Prebiotics and Their Production From Unconventional Raw Materials (Mushrooms)
Hrudayanath Thatoi⁎; Sameer K. Singdevsachan⁎; Jayanta K. Patra† * North Orissa University, Baripada, India
† Dongguk University, Goyang-si, Republic of Korea
Abstract
Mushrooms are the fruiting body of heterotrophic macrofungi which have long been used for both food and medicine. Mushrooms have been studied extensively for improving and maintaining human health and show promise as a source of prebiotics. Mushrooms are rich in prebiotic compounds as they contain carbohydrates like chitin, hemicelluloses, beta and alpha glucans, mannans, xylans, lingocellulose, and galactans. Polysaccharides such as ß-(1 → 3)-d-glucans are well-known prebiotics and the most potent mushroom derived substance. Such polysaccharides and their complexes with protein/peptides are known to be modifiers of biological responses. They contribute significantly to the body’s defense systems by exhibiting immunomodulatory and antitumor activities through the action of immune effecter cells such as dendritic cells (DCs), hematopoietic stem cells, lymphocytes, macrophages, natural killer (NK) cells and T cells. Additionally, other medicinal properties of mushrooms include antioxidant, anticancer, antiinflammatory, cardiovascular, antimicrobial, and antidiabetic characteristics. This paper highlights the information related to prebiotics and nutraceutical values of bioactive substances from mushrooms, an inexpensive and abundant source, for developing new and potential prebiotic(s).
Keywords
Mushrooms; Bioactive compounds; Polysaccharides; Immunomodulating activity; Antitumor activity
1 Introduction
Mushrooms are defined as heterotrophic macrofungi (Ascomycota and Basidiomycota) with characteristic fruiting bodies that are either epigeous (of fruiting bodies above the ground) or hypogeous (of underground fruiting bodies) (Chang and Miles, 1992). Mushrooms have been used as food and medicine since ancient times (Chang and Miles, 2004). Currently both cultivated and wild edible mushrooms are used directly or indirectly as food or medicine (Chang and Miles, 2004). Edible mushrooms are widely consumed in many countries as food for their appealing taste, aroma and nutritional values due to high protein, fiber, vitamins, mineral contents and low/no calories and cholesterol (Thatoi and Singdevsachan, 2014). Approximately 14,000 mushroom species have been identified worldwide, of which 2000 are safe for human consumption and 650, in addition to being edible, possess medicinal properties (Chang and Miles, 2004). Beside their high nutritional value, mushrooms are rich in many bioactive metabolites of high medicinal values such as phenolics and polyphenolic compounds, lectins, polysaccharides, terpenoids, sterols, and various volatile compounds (Ferreira et al., 2010; Singdevsachan et al., 2016). Most of the medicinal mushroom research is in preliminary stages, being based on different tests with crude extracts of the whole mushroom fruiting bodies or mycelia, or with partially purified bioactive compounds.
Today’s foods are not only projected to mollify hunger and provide essential nutrients, but also prevent diseases and improve the health of consumers. Such foods are known as functional foods (Siro et al., 2008). According to the Institute of Medicine’s Food and Nutrition Board, “Functional Foods” are foods or dietary components that provide a health benefit beyond basic nutrition. Prebiotics such as oligosaccharides and polysaccharide (inulin) have emerged to generate great interest as functional food ingredients because they are able to manipulate the composition of colonic microbiota in the human gut by inhibition of exogenous pathogens (Rycroft et al., 2001) and thus improve the health of the host (Roberfroid, 2000, 2002). Recent developments in prebiotics have stressed the need to search for new potential sources of prebiotics. In this context, mushrooms could serve as a potential source for prebiotics as they contain carbohydrates like chitin, hemicellulose, ß and a-glucans, mannans, xylans and galactans. Chitin, a water insoluble polysaccharide, is indigestible to humans and plays a role as a dietary fiber (Kalac, 2009). Several mushroom polysaccharides are credited with prebiotic effects. Crude polysaccharides from Ganoderma lucidum, Lentinus edodes, Pleurotus eryngii, and Flammulina velutipes were found to have significant prebiotic activities (Yamin et al., 2012; Chou et al., 2013). However the bioactivities of water insoluble polysaccharides was less as compared to water soluble polysaccharides (Tao et al., 2006). Most of the polysaccharides from mushrooms are present as linear and branched glucans with different types of glycosidic linkages such as (1 → 3), (1 → 6)-ß-glucans and (1 → 3)-a-glucans. However, some of them are true heteroglycans containing arabinose, mannose, fructose, galactose, xylose, glucose, and glucuronic acids as main side chain components or in different combinations. Even though mushrooms polysaccharides are of different chemical compositions, most of them belong to the group of ß-glucans (Wasser, 2002). Digestive enzymes secreted by the pancreas of brush border of vertebrates, and of mammals in particular, are unable to hydrolyze ß-glucosidic bonds. This makes them resistant to acid hydrolysis in the stomach and they remain nondigestible by human digestive enzymes (Van Loo, 2006). The nondigestible property of mushroom carbohydrate enables it to be considered as a potential source of prebiotic because it meets a part of the definition of prebiotics. However, extensive studies need to be carried out before such a claim could be made due to the fact that all dietary carbohydrates are not necessarily prebiotics (Gibson et al., 2004).
Mushrooms are not only important as prebiotics, but also for other biological properties such as antibacterial, anticancer, antioxidant, antiviral, and antihypoglycemic immunomodulatory activities, as well as being an active medicine in the prevention of cardiovascular diseases (Lemieszek and Rzeski, 2012; Thatoi and Singdevsachan, 2014). Polysaccharides of Lentinus edodes and Pleurotus florida also exhibited antitumor and immunomodulating properties (Yu et al., 2010; Maity et al., 2011). Further, Lentinus edodes is the most studied species with potential antimicrobial activities (Alves et al., 2012a). Additionally, a number of mushroom species, namely Coprinus plicatilis, Lentinus tigrinus, Ganoderma applaunatum, Helvella crispa, Agaricus bisporus, Hypsizygus ulmarius, Calocybe indica, and Flammulina velutipes, have been reported for their antioxidant activity (Babu and Rao, 2013; Thatoi and Singdevsachan, 2014). In a current study, information related to bioactive substances and nutraceutical values of mushrooms for their food and pharmaceutical applications have been discussed.
2 Nutritional Values of Mushrooms
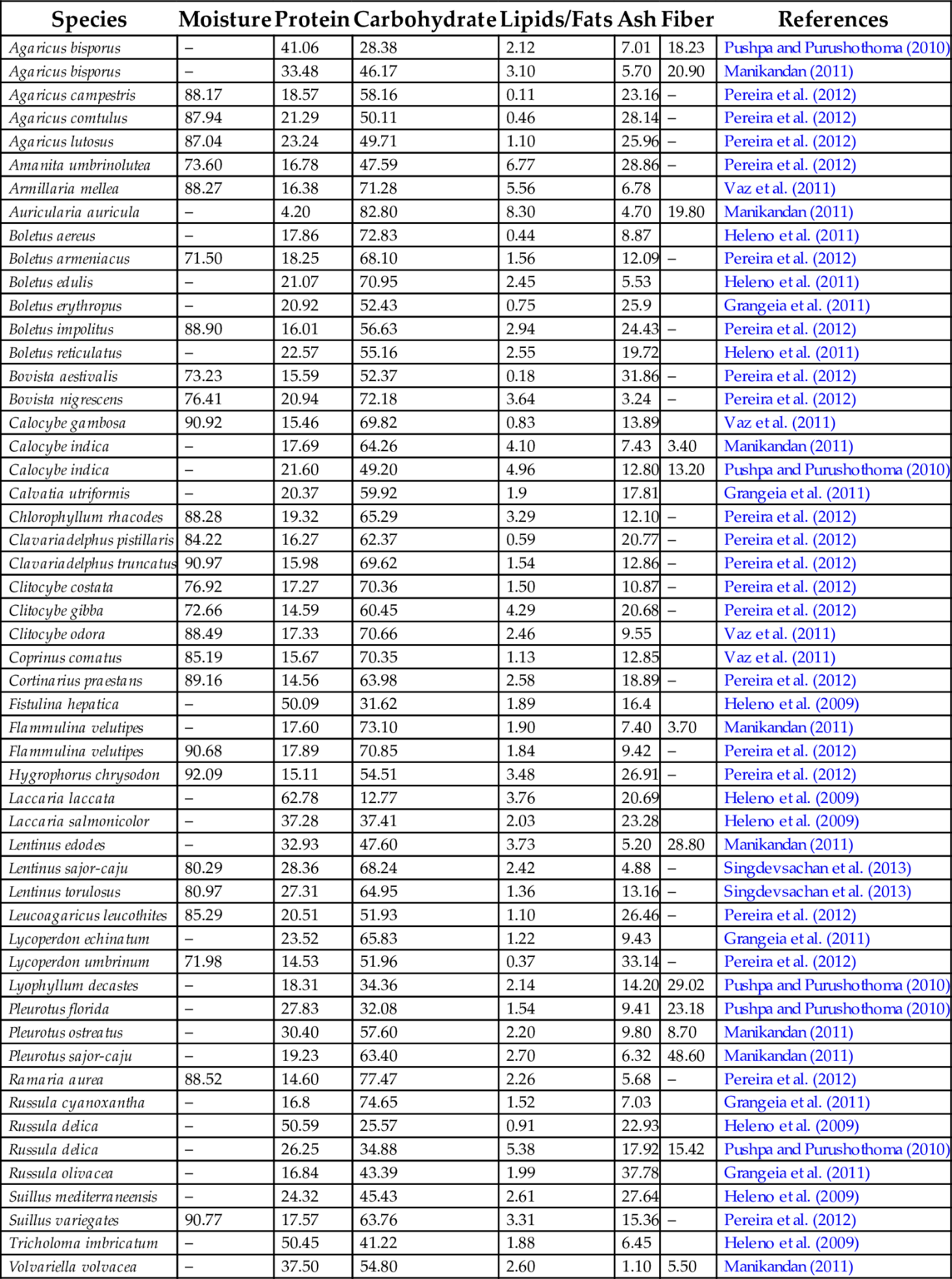
Mushrooms serve as a food with rich nutritional value. While some mushrooms have medicinal value and are used as dietary supplements, some have both properties (Wani et al., 2010; Kalac, 2013). They are quite rich in protein and dietary carbohydrates, with essential amino acids and fiber, poor fat but excellent important fatty acids content (Table 1). In addition, edible mushrooms provide a rich source of vitamins (Mattila et al., 2001; Heleno et al., 2010). Thus, they could be an excellent source for many different nutraceuticals and could be used directly in human diet for promoting health (Vaz et al., 2010; Pereira et al., 2012). During recent years, consumers have been about health and nutritional foods. This has ignited the commercialization of natural foods consumed as dietary supplements. Mushrooms can be considered a functional food as it is a major source of dietary carbohydrates and proteins (Kalac, 2013; Valverde et al., 2015). Functional foods should not be claimed as cures for disease however, there is an increasing number of scientific studies that strongly support functional foods, such as mushrooms, as having a role in disease prevention and in some cases, of bringing about suppression or remission of disease in an individual (Chen et al., 2012a,b; Valverde et al., 2015).
Table 1
| Species | Moisture | Protein | Carbohydrate | Lipids/Fats | Ash | Fiber | References |
|---|---|---|---|---|---|---|---|
| Agaricus bisporus | – | 41.06 | 28.38 | 2.12 | 7.01 | 18.23 | Pushpa and Purushothoma (2010) |
| Agaricus bisporus | – | 33.48 | 46.17 | 3.10 | 5.70 | 20.90 | Manikandan (2011) |
| Agaricus campestris | 88.17 | 18.57 | 58.16 | 0.11 | 23.16 | – | Pereira et al. (2012) |
| Agaricus comtulus | 87.94 | 21.29 | 50.11 | 0.46 | 28.14 | – | Pereira et al. (2012) |
| Agaricus lutosus | 87.04 | 23.24 | 49.71 | 1.10 | 25.96 | – | Pereira et al. (2012) |
| Amanita umbrinolutea | 73.60 | 16.78 | 47.59 | 6.77 | 28.86 | – | Pereira et al. (2012) |
| Armillaria mellea | 88.27 | 16.38 | 71.28 | 5.56 | 6.78 | Vaz et al. (2011) | |
| Auricularia auricula | – | 4.20 | 82.80 | 8.30 | 4.70 | 19.80 | Manikandan (2011) |
| Boletus aereus | – | 17.86 | 72.83 | 0.44 | 8.87 | Heleno et al. (2011) | |
| Boletus armeniacus | 71.50 | 18.25 | 68.10 | 1.56 | 12.09 | – | Pereira et al. (2012) |
| Boletus edulis | – | 21.07 | 70.95 | 2.45 | 5.53 | Heleno et al. (2011) | |
| Boletus erythropus | – | 20.92 | 52.43 | 0.75 | 25.9 | Grangeia et al. (2011) | |
| Boletus impolitus | 88.90 | 16.01 | 56.63 | 2.94 | 24.43 | – | Pereira et al. (2012) |
| Boletus reticulatus | – | 22.57 | 55.16 | 2.55 | 19.72 | Heleno et al. (2011) | |
| Bovista aestivalis | 73.23 | 15.59 | 52.37 | 0.18 | 31.86 | – | Pereira et al. (2012) |
| Bovista nigrescens | 76.41 | 20.94 | 72.18 | 3.64 | 3.24 | – | Pereira et al. (2012) |
| Calocybe gambosa | 90.92 | 15.46 | 69.82 | 0.83 | 13.89 | Vaz et al. (2011) | |
| Calocybe indica | – | 17.69 | 64.26 | 4.10 | 7.43 | 3.40 | Manikandan (2011) |
| Calocybe indica | – | 21.60 | 49.20 | 4.96 | 12.80 | 13.20 | Pushpa and Purushothoma (2010) |
| Calvatia utriformis | – | 20.37 | 59.92 | 1.9 | 17.81 | Grangeia et al. (2011) | |
| Chlorophyllum rhacodes | 88.28 | 19.32 | 65.29 | 3.29 | 12.10 | – | Pereira et al. (2012) |
| Clavariadelphus pistillaris | 84.22 | 16.27 | 62.37 | 0.59 | 20.77 | – | Pereira et al. (2012) |
| Clavariadelphus truncatus | 90.97 | 15.98 | 69.62 | 1.54 | 12.86 | – | Pereira et al. (2012) |
| Clitocybe costata | 76.92 | 17.27 | 70.36 | 1.50 | 10.87 | – | Pereira et al. (2012) |
| Clitocybe gibba | 72.66 | 14.59 | 60.45 | 4.29 | 20.68 | – | Pereira et al. (2012) |
| Clitocybe odora | 88.49 | 17.33 | 70.66 | 2.46 | 9.55 | Vaz et al. (2011) | |
| Coprinus comatus | 85.19 | 15.67 | 70.35 | 1.13 | 12.85 | Vaz et al. (2011) | |
| Cortinarius praestans | 89.16 | 14.56 | 63.98 | 2.58 | 18.89 | – | Pereira et al. (2012) |
| Fistulina hepatica | – | 50.09 | 31.62 | 1.89 | 16.4 | Heleno et al. (2009) | |
| Flammulina velutipes | – | 17.60 | 73.10 | 1.90 | 7.40 | 3.70 | Manikandan (2011) |
| Flammulina velutipes | 90.68 | 17.89 | 70.85 | 1.84 | 9.42 | – | Pereira et al. (2012) |
| Hygrophorus chrysodon | 92.09 | 15.11 | 54.51 | 3.48 | 26.91 | – | Pereira et al. (2012) |
| Laccaria laccata | – | 62.78 | 12.77 | 3.76 | 20.69 | Heleno et al. (2009) | |
| Laccaria salmonicolor | – | 37.28 | 37.41 | 2.03 | 23.28 | Heleno et al. (2009) | |
| Lentinus edodes | – | 32.93 | 47.60 | 3.73 | 5.20 | 28.80 | Manikandan (2011) |
| Lentinus sajor-caju | 80.29 | 28.36 | 68.24 | 2.42 | 4.88 | – | Singdevsachan et al. (2013) |
| Lentinus torulosus | 80.97 | 27.31 | 64.95 | 1.36 | 13.16 | – | Singdevsachan et al. (2013) |
| Leucoagaricus leucothites | 85.29 | 20.51 | 51.93 | 1.10 | 26.46 | – | Pereira et al. (2012) |
| Lycoperdon echinatum | – | 23.52 | 65.83 | 1.22 | 9.43 | Grangeia et al. (2011) | |
| Lycoperdon umbrinum | 71.98 | 14.53 | 51.96 | 0.37 | 33.14 | – | Pereira et al. (2012) |
| Lyophyllum decastes | – | 18.31 | 34.36 | 2.14 | 14.20 | 29.02 | Pushpa and Purushothoma (2010) |
| Pleurotus florida | – | 27.83 | 32.08 | 1.54 | 9.41 | 23.18 | Pushpa and Purushothoma (2010) |
| Pleurotus ostreatus | – | 30.40 | 57.60 | 2.20 | 9.80 | 8.70 | Manikandan (2011) |
| Pleurotus sajor-caju | – | 19.23 | 63.40 | 2.70 | 6.32 | 48.60 | Manikandan (2011) |
| Ramaria aurea | 88.52 | 14.60 | 77.47 | 2.26 | 5.68 | – | Pereira et al. (2012) |
| Russula cyanoxantha | – | 16.8 | 74.65 | 1.52 | 7.03 | Grangeia et al. (2011) | |
| Russula delica | – | 50.59 | 25.57 | 0.91 | 22.93 | Heleno et al. (2009) | |
| Russula delica | – | 26.25 | 34.88 | 5.38 | 17.92 | 15.42 | Pushpa and Purushothoma (2010) |
| Russula olivacea | – | 16.84 | 43.39 | 1.99 | 37.78 | Grangeia et al. (2011) | |
| Suillus mediterraneensis | – | 24.32 | 45.43 | 2.61 | 27.64 | Heleno et al. (2009) | |
| Suillus variegates | 90.77 | 17.57 | 63.76 | 3.31 | 15.36 | – | Pereira et al. (2012) |
| Tricholoma imbricatum | – | 50.45 | 41.22 | 1.88 | 6.45 | Heleno et al. (2009) | |
| Volvariella volvacea | – | 37.50 | 54.80 | 2.60 | 1.10 | 5.50 | Manikandan (2011) |
Since ancient times, mushrooms have been treated as a distinctive kind of food. The Greeks believed that mushrooms provided strength for warriors in battle. Pharaohs regarded mushrooms as a delicacy and the Romans considered mushrooms as a “Food of the Gods” and served them only on special occasions. Chinese treated mushrooms as a healthy food, the “elixir of life.” Indigenous Mexicans used mushrooms as hallucinogens in religious ceremonies and in shamanism, as well as for therapeutic purposes (Chang and Miles, 2004). In recent years, fast-growing mushrooms have received a remarkable amount of interest with the realization that they possess high nutritional values with medicinal properties.
The cultivation procedures may influence the chemical configuration and the nutritive potential of various edible mushrooms. Additionally, significant variations occur both among and within species (Reis et al., 2012; Kalac, 2013). Recently several authors reviewed the nutritional composition of mushrooms and reported that they contain a high moisture percentage that ranges between 80 and 95 g/100 g, approximately (Kalac, 2009, 2013; Thatoi and Singdevsachan, 2014; Valverde et al., 2015). Furthermore, edible mushrooms are a good source of protein, 200–250 g/kg of dry matter; in which leucine, valine, glutamine, glutamic, and aspartic acids are the most abundant amino acids. Mushrooms are low-calorie foods since they provide low amounts of fat. Edible mushrooms contain high amounts of ash, mainly calcium, copper, iron, magnesium, potassium, phosphorus, and zinc. Carbohydrates are found in high proportions in edible mushrooms, 200–800 g/kg of dry matter, including chitin, glycogen, trehalose, and mannitol; as well as containing fiber, ß-glucans, hemicelluloses; and pectic substances (Kalac, 2009, 2013; Thatoi and Singdevsachan, 2014; Valverde et al., 2015). Additionally, glucose, mannitol, and trehalose are abundant sugars in cultivated edible mushrooms, while fructose and sucrose levels are low. Mushrooms are also a good source of vitamins with high levels of riboflavin, niacin, folates, and traces of vitamin C, B1, B12, D, and E (Kalac, 2009, 2013; Thatoi and Singdevsachan, 2014; Valverde et al., 2015). Mushrooms are the only nonanimal food source that contains vitamin D; consequently they are the only natural source of vitamin D available to vegetarians. Specifically wild mushrooms are an excellent vitamin D2 source because, unlike their cultivated counterparts which are usually grown in darkness, they receive the UV-B light needed to produce vitamin D2 (Kalac, 2009, 2013; Thatoi and Singdevsachan, 2014; Valverde et al., 2015).
3 Bioactive Components of Mushrooms
Mushrooms in the 20th century are well known to people all over the world as an important biosource of novel secondary metabolites. Mushrooms contain biologically active compounds (Wasser, 2002). The secondary metabolites of mushrooms are chemically diverse, with wide variety of biological activities. While applications of these compounds are already explored in traditional medicines, they are in new targets of modern medicine. In particular, mushrooms which could play important roles in modern medicine represent an unlimited source of compounds with different medicinal properties, including low-molecular-weight (LMW, e.g., amines, cerebrosides, catechols, isoflavones, organic germanium, quinones, triacylglycerols, sesquiterpenes, steroids, and selenium) and high-molecular-weight compounds (HMW, e.g., glycoproteins, glycopeptides, homo and heteropolysaccharides, proteins, RNA-protein complexes) (Figs. 1 and 2) (Ferreira et al., 2010).
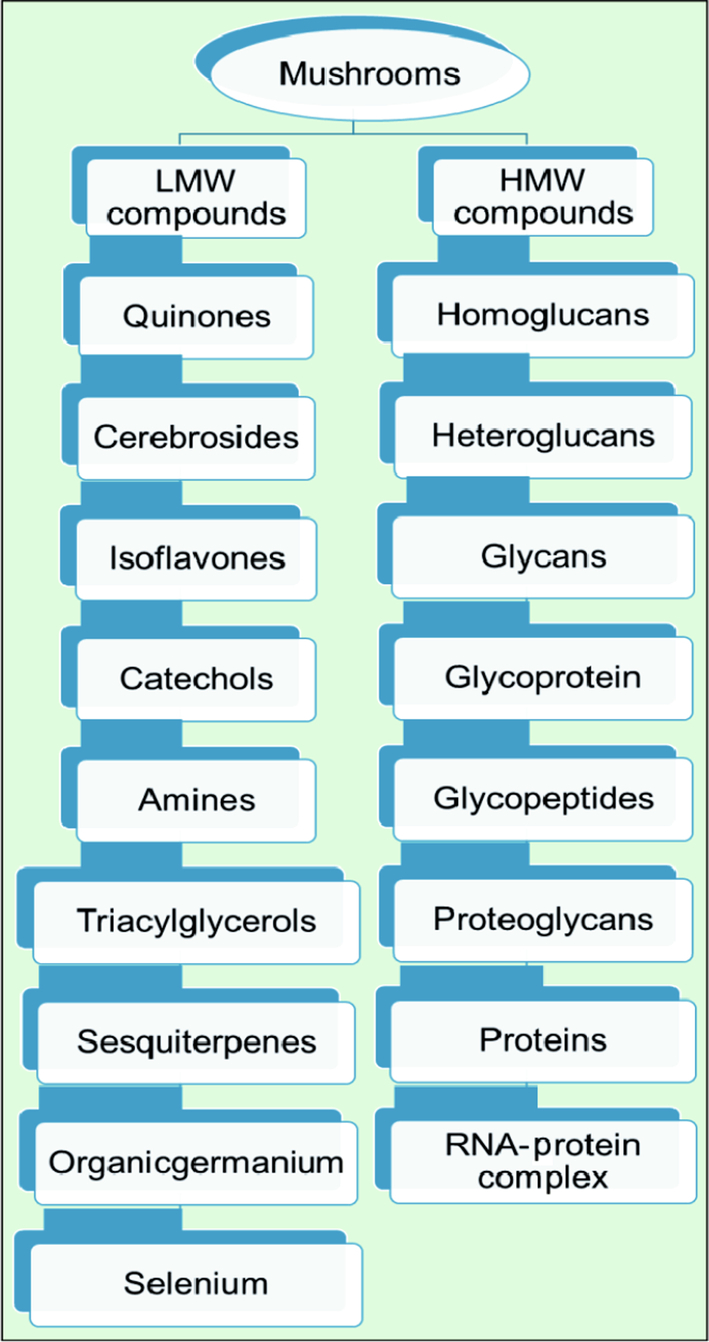

3.1 Low Molecular Weight Compounds of Mushrooms
Mushrooms contain a diversity of complex organic compounds such as phenolic compounds, polyketides, triterpenoids and steroids derived from secondary metabolism (Wasser, 2002; Zaidman et al., 2005). Such compounds have been used in the treatment of many health problems, including free radical related diseases, microbial infections and cancer, to name a few. (Paterson, 2006; Vaz et al., 2010). The secondary metabolites with LMW present in mushrooms include quinones, cerebrosides, isoflavones, catechols, amines, triacylglycerols, sesquiterpenes, steroids, organic germanium, and selenium (Ferreira et al., 2010). The naturally occurring 6-(3,4-dihydroxystyryl)-4-hydroxy-2-pyrone (hispidin) was isolated from the culture broth of Phellinus linteus, Gymnopilus marginatus, G. patriae, G. parvisporus and Inonotus hispidus. Hispidin is a potent inhibitor of PKCß, a protein kinase which plays an important role in angiogenesis (Zaidman et al., 2005). Hispidin synthesized by Gonindard et al. (1997) possesses cytotoxic activity against human keratinocytes (SLC-1 tumor cell line) and human pancreatic duct cells (Capan-1 tumor cell line). Sterols isolated from Cordyceps sinensis were found to have the ability to inhibit the proliferation of K562, Jurkat, WM-1341, HL-60, and RPMI-8226 tumor cell lines (Bok et al., 1999). Ganomycin A and B isolated from Ganoderma pfeifferi, showed antibacterial activity against B. subtilis, M. flavus and S. aureus (Mothana et al., 2000). Agrocybe aegerita, an edible mushroom, is an important source of bioactive secondary metabolites such as indole derivatives with free radical scavenging activity, and cylindan with anticancer activity.
3.2 High Molecular Weight Compounds of Mushrooms
High-molecular weight compounds, including polysaccharides and polysaccharide conjugates, have been isolated from medicinal mushrooms and developed as cytostatic polysaccharide drugs in Japan and approved in other countries for the clinical treatment of cancer patients. These are “Lentinan” from the fruiting bodies of Lentinus edodes, “Schizophyllan” (Sonifilan, SPG) from the culture fluid of Schizophyllum commune, and “Krestin” (PSK), from the cultured mycelium of Trametes versicolor. Lentinan and schizophyllan are pure ß-glucans, whereas Krestin (PSK) is a protein bound polysaccharide. These three compounds possess immunomodulating properties (Mizuno, 1993; Larone, 2002, 2002; Zaidman et al., 2005; Zhang et al., 2007).
3.3 Mushrooms as a Possible Source of Prebiotics
A prebiotic is a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth of one or a limited number of bacteria in the colon (Gibson and Roberfroid, 1995). The concept of prebiotics is laid out by certain criteria such as resistance to gastric acidity, fermentation by intestinal microflora and selective stimulation of the growth, hydrolysis by mammalian enzymes and gastrointestinal absorption, and/or activity of intestinal bacteria associated with host well-being and health (Gibson et al., 2004) The critical criteria for a food ingredient as prebiotic is reviewed in Fig. 3. The first attribute of prebiotics, which is nondigestible or resistant to the upper gut tract, indicates that the prebiotics can withstand digestive processes before they reach the colon, thus effectively stimulating beneficial bacteria like bifidobacteria and lactobacilli (Gibson and Collins, 1999; Wang, 2009). Prebiotics are found in several vegetables and fruits, and are considered as functional food components (Jovanovic-Malinovska et al., 2014). The consumption of prebiotics may have many positive health benefits including but not limited to, improved colonic integrity, immune function, reduced allergic response, improved digestion and elimination.
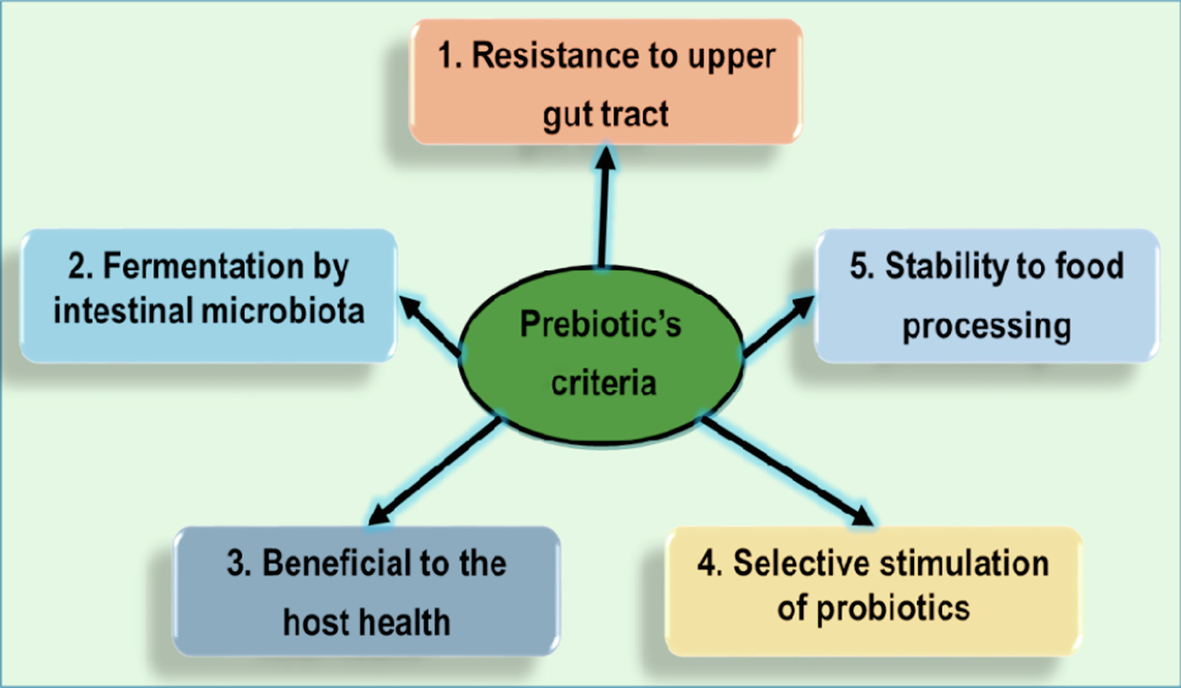
The criteria for the classification of a food ingredient as a prebiotic include selective fermentation by potentially beneficial bacteria in the colon (Gibson et al., 2004; Wang, 2009). The effects of this fermentation may lead to an increase in the appearance or change in the arrangement of short-chain fatty acids, increased fecal weight, reduction in luminal colon pH, decrease in nitrogenous end products and reductive enzymes, increased expression of the binding proteins or active carriers associated with mineral absorption, and immune system modulation (Douglas and Sanders, 2008; Slizewska et al., 2012), which is beneficial to the host health. Selective stimulation of the growth and/or activity of intestinal bacteria potentially associated with health and well-being is considered as one of the typical criteria for prebiotic classification (Gibson et al., 2004). Further, prebiotics are markedly suitable for the growth and activities of probiotics such as bifidobacteria and lactobacilli, while suppressing the growth of clostridia and bacteroides (Wang, 2009).
Gibson (2004) found dietary carbohydrates like fibers and oligosaccharides to be important prebiotics. Stowell (2007) extensively reviewed the existing prebiotics. Inulin, fructooligosaccharides, galactooligosaccharides, lactulose and polydextrose are considered to be “established prebiotics,” whereas isomaltooligosaccharides, xylooligosaccahrides are grouped under “emerging prebiotics.” Other than the aforementioned prebiotic compounds, mannitol, maltodextrin, raffinose, and sorbitol are also prebiotics with proven health properties (Mandal et al., 2009; Vamanu and Vamanu, 2010).
Though soybeans (Crittendan and Playne, 1996), chicory root (Roberfroid, 2000), herbs (Guo et al., 2004), oats (Gokavi et al., 2005), and cereal (Michida et al., 2006) are major source of prebiotics, edible mushrooms are gaining much attention as an alternative source (Guo et al., 2004; Aida et al., 2009). The major components rendering prebiotic function in mushrooms are nondigestible polysaccharides such as glucan, chitin, and hetropolysaccharides. Several mushroom polysaccharides like pleuran, lentinan, schizophyllan, ß and a-glucans, mannans, xylans, galactans, chitin, inulin, and hemicelluloses can be credited to promising prebiotic effects. Chitin are water insoluble polysaccharides that are present in some taxonomical groups of Zygo-, Asco-, Basidio-, and Deuteromycetes (Vetter, 2007). Fructans are members of a larger group of inulins and oligosaccharides. These polysaccharides consist of fructose moieties, which are lined by glycosidic bonds (Kelly, 2008). These polysaccharides help to increase the population of bifidobacteria and lactobacilli in the colon, thereby adding health benefits to the host (Roberfroid, 2007; Olmstead, 2008). d-Glucans are polysaccharides linked by a or ß-glycosidic linkage (Andriya et al., 2009). These polysaccharides are known to activate the population of Lactobacillus rhamnosus, Bifidobacterium bifidium, and Enterococcus (Ruthes et al., 2013; Giavasis, 2014). Grifloan are polysaccharides having a ß-linked glucose molecule with triple helix structure. It has beneficial effects on Bifidobacterium and Lactobacillus, and adverse effects on Salmonella. It helps in increasing glucose consumption and activity of lysosomal enzyme, ß-d-glucourinodase, in macrophages (Andrea et al., 1999).
Most of the mushrooms can be used as prebiotics are Agaricus bisporus, Auriculariajudae, Boletus erythropus, Calocybeindica, Flammulina velutipes, Ganoderma lucidium, Geastrum saccatum, Hericium erianaceus, Lentinusedodes, and Pleurotus ostreatus. Pleuran from oyster (Pleurotus ostreatus) mushrooms and lentinan from shiitake (Lentinus edodes) mushrooms are the most frequently used ß-glucans as prebiotics. Both of them are effective against intestine inflammation (Zeman et al., 2001) and inhibit the development of intestinal ulcers in rats (Nosalova et al., 2001). Lentinan also shows a positive effect on peristalsis in weaned piglets (Van Nevel et al., 2003). Additionally, it has been demonstrated that sclerotial ß-glucans from mushrooms (Pleurotus tuber-regium, Polyporous rhinocerus, and Wolfiporia cocos) can be utilized by human colonic bacteria in vitro (Wong et al., 2005). The colonic fermentation of sclerotial ß-glucans isolated from P. tuber-regium could also enhance the absorption of calcium and magnesium in ovariectomized rats (Wong et al., 2006). Synytsya et al. (2009) gave a positive overview, citing that mushroom glucans of Pleurotus ostreatus and P. eryngii were able to stimulate the growth of probiotics—Lactobacillus sp. (four strains: Lac A–D), Bifidobacterium sp. (three strains: Bifi A–C), and Enterococcus faecium (two strains: Ent A and B)—to a certain degree.
Further, synergetic effects of mushroom polysaccharides have been demonstrated by several researchers for the growth of probiotics. The research studies have indicated that polysaccharides from Pleurotus spp. (Synytsya et al., 2009), Lentinus edodes, Tremella fuciformis (Guo et al., 2004), and Agaricus spp. mushrooms (Giannenas et al., 2011) have prebiotic activity. The effect of three kinds of crude polysaccharides (PSI, PSII, and PSIII) from Agaricus blazei Murill (obtained by diethyl-amino ethanol cellulose column chromatography) on the growth of lactic acid bacteria have been described by Lili and Jianchun (2008). Additionally, mushrooms such as Pleurotus ostreatus and Lentinus edodes can significantly modify intestinal flora composition by promoting the breakdown and propagation of beneficial microorganisms such as Lactobacilli and Bifidobacteria, as well as by hindering the growth of pathogenic bacteria such as E. coli, Clostridium, and Salmonella (Zhou et al., 2011). The effect of crude polysaccharides extracted from Ganoderma lucidum on the growth of probiotics have been studied by Yamin et al. (2012) in batch-culture fermentation of human fecal culture. Growth promotion of Bifidobacterium sp. and Lactobacillus sp. and growth inhibition of Salmonella sp. proved its prebiotic effect. Prebiotic activity of crude polysaccharides from Lentinula edodes stipe, Pleurotus eryngii base, and Flammulina velutipes base were also studied (Chou et al., 2013). Recently, the prebiotic property of edible mushrooms (Auricularia auricula-judae, Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus abalonus, and Volvariella volvacea) was evaluated by Saman et al. (2016) and found that three selected mushrooms (Pleurotus ostreatus, Pleurotus sajor-caju, and Pleurotus abalones) stimulate the growths of bifidobacteria (Bifidobacterium bifidum TISTR 2129, B. breve TISTR 2130) and lactobacilli (B. animalis TISTR 2195 and B. longum TISTR 2194); and could suppress the growth of harmful bacteria in human gut model.
4 Mushroom as Potential Source of Pharmaceuticals
4.1 Antitumor and Immunomodulatory Properties
The active components in mushrooms responsible for anticancer activity include lentinan, krestin, lectin, hispolon, calcaelin, illudin S, psilocybin, Hericium polysaccharide A and B (HPA and HPB), ganoderic acid, schizophyllan, laccase, etc. (Patel and Goyal, 2012). Among the different components, polysaccharides from mushrooms are the best known and most potent substances with antitumor and immunomodulating properties. A novel water-soluble polysaccharide (POPS-1) was isolated from the fruiting bodies of the mushroom, Pleurotus ostreatus by hot-water extraction, ethanol precipitation, and fractionation by DEAE-cellulose ion exchange and Sepharose CL-6B gel filtration chromatography technique by Tong et al. (2009). Cytotoxicity assay showed POPS-1 presented significantly higher antitumor activity against the HeLa tumor cell line in vitro, in a dose-dependent manner. However, it exhibited significantly lower cytotoxicity to human embryo kidney 293T cells than HeLa tumor cells compared with anticancer drug 5-fluorouracil. This finding suggests that the soluble polysaccharide POPS-1 may be considered as a potential candidate for developing a novel low-toxicity antitumor agent (Tong et al., 2009).
Methanol extract of Phellinus linteus and its fractions, including methylene chloride, ethyl acetate, and n-butanol, have shown the potential for antiangiogenic effects through the inhibition of human umbilical vein endothelial cell (HUVECs) proliferation, migration and assembly into capillary-like structures as well as in vivo angiogenesis. The findings of this study indicate the potential role of the mushroom extract in stimulated angiogenesis, such as inflammation and tumor development (Lee et al., 2010). Huang et al. (2011) evaluated the anticancer effect of a mycelial culture of the mushroom P. linteus, and revealed its potential mechanism in vivo conditions. Mushroom extract from Phellinus linteus, when administered daily for 8 weeks in human hepatoma (Hep3B) cell-transplanted mice, resulted in a significant reduction in tumor size and increase in T cell numbers; IL-12, IFN-c and TNF-a secretion; NK cell activity and phagocytic ability were observed. From this, it can be concluded that P. linteus extract exhibits a potential therapeutic use for both antitumor and immunomodulatory effects. Li et al. (2011) studied the possible antitumor effect of glycosylated protein and proteoglycan purified from P. linteus on human cancer cells and mechanisms involved. Studies on cell inhibition assay showed the antiproliferative effect of proteoglycan on human hepatocellular liver carcinoma (HepG-2), human colon adenocarcinoma (HT-29), human lung cancer (NCIH-460) and human breast adenocarcinoma (MCF-7) cells. There was increase in spleen and thymus weights, the plasmatic immunoglobulin receptor pIgR and IgA levels when HT-29-bearing mice were treated with 100 mg/kg proteoglycan. On the other hand, measurement by ELISA showed a significant decrease in plasmatic prostaglandin E2 (PGE2), regenerating islet-derived protein 4 (Reg IV), epidermal growth factor receptor (EGFR), and (protein kinase B) Akt concentrations. The study suggests the immunopotentiator role of proteoglycan that protects T cells from escaping PGE2 attack, enhance the mucosal IgA response, and disrupt the Reg IV/EGFR/Akt signaling pathway (Li et al., 2011).
Akiyama et al. (2011) studied the effects of agaritine, a hydrazine-derivative from hot-water extract of the mushroom Agaricus blazei Murrill, on human leukemic monocyte lymphoma (U937) cells. It was found that Agaritine treatment induced DNA fragmentation, annexin V expression, cytochrome c release and increase in caspase-3, 8, and 9 activities. This suggests that agaritine moderately induces apoptosis in U937 cells. Additionally, Agaricus blazei has been used as an adjuvant in cancer chemotherapy; various types of antileukemic bioactive components have been extracted from it. Apart from this, lentinan, produced from the fruiting bodies of the shiitake mushroom (Lentinus edodes), is a ß-(1 → 3), ß-(1 → 6)-d-glucan which showed effective antitumor and immunopotentiating activity (Vannucci et al., 2013).
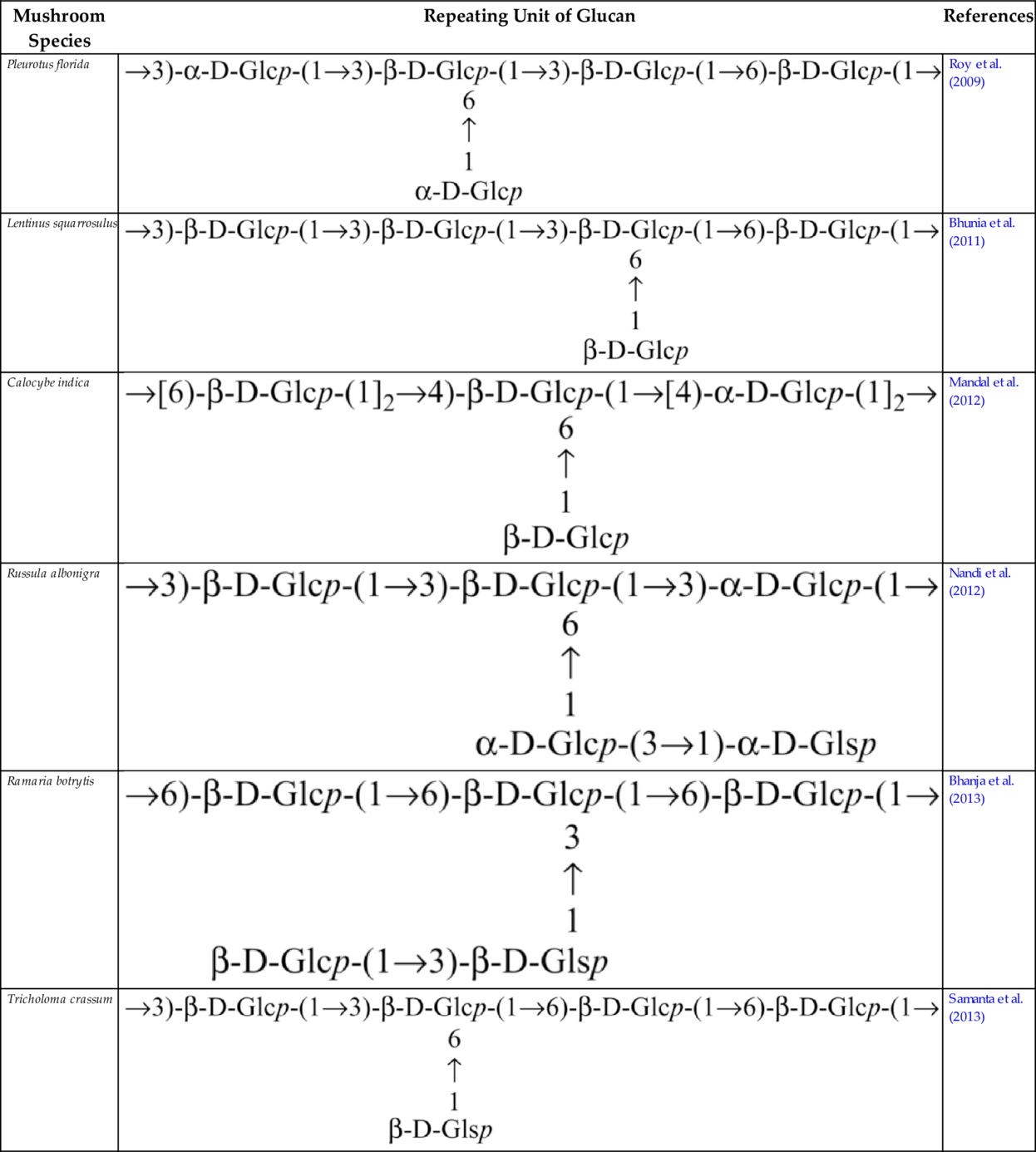
Glucan from edible mushrooms has shown immunomodulatory activity, encouraging stimulation of macrophages, splenocytes and thymocytes. An immunoenhancing water soluble glucan (Table 2) from an edible mushroom, Pleurotus florida, cultivar Assam Florida, has been found to stimulate macrophages, splenocytes and thymocytes (Roy et al., 2009). Glucan (Table 2) from the mushroom, Lentinus squarrosulus consists of (1 → 3,6)-linked, (1 → 3)-linked, (1 → 6)-linked and terminal ß-d-glucopyranosyl moieties in proportions of approximately 1:2:1:1, and showed optimum activation of macrophages as well as splenocytes and thymocytes (Bhunia et al., 2011). Other immunostimulating glucan that have been reported are (1 → 4)-, (1 → 6)-branched glucan (Table 2) from Calocybe indica (Mandal et al., 2012), PS-I (1 → 6-ß-d-glucan) and PS-II (1 → 3,6-ß-d-glucan) from Termitomyces robustus var. (Bhanja et al., 2012). Glucan were also reported from an ectomycorrhizal edible mushroom Russula albonigra (Krombh.) Fr. (Nandi et al., 2012) and from Ramaria botrytis (Table 2) (Bhanja et al., 2013). Glucan (Table 2) isolated from Tricholoma crassum (Berk.) Sacc. showed macrophage activation in vitro by NO production in a dose dependent manner and strong thymocyte and splenocyte immunostimulation in mouse cell culture medium (Samanta et al., 2013).
Table 2
| Mushroom Species | Repeating Unit of Glucan | References |
|---|---|---|
| Pleurotus florida | 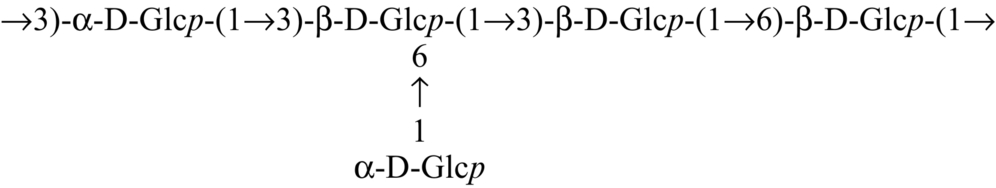 | Roy et al. (2009) |
| Lentinus squarrosulus | 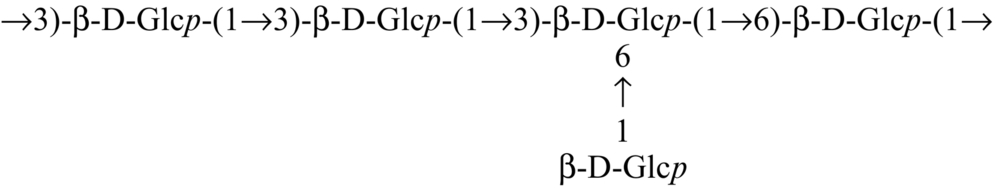 | Bhunia et al. (2011) |
| Calocybe indica | 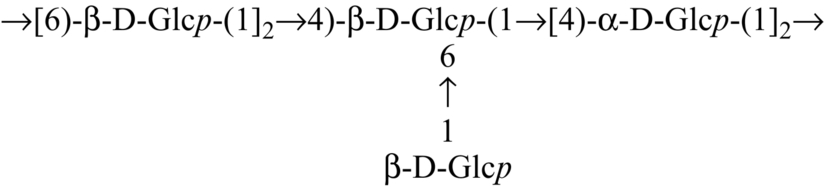 | Mandal et al. (2012) |
| Russula albonigra | 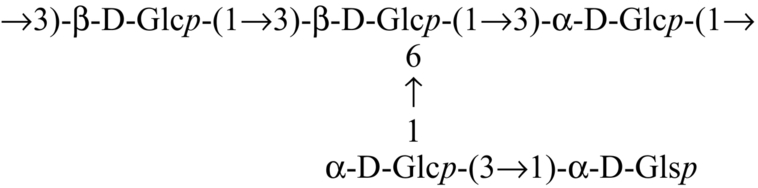 | Nandi et al. (2012) |
| Ramaria botrytis | 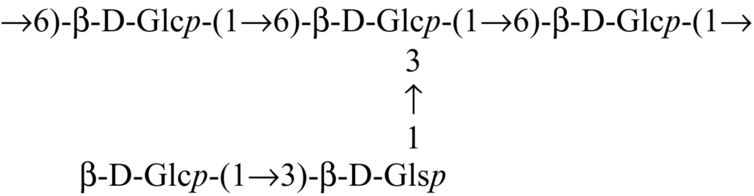 | Bhanja et al. (2013) |
| Tricholoma crassum | 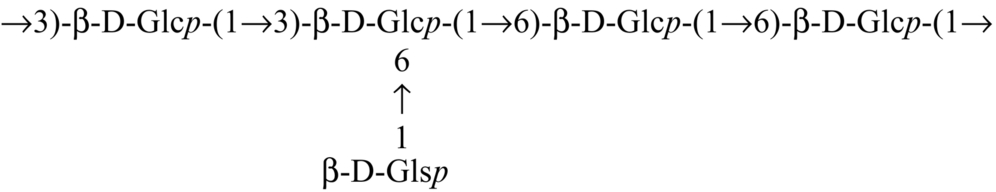 | Samanta et al. (2013) |
Glucan from hybrid mushrooms also exhibits immunomodulatory activity. A (1 → 6)-ß-d-glucan from a somatic hybrid mushroom (PfloVv5FB) of Pleurotus florida and Volvariella volvacea was reported to exhibit significant immunoenhancing properties which could stimulate the macrophages, splenocytes, and thymocytes (Das et al., 2010). Sarkar et al. (2012) reported another (1 → 6)-ß-d-glucan from a hybrid mushroom, obtained by backcross mating between hybrid mushroom PfloVv12 and Volvariella volvacea, which was found to be immunostimulating in nature. Some more examples of such glucan include a water soluble glucan (comprising terminal (1 → 3,6)-linked and (1 → 6)-linked ß-d-glucopyranosyl moieties in a molar ratio of nearly 1:1:3) isolated from an edible hybrid mushroom (Pfle1r) of Pleurotus florida and Lentinula edode (Maji et al., 2012) and a (1 → 3,6)-ß-d-glucan from a hybrid mushroom (PfloVv5FB) (obtained through protoplast fusion between Pleurotus florida and Volvariella volvacea strains) (Maity et al., 2013).
Several heteroglycans have been reported to impart potential immunomodulatory effects. A heteroglycan (Table 3) isolated from the hot water extract of the fruiting bodies of an edible mushroom, Lentinus squarrosulus (Mont.) Singer, contains d-galactose, l-fucose, and d-glucose in a molar ratio of nearly 1:1:5; this polysaccharide is capable of activating macrophages, splenocytes and thymocytes (Bhunia et al., 2010). Heteroglycan (Table 3) from the edible mushroom Calocybe indica, var. APK2, comprises d-glucose, d-galactose, and l-fucose in a molar ratio of nearly 3:1:1 and has been proven to show immunoenhancing and cytotoxic activity upon HeLa cell lines (Mandal et al., 2011). Heteroglycans (Table 3) extracted from the hot aqueous extract of Pleurotus ostreatus cultivar, containing d-glucose and d-galactose in a molar ratio of nearly 7:1, is also an immunostimulant (Maity et al., 2011). Heteroglycans from hybrid mushrooms also exhibit immunoenhancing activity. Some examples of these are (a) heteroglycan (Table 3) from an aqueous extract of the somatic hybrid mushroom (PfloVv1aFB) of Pleurotus florida and Volvariella volvacea, constituted of d-galactose, d-mannose, and d-glucose with the molar ratio of nearly 1:1:4 (Patra et al., 2011); (b) heteroglycan (Table 3) from an alkaline extract of a somatic hybrid mushroom (PfloVv1aFB) of Pleurotus florida and Volvariella volvacea and consisting of d-galactose, d-mannose, and d-glucose with the molar ratio of nearly 1:1:4 (Bhunia et al., 2012); (c) heteroglycan (Table 3) isolated from hot aqueous extract of fruiting bodies of an edible hybrid mushroom Pfle1r of Pleurotus florida and Lentinula edodes and made up of d-glucose, d-mannose, and d-galactose residues in a molar ratio of nearly 1:1:1 (Maji et al., 2013); and (d) an immunostimulating water-soluble heteroglycan (PS-II) (Table 3) isolated from aqueous extract of the fruiting bodies of a hybrid mushroom, Pfls1h of Pleurotus florida and Lentinus squarrosulus (Mont.) Singer., composed of (1 → 6)- and (1 → 2,4,6)-a-d-galactopyranosyl, terminal ß-d-mannopyranosyl and terminal ß-d-glucopyranosyl residues in a relative proportion of approximately 1:1:1:1 (Sen et al., 2013). An immunoenhancing water-soluble hetero-polysaccharide (Table 4) isolated from an alkaline extract of the fruit bodies of an ectomycorrhizal edible mushroom, Tricholoma crassum (Berk.) Sacc., exhibited splenocyte, thymocyte as well as macrophage activations (Patra et al., 2012). Heteropolysaccharide (Table 4), having molecular weight ~ 2.1 × 105 Da, was isolated from hot aqueous extract of the fruit bodies of hybrid mushroom Pfle 1p. The hybrid mushroom Pfle 1p was obtained through intergenic protoplast fusion between Pleurotus florida and Lentinula edodes. The heteropolysaccharide contained d-glucose, d-galactose, and d-mannose in a molar ratio of nearly 4:2:1 and showed in vitro macrophage activation by NO production and also stimulated splenocytes and thymocytes (Maity et al., 2013).
Table 3
| Mushroom Species | Repeating Unit of Heteroglycan | References |
|---|---|---|
| Lentinus squarrosulus | 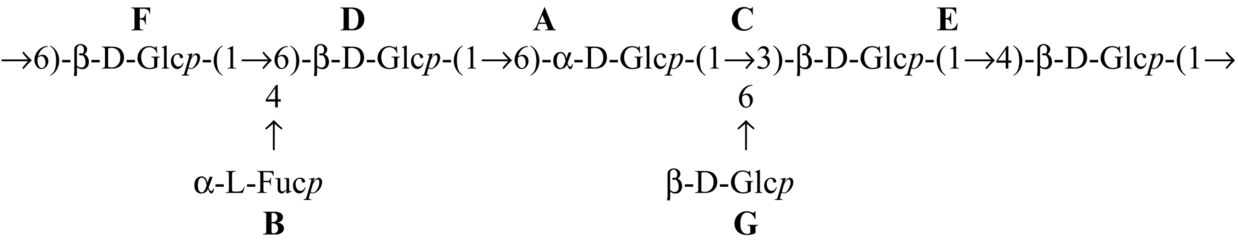 | Bhunia et al. (2010) |
| Calocybe indica | 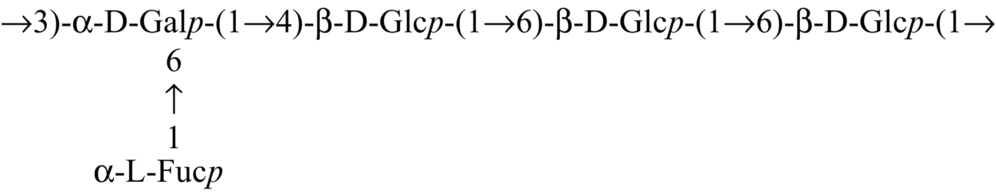 | Mandal et al. (2011) |
| Pleurotus ostreatus | 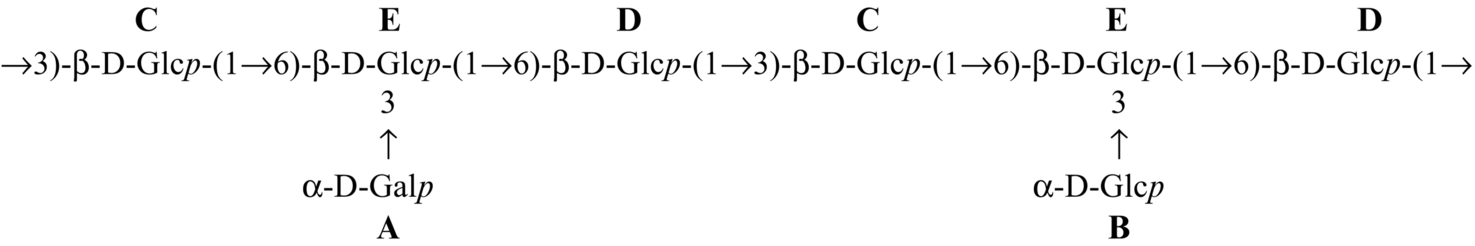 | Maity et al. (2011) |
| Hybrid mushroom (PfloVv1aFB) | 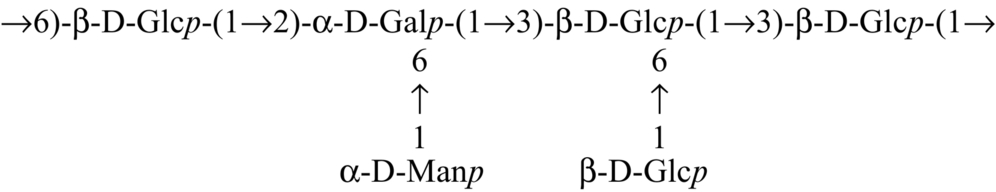 | Patra et al. (2011) Bhunia et al. (2012) |
| Hybrid mushroom (Pfle1r) | 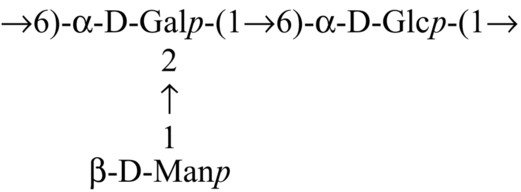 | Maji et al. (2013) |
| Hybrid mushroom (Pfle1r) | 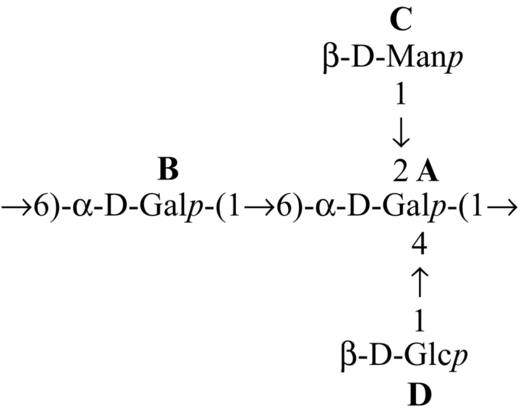 | Sen et al. (2013) |
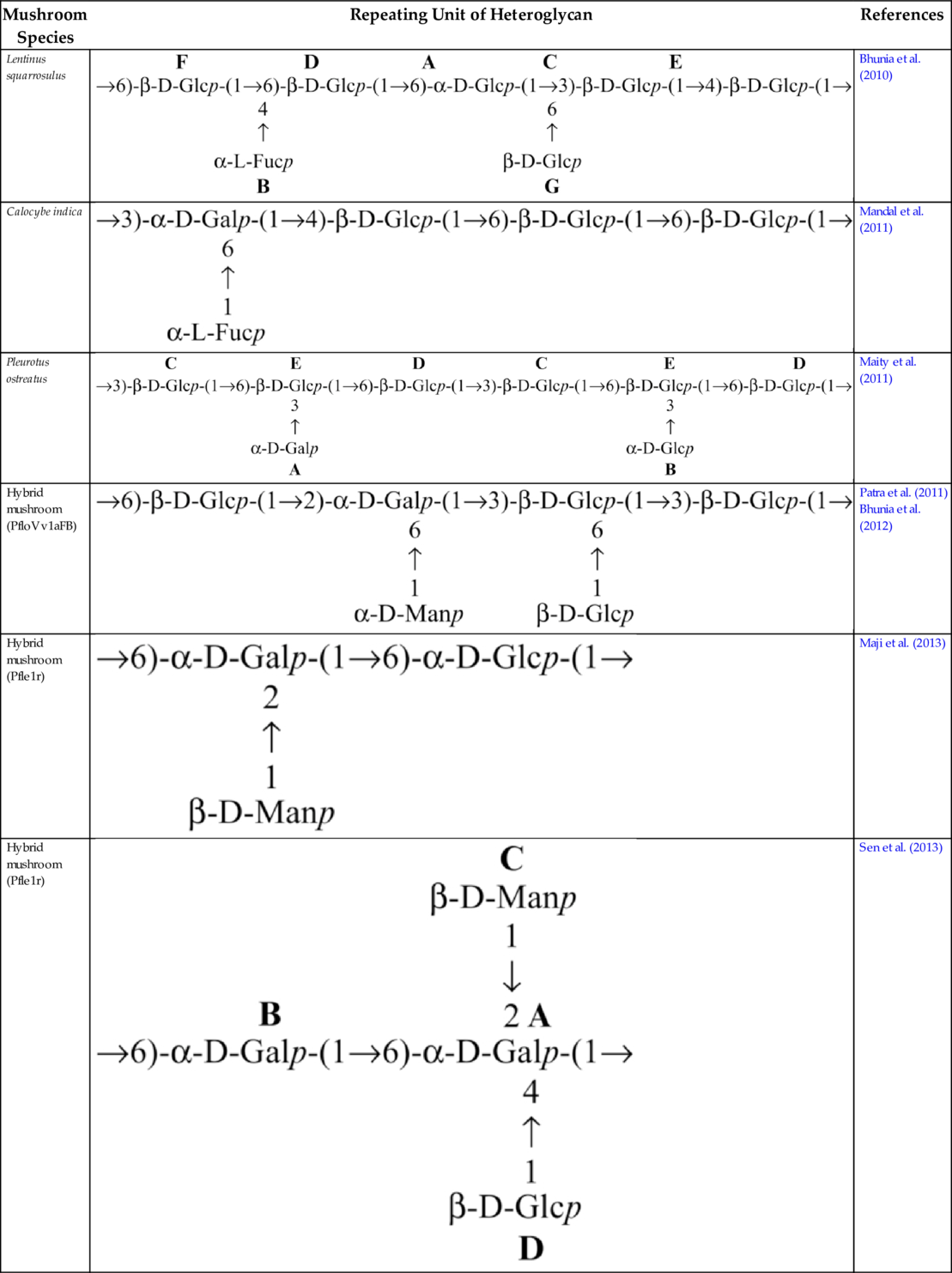
Table 4
| Mushroom Species | Repeating Unit of Heteropolysaccharide | References |
|---|---|---|
| Tricholoma crassum | 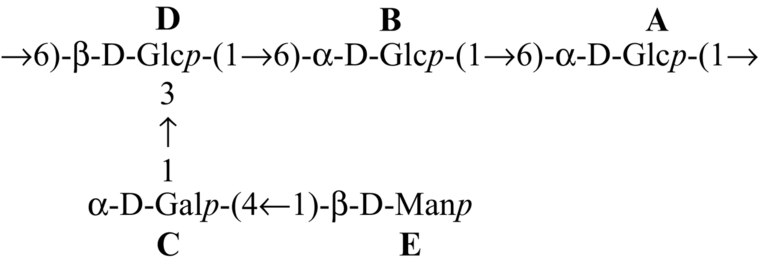 | Patra et al. (2012) |
| hybrid mushroom (pfle 1p) | 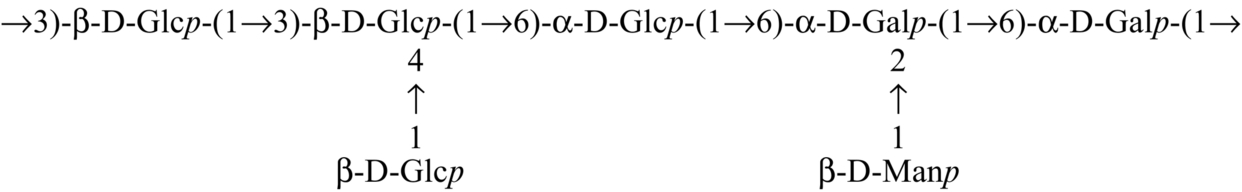 | Maity et al. (2013) |
4.2 Antioxidant Activity
Different wild mushroom species were reported to have antioxidant activity, which mainly related to their phenolic compounds (phenolic acids and flavonoids), followed by tocopherols, ascorbic acid and carotenoids (Ferreira et al., 2009). These molecules were quantified in different species, mainly from Finland, India, Korea, Poland, Portugal, Taiwan, and Turkey. Ferreira et al. (2009) reviewed the values currently available in literature, but expressed in different basis (dry weight, fresh weight, and extract). Helvella crispa from India revealed the highest content of phenolic compounds expressed per gram of extract (34.65 mg/g), while Sparassis crispa from Korea revealed the highest value expressed in a dry weight basis (0.76 mg/g). Auricularia fuscosuccinea (white) from Taiwan (32.46 mg/g of extract), Agaricus silvaticus (3.23 × 10- 3 mg/g of dry weight), and Ramaria Botrytis (2.50 × 10- 4 mg/g of fresh weight) from Portugal, contained the most tocopherols of all the species. Auricularia fuscosuccinea (brown) from Taiwan (11.24 mg/g of extract), Suillus collinitus from Portugal (3.79 mg/g of dry weight) and Agaricus bisporus from Poland (0.22 mg/g of fresh weight) revealed the highest levels of ascorbic acid. Lactarius deliciosus from Portugal revealed the highest content of ß-carotene (0.09 mg/g of extract).
The antioxidant properties of A. brasiliensis were evaluated using methanol, ethanol, dimethyl sulfoxide and water as solvent; extraction was preceded for a variety of times and temperatures. The best conditions for extraction of antioxidants is with methanol as solvent at 60°C for 60 min (Mourão et al., 2011). Mourão et al. (2011) showed that average antioxidant activity for the closed cap group is 24% higher than opened caps. The phenolic composition of methanolic extract from A. bisporus was analyzed by HPLC and it contains rutin, gallic acid, caffeic acid, and catechin. All these contribute to its antioxidant activity (Abah and Abah, 2010). Armillaria mellea, commonly known as the honey mushroom, is pathogenic and grows on living trees and on dead and decaying food material (Zekovic et al., 2010). Armillaria mellea has a strong symbiotic relationship with Gastrodia elata, known as Tian Ma (Lung and Chang, 2011). The antioxidant activity of Armillaria mellea ethanol extract is higher than that of Lycoperdon saccatum which is in accordance with its higher content of the two trace elements, selenium and zinc (Zekovic et al., 2010). Methanolic extracts from dried mycelia, mycelia free broth and hot water extracts from dried mycelia by Armillaria mellea submerged cultures showed low EC50 values (< 10 mg/mL). Their ascorbic acid and total phenol contents are well correlated with the reducing power and the scavenging effect on superoxide anions (Lung and Chang, 2011). A low molecular weight polysaccharide (2.8 × 104 Da) isolated from the fruiting body of Auricularia polytricha exhibited stronger hydroxyl radical scavenging activity than vitamin C at the same concentration (Sun et al., 2010). Furthermore, polysaccharides isolated from water, acidic and alkaline solutions from Lentinus edodes separately showed antioxidant activity through inhibition activity of hydroxyl, ABTS+ radical and lipid peroxidation (Li et al., 2012). Heleno et al. (2011) have screened antioxidant activities of six different mycorrhizal mushrooms: Boletus aereus, B. edulis, B. reticulatus; not edible: B. purpureus, B. satanas, B. rhodoxanthus. Among these, B. aereus showed highest antioxidant activity. Their studies revealed that EC50 value for DPPH scavenging activity (0.43 mg/mL) of the mushroom B. edulis was lower than that of Indian (1.4 mg/mL), Taiwanian (~ 1.5 mg/mL) and Turkish (~ 0.5 mg/mL) specimens (Heleno et al., 2011). Antioxidant activities of ethanolic extracts of B. edulis and B. auranticus have been also identified by Vidovic et al. (2010). However, the total phenol as well as hydroxyl radical scavenging activity of B. auranticus (EC50 0.016 mg/mL) was found to be higher than B. edulis, except reducing power, where B. edulis shows higher activity. Studies by Vidovic et al. (2010) have reported Variegatic acid in both the extracts.
Water extracts of Pleurotus ostreatus with higher phenolic content possessed better antioxidant activities than ethanol extract (Chirinang and Intarapichet, 2009). Kim et al. (2009) studied the antioxidant activities of methanolic extracts of oyster mushrooms. The extract from yellow strain (P. cornucopiae) showed the highest radical scavenging activity, reducing power, ferrous chelating ability and total phenolic contents over the dark-gray strain (P. ostreatus) and pink strain (P. salmoneostramineus). Yim et al. (2010) have also studied the antioxidative potential and total phenolic content of water extract of P. ostreatus and found that the ferric reducing power was significantly higher than BHA and ascorbic acid.
Antioxidant and antiradical activity of methanol extracts from six Termitomyces species (T. titanicus, T. aurantiacus, T. letestui, T. clypeatus, T. microcarpus, and T. eurhizus) growing in Tanzania were studied by Tibuhwa (2012). Results showed highest ability to decrease DPPH radical by T. microcarpus (EC50 < 0.1 mg/mL) followed by T. letestui (EC50 = 0.14 mg/mL), while least ability was shown by T. eurhizus (EC50 = 0.36 mg/mL). The study also showed high antiradical activity (EAU515 1.48) of T. microcarpus followed by T. aurantiacus (EAU515 1.43), while the lowest was observed from T. eurhizus (EAU515 0.7). Additionally, T. microcarpus contained high amount of phenols, flavonoids and ß-carotene (Tibuhwa, 2012). Wang et al. (2012a,b) isolated three polysaccharides from the mushroom Tricholoma lobayense and evaluated their antioxidant activity. A study by Chatterjee et al. (2011) revealed that alcohol extract of T. giganteum possesses better antioxidant activity than that of water and ethyl acetate extract of the same mushrooms.
4.3 Hypoglycaemic/Antidiabetic Activity
Edible and medicinal mushrooms are a potent source of biologically active compounds with antidiabetic effects (Kiho et al., 1994a; Hong et al., 2007). Many mushroom species appear to be effective for both the control of blood glucose levels and the amelioration of the course of diabetic complications. Medicinal mushrooms such as Agaricus bisporus, A. subrufescens, Cordyceps sinensis, Coprinus comatus, Ganoderma lucidum, Inonotus obliquus, Phellinus linteus, Pleurotus spp., Poria cocos and Sparassis crispa have been reported to have antihyperglycemic effects (Silva et al., 2012). Additionally, mushrooms are known to contain compounds which help in proper functioning of the liver (Wani et al., 2010), pancreas and other endocrinal glands, thereby promoting formation of insulin and related hormones, consequently ensuring healthy metabolic functions (Wasser and Weis, 1999; Zhang and Lin, 2004; Chen et al., 2012a,b). Mushroom polysaccharides such as beta glucans have the ability to restore the function of pancreatic tissues through increased insulin output by ß-cells, which causes lowering of blood glucose levels. Polysaccharides have also been shown to improve the sensitivity of peripheral tissues to insulin. In addition to this, the consumption of mushrooms reportedly decreases the lipid levels, including total cholesterol, total triglyceride, and low-density lipoproteins, and increases the level of high-density lipoproteins (Lee et al., 2012).
Studies made by Kiho et al. (1994b) showed that Glucuronoxylomannan (AC) from the fruiting bodies of T. fuciformis exhibited a significant dose-dependent hypoglycemic activity in normal mice, and also demonstrated significant activity in streptozotocin-induced diabetic mice by means of intraperitoneal administration. Exopolysaccharides (EPS) of the mushroom T. fuciformis has reported to exhibit a considerable hypoglycemic effect and improved insulin sensitivity, possibly through regulating PPAR-gamma-mediated lipid metabolism, when evaluated for antidiabetic activities in mice models (Cho et al., 2007). These results indicated that Tremella fuciformis has a potential oral hypoglycemic effect and can be used as a functional food in the management of Diabetes mellitus (DM). The exo-polymer (GAE) produced by submerged mycelial cultures of Ganoderma applanatum and Collybia confluens have shown hypoglycemic effects in streptozotocin (STZ)-induced diabetic rats. The results strongly demonstrated the potential of exopolymer in combating diabetes in experimental animals (Yang et al., 2007). Water-soluble polysaccharide (FA) from fruiting bodies of A. auricula-judae has been reported to show a hypoglycemic effect on genetically diabetic mice (KK-Ay).
The fruit body of Agaricus subrufescens is useful as a health promoting food. Studies preformed on murine models and human volunteers to examine the immune-enhancing effects of the naturally outdoor-cultivated fruit body of Agaricus brasiliensis KA21 (i.e., Agaricus blazei) have shown antitumor, leukocyte-enhancing, hepatopathy-alleviating and endotoxin shock-alleviating effects in mice (Liu et al., 2008). In the human study, percentage body fat, percentage visceral fat, blood cholesterol level, and blood glucose level were decreased and natural killer cell activity was increased (Liu et al., 2008). Beta-glucans and oligosaccharides (AO) of Agaricus blazei Murill showed antihyperglycemic, antihypertriglyceridemic, antihypercholesterolemic, and antiarteriosclerotic activity indicating overall antidiabetic activity in diabetic rats; AO had about twice the activity of beta-glucans with respect to antidiabetic activity (Kim et al., 2005). Further supplement of Agaricus blazei Murill extract has improved insulin resistance among subjects with type 2 DM. The increase in adiponectin concentration after taking Agaricus blazei Murill extract might be the mechanism that brings the beneficial effect (Hsu et al., 2007).
Recent studies have determined that mushrooms such as Hericium spp. may have important physiological functions in humans, including antioxidant activities, regulation of blood lipid levels and reduction of blood glucose levels (Wang et al., 2005). Researchers have found that the hypoglycemic effects of feeding the methanol extract of H. erinaceus to streptozotocin-induced diabetic rats significantly lowered elevation rates of blood glucose levels (Wang et al., 2005). Additionally, the culture broth of Inonotus obliquus also possesses significant antihyperglycemic, antilipid peroxidative, and antioxidant effects in alloxan-induced diabetic mice (Sun et al., 2008).
Crude polysaccharides of Cordyceps sinensis were tested in normal mice and streptozotocin-induced diabetic mice. Oral administration significantly lowered the glucose level in mice (Kiho et al., 1993). A polysaccharide obtained from the cultural mycelium of Cordyceps sinensis showed potent hypoglycemic activity in genetically diabetic mice after intraperitoneal administration. The plasma glucose level was quickly reduced in normal and streptozotocin-induced diabetic mice after intravenous administration (Kiho et al., 1996). Cordyceps, a Chinese herbal medicine with a fruiting body and stem, has been proposed to have multiple medicinal properties. The diabetic rats had significantly lowered weight gain and higher blood glucose response in oral glucose tolerance test than the control rats; these changes were significantly reduced by administrating the fruiting body of Cordyceps. These improvements suggested that the fruiting body of Cordyceps has a potential to be a functional food for diabetes management (Lo et al., 2004). Another study revealed that isolated polysaccharide from Cordyceps sinensis (CSP-1) produced a significant drop in blood glucose level in both STZ-induced diabetic rats and alloxan-induced diabetic mice. This led to the conclusion that CSP-1 may stimulate pancreatic release of insulin and/or reduce insulin metabolism (Li et al., 2006). Researchers evaluated the antidiabetic effect of an alpha-glucan (MT-alpha-glucan) from the fruit body of maitake mushrooms (Grifola frondosa) on KK-Ay mice. These data suggest that MT-alpha-glucan has an antidiabetic effect on KK-Ay mice which might be related to its effect on insulin receptors, that is increasing insulin sensitivity and ameliorating insulin resistance of peripheral target tissues (Hong et al., 2007).
4.4 Antimicrobial Activity
Certain mushrooms have antimicrobial properties which provide control for many human diseases, are generally safe for use and are also effective. Several mushrooms are reported to effectively exhibit both antibacterial and antifungal activity against antibiotic resistant pathogens (Sharma et al., 2014). Agaricus bisporus, the most cultivated mushroom in the world, is in forefront for its antibacterial activity. The methanolic extract revealed MIC = 5 µg/mL against Bacillus subtilis even lower than the standard ampicillin (MIC = 12.5 µg/mL) (Barros et al., 2008a). It also displayed antibacterical activity against Gram positive bacteria Bacillus cereus, Micrococcus luteus, Micrococcus flavus, Staphylococcus aureus and Staphylococcus epidermidis (Tambekar et al., 2006; Öztürk et al., 2011; Ozen et al., 2011) and Gram negative bacteria Escherichia coli, Pseudomonas aeruginosa, Enterobacter aerogenes, Klebsiella pneumoniae, Proteus vulgaris, Salmonella typhi, and Salmonella typhimurium (Tambekar et al., 2006; Ozen et al., 2011). Other Agaricus species (such as Agaricus bitorquis) methanolic extracts also showed inhibitory effect upon all the tested Gram-positive bacteria and Gram-negative bacteria (Öztürk et al., 2011). Agaricus silvicola methanolic extract also revealed antimicrobial properties against Bacillus cereus (MIC = 5 µg/mL), Bacillus subtilis (MIC = 50 µg/mL), and against Staphylococcus aureus (MIC = 5 µg/mL), lower than the standard ampicillin (MIC = 6.25 µg/mL) (Barros et al., 2008a). The mycelium of Agaricus cf. nigrecentulus and Tyromyces duracinus (ethyl acetate extracts) showed activity against only Staphylococcus saprophyticus (Rosa et al., 2003). Conversely, Agaricus essettei, Agaricus silvicola, Agaricus silvaticus, and Agaricus cf. nigrecentulus did not show any antibacterial activity against Gram-negative bacteria (Rosa et al., 2003; Öztürk et al., 2011; Barros et al., 2008a).
The ethanolic extracts of Armillaria mellea mycelium showed antibacterial effect against Sarcina lutea, however no activity was observed upon other Gram-positive bacteria (Kalyoncu et al., 2010). However, ethanolic extract of their fruiting bodies showed broad-spectrum antimicrobial activity (Kalyoncu and Oskay, 2008). Cantharellus cibarius methanolic extract demonstrated good activity against Gram-positive bacteria Bacillus subtilis and Staphylococcus aureus (Barros et al., 2008a,b; Ozen et al., 2011) and Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa (Ozen et al., 2011). Clitocybe alexandri methanolic extract presented significant activity against Bacillus subtilis, Micrococcus luteus, Enterobacter aerogenes, and Escherichia coli (Solak et al., 2006). Kalyoncu and Oskay (2008) tested antimicrobial activity of chloroform and ethanolic extracts from Clitocybe geotropa, the latter showing significant capacity against Bacillus cereus and Proteus vulgaris.
The genus Cortinarius is one of the most diverse genera of mushrooms. Ethyl acetate extracts of C. ardesiacus, C. archeri, C. atrosanguineus, C. austrovenetus, C. austroviolaceus, C. coelopus, C. [Dermocybe canaria], C. clelandii, C. [D. kula], C. memoria-annae, C. persplendidus, C. sinapicolor, and C. vinosipes, plus an additional 47 collection samples not identified at the species level, exhibited IC50 values = 0.09 of mg/mL against Staphylococcus aureus and P. aeruginosa (Beattie et al., 2010).
Ganoderma lucidum is one of the most well known traditional medicinal mushrooms. Various extracts have been found to be equally effective when compared with gentamycin sulfate, the acetone extract being the most effective. The extract of this mushroom demonstrated strong antibacterial activity, mainly against Klebsiella pneumonia, and moderate inhibition against Bacillus subtilis and Staphylococcus aureus (Quereshi et al., 2010), but in the study reported by Sheena et al. (2003), its methanolic extract showed poor antimicrobial activity. Other authors described the capacity of aqueous extract to inhibit 15 types of Gram-positive and Gram-negative bacteria, with the highest inhibition exhibited against Micrococcus luteus (Gao et al., 2005). Ethyl acetate extracts of Phellinus sp., Gloeoporus thelephoroides and Hexagonia hydnoides inhibited Bacillus cereus growth while the same extract of Nothopanus hygrophanus mycelium presented inhibitory activity against Listeria monocytogenes and Staphylococcus aureus. Irpex lacteus mycelium extract was the most effective, presenting a broad spectrum of activity (Rosa et al., 2003).
The antimicrobial activity of Pycnoporus sanguineus has been known since 1946, when Bose isolated poliporin, a compound active against Gram-positive and Gram-negative bacteria and without toxicity in experimental animals. Rosa et al. reported inhibition against Listeria monocytogenes and Staphylococcus aureus (Rosa et al., 2003). Smânia et al. (1995, 1997) showed that this mushroom produces cinnabarine, an orange pigment active against Bacillus cereus, Staphylococcus aureus, Leuconostoc mesenteroides, Lactobacillus plantarum, and several Streptococcus spp. Cinnabarine was more active against Gram-positive than Gram-negative bacteria (Rosa et al., 2003). Additionally, all the tested gram-positive bacteria were susceptible to methanolic extracts of Lactarius species and Tricholoma portentosum (Barros et al., 2007a,b; Ozen et al., 2011). Among Lactarius species (L. piperatus, L. camphorates, L. volemus, L. delicious), L. camphoratus methanolic extract was the one with greater antimicrobial activity (Ozen et al., 2011). Methanolic extracts of Ramaria botrytis and ethanolic extract of Ramaria flava inhibited the growth of Gram-positive bacteria better than Gram-negative bacteria (Gezer et al., 2006). The antimicrobial effect of ethanolic extract of Laetiporus sulphureus was tested by Turkoglu et al. (2007) and strongly inhibited the growth of the gram-positive bacteria tested, including Bacillus subtilis, Bacillus cereus, Micrococcus luteus, and Micrococcus flavus.
Lepista nuda methanolic extract had a good action on Gram-positive bacteria, more specifically on Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus (Dulger et al., 2002; Barros et al., 2008b). Ishikawa et al. (2001) reported the inhibitory activity of Lentinus edodes ethyl acetate extract against Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis. This mushroom (aqueous extract), as well as menthol extract of n-BuOH fraction of Phellinus linteus, demonstrated good activity against MRSA (Hur et al., 2004; Hearst et al., 2009). Furthermore, Streptococcus pyogenes was very sensitive to Lentinus edodes chloroform extract whereas no effect was found against Escherichia coli, Pseudomonas fluorescens, Klebsiella pneumoniae or Camphylobacter jejuni (Hatvani, 2001). The ability of L. edodes extracts to improve oral health has also been extensively researched, because it showed a strong bactericidal effect upon Streptococcus mutans and Prevotella intermedia which are strongly implicated in dental caries and tooth decay (Hirasawa et al., 1999; Signoretto et al., 2011). Mycelium of Leucopaxillus giganteus (methanolic extract) presented antimicrobial capacity, inhibiting only Gram-positive bacteria and in the decreasing order: Staphylococcus aureus > Bacillus cereus > Bacillus subtilis (Barros et al., 2007c).
Methanolic extract of Phellinus rimosus and Navesporus floccosa showed moderate inhibition of Bacillus subtilis and Staphylococcus aureus (Sheena et al., 2003). Pleurotus ostreatus and Meripilus giganteus showed broad-spectrum antimicrobial activity. The maximum effect was shown by ethanolic extracts of Pleurotus ostreatus against Sarcina lutea (Kalyoncu et al., 2010). Ether extract of Pleurotus sajor-caju showed high antibacterial activity against Staphylococcus aureus, whereas Staphylococcus epidermidis showed high sensitivity for ethanol extract (Tambekar et al., 2006).
In vitro antimicrobial activity of the acetone and methanol extracts of the mushrooms Amanita rubescens, Cantharellus cibarius, Lactarius piperatus, and Russula cyanoxantha was studied by Kosanic et al. (2013). The antimicrobial activity was estimated by determining the minimal inhibitory concentration by using micro dilution plate method against five species of bacteria and five species of fungi. Generally, the tested mushroom extracts had relatively strong antimicrobial activity against the tested microorganisms, suggesting that mushrooms may be used for pharmaceutical purposes in the treatment of various diseases. The culture filtrates of 27 edible mushrooms were screened for antimicrobial activity against plant pathogens (Chen and Huang, 2011). It was found that culture filtrates of Lentinula edodes and Clitocybe nuda were able to completely inhibit spore germination of Colletotrichum higginsianum. Three culture filtrates that contained substances having the capacity to completely inhibit spore germination of Alternaria brassicicola were Ganoderma lucidum, L. edodes, and C. nuda. These results suggest that substances from edible mushrooms have the potential to be developed into biocontrol agents for the control of plant diseases (Chen and Huang, 2011). Menaga et al. (2012) found that bioactive compounds from Pleurotus florida mushroom extracts could be used as alternate therapeutics to antibiotics. Additionally, Alves et al. (2012b) have reported that Fistulina hepatica, Russula botrytis, and Russula delica are the most promising mushroom species as antimicrobial agents. It is important to develop new studies with different mushroom species to address the microorganisms so problematic to human health.
5 Conclusion
Mushrooms are an alternate source of nutrients with low fats and calories. In general, proteins of mushrooms contain all nine essential amino acids required by humans. Additionally, they are also a relatively good source of nutrients such as phosphorus, iron, and vitamins, including thiamine, riboflavin, ascorbic acid, ergosterol, and niacin. Mushrooms have also been reported as therapeutic foods which are required to prevent diseases such as hypertension, diabetes, hypercholesterolemia, and cancer. All these functional characteristics are mainly due to the presence of dietary fibers, bioactive components, antioxidants, lectins and antimicrobial agents. Furthermore, edible mushrooms are gaining much attention as an alternative source of prebiotics. The major components rendering prebiotic function in mushrooms are nondigestible polysaccharides such as glucan, chitin and hetropolysaccharides. Several mushroom polysaccharides, like pleuran, lentinan, schizophyllan, ß and a-glucans, mannans, xylans, galactans, chitin, inulin and hemicelluloses, can be credited with promising prebiotic effects. Mushrooms rich in immune-modulating polysaccharides are used as health-promoting food supplements (nutraceuticals). However, the mechanism of action of various secondary metabolites isolated from medicinal and wild edible mushrooms are yet to be discovered. Thus, further research will uncover different applications with respect to the high nutritional and therapeutic potential of mushrooms, namely as functional foods or as a source of nutraceuticals for maintenance and promotion of health and a good quality of life.