In 1985 two radiologists in Goiânia, Brazil, who treated people with cancer with radiation therapy machines containing cobalt-60 and cesium-137, moved to a new office. The physicians planned to take the devices with them, but the owner of their old clinic building claimed that the cesium-137 machine was his and held on to it. The dispute moved to the courts. Over the next year, the owner could not attract other physicians as tenants. The building remained empty, eventually fell into disrepair, and then was partially demolished. Two years later one of the radiologists returned to remove the cesium-137 unit but was stopped by police who had been called into the disagreement. The radiologist warned the owner that someone needed to take responsibility for what could happen with what he called “the cesium bomb,” and he wrote to the director of the Institute for Civil Servants, who had called the police to stop him from removing the machine, alerting him to the potential radiation hazard. The court’s response was to post a security guard at the facility twenty-four hours a day to deter people, especially would-be scavengers, from entering the building. Their presence worked—for four months, anyway.
On September 13, 1987, the daytime guard called in sick so he could go to a movie theater with his family and watch Herbie Goes Bananas. No replacement guard was sent. Two scavengers who had long heard rumors that there was valuable equipment in the building seized the opportunity to enter and saw the huge metal-encased cesium-137 device, which they assumed had value as scrap. When such a machine is intact, the cesium-137 is encased in a tungsten-and-steel capsule surrounded by lead, thus shielding anyone who comes close to it from exposure to the gamma rays that cesium-137 emits. (Someone standing three to six feet in front of such an unshielded source for one to two hours or less could receive a lethal dose of radiation.) The scavengers, unaware of the potential danger of their trophy, spent hours removing the shiny stainless-steel-cased rotating assembly containing the cesium-137, which looked like the most valuable part. It was certainly the most dangerous. The instant they removed the assembly, they were potentially exposed to the cesium-137 beam, as they would have been had the machine been turned on.
They put the assembly into a wheelbarrow, took it about a mile to one of their homes, and placed it under a mango tree in the garden. Because the assembly was no longer shielding the gamma rays emitted by the cesium-137, within forty-eight hours both scavengers suffered from dizziness, vomiting, and diarrhea. They went to a clinic and were told they had either a food allergy or food poisoning. Despite feeling ill, one of the scavengers continued trying to break open the capsule holding the cesium-137, convinced there was something even more valuable inside. Finally he punctured the thick glass window of the orange-sized capsule that held the cesium and scooped some out. He first assumed that the cesium was gunpowder, but he and a couple of coworkers could not ignite it. In touching it, however, they became contaminated; the effects would soon show up as radiation burns on their bodies.
Several days after stealing the device, one of the scavengers sold the dismantled parts to the owner of a scrap yard, who placed them in his home garage. This was when the danger escalated. That night the scrap dealer noticed a blue light emanating from his new acquisition. (The word “cesium” is derived from the Latin caesius, meaning “heavenly blue.” The blue in this instance was fluorescence of cesium rather than radiation itself.) He was immediately enamored of the glowing powder, which he thought was valuable or perhaps even supernatural, and took it into his house to show his family. Over the next three days, he invited friends and other family members to see this wondrous substance and gave them some of it. Not only were they contaminated, but they spread cesium-137 wherever they went afterward. One man took enough of the cesium to paint a cross on his abdomen and carried the rest home to show his family. His six-year-old daughter smeared cesium on her body, proudly showed her mother how she glowed, and then swallowed some that was on her hands when she ate. Contamination was spread further when the scrap dealer sold the remnants of the machine to a second dealer.
Fifteen days after the scavengers stole the machine, the wife of the scrap dealer who had bought the device from them realized that many people in her family and among her friends were getting sick. She went to the scrap yard where the remaining pieces of the radiation device were stored, put them in a plastic bag, and took a bus across town to a medical clinic, leaving a trail of radioactive cesium-137 and potentially exposing thousands of people along the way. At the clinic she placed the bag on the desk of the doctor who saw her and told him that the contents were making her family ill. The doctor, thinking she had a tropical disease, sent her to a hospital, where some of the others who had been in contact with the cesium-137 had already been admitted, with the same diagnosis. The doctor was at first content to leave the bag on his desk, but he then grew apprehensive that it might be dangerous and moved it to a courtyard, where it remained for a day.
One of the physicians at the hospital suspected that so many people with similar skin lesions might have been harmed by radiation. He contacted the Goiânia State Environmental Protection Agency and proposed that a medical physicist look at the items in the bag. Fortunately, such a physicist was visiting Goiânia, and the next day he borrowed a Geiger counter, used for geological measurements, from a government agency and set out for the hospital. He found the readings en route so high, he assumed the device was broken. He returned for a replacement and kept it turned off as he traveled to the hospital.
During the physicist’s absence to obtain the Geiger counter, the hospital physician grew more concerned about the bag’s contents and called the fire department. The physicist arrived just in time to stop the firefighters from throwing the bag into the river. After he turned on the replacement detector, he was stunned to find that whatever was in the bag was emitting radiation millions of times above normal.
News of an accident involving radioactivity spread mass confusion and worry. Authorities alerted the hospitals where the radiation victims had been and tried to find everyone who might have been exposed so that the spread of radiation could be stopped; they confiscated clothes from those known to have touched the cesium-137. The round-up eventually led to the monitoring of more than 110,000 people, many of whom were brought to the town’s soccer stadium for evaluation and triage. There they were given showers to decontaminate themselves and housed in tents. The easiest way to determine who in a large group is in most need of help after a radiation accident is to ask that everyone who has become nauseated to take one step forward. Nausea implies a dose of at least 1,000 millisieverts (more on these soon); this is the level at which, after two days, blood cells affected by radiation die, and complications like anemia, bleeding, and infection arise.
Here the story takes another turn. The Brazilian navy had a secret nuclear program designed to counter a perceived Argentinean effort to build a nuclear weapon, and officials worried that news of a radiation accident might scuttle their program. They gathered employees of a commercial nuclear power facility—the only people in the country with extensive radiation experience—and flew them to Goiânia. There they used Geiger counters to check the thousands of people in the football stadium for radioactive contamination. In all, 249 people were determined to have been in contact with cesium-137. One hundred twenty had slight radioactive residue on their skin or clothing and were quickly decontaminated by thorough washing. The remaining 129 required greater attention: 79 had skin or external exposure that required treatment but not hospitalization, and 50 showed a higher level of exposure; 20 of them were admitted to a hospital. Bone marrow failure (which halts the production of blood cells and is fatal if not reversed) developed in 14, and 10 of them and 4 other patients were secretly transported by plane to Marcílio Dias Naval Hospital (Hospital Naval Marcílio Dias) in Rio de Janeiro.
The doctors in Rio had little experience with a radiation emergency of this magnitude. However, Dr. Daniel Tabak, a hematologist, had worked with one of us. Bob Gale a year earlier had helped treat firefighters and others who received very high doses of radiation when they responded to the explosion and fires at the Chernobyl nuclear power facility. (He has participated extensively in treating victims of almost every major nuclear accident in the past twenty-five years and in assessing the long-term health implications of those accidents, including the one at Fukushima.) Tabak tracked Bob down in Bonn, Germany, where he had just spoken to a parliamentary committee about nuclear issues, and asked if he would come immediately to Rio.
As soon as Bob heard what had happened, he knew—from his experience with the Chernobyl victims and from his work with his colleague David Golde at UCLA—that recently developed hormones called recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF), which were then in clinical trials on people receiving anticancer chemotherapy, would be useful. Bob and his Soviet colleagues had used this drug at the Chernobyl accident (more on this later), and he knew it was not available in Brazil. It stimulates the bone marrow cells to produce granulocytes, the white blood cells that fight infections. The bone marrow of people with severe radiation sickness cannot produce sufficient blood cells to keep them alive, so doctors transfuse them with red blood cells (which carry oxygen) and with platelets (which clot blood), along with antibiotics and antivirus drugs. Granulocytes, however, cannot be effectively given by transfusion—they must be generated within the body.
Bob called Roland Mertelsmann, a colleague in Frankfurt am Main, a hundred miles away, who was testing the hormones. Mertelsmann and Sandoz, the Swiss pharmaceutical company with which he was working, agreed to give Bob some for use in Brazil. He sped to Frankfurt, collected the drug, packed in dry ice in a Styrofoam box, and barely made the last flight of the day.
Bob arrived in Rio with no visa and a box steaming carbon dioxide from the evaporating dry ice. The lack of a visa was not a problem, nor was the vaporous package. But his visibility was. A year after the Chernobyl disaster, Bob was still easily recognizable. The Brazilian navy didn’t want a doctor who was famous for treating radiation victims to be seen in Rio. Tabak met him, whisked him through immigration and customs, then had him lie down in the backseat of a car so that he would not be seen as they drove to a hotel and then the navy hospital.
Because the victims had absorbed cesium-137 by handling it and even by eating and drinking it, their bodies were radioactive (the cesium-137 in the person was radioactive, not the person) and thus were a risk to those who cared for them. No pregnant nurses or nurses of childbearing age were allowed onto the medical team because of the potential radiation damage to their unborn children. To avoid any unnecessary exposure to the radiation emitted from the victims, the doctors and nurses operated behind lead shields. That, however, proved impractical. These were acutely ill people, and it was impossible to care for them with such encumbrance. Bob and the others accepted that they would be exposed to radiation that had a low risk of causing them harm later in life. Fortunately, none have shown ill effects in the ensuing twenty-five years.
Four of the eight people who received the bone marrow hormone survived. The four who died included the wife of the original scrap dealer who had taken the bag of parts to the health center and the young girl who ate and smeared herself with the cesium-137. Radiation killed their white blood cells, which allowed bacteria to take over, and they died of their infections. One victim needed to have a forearm amputated because of severe radiation burns. But ten of the fourteen victims taken to Rio survived their ordeal, as did all those treated in Goiânia. (Bob had a much smaller drama. The admiral of the Brazilian navy in charge of the secret program liked him very much but worried that Bob might expose the program after he left the country, half-jokingly confiscated his passport just before his departure, then returned it.)
A key lesson from this story is that not being aware of the inherent dangers of radiation and radioactivity can prove harmful or even fatal. But another lesson is that the dangers of radiation are not necessarily what you suppose them to be. Sometimes an event that seems to have all the makings of a catastrophe capable of harming hundreds or thousands of people actually harms relatively few. There often is a great difference between what we fear and what is real, and that is a gap we hope to close.
Earth, born more than 4.5 billion years ago, is a radioactive planet in the radioactive solar system in the radioactive universe made by the Big Bang, which happened 9 billion years earlier. Radiation is older than the universe—thorium-232 has a half-life of about 14 billion years, almost three times longer than the age of Earth—yet we have known about it only since 1895, when the German physicist Wilhelm Conrad Röntgen (1845–1923) discovered X-rays. Without radiation, there would be no life on Earth.
All of us are radioactive. We, and our environment, exist by virtue of green plants that capture photons, the basic units of light energy—they are produced by thermonuclear fusion within the Sun. Plants use these photons via photosynthesis to separate water into hydrogen and oxygen. The hydrogen is then combined with carbon dioxide from the atmosphere to produce glucose, which the plant burns to produce energy. The oxygen is released into the atmosphere, and we and virtually all other living creatures breathe it. (There are some organisms that exist without oxygen.) Energy produced by burning glucose is transferred to us when we eat plants, or animals that feed on plants or plant products.
Subatomic particles and electromagnetic waves of radiation flow from the Sun through the universe and collide with Earth (and with us). Photons have the properties of both a particle and a wave but they have no mass, and they and the subatomic particles are much smaller than we can see or even imagine. Electromagnetic waves are categorized not by size but by their frequency (which is the opposite of their wavelength—the distance between two waves) and by their energy, which is proportional to their frequency: if you look at a line showing repeated waves, one wavelength is the distance from the peak of one to the peak of the next. Some waves are so close together that their wavelength is a thousand times smaller than the head of a pin. Other waves are so far apart that their wavelength is longer than half a mile. The shorter the distance between waves, the greater their energy and the greater their potential to harm. When waves or particles from the Sun or other sources strike matter (like us), their energy, or at least some of it, is transferred to that matter. The greater the energy transferred and the greater the radiation dose, the greater the danger to humans. The scale from the least dangerous waves (those with the longest wavelengths) to the most dangerous (those with the shortest) has radio waves at one end and then microwaves, infrared radiation, visible light, ultraviolet (UV) radiation, X-rays, and gamma rays at the other end. The difference between them is vast. An X-ray delivers about 10 million times more energy than a radio wave, which gives a sense of why radio waves don’t hurt us but X-rays can.
Atoms and subatomic particles are at the core of our existence, yet they are astonishingly small. To have a sense of just how small, let’s say a grapefruit is full of nitrogen atoms. If you made each of those atoms the size of a blueberry, the grapefruit would then have to be the size of Earth to hold them. For you to be able to see the nucleus of one atom, a blueberry would have to be the size of a football stadium; the nucleus would be the size of a small marble. It stretches the imagination to grasp just how dense a nucleus is, but try this: mentally, take 6 billion or so cars and squeeze them into a box 1 foot by 1 foot by 1 foot. And that’s just one atom. Even smaller than the nucleus are subatomic particles like protons and neutrons (which give the nucleus most of its mass) and electrons.
Electrons and high-speed electrons (also called beta particles) are atomic particles that are fundamental particles, meaning they cannot be broken into smaller parts (although some recent experiments suggest they can). Neutrons are also particles (composed of quarks, which are also fundamental particles). In contrast to protons (also composed of quarks), which have a positive electrical charge, neutrons have no charge. Two protons and two neutrons that stick together and have mass make up an alpha particle.
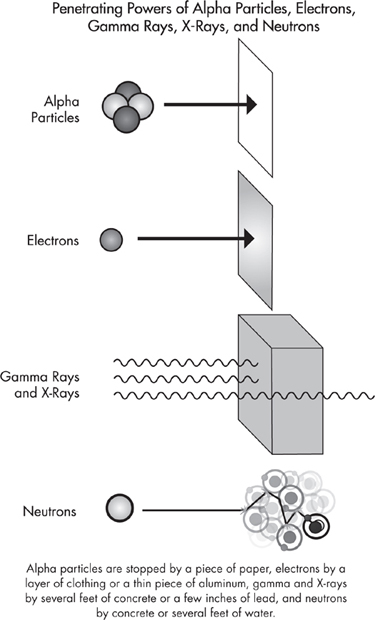
All three types of particles—electrons, neutrons, and alpha particles—can harm humans. Neutrons are the most dangerous because they are very energetic, can penetrate deeply, and deposit large amounts of energy into tissues. Alpha particles are intermediate in their danger to humans because they are comparatively large and they deposit all their energy locally, they do not penetrate as deeply as neutrons. Electrons are the least dangerous because they cannot penetrate very deeply and because they have relatively less energy to deposit into matter. The illustration on this page shows that electrons penetrate deeper than alpha particles, but as a particle moves through matter, an alpha particle deposits much more energy than does an electron. When an atom releases alpha particles, the process can also result in the release of gamma rays (high-energy electromagnetic radiation), which can also harm humans. Sometimes these particles, especially alpha particles, can enter the body in a place where they can cause considerable local damage. For example, people living in areas where there are high concentrations of radon gas inhale large numbers of radon atoms into their lungs, which then release alpha particles into the surrounding tissue. This is an important cause of lung cancer, especially in nonsmokers.
Electromagnetic waves cannot be seen, with one very important exception: visible light. (See the first illustration in the insert.) Visible light, though, is a very small part—less than 1 billion-billionth—of the electromagnetic spectrum, all parts of which are forms of radiation (energy in motion).
We live on a planet that is 93 million miles from the Sun—not too near, not too far, just right for our oxygen-and-carbon-based life. Life on Earth has evolved to exist in the conditions the planet offers. If we were a paltry 50,000 or 100,000 miles closer to or farther away from the Sun, the photons we rely on to sustain life would be either too strong or too weak for life as we know it.
We may think of ourselves as beings of thought and limb, of flesh and blood, but actually we are all atoms and molecules undergoing constant chemical reactions, trillions upon trillions of them every second, encased in the semipermeable membrane that is our skin. These reactions power our brain, our heart, our muscles, and our vision. They lead to growth of old cells or, when we exercise or need to repair an injury, to the birth of new cells; they make us age, and eventually, when they run out of steam, we die. When our chemistry changes, so do we. Cells that perform a function start doing it differently or not at all. Chemical changes in cells can be advantageous; for instance, radiation from the Sun, in the form of ultraviolet rays (UVB), triggers cells in the skin to produce vitamin D from cholesterol. Vitamin D is essential to help us absorb calcium from our diet and maintain our bony skeleton. Something like plants, we capture photons from the sun. But UVB rays can also be dangerous—they can cause mutations in the DNA of skin cells that can end in cancer. Our chemistry is our destiny.

Many forms of radiation, like microwaves and radio waves, have insufficient energy to cause important changes in the cells they strike. But other, more energetic forms can alter the structure of atoms they hit, forcing electrons out and producing a charged particle—an ion. These forms of radiation are termed ionizing radiations and they are everywhere. Some are produced by the natural decay of radionuclides (atoms that are radioactive) remaining from the creation of the universe. Others are man made or man caused, coming from exploding nuclear weapons, burning coal (which releases naturally occurring radionuclides locked inside raw coal), fissioning uranium in nuclear power facilities, and many other sources. Ionizing radiation in sufficient quantities can be a life changer.
Heat is a form of energy. The amount of heat it takes to turn a normal cell into a cancerous one is roughly that contained in a cup of hot chocolate. The difference is that the energy in the hot chocolate is broadly diffused and so warms the whole cup, whereas the energy in ionizing radiation is as focused as a firmly struck cue ball is to a rack of billiard balls. Just as the energy from the cue ball knocks apart the rack, ionizing radiation literally knocks an electron out of an atom and produces ions that can magnify radiation damage.
Atoms contain a nucleus surrounded by a cloud of electrons. Not all nuclei are stable. Unstable nuclei undergo radioactive decay, releasing the ionizing radiations we have been speaking about. Sometimes this nuclear instability is the result of a nucleus that absorbed a subatomic particle. Unstable nuclei can emit combinations of gamma rays, electrons, and subatomic particles. Rather as a wet dog shakes off water, the nucleus wants to be rid of what is not normal for it. In thermodynamic terms, it seeks the most stable configuration. The period of instability varies from substance to substance at a known rate called a half-life—the time it takes for one-half the radioactivity to be emitted. It can last from nanoseconds (copernicium-285) to billions of years (thorium-232, uranium-238). (We will go into the details in chapter 3.) Ionizing radiation can sometimes prevent cells from doing their work as designed. For example, when radiation damages DNA, it is usually repaired quickly. But if the repair is improper, the chemical changes can lead to a cancer, another illness, or death.
Radioactivity is in our food and our water, even in our bodies. It is part of our makeup and causes no known harm. There are several radioactive elements in each of us, among them naturally occurring radioactive forms of potassium (potassium-40) and carbon (carbon-14), as well as man-made isotopes like cesium-137 that result from nuclear fission. Each second, thousands of radioactive atoms in our bodies decay; sleep next to someone, and your bedmate will get a dose of radiation from you. Potassium-40, which to the body looks like normal nonradioactive potassium, is taken up by all cells but especially muscle cells. Men, who usually have more muscle mass than women, are on the whole more radioactive than women because they have more potassium-40.
About half of the radiation we normally receive comes from natural sources called background radiation. There are two major sources of background radiation: cosmic radiation, which comes from the universe, including our Sun (cosmic radiation increases when there are solar flares) and supernovas (that fling out particles when they explode); and terrestrial radiation, which comes from radionuclides in the Earth’s crust. An additional component comes from radiation in our body. We live in a sea of radiation. So when scientists want to analyze radioactivity in a sample of something (or us), they have to shield their radiation detectors with lead or other dense material to block out background radiation. Because the explosion of atomic bombs in the atmosphere released radionuclides that never before existed on Earth, objects made after 1945 contain man-made radioisotopes. In contrast, steel made before 1945 is less radioactive than that manufactured afterward and is prized for making radiation detector shields.
Radon-222, an odorless, colorless gas, is a decay product of radium-226 and a link in the uranium-238 and thorium-232 decay chain that ends in lead-206, which is not radioactive. It is everywhere on Earth (though unevenly concentrated), and it and its decay products (called radon daughters) account for about two-thirds of our annual background radiation dose. Radon-222 further decays into polonium-218 and bismuth-214, among other radioactive elements, both of which emit alpha particles. Because radon-222 is a gas, it can be inhaled, and the alpha particles it releases can be highly damaging to the lungs. Radon-222 gas gets trapped in unventilated basements. In areas with high concentrations of radium in the soil, radon-222 also enters the groundwater and evaporates (especially in hot water), so we inhale it when we shower. Radon-222 and related radionuclides are estimated to be the most common cause of lung cancer deaths in nonsmokers. (Some lung cancer in nonsmokers is attributed to so-called passive smoking, secondhand exposure to cigarette smoke.) The Environmental Protection Agency estimates that radon causes about 21,000 of the approximately 160,000 lung cancer deaths in the United States each year. Smoking is of course the largest cause overall, and radon gas and smoking may interact to increase lung cancer risk.

As we mentioned, the other part of the radiation we receive comes from man-made sources. About 80 percent of it comes from medical procedures like X-rays, CT scans, and nuclear medicine studies using radionuclides such as iodine-131 (thyroid scans), fluorine-18 (PET scans), technetium-99m (liver, spleen, and bone scans). (The “m” stands for metastable, which means that it decays rapidly into gamma rays.) The other roughly 20 percent of man-made radiation comes from televisions, computer screens, smoke detectors, heart pacemakers, porcelain teeth, and the like. Only a small part of our average man-made radiation exposure comes from occupational sources and the residual fallout from atmospheric nuclear weapons tests. About 1 percent of the 20 percent is the result of the nuclear fuel cycle—mining, transport, fissioning, and waste of the nuclear fuels that run nuclear power facilities.
Scientists know that the human body can tolerate a considerable amount of ionizing radiation, because we absorb a great deal that is naturally present in our environment. Still, it is wise to keep your exposure to radiation as low as is reasonably possible.
We aim to examine some of the cancers that ionizing radiations can cause—most commonly but not exclusively cancers of the blood (leukemia), breast, thyroid, lung, and brain; sources of ionizing radiation include uranium-235 (used in nuclear power facilities and the source of energy for the bomb dropped on Hiroshima); plutonium-239 (which originates in nuclear reactors and is produced by the capture of extra neutrons by uranium-238 to form uranium-239, which then undergoes a series of decays to form plutonium-239; it is also used as fuel in nuclear power facilities and was the source of energy in the bomb dropped on Nagasaki); iodine-131 (a radioactive by-product of fission that is released by nuclear power plant facilities and is a cause of thyroid cancer); cesium-137 (used in radiation therapy but deadly if not properly contained); polonium-210 (one of the cancer-causing agents in cigarette smoke); and strontium-90 (another by-product of uranium-238 fission, which settles in the teeth and other bones and can cause bone and other cancers). We will look at the risks associated with other topics of broad concern: radiated food; radiation therapy given to treat cancer; nuclear power facilities; radioactive waste; and nuclear terrorism. At the end of the book, we will answer commonly asked questions.
We also will consider the risks and advantages of using nuclear energy to generate electricity. An understandable response to the accident at the Fukushima Daiichi nuclear power facility and the release of radiation into the air, ground, and sea is to question the wisdom and safety of nuclear energy in general and to ask whether we should abandon its use. In Japan, there is a powerful movement to halt the use of nuclear energy; the last of Japan’s more than fifty commercial reactors, which provided 30 percent of its electricity, was shut down in May 2012. (The next month, however, two reactors were reopened.) In May 2011, the German government voted to shut all of that nation’s nuclear plants by 2022, though it will continue to buy electricity produced by nuclear energy from its next-door neighbor France. Others argue that nuclear energy is essential to power the exponentially growing global need for electricity. In October 2011 the French Alternative Energies and Atomic Energy Commission (CEA) announced plans to construct a sixtieth nuclear reactor and is looking to sell its decades of technological expertise in building nuclear power plants to India, China, Britain, Poland, South Africa, Turkey, and Brazil.
The specter of radiation is so frightening to many people that it eclipses reality. We tend to forget that the 9.0-magnitude earthquake in Japan that triggered the failure at the Fukushima Daiichi nuclear power facility was so unimaginably powerful that it moved the entire 137,893 square miles of the main island of Honshu eight feet eastward; that the tsunami obliterated whole towns, drowned almost 20,000 people, and left more than 300,000 temporarily or permanently homeless; and that although additional radioactive leaks from the crippled nuclear reactors still pose a danger, no one has died from radiation poisoning, and the predictions are that the released radiation may cause only a slight, probably undetectable, increase in cancer risk in the exposed population over the next several decades. The stakes for not making intelligent decisions about radiation are so high that we must equip ourselves with as much knowledge as we can and not let our sometimes illogical fears influence our judgment or allow us to fall victim to the social, economic, and psychological damage they can inflict. But we must also carefully weigh the risks of nuclear technologies and seek the best balance of benefit and risk.