Development of New Probiotic Foods—A Case Study on Probiotic Juices
Veeranjaneya Reddy Lebaka⁎; Young Jung Wee†; Venkatarami Reddy Narala⁎; Vinod Kumar Joshi‡ * Yogi Vemana University, Kadapa, India
† Yungnam University, Gyeongsan, South Korea
‡ Dr. Y. S. Parmar University of Horticulture and Forestry, Solan, India
Abstract
The increasing demand for “healthy” foods (beyond basic nutrition) stems from the awareness people have regarding the relationship between diet and good health. This has led to the development of new and innovative food products throughout the world. In recent years, the concept of functional foods has moved progressively towards the development of dietary supplements which can affect intestinal microbial composition and activities. Among the functional components, probiotics is one of the fastest growing categories of food for which scientific researchers have demonstrated therapeutic evidence. The term probiotics is defined by FAO/WHO as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” Probiotics are increasingly being added to food products in order to develop functional foods with health promoting effects, but until the last decade, dairy products were the primary food group being used for this purpose. Development of nondairy probiotic foods, such as fruit juices, may provide greater choice to consumers and be attractive to those who cannot eat dairy foods. Limited documentation exists concerning the use of probiotics with various food matrices. In this review, the development of fruit juice probiotics, their application in the health and food areas and new trends in probiotic products and processes are presented.
Keywords
Functional foods; Probiotics; Nondairy products; Fruit juices; Encapsulation
1 Introduction
Development of the man-microbe symbiosis during early life is a very intriguing and important biological process. In humans, the intestinal microbiota plays a key role in host physiology and metabolism (Scholtens et al., 2012). The intestinal microbiota is active during the first years after birth. The infant gastrointestinal tract (GIT) is rapidly colonized through events related to the process of birthing (Adlerberth and Wold, 2009; Sela and Mills, 2014; Thum et al., 2012). Exposure to vaginal, fecal, epidermal, and milk microbiota are among the various routes by which microbial inoculation may occur (Cabrera-Rubio et al., 2012; Sela and Mills, 2014). The past decade has witnessed increasing attention and zeal being dedicated to explicate the role of the gastrointestinal microbiota in health and diseases as well as explore and exploit novel ways to investigate and manipulate the gut microbial composition for an improved health and well-being.
The word “probiotic” comes from the Greek word “ρο-βίο” which means “for life”. Probiotics were first introduced in the twentieth century around the year 1900 by the Russian Nobel Prize winner Elie Metchnikoff, who studied the longevity of Bulgarian farmers and suggested a direct link to their daily consumption of fermented milk products that contained large amounts of live nonpathogenic bacteria such as Lactobacillus bulgaricus, which can modify human intestinal flora in favor of microbial species useful to the host organism (Zhang et al., 2005). Later on it was found that yogurt contained the microorganisms required to guard the intestine from the damaging effects of other harmful bacteria. Different microorganisms have been used since then as probiotics in the last century for their ability to prevent and cure diseases. Kollath in 1953, first defined the term “probiotic”, when he used the term to denote all organic and inorganic food complexes as “probiotics,” in contrast to harmful antibiotics, for the purpose of upgrading such food complexes as supplements. Vergio, in his publication “Anti- und Probiotika,” compared the detrimental effects of antibiotics and other antimicrobial substances with favorable factors (“Probiotika”) on the gut microbiology. Lilly and Stillwell proposed probiotics to be “microorganisms promoting the growth of other microorganisms” (Vasudha and Mishra, 2013). Probiotics are defined as live microorganisms with a positive influence on their host with the ability to improve the intestinal microbial equilibrium (Guarner and Schaafsma, 1998). An expert panel was convened in October 2013 by the International Scientific Association for Probiotics and Prebiotics (ISAPP) to discuss the field of probiotics. The FAO/WHO definition of a probiotic—“live microorganisms which when administered in adequate amounts confer a health benefit on the host”—was reinforced as relevant and sufficiently accommodating for current and anticipated applications (Hill et al., 2014). The probiotic market was worth 15.7 billion Euros in 2010, and is expected to increase to 22.6 billion euro by 2015 (BCC Research, 2011).
2 Probiotic Microorganisms
To consider a microorganism as probiotic, the validation of its characteristics, strain identification, health benefits and other characteristics are required (Kailasapathy, 2010). For a long time, a very limited number of microbial strains, then used in food products or as supplements, were considered as probiotics based on their relevant properties (Grattepanche and Lacroix, 2010). The large variety of functional fermented products and modernization of the biochemical and genetic investigations of microorganisms has led to an increase in the number of microorganisms with probiotic potential. A list of various types of microorganisms used as probiotics is given in Table 1.
Table 1
| Lactobacillus Species | Bifidobacterium Species | Yeast and Other Species |
|---|---|---|
| L. acidophilus | B. bifidum | Saccharomyces boulardii |
| L. casei Shirota | B. breve | Saccharomyces cerevisiae |
| L. delbrueckii spp. bulgaricus | B. infantis | Streptococcus thermophilus |
| L. johnsonii | B. longum | Enterococcus faecalis |
| L. reuteri | B. adolescentis | Enterococcus faecium |
| L. rhamnosus | B. animalis | Pediococcus acidilactici |
| L. gallinarum | B. lactis | Lactococcus lactis |
| L. plantarum | Leuconostoc mesenteroides | |
| L. salivarius | Bacillus cereus | |
| L. crispatus | Escherichia coli Nissle 1917 | |
| L. gasseri | Propionibacterium freudenreichii |
Probiotic microorganisms are usually available as culture concentrates in dried or deep-freeze form as food additives for industrial or home use. These may be consumed either as food products (fermented or nonfermented) or as dietary supplements (products in powder, capsule or tablet forms). To be defined as probiotics, these bacteria must also fulfill some specific criteria listed by the European Union (Becquet, 2003):
- ● Detailed definition and typing
- ● Lack of pathogenic effects (i.e., production of enterotoxins and cytotoxins, entero invasiveness, adhesion of pathogens, hemolysis, serological pathogenicity, presence of antibiotic-resistant genes)
- ● Strain reaching its site of action, usually the gut, and thus survive to physiological stress met during its ingestion: acid stomach and gut pH, presence of biliary salts (Butel, 2014)
- ● Ability to adhere to the intestinal epithelium
- ● Ability to colonize the colon
- ● Proven clinical effect on health
- ● Safety (Gorbach, 2000)
- ● Competitive antagonism against pathogenic bacteria
The beneficial effects of probiotics (food with added live microbes) on human health are being increasingly promoted by health professionals. It has been observed that probiotics can play a significant role in many metabolic and immunological functions and also could have a significant effect in alleviating infectious disease in children. Disturbed gut microbial balance has been underscored as an originating factor for various metabolic, lifestyle, and diet-related maladies such as obesity, endotoxemia, insulin resistance, type 2 diabetes (T2DM), metabolic syndrome (MetS), inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), nonalcoholic fatty liver disease (NAFLD), GIT cancers and more. So far, over 900 investigations with human involvement and numerous review articles have been published on the favorable effect of probiotics. The studies were conducted with different probiotic strains on different health benefits and on different target populations (Makinen et al., 2012). Fig. 1 shows the key health benefits bestowed by probiotics.
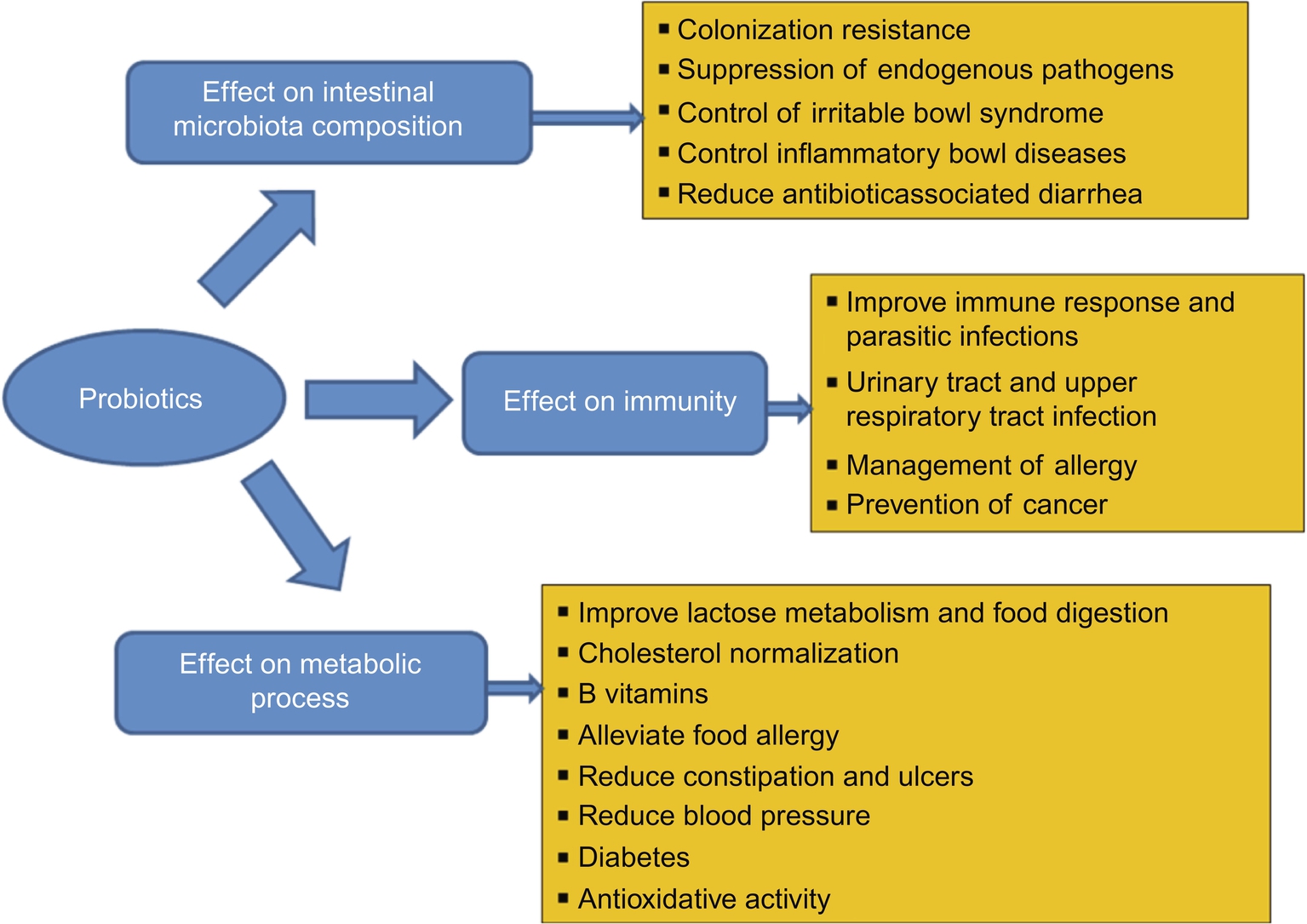
3 Probiotic Products
Classification and types of probiotic foods are given in Fig. 2.
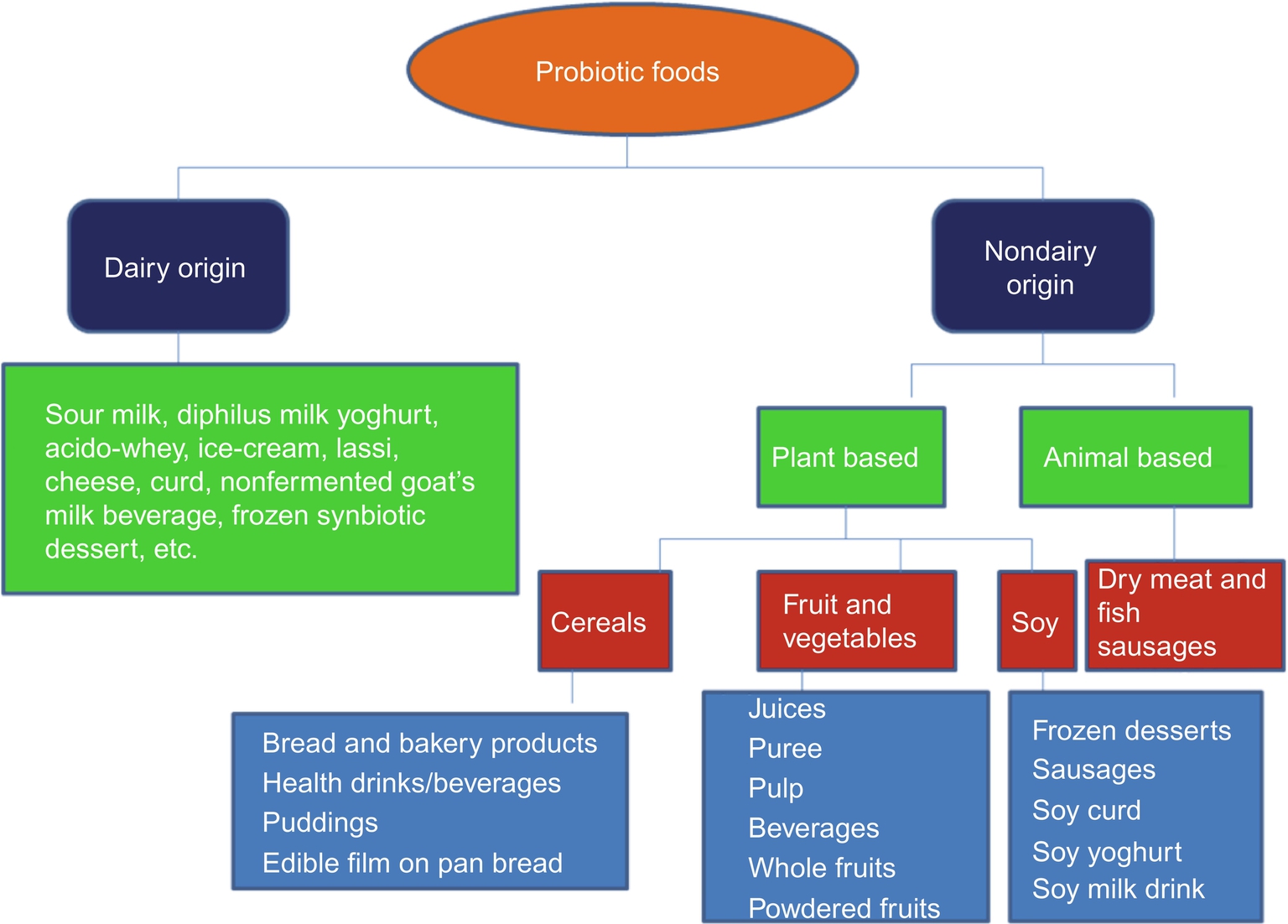
3.1 Dairy Products
Traditionally dairy fermented products have been considered to be the best probiotics carriers because they are easy to manufacture. All the dairy industry products (milk, yogurt, cheese, milk proteins, and milk related desserts) have been utilized for probiotification and consumers have accepted the presence of microorganisms in the dairy products they consume (Boza-Méndez et al., 2012). Dairy-based products account for approximately 43% of the functional beverage market, and is mostly comprised of fermented products (Özer and Kirmaci, 2010). Fermented milks, especially yogurt-style products, are the most popular functional probiotic beverages with kefir in Western Europe and North America and ymer in Denmark being good examples. Probiotics in dairy products were shown to be very promising features for a functional food, because they exhibited excellent conditions for maintaining the viability of probiotic bacteria (Buriti et al., 2007a,b; Souza and Saad, 2009; El-Dieb et al., 2012). When comparing other matrices with dairy matrices, the protective effect of the latter, especially from milk proteins on probiotics in the digestive system, has been discussed in the literature. Proteins are sources of bioactive peptide precursors, which resist passage through the digestive tract. Furthermore, milk has a physicochemical composition rich in protein with considerable amounts of lipids resulting in a protective matrix for probiotics. These characteristics favor the survival of probiotics against adverse conditions of the digestive system. Milk proteins are utilized as a suitable carrier matrix for probiotic bacteria, suggesting that it is effective in allowing probiotic bacteria reach their site of action (Ritter et al., 2009).
3.2 Nondairy Products
In recent years, nondairy probiotic delivery has been attracting more attention due to the increased demand from consumers. This demand is particularly due to an increase in the lactose intolerant population (around 70% in Asia), allergies to milk proteins and the prevalence of high cholesterol. Increase in the consumer vegetarianism throughout the developed countries has also increased the demand for vegetarian probiotic products. These are the major drawbacks related to the fermented dairy products (Heenan et al., 2004; Yoon et al., 2006). Economic and cultural factors may also negatively affect the consumption of probiotic dairy products, since most are fermented foods. Nondairy probiotic beverages are particularly attractive due to their lack of dairy allergens, low cholesterol content and vegan friendly status (Prado et al., 2008). Furthermore, different substrates can provide different combinations of antioxidants, dietary fiber, minerals and vitamins. This has led to the success of biofunctional foods and a desire to expand and provide alternative probiotic beverage choices that are not dairy-based. The non dairy functional/probiotic foods market is projected to have an annual growth rate of 15% between 2013 and 2018 (MarketsandMarkets, 2013). The above fact is highlighted by the trend in the U.S. functional food market, which is developing in a different fashion from that seen in Europe, with its functional food sector more broadly defined as neutraceuticals and consumer interest tending to lie more with botanical dietary supplements rather than fortified of foods.
The above mentioned draw backs of dairy probiotics has led to the search for new and alternative carriers for probiotic microbes. Development of nondairy probiotic products, such as fruits, vegetables and cereals, has been demonstrated as one of the best choices and demand for nondairy probiotics is increasing (Yoon et al., 2006; Prado et al., 2008; Granato et al., 2010a,b). The structural characteristics and composition (nutrients such as minerals, vitamins, dietary fibers, and antioxidants including good amount of sugars) of fruits, vegetables and cereals are suitable and ideal substrates for probiotic microbes (Reddy et al., 2015). Demand in the development of fruit juice based probiotics is increasing and is more attractive due to their taste, nutrient profiles and general acceptance as being healthy and refreshing foods (Nualkaekul et al., 2011).
3.3 Why Fruits are Ideal Choice?
Fruits are among the most important foods of mankind as they are not only nutritive but also play a vital role in maintaining health. Fruits, both fresh and processed, not only improve the quality of diet, but also provide essential ingredients such as carbohydrates vitamins, minerals, antioxidants. Fermentation is a viable technique in the development of new products with modified physicochemical and sensory qualities, especially flavor and nutritional components. Alcohol, acetic and lactic acid fermentations are important for quality in production. Fermented beverages have been known to humankind from time immemorial (Reddy et al., 2013).
Fruit juices have good amounts of sugars, minerals, and vitamins, which generally enhances the survival of probiotics during storage (Ding and Shah, 2008; Sheehan et al., 2007). Fruit juices are also an ideal choice for consumers who are interested in low cholesterol foods or suffer from lactose intolerance (Granato et al., 2010a,b; Prado et al., 2008). The rate of lactose intolerance (LI) incidence in different ethnic groups is given in Table 2. Previous studies have revealed that pH, organic acids levels, dietary fiber, protein, total phenol, and oxygen are the main factors which affect the survival of probiotics in fruit juices (Champagne et al., 2005; Nualkaekul et al., 2011; Saarela et al., 2006; Shah, 2000). It has been suggested that fruit juice is an ideal medium for carrying functional food ingredients such as probiotics because its residence time in the stomach is short, therefore the bacteria are not overexposed to the unfavorable acidic conditions of the stomach.
Table 2
| Sl. No. | Ethnicity/Geographic Region | % Population With LI |
|---|---|---|
| 1 | East Asian | 90–100 |
| 2 | Indigenous (North America) | 80–100 |
| 3 | Central Asian | 80 |
| 4 | African American (North America) | 75 |
| 5 | African (Africa) | 70–90 |
| 6 | Indian (Southern India) | 70 |
| 7 | Indian (Northern India) | 30 |
| 8 | Balkans Region | 55 |
| 9 | Latino/Hispanic | 51 |
| 10 | French (Southern France) | 65 |
| 11 | Anglo (North America) | 21 |
| 12 | Italian (Italy) | 20–70 |
| 13 | German (Germany) | 15 |
| 14 | British (UK) | 5–15 |
Data from de Souza Neves Ellendersen, L., Granato, D., Bigetti Guergoletto, K., Wosiacki, G., 2012. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 12, 475–485.
The protein and dietary fiber present in the fruit juice was shown as favorable for the survival of probiotics during storage in fruit juices (Champagne et al., 2011; Ding and Shah, 2008; Nualkaekul and Charalampopoulos, 2011; Saarela et al., 2006; Sheehan et al., 2007). The cell count of Lactobacillus casei in mare milk (8.59 ± 0.04 log CFU/mL) and in pineapple juice (8.20 ± 0.01 log CFU/mL) are comparable; the implication of this being that fruits are the best media for probiotic growth, in addition to being naturally full of essential nutrients and tasting good (Luckow and Delahunty, 2004a,b; Sheehan et al., 2007; Zhou et al., 2009). Fruit juice is cited as a healthy food product, and is consumed regularly by a large percentage of the population. However, the survival of probiotics in fruit-based matrix is more complex than in dairy products because bacteria usually need protection from the acidic conditions in the media (Shah, 2007). In spite of the challenges involved in the process of nondairy probiotic foods, it was suggested that fruit juice could serve as a good substrate for probiotics (Mattila-Sandholm et al., 2002; Tuorila and Cardello, 2002).
Fresh fruits and vegetables contain mostly cellulose which is not digested by the gastrointestinal system. Use of vacuum impregnation technology for probiotic bacteria were done with apples, showing promising results (Alzamora et al., 2005; Betoret et al., 2003). Thus, fruit matrices are certainly an important area of research and development with great potential for the functional food market. According to Kourkoutas et al. (2005), fruits contain requirements necessary for probiotic adhesion to plant tissue. In this way, fruits such as apples and pears, due to their cellulose content, may exert a protective effect on the probiotic microorganisms during passage through the intestinal tract. Studies conducted by Martins et al. (2013) group showed that fruits, such as apple, guava, banana, and melon, have potential as carriers for probiotic bacteria. The results of scanning electron microscopy showed a positive interaction between the probiotic microorganisms and the fruity tissues, since bacteria strongly adhered to the fruit surface (Martins et al., 2013). Therefore, the intrinsic characteristics of the plant surface microarchitecture, due to the presence of ridges and natural prebiotic compounds (such as oligosaccharides), protect probiotic microorganisms from the acidic environment of the stomach and are a source of nutrients, which positively influences bacterial survival (Ranadheera et al., 2014). However, Ijabadeniyi (2010) indicated the need for further work on the mechanism of internalization of microorganisms in plants. In particular, the way microorganisms fit into openings naturally present on the fruits’ surfaces, such as stomata and lenticels, and in tissue damaged by processing (Martins et al., 2013).
Studies demonstrate that the natural sugars that are present in juices can facilitate the growth of probiotic organisms and taste good. This is true of tomato, pomegranate, pineapple, orange, and cashew-apple juice. These microbes can impact physiochemical aspects, such as increasing the concentrations of flavanones and carotenoids in orange juice, and have shown good survival rates during beverage storage. The final acidity of these beverages is quite high after the fermentation by probiotic Lactobacillus species (Lactobacillus casei, Lactobacillus paracasei, Lactobacillus delbrueckii, Lactobacillus plantarum, and Lactobacillus acidophilus). The enrichment of juices with brewer’s yeast autolysate before fermentation positively impacts the nutritional quality of the final beverage, raising the feasibility of cofermentation by the appropriate combination of bacteria and yeast. Examples of commercially available probiotic-containing fruit juices include Biola and Bioprofit (Priya and Munishamanna, 2013).
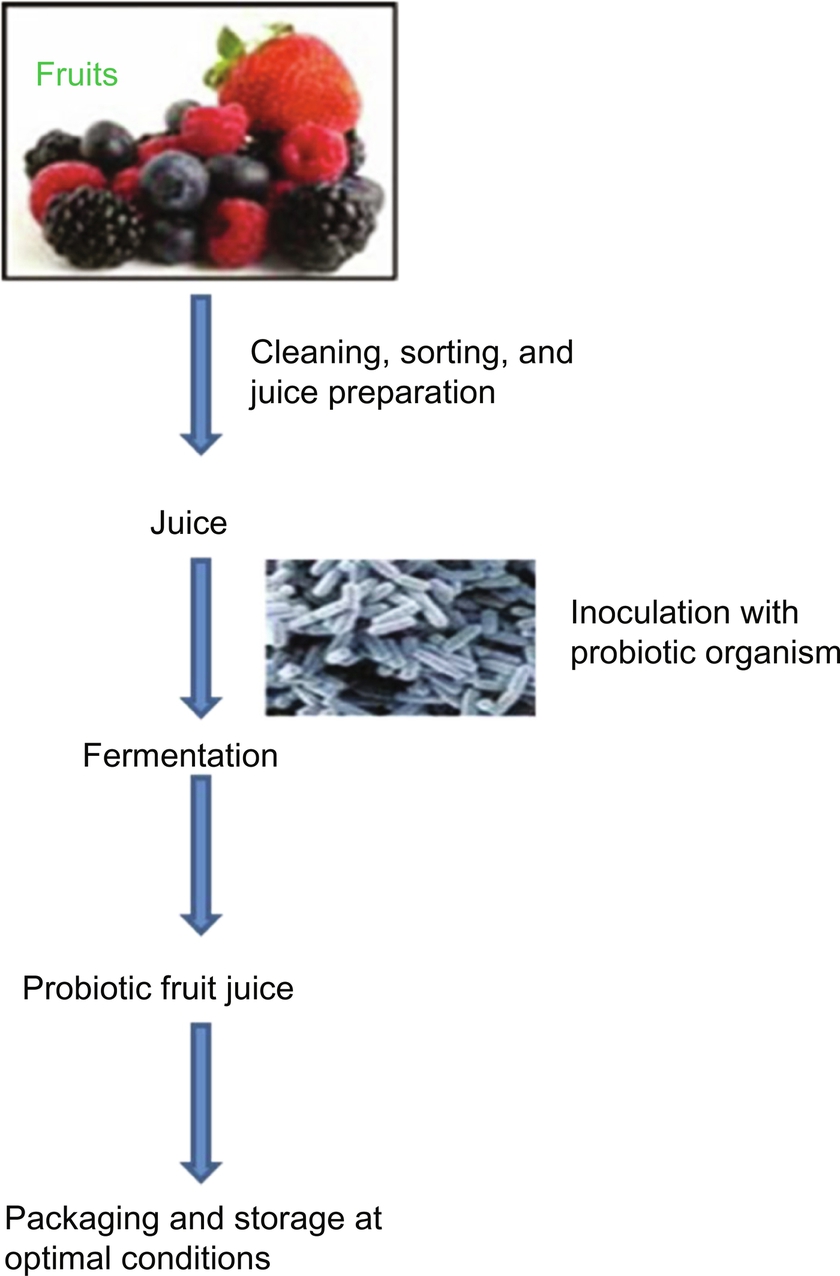
3.4 Preparation of Fruit Juice Probiotics
A flow diagram of preparation of probiotic fruit juice is depicted in Fig. 3.
3.5 Types of Fruit Juice Probiotics
To avoid the disadvantages of dairy-based fermented foods and to get appealing tastes and refreshing profiles, several nontraditional, nondairy-based fermented foods (fruit juices) have been developed (Tables 3 and 4). Some fruits, such as apples, oranges, black currant, banana, blueberry, pineapple, cashew apple, cantaloupe melon, raspberry, pomegranate juice, etc. (Savard et al., 2003; Yoon et al., 2005; Pereira et al., 2011; Nualkaekul et al., 2012; Fonteles et al., 2013; Anekella and Orsat, 2013), mixed with vegetable juice (Nosrati et al., 2014) are being employed for the development of probiotic juices.
Table 3
| Sl. No. | Name of the Fruit | Reference |
|---|---|---|
| 1 | Fermented banana pulp | Tsen et al. (2004) |
| 2 | Fermented banana | Tsen et al. (2009) |
| 3 | Tomato-based drink | Yoon et al. (2004) |
| 4 | Many dried fruits | Betoret et al. (2003) |
| 5 | Green coconut water | Prado et al. (2008a) |
| 6 | Cranberry, pineapple, and orange juices | Sheehan et al. (2007) |
| Kiwi | Sheehan et al. (2007) | |
| 7 | Grape and passion fruit juices | Saarela et al. (2006) |
| 8 | Probiotic banana puree | Tsen et al. (2009) |
| 9 | Nonfermented fruit juice beverages | Renuka et al. (2009) |
| 10 | Blackcurrant juice | Luckow and Delahunty (2004a,b) |
| Pomegranate | Mousavi et al. (2011) | |
| 11 | Plum juice | Sheela and Suganya (2012) |
| 12 | Cashew apple juice | Pereira et al. (2011) |
| 13 | Fruit juices (mango, sapota, grape) | Vijaya Kumar et al. (2013) |
| Peach | ||
| 14 | Guava | Dipjyoti et al. (2015) |
| 15 | Mixture of pineapple, apple, and mango juices | Mashayekh et al. (2015) |
| 16 | Clarified apple juice | Pimentel et al. (2015a) |
| 17 | Clarified apple juice with oligofructose or sucralose | Pimentel et al. (2015b) |
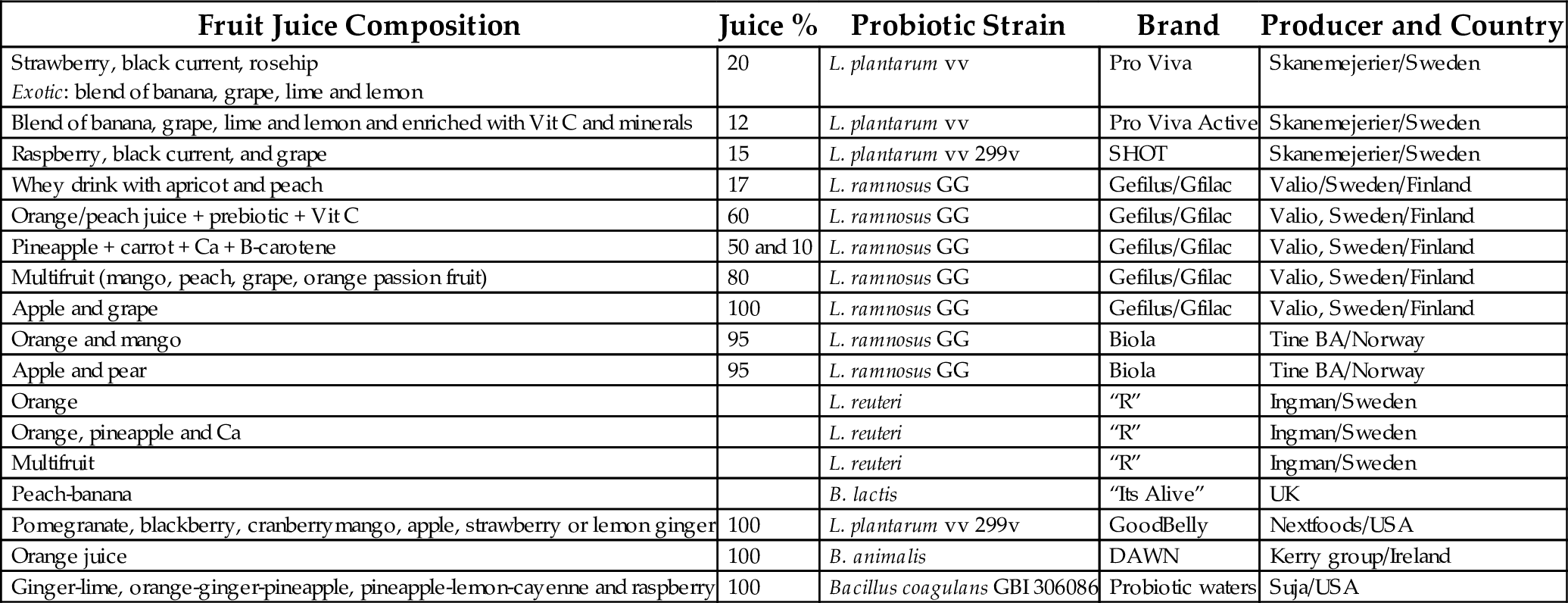
Table 4
| Fruit Juice Composition | Juice % | Probiotic Strain | Brand | Producer and Country |
|---|---|---|---|---|
| Strawberry, black current, rosehip Exotic: blend of banana, grape, lime and lemon | 20 | L. plantarum vv | Pro Viva | Skanemejerier/Sweden |
| Blend of banana, grape, lime and lemon and enriched with Vit C and minerals | 12 | L. plantarum vv | Pro Viva Active | Skanemejerier/Sweden |
| Raspberry, black current, and grape | 15 | L. plantarum vv 299v | SHOT | Skanemejerier/Sweden |
| Whey drink with apricot and peach | 17 | L. ramnosus GG | Gefilus/Gfilac | Valio/Sweden/Finland |
| Orange/peach juice + prebiotic + Vit C | 60 | L. ramnosus GG | Gefilus/Gfilac | Valio, Sweden/Finland |
| Pineapple + carrot + Ca + B-carotene | 50 and 10 | L. ramnosus GG | Gefilus/Gfilac | Valio, Sweden/Finland |
| Multifruit (mango, peach, grape, orange passion fruit) | 80 | L. ramnosus GG | Gefilus/Gfilac | Valio, Sweden/Finland |
| Apple and grape | 100 | L. ramnosus GG | Gefilus/Gfilac | Valio, Sweden/Finland |
| Orange and mango | 95 | L. ramnosus GG | Biola | Tine BA/Norway |
| Apple and pear | 95 | L. ramnosus GG | Biola | Tine BA/Norway |
| Orange | L. reuteri | “R” | Ingman/Sweden | |
| Orange, pineapple and Ca | L. reuteri | “R” | Ingman/Sweden | |
| Multifruit | L. reuteri | “R” | Ingman/Sweden | |
| Peach-banana | B. lactis | “Its Alive” | UK | |
| Pomegranate, blackberry, cranberrymango, apple, strawberry or lemon ginger | 100 | L. plantarum vv 299v | GoodBelly | Nextfoods/USA |
| Orange juice | 100 | B. animalis | DAWN | Kerry group/Ireland |
| Ginger-lime, orange-ginger-pineapple, pineapple-lemon-cayenne and raspberry | 100 | Bacillus coagulans GBI 306086 | Probiotic waters | Suja/USA |
Data from Mousavi, Z.E., Mousavi, S.M., Razavi, S.H., Emam-Djomeh, Z., Kiani, H., 2011. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World. J. Microbiol. Biotechnol. 27(1), 123–128.
3.5.1 Apple
Manufacturing of probiotic apple juice by Lactobacillus casei fermentation was studied. For optimum production pH (4.6), temperature (30°C) inoculum (4.87 log CFU/mL) and incubation period (16 h) were selected as effecting factors. During fermentation and storage, yellowness increased and redness reduced. The initial pH and temperature was demonstrated to have an effect on fermentation and the growth of microorganisms varied in accordance to the species and substrate used. The effect of temperature on the growth of Lactobacillus casei was greater than that of pH value. Initial pH had no significant effect on the biomass. A good number of living cells was observed at a mild temperature (~ 30°C) as higher temperatures diminished the viability of Lactobacillus casei. The best viability was observed at a pH 6.4 and a temperature of 30°C. Biomass production was increased in apple juice that was inoculated with 7.48 log CFU/mL. Apple juice produced by using optimum conditions was then refrigerated for 42 d in order to examine the bioavailability of Lactobacillus casei. After 3 weeks of storage, the viable cell number increased from 8.41 to 8.72 log CFU/mL and then decreased to 8.62 on day 35. The number of viable cells decreased at the end of storage period to a range quite acceptable (8 CFU/mL) for probiotic products (Gökmen et al., 2003). Optimization of culture conditions to develop the probiotic apple beverage was studied using response surface methodology. It was found that 10 h of fermentation at 37°C in Gala apple juice is best. Sensory evaluation of the prepared-fresh, fermented probiotic apple beverage determined it to have a thick texture and sweet taste while the probiotic apple beverage stored for 28 days at 7°C showed a thick texture and acidic taste (Table 5). Finally, when the fermented probiotic beverage was tested by potential consumers, it showed an acceptance index of 96% (de Souza Neves Ellendersen et al., 2012).
Table 5
| Samples | |||
|---|---|---|---|
| Attributes | Gala Apple Juice | Fresh Probiotic Apple Beverage | Probiotic Apple Beverage (Stored for 28 days) |
| Appearance | |||
| Caramel colorc | 7.83a (± 0.94) | 3.54b (± 2.28) | 5.84b (± 2.10) |
| Aroma | |||
| Applec | 7.53a (± 0.78) | 3.32b (± 2.44) | 3.80b (± 2.07) |
| Taste | |||
| Acidicd | 4.30a (± 2.53) | 3.32a (± 2.53) | 6.13b (± 2.57) |
| Applec | 6.49a (± 1.40) | 3.60b (± 2.20) | 4.04b (± 2.35) |
| Sweete | 6.43a (± 2.02) | 6.21a (± 2.05) | 4.56b (± 2.45) |
| Texture | |||
| Thickd | 0.99a (± 2.23) | 5.81b (± 2.01) | 6.31b (± 2.40) |
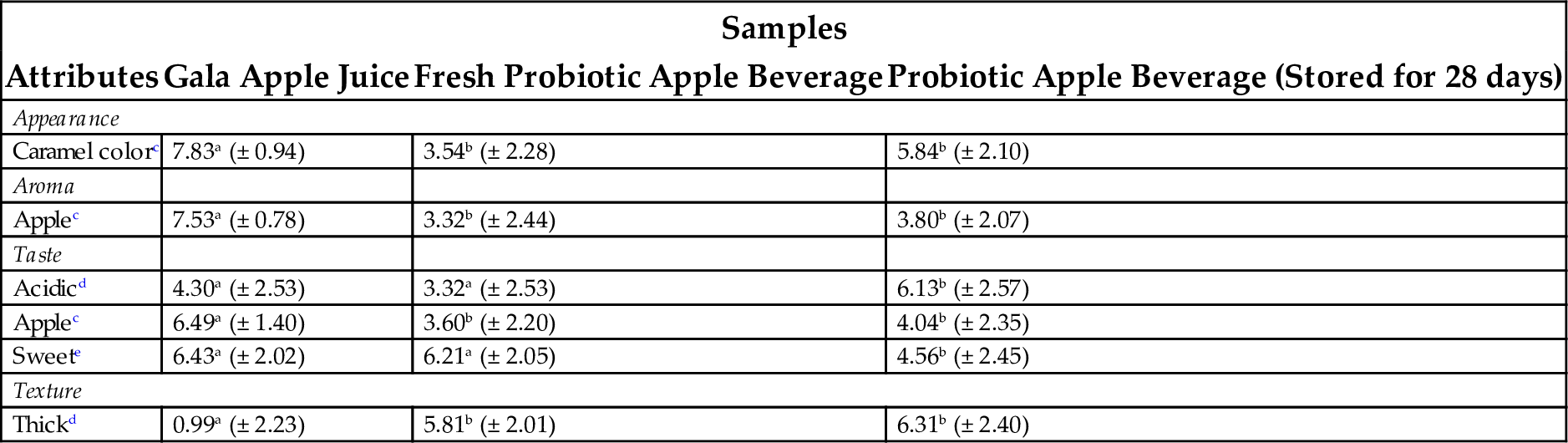
Source: de Souza Neves Ellendersen, L., Granato, D., Bigetti Guergoletto, K., Wosiacki, G., 2012. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 12, 475–485.
Means followed by the same letter, on the same line, did not differ significantly from each other (P > .05).
c Average of 9 tasters.
d Average of 7 tasters.
e Average of 10 tasters.
Pimentel et al. (2015a) have studied the physicochemical characteristics, probiotic viability and acceptability of Lactobacillus paracasei fermented clarified apple juice with oligofructose. No change in the physicochemical characteristics, acceptability or stability in storage, including enhanced probiotic survival, was observed. They also tested the effect of packaging material (probiotic juice in plastic or glass) on storage (4°C for 28 days) and suggested that viability of the probiotic culture in glass package was greater than in plastic and also clarified that packaging material (glass or plastic) have no influence on the physicochemical characteristics and consumer acceptability of juices. In another study Pimentel et al. (2015b) investigated the effect of adding oligofructose or sucralose as sugar substitutes as well as a probiotic on sensory quality and acceptance of clarified apple juice fermented with Lactobacillus paracasei. The study showed that oligofructose (20 g/L) substituted juices were less sweet than those with sucrose (20 g/L). However, both oligofructose and sucralose contributed to increased acceptance (taste and overall impression) of the pure juices. The probiotic supplementation increased the turbidity of the juice but acceptance (appearance, aroma, flavor, texture, and overall impression) did not diminish. The above studies demonstrate that it is possible to develop a synbiotic apple juice that has a similar sensory profile (excepting the presence of particles and turbidity) and acceptance to that of the sucrose-added juice by adding Lactobacillus paracasei as a probiotic culture and oligofructose as a sugar substitute and prebiotic.
3.5.2 Banana
Banana (Musa spp.) is an important food crop cultivated widely in tropical and subtropical areas and is one of the major fruits in the world (Gowen, 1995). It was processed widely for various products including banana puree, banana pulp, banana figs, banana flour or powder, banana chips, canned banana slices, banana jam, banana vinegar, banana wine, and banana juice, etc. Application of banana as a medium for LAB fermentation has also been studied (Aegerter and Dunlap, 1980; De Porres et al., 1985). Probiotic banana product with Lactobacillus acidophilus and certain other fruits (as prebiotic) have been prepared and might provide functional benefits (as synbiotic) (Prajapati et al., 1987). Stability and viability of free and immobilized Lactobacillus acidophilus by n-carrageenan entrapment was evaluated. Results revealed that entrapment enhanced stability and viability along with fermentation efficiency (Tsen et al., 2004). After the completion of fermentation, more viable cells in gel beads (108 CFU/(mL gel)) than in free cells (106 CFU/mL) were observed. Immobilized cells survive under adverse conditions and fermented efficiently compared to free cells. Immobilized Lactobacillus acidophilus fermented banana medium was found to possess synbiotic properties and resulted in desirable viable cell counts of 108 CFU/mL in the final product (Tsen et al., 2004). Yogurt with 15% banana marmalade (BM) was also prepared to study the effect of fermentation conditions on its sensory properties. Results from this study have potentially contributed to the use of probiotic cultures in fruit-flavored yogurt production. 106 log CFU/g viable cells were observed at 1 week of storage at 4°C and then began to decrease in both viable cell count and sensory qualities. The best sensory scores were recorded in the yogurts produced in 15% BM fermented with Bifidobacterium bifidum (Songül et al., 2012).
3.5.3 Blackberry
Rubus fruticosus, commonly referred to as the blackberry, has a high abundance of healthy antioxidants and nutrients such as anthocyanins, proanthocyanidins and other flavonoids, salicylic acid, ellagic acid, and fiber (Hager et al., 2008a,b; Rommel et al., 1992; Wang and Lin, 2000). Many of these compounds have been recognized for their anticancer properties. Commercially probiotic blackberry fruit juice is prepared with 108 CFU/mL by Next foods USA (Vattem et al., 2005).
3.5.4 Blueberry
Blueberries species are widely distributed in North America, Europe, Asia, and Africa, and they have the highest antioxidant capacity. Zhu et al. (2016) clearly demonstrated that the combination of blueberry juice and probiotic bacteria has a synergistic effect against the progression of AFLD. Blueberry juice can reduce the damage and apoptosis of AFLD by improving the activity of SIRT1 and promote activity of liver cells against oxidative damage by increasing the activities of related enzymes to remove oxygen free radicals, protect liver cells, effectively prevent lipid peroxidation, and regulate lipid metabolism.
3.5.5 Black Current Fruit
Luckow and Delahunty (2004a,b) evaluated the consumer’s acceptance for the appearance, aroma, texture, and flavor of the probiotic fruit juices. Novel blackcurrant juices containing the probiotic cultures were compared with the conventional blackcurrant juices by means of descriptive analysis. The probiotic juices contained aroma and flavors characteristic of the functional ingredients. Subsequent testing took place in a local shopping center where the consumers were presented with two randomly coded blackcurrant juice samples. One of the products was a natural blackcurrant juice and the other was a commercially processed blackcurrant juice containing probiotic cultures. The consumers were instructed that one of the juice samples contained “special ingredients” designed to improve their health. They were asked to assess their overall impression of both the juices and to rate their acceptance of the sensory characteristics. Additionally, based on their overall impressions and guided by their individual expectations, the consumers were asked to identify the juice they perceived to be the “healthiest” (e.g., containing the “special ingredients”). The juice preference was dependent on the gender and the age. In general, the consumers selected their most preferred juice product as the “healthiest” sample.
3.5.6 Cranberry Bush Fruit
Cranberrybush (Viburnum opulus L.) is the fruit of a deciduous shrub, which belongs to Caprifoliaceae family native to Europe, North Africa and North Asia and also often found in the central zone of western Russia. It is commonly used for ornamental purposes (Sedat Velioglu et al., 2006; Anonymous, 2008). The bark and fruit of the European cranberrybush tree are widely used in pharmacology and known for their antispasmodic and antimicrobial properties, relief of asthma, cold, fever, nervousness, water retention problems, cough, cramps, stomachache, menstrual cramps, uterine infections, blood pressure, and infertility (Anonymous, 2008; Nellessen, 2006). In Turkey, after harvesting the fruits in autumn, a fermented juice product is produced via spontaneous fermentation by putting the fruit in plastic jars topped with water and left for 3–5 months. This juice is not very palatable due to its strong sour flavor. The local people who relish its consumption believe the astringent taste of the juice can be reduced with a longer fermentation (Sedat Velioglu et al., 2006; Sagdic et al., 2014). 332 isolates of lactic acid bacteria (LAB) strains and Leuconostoc species isolated from traditional fermented gilaburu fruit juice were evaluated their probiotic potential. Characterization and probiotic potentials of the LAB isolated from fermented gilaburu (V. opulus) juice were studied just once and further research needs to be done on their qualities in similar food formulations as a probiotic (Sagdic et al., 2014).
3.5.7 Cashew Apple
Anacardium occidentale L. is a tropical tree native to the northern and northeastern regions of Brazil. Cashew apple is pseudofruit and is the part of the tree that connects it to the cashew nut, the tree’s true fruit. The cashew apple is very popular and highly consumed as a drink and as concentrated juice. Cashew apple is rich in fructose, glucose, minerals, several amino acids and is considered a good antioxidant with high ascorbic acid and phenols. (Zepka et al., 2009; Rabelo et al., 2009).
Pereira et al. (2011) studied the probiotification of cashew apple juice by Lactobacillus casei NRRL B-442. The optimum conditions for probiotic cashew apple juice production were an initial pH 6.4, a fermentation temperature of 30°C, an inoculation level of 7.48 log CFU/mL (Lactobacillus casei) and 16 h of fermentation process. It was observed that the Lactobacillus casei grew during the refrigerated storage. Viable cell counts were higher than 108 CFU/mL throughout the storage period (42 days). The juice’s lightness and yellow tint increased, resulting in a total color change from the initial redness of the juice which was substantially reduced during fermentation and storage. The above observations clearly indicate that cashew apple juice is as good a food matrix for Lactobacillus casei growth as dairy products and probiotic cashew apple juice fermented with Lactobacillus casei is a excellent functional health drink. The probiotic juice was stable up to 42 days of refrigerated storage without considerable viability losses and the characteristic color of the juice (yellowness) was enhanced along fermentation and storage. Enzyme browning, which is characteristic of cashew apple juice, is usually prevented using chemical products such as sodium metabissulfite, was avoided with just the application of fermentation technology. Besides taste, color is an important factor in food acceptance and the maintenance of the characteristic color without preservatives is a technological advantage. Therefore, cashew apple juice fermented with Lactobacillus casei is a healthy alternative of functional foods containing probiotics.
3.5.8 Green Coconut Water
Soccol et al. (2007) developed probiotic green coconut water fermented by Lactobacillus plantarum AC-1, Lactobacillus plantarum B-7, and Bifidobacterium animalis subsp. lactis BFL-9. The supplementation of the beverage with yeast extract, hydrolyzed soy protein, and sucrose produced maximum yield to a mixed culture with 8 h of fermentation. After 28 days of cold storage at 4°C, lactobacilli and Bifidobacterium animalis subsp. lactis presented 8 and 7 log CFU/mL, respectively. The fermented beverage added sugar and coconut aroma presented good sensorial characteristics and acceptability from potential consumers. Furthermore, the beverage could be classified as “low calorie” delivering only 33.5 kcal/100 mL.
3.5.9 Dates
An economically cheap medium is critical for industrial scale production of probiotic microorganisms (Oh et al., 1995). In this context, high amounts of sucrose as well as reducing sugars (especially glucose and fructose) present in date palm (Phoenix dactylifera) fruit will offers potential for convenient and inexpensive biomass production (Al-Shahib and Marshall, 2003). Date syrup and pits were reported to have positive influence as nutrients for the cultivation of Lactococcus lactis and consequently, were suggested as a suitable substrate for the cultivation of microorganisms (Khiyami et al., 2008). Date powder was used for the first time as a low-cost substrate during the optimization of culture conditions for the economic production of a probiotic bacterium, Lactobacillus casei ATCC 334. The effect of 11 factors on bacterial growth was investigated using the Taguchi experimental design and three factors, including palm date powder (38 g/L), tryptone (30 g/L) and agitation rate (320 rpm), were found to be the most significant parameters by response surface methodology (Shahravy et al., 2012).
3.5.10 Guava
Guava (Psidium guajava L.) is a widely cultivated fruit plant among the Psidium species. It is distributed worldwide in the tropical and subtropical areas. The fruit has a light yellow or pink pulp, is eaten fresh or as preserves, and is processed for use in dairy and baked products. It is rich in vitamin C, carbohydrates, proteins, calcium phosphorus, vitamin A, pantothenic acid, riboflavin, and thiamin (Diwan and Shukla, 2004; Reddy and Reddy, 2011).
The suitability of probiotic guava fruit juice beverage fermented with lactic acid bacteria isolated from milk, curd, whey and Lactobacillus plantarum has been studied by Dipjyoti et al. (2015). In this study the probiotic strains isolated from dairy products are used. The formulated guava fruit beverage was inoculated with these isolates and incubated at 30°C and measured for pH, acidity, sugar content, and viable cell count changes during fermentation under controlled conditions. The pH of the guava fruit beverage was initially 5.5 at °C and decreased gradually to a suitable range. The titrable acidity at 30°C was 0.28%–0.32% and found to increase with further fermentation. The sugar at 30°C was initially 15%–22%. The viable cell counts at 30°C were estimated, ranging between 5.6–8.9 × 106. The fruit beverage was assessed for acceptability through sensory evaluation. Molecular confirmation of isolate no. 8 through 16 s rDNA sequence has been done and was found to be Lactobacillus coryneformis. The final product resulted in suitable pH, acidity, sugar content and ideal number of viable cell counts. The probiotic guava fruit beverage could serve as a healthy beverage for consumers with dairy allergies, providing benefits to gut health, prevention of diarrhea and an excellent nutrient source for populations vulnerable to undernourishment.
3.5.11 Mango
Mango (Mangifera indica L.) belongs to the Anacardiaceae family, comprises > 70 genera, and is one of the most economically important fruits. According to the historical records, its cultivation began as a fruit tree in India around 4000 years ago. It is reputedly called the “king of fruits.” Mango occupied fifth rank in the total production of fruit crops worldwide and is produced in over 90 countries. Asia accounts for approximately 77% of global mango production. Mango is an important source of antioxidants, vitamins, minerals, and dietary fiber. Unlike many other fruits mangos do not contain any allergic proteins, and are a healthy alternative to dairy products for probiotification (Reddy et al., 2015; Vijay Kumar et al., 2015).
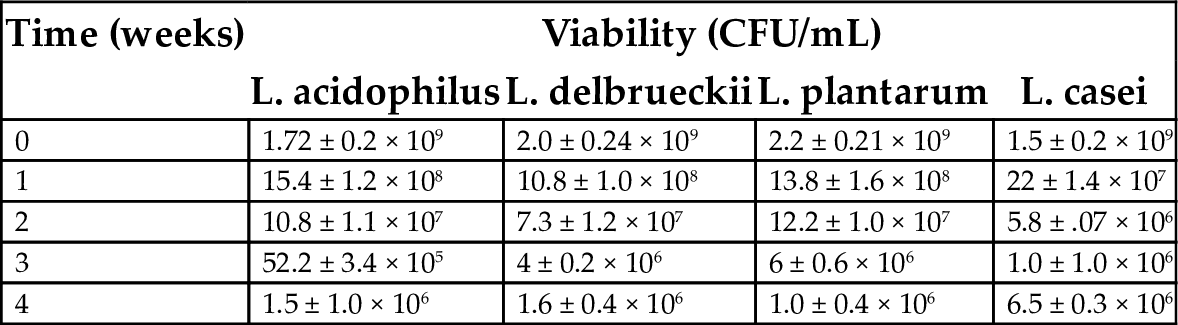
Probiotification of mango juice was carried out by four lactic acid bacteria (Lactobacillus acidophilus (MTCC10307), Lactobacillus delbrueckii (MTCC911), Lactobacillus plantarum (MTCC9511) and Lactobacillus casei) fermentation (Reddy et al., 2015). Mango juice fermentation was performed at 30°C for 72 h under microaerophilic conditions. Microbial population, pH, titrable acidity, sugar, and organic acid metabolism were measured during the fermentation period and the viability of the strains was determined under the storage conditions at 4°C for 4 weeks. The four lactic acid bacteria used in this study showed good growth in mango juice and were found capable of rapidly utilizing the juice for cell synthesis and lactic acid production without nutrient supplementation. The lactic acid cultures rapidly fermented mango juice and reduced the level of sugar. Lactobacillus plantarum consumed the sugar at a much faster rate than the other three strains. The lactic acid bacteria reduced the pH to as low as 3.2 from 4.5 within 72 h of fermentation (Fig. 4). Substrate concentration was reduced to 5.8% (w/v) from 12% (w/v). Lactobacillus plantarum showed the faster utilization of sugar and reduction of pH of the mango juice compared to the other strains used. The viability of cells maintained at 1.0 × 107 CFU/mL throughout the storage period. The effect of cold storage on the viability of lactic acid bacteria species in fermented mango juice is presented in Table 6. From this investigation, it can be concluded that mango juice is suitable for the production probiotic beverage. The total phenolic content (TPC), antioxidant and antimicrobial activity of probiotic mango juice was investigated by Kumar et al. (2015). TPC was increased significantly in 72 h of fermented probioticated juice and DPPH radical scavenging activity was significantly higher in probioticated mango fruit juice than in nonprobioticated fruit juice. No significant difference between the sensory scores of probioticated and nonprobioticated products was noticed and the influence of fermentation on juice texture, taste, flavor and overall acceptance was not significant. From these results, it can be concluded that probiotic mango fruit juice can be utilized to deliver probiotic LAB to lactose-intolerant people and those who are allergic to milk-based products.
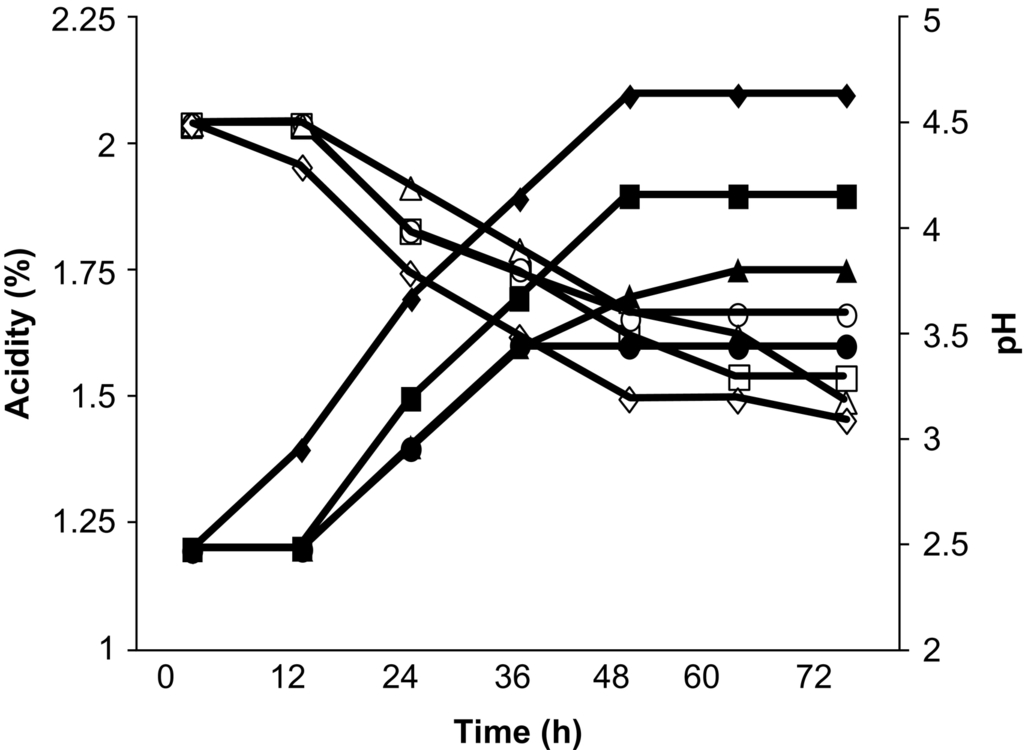
Table 6
| Time (weeks) | Viability (CFU/mL) | |||
|---|---|---|---|---|
| L. acidophilus | L. delbrueckii | L. plantarum | L. casei | |
| 0 | 1.72 ± 0.2 × 109 | 2.0 ± 0.24 × 109 | 2.2 ± 0.21 × 109 | 1.5 ± 0.2 × 109 |
| 1 | 15.4 ± 1.2 × 108 | 10.8 ± 1.0 × 108 | 13.8 ± 1.6 × 108 | 22 ± 1.4 × 107 |
| 2 | 10.8 ± 1.1 × 107 | 7.3 ± 1.2 × 107 | 12.2 ± 1.0 × 107 | 5.8 ± .07 × 106 |
| 3 | 52.2 ± 3.4 × 105 | 4 ± 0.2 × 106 | 6 ± 0.6 × 106 | 1.0 ± 1.0 × 106 |
| 4 | 1.5 ± 1.0 × 106 | 1.6 ± 0.4 × 106 | 1.0 ± 0.4 × 106 | 6.5 ± 0.3 × 106 |
3.5.12 Noni Fruit
Noni (Morinda citrifolia) is a tropical and subtropical plant that grows on the Pacific islands. It contains good natural medicinal qualities (Dixon et al., 1999; McClatchey, 2002) and has been cultivated for over 2000 years. All the parts of the plant (fruit, leaves, bark, and root) have been known to contain active compounds (Chan-Blanco et al., 2006). Most noni fruit is consumed as fresh juice, which is traditionally made by the natural fermentation of noni fruit in sealed containers for 4–8 weeks at ambient temperature (Wang et al., 2009). Wang et al. (2009) assessed the feasibility of the noni fruit as a substrate for the development of probiotic juice by Lactobacillus casei, Lactobacillus plantarum, and Bifidobacterium longum. All tested strains grew well in noni juice. After 4 weeks of storage at 4 °C, Bifidobacterium longum and Lactobacillus plantarum survived under low pH conditions in the fermented juice. Lactobacillus casei produced less lactic acid than Bifidobacterium longum and Lactobacillus plantarum. After 4 weeks of cold storage at 4°C. They found that Bifidobacterium longum and Lactobacillus plantarum are optimal probiotics for fermentation with noni juice.
3.5.13 Peach
Peaches (Prunus persica) are rich source of minerals, vitamins and contain a good amount of sugar (Pakbin et al., 2014). They are rich in phytochemicals, dietary fiber and polyphenols that provide health benefits to the consumers (Magerramov, 2006). Probiotic peach juice was prepared by Pakbin et al. (2014) using three different LAB species (Lactobacillus casei, Lactobacillus delbrueckii, and Lactobacillus plantarum) and it was determined that all species adequately fermented the peach juice and showed positive growth (reached 1.12 × 109 CFU/mL after 48 h at 30°C). The viable cell counts of Lactobacillus plantarum and Lactobacillus delbrueckii in fermented peach juice were 7.2 × 105 and 1.7 × 107 CFU/mL after 4 weeks in storage at 4°C. However, Lactobacillus casei was unable to survive at low pH and high acidity conditions and in 4°C cold storage for 1 week it completely lost cell viability.
3.5.14 Pear
The Korean pear (Pyrus pyrifolia Nakai) is one of the most abundantly produced fruits in South Korea and is mainly composed of 85%–88% water, 10%–13% carbohydrates, 0.3% proteins, and 0.2% lipids (Kim et al., 2010a,b). It is mostly consumed as a fresh fruit. Kim et al. (2010a,b) have studied the suitability and potential effects of Korean pear puree on probiotic microorganism Leuconostoc mesenteroides. The pH and titratable acidity of the pear puree were 4.06% and 0.66%, respectively, after 12 h of fermentation. The viable cell count of Lactobacillus mesenteroides 51-3 rapidly increased to 3.7 × 109 CFU/g after 12 h of cultivation. The content of lactic acid and acetic acid was determined to be 0.138% and 0.162%, respectively, after 12 h of fermentation. When the fermented pear puree was stored at 4°C, the pH, titratable acidity and viable cell count (109 CFU/g) remained fairly constant for 14 days.
3.5.15 Pineapple
Probiotic pineapple juice beverage was produced from sonicated pineapple juice by Lactobacillus casei NRRL B442 fermentation. Maximum microbial viability was found at 31°C and pH 5.8 (optimized conditions). After 42 days of storage under refrigeration (4°C), the microbial viability was 6 × 106 CFU/mL in the nonsweetened sample and 4.7 × 106 CFU/mL in the sweetened sample. The pH of both samples during storage was decreased due to postacidification. Browning of juice was not observed and color of the juice was maintained throughout the storage period. From this it can be concluded that the sonicated pineapple juice is suitable for Lactobacillus casei cultivation and as well as good substrate for the development of an alternative nondairy probiotic beverage.
The survival of probiotic lactic acid bacteria, belonging to Lactobacillus plantarum and Lactobacillus fermentum species, was monitored on artificially inoculated pineapple pieces throughout storage. The main nutritional, physicochemical, and sensorial parameters of minimally processed pineapples were monitored. Finally, probiotic Lactobacillus were further investigated for their antagonistic effect against Listeria monocytogenes and Escherichia coli O157:H7 on pineapple plugs. These results suggested that at 8 days of storage, the concentration of Lactobacillus plantarum and Lactobacillus fermentum on pineapples pieces ranged between 7.3 and 6.3 log CFU/g, respectively, without affecting the final quality of the fresh-cut pineapple. The antagonistic assays indicated that Lactobacillus plantarum was able to inhibit the growth of both pathogens, while Lactobacillus fermentum was effective only against Lactobacillus monocytogenes. This study suggests that both Lactobacillus plantarum and Lactobacillus fermentum could be successfully applied during processing of fresh-cut pineapples, contributing at the same time to inducing a protective effect against relevant food borne pathogens.
An examination of the fermented functional drink production was done based on the mixture of pineapple, apple and mango by Lactobacillus casei PTCC 1608. To produce a probiotic fermented drink based on the mixture of pineapple, apple and mango, microbe suspension with initial concentration 106, 107 CFU/mL is provided and inoculated to the concentrated mixture of juice (concentration of 20%, 30%, and 40%) and fermentation process is performed for 72 h at a temperature of 37°C. During fermentation in all treatments, the population of probiotic bacteria was increased due to using sugar and nutrients in juice, acidity is increased and sugar is reduced. Based on the results, F2T2 treatment with concentration of 30% of juice (including 15% pineapple juice, 7.5% apple juice and 7.5% mango juice) and density 107 CFU/mL is the best treatment and with the highest bacteria measured after 28 days. The results of the study suggest that the mixture of pineapple, apple and mango juice is a good medium for the growth of lactic acid bacteria and functional drink production.
3.5.16 Pomegranate
Pomegranate (Punica granatum, Punicaceae) is a well known tropical fruit produced worldwide. The fresh juice contains 85.4% water and considerable amounts of total soluble solids (TSS), total sugars, reducing sugars, along with bioactive secondary metabolites (anthocyanins, ellagic acid derivatives, and hydrolyzable tannins) and nondigestible carbohydrates (prebiotics). These antioxidants are more potent, on a molar basis, than many other antioxidants including vitamin C, vitamin E, coenzyme Q-10 and alpha-lipoic acid (Aviram et al., 2002). The antioxidant level of pomegranate juice was found to be higher than green tea and red wine (Gil et al., 2000). It has also been reported that pomegranate contains considerable health-promoting properties with antimicrobial, antiviral, anticancer, antioxidant and antimutagenic effects (Negi et al., 2003).
Attempts were made to produce a nondairy probiotic drink based on pomegranate juice which already possesses many inherent health benefits. Probiotic pomegranate juice was produced through its fermentation by four LAB strains (Lactobacillus plantarum, Lactobacillus delbruekii, Lactobacillus paracasei, Lactobacillus acidophilus) (Mousavi et al., 2011). Fermentation was carried out at 30°C for 72 h under microaerophilic conditions. Lactobacillus plantarum and Lactobacillus delbruekii showed better microbial growth and sharp pH increment at the initial stages of fermentation in comparison with other strains. The most significant acid present in pomegranate juice is citric acid which was consumed considerably by all probiotic lactic acid bacteria. Higher viability during the storage time was observed in Lactobacillus plantarum and Lactobacillus delbruekii. There was no loss in viability of cells up to 2 weeks, but it decreased dramatically after 4 weeks. The characteristic low pH value of pomegranate juice could be adjusted by mixing with other fruits or by protecting probiotics from acidic environment by microencapsulation (Mousavi et al., 2011). It can be concluded that pomegranate juice was proven to be a suitable media for the production of probiotic drink.
3.5.17 Sapota
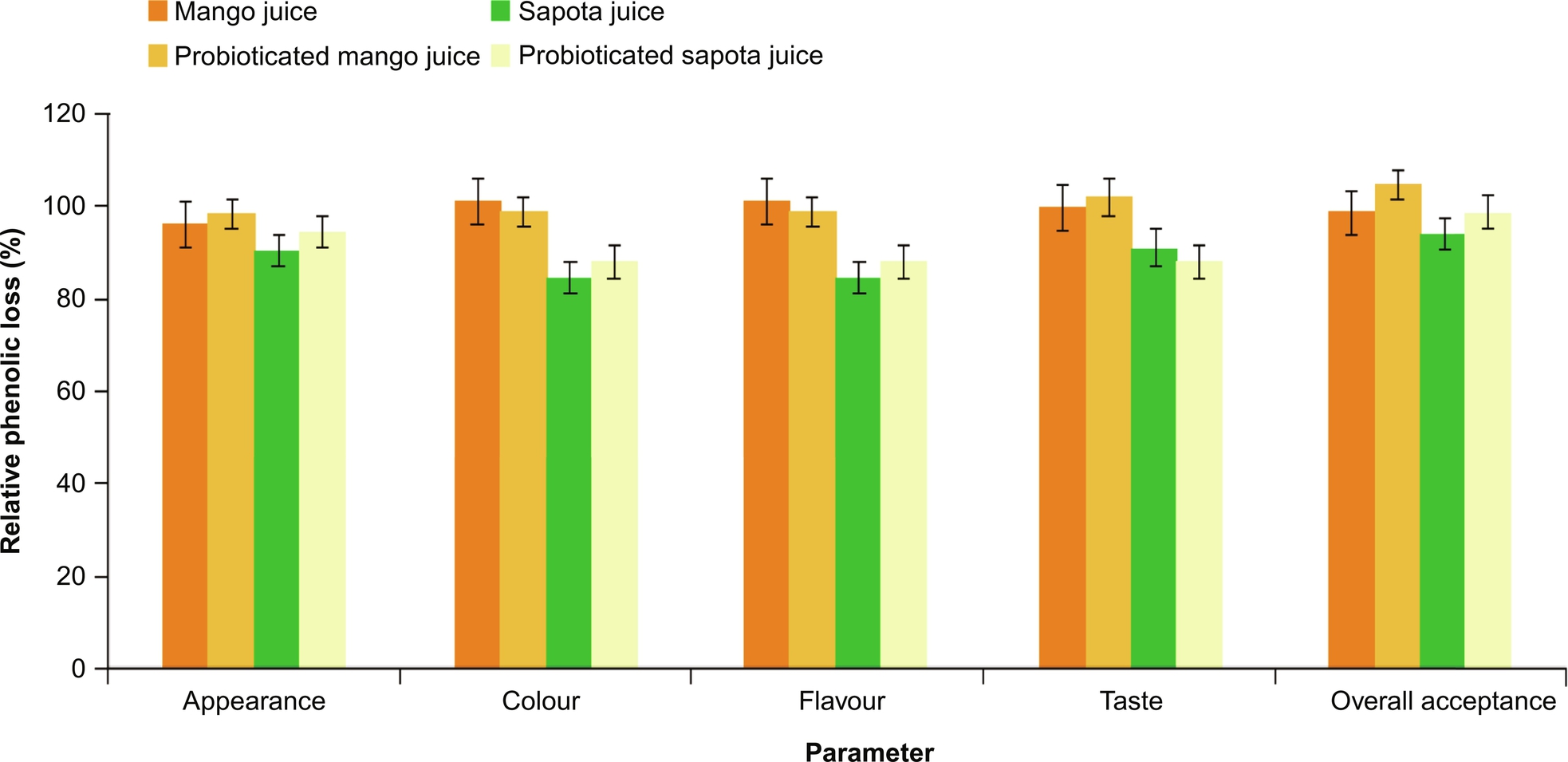
Sapota (Achras sapota L. or Manilkara zapota L.) is one of the major fruit crops in India, Mexico and Venezuela. Sapota fruit contains significant fermentable sugars as well as protein, phenolics carotenoids, ascorbic acid, minerals (potassium, copper and iron), and vitamins (A, C, folate, and pantothenic acid). Because of these properties, sapota is considered a healthy fruit to alleviate micronutrient malnutrition. Recently, it was also reported that a methanolic extract of sapota fruit inhibits tumor growth (Srivastava et al., 2014). There are about 40 varieties of sapota grown in different parts and “Kalipatti” is the leading cultivar in the India. Vijay Kumar et al. (2015) developed a probiotic sapota juice and studied the growth kinetics of LAB, antioxidant, antimicrobial and sensory quality. From this investigation, it can be concluded that sapota juice is also suitable for the production probiotic beverages with a high cell count (8 × 108 CFU/mL). Total phenolic content (TPC), antioxidant and antimicrobial activity of probiotic sapota juice was investigated. TPC was increased significantly in 72 h fermented probioticated juice and DPPH radical scavenging activity was significantly higher in probiotic sapota fruit juice than in nonprobiotic fruit juice (Fig. 5). No significant difference between the sensory scores of probioticated and nonprobiotic products was noticed and the influence of fermentation on juice texture, taste, flavor and overall acceptance was not significant. From these results it can be concluded that probiotic sapota fruit juice can be use to deliver probiotic LAB to lactose-intolerant people and those who are allergic to milk-based products.
3.5.18 Tomato
Yoon et al. (2004) have studied the suitability of the tomato juice as a substrate for the production of probiotic juice by four different LAB (Lactobacillus acidophilus LA39, Lactobacillus plantarum C3, Lactobacillus casei A4, and Lactobacillus delbrueckii subsp. bulgaricus D7). After 72 h of fermentation the biomass concentration maximally reached to 9 × 109 in Lactobacillus acidophilus LA39, pH was reduced to 4.1 and acidity was increased to 0.65%. Lactobacillus plantarum consumed the sugar at a much faster rate than the other three LAB. The viability remains in a range of 6–8 log CFU/mL. During the storage Lactobacillus delbrueckii showed the highest viability compared to other three. The lactic acid cultures significantly fermented tomato juice and reduced the level of sugar. The effect of potential probiotics and autochthonous LAB on health-promoting and sensory properties of tomato juice was studied by Di Cagno et al. (2013). Compared to unfermented tomato juice or tomato juice fermented with allochthonous strains, the values of viscosity, color, total antioxidant activity, concentration of ascorbic acid, glutathione, and total free amino acid increased when the tomato juice was fermented with autochthonous starters. From these results, it can be concluded that the fermented tomato juice could be utilized as a substrate for probiotification, and the product could serve as a health beverage for consumers like vegetarians and individuals allergic to dairy products.
3.6 Yeast Probiotic Juices
The probiotic characteristics of the Saccharomyces cerevisiae var. boulardii (S. boulardii) were confirmed by double-blind studies (Czerucka et al., 2007). In 1920 Henri Boulard isolated it from lychee fruit. S. boulardii was used as a preventative and therapeutic agent for the curing of diarrhea (McFarland, 2009; Rajkowska et al., 2012). The volume of studies reporting significant numbers of yeast in traditional fermented beverages indicates their importance in these fermentations. Yeasts in dairy generate desirable aromatic compounds, proteolytic and lipolytic activities and can aid bacterial growth by producing amino acids, vitamins and other metabolites, and contribute to the final composition of the product by producing ethanol and carbon dioxide. In particular, studies have demonstrated that yeast can exert a positive effect on the abundance of Lactobacillus in fermented environments (Gadaga et al., 2001), and this might be a key function in such symbioses, as well as preventing the growth of undesirable species. In addition the previously mentioned benefits, it also helps protect health like other probiotic bacteria strains through the production of enzymes like phytase (useful in chelate degradation) and short chain fatty acids, degradation of pathogenic toxins and improves the immune system (Czerucka et al., 2007; McFarland, 2009). It can increase the availability of nutrients in fermented food systems via change of food components and biofortification of folate (Rajkowska et al., 2012). These positive effects are dependent on the amount of S. boulardii in the diet. However, above 103 CFU/g causes an undesirable taste and texture due to alcoholic fermentation. Therefore, S. boulardii is still under research as a food additive and starter culture (Joshi and Thorat, 2011). While yeast only comprise < 0.1% of the gut microbiota, they are 10 times larger than prokaryotes and can thus impede colonization of pathogenic bacteria (Czerucka et al., 2007). Some species of Saccharomyces and Candida yeasts are common to both fermented beverages and the gut microbiota. As such, these species could be investigated with a view to their contribution to fermentations and optimizing health-promoting potential. However, to date Saccharomyces boulardii is the only recognized probiotic yeast. Success has been made in incorporating them in commercial fermented milk products, but excessive gas production during storage can be an issue.
A new functional beverage by the fermentation of probiotic yeast Saccharomyces cerevisiae boulardii was formulated using tomato juice (Fratianni et al., 2013). The different culturing media did not negatively affect the yeast resistance to the in vitro passage through acidic and pancreatic environments. Several biochemical parameters of the fermented juice, its polyphenol and lycopene content, as well as its DPPH free radical scavenging capability, were evaluated during storage. Fermentation of the juice with S. boulardii caused only a slight decrease of total polyphenols (0.442 mol/L vs. 0.474 mol/L, respectively) and lycopene (13.38 g/mL vs. 16.76 g/mL, respectively), compared to raw juice. On the other hand, after passage through in vitro double gastric + pancreatic conditions, the amount of such biocomponents was constant during storage until 28 days, and only decreased after 56 days. The amount of fermented juice required to inhibit the DPPH activity of 50% increased three times. The developed product might be an alternative for delivering probiotics, especially for individuals suffering from lactose intolerance to dairy products. Fermentation of pomegranate juice as single or mixed substrate with orange juice, without addition of extra nutrients, using kefir grains (mixture of yeast and LAB) was studied. During storage at 4°C for 4 weeks, sugar consumption and ethanol production were monitored as was the survival of lactic acid bacteria. The results showed that addition of orange juice improved the ability of kefir grains to ferment pomegranate juice and increased the survival rates of LAB contained in kefir grains during storage. It is worth noting that 75% of cells survived (6.48 log CFU/mL) after 4 weeks of storage in the fermented substrate (24% pomegranate juice). Lactic acid formation was observed in all products, being especially high (1.3–1.9 g/L) in mixed substrates, indicating metabolic activity of microbes during storage. Sensorial test results of the product showed consumer acceptance for all the fermented juices. These results suggest that there is a possibility to produce low alcoholic nutrient fruit beverages with potential antioxidant (fruit constituents) and probiotic properties.
4 Challenges
4.1 Survivability and Stability
The health benefits of probiotics mainly depend on their quantity in foods and their survivability in the gastrointestinal tract. The viability of probiotics is strain-dependent and different from one strain to another (do Espirito Santo et al., 2011; Tripathi and Giri, 2014). In the final product the number of probiotics should be at least 106 or 107 CFU/mL at the end of storage which corresponds to 109 CFU per portion (Nualkaekul and Charalampopoulos, 2011).
A good amount of research has been carried out and usable data is available on enhancing the storage stability of probiotics. The interpretation of this data is hindered by certain factors such as the probiotic process conditions prior to storage and many studies lack kinetic data. Kinetic data, along with storage temperatures and aw, would allow better interlaboratory comparison of results, and improve predictions of probiotic survival in different storage conditions. Following this train of thought, it is not enough to merely secure viable cell counts that meet the minimal requirement at end of the storage period (typically 106–107 cells/g) but also to limit the magnitude of viability loss during shelf life. This could potentially avoid the overdosing of probiotic cells in the product at initial stage and keep the cost of production at an economically reasonable level (Makinen et al., 2012).
In addition to the essential nutrients (minerals, vitamins, dietary fibers, antioxidants), juices contain several strong components that could limit probiotic survival in juices (Perricone et al., 2015). According the Tripathi and Giri (2014), these can divide into three groups:
- 1. Food parameters: pH, titratable acidity, molecular oxygen, water activity, presence of salt, sugar and chemicals, like hydrogen peroxide, bacteriocins, artificial flavoring, and coloring agents;
- 2. Processing parameters: heat treatment, incubation temperature, cooling rate, packaging materials and storage methods, oxygen levels, volume;
- 3. Microbiological parameters: strains of probiotics, rate and proportion of inoculation.
pH is one of the very important factors affecting the viability of probiotics. Juices contain a significant amount of organic acids resulting in the low pH. Consequently the juices could have the combined effect of acidic conditions and the intrinsic antimicrobial effect of acids. A few major probiotics (Lactobacilli and bifidobacteria, Lactobacilli) are resistant from pH 3.7 to 4.3 and can survive in fruit juices. Bifidobacteria are less acid tolerant, and a pH of approximately 4.6 is detrimental for their survival (Tripathi and Giri, 2014; Reddy et al., 2015). In contrast, the above probiotics showing good viability in low pH of fruit juices in these cases pH cannot explain the trends experienced by some probiotics. Nualkaekul et al. (2011) investigated the factors that affected the survival of Bifidobacterium longum in model solutions and in fruit juices (orange, grapefruit, blackcurrant, pineapple, pomegranate, and strawberry). They reported that after storage at 4°C for 6 weeks in orange, grapefruit, blackcurrant, and pineapple juices, bifidobacteria decreased not < 0.8 log CFU/mL and orange and pineapple juice supported the highest cell count. Furthermore, they found some controversial data on the effects of pH, as the decrease in grapefruit was only 0.5 log CFU/mL, despite the low pH (3.21) and the high concentration of citric acid (15.3 g/L). On the other hand, the probiotic viability was below the detection limit in pomegranate after 1 and 4 weeks in strawberry juice. These results suggest that survival was the outcome of synergistic and antagonistic action of some factors in that phenolic compounds could play a significant role. Generally, pH exerts a detrimental effect, but protein and dietary fiber could protect cells from acidic stress; the role of citric and malic acids is controversial, as they seemed to protect probiotics, whereas phenols could cause a strong viability loss (Tripathi and Giri, 2014). Although the pH is a drawback for probiotic survival in juices, Ranadheera et al. (2014) observed that the incorporation of lactic acid bacteria into fruit juices with low pH enhanced the resistance of bacteria to subsequent stressful acidic conditions, such as those found in gastrointestinal tract.
4.2 Sensory Traits
Another critical challenge for probiotification of fruit juices is consumer acceptance (Luckow and Delahunty, 2004a,b; de Souza Neves Ellendersen et al., 2012). It has been reported that probiotification of fruit juice can result in flavors described as “dairy,” “medicinal,” “acidic,” “salty,” “bitter,” “astringent,” “artificial,” or “earthy” (Granato et al., 2010a,b; Luckow and Delahunty, 2004a,b; Saeed et al., 2013). However, it is unclear whether all probiotic cultures give the product the same flavor at the same levels of intensity (Luckow et al., 2006). The probiotic effects on the sensory characteristics of juices depend on the type of fruit, probiotic organism, and the storage temperature and supplementation of prebiotics and protectants. Some researchers have shown that probiotics did not affect the overall acceptance of certain fruit juices. For example Perricone et al. (2015) demonstrated no adverse change in flavor for pineapple juice containing Lactobacillus reuteri; and Tapia et al. (2007) for a fresh apple beverage fermented by Lactobacillus casei, and de Souza Neves Ellendersen et al. (2012) using apple juice.
A possible solution for unpleasant flavor outcomes in probiotic juices is masking, in other words, the addition of pleasant aroma and volatile compounds able to “mask” the presence of probiotics. Luckow et al. (2006) reported that the addition of tropical fruit juices such as pineapple, mango or passion fruit (10%, v/v) might positively contribute to the aroma and flavor of the final product. Finally, Ranadheera et al. (2014) confirmed that some fruit juices could naturally mask the “medicinal” taste of probiotics. However, the addition of probiotic cultures to fruit juices presents numerous technological challenges, due to their acidity, the presence of oxygen, and inherent differences among fruits (Saeed et al., 2013; Vasudha and Mishra, 2013).
5 Possible Remedies
To overcome initial challenges, many authors have proposed successful strategies to improve the survival of probiotics in juices. In this section authors provide details of some compelling solutions.
5.1 Supplementation of Growth Promoters and Protectants
An easy way to improve probiotic stability in fruit juice could be the fortification of juice with some growth promoters and protectants (oligosaccharides, cellulose and dietary fiber) or with some ingredients able to exert a protective effect. Saarela et al. (2006) reported that apple juice fortified with glucans, for example oat flour (with 20% of β-glucan) could protect Lactobacillus rhamnosus during refrigerated storage. Oligofructoses could increase the viability of probiotic cultures during processing and storage of the products because they are substrates available for the metabolism of these microorganisms (Donkor et al., 2007) and, could therefore increase the stability of probiotics in fruit juices during storage. Furthermore, oligofructoses have a sweet taste similar to sucrose and may be used as sugar substitutes (Renuka et al., 2009; Yousaf et al., 2010).
Pimentel et al. (2015a) have produced probiotic apple juice with oligofructose fermented with Lactobacillus paracasei. After fermentation they have evaluated the physicochemical characteristics, probiotic viability and acceptability after refrigerated storage (4°C for 28 days) in plastic or glass packages. Results suggested that the oligofructose addition did not change any physicochemical characteristics and storage stability of the products. Rakin et al. (2007) fermented beetroot and carrot juices with yeast autolysate before lactic acid fermentation with Lactobacillus acidophilus; enhanced growth of Lactobacillus acidophilus, reduced fermentation time, enriched the juices with amino-acids, vitamins, minerals and antioxidants and a positive effect on the survival of probiotics was observed with the supplementation of autolysate.
Few studies have evaluated the effect of supplementation of nonfermented juices with probiotics (Champagne and Gardner, 2008; Ding and Shah, 2008; Saarela et al., 2011; Sheehan et al., 2007; Sohail et al., 2012) or prebiotics (Renuka et al., 2009; Yousaf et al., 2010; Martinez-Villaluenga et al., 2006). It has also been shown that supplementation of a model fruit juice with green tea extract stabilizes probiotics for 6 weeks of refrigerated storage (Shah et al., 2010). Furthermore, the addition of oat or barley fibers to fruit juice improved the survival of Bifidobacterium breve added as a frozen concentrate to the juice (Saarela et al., 2011).
5.2 Adaptation
Gobetti et al. (2010) reported that the exposure of probiotics to a sublethal stress conditions could induce resistance. Perricone et al. (2014) have evaluated the viability of Lactobacillus reuteri DSM 20016 in pineapple, orange, green apple, and red fruit juices and found that the probiotic experienced a strong viability loss in red-fruit juice, probably due to a combined effect of low pH and phenols. Prolongation of the viability was achieved by two different strategies: (1) strain cultivation in a lab medium containing different amounts of red fruit juices (up to 50%); and (2) supplementation of the medium with vanillic acid (phenol stress) or acidified to pH 5.0 (acid stress). These approaches resulted in Lactobacillus reuteri by 5 (phenol stress) or 11 days (pH stress). Saarela et al. (2011) improved the survival of Bifidobacterium breve by UV mutation and with cultivation of probiotic organism at a sub-lethal pH level.
5.3 Induction of Resistance
5.3.1 Mutagenesis
UV light or chemicals have been commonly used to obtain strains with altered characteristics or to study different microbial processes. This technique has been used successfully in probiotics research for increasing the stability of Bifidobacterium breve and Bifidobacterium animalis in low pH products (Saarela et al., 2011). This approach has also been used to improve the stability of the product in terms of sensorial attributes, for instance, metabolic activity of Bifidobacteria during manufacture or storage of food is often not desirable, since the production of large amounts of acetic acid may result in undesirable flavors. Novel Bifidobacteria strains producing low amounts of acetic acid have been obtained by UV mutagenesis; these strains would make possible the elaboration of stable and organoleptically acceptable products (Sánchez and Margolles, 2012).
5.3.2 Selective Pressure
Selective pressure (stress factor) has also been employed to obtain resistant probiotic strains. Usually strains obtained using this technique present stable phenotypes and cross-resistance to other stresses (acid and temperature). Both Lactobacilli and Bifidobacteria were improved with increased heat, oxygen (Li et al., 2010), or acid (Collado and Sanz, 2006) tolerance (Berger et al., 2010) by selective pressure technique. Though the use of these stress-resistant strains can be useful for improving stability in industrial processes, care should be taken as the stress adaptation may alter other properties of the strain (Gueimonde et al., 2007). It was also reported that the use of stress-resistant strains in probiotification do not promote significant changes in the behavior of starter cultures and the sensory properties of fermented milks (Sánchez et al., 2010). Hence these adapted strains could be a potential option in developing stable probiotic products.
5.3.3 Genetic Modification of the Strains
Genetic modification is an alternative tool for improving stability and survivability of probiotic microorganisms. However, this is not viable in all the countries, for example in Europe, GMOs are not well accepted by consumers. There are two different basic approaches that can be pursued:
- (1) Homologous expression—modify the expression/production of genes already present on the microorganism.
- (2) Heterologous expression—introduce genes from other microbial species.
Examples of both do exist, for first one—over expression of a chaperone in Lactobacillus paracasei was found to increase the strain stability (Desmond et al., 2004), and heterologous expression of the betaine uptake system (BetL) from Listeria into Lactobacillus salivarius was found to increase tolerance to acid and high osmolar conditions (Sheehan et al., 2006).
5.3.4 Encapsulation/Microencapsulation
A number of techniques have been developed and evaluated for the reduction of fatal effects of the gastrointestinal system on probiotic microorganisms. Among these, encapsulation technique is one of the most promising. The technology of encapsulation of probiotic living cells adopted from the immobilization technology is used for both enzymes and whole cell culture in the biotechnology industry. It is a process by which bioactive materials are coated with other protective materials, or their mixtures, and sealed contents can release at controlled rates under the influences of specific conditions. Microencapsulation protects the bioactive component from environmental stresses such as oxygen, high acidity, and gastric conditions and can be used for passing through the stomach with little damage (Huq et al., 2013). Protection of the microencapsulated bioactive component when passing through the stomach, could be increased using water insoluble wall materials. In recent years, many studies have been carried out on preservation of probiotic microorganisms by microencapsulation during food processing and storage (Lapsiri et al., 2012). Proteins, polysaccharides, sugars and their combinations, or some liquid food matrices can be used to encapsulate probiotics (Chavez and Ledeboer, 2007; Pispan et al., 2013).
Microencapsulation of probiotic organisms is of interest to the probiotic food industry as the best method to maintain the potency of probiotic microorganisms being delivered to the gastrointestine (Siuta-Cruce and Goulet, 2001). When considering encapsulation, we need to maintain two things: their size (typically between 1 and 5 μm diameter), which immediately excludes nanotechnologies; and the fact that they must be kept alive. Main reasons for using this method for protection of probiotics are as follows:
- ● Improving viability and stability of probiotic cultures during production, storage and passage through the gastrointestinal tract (Kailasapathy, 2002; Krasaekoopt et al., 2003; Sultana et al., 2000)
- ● Providing a controlled and efficient release of probiotic bacteria in GIT (Crittenden et al., 2006; Kailasapathy, 2002)
- ● Easier handling of the cultures (Picot and Lacroix, 2003)
- ● Limited effects on sensory properties of the product containing microcapsules (Picot and Lacroix, 2003)
Encapsulation technology is usually followed a three stages process. The first step consists of incorporating the bioactive component in a matrix which can be liquid or solid. In the case of the core being liquid, incorporation will be a dissolution or a dispersion in the matrix, whereas if the core is solid, the incorporation will be an agglomeration or an adsorption. For the second step, the liquid matrix is dispersed while a solution is pulverized on the solid matrix. The last step consists of stabilization by a chemical (polymerization), a physicochemical (gelification) or a physical (evaporation, solidification, coalescence) process (Picot and Lacroix, 2003).
There are several microencapsulation methods for probiotics including spray drying, freeze drying, fluidized bed drying, extrusion, emulsion, coacervation, and phase separation (Kailasapathy, 2002). However, two widely used encapsulation techniques are extrusion and emulsion (Krasaekoopt et al., 2003). A variety of materials have been used for the microencapsulation of probiotics including alginate, starch, alginate-starch, cellulose acetate phthalate, κ-carrageenan, gelatin, xanthan-gellan, chitosan and whey protein (Doleyres and Lacroix, 2005; Krasaekoopt et al., 2003). It is also difficult to achieve viable probiotics at ambient temperatures without effecting the sensory attributes. One means to achieve stability is through the separation of the bacteria from the fruit juice after the fermentation and then mix them at the point of consumption. In the case of the Probiotic Straw by BioGaia, dried probiotic cells are immobilized in the inner wall of the straw. This seems to be a good vehicle for delivering probiotics.
6 Future Perspectives
The utilization of nondairy foods, fruit juices, as probiotics carriers presents potential advantages and valuable alternatives for the food industry. Because several probiotic bacteria observed impressive viability in nondairy foods, there is great potential in using fruits, as substrate or as active ingredients (prebiotic), during manufacture of probiotic foods. In order to improve the survival rates of probiotic microorganisms during food production, the process of microencapsulation shows promise. There are some challenges to be resolved for the safe launch of fruit juice probiotics, in other words the survivability/stability and the effects on the sensory traits; however promising possible solutions exist. Some probiotic juices are already available to consumers and many other products will be launched on the market in the near future. Ingredients used in encapsulation materials are recognized as being safe and may be used in food applications. Therefore, there is of widespread interest in the improvement of the physical and mechanical stability of the polymers use in probiotics encapsulation, to ensure high population of probiotics not only in food during storage but also after gastrointestinal digestion. Existing probiotics generally belong to the genus Lactobacillus. However, few strains are commercially obtainable for probiotic function. Gene technology and relative genomics will play a role in upcoming research and the development of new strains, with gene sequencing allowing for an increase in appropriate mechanisms and the functionality of probiotics. More research is warranted on the effect of storage on the functional properties of probiotics. It remains to be determined to what extent the age of the stored cultures influences their health benefits. It is indisputable that fruit and vegetable matrices will be an important research and development area for future probiotic/functional food markets. Comprehensive analysis of the effects of these functional foods on human health is highly advantageous.