CHAPTER 60
Malignant Hyperthermia
Introduction
Malignant hyperthermia (MH) is an inherited disorder that is manifested by exposure to potent volatile anesthetic gases and succinylcholine. The name was derived from early observations of patients who developed a rapid rise in body temperature during anesthesia. Previous estimates of patient mortality associated with MH approached 80%. Fortunately, over the past half century, the mortality from MH has declined to less than 5%. The challenge for anesthesia providers and the anesthesia team has been to recognize MH in its earliest stages and initiate treatment promptly. Any delays in diagnosis or treatment have been shown to increase the risk of patient injury or death. The anesthesia technician plays a critical role in preparing for and implementing the resource-intensive, rapid treatment required for this emergency unique to anesthesia.
Epidemiology
Cases of MH have been reported from almost every country and within most ethnic groups. Based on genetic studies, the prevalence of MH has been found to be as high as 1 in 500 individuals. This means that 1 in every 500 individuals may carry the genetic mutation that predisposes to MH. Patients with this condition are referred to as MH susceptible (MHS). However, the reported incidence of MH events is much lower. Some studies report the incidence of MH at 1 in 5,000 anesthetics, while others estimate the incidence at 1 in 100,000 surgical procedures, with a mortality of approximately 12%, although mortality rates may be higher in a subset of patients experiencing an MH event outside the hospital. Associated risk factors that predict increased morbidity and mortality include patients with a muscular build and failure to detect the condition in a timely fashion. Based upon data from the North American Malignant Hyperthermia Registry and the Malignant Hyperthermia Association of the United States (MHAUS), about 600 cases of MH occur each year in the United States.
Pathophysiology
The underlying cause for MH susceptibility focuses on a protein channel in the muscle cell, the ryanodine receptor, that regulates intracellular calcium release into the muscle cell cytoplasm. The release of calcium leads to muscle contraction; calcium is then taken up from the muscle cell cytoplasm and the muscle relaxes. The process of contraction and relaxation of myofibrils consumes energy and generates heat. Under ordinary circumstances, the generation of heat is not significant.
In an MHS patient, the ryanodine receptor is abnormal due to a RYR1gene defect located on chromosome 19q13.1. There are 35 known causal mutations for MH with many other genetic variants of unknown significance. The resulting defective calcium channel allows for unregulated release of calcium, and the cellular processes involved in reuptake of calcium are quickly overwhelmed. The presence of excess calcium constantly stimulates muscle activity. This hypermetabolic state generates heat and consumes all the available oxygen, nutrients, and energy, resulting in cellular damage and a mixed respiratory and metabolic acidosis.
Eventually, the cellular mechanisms that maintain homeostasis begin to fail and cellular integrity becomes compromised. Potassium may leak from the cell into the bloodstream, leading to dangerously elevated potassium levels. The weakening and eventual breakdown of muscle cells releases myoglobin into the bloodstream (rhabdomyolysis). The presence of brown or cola-colored urine may serve as an indicator of free myoglobin in the blood, which is toxic to the kidneys, necessitating a high urine output to avoid kidney failure. Hence, if MH is not recognized and aggressively treated, the patient may suffer severe complications associated with high body temperatures, extreme acidosis, rhabdomyolysis, and elevated serum potassium, which may lead to arrhythmias and cardiac arrest. These changes may occur over a period of only 10-20 minutes.
Clinical Presentation
The vast majority of patients who are MHS have no signs or symptoms of being at risk for MH. Some may, in retrospect, have experienced significant muscle cramping or even have suffered heatstroke, but these are nonspecific signs. Patients should be asked about hereditary muscle disorders and a personal or family history of unusual anesthesia complications: these can help assess the risk for MH, but not exclude risk completely. In fact, over half of the patients who experienced MH had two or more general anesthetics prior to their first MH crisis.
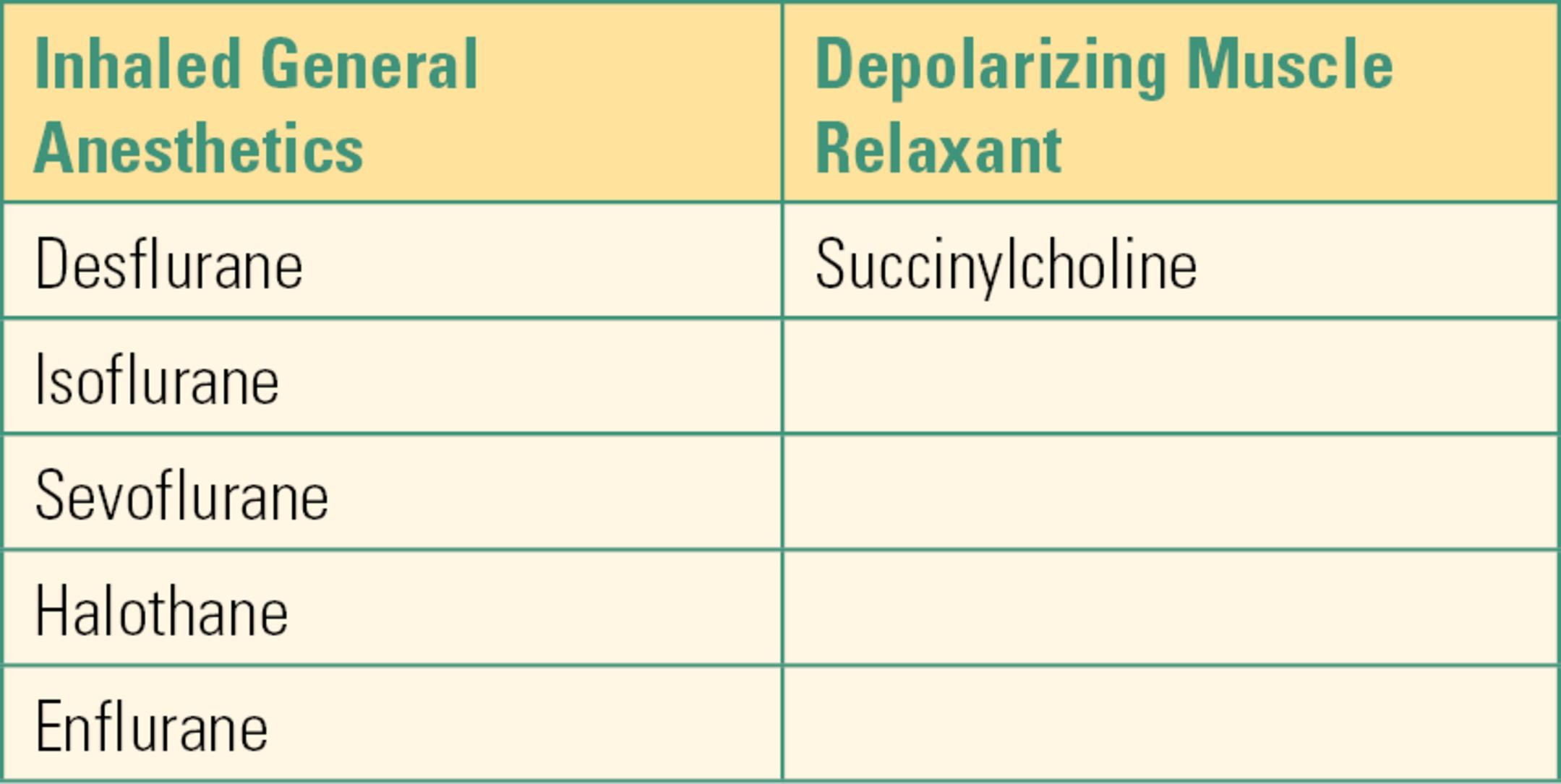
The onset time of MH has been found to vary based on the choice of halogenated anesthetic gas and the use of succinylcholine. The quickest onset of MH was elicited by a combination of halothane and succinylcholine on induction of anesthesia. However, halothane, the most potent inhalational trigger, is no longer available for human use in many countries. In addition, the FDA issued a black box warning against the routine use of succinylcholine, the only depolarizing muscle relaxant, resulting in a significant decline in its use. Nevertheless, MH may trigger with any of the halogenated volatile anesthetics (Table 60.1). The onset time of MH has been reported to occur after many minutes or hours of anesthetic administration or even in the early recovery period. In rare instances, environmental and physiologic stressors, such as heat or physical exertion, have triggered an MH event.
Table 60.1. Malignant Hyperthermia Triggering Agents
The clinical presentation of MH under anesthesia may be subtle or dramatic (Table 60.2). One of the earliest signs of MH is tachycardia. However, the earliest specific sign is a rapid rise in end-tidal CO2 despite attempts at hyperventilation. This will manifest as increasing levels of carbon dioxide on the capnograph, which is unresponsive to changes in minute ventilation. This is due to the accelerated production of CO2 during the hypermetabolic phase.
Table 60.2. Clinical Presentation

Muscle rigidity may be present in over 50% of MH cases if the syndrome is allowed to continue and is considered pathognomonic for MH when associated with an unresponsive increase in end-tidal CO2. The earliest sign of muscle rigidity may be masseter muscle rigidity (clenching of the jaw) with the administration of succinylcholine to help secure the airway. The classic “jaws of steel” make it impossible to open the mouth for laryngoscopy or placement of an airway. This rigidity may last several minutes and is NOT responsive to nondepolarizing muscle relaxants, such as vecuronium or rocuronium. A rapid rise in body temperature usually follows the other signs described, but once temperature begins to rise, core temperature may increase 1°C-2°C every 5-10 minutes, particularly in muscular individuals.
Laboratory Findings
Elevated end-tidal carbon dioxide is an early sign of MH. Initially, respiratory acidosis predominates, then as the syndrome progresses, metabolic acidosis becomes more prominent. This should prompt the anesthesia provider when considering MH to obtain an arterial blood gas for confirmation of the presence or absence of a respiratory and/or metabolic abnormality and its severity. Hypoxemia is usually NOT a problem, although in severe cases, mild desaturation may be noted. Potassium levels should be assessed with each blood gas sample as life-threatening hyperkalemia may occur during an MH crisis. Creatine kinase (CK or creatine phosphokinase, CPK) is a marker of muscle damage with serum concentrations reflecting the extent of tissue injury. The CK levels should be monitored until the concentration decreases to within normal values. Serum myoglobin should also be assayed as well as urine myoglobin. Since testing for myoglobin levels may not be readily available or the results quickly obtained, another simple and rapid test for myoglobin can be performed by testing the patient’s urine sample for hemoglobin. The use of a urine hemoglobin test strip is based on the shared positive reaction to the presence of myoglobin in the urine. If positive and there are no red blood cells reported in the microscopic examination of the urine, then the test is presumed positive for the presence of myoglobin.
Coagulation studies for disseminated intravascular coagulation should be ordered as well, especially in cases where the body temperature has risen to a critical temperature of greater than 41.5°C. Testing for serum calcium levels is unnecessary, since serum concentrations are generally unchanged. After the acute episode is controlled, serial CK levels should be followed every 6-12 hours until the results plateau and begin to return to normal. If the syndrome continues, then blood gases and electrolytes need to be monitored at frequent intervals. In addition, body temperature should be continuously monitored.
Intraoperative Management
Successful management of an MH crisis requires a carefully coordinated plan involving many individuals, including operating room nurses, surgeons, anesthesia providers, anesthesia technicians, and a variety of ancillary personnel. Time and effective resource utilization are essential for increasing the chances for a good patient outcome. MH simulations have repeatedly demonstrated that the anesthesia provider is rapidly overwhelmed with the number of tasks that must be accomplished. A well-trained anesthesia technician can be invaluable during an MH crisis.
Once the diagnosis of MH is made, the anesthesia provider will need to discontinue the anesthetic gas and begin hyperventilating the patient with 100% oxygen. If surgery is not completed, the situation will require switching to an intravenous (IV) anesthetic technique. This will require multiple infusion pumps for the individual drugs. Also, the fresh gas flow of oxygen will need to be increased to the highest level to help decrease the anesthetic gas concentration within the anesthesia machine, although significant amounts may be stored and redistributed from the patient’s body. MHAUS does not recommend abandoning the contaminated anesthesia machine and switching to another anesthesia machine or to manual ventilation with a different oxygen source and bag-valve-mask resuscitator.
The most critical step in MH management is to begin treatment with dantrolene. The surgeon must also be notified to help coordinate a timely ending to the surgery. In addition, if not already being monitored, core temperature monitoring must be established: esophageal, rectal, bladder, and as a last resort axillary. Skin temperature is unreliable in an MH crisis. Active cooling of the patient can be achieved with ice packs applied to the head, axilla and groin; the forced air machine set at lowest temperature setting; intravenous administration of cold saline solution; and irrigation of body cavities, such as the bladder or open abdomen with cold sterile solution.
The declaration of an MH crisis should mobilize all available personnel, along with an MH cart containing dantrolene sodium.
Dantrolene is the only antidote for treating MH. The older drug formulation is relatively insoluble and requires the assistance of many individuals to mix the dantrolene and prepare it for injection. It is packaged in a glass vial in the form of a lyophilized, orange powder (Fig. 60.1). Each vial contains 20 mg of dantrolene and requires 60 mL of preservative-free sterile water for diluent producing a light orange solution. Only sterile water (not other fluids containing glucose or saline, which may be more readily available) can be used. The newer formulation is a lyophilized orange cake of nanoparticles packaged in a glass vial requiring 5 mL of preservative free sterile water for diluent constituting 250 mg of dantrolene (Fig. 60.2). Complete dissolution of dantrolene is visually confirmed by an orange suspension. The initial dose is 2.5 mg/kg. Each facility should stock a minimum of 36 vials of the older form dantrolene or 3 vials of the new nanocrystalline dantrolene. For a patient weighing 80 kg, 10 vials of the older formulation will be required as each vial contains only 20 mg; however, only 1 vial is needed for the newer formulation. Preparation of dantrolene for injection is often labor intensive requiring 20 seconds or more of agitation to dissolve each vial of the older formulation, while the new formulation of dantrolene takes less than 10 seconds to dissolve into solution. Setup for mixing dantrolene should be planned prior to an MH crisis. Mixing stations can be simple or complex, especially for the older formulation (Figs. 60.3 and 60.4).

FIGURE 60.1. The traditional formulation of dantrolene in 20-mg vials before and after reconstitution with sterile water; 36 vials are considered an adequate supply.
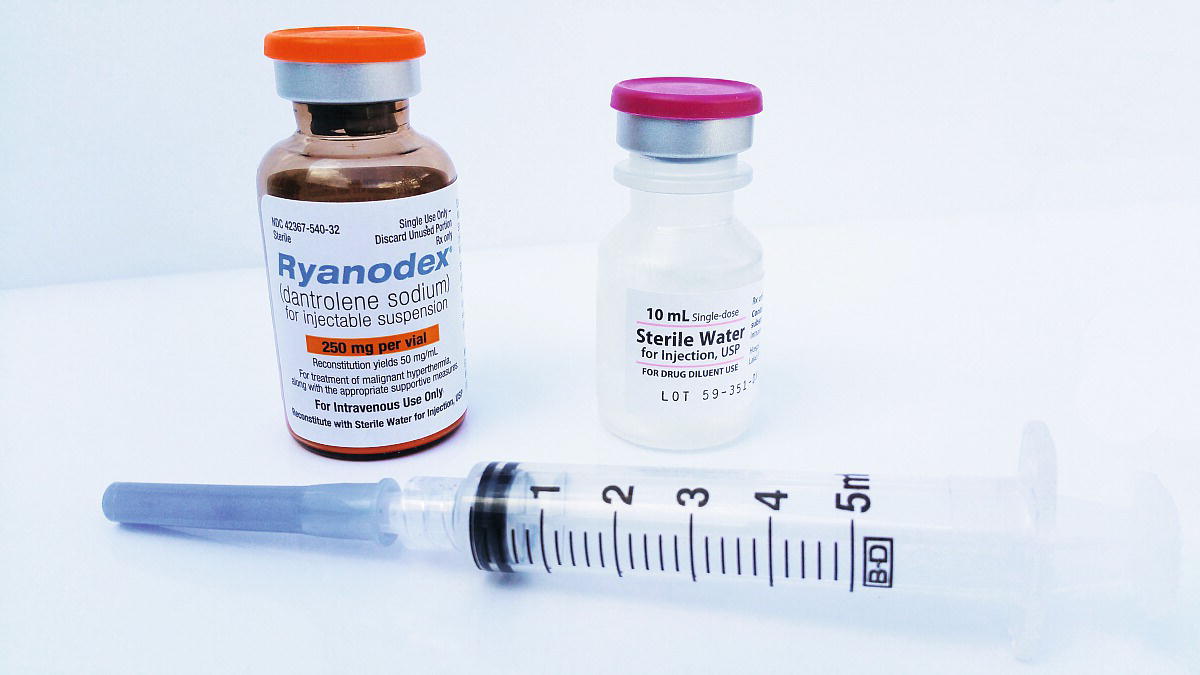
FIGURE 60.2. The new nanocrystalline formulation of dantrolene in 250 mg vial requiring 5 mL of sterile water for reconstitution.
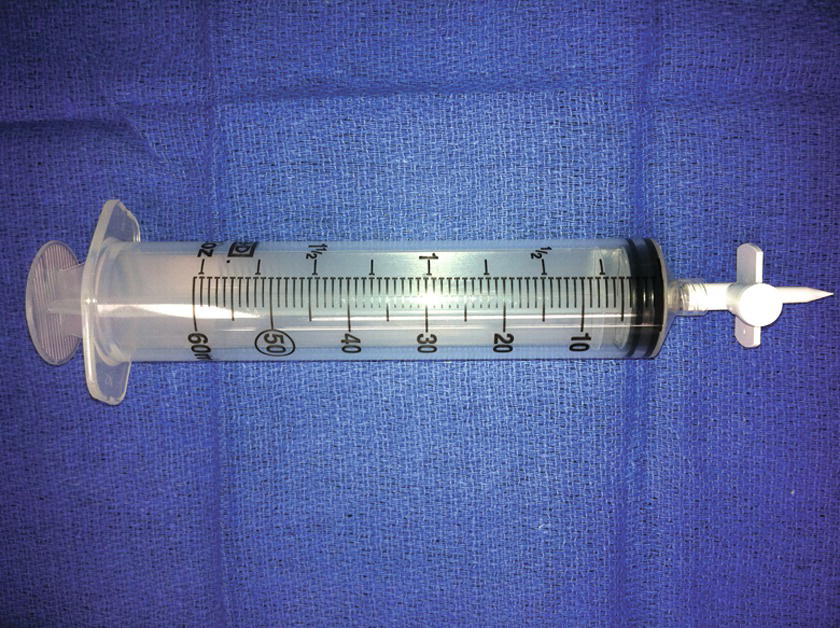
FIGURE 60.3. Simple setup for mixing and dissolving the original dantrolene. Multiple syringes and spikes will be required for an adequate adult dose.
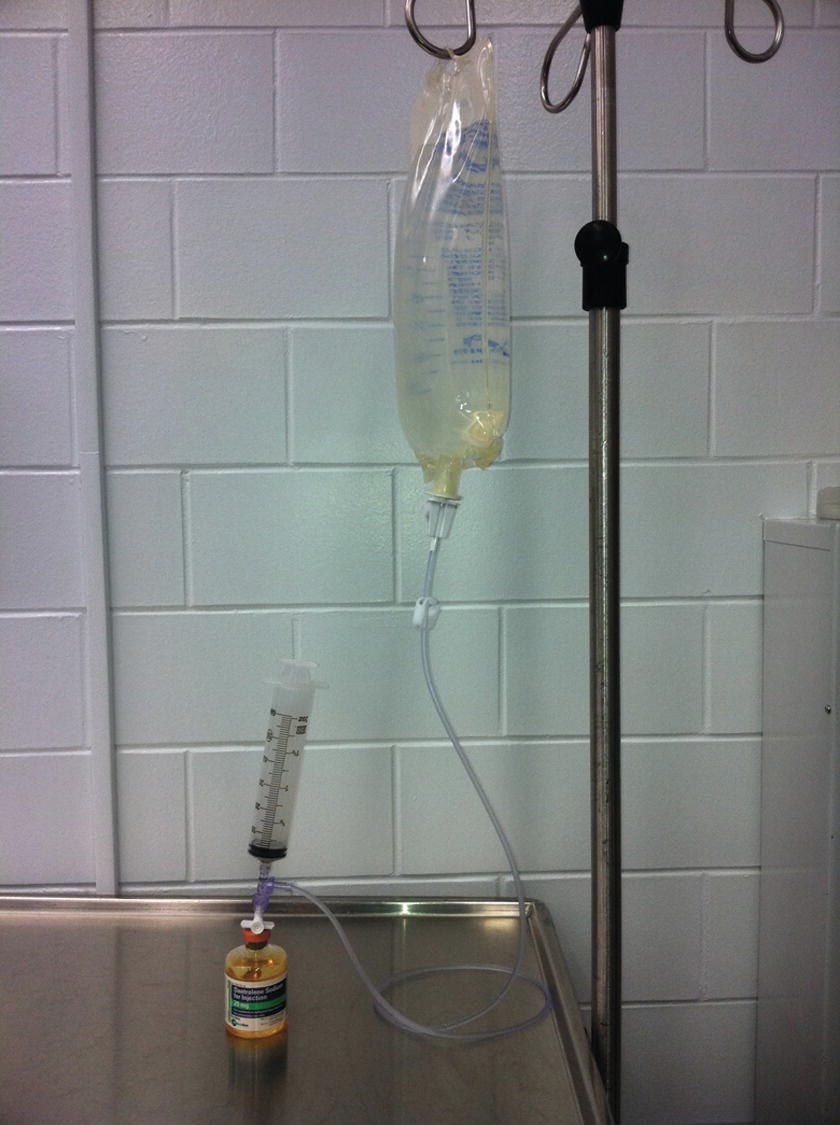
FIGURE 60.4. Complex setup for mixing and dissolving the original dantrolene. Again, multiple syringes and vials required.
Additional doses of 2.5 mg/kg of dantrolene may be required based on clinical findings and normalization of acid-base balance. Once treatment is initiated, it is recommended that the drug be continued for at least 36 hours as determined by the continued laboratory and clinical manifestations of MH. Other supplies that should be readily available, as well as their purpose, can be noted on the Malignant Hyperthermia Supplies/Cart List (Table 60.3).
Table 60.3. Malignant Hyperthermia Suppliesc

aInitial dose of dantrolene 2.5 mg/kg. Each vial of old formulation contains 20 mg of dantrolene and 3 g of mannitol. Mix each vial with 60 mL of sterile water (bacteriostatic-free) for injection. May need vigorous agitation for 20 seconds or less with new formulation. Newer nanoparticle formulation contains 250 mg of dantrolene and 125 mg mannitol. Each vial is mixed with 5 mL of sterile water (bacteriostatic free).
bLidocaine is standard in the MHAUS cart, but amiodarone is listed as acceptable alternative; lidocaine remains part of MHAUS protocol, but is no longer part of advanced cardiac life support (ACLS) protocol. MHAUS recommends that MH patients be treated according to ACLS guidelines. Lidocaine is not for use in wide QRS complex arrhythmias.
chttps://www.mhaus.org/healthcare-professionals/be-prepared/what-should-be-on-an-mh-cart/
ABG, arterial blood gas; CBC, complete blood cell count; CK, creatine kinase; INR, international normalized ratio; IV, intravenous; LDH, lactate dehydrogenase; NG, nasogastric; PT, prothrombin time; PTT, partial thromboplastin time; SMA, serum metabolic assay.
If the patient is hyperthermic, active cooling should be instituted to reduce the body temperature to less than 38°C, after which cooling can be stopped. Large-bore IV catheters and/or a central venous catheter will be necessary. An arterial line must be placed to obtain frequent blood samples and monitor the patient’s fluid status and hemodynamics. Blood gases will need to be drawn frequently and at regular intervals to assess treatment for acid-base imbalance, electrolyte abnormalities, hematology indices, and coagulation status. Also, determination of CK and myoglobin levels will be needed to monitor the extent of tissue damage and potential renal impairment. A bladder catheter will need to be placed, if not already in place, to monitor urine output and to sample the urine for the presence of myoglobin (often noted by brown or cola-colored urine). It should be noted that the older formulation of dantrolene contains 3 g of mannitol per 20 mg vial, which may contribute toward an osmotic diuresis, while the new dantrolene formulation contains only 125 mg of mannitol. Additional recommendations for monitoring and treatment may be obtained by consulting an MH expert through the hotline number (1-800-MH-HYPER) of MHAUS.
Postoperative Care
The management of MH is not over once the crisis is controlled. Recurrence of the syndrome, even with dantrolene treatment, may occur in 25% of patients. Therefore, patients require management in an intensive care unit for 48-72 hours after the initial clinical presentation, and they will need to continue therapy with dantrolene at 1 mg/kg every 4-6 hours whether or not they demonstrate signs of MH. In addition, they will continue to need frequent laboratory tests to monitor and treat acid-base derangements; electrolyte abnormalities such as hyperkalemia, rhabdomyolysis, renal impairment; and coagulation abnormalities.
Patients experiencing an MH crisis in an outpatient or ambulatory setting outside a hospital pose a unique set of problems. Protocols for emergency transfer of patients experiencing an MH event should be in place prior to any patient undergoing general anesthesia or being exposed to any MH-triggering drug. Proper transfer of a patient experiencing an MH crisis outside of a hospital facility requires at a minimum that the patient’s condition be stable before transfer. Therefore, the patient should not be moved unless there is a reduction of temperature to less than 100°F, cardiovascular stability, and end-tidal carbon dioxide close to normal. The patient must show signs that the crisis is resolving. Dantrolene, along with cardiovascular monitors as well as an experienced person who can analyze changes in vital signs and administer dantrolene, should accompany the patient to the hospital. MHAUS recommends that each facility that provides anesthesia care know which hospital(s) in their vicinity have the capability to treat and manage an MH crisis.
Testing
Confirmation of MH susceptibility, because of a clinical event or family history, can be determined by laboratory tests. There are two tests available. The one that is most sensitive and specific is the muscle biopsy contracture test, in which a sample from the quadriceps muscle is harvested using local anesthesia or a nontriggering anesthetic technique in the operating room. The muscle is dissected into fine strips to be tested for contractile force on exposure to incremental doses of halothane and caffeine; contracture is observed in those who are MHS. The drawback to the test is that the patient needs to be present at the testing center since the test must be performed right away. There are only four muscle biopsy testing centers in North America. This test has a sensitivity of 99%, with a specificity of 78%. Within the past several years, a DNA-based test has been introduced. This genetic test is still under refinement, but it has the advantage of requiring only a blood sample or a mouth swab, which may be sent to one of four genetic testing centers. Because there are many mutations that may lead to MH susceptibility and many more that are yet to be determined, the test will only detect about 30% of those who are MHS. However, if the patient does have one of the mutations, he or she is definitely susceptible and other members of the family should be tested for this mutation as well.
MH-Susceptible Patients
MHS patients having surgery require a nontriggering anesthetic technique, that is, free from potent anesthetic gases and succinylcholine. A local, regional, spinal, or epidural block is preferable because all local anesthetics are nontriggering agents. Another technique is total intravenous anesthesia (TIVA), which utilizes intravenous anesthesia medications for achieving hypnosis/sedation, analgesia, amnesia, and muscle relaxation in the surgical patient. TIVA is usually needed for MHS children as they will not typically tolerate being awake during a surgical procedure. This technique requires several pieces of equipment in the form of infusion pumps for accurately delivering medications plus a carrier fluid to ensure delivery of medications into the body. Nitrous oxide is a nontriggering gas and may be used with any technique. Prophylactic administration of dantrolene to MHS patients is discouraged due to the associated adverse side effects of the medication and the availability of proven, safe alternative anesthetic techniques.
Controlled ventilation with a secure airway may require use of an anesthesia machine to ventilate the patient. Preparation of the anesthesia machine has recently been reevaluated with the introduction of newer, more complex anesthesia delivery systems. Initially, the recommendation for anesthesia machine preparation for an MHS patient consisted of high flows of oxygen (10 L/min) through a machine with the ventilator cycling for 20 minutes, as well as replacing the breathing circuit and soda lime. Ironically, modern innovation in breathing systems with decreased internal volumes and improved gas handling and ventilator design have contributed to increasing the time to purge residual anesthetic gases. Comparisons between old and new and different designs of anesthesia machines demonstrate that a general recommendation for purging anesthesia machines is no longer valid. Machine preparation must be individualized according to manufacturer specifications. In addition to recommendations found in published studies and at the MHAUS website, it is advised to check with the anesthesia machine manufacturer and devise an institutional protocol for machine preparation (Table 60.4).
Table 60.4. Summary of Study Recommendations for Preparing Anesthesia Machines for Malignant Hyperthermia-Susceptible Patients

aCharcoal filter (QED® or Quick Emergence Device, Anecare Laboratories, Salt Lake City, UT) on inspiratory limb; FGF ≥ 10 L/min for 5 min with filter “off,” then for 5 min with filter “on” and FGF ≥ 10 L/min for first 5 min of case, and then FGF ≥ 2 L/min for at least 6 h.
FGF, fresh gas flow; I:E, inspiratory to expiratory ratio; PEEP, positive end-expiratory pressure; ppm, parts per million; RR, respiratory rate; TV, tidal volume.
Reprinted from Kim TW, Nemergut ME. Preparation of modern anesthesia workstations for malignant hyperthermia-susceptible patients: a review of past and present practice. Anesthesiology. 2011;114(1):211, with permission.
A new development in machine preparation for the MHS patient is the introduction of activated charcoal filters that when connected to the inspiratory and expiratory ports of the ventilator will rapidly lower the anesthetic gas concentration in the machine (see Figs. 60.5 and 60.6). The reduction of anesthetic gas concentration to less than 5 ppm takes less than 2 minutes and is sustained with fresh gas flow rates lower than 10 L/min protecting against the rebound effect when gas flows are reduced. As well, the filters have a reported efficacy of 12 hours using a minimum fresh gas flow of 3 L/min, provided the machine is first prepared by flushing with high fresh gas flows for 90 seconds prior to placement of the filters. However, when used emergently for a patient exposed to a volatile triggering agent, the canisters must be changed every 60 minutes to avoid exceeding 5 ppm of halogenated volatile anesthetic gas. Also, the filters may assist in rapidly decreasing the concentration of anesthetic gas within the patient during an MH crisis. Unfortunately, there are only anecdotal reports of using the filters in a clinical setting. More studies are needed to establish their efficacy and safety.

FIGURE 60.5. Two activated charcoal filters packaged as a pair; one filter for the inspiratory limb of the anesthesia circuit, and one for the expiratory limb.

FIGURE 60.6. Two activated charcoal filters attached to the inspiratory and expiratory limbs of the anesthesia circuit.
Support for MH-Susceptible Patients
The MHAUS was formed in 1981 to provide education and guidance to patients and clinicians in the management of MH. The organization has a Web site, www.mhaus.org, with a great deal of information that is available at no cost. Also, there is an MH hotline available 24/7 at no charge to the user that puts a clinician in immediate contact with an experienced consultant knowledgeable in MH. Over 1,000 calls per year are logged on the hotline. There are other more in-depth programs available for a nominal charge.
The North American Malignant Hyperthermia Registry of MHAUS was formed in the late 1980s as a repository for patient-specific information related to MH. Data are submitted by clinicians or patients. The data have been invaluable for understanding the clinical presentations of MH and other disorders related to MH.
Summary
In summary, the morbidity and mortality associated with MH may be dramatically reduced through early diagnosis and treatment, which must include withdrawal of all triggering agents and administration of dantrolene sodium. The management of MH is an intense practice in crisis resource management requiring effective and efficient use of all personnel, equipment, and drugs. Anesthesia technicians play a critical role in the management of MH in the perioperative period. MHAUS maintains an MH hotline (1-800-MH-HYPER) to assist anyone with questions regarding MH or the management of a patient.
Review Questions
1. Which of the following agents is SAFE to use in an MH patient?
A) Succinylcholine
B) Nitrous oxide
C) Sevoflurane
D) Halothane
E) Desflurane
Answer: B
Succinylcholine and all of the potent inhaled anesthetics can trigger MH. Nitrous oxide is safe to use.
2. Which of the following is an indicator of an MH crisis?
A) Jaw muscle relaxation
B) Low end-tidal CO2 levels
C) Bradycardia
D) Cola-colored urine
E) Symptoms that begin 2 days after surgery
Answer: D
Muscle breakdown in an MH crisis releases the muscle protein myoglobin into the bloodstream where it is filtered by the kidneys. This can turn the urine a brownish color. Jaw muscle rigidity, tachycardia, and high end-tidal CO2 levels are signs of MH. Although MH can present in the recovery room, it is rare. Most late cases of MH present within an hour of surgery.
3. Which of the following supplies are not required during an MH crisis?
A) 36 vials of older-formulation of dantrolene or 3 vials of nanocrystalline dantrolene and bacteriostatic-free sterile water
B) Extra personnel
C) Arterial line setup
D) Vascular access setup (peripheral and/or central)
E) None of the above (all of the supplies may be needed)
Answer: E
The administration of dantrolene is the only effective treatment for MH. Thirty-six vials need to be readily available to treat the initial crisis. The patient should be continually monitored in an intensive care setting, because MH has been documented to recrudesce up to 36 hours after the initial treatment. Dantrolene is packaged in a lyophilized form that requires reconstitution with bacteriostatic-free sterile water.
4. Which of the following is FALSE regarding the anesthesia machine and MH?
A) It is necessary to switch out the anesthesia machine immediately during an MH crisis.
B) Preparing modern anesthesia machines for an MHS patient are machine specific.
C) Anesthesia machine preparation requires flushing with oxygen at 10 L/min for between 20 and 50 minutes.
D) New activated charcoal filters attached to the inspiratory and expiratory ports of the anesthesia machine can achieve rapid reductions in halogenated agents.
E) All of the above.
Answer: A
Although the halogenated agent should be immediately turned off and high fresh gas flows initiated, MHAUS does not recommend switching out the anesthesia machine as a first priority during an MH crisis. Modern anesthesia machines require a variable time of flushing with 100% oxygen at 10 L/min to achieve acceptably low concentrations of halogenated agents. Most can be prepared within 20-50 minutes. New activated charcoal filters may be used to prepare the anesthesia machine or be used during an MH crisis to reduce the patient’s exposure to halogenated agents.
5. Dantrolene is
A) Always dosed at 2.5 mg/kg
B) Drawn up only in sterile water
C) Discontinued when patients leave the operating room
D) Available in two formulations, one of which works better
E) Required for hospital operating rooms; office-based and out-of-OR anesthesia locations should always know where the nearest dantrolene is available in case a patient needs to be transferred
Answer: B
Dantrolene must be drawn up only in sterile water and not saline, lactated Ringer’s, or glucose-containing fluids. Its starting dose is 2.5 mg/kg, but it may be repeated with additional doses of 2.5 mg/kg up to a total of 10 mg/kg. It is not discontinued when patients leave the operating room, but continued at 1 mg/kg every 4-6 hours in the intensive care unit postoperatively. It continues to be available in two formulations; both work well, but one dissolves much faster than the other, thus using personnel resources more efficiently in a crisis. Dantrolene must be available in every anesthetizing location; office-based anesthesia locations and out-of-OR anesthesia locations in the hospital must stock dantrolene for immediate on-site administration. Preparation for MH crisis includes planning for MH expertise and ICU transfer after a patient is stabilized.
6. Testing for MH is recommended before anesthesia for all patients who might be MH susceptible:
A) True.
B) False.
Answer: B
Testing with a muscle biopsy requires travel and a minor surgical procedure, and not all those who have a positive result are MHS. Genetic testing only detects 30% of those who are MHS, since not all genes are known. When an MH patient tests positive for a known gene, it is recommended other family members can be tested. Safe nontriggering techniques (regional or TIVA) are available when there is a high clinical suspicion that a patient may be MHS.
SUGGESTED READINGS
Baker KR, Landriscina D, Kartchner H, et al. The Icarus effect: the influence of diluents warming on dantrolene sodium mixing time. AANA J. 2007;75:101-106.
Bilmen JG, Gillies RL. Clarifying the role of activated charcoal filters in preparing an anaesthetic workstation for malignant hyperthermia—susceptible patients. Anaesth Intensive Care. 2014;42(1):51-58.
Birgenheier N, Stoker R, Westenskow D, et al. Activated charcoal effectively removes inhaled anesthetics from modern anesthesia machines. Anesth Analg. 2011;112:1363-1370.
http://www.dynasthetics.com/Vapor-Clean/Vapor-Clean-IFU.pdf. Accessed July 20, 2016
Kim TW, Nemergut ME. Preparation of modern anesthesia workstations for malignant hyperthermia-susceptible patients: a review of past and present practice. Anesthesiology. 2011;114(1):205-212.
Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg. 2010;110(2):498-507.
Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015;10:93.
Rosero EB, Adesanya AO, Timaran CH, et al. Trends and outcomes of malignant hyperthermia in the United States, 2000 to 2005. Anesthesiology. 2009;110(1): 89-94.
Resources for Management and Education:
The Malignant Hyperthermia Association of the United States
Nonemergent calls: 1-607 674 7901
Hotline calls: 1-800 MH Hyper
Address: PO Box 1069
Sherburne, NY 13460
Resources Available:
Educational material
In-service training kit
Wall posters for emergency management
Transfer of care protocol
MH mock drill kit
The North American Malignant Hyperthermia Registry
UPMC Mercy Hospital
8th Floor, Ermire Building (B)
Room 8522-3
1400 Locust Street
Pittsburgh, PA 15219
Toll Free Number: 1-888-274-7899