CHAPTER 26
Vaporizers
Introduction
Inhalational agents are drugs with anesthetic properties administered in the form of a gas. For a drug injected intravenously, the dosing relates to the mass the patient receives (e.g., in grams) in the form of a specific volume at a specific concentration. Inhalational agents, however, are delivered as a concentration in a volume of gas. The “volume” is being continuously delivered with each breath the patient receives. As these agents normally exist as liquids at room temperature and atmospheric pressure, a vaporizer is used to turn the liquid into a gas that the patient can inhale.
In the middle of the 19th century, the first available “vaporizers” were merely devices that allowed the patient to breathe evaporated liquid agents. The device used by William Morton in the first public demonstration of ether anesthesia in 1846 was a container that contained a sponge soaked with ether (Fig. 26.1). The patient breathed in the agent as it evaporated off the sponge.
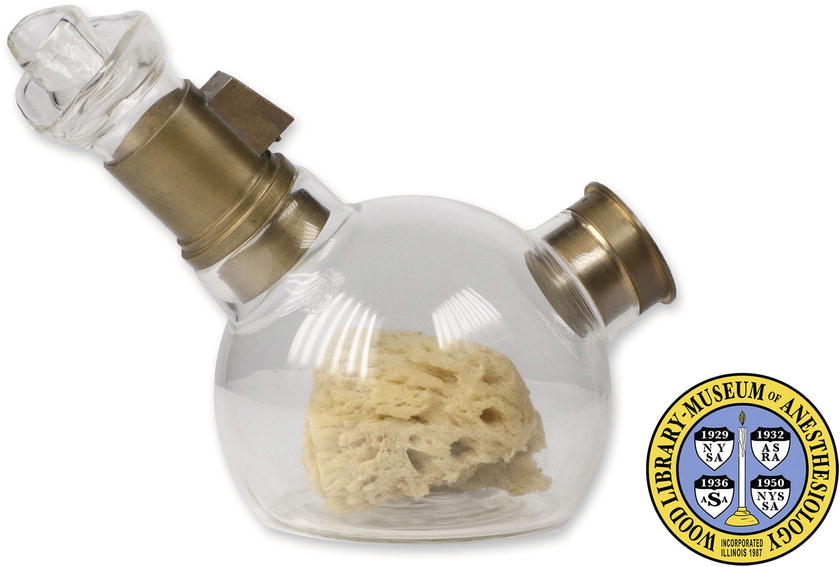
FIGURE 26.1. Early vaporizer. A replica of the inhaler used by Dr. William Morton to demonstrate the use of ether anesthesia in 1846. An ether-soaked sponge was placed inside, and the patient breathed in the evaporated vapor. (Photo courtesy of the Wood Library-Museum of Anesthesiology, Park Ridge, IL [http://www.woodlibrarymuseum.org/museum_view.php?id=2].)
Later, chloroform was administered by dropping the liquid agent using special dropper bottles (Fig. 26.2) over a cloth that was placed either directly over the patient’s mouth or draped over a wire mask. Although such devices allowed the liquid to evaporate into a gaseous form, the concentrations of the agent could not be controlled. Modern vaporizers were thus developed to deliver a precise and constant concentration of the agent.

FIGURE 26.2. Chloroform dropper. From left to right: a chloroform drop flask, a drop bottle with a control valve, and an alembic flask. Such devices allowed careful titration of the liquid agent. (Photo courtesy of the Wood Library-Museum of Anesthesiology, Park Ridge, IL [http://www.woodlibrarymuseum.org/museum_view.php?id=25].)
Physical Chemistry
To understand the basic principles of how modern vaporizers work, we need to review some principles of physical chemistry: the concepts of vapor, vapor pressure, and gas concentrations.
Vapor and Vapor Pressure
A vaporizer turns the liquid anesthetic agent from a liquid form to a gas or vapor. All substances can exist in liquid, solid, or gas forms, depending on the pressure and temperature of the substance. As a gas is compressed under increasing pressure, the particles are pushed closer together until the gas turns into a liquid. For example, when nitrogen gas is compressed enough, it turns into liquid nitrogen. For some gases, there is a critical temperature above which a gas cannot exist as a liquid, no matter how much pressure is applied.
A vapor is a substance in the gaseous phase at a temperature below its critical point. That is, it is a gas that has the potential to become a liquid when compressed, or subjected to a higher pressure. When a volatile liquid is placed in a closed container, a certain percentage of the liquid molecules evaporate to become vapor. This vapor creates a pressure, called the vapor pressure. As more heat is applied, more molecules enter the gaseous phase, resulting in a greater pressure. As such, the vapor pressure of any substance increases with temperature. The concentration of an agent delivered by a vaporizer depends on the vapor pressure of the agent. Because different agents have different vapor pressures, each modern vaporizer is calibrated for use with a specific agent. Of note, desflurane has a much higher vapor pressure at room temperature than other agents and thus requires a vaporizer with unique features (see below).
Gas Concentration: Partial Pressure and Volume Percent
The concentration of a vapor can be expressed as either a partial pressure or a volume percent. In a mixture of gases, each gas independently contributes part of the total pressure, which is the sum of the partial pressures of all gases present. The portion of the total pressure created by any given vapor is called the partial pressure of that gas. Although the partial pressure of the gas is what actually corresponds to the clinical effect of an anesthetic gas in the body, concentrations delivered by a vaporizer are commonly expressed as a volume percent for practical convenience. Volume percent is the fraction of the total pressure attributable to the gas of interest expressed as a percent (partial pressure of gas/total ambient pressure × 100). This term may be a slight misnomer as the gas molecules are mixed together in a shared volume. Nonetheless, because the volume of a gas is proportional to the number of particles, given a constant pressure and temperature, the volume percent of an agent can be thought of as a percentage of the total number of gas molecules delivered to the patient.
Principles of Modern Vaporizers
Vaporizers vary greatly in their design and construction. Figure 26.3 shows one common type of modern vaporizer. All modern (concentration-calibrated vaporizers) are placed out of circuit—that is, between the flowmeter and the common gas outlet, rather than within the breathing system or between the common gas outlet and the breathing system. The intent of this chapter is not to go over the specifics of the operation of any specific vaporizer model but to provide an overview of the principles underlying the operation of modern vaporizers.

FIGURE 26.3. From left to right, a desflurance, a sevoflurane, and an isoflurane vaporizer.
Variable Bypass Vaporizers
As mentioned previously, the basic purpose of a vaporizer is to deliver a set concentration of anesthetic gas in a volume of inert gas, such as oxygen. Figure 26.4 shows the general schematic of a vaporizer. In this schematic, fresh gas flow enters from the top left, corresponding to the vaporizer inlet. The inert gas can then flow across the top bypass chamber, without being exposed to any volatile agent. Alternatively, some of the fresh gas flow can be diverted down into the vaporizing chamber, where it becomes saturated with a certain concentration of the volatile agent, as determined by the partial pressure of the agent. The concentration dial can vary the percentage of gas, called the splitting ratio that bypasses the vaporizing chamber; this construction is thus called the variable bypass vaporizer.
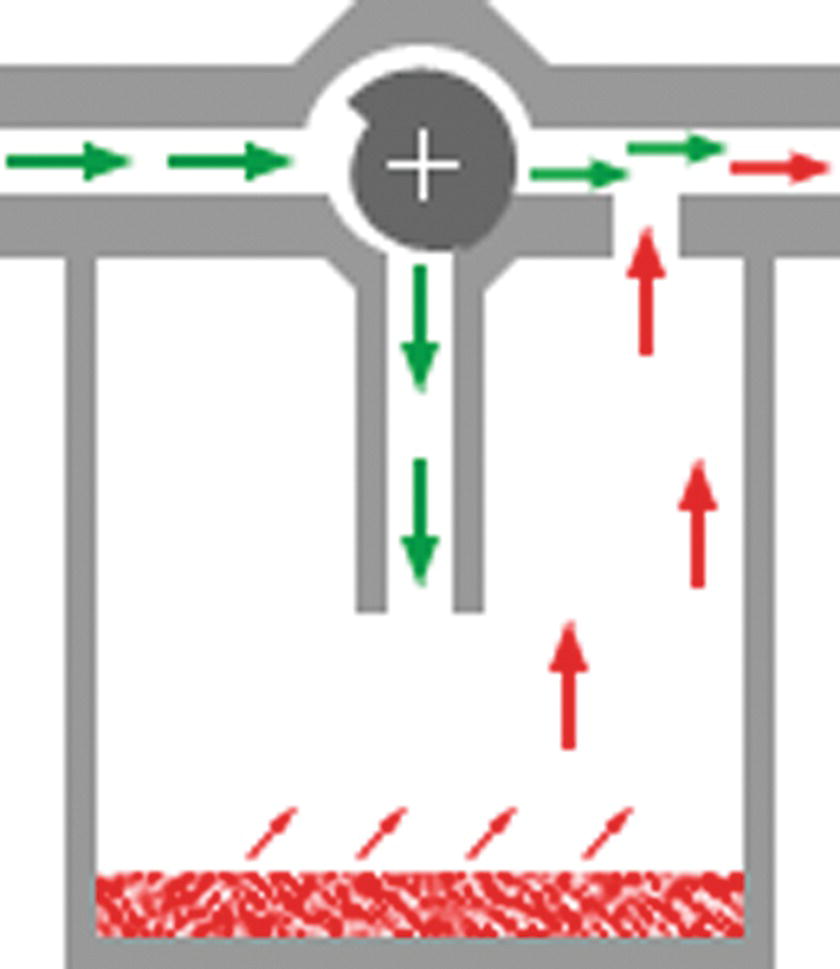
FIGURE 26.4. Schematic of a variable bypass vaporizer. Simple schematic of modern vaporizer: Fresh gas flow enters the vaporizer inlet (top left) and is directed either down into the vaporizing chamber to become saturated with vapor or into a bypass chamber across the top. By varying the ratio of the split, the output vaporizer concentration (top right) can be changed. (Figure courtesy Dr. Guy Watney [http://www.asevet.com/resources/vaporizer/index.htm].)
The end result is that the partial pressure of the volatile agent in the vaporizing chamber is diluted by the fresh gas flow through the bypass to obtain the desired concentration of anesthetic. The gas with the desired vapor concentration exits through the outlet of the vaporizer, shown at the top right of the schematic. In an older type of vaporizer, called the copper kettle, the gas flows for both the flow directed to the vaporization chamber and for the flow that would skip the chamber had to be manually adjusted to achieve a desired output gas concentration. Modern variable bypass vaporizers do this automatically when the concentration dial is set to a desired concentration.
Although the input fresh gas flow rate theoretically can increase the output gas concentration, this effect is minimal with most modern vaporizers. The input gas composition (i.e., if nitrous oxide is used in addition to oxygen) can also affect the concentration. To account for these effects, many electronic vaporizers have a feedback system that adjusts its internal settings based on the actual gas output. In other types of vaporizers, such as the Tec 6 used for desflurane, there are two separate input circuits, rather than a single fresh gas flow that is split.
Vaporization Method
Older vaporizers such as the copper kettle bubbled the carrier gas up through the liquid anesthetic to saturate the carrier gas with the anesthetic. Most modern vaporizers have the carrier gas flow over the liquid agent where it takes up the anesthetic. Increasing the surface with internal wicks and baffles makes the vaporization and uptake of the anesthetic more efficient. To accommodate the higher vapor pressure of desflurane, the Tec 6 vaporizer uniquely uses a gas/vapor blender in which the desflurane is heated to a constant temperature to produce a vapor that is then injected into the gas flow in a regulated fashion (Fig. 26.5).
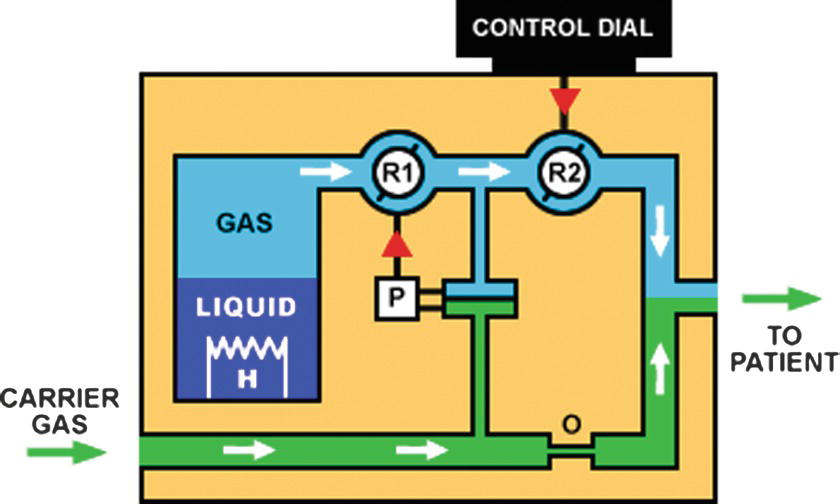
FIGURE 26.5. Schematic of Tec 6 desflurane vaporizer. The liquid desflurane (liquid) is heated (H) to a vapor form (gas), which is then mixed with the carrier gas to produce a vaporizer output of the desired concentration. (Figure courtesy Dr. Guy Watney [http://www.asevet.com/resources/vaporizer/index.htm].)
Temperature Compensation
Since vapor pressure depends on the temperature of the gas, the output vapor concentration of older vaporizers was often dependent on temperature. Modern vaporizers have an automatic mechanism built in that regulates the variable bypass to compensate for changes in temperature, keeping the gas concentration roughly constant over standard operating temperatures. The degree of temperature compensation depends on the specific vaporizer; some vaporizers have gas outputs that slightly increase as ambient temperature increases (Table 26.1).
Table 26.1. Properties of Various Vaporizers

aTec 4, 5, 7, SevoTec, Aladin (ADU); Vapor 19, Vapor 2000. Table adapted with permission courtesy Dr. Michael Dosch (https://www.udmercy.edu/about/people/university/chp-mson/crna/michael-dosch).
Operation of Vaporizers
Anesthesia technicians know how to install and remove, transport, fill, operate, and drain vaporizers. It is important to be familiar with safety features and potential problems associated with each process.
Installation of a Vaporizer
Vaporizer mounting systems can be permanent or detachable. Permanent mounting systems have the advantage of less risk of physical damage and leaks, but the disadvantage is that vaporizers cannot be swapped out if a different agent is needed or if a vaporizer malfunctions. Most modern anesthesia machines have detachable mounting systems, in which vaporizers can be mounted or removed without tools. In general, each vaporizer position has an input and output port valve, each with O-rings to prevent an air leak between the mounting system and the vaporizer. Missing or broken O-rings or improper mounting of the vaporizer are potential causes of a vaporizer leak. Before mounting or detaching a vaporizer, it and all adjacent vaporizers should be turned off. The “travel” setting on the vaporizer should be used, if present, to prevent the agent from filling the bypass chamber (Fig. 26.6). Even with the travel setting in use, care should be taken to transport all vaporizers in an upright position to prevent liquid agent from entering inappropriate compartments.

FIGURE 26.6. The concentration dial must be in the Travel (T) position of the Vapor 2000 vaporizer before the vaporizer can be unlocked from the machine. This isolates the vaporizer chamber to prevent liquid from entering the bypass chamber.
Filling the Vaporizer
As vaporizers are calibrated for use with specific agents with given partial pressures, an incorrect gas concentration will be delivered to the patient if an incorrect agent is used to fill the vaporizer. Because of this, the filling systems of modern vaporizers are designed to allow a vaporizer to be filled with only a specific agent. In a bottle-keyed system, each agent comes in a specifically designed bottle that may itself be used to fill the vaporizer or that uses an attachment (filler) that uniquely connects to the bottle of the agent and the filling port of the corresponding vaporizer. A common bottle-keyed system (Easy-Fill System) uses a bottle collar that is color coded according to the anesthetic agent. The filling attachment of the same color as the bottle collar is designed in such a way that it can only screw onto the appropriate bottle (Figs. 26.7 and 26.8). There may be a metal block in some vaporizers that must be removed prior to attachment of the filling device. After the bottle-filler assembly is inserted into the vaporizer, some vaporizers have a latch that must be opened, after which the bottle is rotated up to fill the vaporizer as shown in Figure 26.9.

FIGURE 26.7. As an example of the bottle-keyed system, the collar of the sevoflurane bottle (left) has a color and spacing of protrusions that match an agent-specific filling attachment (right) with appropriately spaced indentations.
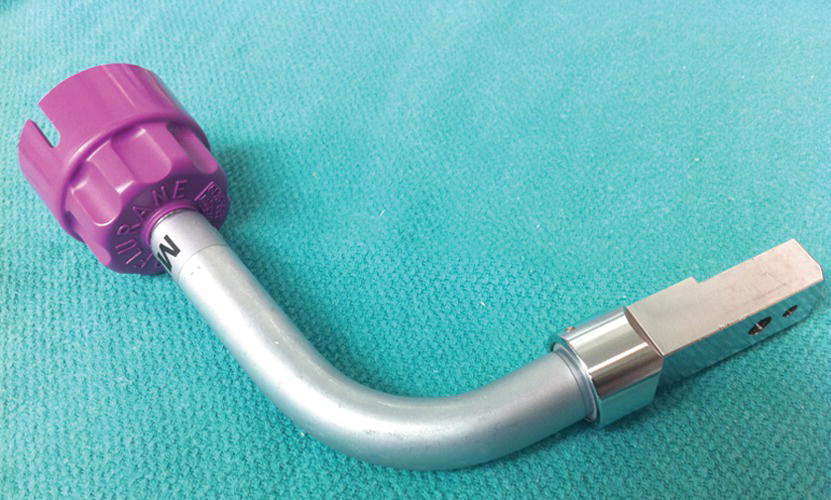
FIGURE 26.8. Filling attachment for use with a keyed filling system. The color-coded base attaches uniquely to the collared bottle, and the rectangular filling block is grooved to uniquely fit into a specific vaporizer.

FIGURE 26.9. After insertion of the filling attachment with the bottle upright, a latch is pulled to secure the attachment, and the bottle is rotated 180 degrees to an inverted position to fill the vaporizer.
Funnel-fill systems are less frequently used but exist in some older vaporizers; a screw-in plug is removed to expose the opening of the funnel, into which the liquid agent is poured. A screw-in adaptor can be attached to the funnel, for use with the bottle-keyed system. Desflurane vaporizers often use a Quick-Fill System in which the grooved filling attachment is permanently attached to the bottle. This is an extra measure to prevent desflurane from being poured into the wrong vaporizer (Fig. 26.10).

FIGURE 26.10. A desflurane bottle with a permanently attached filling attachment that uniquely fits into a desflurane vaporizer.
The level of liquid agent remaining in the vaporizer may be displayed electronically on the anesthesia machine, or more commonly by a liquid fill indicator. The level of anesthetic agent remaining should be checked prior to the start of every case. Each type of vaporizer will have a different rate of consumption based on its efficacy, but a rough estimate of how long the liquid anesthetic should last is given by the following formula:
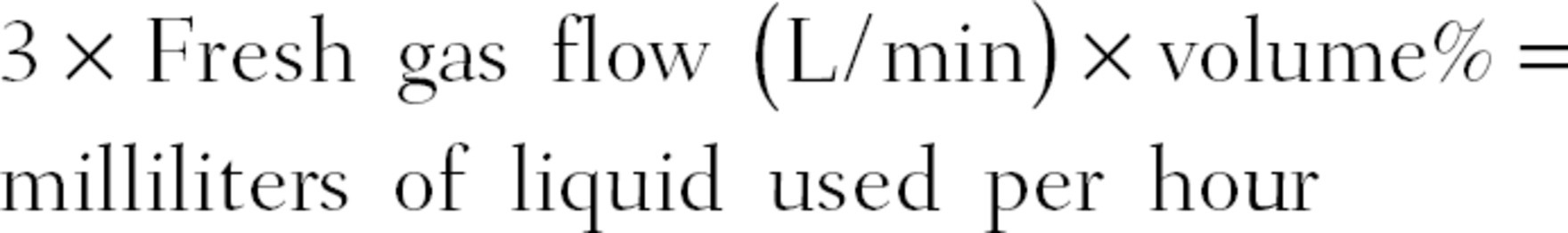
Thus, a case using sevoflurane at 1.8% at 2 L/min fresh gas flow yields an hourly consumption of 10.8 mL. The capacities of vaporizers vary greatly from roughly 100-400 mL, but based on the equation above, a vaporizer filled to 200 mL would last about 18.5 hours.
Gloves should always be used when filling the vaporizer, as the liquid agents can be caustic to skin. Whichever system is used, the cap on the vaporizer filling receptacle or the metal block must be replaced snugly. This is a very common oversight that can create a leak in the system, resulting in anesthetic gas leaking out to the atmosphere when the vaporizer is turned on. Most modern vaporizers have a system to prevent the vaporizer from being overfilled, but care should nonetheless be taken to not fill the vaporizer above the fill line.
Delivery of Anesthetic
Modern vaporizers generally have a dial calibrated in terms of volume percent; a counterclockwise rotation of the concentration dial universally increases the concentration. Some vaporizers have a button on the dial that needs to be depressed while the gas is turned on as an additional safety feature. All vaporizers have a vapor exclusion, or interlock, system that mechanically prevents more than one vaporizer from being turned on at the same time. As mentioned earlier, there may be a discrepancy between the concentration dial setting and the actual vaporizer output as the vaporizer function may be affected by the fresh gas flow, the temperature of the gas, and the vaporizer itself. The gas composition (whether nitrous oxide is present in the fresh gas flow) may also slightly affect the vaporizer output.
In addition to monitoring the concentration of the gas delivered to the patient, “end-tidal agent monitoring” is now standard of care. This measures the concentration of anesthetic gas released from the patient at the end of a breath (often together with the end-total carbon dioxide level), via the fresh gas sampling line attached to the circuit near the patient. This expired concentration is initially significantly lower than the inspired concentration because of uptake of the gas by the body; as steady-state equilibrium is achieved, the expired and inspired concentration becomes more closely approximated. The end-tidal gas concentration helps confirms that the patient’s body has absorbed (and is also now releasing) a given concentration of agent, and it is thus what is clinically used to titrate the delivery of anesthetic gas.
Removal of Anesthetic
The method of draining an agent from a vaporizer varies according to the vaporizer model. In general, there is a drain valve or nozzle that may simply require removal of a plug or insertion of a drain attachment (as with the desflurane Quick-Fill System). The removed agent can theoretically be set to evaporate in an area that people will not be exposed to the vapor. (Should small accidental spills occur, be reassured that modern anesthetic gases are not thought to be harmful, and are not sedating to bystanders in this low concentration as they evaporate). Alternatively, the agent could be poured into a container connected to the vacuum system, which will remove the vapor as the agent evaporates.
Troubleshooting
An anesthesia technician will be called upon to help troubleshoot vaporizers when they malfunction. In diagnosing an issue, it is helpful to categorize the problem as one in which the vaporizer is delivering higher than expected vapor output or lower than expected output. These abnormalities can be detected when an agent monitor is utilized and the detected agent appears to be higher or abnormally lower than the concentration selected on the vaporizer dial.
Higher than Expected Vapor Output
If the vaporizer is overfilled, or if the vaporizer is tipped, the liquid agent can spill into the bypass chamber. When this happens, the normally agent-free diluent (fresh) gas that “bypasses” the agent is now exposed to the anesthetic and picks up some vaporized agent. The result is that the vaporizer output contains more vapor than the vaporizer dial has been set for. As mentioned above, the “travel” setting on some vaporizers seals the vaporizing chamber during transport to prevent liquid from entering the bypass chamber.
A failure of the vaporizer interlock system may also result in more than one vaporizer being turned on at the same time. This problem can be detected by an agent monitor (see Chapter 30, Gas Analyzers). The monitor will display an error message or the presence of multiple volatile agents. Although a properly mounted vaporizer should not have this problem, the reversal of flow (i.e., fresh gas flow entering through the exit port) through a vaporizer can, in some vaporizers, cause excessive vapor pressure. Another cause of higher than expected agent readings occurs when an agent is used incorrectly in a vaporizer meant for use with an agent with a lower vapor pressure. In this case, the output gas concentration will be higher than expected. The keyed filling systems meant to prevent this problem can be circumvented (Fig. 26.11). This practice must be strictly avoided. The clinical effect of the increased concentration will depend on the relative potencies of the two agents, which is reflected by the minimum alveolar concentration (MAC) values.
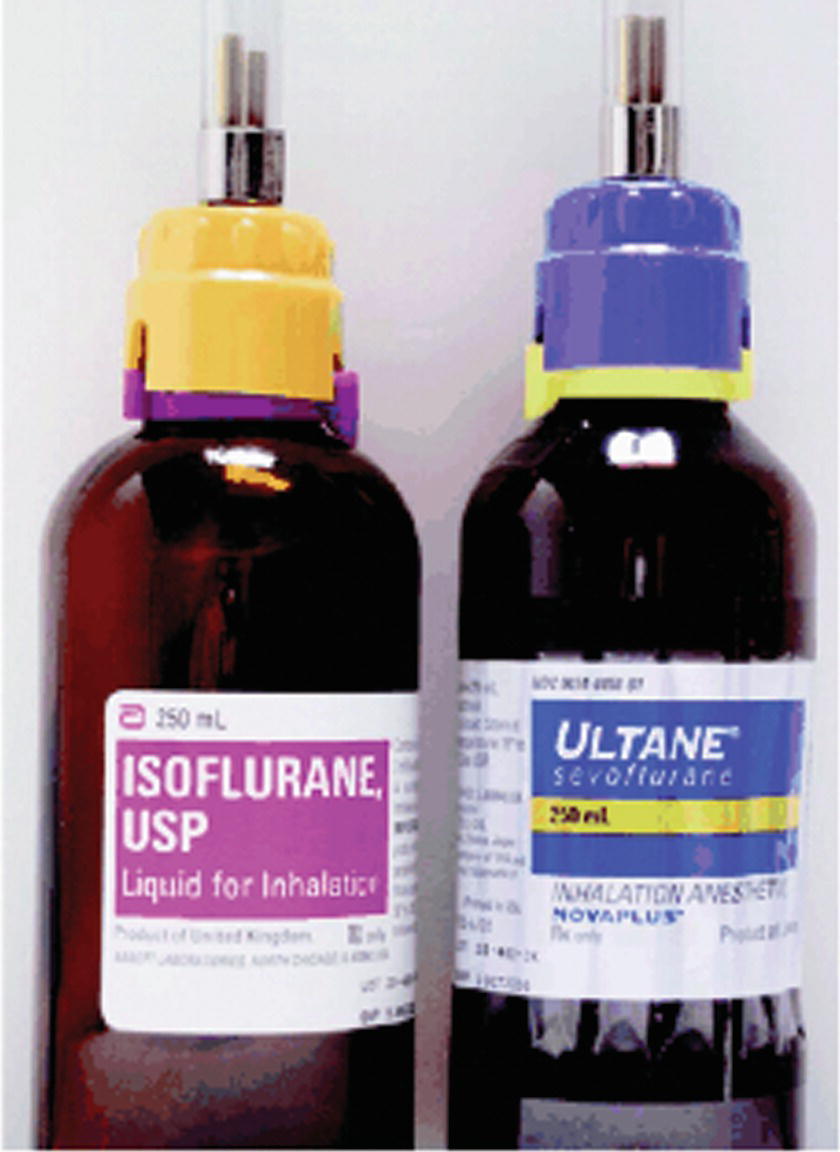
FIGURE 26.11. The yellow sevoflurane-keyed filling device is incorrectly screwed onto an isoflurane bottle and a purple isoflurane-keyed filling device is incorrectly screwed onto a sevoflurane bottle. (From Keresztury MF, et al. A surprising twist: an unusual failure of a keyed filling device specific for a volatile inhaled anesthetic. Anesth Analg. 2006;103(1):124-125.)
Lower than Expected Vapor Output
A simple and common cause of decreased vapor output is absent or low anesthetic agent levels in the vaporizer. Checking the agent level is a quick first step in troubleshooting decreased vapor output. Many modern vaporizers will sound an alarm when the agent level is low. Another cause of lower than expected output is if a vaporizer leak is present. The most common cause of a leak is a missing or loose cap on the filling port. Other sources of leaks are vaporizer misalignment (i.e., resulting from a foreign object wedged under the vaporizer), a damaged or missing O-ring in the vaporizer, or internal mechanical failure (e.g., from physical damage). A leak in the downstream circuit may also result in a lower agent level being detected by the agent monitor. If a leak is suspected, the gas sampling line can be used to “sniff” around the vaporizer and circuits to detect the source of the leak.
Low-Pressure Circuit Leak
The vaporizer is part of the low-pressure circuit, which includes the flowmeter, vaporizer, check valves (which may be internal to the vaporizer), and the common gas outlet. Historically, on older anesthesia machines, a “low-pressure” leak test could be manually performed. A collapsed suction bulb would be attached to the common fresh gas outlet, and with the vaporizers off, the bulb is confirmed to stay collapsed for more than 10 seconds. Each vaporizer is then turned on; reinflation of the bulb when the vaporizer is turned represents a “leak” within the vaporizer system, such as a missing port O-ring on the manifold, a partially open filling port, or a faulty internal check valve. With many modern anesthesia machines, the negative pressure leak check process is part of the automatic electronic checkout process; in fact, the manual attachment of a suction bulb may actually not be possible due to inaccessibility of the actual common gas outlet. Nonetheless, it is helpful in the conceptual approach to troubleshooting circuit leaks to recognize the role of the vaporizer within the low-pressure system.
Other Problems
A physically damaged vaporizer or particulate contaminants in the vaporizer may cause a multitude of nonspecific problems, and the vaporizer should be immediately set aside and sent out for appropriate repair. Regular maintenance should be performed according to the manufacturer’s guidelines and institutional protocols; in general, semiannual preventative maintenance with annual servicing would be appropriate.
Summary
Anesthetic agent vaporizers are a critical component of anesthesia machines. The laws of physics govern how liquid agents vaporize, and this determines how liquid anesthetic agents function within a vaporizer. Modern vaporizers add anesthetic vapor to the fresh gas flow to deliver a desired concentration of anesthetic gas. A thorough knowledge of the inner workings of vaporizers will help an anesthesia technician troubleshoot vaporizer problems.
Review Questions
1. Which of the following statements about filling an isoflurane vaporizer with sevoflurane are TRUE?
A) Agent monitors cannot detect different anesthetic agents.
B) The output of the vaporizer would be unchanged.
C) The output of the vaporizer would change.
D) The vaporizer would not output any vapor.
E) None of the above.
Answer: C
Sevoflurane has a much lower vapor pressure than isoflurane. Each vaporizer is calibrated for the specific vapor pressure of the agent intended for the vaporizer. If the vaporizer is filled with an agent of a different vapor pressure, the output will differ from what is set on the dial. Agent monitors are specifically designed to detect the amount of agent in the circuit and are the best way to determine if there is a discrepancy between the dial setting and the vaporizer output.
2. The majority of modern vaporizers have the following characteristics EXCEPT
A) Agent specific
B) Variable bypass
C) Temperature compensated
D) In-circuit
E) Flow over
Answer: D
Modern vaporizers are placed such that the agent is introduced into the system prior to the common gas outlet. Very old vaporizers injected agent directly into the breathing circuit (in-circuit design). All of the answers describe features of modern vaporizes.
3. Which of the following would be least likely to cause an overdose of anesthetic agent?
A) Damaged O-ring on the vaporizer-mounting bracket
B) Filled vaporizer that has been tipped over
C) Vaporizer usage in an especially hot environment
D) Failed interlock system
E) All of the above.
Answer: A
Damaged O-rings typically cause a leak with some of the gas containing anesthetic escaping (not added to the fresh gas). This will result in lower than expected vaporizer output. Vaporizers that have been tipped over can introduce the agent into the bypass circuit, causing the bypass gas to pick up the agent, resulting in a higher than expected vaporizer output. As temperature increases, the vapor pressure of liquids increases, which could increase the amount of agent added to the fresh gas. Most modern vaporizers compensate for temperature variations within a set range. A failed interlock system may allow multiple vaporizers to contribute agents to the fresh gas flow, potentially resulting in an overdose.
4. What is the volume percent of sevoflurane if the partial pressure is 19 mm Hg and atmospheric pressure is 633 mm Hg?
A) 0.3%
B) 12%
C) 3%
D) 1.2%
Answer: C
using the equation (partial pressure of gas/total ambient pressure × 100), we can determine that the percentage of sevoflurane being delivered is 3%. 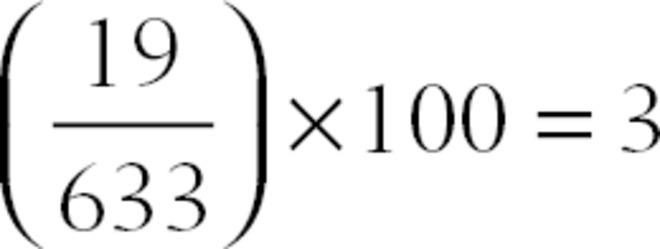 .
.
5. Your workroom has run out of sevoflurane for the day. You notice that each room has a full bottle backup available to refill its vaporizer if needed. If the providers deliver an average of 2.1% sevoflurane and fresh gas flows of 2 L/min, do you have enough agent to make it through the day?
A) Yes
B) No
Answer: A
Using the equation 3 × Fresh gas flow (L/min) × volume% = milliliters of liquid used per hour, you will use 12.6 mL of sevoflurane per hour. Assuming all of the sevoflurane bottles hold the standard 250 mL, you have enough sevoflurane on hand to last just over 19 hours. Unless your vaporizers were empty to start the day or your facility runs cases 24 hours a day nonstop, you have enough to last the day. You should, however, notify your providers that you are low on agent and order more ASAP.
6. All vaporizers will alert you to a low agent fill level.
A) True
B) False
Answer: B
Many modern vaporizers such as the Aladdin cassettes found in GE machines can alert you to low agent level because they are electronically controlled. Similarly, the Tec 6 desflurane vaporizer has an alert because it is powered. The majority of sevoflurane and isoflurane vaporizers in use do not have power for alarms and only have a sight glass to visually gauge fill level.
SUGGESTED READINGS
Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 8th ed. Philadelphia, PA: Wolters Kluwer; 2017.
Dorsch J, Dorsch S. Vaporizers in Understanding Anesthesia Equipment. 5th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Williams; 2008:121-190.
Ehrenwerth J, Eisenkraft JB, Berry JM, eds. Anesthesia Equipment: Principles and Applications. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2013.
Feldman JM, Keresztury MF, Newman AG, et al. A surprising twist: an unusual failure of a keyed filling device specific for a volatile inhaled anesthetic. Anesth Analg. 2006;103(1):124-125.