CHAPTER 52
MRI Safety
Introduction
Magnetic resonance imaging (MRI) has expanded dramatically over the past decade. Unlike CT scan or x-ray, MRI does not employ ionizing radiation; its images are produced by a fixed powerful magnetic field and radiofrequency (RF) pulses, which elicit signals from the hydrogen atoms found within human tissues. Since MRI does not use ionizing radiation to produce an image, it is often one of the safest diagnostic tools available for a patient. The successful execution of MRI, however, requires that the patient remain completely still, often for long periods of time. To achieve this, some patients will require either deep sedation or general anesthesia. Thus, it is essential for you as an anesthesia technician to be familiar with the unique MRI environment, which may be isolated from the operating room and which has unique hazards for providers and patients generated by its powerful magnetic fields.
Patients needing anesthesia services for MRI include children, uncooperative or claustrophobic adults, critically ill individuals, and patients with comorbid illnesses requiring either sedation for stillness or specialized monitoring for safety during the long scan. Though the magnetic field associated with MRI has not been shown to produce any significant harmful physiologic effects, there are a number of potential hazards, which are unique to the MRI environment that impact both patients and health care personnel.
The MRI Scanner Magnet
A powerful magnet, the core component of the MRI scanner, creates a strong magnetic field. When this reaches the body, it excites hydrogen atoms within the tissues to be examined; each tissue’s hydrogen atoms emit slightly different energies. The energy emitted by the hydrogen atom as it responds to the magnetic field is sensed by the MRI equipment and is ultimately transformed into high-resolution images. These images are often superior to other diagnostic imaging modalities (e.g., simple x-ray, CT scanning, ultrasound), because a 3-D image that shows each of the different tissues of the body can be seen.
During installation of an MRI system, the magnet (a cylinder that is filled with cryogens) is positioned so that it surrounds the patient who lies within the bore of the scanner during an MRI exam. An electric current is then applied to the magnet, which ramps up the magnetic field to the designated strength. Once this process is completed, the magnet remains “on”—even when the MRI scanner is not in use. An MRI scanner cannot be turned off. This unique feature of MRI equipment requires that all personnel be mindful of the risks imposed by the powerful magnetic field, even when the scanner is unoccupied. In extreme emergencies, the magnetic field can be turned off by a procedure called “quenching.” However, this emergency maneuver should only be initiated after careful consideration and in a controlled manner. Quenching can cause potentially lethal harm to personnel. It can also cause significant damage to the MRI scanner magnet and to surrounding equipment and the MRI scan room. Even if no harm comes to the magnet, the room, or personnel, costly cryogen gases are wasted into the atmosphere.
The strength of a magnetic field is measured in Tesla units. To understand the order of magnitude of a Tesla (T), the magnetic field generated by the earth is 1/20,000 of a Tesla = 0.00005 T. Another unit of measure of magnetic field strength is the gauss (G), where 10,000 G = 1 T. Thus, the magnetic field strength of the earth is also equivalent to 0.5 G. Typical MRI scanning equipment utilizes magnets with field strengths ranging between 1.5 and 3 T, or 30,000 and 60,000 times the magnetic field generated by the Earth.
Every MRI suite has a designated 5 G line (see Fig. 52.1). This is the point at which any ferrous metallic object can become a projectile and be drawn into the bore of the magnet. In addition, electronic devices that are not MRI approved will also interact with the magnetic field within the 5 G line and malfunction. Ordinarily, one can expect that the 5 G line will be encountered within the room housing the MRI scanner, but in some instances, it can protrude outside of this room. The closer you approach the magnet, the higher the intensity of the magnetic field.
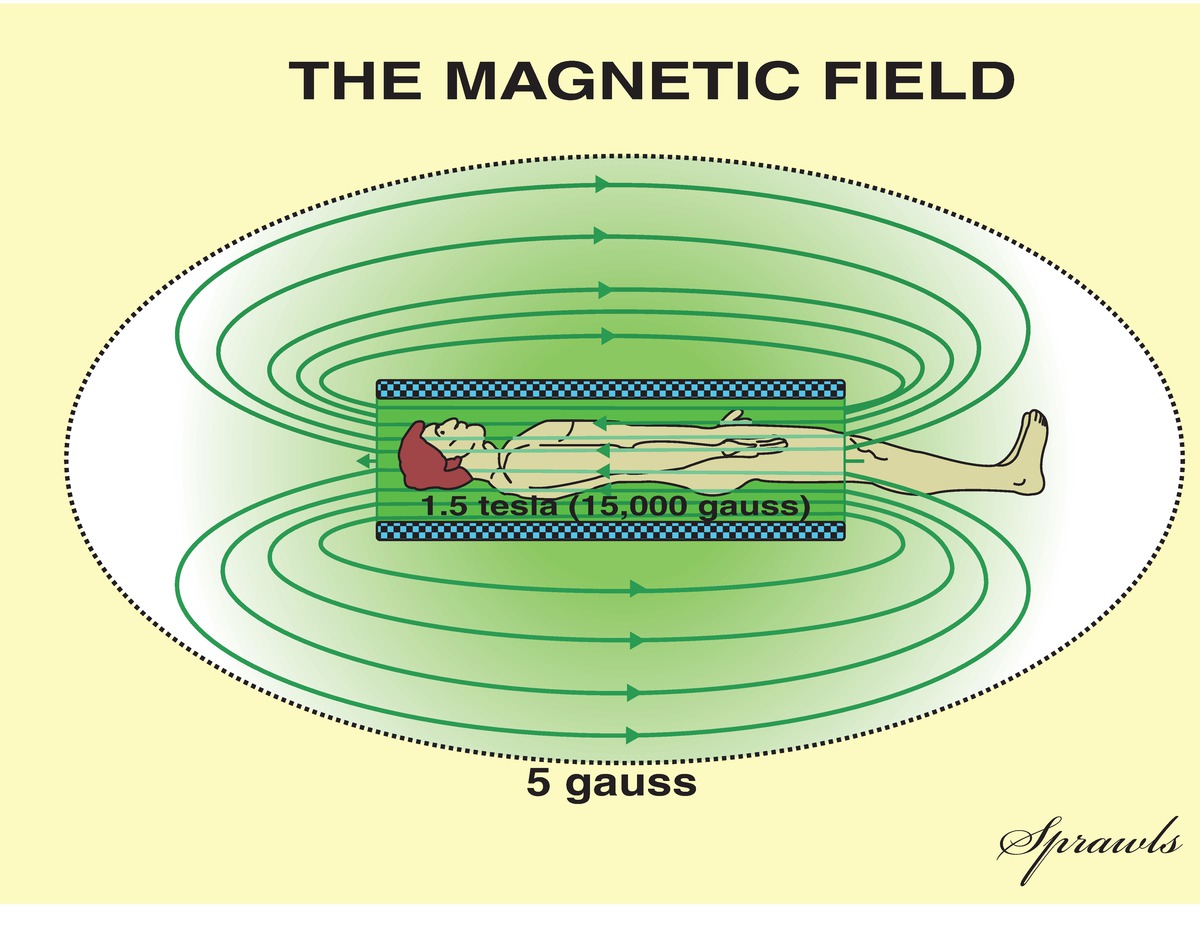
FIGURE 52.1. Diagram demonstrating the attenuation of magnetic field strength as one moves away from the MRI scanner magnet. Once inside the 5 gauss line, ferrous objects can become projectiles and electronic equipment will malfunction. (Redrawn from Sprawls, P. Magnetic Resonance Imaging: Principles, Methods, and Techniques. 1st ed. Madison, WI: Medical Physics Publishing; 2000. Available from http://www.sprawls.org/mripmt/MRI02/index.html#The_Magnetic_Field. Accessed April 14, 2017. Reprinted by permission of Medical Physics Publishing.)
The MRI Suite
The layout of an MRI environment is comprised of four zones based upon (1) type of activity; (2) approved objects and equipment; and (3) individuals who are permitted access to the area (see Fig. 52.2).
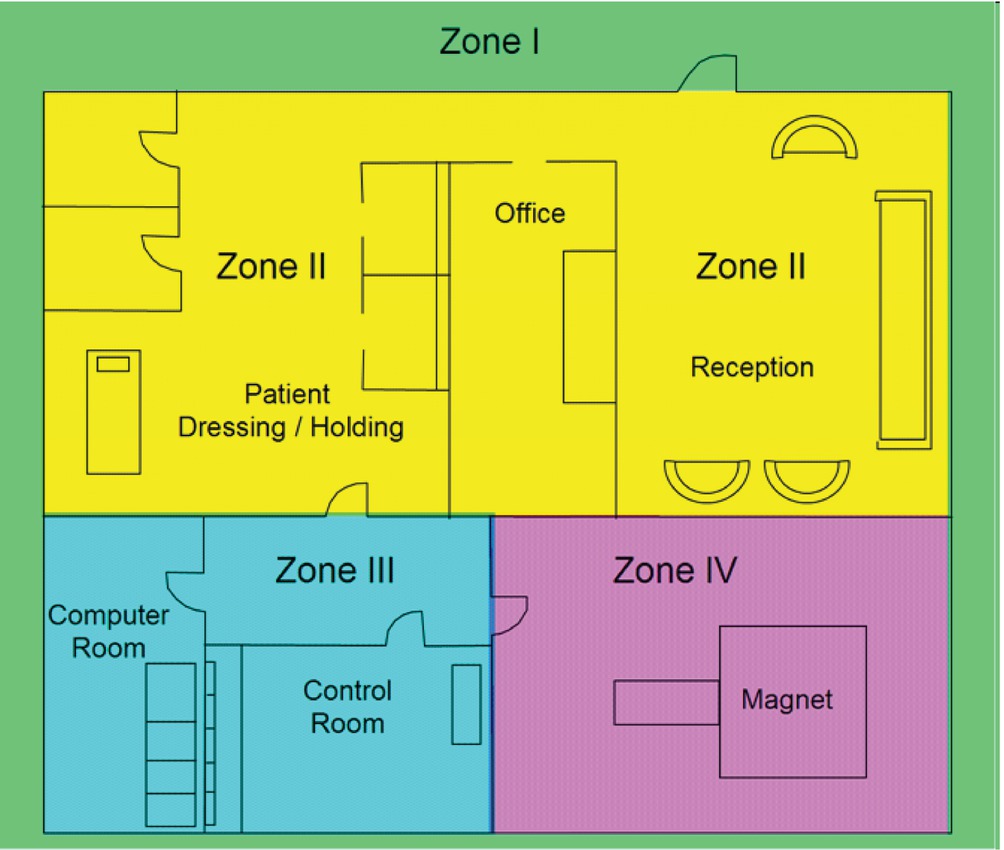
FIGURE 52.2. Schematic layout of an MRI suite. (From Bushberg JT, Seibert JA, Leidholdt EM. Essential Physics of Medical Imaging. Philadelphia, PA: Wolters Kluwer Health; 2011:498, with permission.)
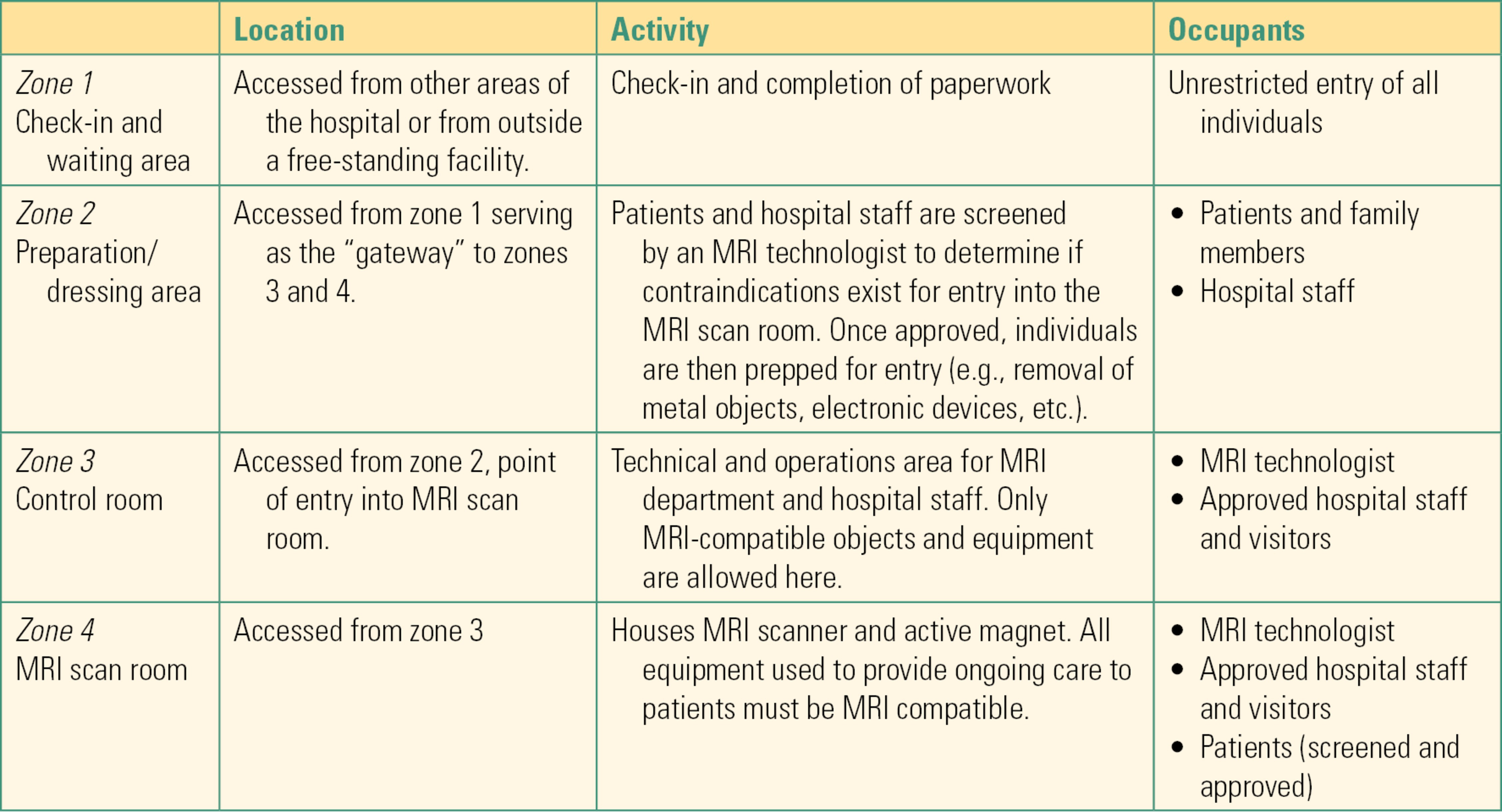
As one moves through these zones, restrictions become more rigorous in order to mitigate the risks posed by the powerful magnetic field emanating from the MRI scanner. A description of these zones is summarized in Table 52.1. Most institutions require the completion of specialized training, which covers MRI safety before they are allowed to work in an MRI environment. This includes all staff members who will need access to a patient undergoing an MRI exam, including anesthesia providers and technicians.
Table 52.1. Zones of the MRI area
Potential Hazards in the MRI Suite and Their Anesthetic Implications
Ferromagnetic objects that are commonplace in the hospital environment as well as in possession by hospital personnel (e.g., scissors, pens, hemostats, etc.) are one of the greatest hazards in the MRI suite. These objects have become projectile (drawn into the bore of the MRI magnet), some of which have resulted in significant patient injury as well as death. Thus, patients and all staff members must remove metallic objects before entering zones 3 and 4. Patients and staff members with implanted devices or objects must also be identified and carefully screened by MRI department personnel in order to prevent serious and possibly catastrophic injury that may result from exposure to the powerful magnetic field. Implants that are contraindicated include automated implanted cardiac devices (AICDs) such as pacemakers and defibrillators; heart loop recorders; insulin pumps; nerve stimulators; intracranial aneurysm clips and coils; deep brain stimulators; and certain types of mechanical heart valves, ocular implants, and intrauterine devices (IUDs). Hearing aids, glasses, and dentures that contain a magnet to remain in place must also be removed.
Finally, it is important to note that a strong magnetic field may erase data stored on the magnetic strips found on credit/debit cards and also on devices that are used for electronic storage of data (e.g., flash drives). Exposure to a strong magnetic field may also affect the functionality of media that employ an RF identification chip such as an ID badge. Electronic devices commonly utilized by staff such as smartphones, pagers, and watches may malfunction and be permanently damaged if exposed to a strong magnetic field. All these materials and devices must be removed and secured prior to entering high-risk areas.
Many components of “routine” monitoring equipment such as monitoring cables, EKG electrodes, and oxygen saturation probes can become injurious to the patient when exposed to RF currents as they may become dangerously hot when subjected to strong magnetic fields. Only MRI-compatible cables, electrodes, and patient monitoring equipment should be used to avoid thermal injuries. All unapproved cables and wires must be removed from the patient. A varying magnetic field will also produce a current in any conductive material that is touching the patient or forms a loop: even IV tubing should remain uncoiled and avoiding direct contact with the patient’s skin. In addition, proper padding should be placed between any areas of skin-to-skin contact. RF heating can occur in patients who are not properly padded and isolated from the interior of the scanner. In order to reduce the risk of RF heating, patients should be positioned within the scanner so that they do not touch its inside walls and maintain at least a 2 cm distance from the bore of the magnet. Patients who are awake will complain of heating of any cable, IV tubing, or contact with the scanner; anesthetized patients will not, and additional caution must be taken.
The varying magnetic field and delivery of RF pulses during an MRI scan produce very loud and repetitive sounds. The maximum noise level inside an MRI scanner during an exam that is considered “safe” by the FDA is 105 dBA (listening to a rock band indoors is rated at 110 dBA). Exposure to noise levels at or above 85 dB is known to cause hearing loss, particularly if that exposure is prolonged. In order to protect patients from noise-induced hearing loss, hearing protection (usually MRI-compatible ear plugs) must always be provided prior to the start of an MRI scan—this includes anesthetized or sedated patients. Any staff who remains in the scan room during an exam must also wear hearing protection.
Electronic equipment commonly utilized during anesthesia care must be shielded from RF pulses in order to prevent interference and assure proper functioning. Thus, anesthesia machines, electronic monitoring systems, gas analyzers, and infusion pumps, all common fixtures in anesthetizing sites (operating rooms, interventional radiology, GI labs, etc.) must be modified in order to be compatible with the MRI environment. Some medical equipment, such as an anesthesia gas machine, may have a gauss meter that will activate an alarm if subjected to a magnetic field above its approved gauss threshold. Thus, it is important to identify the 5 G line and to position equipment whenever possible in a location with the lowest gauss rating to ensure its proper functioning. Available MRI-compatible devices often have significant differences in configuration and user interface when compared to standard equipment. Anesthesia providers and technicians must receive formal orientation in the use and care of this specialized equipment, and maintain sufficient proficiency to safeguard patient safety.
The American Society for Testing and Materials (ASTM) has standardized the labeling process, which identifies items suitable for use in an MRI environment (see Fig. 52.3). Anesthesia providers and technicians should familiarize themselves with this classification system so that they can readily identify approved and unapproved equipment. An MRI technologist should be consulted if an item is unlabeled or if there is any question surrounding the safety of its use before bringing it into zone 3 or zone 4. In order to enable the use of an anesthesia cart beyond zone 2, it must be constructed of materials approved for use in an MRI environment. Best practice is that even a cart designated to zone 2 be entirely MRI compatible; anesthesia carts are often requested in emergencies, when MR safety may not be the primary concern of the anesthesia team. They are among the many large pieces of equipment that have been pulled into the bore of machines. All items contained within the cart must also meet this standard; you can easily imagine a laryngoscope blade or other piece of equipment brought urgently into zone 4 in an emergency. Thus, the anesthesia technician must ensure that “routine” items that are potentially ferromagnetic such as laryngoscope handles and blades are removed from the anesthesia cart and replaced by their MRI-compatible counterparts. Failure to do so could result in what would normally be considered an innocuous item now converted into a dangerous projectile if it were to be drawn into the bore of the MRI scanner. The best practice is to designate an anesthesia cart, anesthesia supplies, and resuscitation equipment for use only in an MRI environment and have it maintained by anesthesia department staff who have completed training for MRI safety.

FIGURE 52.3. ASTM Standard F2503-05 recommended MR safety labeling. A: On the left, MR safe materials that pose no known hazard in MRI environments. B: In the middle, MR unsafe materials which are known to pose hazards in MRI environments and should not be used beyond zone 2. C: On the right, MR conditional items which MAY pose a hazard in an MRI environment—consultation with the MRI technologist is required. (From Bushberg JT, Seibert JA, Leidholdt EM. Essential Physics of Medical Imaging. Philadelphia, PA: Wolters Kluwer Health; 2011:495, with permission.)
Medical gas cylinders are normally composed of ferromagnetic material. Incidents where they have become projectile in MRI environments with resulting patient injury and/or damage to MRI equipment have been documented. Only MRI-approved medical gas cylinders and flowmeters can be used in zone 3 or zone 4. If these are unavailable, then alternative plans must be made to tether a medical gas supply into these patient care areas.
Local MRI personnel receive exhaustive training in MRI safety. An important part of their role is supervision of all staff and patients entering zone 4 to ensure that no ferromagnetic projectile approaches the magnet. Anesthesia personnel, including you, introduce hazards into this environment. You are a visitor, and many of the tools of your work are ferromagnetic: pagers, badge clips and keys, scissors, gas tanks, wrenches, anesthesia machines, pumps, monitor cables; the list is long. The MR technologist is not suspicious of you personally or because of your field: it is their job. An MRI magnet is like a gun. A bullet fires out of a gun, driven by an explosion of gunpowder. In the MR environment, the “bullet” (any ferromagnetic object) is pulled into the magnet, driven by a force 30,000 times as strong as the magnetic field of the earth. The force becomes stronger the closer to the magnet it is. It is everyone’s responsibility to prevent ferromagnetic objects from entering zone IV, where they can be pulled into the magnet. If an object is pulled into the magnet, the object may damage the magnet; staff (including you) can be injured even during room setup; if a patient is present when a ferromagnetic object is pulled toward the magnet, they can be injured or killed.
Summary
Providing anesthesia care in an out-of-OR setting brings additional concerns that are not encountered in the operating room. These issues can adversely impact workflow and patient safety. MRI has evolved into an important diagnostic modality spanning multiple medical specialties such as neurosurgery/neurology, orthopedics, oncology, and interventional radiology. A significant proportion of patients who must undergo an MRI exam will require anesthesia services. Managing these patients presents additional challenges, many of which are unique among out-of-OR environments. This is particularly true in the provision of equipment and the interaction of equipment with anesthesia practice. With a full understanding of what it means for all equipment to be “MRI compatible,” the anesthesia technician can play a central role in safe MRI anesthesia practice.
Anesthesia providers and technicians must adapt their practice within the MRI environment in order to safeguard patient safety. Some important considerations include the following:
- Acquisition of sufficient knowledge involving MRI technology and safety
- The need for specialized MRI-compatible anesthesia and patient monitoring equipment
- Altered procedures for induction of anesthesia that often will occur in a remote location (e.g., zone 2 or outside of the MRI suite), before moving a patient to the MRI scan room (zone 4)
- Unique patient positioning requirements within the MRI scanner which may result in the patient not being easily accessible to the anesthesia provider
- Planning for safe emergence from anesthesia which often requires moving the patient out of zone 4 while they remain anesthetized and monitored
- Transport of patients after emergence of anesthesia (or while they are still sedated) to a remote location such as the PACU or other specialized unit for the recovery of patients who have received sedation or anesthesia
- Development of sound communication skills in order to enhance working relationships between MRI department staff members who may be unfamiliar caring for anesthetized patients and anesthesia staff who may be unfamiliar working in the hazardous MRI environment.
The anesthesia provider needs your support in this challenging environment, and the technician plays an essential role in the anesthesia care of patients in the MRI suite. Your contribution supports them with the optimization of workflow and assists in protecting the patient from the hazards associated with MRI.
Review Questions
1. Which of the following poses the greatest hazard to both patients and staff in the MRI suite?
A) Ionizing radiation
B) A strong magnetic field
C) Nonferromagnetic materials which can become projectiles
D) Loud noise levels
Answer: B
Ionizing radiation is not employed in MRI scanning. The strong magnetic field required in MRI can cause ferromagnetic objects to become projectile and be drawn into the bore of the MRI scanner, resulting in injury. Nonferromagnetic materials such as plastic and aluminum are compatible with MRI safety. Loud noises are a hazard to hearing but are not as potentially hazardous to patients with the simple use of earplugs. The strong magnetic field is a significant risk as it can cause ferromagnetic devices to become projectiles and can cause electronic equipment to malfunction. The magnetic field does not cause direct patient injury.
2. Why is it important to be able to identify the 5 gauss (G) line?
A) Once inside the 5 G line, ferrous metallic objects can become projectiles.
B) All non-MRI compatible electronic equipment should be placed within this boundary.
C) The 5 G line demarcates the outer boundary of zone 4.
D) It represents the highest level of the magnetic field and is located closest to the MRI magnet.
Answer: A
The 5 G line represents the point in which any ferrous metallic object can become a projectile. Electronic devices that are not MRI approved that are placed within the 5 G line will malfunction. Though in general the 5 G line is encountered within the MRI scan room (zone 4), it can protrude outside of this room into the MRI control room (zone 3). As one moves away from the bore of the MRI scanner, the strength of the magnetic field will diminish—the 5 G line being located at some distance away from it, dependent upon the strength of the MRI magnet. For this reason, all MRI-approved electronic devices that must be located within the 5 G line should be placed as close to it as possible.
3. Screening and preparation of patients and staff for entry into the MRI scanner is done in which zone of the MRI suite?
A) Zone 1
B) Zone 2
C) Zone 3
D) Zone 4
Answer: B
Entry into zone 1 is unrestricted. Entry into zone 3 and zone 4 should only occur after one is screened by an MRI technologist to identify any contraindications for exposure to a strong magnetic field and removal of all non-MRI approved objects and devices.
4. Which of the following actions is the best course of action if the anesthesia technician is considering whether to bring a piece of equipment bearing the label seen below into the MRI scan room?

A) It is permissible to bring the item into the MRI scan room as there are no contraindications for using the item in zone 3 or zone 4.
B) The item is not MRI compatible; therefore, it should not be brought into the scan room.
C) Consult with an MRI technologist to determine if it may be brought into the MRI scan room.
D) Apply a magnet to the object to determine if it has ferromagnetic properties.
Answer: C
This ASTM standard label is placed on an MR “conditional” item and indicates that the item may pose no hazard only if it is used within a specified MR environment. The MRI technologist must be consulted to determine if these requirements are met prior to bringing the item into zone 3 or zone 4.
5. Which of the following statements is correct?
A) The anesthesia gas machine which is used in the out-of-OR environment should also be appropriate for use in the MRI scan room.
B) Standard portable infusion pumps can be used in zone 4 of the MRI suite as long as they are outside the 5 G line.
C) Standard patient monitoring systems may be used in the MRI scan room as long as they are firmly fixed to the MRI-compatible anesthesia machine and as close to the 5 G line as possible.
D) The anesthesia cart used in an MRI suite must be constructed from MRI-compatible materials and must contain only MRI-approved supplies and anesthesia equipment.
Answer: D
The anesthesia machine, all infusion pumps (including syringe pumps), and patient monitors used during MRI must be shielded from RF pulses and designed to function when exposed to strong magnetic fields. They have an MRI-specific design. The level of approved magnetic field strength, if one exists, should be identified for each piece of equipment; it should be placed as close to the 5 G line as possible to avoid exceeding the approved threshold. The anesthesia cart should be MRI compatible, designated for use only in an MRI environment, and stocked only with MRI-compatible anesthesia supplies and resuscitation equipment.
6. Which of the following statements is true?
A) The anesthesia provider cannot stay in the scan room because it will damage their hearing.
B) Standard IV tubing can be used in the MRI suite as long as it is checked for patency before the scan is started.
C) A standard anesthesia cart can be used in zone 2 of the MRI suite.
D) Standard anesthesia monitors can be used in zone 2 of the MRI suite.
Answer: D
IV tubing must be checked to ensure that no loops are present which can heat up due to magnetic fields and that tubing is not in direct contact with patient skin. ASA standards permit the anesthesia provider to leave the MRI scan room provided that visual contact with the patient is maintained along with all other standard monitors. However, the anesthesia provider may choose to remain in the scan room with hearing protection. Zone 2 of the MRI suite is not an area where RF interference with pumps or monitors is likely or where ferromagnetic projectiles pose a hazard. However, the anesthesia cart is mobile as are its components, and use of ferromagnetic materials in this area is high risk and not considered best practice. Monitors that do not travel into zone 4 with the patient can be used outside the 5 G line.
SUGGESTED READINGS
Bell C. Anesthesia in the MRI Suite. Anesthesia Patient Safety Foundation (APSF); September 22, 2010. Available from: www.apsf.org/resources_safety_suite.php. Accessed February 28, 2014.
Bushberg JT, Seibert JA. Essential Physics of Medical Imaging. 3rd ed. Philadelphia, PA: Wolters Kluwer Health.
Chaljub GKL. Projectile cylinder accidents resulting from the presence of ferromagnetic nitrous oxide or oxygen tanks in the MR suite. Am J Roentgenol. 2001;177(1): 27-30.
Gilk T, Latino R. MRI Safety 10 Years Later. 2009. Available on February 7, 2015, from PSQH: Patient Safety & Quality Healthcare: http://psqh.com/mri-safety-10-years-later
Gooden C. Anesthesia for magnetic resonance imaging. Curr Opin Anesthesiol. 2004;17:339-342.
Kanal E, Barkovich J, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501-530.
Veenith T, Coles J. Anaesthesia for magnetic resonance imaging and positron emission tomography. Curr Opin Anesthesiol. 2011;24:451-458.