrounding alveoli, but the alveoli are not ventilated. Shunt primarily affects arterial oxygenation and, to a much lesser extent, carbon dioxide elimination. The poorly oxygenated blood in the capillaries travels past the alveoli that are not ventilated and returns to the heart. The shunted blood has significantly lower oxygen content (75% vs 100% hemoglobin oxygen saturation) and slightly higher carbon dioxide partial pressure (45 vs 40 mm Hg) than blood that participated in gas exchange in the lungs (Fig. 7.12).
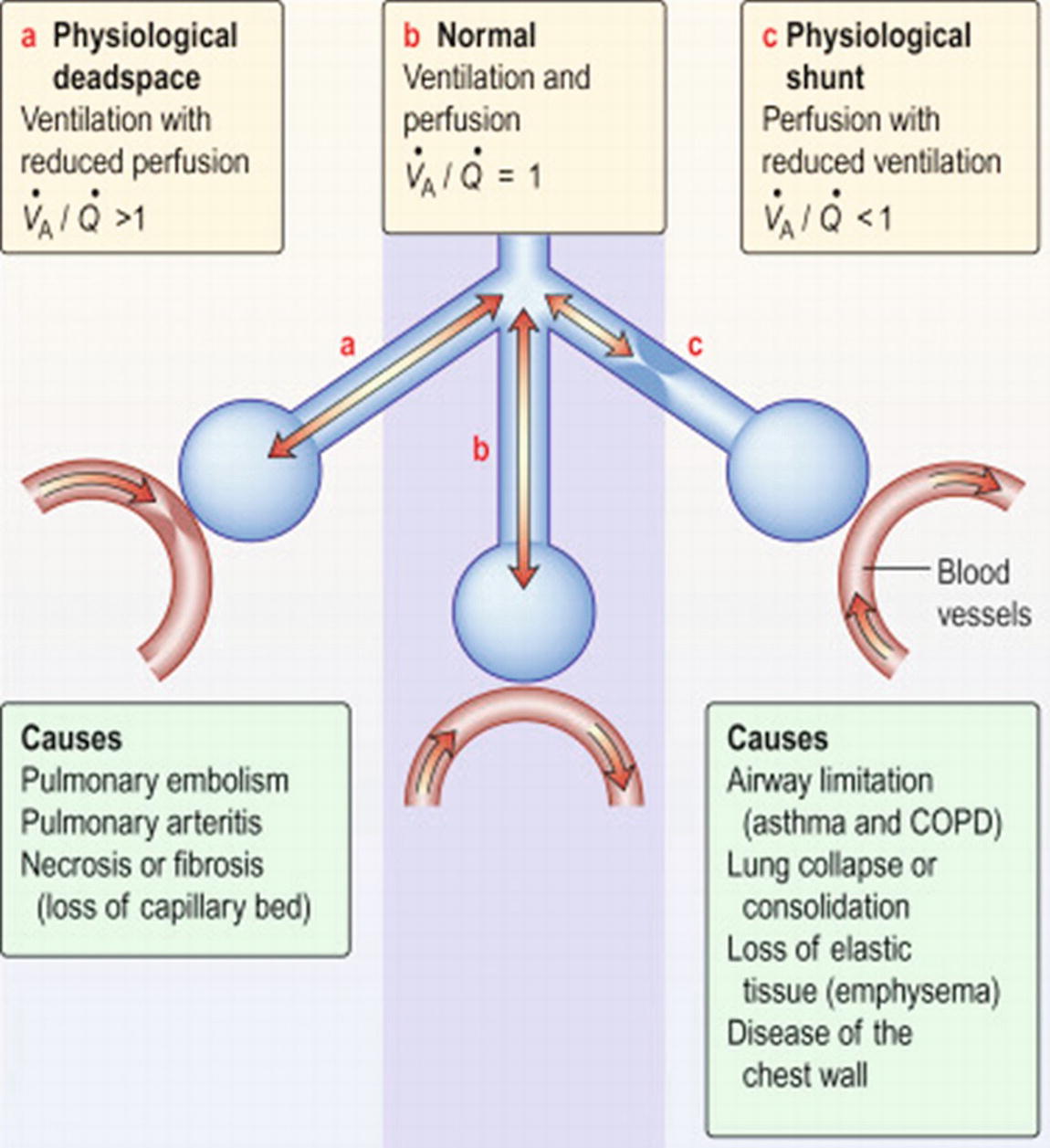
FIGURE 7.12. Ventilation-perfusion matching. VA, ventilation; Q, perfusion. In the center is the normal lung, in which ventilation and perfusion are matched. On the left is physiologic dead space, where ventilation happens, but not perfusion. On the right is shunt, where perfusion happens, but not ventilation. (Reprinted from Frew AJ, Doffman SR, Hurt K, Thomas RB. Kumar and Clark's Clinical Medicine. 9th ed. 2016;1057-1137, Figure 24.8. Copyright ©2016 Elsevier. With permission.)
True shunt occurs when inspired gases cannot reach alveoli due to a physical blockage; for example, a mucus plug, foreign body, or endotracheal tube inserted past the carina. Although rare, clinically significant shunt can also occur through a defect in the heart so that blood flows directly from the right side of the heart to the left side of the heart bypassing the lungs. Shunt “effect” is when alveoli are poorly ventilated (not completely lacking in ventilation) and gas exchange is impaired. Shunt effect occurs when left heart failure or acute lung injury prevents effective ventilation of perfused alveoli. In this instance, there is fluid overload in the alveoli (from alveolar-capillary membrane disruption), with subsequent impairment of adequate gas exchange. Shunting may also be caused purposefully by the anesthesia provider during lung, esophageal, or aortic surgery by placement of a double-lumen tube or endobronchial blocker to allow the lung on the operative side to be deflated in order to facilitate surgical exposure or accidentally by misplacement of the endotracheal tube in a mainstem bronchus as opposed to the trachea.
Even under normal circumstances, some portions of the lung are better ventilated than others, and some portions are better perfused than others. This occurs primarily due to the effects of gravity. For example, think of a patient standing upright and consider the effects of gravity on the lungs and blood flow to the different levels of the lungs. The top, or apex, of the lungs is stretched open because of its attachment to the rest of the lung, and due to gravity, the rest of the lung is being pulled toward the ground. The heart is below the apex of the lung, and so the blood must be pushed up to reach the lung apices, which may result in less blood flow to the apices of the lungs. The amount of ventilation to the apices of the lungs is more than the available perfusion to the apices of the lungs, so a ventilation-perfusion mismatch occurs. At the bottom (gravity dependent), the weight of the lungs causes alveoli to collapse and ventilation is less than ventilation to the apices of the lungs. Perfusion to the base of the lungs is much more robust than perfusion to the apices of the lungs because blood flow to the lungs is gravity dependent. Ventilation-perfusion mismatch occurs at the base of the lungs, causing a relative shunt to occur.
Control of Breathing
Breathing occurs through a combination of automatic reflexes and conscious control in the brainstem, brain, lungs, and central blood vessels (Fig. 7.13). Fortunately, breathing is largely an involuntary action governed by the brainstem (you still breathe when you are asleep); however, you have some voluntary control and can take a deep breath if you want to. Breathing is largely controlled by the respiratory center in the brainstem. Many factors can influence the respiratory center to alter breathing. Minute ventilation is defined by how much gas moves into and out of the lungs during 1 minute of breathing. It is determined by multiplying the respiratory rate and the tidal volume in 1 minute. Minute ventilation may increase by increasing the respiratory rate (number of breaths per minute) or by increasing the volume of each breath. Minute ventilation increases in response to an increase in carbon dioxide production (higher blood levels), decreased oxygen saturation, or increased amount of acid in the blood. The ability to change minute ventilation is important. For example, trauma patients, patients with fever, and people engaging in exercise increase the amount of carbon dioxide they produce. They need to increase their minute ventilation to remove the extra carbon dioxide from the blood. During anesthesia, the drivers for breathing are altered and reflexes are blunted. Patients may be paralyzed, so that their breathing needs to be fully controlled. Carbon dioxide could even be added to their system for laparoscopy. All these mean that the anesthesia provider will need to adjust the ventilation of the patient to respond to changes in carbon dioxide production or increased oxygen demand.
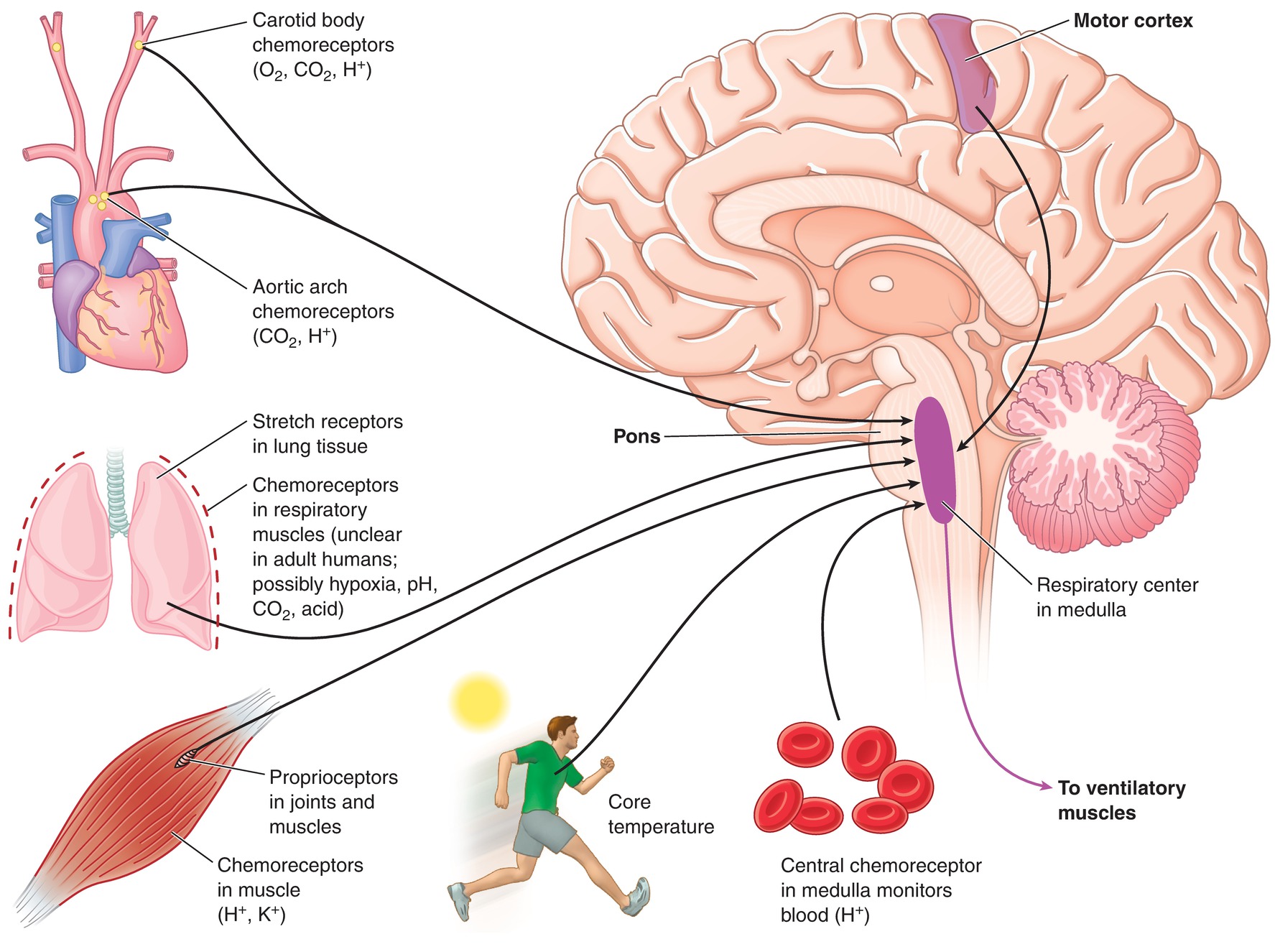
FIGURE 7.13. The respiratory control center is located within the medulla. The respiratory control center adjusts pulmonary ventilation to meet the body’s needs due to feedback from chemoreceptors and proprioceptors. (From Kraemer WJ, Fleck SJ, Deschenes MR. Exercise Physiology. Philadelphia, PA: Wolters Kluwer Health; 2011, with permission.)
Mechanics of Breathing
Gases move across a pressure gradient from an area of higher pressure to an area of lower pressure. Gases move into the lungs when the pressure in the thoracic cavity, and thus in the lungs, is lower than the pressure in the atmosphere (or breathing circuit). Inspiration ceases when the pressure in the lungs and thoracic cavity equals the pressure in the atmosphere. Expiration begins when the pressure in the lungs is greater than the pressure in the atmosphere.
Inspiration is usually an active process. When the diaphragm contracts, it causes the rib cage to move up and out, and it pushes the abdominal contents down. This expands the chest and lowers the pressure inside the rib cage. The decrease in the intrathoracic pressure creates a pressure gradient and gas flows into the lungs from the outside. The strap muscles of the neck and chest muscles can be used to increase the force of inspiration. All of these muscles help elevate the ribs, which expands the chest, and further decreases the pressure. In patients with respiratory distress, noninvasive ventilation techniques (CPAP or BPAP) can be used, as these provide extra inspiratory pressure. They require the patient to generate less negative pressure on his own, decreasing the work of breathing.
Expiration is usually a passive process owing to the tendency of the lungs to want to collapse after inspiration. During expiration, the elastic energy in the lungs causes the lungs to contract. These forces increase the pressure in the lungs above the pressure of the atmosphere and gases move out of the lungs and into the atmosphere. Although expiration is normally passive, it can be augmented by contraction of muscles in the abdominal wall that results in displacement of abdominal contents superiorly, decreasing lung volume by increasing pressure around the lungs. Chest wall muscles can also be activated to cause the ribs to descend and actively increase the intrathoracic pressure.
Patient Position, Anesthesia, and Respiratory Mechanics
Patient position may affect lung mechanics and ventilation-perfusion matching. In the standing or reverse Trendelenburg (feet tilted downward) position, the abdominal contents fall away from the diaphragm due to gravity. In the supine position, the abdominal contents tend to press up on the thorax and compress the lungs. Patients lying prone have increased abdominal pressure and an even greater tendency for the abdominal contents to compress the lungs. The prone position also puts direct pressure on the chest, further compressing the lungs. To offset these effects, patients who are positioned prone have supports placed across their pelvis and superior portion of the thorax, as well as the lateral edges, to decrease pressure on the abdomen and chest (see Chapter 20, Patient Positioning).
Position not only affects lung volumes but also blood flow to the different lung regions. Gravity influences blood flow to the different lung regions. If patient position causes sufficient ventilation-perfusion mismatch, oxygenation can be affected.
General anesthesia has several effects on lung mechanics. Both inhaled anesthetics and intravenous anesthetics result in muscular relaxation, compression of the lungs, and decreased FRC. Intravenously administered neuromuscular blockers (paralytic agents) result in further muscle relaxation and an even smaller FRC. Decreased blood pressure associated with the inhalational and intravenous anesthetics causes ventilation-perfusion changes and increased dead space ventilation as well as increased intrapulmonary shunt. Thus, a patient who is supine and awake may exhibit an oxygen saturation of hemoglobin measured by pulse oximetry of 99%-100%. After induction of general anesthesia, the oxygen saturation may decline unless the effects of anesthesia on pulmonary function are counteracted.
Summary
The pulmonary system is a complex system involving many different organs including the lungs, heart, blood vessels, brain, and musculoskeletal system. The anesthesia provider must have intimate knowledge of the interactions between the various systems in order to provide safe anesthesia care to the patient. Knowledge of these systems will allow the anesthesia technician, especially in emergency situations, to anticipate the needs of the anesthesia provider and understand the thought process behind the selection of various tools and anesthetic techniques.
Review Questions
1. Which of the following is the MAIN function of the respiratory system?
A) Gas exchange
B) Gas humidification
C) Acid-base balance
D) Immunologic defense
E) Production of surfactant
Answer: A
The main function of the respiratory system is gas exchange, which provides oxygen to the blood for delivery to cells and removes carbon dioxide that is generated by cell metabolism.
2. A transtracheal nerve block is performed by piercing which structure?
A) Thyroid cartilage
B) Cricoid cartilage
C) Cricothyroid membrane
D) Arytenoid cartilage
E) Thyrohyoid membrane
Answer: C
The recurrent laryngeal nerve (subglottic area) may be anesthetized by application of topical anesthetics usually through the fiberoptic scope or via transtracheal block performed by injection of local anesthetic through the cricothyroid membrane directly into the trachea.
3. Which of the following is NOT a part of the lower airway?
A) Trachea
B) Bronchi
C) Alveoli
D) Vocal cords
E) Bronchioles
Answer: D
The lower airway begins at the level of the trachea, just below the glottic opening (vocal cords) and includes the trachea, bronchi, bronchioles, respiratory bronchioles, and alveoli.
4. Which of the following participates in gas exchange with blood?
A) Trachea
B) Bronchi
C) Bronchioles
D) Alveoli
E) Type II pneumocytes
Answer: D
The trachea, bronchi, and bronchioles are conducting airways and do not participate in gas exchange between the airway and blood. Type II pneumocytes produce surfactant.
5. When air accumulates in the pleural space, it is referred to as:
A) Empyema
B) Pneumothorax
C) Hemothorax
D) Hemopneumothorax
E) Pleural effusion
Answer: B
Air in the pleural space is referred to as a pneumothorax. When blood accumulates, it is a hemothorax. Pus in the pleural space is an empyema. Other fluid in the pleural space is an effusion.
6. At the end of normal expiration, the volume of gas remaining in the lungs is referred to as:
A) Total lung capacity
B) Inspiratory capacity
C) Functional residual capacity
D) Forced vital capacity
E) Closing capacity
Answer: C
The functional residual capacity (FRC) is the volume of gas in the lungs at the end of a normal exhalation. Functional residual capacity, serving as a reserve source of oxygen and a patient’s ability to tolerate periods of apnea, is a very important concept to anesthesia providers.
7. Which of the following places the patient at risk for aspiration?
A) High blood pressure
B) COPD
C) Obesity
D) Kidney failure
E) Thyroid disease
Answer: C
Patient factors that slow gastric emptying (e.g., diabetes, obesity) or in whom the muscular valve between the stomach and esophagus is dysfunctional (e.g., gastroesophageal reflux) are at increased risk for aspiration during anesthesia.
8. Which of the following conditions results in increased resistance to gas flow?
A) High blood pressure
B) COPD
C) Obesity
D) Kidney failure
E) Thyroid disease
Answer: B
Medical conditions, such as asthma and COPD, reduce the cross-sectional area in the bronchi and bronchioles, thus increasing the resistance to gas flow. This becomes important to the anesthesia provider as the patients may require longer inspiratory and expiratory times in order to achieve the same amount of gas flow and complete exhalation of inspired gases.
9. Which patient position allows for an improvement in respiratory mechanics?
A) Prone
B) Lateral decubitus
C) Supine
D) Reverse Trendelenburg
E) Trendelenburg
Answer: D
Patient position may affect lung mechanics and ventilation-perfusion matching. In the standing or reverse Trendelenburg position, the abdominal contents fall away from the diaphragm due to gravity. The other positions allow for abdominal contents to be displaced cephalad and possibly impair respiratory mechanics.
SUGGESTED READINGS
Butterworth J, Mackey D, Wasnick J, eds. Respiratory physiology and anesthesia. In: Morgan & Mikhail’s Clinical Anesthesiology. 5th ed. New York, NY: McGraw-Hill Medical; 2013:Chapter 23.
LeBlond RF, Brown DD, DeGowin RL. The chest: chest wall, pulmonary, and cardiovascular systems; the breasts. In: LeBlond RF, Brown DD, Suneja M, Szot JF, eds. DeGowin’s Diagnostic Examination. 10th ed. New York, NY: McGraw- Hill Medical; 2015:260-307.
West JB. Pulmonary Pathophysiology: The Essentials. 8th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2013.
West JB, Luks AM. West's Respiratory Physiology: The Essentials. 10th ed. Philadelphia, PA: Wolters Kluwer; 2016.