Selection of New Probiotics: The Case of Streptomyces
Sneha Hariharan⁎; Selvakumar Dharmaraj† * University of Madras, Chennai, India
† Karpagam Academy of Higher Education, Coimbatore, India
Abstract
Probiotics are live organisms which can benefit host immunity by providing essential nutrients as growth supplements and increasing resistance to infectious diseases in fish and shellfish. Currently there are several commercial probiotic products prepared from various bacteria and yeast sources, such as Bacillus sp., Lactobacillus sp., Enterococcus sp., Carnobacterium sp., and Saccharomyces cerevisiae, and their applications are managed by careful recommendations. Use of these probiotic products is quite satisfactory, yet new diseases continue to arise, leading to the search for new probiotics essential in improving the host’s resistance to fight against emerging diseases in aquaculture. Marine Streptomyces has been designated as a drug for a long time. Additionally, to date, a large number of bioactive substances have been isolated. Despite many significant beneficial features which make them good probiotics, marine Streptomyces have rarely been used in aquaculture. However, this group of bacteria promises to supply the most potential probiotics in the near future.
Keywords
Marine Streptomyces; Probiotics; Aquaculture; Gut microbes; Antimicrobial agents
1 Introduction
1.1 Aquaculture Status of the World
Feeding an expected global population of 9.6 billion by 2050 is a daunting challenge that is engaging researchers, technical experts, and leaders the world over. A relatively unappreciated yet promising fact is that fish can play a major role in satisfying the palates of the world’s growing middle income class while also meeting the food security needs of those with less income. Already, fish represents 16% of all animal protein consumed globally, and this proportion of the world’s food basket is likely to increase as consumers with rising incomes seek higher value seafood, while aquaculture steps up to meet increasing demand. Aquaculture has grown at an impressive rate over the past decades. It has helped to produce more food fish, kept the overall price of fish down, and made fish and seafood more accessible to consumers around the world (Word Bank Report, 2013). For this reason, greater investment is needed in the industry for new and safer technologies, their adaptation to local conditions, and their adoption in appropriate settings. Fisheries and aquaculture are sources of not just health, but also of wealth. Employment in the sector has grown faster than the world’s population, providing jobs to tens of millions and supporting the livelihoods of hundreds of millions. Fish continues to be one of the most-traded food commodities worldwide. It is especially important for developing countries, sometimes worth half the total value of their traded commodities. Global fish production continues to outpace world population growth, and aquaculture remains one of the fastest-growing food producing sectors (FAO, 2016). FAO reports concerning aquaculture status of the world for the years 2015 and 2016 are still in development. The FAO report for the year 2014 is complete and clearly discussed below.
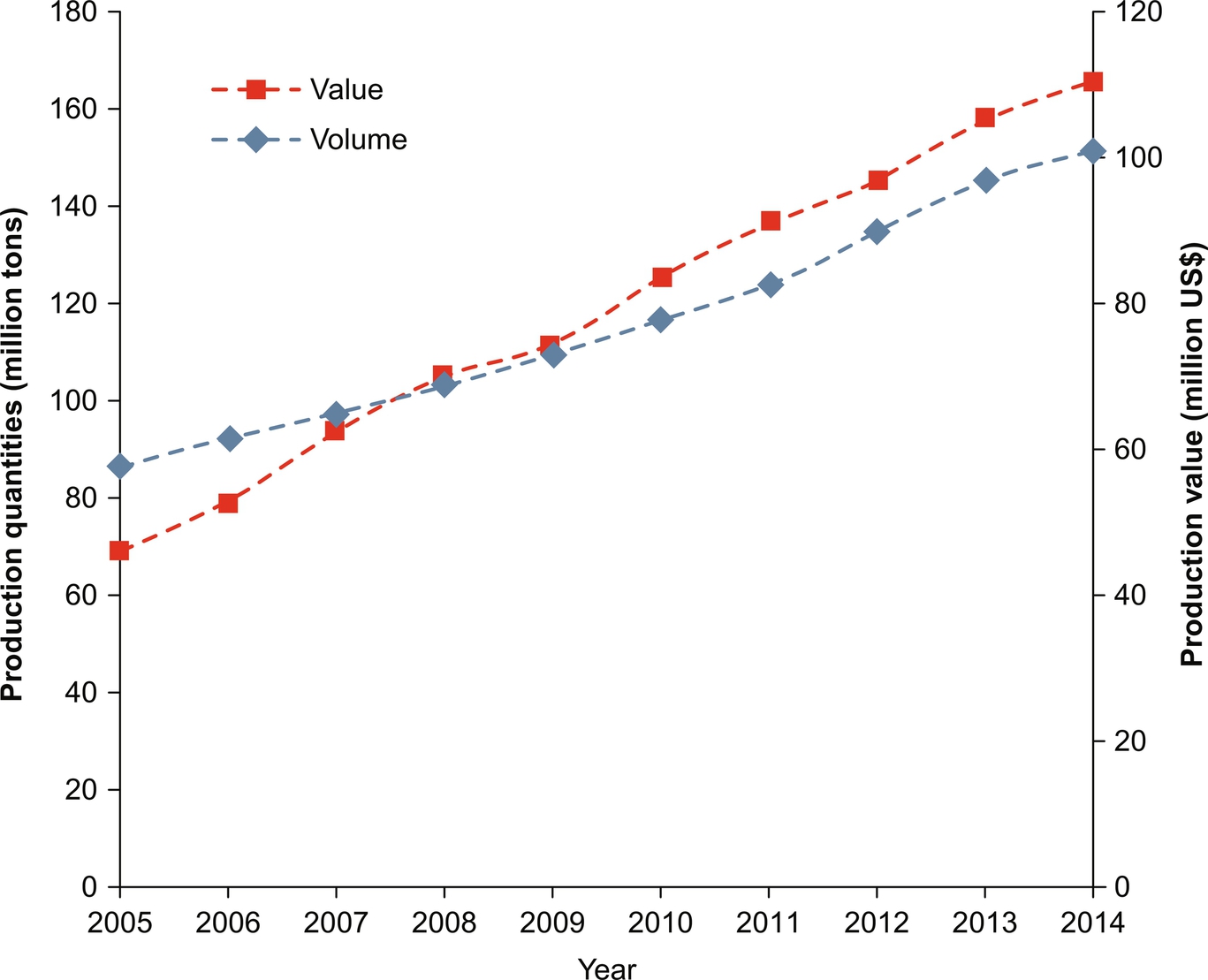
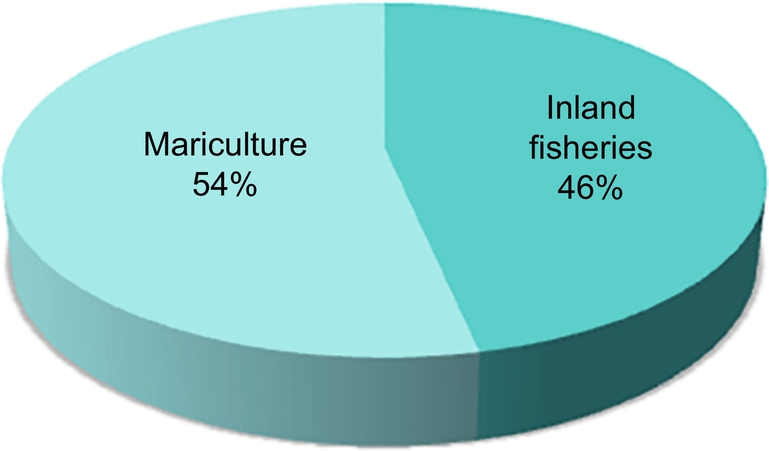
Global aquaculture production has experienced tremendous growth over past 50 years, starting from a production of less than a million tons in the early 1950s to over 167.2 million tons in 2014 (Fig. 1) and is expected to rise in accordance with the demands of the world’s growing population. Individually, the inland fisheries and mariculture alone contribute around 47.19 and 53.90 million tons in the year 2014 (Fig. 2). Aquaculture production has increased at an average annual growth rate of 5.8%, from 44.3 million tons in 2005 to 73.8 million tons in 2014. The value of aquaculture production is estimated at USD $160.2 billion in 2014, and may continue to rise in the future (FAO, 2016).
World food fish aquaculture production in 2014 consisted of 49.9 million tons of finfish (68%), 16.1 million tons of mollusks (22%), 6.9 million tons of crustaceans (9%), and 0.9 million tons of other aquatic animal species (1%). Inland aquaculture produced 43.6 million tons of finfish, representing 59% of world food fish aquaculture in 2014. The above quantities do not include production of aquatic plants, mostly seaweeds, that reached 28.5 million tons in 2014, of which aquaculture produced 27.3 million tons (96%). Carrageenan seaweeds (including Kappaphycus alvarezii and Eucheuma spp.) are the main cultured seaweeds (11 million tons), followed by Japanese kelp (7.7 million tons) (FAO, 2014).
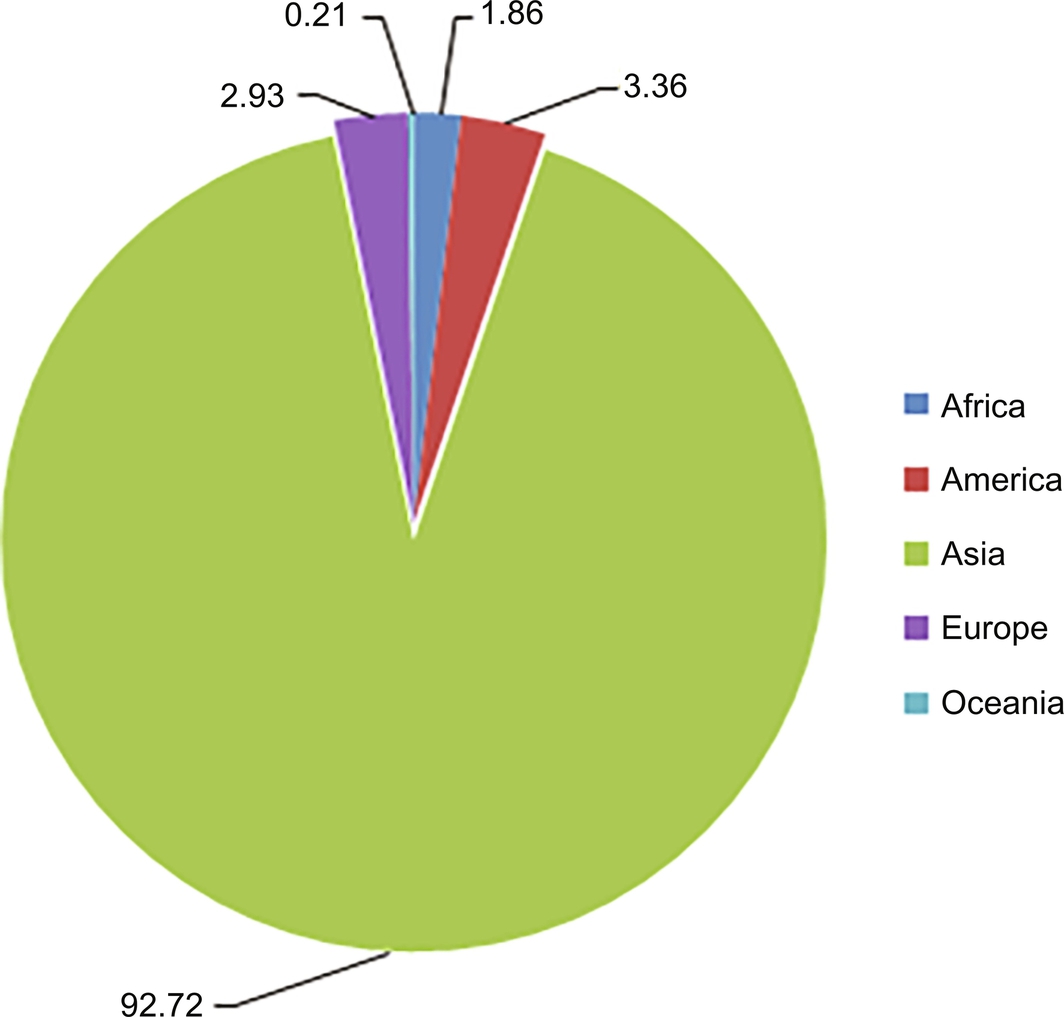
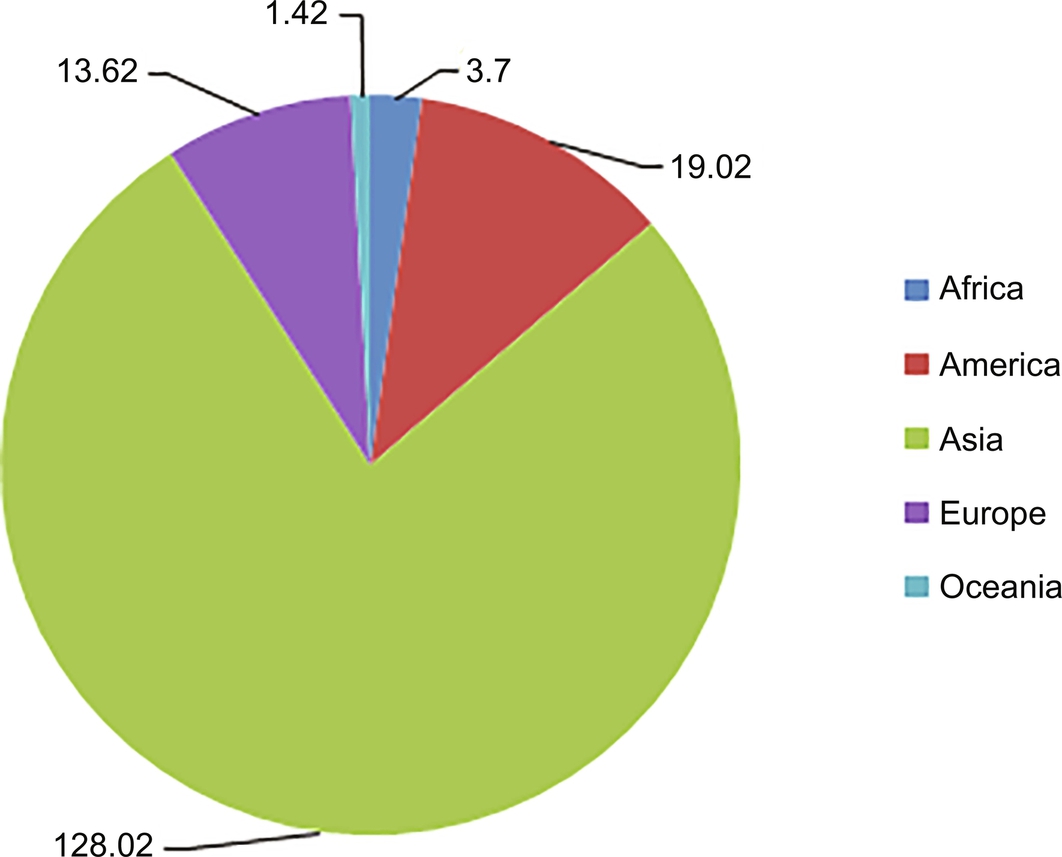
Aquaculture experienced high average annual growth of 10.8% in the 1980s and 9.5% in the 1990s. However, the growth rate eased to an average of 5.8% in the period of 2005–14. It has been estimated that Asia has the highest aquaculture production (quantitative and value) when compared with rest of the continents (Figs. 3 and 4). The contribution of aquaculture to total fish production has risen steadily, reaching 44% in 2014. Asia has produced more farmed fish than wild fish since 2008, with aquaculture reaching a share of 55% of its total production in 2014. In the same year, the contributions from aquaculture were between 17% and 18% for other continents, except Oceania (13%). In 2014, the top ten aquaculture producers (excluding aquatic plants and nonfood products) were China (45.5 million tons), India (4.9 million tons), Indonesia (4.3 million tons), Vietnam (3.4 million tons), and Bangladesh (2 million tons), followed by Norway, Chile, Egypt, Myanmar, and Thailand. They contributed 89% of the world’s production (FAO, 2014).
Many millions of people around the world find a source of income and livelihood in the fisheries and aquaculture sector. In 2014, about 56.6 million people were engaged in the primary sector of capture fisheries and aquaculture and, of this total, 36% were engaged full time, 23% part time, and the remainder were either occasional fishers or of unspecified status. For the first time since the period 2005–10, the total engagement in fisheries and aquaculture did not increase (FAO, 2016).
1.2 Implementation of Probiotics Usage in Aquaculture
Although aquatic food production through aquaculture is the fastest growing sector, and vaccines are being developed and marketed in aquaculture, disease is still a major problem in the aquaculture industry (Bondad-Reantaso et al., 2005). During the last decades, chemical additives and veterinary medicines, especially antimicrobial agents, were used to prevent and control diseases that occur in aquaculture (Wang and Xu, 2004; Cabello, 2006; Lupin, 2009). However, the risks associated with the transmission of resistant bacteria from aquaculture environments to humans, and the introduction in the human environment of nonpathogenic bacteria, containing antimicrobial resistance genes, and the subsequent transfer of such genes to human pathogens existed according to FAO (2005). Consequently, serious loss because of the spread of diseases has been increasingly recorded. This is a significant constraint on aquaculture production and trade, and negatively affects economic development in many countries.
The utility of antimicrobial agents as a preventive measure has been questioned, given extensive documentation of the evaluation of antimicrobial resistance among pathogenic bacteria. On the other hand, antibiotics inhibit or kill beneficial microbiota in the gastrointestinal (GI) ecosystem, but also made antibiotic residue accumulate, resulting in fish products that are harmful for human consumption (WHO, 2006). Because of the health risks associated with the use of antibiotics in animal production, there is a growing awareness that antibiotics should be used with more care (Defoirdt et al., 2011).
Resistance mechanisms can arise one of two ways: chromosomal mutation or acquisition of plasmids. Chromosomal mutations cannot be transferred to other bacteria, but plasmids can transfer resistance rapidly (Lewin, 1992). Several bacterial pathogens can develop plasmid-mediated resistance. Plasmids carrying genes for resistance to antibiotics have been found in marine Vibrio species and they could be the bacteria found in aquaculture ponds, transfer via plasmids, transduction via viruses, and even direct transformation from DNA absorbed from the particles in the water, or on sediment surfaces, could all be likely mechanisms for genetic exchange (Moriarty, 1997). For example, transference of multi-drug resistance occurred in Ecuador during the cholera epidemic (1991–94) in Latin America, beginning among persons who were working on shrimp farms. Although the original epidemic strain of Vibrio cholerae 01 was susceptible to the 12 antimicrobial agents tested, in coastal Ecuador it became multidrug resistant by the transference of resistance genes of noncholera vibrios that are pathogenic to shrimp (Weber et al., 1994). In addition, other evidence of the transmission of resistance between aquaculture ecosystems and human has been demonstrated, when a novel florofenicol resistance gene floR, in Salmonella typhimurium DT104, which also confers resistance to chloramphenicol and is almost identical by molecular sequence to the florofenicol resistance gene was first described in Photobacterium damsela, bacterium found in fish (Angulo, 2000).
A significant issue affecting production is the loss of stock through disease. Diseases caused by Vibrio spp. and Aeromonas spp. are commonly implicated in episodes of mortality. When faced with disease problems, the common response has been to turn to antimicrobial drugs. The livestock and aquaculture industries have experienced widespread use of antimicrobial drugs in their practices. While the use of such products has an obvious benefit to treat animals infected by bacterial disease, the use of antimicrobial drugs has been either prophylactic (preventative), or for growth enhancement (Van den Bogaard and Stobberingh, 2000). Certain antimicrobial drugs have been shown to positively influence growth of livestock and are used widely (Acar et al., 2000; Phillips et al., 2004). Given this, and the desire to prevent establishment of pathogenic bacteria, it is argued that antimicrobial drugs have been widely overused (Aarestrup, 1999). Schwarz et al. (2001) provided a good overview of antimicrobial drugs used in animals and the associated potential hazards. In aquaculture, this was felt most dramatically in the shrimp industry where massive increases in production, overcrowding of animals and unchecked antibiotic usages led to the emergence of numerous antibiotic resistant bacteria and production crashes in many Asian countries (Karunasagar et al., 1994; Moriarty, 1999). For example, production figures for shrimp in the Philippines dropped by 55% in 2 years, from 90,000 to 41,000 tons between 1995 and 1997. In fact, it has never recovered and, in 2002, a mere 37,000 tons was produced. An industry previously worth USD $760 million is now worth only USD $240 million (FAO, 2007). Similarly, Thai shrimp production dropped by 40% between 1994 and 1997 due to disease problems, bacterial pathogens, and shrimp viruses. Within aquaculture, there are numerous reports of antibiotic resistant bacteria of farm origin. However, the risk is not just the potential loss to the farmer. The emergence of antibiotic resistant bacteria on aquaculture farms could pose a risk to human health. There are many reports illustrating the transferal of resistant genes between bacteria. This process means antibiotic-resistant bacteria originating from a shrimp farm could potentially transfer plasmids to bacteria involved in human health problems. Studies point to a farm animal origin in certain antibiotic-resistant bacteria genes that have made their way into human bacteria. However, recent reports argue against this phenomenon. The argument is based on the view that, although antibiotic-resistant bacteria have arisen in animal husbandry through the use of antimicrobials, there is insufficient data to show a link to resistant gene transferal to humans. They argue in favor of the beneficial role antibiotics play in farming, and caution against premature, unscientific decisions to restrict antibiotic use. Regardless of which argument is true, governments and organizations have introduced much tighter restrictions for antibiotic usage in animal production (Molina-Aja et al., 2002; Sahul Hameed et al., 2003; Alcaide et al., 2005).
In 1997, the European Union (EU) initially banned the use of avoparcin, and in 1999, included virginiamycin, spiramcin, tylosin, and bacitracin as banned growth promoters in animal feed and also implemented a ban on the use of all nontherapeutic antimicrobials in animal production (Delsol et al., 2005). The United States has been less stringent. There was a proposal in 2000 to introduce a ban on the use of fluoroquinolone, and there was concern also about the use of virginiamycin (Nawaz et al., 2001). More recently a bill called “Preservation of Antibiotics for Medical Treatment Act of 2005” was presented in the US congress. This act would see a ban on the nontherapeutic use of any drug intended for human use as well as in the production of feed animals (Martin, 2005). Other countries which currently have less antibiotic control, such as many of the Asian countries, are likely to be pressured through foreign restrictions, via the export markets which are being tightly controlled for antibiotic-contaminated products. Despite chloramphenicol being banned in Thailand since 1999 as a result of worldwide concern over its use in animal production, trace levels are still detected in shrimp from Thailand, causing a temporary ban of Thai shrimp by the EU (Heckman, 2004). Chloramphenicol has also been detected in shrimp from Myanmar, India, Pakistan, and Vietnam, highlighting the continued misuse of antimicrobial drugs in Asian shrimp farming. A leading example in the eradication of antibiotic use can be seen in the Norwegian salmon industry. After concern about the use of antibiotics in the late 1980s, there has been a 95% drop in usage from 50 to 1 ton annually. During the same period, salmon production has increased 10-fold from about 5500 to 55,000 tons. The turnaround has been attributed to the use of vaccines, better husbandry, and selective breeding programs (Maroni, 2000). There is a developing social attitude against unnecessary use of antimicrobial drugs and where possible, farmers now seek to move away from nonessential antimicrobial drug use. Several alternative methods have been considered to improve the quality and sustainability of aquaculture production (Li et al., 2006).
Science-based knowledge on probiotics and prebiotics has increased in recent years (Subasinghe et al., 2009; FAO, 2006). The use of probiotics or beneficial bacteria, which control pathogens through a variety of mechanisms, is increasingly viewed as an alternative to antibiotic treatment. The use of probiotics in human and animal nutrition is well documented (Rinkinen et al., 2003), and recently, they have begun to be applied in aquaculture (Gatesoupe, 1999; Gomez-Gil et al., 2000; Irianto and Austin, 2002a,b). While probiotic research in aquaculture first focused on fish juveniles, much attention has recently been given to larvae of fish, shellfish, and live food organisms in aquaculture as they are easily prone to diseases, and they provide health benefits in several ways. They are important sources for C, N, and S cycles, and any imbalance in the microflora of systems leads to pathogenesis (Rengpipat, 1996).
Now, numbers of preparations of probiotics are commercially available and have been introduced to fish, shellfish, and mollusk farming as feed additives, or are incorporated in pond water (Prado et al., 2010). According to the producers’ claims, these products are effective in supporting the health of aquatic animals and are also safe. However, there are doubts with regard to the general concept of probiotics and to these claims. Indeed, the current explanations and principles are still not enough to describe what probiotics actually are, where they come from, and what they can do (Wang et al., 2008). Thus, there is clearly a need for increasing our knowledge of aquaculture animals and of effective preparation, technological applications, and safety evaluation of probiotics. The importance of aquaculture products is set to increase dramatically as a result of overfishing of the world's waters and a continuing increase in demand for seafood. With increasing demand for environment-friendly aquaculture, alternatives to antibiotic growth promoters in fish nutrition are now widely accepted. The chapter mainly focuses on the importance of marine Streptomyces which can be an alternative and replacement food. This chapter also discusses the prospects of using marine Streptomyces as probiotics and their limitations in aquaculture.
2 Probiotics
2.1 Definition
Probiotics are beneficial microorganisms, or their products, that provide health benefits to the hosts. These probiotics has been used in aquaculture as disease control agents, or as feed supplements, to improve growth and in some cases, as a means of replacing antimicrobial compounds (Moriarty, 1997; Dharmaraj and Dhevendaran, 2010). Much research has been carried out in the field of probiotics over the past 30 years, but the original idea was possibly formed by Metchnikoff in the early 1900s. Metchnikoff (1907) theorized that human health could be aided through the ingestion of fermented milk products. The word “probiotic” was introduced by Parker (1974), who defined it as “organisms and substances which contribute to intestinal microbial balance.” The term probiotic means “for life,” originating from the Greek words “pro” and “bios” (Gismondo et al., 1999). According to Browdy (1998), one of the most significant technologies that evolved in response to disease control problems is the use of probiotics. Probiotics are live microbes that can be used to improve the host intestinal microbial balance and growth performance. Development of probiotics in aquaculture management will reduce the prophylactive use of antimicrobial drugs, as the recent overdependence on the antimicrobial drugs poses potential hazards to people who consume them (Salminen et al., 1999).
Fuller (1989) proposed the widely accepted definition for probiotics, which he gives as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.” Fuller's definition was a revision of the original probiotic concept which referred to protozoans producing substances that stimulated other protozoans (Lilly and Stillwell, 1965). Several modifications were put forward to shorten the definition for probiotics (Gram et al., 1999; Irianto and Austin, 2002a,b). Verschuere et al. (2000) proposed a definition which states that “a live microbial adjunct which has a beneficial effect on the host by modifying the host associated or ambient microbial community, by ensuring improved use of the feed or enhancing its nutritional value, by enhancing the host response towards disease, or by improving the quality of its ambient environment.” Kesarcodi-Watson et al. (2008) defined it as “it should benefit the host either nutritionally or by changing its immediate environment.” Current probiotic applications and scientific data on mechanisms of action indicate that nonviable microbial components act in a beneficial manner, and this benefit is not limited just to the intestinal region (Salminen et al., 1999). The concept of probiotic activity has its origins in the knowledge that active modulation of the gastrointestinal tract (GIT) could confer antagonism against pathogens, help development of the immune system, provide nutritional benefits, and assist the intestinal mucosal barrier (Vaughan et al., 2002).
Today probiotics are quite commonplace in health-promoting “functional foods” for humans, as well as therapeutic, prophylactic, and growth supplements in animal production and human health (Senok et al., 2005). Multiple ways exist in which probiotics could be beneficial, and these could act either singly or in combination for a single probiotic. These include: inhibition of a pathogen via production of antagonistic compounds, competition for attachment sites, competition for nutrients, alterations of enzymatic activity of pathogens, immune-stimulatory functions, and nutritional benefits such as improving feed digestibility and feed utilization (Bomba et al., 2002). It is often reported that a probiotic must be adherent and colonize within the GIT, replicate to high numbers, produce antimicrobial substances, and withstand the acidic environment of the GIT (Dunne et al., 1999; Mombelli and Gismondo, 2000). However, these descriptions are misleading. These beliefs are based on the understanding that a probiotic must become a permanent member of the intestinal flora. While bacteria with this capacity are common, and much probiotic research focuses on attachment capacity of bacteria, it has actually been demonstrated that transient bacteria can also exert beneficial effects (Isolauri et al., 2004). Additionally, contrary to the requisite of being able to attach to mucus and produce antimicrobial substances, a probiotic need only possess one mode of action. Multistrain and multispecies probiotics have proven that it is possible to provide synergistic bacteria with complementary modes of action to enhance protection (Timmerman et al., 2004).
2.2 Mode of Action
There are three general modes of probiotic actions that have been classified as follows:
- (1) Probiotics might be able to modulate the host’s gut defense mechanism, including the innate as well as the acquired immune system, and this mode of action is most likely important for the prevention and therapy of infectious diseases, as well as for the treatment of inflammation of the digestive tract or parts thereof.
- (2) Probiotics can also have a direct effect on other microorganisms, commensal and/or pathogenic ones, and this principle is of importance in many cases for the prevention and therapy of infections and restoration of the microbial equilibrium in the gut.
- (3) Finally, probiotic effects may be based on actions affecting microbial products, host products and food ingredients and such actions may result in inactivation of toxins and detoxification of host and food components in the gut.
According to the above postulates, all three modes of probiotic actions are likely associated with gut and/or gut microbiota (Oelschlarger, 2010). Therefore, the fact (probiotics may affect the microbial products, host products and food ingredients, which may result in inactivation of toxins, detoxification of host and food components in the gut) that has apparently dealt with is another “organ,” the so-called “microbiotic canal” with the increased knowledge of the specific activity of the gut microbiota (Wolf, 2006). In general, the gut microbiota remain relatively stable throughout life once established, although they can be influenced by several factors such as mode of delivery, hygiene, and the use of antibiotics. The gut microbiota with the epithelium and mucosal immune system orchestrate a network of immunological and nonimmunological defenses, providing both protection against pathogens and tolerance to commensal bacteria and harmless antigens (Sanz and Palma, 2009). The important role of commensal bacteria in development of an optimally functioning mucosal immune system was demonstrated in germ-free animals (Tlaskalová-Hogenová et al., 2004). Therefore, the imbalance of gut microbiota has been linked to several diseases including inflammatory bowel diseases, periodontal disease, rheumatoid arthritis, atherosclerosis, and allergies. So, probiotics, that is, microbial strains that have beneficial effects on the host, are thought to benefit this intestinal ecosystem (Julio and Marie-Joséé, 2011).
In addition, some probiotics strains also induce the secretion of multiple antimicrobial materials by intestinal Paneth cells through cell-autonomous MyD 88-dependent toll-like receptor activation (Vaishnava et al., 2008) and regulate the alterations of permeability related with infections, stress, and inflammatory conditions (Lutgendorff et al., 2008). As for the aquatic animals such as fish and shrimp, the colonization of the gastrointestinal tract starts immediately after hatching and is completed within a few hours to modulate expression of genes in the digestive tract, thus creating a favorable habitat for them and preventing invasion by other bacteria introduced later into the ecosystem (Balcázar et al., 2006). This is attributed to competitive exclusion mechanisms and improved immune system development and maturation. Intake of probiotics has been demonstrated to modify the composition of the microbiota, and therefore assist in returning a disturbed microbiota (by antibiotics or other risk factors) to its normal beneficial composition (Gómez and Balcázar, 2008). As for the mechanisms during this physiological process, the production of antimicrobial substances, competition for nutrients or adhesion receptors, inhibition of virulence gene expression, and enhancement of the immune response are all included (Irianto and Austin, 2002a,b; Vine et al., 2004; Kim and Austin, 2006; Balcázar et al., 2007). However, the exact mechanism by which these probiotics do this is not known. Advances in the understanding of the mechanisms between gut microbiota and probiotics and how the immune system of aquatic animals generally responds to gut microbiota would be of great help to identify the molecular targets of probiotics and the biomarkers of their effects, and to provide sounder evidences on their benefits on physiologic conditions and immune-mediated disorders.
Also proposed are other possible modes of action of probiotics that include the inhibition of a pathogen through the production of bacteriocin like compounds, competition for attachment sites, competition for nutrients (particularly iron in marine microbes), alteration of enzymatic activity of pathogens, immuno-stimulatory functions, and nutritional benefits such as improving feed digestibility and feed utilization (Kesarcodi-Watson et al., 2008; Martinez Cruz et al., 2012). In order to achieve this probiotic status, the microbes have to follow certain number of criteria in terms of their biosafety and function. The advantageous characteristics of a potential probiotic include (i) should not be destructive to the host, (ii) proper transportation to the active site and their ability to survive in that environment, (iii) their potential in colonizing and propagating in the host, and (iv) virulence genes or antibiotic resistance genes should not be expressed (Hai, 2015).
2.3 Common Microorganisms Used as Probiotics
Probiotics currently used in aquaculture industry include a wide range of taxa—from Lactobacillus, Bifidobacterium, Pediococcus, Streptococcus, Carnobacterium spp., Bacillus, Flavobacterium, Cytophaga, Pseudomonas, Alteromonas, Aeromonas, Enterococcus, Nitrosomonas, Nitrobacter, Vibrio spp., and yeast (Sahu et al., 2008; Hemaiswarya et al., 2013). While using some beneficial probiotic bacteria for fish, some might be highly pathogenic, Vibrio alginolyticus for example, will lead to destructive effects in the aquaculture systems. Therefore, it is necessary to take care in the choice of probiotic before administration. The best-known probiotic strains such as Bifidobacteria, Lactobacilli, and Streptococcus thermophilus are employed as the dietary supplementation in the aquaculture industry and they will increase the efficiency and sustainability of aquaculture production (Kim et al., 2007).
Typically, the lactic acid bacteria (LAB) have been widely used and researched for human and terrestrial animal purposes, and LAB are also known to be present in the intestine of healthy fish (Hagi et al., 2004). LAB mainly focused on the fact that they are natural residents of the human gastrointestinal tract (GIT) with the ability to tolerate the acidic and bile environment. LAB also functions to convert lactose into lactic acid, thereby reducing the pH in the GIT and naturally preventing the colonization by many bacteria (Klewicki and Klewicka, 2004). The most widely researched and used LAB is lactobacilli and bifidobacteria (Ross et al., 2005).
Other commonly studied probiotics include the spore forming Bacillus spp. and yeasts. Bacillus sp. have been shown to possess adhesion abilities, produce bacteriocins (antimicrobial peptides) and provide immune-stimulation (Cladera-Olivera et al., 2004; Barbosa et al., 2005). The strains appear to be effective probiotics and commercial products containing such strains have been demonstrated to improve shrimp production to a level similar to that when antimicrobials are used (Decamp and Moriarty, 2006). Bacillus spp. hold added interest in probiotics as they can be kept in the spore form and therefore stored indefinitely on the shelf (Hong et al., 2005). The yeast, Saccharomyces cerevisiae, also has been commonly studied, whereby immune-stimulatory activity was demonstrated, and production of inhibitory substances was shown (Van der Aa Kühle et al., 2005).
2.4 Application of Probiotics in Aquaculture
When looking at probiotics intended for an aquatic usage it is important to consider certain influencing factors that were fundamentally different from that of terrestrial based probiotics (Kesarcodi-Watson et al., 2008; Gatesoupe, 1999). A fairly constant habitat of resident microbiota in the gastrointestinal tract of terrestrial livestock is important, whereas most microbiota is transient in aquatic animals (Moriarty, 1999). A shift in the intestinal microflora of Atlantic halibut (Hippoglossus hippoglossus) larvae during first feeding was studied and the results showed the transition from a prevailing Flavobacterium spp. intestinal flora to an Aeromonas spp./Vibrio spp. Dominant flora occurred when first feeding commenced (Bergh et al., 1994). It indicated that the gut microbiota of aquatic animals may change rapidly with the intrusion of microflora from water, live food, and an artificial diet. In addition, aquatic animal and microorganisms share the same ecosystem in the aquatic environment, and it suggested an interaction between the microbiota, including probiotics, and the host is not limited to the intestinal tract.
Most probiotics used in aquaculture belong to the lactic acid bacteria, of the genus Bacillus, to the photosynthetic bacteria or to the yeast, bacteriophages, microalgae, although other genera or species have also been mentioned (Lauzon et al., 2008). The wide applications belong to endospore-forming members of Bacillus genera (Hong et al., 2005), in which Bacillus subtilis is commonly used in aquaculture. One of the first evaluations of commercial products focused on a bacterial preparation called Biostart that is derived from Bacillus isolates. It was used during the production of cultured catfish studying the effect of inoculum concentration (Queiroz and Boyd, 1998). In 1998, Moriarty (1998) reported that the use of commercial probiotic strains of Bacillus spp. increased the quality and viability of pond-raised shrimp. Meanwhile, Chang and Liu (2002) evaluated the effect of Enterococcus faecium SF68 and Bacillus toyoi isolates present in Cernivet LBC and Toyocerin, respectively, to decrease the mortality of the European eel because of the edwardsielosis, ensuring greater efficiency with E. faecium SF68. It is relevant to note that E. faecium has long been known as a probiotic for humans, whereas B. toyoi has been used with terrestrial animals. Moreover, a B. subtilis strain combined with hydrolytic enzymes to produce Biogen, was used to supplement the feed of Oreochromis niloticus, obtaining significant increases in productivity (El-Haroun et al., 2006).
Many studies have reported promising results using a single beneficial bacterial strain as probiotic in the culture of many aquatic species (Tovar-Ramírez et al., 2010; Wang and Gu, 2010; Zhou et al., 2010; Wang, 2011). It is important to consider the possibility of using different species, as suggested by Noh et al. (1994) and Bogut et al. (1998). The effect of probiotics, photosynthetic bacteria (Rhodobacter sphaeroides) and Bacillus sp. (B. coagulans) on growth performance and digestive enzyme activity of the shrimp, Penaeus vannamei, was investigated and the results showed that the effects were related to supplementation concentrations of probiotics and thus use of a 10 g/kg (wet weight) supplement of probiotics in shrimp diet was recommended to stimulate productive performance (Wang, 2007). A mixture of Bacillus probiotic bacteria (Bacillus subtilis, Bacillus licheniformis and Bacillus pumilus) was also evaluated in the gilthead sea bream (Sparus aurata) larviculture focusing on their effect on survival, growth, and general welfare (Avella et al., 2010). The data generated in this study show the benefit of administration of Bacillus probiotic mixture in terms of stress response and growth, and provide scientific and technical support for the implementation of sustainable development of sea bream aquaculture. Similar results were also observed in olive flounder supplemented with Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus sakei, Bacillus subtilis, and Saccharomyces cerevisiae as individual and mixed enriched diet (Harikrishnan et al., 2011a). Lactobacilli probiotics individually or mixed with a Sporolac-enriched diet were used to enhance the immune status, thereby improving the disease resistance in lymphocystis disease virus infected olive flounder (Paralichthys olivaceus) and the results demonstrated better innate immune response and disease resistance were found in groups supplemented with mixed probiotics (Harikrishnan et al., 2010). However, feeding experiments conducted on O. niloticus using the diets containing single or mixed isolates of probiotic bacteria show different results in survival rates and highest with fish fed diets supplemented with B. pumilus was observed, followed by a mixture of probiotics (B. firmus, B. pumilus and C. freundii in equal numbers), and then C. freundii (Aly et al., 2008). This indicates that the beneficial effects of probiotics fed aquatic animals are associated with probiotic strains, isolation species, culture animals, and water quality. Altogether, the data reported above may well explain the current trend to prefer alternative probiotics for the application in aquaculture.
The human probiotic, Lactobacillus rhamnosus ATCC (American Type Culture Collection, Rockville, MD, USA), was used in rainbow trout for 51 days to reduce mortality by Aeromonas salmonicida, the causative agent of the fish disease “furunculosis” (one of the major fish diseases in many parts of world). Mortality was reduced from 52.6% to 18.9% when 109 cells g− 1 were administered with feed, when probiotic dose was increased to 1012 cells g− 1 of feed the mortality reached 46.3% (Nikoskelainen et al., 2001). Apparently, increasing dosage does not necessarily improve protection. Abasali and Mohamad (2010) increased the gonadosomatic index and the production of fingerlings in females of reproductive age, using mixed cultures consisting of L. acidophilus, L. casei, E. faecium, and B. thermophilum (Primalac).
Additionally, a large number of studies have combined probiotics with prebiotics, a selectively fermented ingredient that allows specific changes both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health (Gibson et al., 2004). Thus, the synbiotics, as a combination of probiotics and prebiotics, have been studied with synergistic effects anticipated. Currently, there are several recognized functional prebiotic oligosaccharides such as fructooligosaccharides (FOS), mannan oligosaccharides (MOS), insulin, ß-glucan, and xylooligosaccharides (XOS) in use around the world. The effect of dietary application of a commercial probiotic (Bacillus spp.) and MOS, used singularly and combined, on the survival, growth performance and feed cost-benefit of larval European lobster (Homarus gammarus) was assessed and the results strongly suggest that the dietary combination of Bacillus spp. and MOS is cost effective when used to promote survival and provides the added benefits of improved growth performance, compared to their individual supplementation (Daniels et al., 2010). It suggested that the combined application of probiotics and prebiotics has promising prospects in replacing growth-promoting chemotherapeutics in the aquaculture industry and could be a useful tool in the rearing of certain aquatic animals. Recently, herbs and probiotics are combined in diet and treated as one of the promising alternative tools to supplement and supplant antibiotics, chemicals or vaccines (Sahu et al., 2008; Nayak, 2010). According to Harikrishnan et al. (2011b), administration of probiotics (Lactobacillus sakei BK19) and Chinese skullcap (Scutellaria baicalensis) can effectively minimize the mortality and restore the altered hematological parameters, enhancing the innate immunity in O. fasciatus against Edwardsiella tarda, which indicates a promising role as a preventative in disease and disease outbreaks in aquaculture.
Aquatic animals have a much closer relationship with their external environment. Potential pathogens are able to maintain themselves in the external environment of the animal (water) and proliferate independently of the host animal (Verschuere et al., 2000). These potential pathogens are taken up constantly by the animal through the processes of osmoregulation and feeding. A study of Atlantic halibut, Hippoglossus hippoglossus, showed the transition from a prevailing Flavobacterium spp. intestinal flora to an Aeromonas spp./Vibrio spp. dominant flora occurred when first feeding commenced (Bergh et al., 1994). This study highlighted the impact that the external environment and feeding had on the microbial status of the fish. However, the same study also found that the larvae did maintain a specific intestinal flora different from that of the external tank flora. This showed that, although there were ever-present external environmental factors influencing the microbial flora inside an aquatic animal, they could still maintain a host specific flora at any given time. It was suggested that this ability did not apply to bivalve larvae (Jorquera et al., 2001). Their work demonstrated that the transit time of bacteria in bivalve larvae was too short to allow the establishment of a bacterial population different from that of the surrounding water.
Based on the intricate relationship an aquatic organism has with the external environment when compared with that of terrestrial animals, the definition of a probiotic for aquatic environments needs to be modified. Apart from the requirement of the probiotic to be a live culture, this definition is a lengthy way of describing a probiotic as defined by Irianto and Austin (2002a,b) thus “a probiotic is an entire or components of a microorganism that is beneficial to the health of the host.” The latter definition is in accordance with that given by Salminen et al. (1999). The nonrequirement of being a live culture would allow for certain suggested immunostimulants (Itami et al., 1998; Smith et al., 2003), which are bacterial derivatives such as peptidoglycan and lipopolysaccharides, to be included as probiotics. Although there is some dispute about what an aquatic probiotic actually is, all definitions differ from that of Fuller (1989) in that there is no longer the requisite for the probiotic to be acting in the GIT. Therefore, modes of action such as competition for nutrients and production of inhibitory substances could occur in the culture water. Additional effects of probiotic action should also be considered, given the modified definition, including change of the water quality and interaction with phytoplankton (Verschuere et al., 2000). Phytoplankton capable of producing substances toxic to other bacteria could potentially act in a beneficial manner. For example, Skeletonema costatum, a common microalga used in mollusk and crustacean larviculture, has been shown to produce an organic extract capable of inhibiting the growth of Listonella anguillarum and three other vibrios (Naviner et al., 1999). Another study has shown a microalga, Caulobacter sp., produced the antibiotic thiotropocin (Kawano et al., 1997). This compound was shown not only to be inhibitory towards the fish pathogen Lactococcus garvieae, but also had antimicrobial activity against Skeletonema costatum and Heterosigma akashiwo. Perhaps of more importance is the consideration of what effect adding a probiotic bacterium will have upon phytoplankton. Microalgae are required for most larviculture in aquaculture and, in fact, certain bacteria can stimulate microalgal growth (Suminto Hirayama, 1996, 1997; Fukami et al., 1997). Thus, probiotics could be specifically targeted for microalgae production; however, the subsequent effects of such bacteria towards the larvae must be established. The more realistic approach is using probiotics aimed at improving the health of the larvae and then determining whether this bacterium has an effect upon the microalgae. It would be very desirable to discover a probiotic that benefited the larvae and was either beneficial to or did not impair the microalgae. Consequently, the bacteria could be cocultured with the microalgae as the entry-point into the larviculture system. This was done by Gomez-Gil et al. (2002), who found the shrimp probiotic C7b could be cocultured with shrimp larvae food, Chaetoceros muelleri, without affecting the microalga. Similarly, Avendaño and Riquelme (1999) investigated the growth of seven bacterial strains with Isochrysis galbana. Four of these strains did not affect growth of the microalgae, while coculture significantly improved ingestion of bacterium C33 by larval scallop, Argopecten purpuratus. Probiotics in aquaculture may act in a manner similar to that observed for terrestrial animals. However, the relationship of aquatic organisms with the farming environment is much more complex than the one involving terrestrial animals. Because of this intimate relationship between animal and farming environment, the traditional definition of probiotics is insufficient for aquaculture.
In this sense, Verschuere et al. (2000) suggest a broader definition: “It is a microbial supplement with living microorganism with beneficial effects to the host, by modifying its microbial community associated with the host or its farming environment, ensuring better use of artificial food and its nutritional value by improving the host's response to diseases and improving the quality of the farming environment.” The microorganisms present in the aquatic environment are in direct contact with the animals, with the gills and with the food supplied, and have easy access to the digestive tract of the animal. Among the microorganisms present in the aquatic environment are potentially pathogenic microorganisms, which are opportunists; in other words, they take advantage of an animal’s stressed situation (high population density, poor nutrition) to cause infections, worsening in zootechnical performance and even death. For this reason, the use of probiotics for aquatic organisms aims not only to directly benefit the animal, but the farming environment as well. Bergh et al. (1994) observed that, when starting its first feeding, the intestinal flora of the Atlantic halibut (Hippoglossus hippoglossus) changed from a prevalence of Flavobacterium spp. to Aeromonas spp./Vibrio spp. showing the influence of the external environment and food on the microbial community of this fish. Vibrio spp., Plesiomonas shigelloides, and Aeromonas spp. are the main causative agents of diseases in aquaculture, and may even cause food infections in humans. The interaction between the environment and the host in an aquatic environment is complex. The microorganisms present in the water influence the microbiota of the host's intestine and vice versa. Makridis et al. (2000) demonstrated that the provision of two strains of bacteria via food directly into the farming water of the incubators of turbot larvae (Scophthalmus maximus) promoted the maintenance of the bacteria in the environment, as well as the colonization of the digestive tract of the larvae. Changes in salinity, temperature, and dissolved oxygen variations change the conditions that are favorable to different organisms, with consequent changes in dominant species, which could lead to the loss of effectiveness of the product. Accordingly, the addition of a given probiotic in the farming water of aquatic organisms must be constant, because the conditions of environment suffer periodic changes.
Currently, commercial products are available in liquid or powder form, and various technologies have been developed for improvement in the case of fermentation processes which has been focused on optimizing the conditions to increase the viability and functionality of probiotics (Lacroix and Yildirim, 2007). The production is generally carried out in batch cultures due to the difficulty of industrial scale operation of continuous systems (Soccol et al., 2010). More recently, systems have been developed for immobilization of probiotics, especially using microencapsulation. Microbial cells at high density are encapsulated in a colloidal matrix using alginate, chitosan, carboxymethylcellulose, or pectin to physically and chemically protect the microorganisms. The methods commonly used for microencapsulation of probiotics are the emulsion, extrusion, spray drying, and adhesion to starch (Rokka and Rantamaki, 2010). Focused on the application to aquaculture, Rosas-Ledesma et al. (2012) have effectively encapsulated cells of Shewanella putrefaciens in calcium alginate, demonstrating the survival of encapsulated probiotic cells through the gastrointestinal tract of sole (Solea senegalensis). Encapsulation in alginate matrices protects bacteria from low pH and digestive enzymes; this protection helps to release the probiotic into the intestine without any significant damage (Morinigo et al., 2008). Currently, the lyophilized commercial preparations have advantages for storage and transport. However, conditions for reconstitution of these preparations such as temperature, degree of hydration, and osmolarity of the solution are vital to ensure the viability of bacteria (Muller et al., 2009). It is important to emphasize that these products must provide a health benefit to the host; for this, it is necessary that contained microorganisms have the ability to survive storage conditions, and after that, in the digestive tract of aquatic species, remaining viable and stable, and finally improving production (Wang et al., 2008). According to the opinion of the producers, these preparations are safe to use and effective in preserving the health of aquatic animals (Irianto and Austin, 2002a,b).
Thus, the variety of microorganisms present must therefore be considered in the choice of probiotic to be used in aquaculture. Intensive farming systems utilize high stocking densities among other stressors (e.g., management), which often end up resulting in low growth and feed efficiency rates, in addition to weakening the immune system, making these animals vulnerable to opportunistic pathogens present in the environment. In this sense, the effect of probiotics on the immune system has led to a large number of studies with beneficial results on the health of aquatic organisms, although it has not yet been clarified how they act. In addition, probiotics can also be used to promote the growth of aquatic organisms, whether by direct aid in the absorption of nutrients, or by the nutrient supply. However, less attention has been put on the use of Actinobacteria as probiotics in aquaculture despite being widely known as prolific a producer of secondary metabolites, particularly the genus Streptomyces (Butler, 2008). The genus Streptomyces demonstrated promising results as probiotics in aquaculture (Das et al., 2010; Dharmaraj and Dhevendaran, 2010; Augustine et al., 2015). This chapter discusses the prospect of using marine Streptomyces as a probiotic candidate in aquaculture.
3 Prospect of Using Marine Streptomyces as Probiotics
3.1 Life Cycle of Marine Streptomyces
Streptomycetes are highly abundant in nature and remain dormant as spores for long periods until conditions become favorable for growth. The life cycle of a Streptomyces species has been described in the subsequent manner: (1) initial nuclear division phase, (2) primary mycelium, (3) secondary mycelium (including aerial), and (4) the formation of spores. The life cycle of Streptomyces is illustrated in Fig. 5. Once a spore encounters conditions favorable for growth, it germinates. Once a spore settles in a nutrient rich medium, it is stimulated to exit its dormant state and undergo germination and form germ tubes. This is the first step of several morphological differentiations in its life cycle. A germ tube grows out from the spore and elongates into long branching filamentous cells during vegetative growth, forming a mesh of hyphae called the substrate or vegetative mycelium, which grows deep into the solid growing medium. Elongation of hyphae is accomplished through insertion of new cell wall material at the hyphal tip. Infrequent crosswalls separate the hyphae into cellular compartments. Each compartment contains multiple copies of the chromosome and DNA is spread throughout the whole compartment with little separation of individual nucleoids. When growth of the vegetative mycelium has given rise to a colony, nutrient limitation and probably cell density signals contribute to trigger formation of an aerial mycelium on the colony surface (Elliot et al., 2008; Flärdh and Buttner, 2009; Dharmaraj and Dhevendaran, 2016). The aerial hyphae represent reproductive structures and are transformed into pigmented spore chains that mature and eventually release separated spores. Sporulation in the aerial hyphae is restricted to the apical compartment (also referred to as the sporogenic cell) in which a high level of DNA replication takes place generating several copies or more of uncondensed, evenly distributed chromosomes (Ruban-Osmialowska et al., 2006). A developmentally controlled form of cell division (sporulation septation) compartmentalizes the sporogenic cell into prespores. In coordination with this septation, the chromosomes are positioned and segregated so that each prespore compartment receives one copy of the genome (Flärdh, 2003). After the completion of septation, nucleoids are condensed, the prespores become rounded or ovoid, synthesize a thick spore wall, pigment, and the spores are separated.

3.2 Taxonomical Classification of Marine Streptomyces
Actinobacteria represents one of the largest taxonomic units among the 18 major lineages currently recognized within the domain bacteria, including five subclasses and 14 suborders (Stackebrandt, 2000). Among the five subclasses, actinobacteria—bacteria belonging to the Order Actinomycetales (commonly called actinomycetes)—account for approximately 7000 of the metabolites reported in the Dictionary of Natural Products. Actinomycetes have a high GC content in their deoxyribonucleic acid (DNA) and grow as aerial/substrate mycelia (Yoshida et al., 2008). They are responsible for the production of about half of the discovered secondary metabolites (Bull, 2004; Berdy, 2005; Dharmaraj and Alagarsamy, 2009), notably antibiotics (Strohl, 2004), antitumour agents (Olano et al., 2009), anticancer compounds (Dharmaraj, 2011a,b), immunosuppressive agents (Mann, 2001) and enzymes (Pecznska-Czoch and Mordarski, 1988; Oldfield et al., 1998). A large number of actinomycetes have been isolated and screened from terrestrial habitat in the past few decades (Williams et al., 1989). Recently, the rate of discovery of new metabolites from terrestrial actinomycetes has decreased, whereas the rate of re-isolation of known compounds has increased (Fenical et al., 1999; Fenical and Jensen, 2006). Thus, it is crucial that new groups of actinomycetes from unexplored or underexploited habitats be pursued as sources of novel secondary metabolites.
Indeed, the marine environment is virtually untapped as a source of novel actinomycete diversity (Stach et al., 2003; Magarvey et al., 2004) and therefore, of new metabolites (Bull et al., 2005; Fiedler et al., 2005). This is partly caused by the lack of effort spent in exploring marine actinomycetes, whereas terrestrial actinomycetes have been, until recently, a successful source of secondary metabolites. Furthermore, skepticism regarding the existence of indigenous populations of marine actinomycetes arises from the fact that the terrestrial bacteria produce resistant spores that are known to be transported from land into sea, where they can remain available but dormant for many years (Bull et al., 2000). Thus, it has been frequently assumed that actinomycetes isolated from marine samples are merely of terrestrial origin.
Recent data from culture-independent and culture-dependent studies have shown that indigenous marine actinomycetes indeed exist, and it was confirmed that they are autochthonous flora in the marine environment (Moran et al., 1995; Mincer et al., 2002; Jensen et al., 2005; Lam, 2006; Das et al., 2008). A series of papers describing the distribution of actinomycetes in the marine environment, published in the dedicated volume of the Antonie van Leeuwenhoek journal (Bull and Goodfellow, 2005), confirmed the indigenous nature of marine actinobacteria. This view was best supported by the discovery of the first new obligate marine actinomycete genus, Salinispora (formerly known as Salinospora) (Mincer et al., 2005). While early research estimated low numbers (Jensen et al., 1991) and patchy distribution (Mincer et al., 2002) of actinomycetes in the marine environment, more recent studies suggested higher abundance and diversity of actinobacteria with numerous novel taxa (Gontang et al., 2007). Thus, bona fide actinomycetes not only exist in the oceans, but they are also widely distributed in different marine ecosystems (Lam, 2006; Dharmaraj, 2011a,b).
In the marine environment, the representatives from six families have been listed based on molecular studies. The representative families are Micromonosporaceae, Nocardiaceae, Nocardiopsaceae, Pseudonocardiaceae, Streptomycetaceae, and Thermonosporaceae. However, greater sequence coverage or improved DNA extraction efficiencies may be required to detect the rare phylotypes. Besides, new strategies need to be developed for the cultivation of frequently observed but yet-to-be-cultured marine actinobacteria (Jensen and Lauro, 2008). Actinomycete genera identified by cultural and molecular techniques from different marine ecological niches include Actinomadura, Actinosynnema, Amycolatopsis, Arthrobacter, Blastococcus, Brachybacterium, Corynebacterium, Dietzia, Frankia, Frigoribacterium, Geodermatophilus, Gordonia, Kitasatospora, Micromonospora, Micrococcus, Microbacterium, Mycobacterium, Nocardioides, Nocardiopsis, Nonomurea, Psuedonocardia, Rhodococcus, Saccharopolyspora, Salinispora, Serinicoccus, Solwaraspora, ---------Streptomyces, Streptosporangium, Tsukamurella, Turicella, Verrucosispora and Williamsia (Stach et al., 2004; Jensen et al., 2005; Ward and Bora, 2006; Das et al., 2006a,b). More actinobacterial genera are expected to be discovered and reported with culture-independent studies in the years to come. However, regardless of the geographical origin, marine actinomycetes were shown to follow a well-documented pattern in secondary metabolite production (Jensen et al., 2007). It is surmised that marine actinomycetes have different characteristics from those of their terrestrial counterparts and therefore, might produce different types of secondary metabolites. Over the past decade, information on the diversity of actinomycetes in marine habitats has grown considerably, while the somewhat longer held interest in their ability to produce secondary metabolites has continued quite strongly (Stackebrandt et al., 1997).
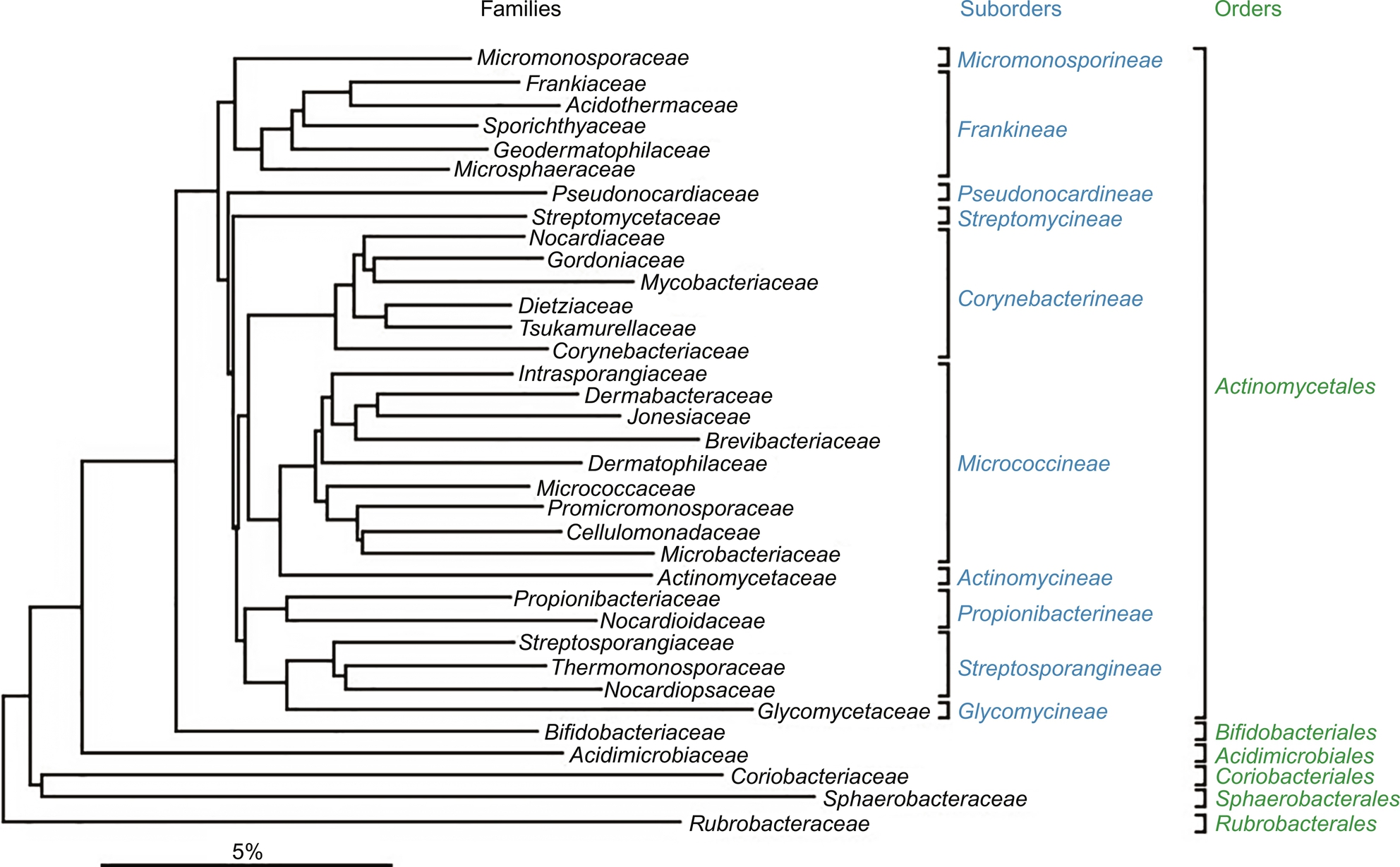
Marine actinomycetes are a prolific source of secondary metabolites and the vast majority of these compounds are derived from the single genus Streptomyces. Streptomyces species are distributed widely in marine and terrestrial habitats (Pathom-aree et al., 2006) and are of commercial interest due to their unique capacity to produce novel metabolites. It was also expected that Streptomyces species will have a cosmopolitan distribution, as they produce abundant spores that are readily dispersed (Antony-Babu et al., 2008). These filamentous bacteria are well adapted to the marine environment and are able to break down complex biological polymers. Streptomyces are classified within the Gram-positive bacteria and form together with the genera Kitasatospora (Omura et al., 1982) and Streptacidiphilus (Kim et al., 2003), the family Streptomycetaceae. The latter family constitutes a separate phylogenetic branch within the phylum Firmacutes, class Actinobacteria, order Actinomycetales, suborder Streptomycineae, based on 16S rRNA gene sequence analysis, with high DNA G + C content of 69–78 mol%. The taxonomy of Streptomyces is shown in Fig. 6 (Stackebrandt et al., 1997; Anderson and Wellington, 2001). In fact, the genus Streptomyces alone accounts for a remarkable 80% of the actinomycete natural products reported to date; a biosynthetic capacity that remains without rival in the microbial world (Watve et al., 2001).
3.3 Morphological Identification of Marine Streptomyces
Marine Streptomyces exhibit different cultural characterization in various types of culture media, which is one of the important criteria in the classical identification. The main characteristics include spore formation, aerial hyphae, with or without color and the soluble pigment, and different growth conditions on various media. The morphological differentiation of marine Streptomyces is generally controlled by relevant genes. Marine Streptomyces display the greatest morphological differentiation among Gram-positive bacteria; however, the cell structures are typical as prokaryotes and totally different from fungi.
The whole structure of a hyphae cell corresponds to bacterial organization: the cytoplasm contains genomic DNA regions, ribosomes, and various inclusions, presumably reserve substances such as polyphosphates, lipids, or polysaccharides. According to the difference of morphology and function, the mycelia can be divided into vegetative mycelium and aerial mycelium. Known as vegetative mycelium or primary mycelium, the substrate mycelium grows into the medium or on the surface of the culture medium. The main function of the substrate mycelium is the absorption of nutrients for the growth of marine Streptomyces. Under the microscope, the substrate mycelia are slender, transparent, phase-dark, and more branched than aerial hyphae. The single hyphae is about 0.4–1.2 μm thick, usually does not form diaphragms and fracture, and is capable of developing branches. The substrate mycelia are white, yellow, orange, red, green, blue, purple, brown, black, and other colors; some hyphae can produce water-soluble or fat-soluble pigment. The water-soluble pigment can seep into culture medium, which changes the medium to the corresponding color. The nonwater-soluble (or fat-soluble) pigment makes the colony the corresponding color. The color of the substrate mycelia, and whether there are soluble pigments, provide important references in the determination of new species.
Aerial mycelium is the hyphae that the substrate mycelium develops to a certain stage and grows into the air. Sometimes, aerial hyphae and substrate mycelia are difficult to distinguish. It can be easily distinguished by an impression preparation on a cover slip viewed in a dry system with a light microscope: substrate hyphae are slender, transparent, and phase-dark; aerial hyphae are coarse, refractive, and phase-bright.
In all kinds of marine Streptomyces, the formation of aerial hyphae is dependent upon the species characteristics, nutritional conditions, or environmental factors. At a certain stage aerial hyphae of marine Streptomyces get differentiated and can form reproductive hyphae called spore-bearing mycelium. Marine Streptomyces has classical polyspores, which form long chains frequently having more than 50 spores. The spores of marine Streptomyces are often called arthrospores (Cross, 1970). The sporulating aerial hyphae of marine Streptomyces can be differentiated into the following main types: (A) Rectiflexibiles type, straight or flexuous spore chains, partly in fascicles; (B) Retinaculiaperti type, spore chains with hooks, open loops or short, irregular spirals having 1–4 turns; (C) Spira type, spore chains in spirals demonstrating two different subtypes: (a) closed, compact spiral and (b) open, loose, and stretched spirals; (D) Verticillati type, spore chains formed in whorls and branched in umbels.
The length, shape, position, and color of marine Streptomyces spore chains are an important basis for classification. Spore chains of the marine Streptomyces have various types of spore-bearing structures: straight, flexous, fascicied, monoverticillate (no spirals), open loops (primitive spirals hooks), open spirals, closed spirals, monoverticillate (with spirals), biverticillate (no spirals), biverticillate (with spirals). Mature spores show a variety of colors such as white, gray, yellow, pink, lavender, blue, or green, and so on.
Hyphal growth of marine Streptomyces shows much similarity with filamentous fungi (Xiang and Morris, 1999). Marine Streptomyces apical hyphal growth was observed using fluorescence microscopy (Flärdh, 2003). The apical cell extends its cell wall only at the tip (green). Once this cell has divided by forming a new hyphal cross wall, the subapical daughter cell is unable to grow and eventually switches its polarity to generate a lateral branch with a new extending tip. A consequence of tip growth is that DNA, which replicates along most of the hyphal length, has to move towards the tip and into new branches by means of a process proposed to designate nucleoid migration. For clarity, few schematic nucleoids are drawn (red), and they are not meant to reflect the actual number of chromosomes per cell. Furthermore, individual nucleoids are typically not observed in vivo as separated bodies in growing hyphae (Fig. 7). In general, when nutrients become limited, a developmental switch occurs during which hyphae start to escape the moist environment and grow into the air. These so-called aerial hyphae can further differentiate into long chains of spores, which can withstand adverse conditions. Following their dispersal, these spores will reinitiate growth in suitable environments.

Some of the key processes involved in the formation of aerial hyphae by marine Streptomyces appear to be very similar to fungi. Both groups secrete highly surface-active molecules that lower the surface tension of their aqueous environment enabling hyphae to grow into the air. In the case of filamentous marine Streptomyces, small peptides (i.e., Sap B and streptofactin) are secreted, while filamentous fungi use proteins known as hydrophobins to decrease the water surface tension. Although these fungal and bacterial molecules are not structurally related, they can, at least partially, functionally substitute each other (Wosten and Willey, 2000).
The bld cascade (for bald, meaning unable to form aerial hyphae) controls the checkpoints that (eventually) lead to the onset of aerial growth, resulting in the formation of surface-active molecules that lower the water surface tension and enable hyphae to grow into the air. Moreover, the bld cascade seems to potentiate hyphae to undergo full development (Kelemen and Buttner, 1998; Willey et al., 2006). Another regulatory pathway is the shy pathway (Claessen et al., 2006), which controls the expression of the chaplin and rodlin genes. These genes encode proteins that assemble into a rodlet layer that provides surface hydrophobicity to aerial hyphae and spores. Both pathways control the production of structural proteins that are involved in the formation of aerial hyphae. When hyphal growth is limited, much of the biomass becomes converted into spores through the extraordinary parasitic growth of a fluffy white aerial mycelium. The syncytial aerial hyphal tips (which may contain more than 50 copies of the genome) undergo multiple cell divisions to generate a string of unigenomic compartments, destined to become tough, desiccation-resistant spores (Flärdh et al., 1999). Thus, substantial growth is interpolated between the first sporulation related decisions, made in the substrate mycelium, and the decisions involved in the formation and maturation of the spore compartments themselves (Chater, 2001). Additionally, the marine Streptomyces spore wall synthesizing complex (SSSC) does not only direct synthesis of the peptidoglycan layer but is also involved in the incorporation of anionic spore wall glycopolymers, which contribute to the resistance of spores. The SSSC also contains eukaryotic type serine/threonine kinases which might control its activity by protein phosphorylation (Sigle et al., 2015).
3.4 Applications of Marine Streptomyces as Probiotics in Aquaculture
Marine Streptomyces are widely distributed in various biological sources such as fish, mollusks, sponges, seaweeds, mangroves, corals as well as in seawater and sediments. These microorganisms are gaining importance not only for their taxonomic and ecological perspectives, but also have been widely recognized as industrially significant. Their potential for producing diverse range of novel secondary metabolites includes antibiotics, antitumor agents, antiparasitics, antioxidants, immunosuppressive agents, enzymes, enzyme inhibitors, and food grade pigments (Dharmaraj et al., 2009; Dharmaraj, 2010; Pimentel-Elardo et al., 2010; Manivasagan et al., 2013; Janardhan et al., 2014; Tan et al., 2015). They have produced wide-variety chemical compounds and have the advantage of producing antagonistic and antimicrobial compounds that can be as valuable probiotics in aquaculture. The ability of producing antagonistic compounds may help the probiotics to compete for nutrients and attachment sites in the host. There have been reports that probiotics which were used for aquaculture also have the ability to synthesize compounds like bacteriocins (Desriac et al., 2010), siderophores (Lalloo et al., 2010), enzymes [protease, amylase, lipase] (Augustine et al., 2015), hydrogen peroxide (Sugita et al., 2007) and organic acids (Sugita et al., 1997). Table 1 summarizes all the features and mechanisms of actions of the probiotic effects evidenced in the marine Streptomyces. Schematic representation detailing the various applications of marine Streptomyces as probiotics in aquaculture has been shown in Fig. 8. You et al. (2007) reported the activity of marine Streptomyces as a potential organism against biofilms produced by Vibrio spp. These organisms synthesize siderophores and it has been suggested that their use can influence the growth of pathogenic Vibrio sp. by competition for iron in the aquatic environment. Probiotics with the capability of producing siderophores are believed to outcompete the pathogens by limiting the bioavailability of iron (Ahmed and Holmstrom, 2014), resulting in growth attenuation of the pathogens due to the fact that iron is essential for growth as well as biofilm formation (Weinberg, 2004). They inhibited the biofilm formation of Vibrio harveyi, Vibrio vulnificus, and Vibrio anguillarum at a concentration of 2.5% (v/v). They dispersed the mature biofilm and inhibited the quorum sensing system of V. harveyi by attenuating the signal molecules, N-acylated homoserine lactones’ activity. The strains have the ability to attenuate the biofilms and also inhibit their quorum-sensing system (Iwatsuki et al., 2008). It is suggested that the strain is a promising candidate for use in marine aquaculture and is also helpful in the prevention of diseases caused by Vibrio spp. You et al. (2005) described the potential of marine Streptomyces against the shrimp pathogen Vibrio spp. and recommended marine Streptomyces as potential probiotic strains due to their ability to degrade macromolecules such as starch and protein in culture pond water, to produce antimicrobial agents and form heat and desiccation-resistant spores. More recently, there have been studies on the possible use of marine Streptomyces in disease prevention against aquatic pathogens.

Table 1
| Mode of Actions | Marine Streptomyces as Probiotics | Outcomes | References |
|---|---|---|---|
| Growth enhancing effect | Streptomyces fradiae and Streptomyces sp. |
(1) Improved growth of postlarval shrimp P. monodon and ornamental fish, Xiphophorus helleri. (2) Produced growth promoting hormone, indole acetic acid which enhanced growth of X. helleri. | Dharmaraj and Dhevendaran (2010, 2013) and Aftabuddin et al. (2013) |
| Single cell protein | Streptomyces sp. |
(3) Used as a protein source for host which increased food conversion rate and food conversion efficiency, enhanced growth performance | Dharmaraj and Dhevendaran (2010, 2015), Suguna and Rajendran (2012), and Dharmaraj et al. (2013) |
| In vivo experiment | Streptomyces strains (CLS-28, 39 and 45) |
(4) Protection of Artemia against V. harveyi—At a concentration of 106 CFU/mL killed all Artemia nauplii in 72 h and on addition of Streptomyces strains [at 1% (v/v)] increased the survival of Artemia nauplii by 67% and adults by 61% after 72 h of exposure at same concentrations of cells (5) Protection of P. monodon against V. harveyi—At a concentration of 107 CFU/mL killed 55% of P. monodon after 5 days exposure and on supplementation Streptomyces CLS-28 incorporated feed increased the survival of P. monodon by 67% compared | Das et al. (2008, 2010) |
| Exoenzyme production | Streptomyces strains (CLS-28, 39 and 45) |
(6) The cultures possess amylolytic, proteolytic, and lipolytic properties which helps in the feed utilization, digestion and resulting in increased weight for the Penaeus monodon | Das et al. (2008, 2010) |
| Improvement in water quality | Streptomyces fradiae Streptomyces sp. Streptomyces CLS-28 |
(7) The strains reduced the level of ammonia in the culture system and there is an increase in the total heterotrophic bacterial populations which helped to accelerate the decomposition of waste materials | Das et al. (2006a,b, 2010) and Aftabuddin et al. (2013) |
| Antibacterial and viral activity | Streptomyces sp. |
(8) The cultures which exhibited probiotic effect have been initially screened for antiviral activity against fish and shellfish pathogens (Aeromonas hydrophila, Serratia sp., and Vibrio spp [V. alginolyticus, V. harveyi, V. parahaemolyticus]) which cause deleterious effects | Dharmaraj and Dhevendaran, (2011,2016) |

Besides displaying the inhibitory effect on bacterial pathogens in aquaculture, marine Streptomyces also has been reported to exhibit antiviral activity, specifically against the white-spot syndrome virus. Very few studies have been carried out on the antiviral property of marine Streptomyces against White Spot Syndrome Virus (WSSV) in penaeid shrimps (Jenifer et al., 2015). WSSV infection can cause cumulative mortality up to 100% within 3–10 days, thereby causing considerable economic loss to the shrimp farmers. In a report by Kumar et al. (2006), antibiotic extracts were obtained from the fermentation broth of twenty-five isolates of marine Streptomyces (isolated from the coastal waters off the Southwest coast of India), incorporated in the formulated feed and supplemented to the postlarvae (PL-20) of the black tiger shrimp Penaeus monodon for 2 weeks to treat against WSSV. The pattern of posttreatment survival % (PCS %) in the 27 treatments (25 experimental and two controls) exhibited a wide range of variation from 11 to 83% during the course of the experiment. PCS % was lowest in the controls (C1-4.3%, C2-5.2%) on day 7. However, six probiotic feeds (SA 2, SA 8, SL 27, SL 33, SL 39, and SL 85) supplemented to postlarvae shrimp recorded the highest PCS percent ranging between 50% and 83%. Also, severity of the infection observed on days 3, 4, and 5 in postlarvae shrimp fed with other diets was not visible. In this case, positive effect was obtained by the antibiotic extract incorporated in the feed against WSSV infected penaeid shrimps.
Marine Streptomyces are primarily saprophytic, living in diverse habitats with the development of branching hyphal filaments (Flärdh and Buttner, 2009). This unique growth adaptation allows marine Streptomyces colonization of the solid substrates by adhering and penetrating to gain access to insoluble organic materials of the host environment. Different hydrolytic enzymes such as amylase, protease and lipase can be produced by marine Streptomyces to break down the insoluble organic materials to provide nutrients for the formation of densely packed substrate mycelium which is reused to fuel the reproductive phase of aerial growth in producing chains of spores (Chater et al., 2010). These unique physiological adaptations of marine Streptomyces are believed to make them potential probiotics, such as the secretion of exoenzymes which may be helpful in facilitating the feed utilization and digestion once they colonize the host intestine in aquaculture. Das et al. (2010) demonstrated that the feed incorporated with marine Streptomyces increased the weight of Penaeus monodon shrimp, suggesting that these marine Streptomyces sp. secreted hydrolytic exoenzymes to improve the amylolytic and proteolytic activity in the shrimp digestive tract for more efficient use of the feed. The feed supplemented with marine Streptomyces fradiae isolated from mangrove sediment was also shown to enhance the growth of the postlarval P. monodon (Aftabuddin et al., 2013).
The formation of enzymatic digestion, sonic vibration and desiccation-resistant spores demonstrated by marine Streptomyces constitute some of the attractive features for this genus of bacteria to resist the harsh environment conditions, thereby allowing them to retain longer shelf life in the aquaculture ponds before being taken up or to resist the low pH in the gastro intestinal tracts of the animals. However, it should be noted that marine Streptomyces spore is only resistant to moderately high temperature as compared to the highly heat resistant endospores of Bacillus sp. which is compositionally and physiologically different from the Streptomyces spore (McBride and Ensign, 1987).
Das et al. (2006a,b) reported a preliminary study on the use of marine Streptomyces incorporated feed as a probiotic source for the growth of black tiger shrimp. Cells of marine Streptomyces were incorporated at different concentrations (0, 2.5, 5.0, 7.5, and 10.0 g/kg feed) in the formulated feeds, supplemented for 25 days and growth was monitored. At a concentration of 10 g, shrimp fed with marine Streptomyces incorporated feed showed high growth in terms of length (15.79%) and weight (57.97%) when compared with the control [length (4.08%) and weight (32.77%)]. The growth of the tiger shrimp, Penaeus monodon, also increased with an increase in the concentration of marine Streptomyces in the supplemented feed. Das et al. (2010) isolated marine Streptomyces strains from the sediment of the shrimp culture system which has the ability to reach the digestive system of the shrimp, therefore allowing easier establishment and growth of the probiotics in the host. These findings indicate that the spore-forming capacity of marine Streptomyces with high acidity and bile acids tolerance makes them a more practical alternative than those bacteria with nonspore forming capability. Further, Das et al. (2010) studied the potential of marine Streptomyces as probiotic by conducting in vivo experiment which successfully demonstrated the protection effect of marine Streptomyces on both juvenile and adult Artemia (15 days old) from Vibrio pathogens. The study demonstrated that the marine Streptomyces at 1% concentration (v/v) resulted in higher survival rates than the untreated control group of Artemia after challenged with V. harveyi or V. proteolyticus at 106 CFU/mL. The protective response shown by the study suggests that marine Streptomyces could be administrated to target organisms through bio-encapsulation in Artemia as a vector for supplementing the beneficial marine Streptomyces as probiotics in aquaculture. Previously many reports were recorded on the use of bioencapsulated probiotics in live food such as Artemia and rotifers to be more effective in the delivery of the probiotics to the digestive tract of the target aquaculture organisms (Gatesoupe, 2002; Suzer et al., 2008). The study also further evaluated the efficacy of the marine Streptomyces in protecting the shrimp P. monodon from the Vibrio pathogens. The feed supplemented with marine Streptomyces sp. CLS-28 for 15 days was found to exert protective effects on shrimp P. monodon against the 12 h challenge of V. harveyi (LD50 at 106.5 CFU/mL). A recent study reported that marine S. rubrolavendulae M56 was found to exhibit antagonistic activity against all four Vibrio species including V. harveyi, V. alginolyticus, V. parahaemolyticus, and V. fluvialis in an in vitro coculture experiment. In order to confirm the in vitro findings, the bio granules of marine Streptomyces rubrolavendulae M56 resulted in lower percentage of mortality of P. monodon postlarvae with the reduction of viable Vibrio sp. in the culture system after 28 days (Augustine et al., 2015).
Besides showing good growth promoting effects in shrimp, all the feeds supplemented with marine Streptomyces were shown to improve growth performance of the ornamental fish, Xiphophorus helleri (red sword tail fish) after a 50 day feeding trial when compared to control without the marine Streptomyces sp. (Dharmaraj and Dhevendaran, 2010). In the same study, marine Streptomyces strains exhibited the production of growth-promoting hormone, indole acetic acid, which could have attributed to the better growth rate of the fish Xiphophorus helleri fed with marine Streptomyces supplemented feeds (Dharmaraj and Dhevendaran, 2010, 2013). It has also been reported that dietary supplementation of microbial carotenoids to the ornamental fishes not only resulted in the recovery of colouration to the fishes; it also showed improved growth of the fishes (Dharmaraj and Dhevendaran, 2011).
The major problem which substantially affects the health of the aquaculture livestock is due to the poor water quality which results in high level accumulation of metabolic waste by the cultured organisms, particularly the rise of ammonia and nitrite as well as the decomposition of residual feed. The probiotic marine Streptomyces was also found to regulate the microflora of the aquaculture water, besides controlling the pathogenic microorganisms, resulted in better pond conditions. Previous studies showed that the application of probiotic product did not adversely affect the microflora of aquaculture, in turn they increased the abilities of protein mineralizing and ammonifying bacterial population which help to accelerate the decomposition process of the accumulated wastes materials (Devaraja et al., 2002). Many reports also exhibited similar type of results indicating the reduction of ammonia level and an increase in the total heterotrophic bacteria in the ponds/tanks treated with the probiotic marine Streptomyces as compared to control ponds/tanks (Aftabuddin et al., 2013). These all establish that marine Streptomyces could be applied as probiotics in aquaculture which may directly or indirectly improve the water quality as well as the growth performance and yield of the particular cultured organisms.
In aquaculture, essential and expensive components of the feed are proteins, especially fish meal. Since the supply of fish meal has become uncertain and the prices have increased rapidly, the need for cheaper alternative protein sources has increased. Among unconventional protein sources, microbial origin appears to be a promising substitute for fish meal, replacing up to 25%–50%. In the present contest, microbial single cell protein of marine Streptomyces is one of the alternative sources of protein and has been utilized and evaluated for better food conversion efficiency and growth for fish and shrimp (Manju and Dhevendaran, 1997; Suguna and Rajendran, 2012; Selvakumar et al., 2013).
Marine Streptomyces are known to produce secondary metabolites that enhance the growth of juvenile fish, shrimp and prawn. There have been reports on the application of marine Streptomyces as a SCP source, incorporated in the formulated feed and supplemented to juvenile prawns weighing about 0.130–0.160 g/body weight. The ingredients of the control feed consisted of fishmeal (14.76 g), groundnut oil cake (14.76 g) and rice bran (35.24 g) with tapioca flour (35.24 g) as binder, whereas the SCP incorporated feed consisted of fishmeal + Streptomyces cells (14.76 g + 165 mg), the rest being the same as the control feed. After 50 days of feeding trials, growth parameters such as feed conversion efficiency, feed conversion ratio and protein content were analyzed. Prawns fed with marine Streptomyces incorporated feed showed improved growth (140.54%), feed conversion efficiency (45%), and higher protein content (54.72%), whereas the prawns fed with control feed showed less growth (89.52%), food conversion efficiency (20%) and protein content of 35.02%. The feed conversion ratio was less for the SCP fed-prawns (2.217) than the control (5.015). Therefore, among unconventional protein sources, single cell protein (SCP) of microbial origin appears to be a promising substitute for fishmeal, and can replace up to 25%–50% fishmeal in aquaculture operations (Manju and Dhevendaran, 1997). It has been reported that the use of marine Streptomyces not only demonstrated beneficial effects as probiotic in aquaculture, the incorporation of Streptomyces in the feed is a cost-effective approach as the probiotic bacteria replaced up to 30%–40% of the fishmeal used in the feed preparation. Reports clearly proved that marine Streptomyces can be a cheaper alternative protein source in aquaculture feed (Dharmaraj and Dhevendaran, 2010, 2015; Dharmaraj et al., 2013).
3.5 Selection of Efficient Strains of Marine Streptomyces as Probiotics
To select efficient strains which can be used as probiotics, particularly marine Streptomyces, there are certain steps which must be followed: (1) preliminary information about sampling collection sites, (2) isolation and identification of probiotic strains, (3) various methods to be adopted to characterize the strains to be potent probiotics, like survivability tests (resistance to low pH, pepsin, bile, and pancreatin), colonization (autoaggregation, hydrophobicity, and coaggregation) and safety (antibiotic susceptibility test and nonhemolytic activity), (4) evaluating the potent probiotic strains ability to fight against pathogens prevailing in a particular environment, (5) assessing the effects of the probiotic strains in the host, (6) knowledge on cost effective benefits of the probiotics (Gomez-Gil et al., 2000; Sheeja et al., 2011).
Many researchers described isolation of probiotic strains from different sampling sites. Marine Streptomyces strains can be isolated from samples such as natural seawater where larvae are growing, culture water where larvae are densely reared in the laboratory, sediments from the marine environment associated with bottom dwelling animals, and from digestive gut of marine fishes (Maeda et al., 1997). It is quite obvious to recommend the site from the native environment, where the survivability of the strains is already established. Microbial food networks are an essential part of marine aquaculture and have a direct impact on yields (Moriarty, 1997). The probiotic strains screened all play an important role in productivity, the nutrition of the cultured animals, disease control, water quality, and environmental impacts (Wang et al., 2008).
In support of the marine Streptomyces, in particular, as probiotics, the comprehensive screening procedures and experimental observations can be followed, which have been shown in Table 2. Marine Streptomyces usage as a component of formulated feed ingredients which can be supplemented to fish has been summarized in Fig. 9. Marine Streptomyces can be mass-cultured, harvested, lyophilized and used to fortify feed (Dharmaraj and Dhevendaran, 2010). In some studies, the supernatant of the broth culture of the strain has been collected, concentrated, and added to the feed. In this particular case, the positive effects may be observed, although it may contravene the definition of probiotics, as there is no addition of live microbial supplement but only cellular components (Kumar et al., 2006). At last the probiotic strains selected should possess certain properties: (1) it should be nonpathogenic to the host; (2) it should be accepted by the host, may be through ingestion, potential colonization, and replication within the host; (3) it should reach the location where the effect is required; (4) it should actually work in vivo as similar to in vitro findings; and (5) it should not preferably carry any virulence resistance genes or antibiotic resistance genes (Verschuere et al., 2000).

Table 2
| Si. No. | Parameters | Methods | Observations |
|---|---|---|---|
| 1 | Preliminary screening |
(a) Isolation of marine Streptomyces from various marine sources like sediments, seawater, sponges, seaweeds, mangroves, fish and shellfish by using different media like Actinomyces isolation agar, Glycerol asparagine agar, Starch casein agar, Kuster’s agar, and Yeast Malt Extract agar (b) Preliminary identification and characterization of the strains has to be carried out using parameters like Morphological (mycelial colouration, soluble pigments, melanoid pigmentation, spore morphology), Biochemical (carbon utilization, amino acids influence, sodium chloride tolerance), physiological (pH, temperature) and chemo-taxonomical (c) In vitro antimicrobial activity has to be carried out by agar well diffusion and disc diffusion methods (d) To test the strains for the extracellular enzymes activities like amylase, protease (e) The strains which should be supplementation to the fish and shellfish have to be mass cultured in a suitable medium (it can be broth or solid surface) |
(1) Marine Streptomyces were found to possess filamentous hyphae, they form tough and leathery colonies which stick on to the various media used (2) Identification of potent strains can be done by these conventional methods—both species and genus level as described (Kampfer, 2006) (3) The strains which exhibited good antagonism against fish and shellfish pathogens which affect their immune system are to be selected (4) The strains which exhibit the maximal enzymatic activities are to be selected (5) The growth of the strains can be observed on the surface of the medium in case of static condition but in broth they grow like a mat which can be harvested by centrifugation and stored at 4°C |
| 2 | Experimental screening |
(f) In vivo evaluation of probiotic strain: (1) The cultured strains can be lyophilized and mixed with feed ingredients, if it is supplemented as live feed (2) It can be dried and mixed with feed ingredients, can be supplemented as SCP source (3) The supernatant extract or lyophilized cells can be added directly to the culture water (g) Check for the strains adhering to the gut of the fish or shellfish and colonized. This can be done by plating the gut extract to the suitable media for the growth of particular strain (h) In vitro calibration of probiotic potentials of the strains: screening for survivability (resistance to low pH, pepsin, bile, and pancreatin), colonization (autoaggregation, hydrophobicity and coaggregation) and safety (antibiotic susceptibility and nonhemolytic activity) (i) Dose optimal: The effective way of introduction and optimum dose to be determined. Since marine Streptomyces is a very slow grower, the lag period and the doubling time would also be determined (j) If the cultures mixed with feed ingredients then proximate compositions like moisture content, crude protein, crude fat, crude fiber, ash, nitrogen-free extract has to be assessed in the feed prepared, as well as the excreta and the gut of the fish and shellfish before and after feed supplementation (k) Statistical analysis: At least triplicate experiments has to be analyzed using various tools like one way or two-way ANOVA, Regression, T-test, Duncan’s multiple range test and Tukey test |
(6) All the growth, survival related parameters have to be analyzed, for example SGR, RGR, AGR, FCR and FCE. Water quality analysis has to be carried out and the strains should not promote algal growth in the culture system (7) To observe whether the strain is retaining and support the adhesion properties in the gut and get colonized in the host or to be supplemented regularly (8) The isolates should be susceptible to range of different antibiotics and they should not be exhibiting hemolytic activity. The cultures should exhibit excellent viability at low pH range and they should be acid-tolerant, resistance to pepsin, bile and pancreatic (9) Marine Streptomyces is nonmotile aerobic, grows as mat in broth (in static condition) and as clump (in shaker culture). The harvested cells has to be centrifuged, lyophilized and at last packed. Supplementation level of the cultures has to be optimized which can be assessed by MIC protocol. Prevention of fungal and algal contaminants (10) The proximate composition value should be of permissive level prescribed by AOAC (2000). It is assessed accordingly to the body weight of fish and shellfish (11) Comparison of the various treatments significance level (P < .05) |
| 3 | Postexperimental screening |
(l) Further, confirmation on the molecular identification of the cultures used (gene sequencing and 16S rRNA phylogenetic analysis) (m) Parameters for mass scale culture and optimum culture conditions to be determined since the metabolic processes are controlled by various sources like carbon, nitrogen, phosphorous, metals, induction, feedback regulation, growth rate, and enzyme decay (n) Finally, a cost-benefit analysis will also have to be carried out |
(12) This is done either for the confirmation of strains until species level (if any novel strains) and also deposition of the strains to the culture centers (13) These parameters help in regular usage of the strains to possess the probiotic property and maximal yield effectively (14) This may help in commercialization of the probiotic product for the usage in aquaculture |

3.6 Possible Limitations in the Usage of Marine Streptomyces as Probiotics
There are three possible setbacks in the usage of marine Streptomyces as probiotics in aquaculture as follows:
- (1) Difficulties in culturing methods
- (2) Risk of lateral gene transfer
- (3) Production of awful odor compounds.
Marine Streptomyces occur in different biological sources such as fish, mollusks, sponges, seaweeds and mangroves as well as seawater and sediments. They are randomly distributed, many difficulties arise in culturing methods, there are currently no proposed standardized media, the organism itself has a slow growth rate of approximately 14 days, and identification requires tedious laboratory procedures in culture-dependent studies.
There is substantial risk in the use of marine Streptomyces as a probiotic in aquaculture were the lateral gene transfer of antibiotic resistance genes that produce efflux pumps, ribosomal protection proteins, and modifying enzymes, by which the organism itself protects from its own antibiotics. It is preferable that the probiotic strains not contain any virulence resistance genes or antibiotic resistance genes because of the emergence of multidrug-resistant pathogens in aquaculture. There have been recent reports on the antibiotic resistance developed by most of the commonly used probiotics such as Lactobacillus sp. (Sharma et al., 2015), Bifidobacterium sp. and Bacillus sp. (Gueimonde et al., 2013). Extensive use of antibiotics in aquaculture has caused antibiotic-resistant bacteria to be widespread (Furushita et al., 2003), which may lead to the condition that the aquatic environment may serve as a reservoir of antibiotic resistance (Biyela et al., 2004). Antibiotic resistant genes causing agents like ribosomal protection proteins and antibiotic modifying enzymes have originated from marine Streptomyces or any other antibiotic producing microbes by lateral gene transfer (Chopra and Roberts, 2001). Recent studies reported that the antibiotic resistant phenotypes displayed by the probiotic marine Streptomyces strains were generally conferred by their intrinsic resistant properties (Das et al., 2010; Latha et al., 2015). Therefore, systematic screening for potential antibiotic resistance gene determinants in a potential probiotics genome must be conducted to assess the potential risks and mobility. Even when marine Streptomyces was not in use as a probiotic, these lateral gene transfer occur naturally. In addition, most studies to date suggest that the probable cause for antibiotic resistance patterns found in aquatic microorganisms is due to increased use of synthetic antibiotics and drugs (Dang et al., 2008). Furthermore, remedial strategies can be a valuable tool to remove the genetic elements that harbor the antibiotic resistance from the relevant probiotic strains (Morelli and Campominosi, 2002; Rosander et al., 2008). Reports on successful establishment of protoplast formation remedial methods were adopted to remove the two resistant plasmids from the parent Lactobacillus reuteri (ATCC55730) without affecting the probiotic properties of the strain (Rosander et al., 2008). For forward, in-depth studies can bring to light any significant disputes in the future.
A final limitation is the production of awful odor causing agents such as Geosmin (trans-1,10,-dimethyl-trans-(9)-decalol) and MIB or 2-methyl-isoborneol (exo-1,2,7,7-tetramethyl-[2.2.1]heptan-2-ol) by the marine Streptomyces. Planktonic cyanobacteria, bacteria, and several genera of fungi also produce geosmin and MIB (Wood et al., 2001). In freshwater aquaculture and in the recirculation water system, these two compounds which are semivolatile terpenoids, impart an earthy-musty taste and odor to the water, as well as to the cultured fish (Klausen et al., 2005; Guttman and Van Rijn, 2008). They reduce the palatability of the cultured livestock and negatively impact aquaculture industries (Auffret et al., 2011). These off-flavor compounds are known to be absorbed and bioaccumulated in the gills, skin and flesh of fish up to 200- to 400-fold as compared to the ambient concentration, resulting in a lower commercial value of the fish (Howgate, 2004). Many efforts have been carried out for the removal of these earthy odor compounds by the use of powdered activated carbon, ozonation, and biofiltration (Elhadi et al., 2004). Among these technologies, ozonation is suggested to be effective in the use of Streptomyces as the probiotics in aquaculture. Ozone has been known to remove odorants such as geosmin and MIB via oxidation (Gonçalves and Gagnon, 2011). Existing studies reported on the combined effect of ozonation (at0.3mgO3/L ROC) and Bacillus sp. S11 probiotic diets can protect shrimp P. monodon from Vibrio sp. challenge test without harm to the shrimp or he probiotic bacteria in their internal system (Meunpol et al., 2003).
Several microorganisms have been implicated in the biodegradation of MIB (Pseudomonas spp., Pseudomonas aeruginosa, Pseudomonas putida, Enterobacter spp., Candida spp., Flavobacterium multivorum, Flavobacterium spp., Slaviensisbacillus spp., Bacillus subtilis) and geosmin (Bacillus cereus, Bacillus subtilis, Arthrobacter atrocyaneus, Arthrobacter globiformis, Rhodococcus moris, Chlorophenolicus strain N-1053, Rhodococcus wratislaviensis). Other ways by which geosmin can be eliminated is through the insertion of genetic mutation in marine Streptomyces so that it can no longer produce geosmin. Recent research with marine Streptomyces has shown that the Cyc2 protein (specifically the N-terminal domain) is required for geosmin biosynthesis. The Cyc2 gene of marine Streptomyces can be made to be nonfunctional or even removed entirely by PCR and a double crossover event. This process can be explained via flowchart (Fig. 10). The ability to mutate Streptomyces genes to prevent the synthesis of geosmin may provide an opportunity to introduce the organism onto a bio-filter without impacting the removal of metabolic waste products while eliminating the synthesis of off-flavors.

4 Conclusion
Marine Streptomyces exhibits promising results as a probiotic application in aquaculture but very few studies have been carried out to establish its efficacy. Further widespread understanding and experimentation is still required to launch marine Streptomyces as a probiotic in disease prevention and growth enhancement for aquaculture animals. Much research about the organism has been done relating to the bioactive compound synthesis. Additionally, more detailed research focusing on the usage of molecular and other techniques in order to clarify the possible underlying mechanism of marine Streptomyces as probiotics in aquaculture environment. Overall, marine Streptomyces has potential as a candidate for probiotics and as well as an alternative to antibiotics in maintaining a sustainable aquaculture.