CHAPTER 38
Infusion Pumps
Introduction
Medication errors account for almost 20% of all medical injuries. Administration is the stage of the medication process most vulnerable to error, and the intravenous (IV) route of drug administration often results in the most serious outcomes of medication errors. IV infusion errors, which involve high-risk medications delivered directly into a patient’s bloodstream, have been identified as having the greatest potential for patient harm.
For the anesthesia technologist, an understanding of the function, components, and use of medication pumps is essential. Medication pumps are one of the most widely used medical technologies in health care and are commonly used in the operating room (OR), obstetric suite (OB), and postanesthesia care unit (PACU). Medication pumps are a normal part of the anesthesia care routine. Infused medications include vasopressors, antibiotics, chemotherapy, local anesthetics, sedatives, hypnotics, and muscle relaxants. Properly working infusion pumps support the “five rights” of medication safety—right medication, right dose, right time, right route, and right patient. Advances in the technology of these pumps have led to the designation of “smart pumps”; these infusion pumps have many features that have been added to increase patient safety. Recent or second-generation improvements include flow check at start of infusion, bidirectional wireless communication, log analysis software for quality improvement analysis, easy-to-read color displays, quick drug find libraries, and onboard digital help screens. Additionally, these units are smaller and have replaceable battery packs to facilitate ease of transport.
The anesthesia technologist will be called upon to deliver pumps to the OR suite or off-site location and should be able to properly set up each device used in their facility. While the infusion pump is in use. the technologist may need to troubleshoot alarms and nonfunctioning devices and be able to determine if the device needs replacement or service. Individual institutions determine the degree of involvement for the anesthesia technologist with setup and troubleshooting of pumps. Since the majority of anesthesia technologists will be involved in these tasks, anesthesia technologists should avail themselves of the opportunities for in-service training, particularly when new pumps are introduced into the department. The individual vendors are a great source of information and instruction on the use and maintenance of the medication pumps used at your facility. In addition, anesthesia technologists will be involved in the maintenance of pump units stored in the department. The anesthesia technologist must ensure that “smart pumps” are updated on a predetermined basis by the pharmacy and that they meet all standards set by governing entities. The technologist should be sure that after the pump is used, it is returned to the workroom and cleaned according to the protocol in place at the facility.
Medication Pump Safety
A major goal in the campaign to improve medication safety is to develop safer systems for the monitoring and delivery of drugs. IV medications administered through a pump must be delivered with precision due to the nature of these medications and the IV route of administration. IV administration results in more rapid onset of drug effect; therefore, harmful side effects or the effects of drug toxicity may be more severe than when the same medication is administered orally. In addition, medication pumps deliver continuous infusions of medications. Thus, any error in dosing or formulation can be compounded over time. These issues effectively reduce the safety margin when medications are administered by IV infusion.
The Joint Commission (TJC) has noted that infusion pumps were frequently involved in medication errors that lead to serious patient injury. Experts reviewing these incidents have identified several human and mechanical errors. Among the most common problems identified was the use of pumps that do not provide protection from the free flow of IV fluid/medication into the patient. In addition, problems can occur when the wrong drug concentration is administered or the wrong rate is set. To address this issue, manufacturers of medication pumps have developed a number of innovations. For example, medication “smart pumps” were introduced in 2002 by ALARIS Medical Systems, Inc. These computerized pumps for large-volume infusion and medication delivery utilize traditional infusion pump technology based on predetermined clinical guidelines. Smart pump design improves medication safety by making it harder for the clinician to inadvertently enter the wrong information when programming the pump. This class of pumps utilize drug libraries that include dosage-limiting parameters and alerts, requiring the clinician to intervene if the medication to be delivered is outside the dose recommended by the pharmacy and clinical advisory teams. Beyond the smart pump, a good system connects infusion pumps, bedside computers, and nursing station computers into a central database, which then connects to the electronic medical record (EMR). Prior to smart pumps, most hospitals did not have drug-dosing limits built into medication pumps, and drug information guidance on high and low limits for IV medications was not available at the point of care.
Other safety features of newer generation medication pumps include dosing alerts, continuously updated drug libraries, and treatment unit-specific programming. Requiring the provider to actively override alerts improves patient safety while allowing flexibility in treating unique patient situations. For example, the pump may alert the provider that the programmed medication should be administered through a central venous line. The provider can override the alert based on clinical circumstances. Linking the smart pump to a computer network allows for drug library information to automatically upload to the device as changes in practice, medication libraries, alert or dosing limits, and practice guidelines occur. Medication pumps can be configured to fit the needs of the patient according to the unit in which he or she is receiving care, such as the OR, PACU, and the adult, pediatric, and neonatal ICUs. Alert limits and drug libraries can be tailored for each specific treatment unit. When a patient is transferred to a different treatment unit, care must be taken to exchange the pump for one that is appropriate to the new treatment unit or, if possible, reset the pump for the new area. For example, when a patient is transferred to the OR from the floor, the existing medication pumps may need to be reset to OR settings to allow the anesthesia provider to access drug libraries and utilize alarm parameters that are appropriate to the OR and delivery of medications by anesthesia personnel. New technologies incorporate systems that will allow a single pump to follow the patient throughout the hospital stay; by entering the hospital department into the “smart pump” via the data screen, the pump will change the drug library and parameters particular to that department’s protocols.
An additional safety feature of modern medication pumps is the capability of continuous data recording similar to an airplane “black box.” The pump software allows the data to be retrieved for quality improvement purposes. This information can be used to review programming errors or identify treatment processes that could be improved. This information was unavailable prior to the introduction of smart pumps.
One critical error that cannot be detected by pump safety features is the attachment of the wrong medication or concentration to the pump. For example, a provider wants to administer a normal saline and an epinephrine infusion. The pump is properly programmed to deliver a normal saline infusion at 100 mL/h and the epinephrine at 0.15 μg/kg/min; however, the bag of epinephrine is inadvertently attached to the tubing going to the pump for the normal saline and the normal saline is attached to the pump for the epinephrine. This situation could lead to a life-threatening reaction in the patient due to an overdose of epinephrine. Bar coding is gaining popularity and could be used on drugs, drug bags, pumps, and patients to ensure correct matches, reducing the opportunity for error.
Types of Medication Pumps
Manufacturers produce medication pumps that meet the needs of a broad range of settings, including delivery of medications from IV bags and/or syringes. This section will give examples of several different types of medication pumps and emphasize the features that are common to that class of pump; however, a comprehensive list of all currently available medication pumps is beyond the scope of this text.
Analog Pumps
Analog pumps have a simple user interface controlled by dials. For the majority of these pumps, the only allowable control is to adjust flow rate. Some allow entry of patient weight and dose of drug to be delivered per minute. Bag infusion and syringe pump systems are no longer using this technology (e.g., Baxter Bard Infus O.R. Syringe pump). These devices are mechanical and do not have the built-in safety systems or ability to make calculations regarding drug concentration and measure of drug delivery.
Microcomputer Pumps
Microcomputer-controlled pumps contain a limited microprocessor that controls pump function and can perform calculations. They can be syringe pumps or pumps designed for medication bags. Examples include the Medfusion 4000 syringe pump and the Alaris® PC point-of-care system (Fig. 38.1). The vast majority have a liquid crystal display (LCD) and keypad. The user interface usually allows entry of drug concentration, patient weight, dose of drug to be infused or rate, and total volume to be infused. The microprocessor can perform calculations, which gives the user some flexibility in entering data to achieve a desired infusion or dose. Most of these devices allow the user to program an optional loading dose (an initial rate or amount of drug delivery) followed by a continuous infusion (maintenance dose). In addition to the above features, these devices usually have an internal memory device for storing drug libraries and data. The user can select medications from the drug library and then confirm concentration and dosing information prior to delivery. The unit will alert the user to concentrations, doses, or rates outside of preset parameters in the library. The majority of infusion pumps for bags or syringes are microcomputer-controlled pumps. In addition to inpatient use, sophisticated microcomputer controlled pumps are available for temporary home use or for implantation in the body (e.g., insulin pumps).

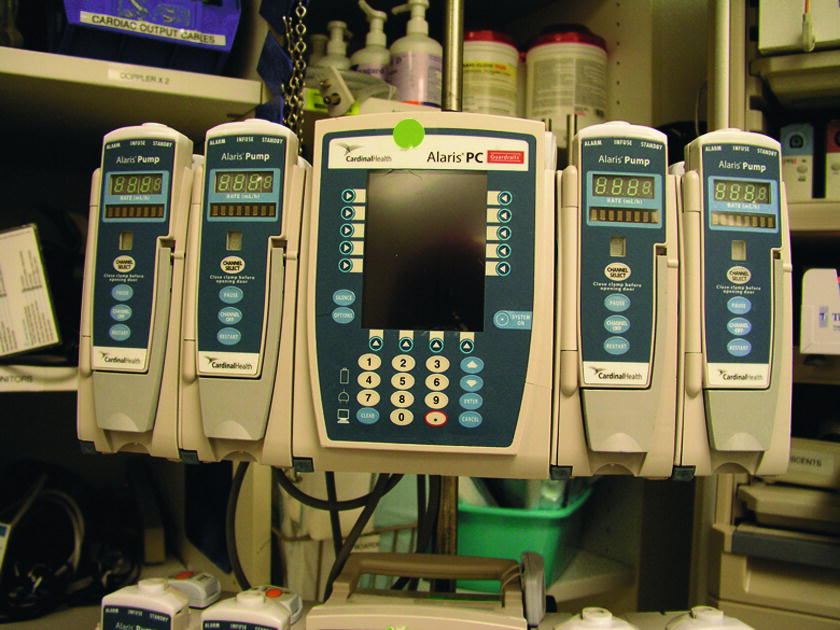
FIGURE 38.1. Microcomputer-controlled pumps.
Patient-Controlled Pumps
Patient-controlled pumps use a syringe or medication bag for the source of medication and include a device to enable the patient to control the infusion or give boluses of medication (Fig. 38.2). These devices are often used for postoperative and labor pain. Key features of these devices include the ability for the provider to set parameters that control basal infusion rates, the maximum amount and timing of bolus administrations triggered by the patient, and a “lockout” device to prevent tampering with the medication by unauthorized personnel.

FIGURE 38.2. Patient-controlled pumps.
Continuous Infusion Pumps
The desire to send patients home with a temporary continuous infusion of medication led to the development of multiple simple, low-cost pumps. Many of the early “home” pumps were simple devices with an elastic chamber that could be filled with a medication solution. These “elastomeric” pumps could then deliver a constant infusion rate that would last until the device was removed or the chamber was empty. Subsequent designs allowed adjustment of the basal flow rate and an on-demand bolus with a timed lockout (e.g., On-Q with Select-A-Flow). These pumps are frequently used to deliver a continuous infusion of local anesthetic or a custom combination of local anesthetic and narcotic, for postoperative pain control. The infusion catheter can be placed directly in a wound or joint space or in proximity to peripheral nerves that supply the painful region.
General Operating Principles for Inpatient Microcomputer Pumps
Before using a pump, the anesthesia technologist will first verify that it has been charged. Since these pumps all have a battery backup system, they should be stored in an area where they can remain plugged in and ready for use. There should be a battery or charge light indicator on the front of the pump. If the pump has not been plugged in, turn on the device and determine battery life, before taking it into the OR. In addition to the pump, operation of the pump may require special syringes (20, 30, or 60 mL), infusion tubing (pump tubing or microbore tubing), or smaller IV bags in which medication can be added (50 or 100 mL normal saline or D5W). Some medications require special nonabsorbent infusion tubing (e.g., nitroglycerine). If multiple medications are to be administered, a stopcock or manifold assembly may be helpful. In the OR, the technologist should ensure that the pump is attached to a designated IV pole, taking care that the IV pole is balanced and will not easily tip over. Plug the pump into a wall outlet. If an outlet is not available, plug the unit into a hospital grade multiport outlet extension. These are often attached to the side of the IV pole. Take care to position power cords out of the way so that OR personnel do not trip on the cords. In addition, position the pump or IV pole on the side of the patient where the anesthesia provider will attach the infusion.
Depending on the facility, the anesthesia technologist may assist the anesthesia provider in setting up the pump. Consult with the provider to determine which drug(s) or fluids will be administered. The operator can enter the patient information required by the specific pump (e.g., patient name or initials, patient weight, medical record number). Most pumps will have a front panel with hard or soft keys to navigate through menus. In addition, a keypad can be used to enter numerical data. For fluid administration, the operator will be required to enter an infusion rate and a volume to be infused. For drug administration, the operator can select the drug from the drug library. If a drug is commonly administered in different concentrations, most drug libraries include choices for selecting the concentration for the drug. Once a drug and a concentration are selected, the operator can select the infusion rate in milliliters per hour or the dosage (e.g., μg/kg/min). The medication infusion tubing should be flushed (primed) and clamped off. The infusion tubing can then be inserted into the pump. In most pumps, insertion of manufacturer-specific infusion tubing is accomplished either by opening a door through which the tubing is threaded or by installation of a “cassette.” Syringe pumps require attaching the syringe and plunger into a cradle. Some manufacturers require that tubing is flushed completely before starting the pump. Many will alarm if air or bubbles are detected as they pass through the pump. Other pumps have a priming function that will allow the operator to flush the tubing after it is inserted into the pump. Do not forget to open the roller clamp or other device that is occluding the tubing before flushing or beginning an infusion. Many pumps will detect an occlusion (high pressure) in the line and sound an alarm (e.g., clamp closed, kink in the line, closed stopcock). Once the infusion tubing has been inserted into the pump and the line primed, the anesthesia technologist must verify that all settings are correct. In addition, if multiple pumps are in use, the technologist should ensure that the correct medication is attached to the correct pump. The tubing can then be connected to the patient. Medication infusions should be attached to a port in the IV tubing as close to the patient as possible. This will allow changes in medication dose to reach the patient quickly. It is good practice to verify that the IV line into which the infusion tubing is attached is not infiltrated and runs well. Many medication infusions will require a “carrier” infusion to be flowing at a minimum rate, usually at least 75 mL/h. This is because the medication infusion itself may only run at a few milliliters per minute. The carrier infusion will flush (carry) the medication through the IV tubing to the patient quickly. It is important to make sure that all medications that are infused through the same IV line are compatible with each other and the carrier fluid. If the pump has been correctly set up and verified by the provider, the technologist will place the pump in standby mode. If the medication is needed immediately, the provider will press start to begin the infusion. Before starting the infusion, make sure all clamps or other devices are not obstructing flow to the patient. A common safety practice is to leave the infusion line clamped when not in use. This is because if the tubing is attached to the patient when the tubing is removed from the pump, it may be possible for infusion to be “wide open” and administer a dangerous amount of medication into the patient. Most modern pumps occlude the infusion tubing with a special device when the tubing is removed from the pump to prevent this occurrence. The flow restriction must be opened manually to allow free flow of fluid through the infusion tubing.
Troubleshooting
Most pumps have alarms for different conditions and will display an error message on the screen. Common problems that can cause alarms during use include the following:
- Air in the tubing (air detection alarm): Make sure the medication bag is not empty. Make sure the drip chamber is appropriately filled (fill to the appropriate level if necessary). Make sure no air or bubbles are in the line. If air or bubbles are found, a clamp should be applied to prevent flow into the patient, the infusion tubing removed from the pump, and the air or bubbles removed from the tubing by aspirating from a distal port. It may even be necessary to detach the infusion tubing from the patient and reflush the tubing.
- High pressure in the line: Check for valves, clamps, or stopcocks that are closed, kinked tubing, infiltrated IV, or improperly installed infusion tubing in the pump.
- Medication dosing outside of usual parameters: Verify the settings with the anesthesia provider. Most pumps allow the provider to override the warning and continue the infusion.
- Low battery: Make sure the pump is plugged in.
Summary
Medication infusion pumps are commonplace in hospitals and outpatient centers alike. The anesthesia technologist plays an important role in the setup, operation, troubleshooting, and maintenance of these devices. Anesthesia technologists will also be responsible for making sure device maintenance is in keeping with regulatory requirements. Due to the complexity of these devices and the potential for severe complications when the wrong medication or the wrong dose is administered by IV infusion, anesthesia technologists should be thoroughly familiar with pump operation and troubleshooting. Technology is quickly advancing in the type and function of pumps; this complexity has also led to increasing regulatory oversight. Anesthesia technologists must attend in-services offered by the manufacturers and facility educators to remain compliant.
Review Questions
1. Medications NOT commonly used in drug pumps include
A) Vasopressors
B) Heparin
C) Antiemetics
D) Muscle relaxants
E) All of the above
Answer: C
Most drugs with a short duration of action or whose action the provider would like to have come on and off rapidly are ideal to be given via infusion. Antiemetics are typically long-acting drugs (since preventing nausea is always a long term goal), and thus, they are not typically given via IV infusion.
2. What are the five rights of medication safety?
A) Right medication, right dose, right time, right patient, right provider
B) Right medication, right time, right concentration, right patient, right dose
C) Right medication, right dose, right time, right route, right patient
D) Right medication, right time, right patient, right dose, right program
Answer: C
Ensuring that all of these factors are correct is crucial to preventing critical and potentially deadly medication errors.
3. What differentiates a smart pump from any other IV pump?
A) Drug libraries that include dosage-limiting parameters and alerts
B) Connection to a central database and EMR
C) Medication sensors to prevent errors
D) Clinician overrides to bypass safety systems
E) All of the above
Answer: A
There is not a pump on the market that can sense the medication being run through it. The other options can be nice features of an advanced smart pump, but the bare minimum a pump needs to be considered a smart pump is drug libraries and dosing parameters with limits.
4. Medication pumps are configured generically to fit the average needs of the patient in any area of the hospital.
A) True
B) False
Answer: A
Most pumps are configured with pharmacy-recommended dosages and concentrations based on generic populations.
5. If a pump sounds an alarm, the technologist should check all of the following EXCEPT
A) The data screen for message alerts
B) Whether the medication is appropriate for this patient
C) Air in the tubing
D) Whether a roll valve or clamp on the IV tubing has not been opened
E) Whether the tubing is properly installed
Answer: B
The anesthesia technologist may check any of the possible reasons for alarm other than if the medication is appropriate for the patient. The anesthesia provider is responsible for making sure that is the case. The machine is incapable of determining if the correct medication is being administered and would not alarm for that reason. If the anesthesia technologist has any reason to suspect that the wrong medication is being infused, the technologist should bring it to the anesthesia provider’s attention immediately.
6. Using the keypad, the following data may NOT be entered into the drug pump.
A) Patient’s weight
B) Volume to be infused
C) Patient’s gender
D) Desired dosage
Answer: C
The patient’s gender is not needed in order to utilize any drug pumps.
7. The leading cause of patient harm is medication errors.
A) True
B) False
Answer: A
Approximately 20% of medical errors are caused by medication errors, making it the single leading cause of harm to patients.
8. Medication pumps are one of the most widely used technologies in the health care environment.
A) True
B) False
Answer: A
Medication pumps are a normal part of the anesthesia care routine and one of the most widely used medical technologies in health care; they are commonly used in the OR, OB, and PACU.
SUGGESTED READINGS
Eskew JA, Jacobi J, Buss W, et al. Using innovative technologies to set new safety standards for the infusion of intravenous medications. Hosp Pharm. 2002;37:1179-1189.
FDA Infusion Pump Improvement Initiative. Available from: FDA.Gov
Hatcher I, Sullivan M, Hutchinson J, et al. An intravenous medication safety system: preventing high-risk medication errors at the point of care. J Nurs Admin. 2004;34:437-439.
Hicks RW, Cousins DD, Williams RL. Summary of Information Submitted to MEDMARX in the Year 2002. The Quest for Quality. Rockville, MD: USP Center for the Advancement of Patient Safety; 2003.
Joint Commission. National patient safety goals. Available from: www.jointcommission.org
Weinger MB, Wiklund MR, Gardner-Bonneau DJ, eds. Handbook of Human Factors in Medical Device Design. Boca Raton, FL: CRC Press; 2010.
Williams C, Maddox RR. Implementation of an IV medication safety system. Am J Health Syst Pharm. 2005;62:530-536.
Wilson K, Sullivan M. Preventing medication errors with smart infusion technology. Am J Health Syst Pharm. 2004;61:177-183.