All About the SAT Chemistry Test
The SAT Subject Tests
What Are the SAT Subject Tests?
The SAT Subject Tests (formerly called the SAT II tests and the Achievement Tests) are a series of college entrance tests that cover specific academic subject areas. Like the better-known SAT, which measures general verbal and math skills, the SAT Subject Tests are given by the College Entrance Examination Board. Colleges and universities often require applicants to take one or more SAT Subject Tests along with the SAT.
SAT Subject Tests are generally not as difficult as Advanced Placement tests, but they may cover more than is taught in basic high school courses. Students typically take an SAT Subject Test after completing an Advanced Placement course or an Honors course in the subject area.
How Do I Know if I Need to Take SAT Subject Tests?
Review the admissions requirements of the colleges to which you plan to apply. Each college will have its own requirements. Many colleges require that you take a minimum number of SAT Subject Tests—usually one or two. Some require that you take tests in specific subjects. Some may not require SAT Subject Tests at all.
When Are SAT Subject Tests Given, and How Do I Register for Them?
SAT Subject Tests are usually given on six weekend dates spread throughout the academic year. These dates are usually the same ones on which the SAT is given. To find out the test dates, visit the College Board Web site at www.collegeboard.org. You can also register for a test at this Web site. Click on the tabs marked “students” and follow the directions you are given. You will need to use a credit card if you register online. As an alternative, you can register for SAT Subject Tests by mail using the registration form in the SAT Registration Bulletin, which should be available from your high school guidance counselor.
How Many SAT Subject Tests Should I Take?
You can take as many SAT Subject Tests as you wish. According to the College Board, more than one-half of all SAT Subject Test takers take three tests, and about one-quarter take four or more tests. Keep in mind, though, that you can take only three tests on a single day. If you want to take more than three tests, you’ll need to take the others on a different testing date. When deciding how many SAT Subject Tests to take, base your decision on the requirements of the colleges to which you plan to apply. It is probably not a good idea to take many more SAT Subject Tests than you need. You will probably do better by focusing only on the ones that your preferred colleges require.
Which SAT Subject Tests Should I Take?
If a college to which you are applying requires one or more specific SAT Subject Tests, then of course you must take those particular tests. If the college simply requires that you take a minimum number of SAT Subject Tests, then choose the test or tests for which you think you are best prepared and likely to get the best score. If you have taken an Advanced Placement course or an Honors course in a particular subject and done well in that course, then you should probably consider taking an SAT Subject Test in that subject.
When Should I Take SAT Subject Tests?
Timing is important. It is a good idea to take an SAT Subject Test as soon as possible after completing a course in the test subject, while the course material is still fresh in your mind. If you plan to take an SAT Subject Test in a subject that you have not studied recently, make sure to leave yourself enough time to review the course material before taking the test.
What Do I Need on the Day of the Test?
To take an SAT Subject Test, you will need an admission ticket to enter the exam room and acceptable forms of photo identification. You will also need two number 2 pencils. Be sure that the erasers work well at erasing without leaving smudge marks. The tests are scored by machine, and scoring can be inaccurate if there are smudges or other stray marks on the answer sheet.
Any devices that can make noise, such as cell phones or wristwatch alarms, should be turned off during the test. Study aids such as dictionaries and review books, as well as food and beverages, are barred from the test room.
The SAT Chemistry Test
What Is the Format of the SAT Chemistry Test?
The SAT Chemistry test is a one-hour exam consisting of 85 multiple-choice questions. According to the College Board, the test measures the following knowledge and skills:
• Familiarity with major chemistry concepts and ability to use those concepts to solve problems
• Ability to understand and interpret data from observation and experiments and to draw conclusions based on experiment results
• Knowledge of laboratory procedures and of metric units of measure
• Ability to use simple algebra to solve word problems
• Ability to solve problems involving ratio and direct and inverse proportions, exponents, and scientific notation
The test covers a variety of chemistry topics. The following chart shows the general test subject areas, as well as the approximate portion of the test devoted to each subject.
SAT Chemistry Subject Areas

When you take the SAT Chemistry test, you will be given a test booklet that includes a periodic table of the elements. The table will show only the element symbols, atomic numbers, and atomic masses. It will not show electron configurations or oxidation numbers. You may not use your own reference tables or a calculator.
What School Background Do I Need for the SAT Chemistry Test?
The College Board recommends that you have at least the following experience before taking the SAT Chemistry test:
• One-year chemistry course at the college preparatory level
• One-year algebra course
• Experience in the chemistry laboratory
How Is the SAT Chemistry Test Scored?
On the SAT Chemistry test, your “raw score” is calculated as follows: You receive one point for each question you answer correctly, but you lose one-quarter point for each question you answer incorrectly. You do not gain or lose any points for questions that you do not answer at all. Your raw score is then converted into a scaled score by a statistical method that takes into account how well you did compared to others who took the same test. Scaled scores range from 200 to 800 points. Your scaled score will be reported to you, to your high school, and to the colleges and universities that you designate to receive it.
Scoring scales differ slightly from one version of the test to the next. The scoring scales provided after each practice test in this book are only samples that will show you your approximate scaled score.
When Will I Receive My Score?
Scores are mailed to students approximately three to four weeks after the test. If you want to find out your score a week or so earlier, you can do so for free by accessing the College Board Web site or for an additional fee by calling (866)756-7346.
How Do I Submit My Score to Colleges and Universities?
When you register to take the SAT or SAT Subject Tests, your fee includes free reporting of your scores to up to four colleges and universities. To have your scores reported to additional schools, visit the College Board Web site or call (866)756-7346. You will need to pay an additional fee.
SAT Chemistry Question Types
The SAT Chemistry test consists entirely of multiple-choice questions. Most are the regular five-answer-choice format that you will be familiar with from taking other standardized tests. The questions in Sections A and B, however, have special formats that do not appear on other tests and that you need to be aware of. The College Board calls these formats “classification sets” and “relationship analysis questions.” Review the following examples before you tackle the Diagnostic Test.
PART A: CLASSIFICATION SETS
In a classification set, you are given five answer choices lettered A through E. The choices may be chemistry principles, substances, numbers, equations, diagrams, or the like. The choices are followed by three or four numbered questions. Your task is to match each question with the answer choice to which it refers. Here are sample directions for a classification set, followed by a sample of this question format.
Questions 3–5:
(A) Coordinate covalent bonding
(B) Ionic bonding
(C) Nonpolar covalent bonding
(D) Metallic bonding
(E) Hydrogen bonding
3. HF
4. N2
5. KI
3. The correct answer is choice E. The bond between the atoms of hydrogen and fluorine is a polar covalent bond, a choice that is not present in the choices above. Now look at the bonding between the molecules of HF. HF can exhibit dipole forces between its molecules, yet another choice that is not present. HF can, however, exhibit hydrogen bonding, a choice that is present.
4. The correct answer is choice C. Nitrogen gas has no difference in electronegativity between the nitrogen atoms. The two nitrogen atoms will form a nonpolar covalent bond. The type of bonding present between the molecules of nitrogen gas will be dispersion forces, the forces present between nonpolar molecules.
5. The correct answer is choice B. Potassium iodide is formed from a metal, potassium, and a nonmetal, iodine. The type of bonding that forms between metals and nonmetals is ionic bonding.
THINK ABOUT THIS 
PART B: RELATIONSHIP ANALYSIS QUESTIONS
Relationship analysis questions are probably not like any question type that you have seen before. Each question consists of two statements labeled I and II with the word BECAUSE between the two statements. For each question, you have three tasks. You must:
• Determine if statement I is true or false.
• Determine if statement II is true or false.
• Determine if statement II is the correct explanation for statement I.
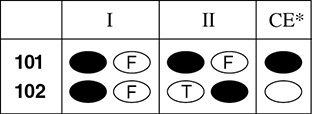
On the answer sheet, you will mark true (T) or false (F) for each statement, and you will mark “correct explanation” (CE) ONLY if statement II is a correct explanation of statement I. Here are sample directions for this kind of question, followed by two examples and a sample of a correctly marked answer sheet.


Here is how you would mark these answers on the answer sheet:
PART C: REGULAR MULTIPLE-CHOICE QUESTIONS
On the SAT Chemistry test, most of the questions are in the regular five-
answer-choice format that is used on standardized tests such as the SAT. Here is an example:
1. Which oxidation half reaction below demonstrates conservation of mass and charge?
(A) Mg2+ + 2e– → Mg
(B) Cl1− + 1e– → Cl2
(C) 2Ag1+ → 2Ag +1e–
(D) Mg → Mg2+ + 2e–
(E) F2 + 2e– → 2F1−
The correct answer is choice D. Note that with this question, as with many other questions on the test, you can find the correct answer by using the process of elimination. The half reactions shown in choices A, B, and E are all reduction half reactions, so those choices can be eliminated. Both remaining choices, C and D, show oxidation and a loss of electrons. But choice C does not demonstrate conservation of charge and mass; if it did, there would have to be two electrons on the left side of the reaction. So the correct answer must be choice D.
You will see a variation of this basic format in which you are offered three choices indicated by the Roman numerals I, II, and III. Your task is to decide which combination of the three choices answers the question. Here is an example:
2. Which of the following indicates an acidic solution?
I. Litmus paper turns blue.
II. Phenolphthalein turns pink.
III. Hydronium ion concentration is greater than hydroxide ion concentration.
(A) I only
(B) II only
(C) III only
(D) I and II only
(E) I, II, and III
The correct answer is choice C. First, review the choices. Choices I and II indicate a basic solution. If they were acidic, then the solutions would be red for litmus and clear for phenolphthalein. Only choice III holds true for an acidic solution. In an acidic solution the concentration of hydronium ions exceeds that of hydroxide ion concentration.
Strategies for Top Scores
When you take the SAT Chemistry test, you’ll want to do everything you can to make sure you get your best possible score. That means studying right, building good problem-solving skills, and learning proven test-taking strategies. Here are some tips to help you do your best.
Study Strategies
• Get to know the format of the exam. Use the practice tests in this book to familiarize yourself with the test format, which does not change from year to year. That way, you’ll know exactly what to expect when you see the real thing on test day.
• Get to know the test directions. If you are familiar with the directions ahead of time, you won’t have to waste valuable test time reading them and trying to understand them. The format and directions used in the practice exams in this book are modeled on the ones you’ll see on the actual SAT Chemistry exam.
• Get to know what topics are covered. Get to know what specific topics are covered on the exam. You’ll find all of them in the review material and practice exams in this book. The following are the “hot topics” on the SAT Chemistry exam:
• Structure and theories of the atom
• Periodic trends and the chemical and physical properties of the elements
• Bonding between atoms and molecules
• Molecular geometries
• Nuclear chemistry
• Kinetic molecular theory and the gas laws
• Liquids, solids, and changes of phase
• Concentration
• Solubility and precipitation of compounds
• Electrolytes and conductivity
• Solution chemistry
• Colligative properties
• Redox reactions and reactions of acids and bases
• Mole relationships and Avogadro’s number
• Molecular and empirical formulas
• Percent composition by mass
• Limiting and excess reagents
• Rates of reaction
• Le Châtelier’s Principle
• Equilibrium constant expressions for gases and slightly soluble salts
• Energy in chemical and physical changes
• Hess’s Law
• Entropy and Gibbs free energy
• Organic and environmental chemistry
• Laboratory safety, procedures, skills, and setups
• Study hard. If possible, plan to study for at least an hour a day for two weeks before the test. You should be able to read this entire book and complete all five practice exams during that time period. Be sure to write notes in the margins of the book and paraphrase what you read. Make study cards from a set of index cards. Those cards can “go where you go” during the weeks and days before the test. If you are pressed for time, focus on taking the five practice exams, reading the explanations, and reviewing the particular topics that give you the most trouble.
Problem-Solving Strategies
• Solve problems in whatever way is easiest for you. There are usually several ways to solve any problem in chemistry and arrive at the correct answer. For example, when converting units some students prefer to use a dimensional analysis whereas others prefer to set up a proportion. Do what is easiest for you. Remember that the SAT exam is all multiple choice. That means that no one is going to be checking your work and judging you by which solution method you chose. So solve the problem any way you like.
• Build good problem-solving skills. When you tackle SAT Chemistry problems, try following this three-step process:
1. When you first read a question, make a list of the given values and variables and the units for the variables.
2. Ask yourself, “What do I have and what do I need to get?” The link between what you have and what you need to get is either an equation that you should be familiar with or certain specific steps to follow to solve particular types of problems.
3. Solve the problem and see if the answer makes sense. For example, if you know that one variable should be much larger than another, make sure your answer reflects that relationship. You’ll see how this works with many of the problems in this book.
• Make sure you know what the question is asking. The questions on the SAT Chemistry test are not deliberately designed to trick you, but it is still important that you look closely at each one to make sure you know what it is asking. If a question asks which compound has the lowest hydrogen ion concentration, don’t pick the answer choice with the highest concentration. Pay special attention to questions that include the words NOT or EXCEPT. You may want to circle these words to make sure you take them into account as you choose your answer.
Test-Taking Strategies
• Answer all the easy problems first, then tackle the harder ones. Keep in mind that the test is only one hour long. There isn’t much time to spend trying to figure out the answers to harder problems, so skip them and come back to them later. There are three reasons why you should do this. The first reason is that every question counts the same in the scoring of the exam. That means that you are better off spending time answering the easier questions, where you are sure to pick up points. The second reason to skip past harder questions is that later on in the test you might come to a question or a set of answer choices that jogs your memory and helps you to go back and answer the question you skipped. The third reason is that by answering the easier questions, you’ll build your confidence and get into a helpful test-taking rhythm. Then when you go back to a question you skipped, you may find that it isn’t as hard as you first thought.
• Use the process of elimination. Keep in mind that on the SAT Chemistry test, like any other multiple-choice test, the answer is right in front of you. Try eliminating answer choices that you know are incorrect. Often this can help you select the correct answer.
• If you must guess, make an educated guess. The SAT has a one-quarter-point penalty for wrong answers to discourage random guessing. So if you have absolutely no idea how to answer a question, you are better off skipping it entirely. However, you may be able to eliminate one or more answer choices. If you can do that, you can increase your odds of guessing the correct answer. If you can make this kind of educated guess, go ahead. If you guess correctly, you’ll earn another point.
• Be wary of answer choices that look familiar but are not correct. Sometimes in the set of answer choices there will be one or more wrong answers that include familiar expressions or phrases. You might be tempted to pick one of these choices if you do not work out the problem completely. That is why it is important to work through each problem thoroughly and carefully to make sure that you pick the correct answer choice.
• You don’t have to answer every question. If you do not know the answer to a question and cannot eliminate any answer choices, skip it and go on. It is better to do that than to risk losing one-quarter of a point for a wrong answer. If you have time at the end of the test, you can return to skipped questions and try to make an educated guess. But you do not have to answer every question to get a good score.
Tips for Test Day
• Don’t panic! Once test day comes, you’re as prepared as you’re ever going to be, so there is no point in panicking. Use your energy to make sure that you are extra careful in answering questions and marking the answer sheet.
• Use your test booklet as scratch paper. Your test booklet is not going to be reused by anyone when you’re finished with it, so feel free to mark it up in whatever way is most helpful to you. Circle important words, underline important points, write your calculations in the margins, and cross out wrong answer choices.
• Be careful when marking your answer sheet. Remember that the answer sheet is scored by a machine, so mark it carefully. Fill in answer ovals completely, erase thoroughly if you change your mind, and do not make any stray marks anywhere on the sheet. Also, make sure that the answer space you are marking matches the number of the question you are answering. If you skip a question, make sure that you skip the corresponding space on the answer sheet. Every 5 or 10 questions, check the question numbers and make sure that you are marking in the right spot. You may want to mark your answers in groups of 5 or 10 to make sure that you are marking the answer sheet correctly.
• Be especially careful when marking the answers to Chemistry Test questions 101–115. On the SAT Chemistry test, questions 101 through 115 comprise a special section. The questions in this section have their own format. (For more about these questions, see the section of this book titled “SAT Chemistry Question Types.”) On the answer sheet, you must mark your answers to these questions in a special section labeled “Chemistry” at the lower left corner. Make sure that you locate this answer sheet section and mark the answers to these questions in the proper place.
• Watch the time. Keep track of the time as you work your way through the test. Try to pace yourself so that you can tackle as many of the 80 questions as possible within the one-hour time limit. Check yourself at 10- or 15-minute intervals using your watch or a timer.
• Don’t panic if time runs out. If you’ve paced yourself carefully, you should have time to tackle all or most of the questions. But if you do run out of time, don’t panic. Make sure that you have marked your answer sheet for all the questions that you have answered so far. Then look ahead at the questions you have not yet read. Can you answer any of them quickly, without taking the time to do lengthy calculations? If you can, mark your answers in the time you have left. Every point counts!
• Use extra time to check your work. If you have time left over at the end of the test, go back and check your work. Make sure that you have marked the answer sheet correctly. Check any calculations you may have made to make sure that they are correct. Take another look at any questions you may have skipped. Can you eliminate one or more answer choices and make an educated guess? Resist the urge to second-guess too many of your answers, however, as this may lead you to change an already correct answer to a wrong one.