Probiotics in the Rescue of Gut Inflammation
Asit Ranjan Ghosh VIT University, Vellore, India
Abstract
Inflammation is the root cause of many diseases. Chronic inflammation in the gut leads to many inflammatory diseases, primarily due to dysbiosis. Evolution of the gut among mammals is host to a large number of microbiota and it appears their number and quantity are quite significant in maintaining the physiological equilibrium, for homeostasis in general and especially the gut, in particular . The evolutionary symbiosis between host and allochthonous microbiota can restore peace and balance in a system overcoming immunological intolerance. This is important to understanding the cause of balance in the gut. Specific probiotic characteristics of microbiota are required to maintain homeostasis. The inflammation of the gut may start from a mere infection, antibiotic use/abuse, dysbiosis, or the upregulation of proinflammatory molecules that comes with aging. Probiotics offer great potential to support gut health and regulate the causes of inflammation. The active surface components, the secondary metabolites, or/and biohydrogenation capabilities of probiotics in the gut reduce or remove the causes of inflammation, and thus relieve the host.
Keywords
Microbiota; Dysbiosis; Inflammation; Probiotics; Biohydrogenation; Gut
1 Introduction
The gut is considered to be the second brain of the body. The gut-brain axis explains the link between digestion, mood, health, behavior, and even psychological state. The enteric nervous system (ENS) of the gut and central nervous system (CNS) of the brain are highly connected in the process of signal transduction including the gut flora (Mayer et al., 2014). Multiple research articles and reviews have refreshed our conceptual understanding regarding the connection of health in relation to gut flora. The type and number of floral members are found to be instrumental in maintaining good health (Lozupone et al., 2012). Current research has recently overwhelmingly established that bacterial gut flora are specific to good health, leading to the bacterial ecology of the gut. This beneficial and health supportive bacterial ecology is sometimes jeopardized or impaired, leading to a condition called dysbiosis (Maynard et al., 2012).
Inflammation is a pervasive condition which is characterized by significant symptoms including pain, redness, swelling, heat, and loss of function. Inflammation is an immunological strategy to eliminate the cause of cell injury, clear necrotic cells, and damaged tissues, and to initiate in tissue repair. However, chronic inflammation appears to be the cause of several inflammatory diseases such as rheumatoid arthritis, diabetes, various cardiovascular diseases, and obesity (Lescheid, 2014; Festi et al., 2014). Gut flora in the state of dysbiosis is affected, which results chronic inflammation by activation of T cells subsets (Th1, Th17, γδT) and suppression of regulatory mechanisms by reducing IgA synthesis, lowering levels of suppressive cytokines IL-10, TGF-β, and reducing Treg cells (Honad and Litman, 2012).
The present treatment of inflammation in the gut (as well as the resultant diseases) is the normalization of gut microbial population by the introduction of beneficial members of the gut microbiota. Fecal microbiota transplantation (FMT) used in alleviating many infectious and inflammatory diseases where fecal microbiota of a healthy individual is transplanted strategically to the gut impaired individual (Bakken et al., 2011). By 2013, the Food and Drug Administration (FDA) approved the legal use of feces as a drug for human health (Ratner, 2014). However, beneficial microbiota of diverse origin also shows promise in offering beneficial effects to human health. Fermented food and nutraceuticals are also recommended for reinstating gut flora (Selhub et al., 2014; Dubey et al., 2015, 2016). “Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” are probiotics (FAO/WHO) (Marteau, 2006). In the following review, the main focus of discussion is to identify the role played by probiotics in the mitigation of inflammation of the gut.
2 Gut Microbiota, Probiotics, and Dysbiosis
The gut is a hollow tubular structure into which nutrient-rich food is pushed, processed, and absorbed, then wastes are expelled as it occurs in even the most primitive hydra. This process occurs sequentially in the buccal cavity, esophagus, stomach, and intestines in humans. The large intestine offers a home for a large number of microbes called gut microbiota or/and commensal microflora. In the beginning of life, prenatal maternal exposure plays a vital role in post-natal microfloral colonization, gut-associated lymphoid tissue (GALT) development, and maintenance of integrity of the mucosal barrier (Daliri and Lee, 2015). In adults, bacterial phyla like bacteroidetes and firmicutes are common with less abundance of actinobacteria, proteobacteria, and verrucomicrobia (Eckburg et al., 2005; Hakansson and Molin, 2011). The beneficial microbes are called probiotics with special characteristics (Fig. 1) (Pande et al., 2012). The undigested metabolic ingredients of the host which support growth of probiotics are termed “prebiotics.” Together, probiotics and prebiotics constitute the functional food, called synbiotics. The effectiveness of probiotics is strain-specific, and each strain may contribute to host health through different mechanisms (Pande et al., 2012).
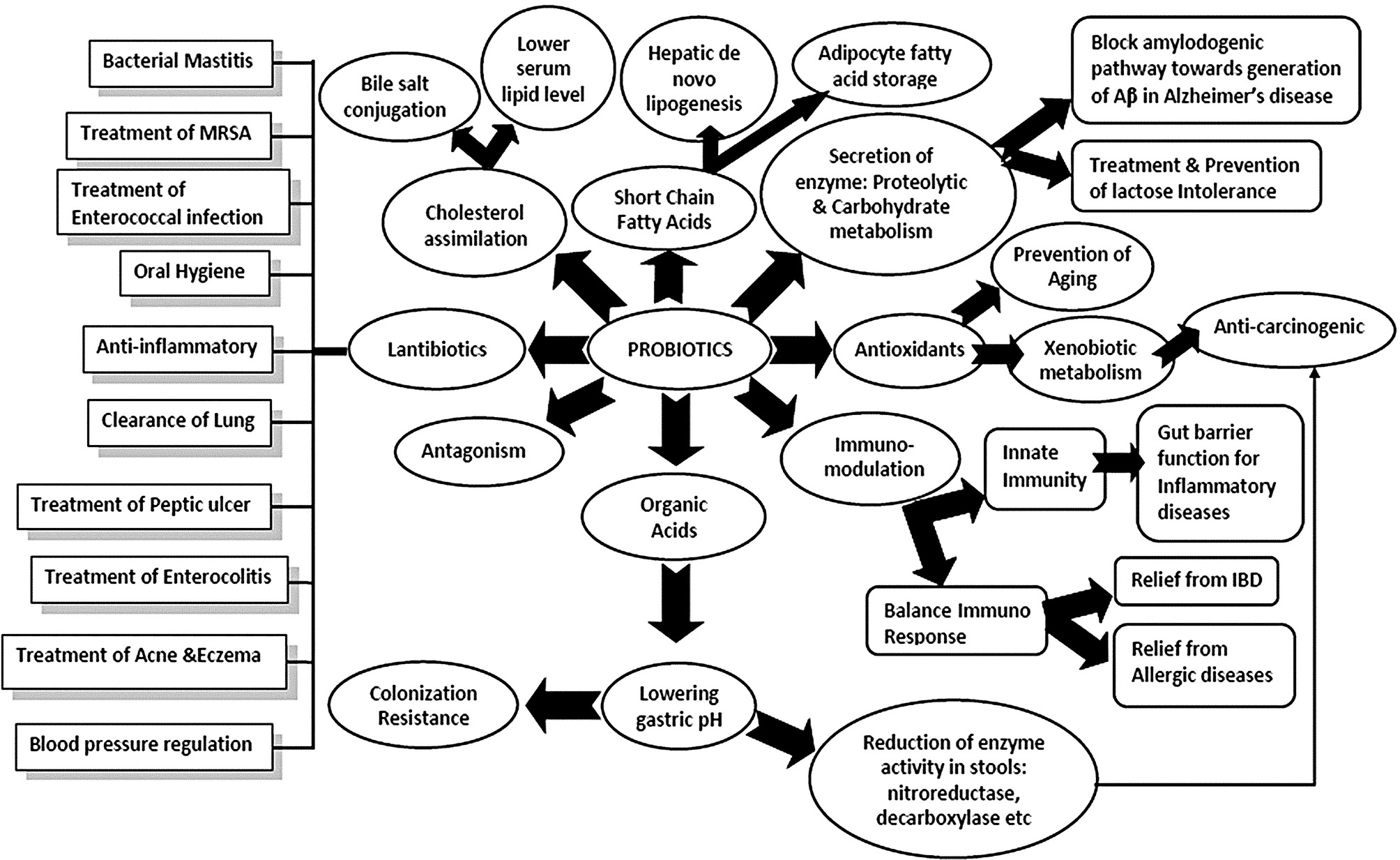
Probiotics commonly stem from the category of lactic acid bacteria (LAB) which are Gram positive, nonspore forming, catalase negative, acid tolerant, and strictly fermentative with lactic acid as the major end product during sugar fermentation. LAB with probiotic potentials are known to exert positive influence on host health and physiology (Marteau, 2006; Pande et al., 2012). Probiotics can be bacteria, molds or yeasts, but most are lactic acid bacteria and consist of a number of heterogenous bacterial genera within the phylum Firmicutes. Among all bacteria, genera such as Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Lactosphaera, Leuconostoc, Melissococcus, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus, and Weissella are recognized as LAB (Lozupone et al., 2012; Lescheid, 2014; Festi et al., 2014). Some are homofermentative, such as Lactococcus and Streptococcus, as they yield two lactates from one glucose molecule, whereas others are heterofermentative (Leuconostoc and Weissella) transform a glucose molecule into lactate, ethanol, and carbon dioxide. All probiotic bacteria are LAB, but all LAB are not probiotic. This phenomenon was observed in a study by Shruthy et al. (2011). LAB constitute an integral part of the healthy gastrointestinal (GI) micro ecology and is critically involved in host metabolism. Gut microbiota ferment various substrates like lactose, biogenic amines, allergenic compounds, and convert them into short chain fatty acids (SCFA), organic acids, gases with enzymes, vitamins, antioxidants, and bacteriocins (Fig. 1) (Pande et al., 2012). In a study by Gowri and Ghosh (2010a,b) several probiotic strains were shown to produce bacteriocin-like Pediocin from Pediococcus pentosaceus GS4 with a molecular weight of 9.9 kDa (unpublished data) that inhibits the growth of Staphylococcus aureus (ATCC 25923), Listeria monocytogens (ATCC 15313) and diarrhoeagenic bacteria including Shigella dysenteriae type1, Shigella sonnei, Salmonella typhimurium, Vibrio cholerae O1 and O139. Conjugated linoleic acid (CLA) is a natural ligand of PPARγ (peroxisome proliferator-activated receptor gamma). Some probiotics have the property of biohydrogenation of linoleic acid to CLA and thus, can regulate inflammation (Dubey et al., 2012, 2015).
Selection criteria for probiotics: Probiotics have attained considerable interest and importance for a variety of medical conditions, and millions of people around the world consume probiotics daily for perceived health benefits. A probiotic strain must be able to survive in the extremely harsh conditions of the digestive tract of the host, such as high acidity in the stomach and concentrated bile found in the small proximal of the intestine. An effective probiotic should be capable of gastrointestinal tract transition, influencing metabolic activities like cholesterol assimilation, lactase activity and vitamin production, overcoming effects of peristalsis, and possession the capacity for colonization. In addition, it must also be safe, commercially feasible, and technologically compatible and must remain viable in storage while maintaining acceptable sensory attributes (Saarela et al., 2000; Bagad et al., 2012; Dubey et al., 2015, 2016).
Gut microbiota is so unique that it has potential to be distinct in populations and as well as in population units (Lozupone et al., 2012). It also lays the foundation to establish the state of human health. Research data confer that several factors like gene, environment, and diet play a major role in having specific gut microbiota (Filippo et al., 2010; Arumugam et al., 2011; Wu et al., 2011). Diet discrepancy and floral diversity remain a key to observation in human microbiome research. For example, dominance of Prevotella and the plant based diet of healthy children in Africa differs with Bacteroides and animal protein-rich diet among healthy adults in the United States (Arumugam et al., 2011; Wu et al., 2011). Differences in microbial composition have been observed in a disease state (Sun et al., 2010; Knights et al., 2011), with special mention of antibiotic-associated diarrhea (Young and Schmidt, 2004), ulcerative colitis (UC) (Frank et al., 2007), Crohn’s disease (CD) (Dicksved et al., 2008), irritable bowel syndrome (IBS) (Carroll et al., 2011), Clostridium difficile-associated diarrhea (Chang et al., 2008), and with drug (paracetamol) metabolism (Clayton et al., 2009). Therefore, gut microbiota establish a balance being influenced by several factors, which in turn determine the state of health. Disturbances potentially lead to dysbiosis when the immune-regulatory network is ruffled.
Dysbiosis brings change in gut flora and becomes the etiology of many inflammatory diseases (Maynard et al., 2012) like CD, UC, IBS, IBD, necrotizing enterocolitis, and extraintestinal diseases like rheumatoid arthritis, multiple sclerosis, diabetes, atopic dermatitis, asthma, obesity, and metabolic syndromes (Maynard et al., 2012). Research findings are rather convincing in establishing these inflammatory pathologies with dysbiosis; so reshaping the floral diversity with the application of beneficial bacteria (probiotics) is in the forefront of medical research.
3 Gut Immunity
A healthy gut is the natural home of a healthy microbiota. Normal gut function is closely linked with restoring the health of the mucosal layer lining its length and associated lymphoid tissues. The gut is the site of the largest microbial populations and the biggest source of immunological stimulations (Delcenserie et al., 2008). It is necessary to understand how the immunological complexity is overcome and restore homeostasis.
The largest mass of lymphoid tissues are assembled in human intestine and immune cells like phagocytes (neutrophils, monocytes, macrophages, even natural killer—NK cells), dendritic cells, and lymphocytes (T and B) are stored to combat any attack by innate and adaptive immune systems (Salminen et al., 1998). Together, with immunological function, the human intestine has three primary physiological functions: digestion of food, absorption of nutrients, and keeping toxins and toxic elements out of the body (Jakoby and Ziegler, 1990). Failure in any of these functions results in defective energy production, faulty energy need, and waning of body’s reserve, eventually culminating in a disease. However, the gut epithelia are central to the orchestration of intestinal defenses and possess multitasking abilities. They are composed of five different types of epithelium: absorptive enterocytes, goblet cells, Paneth cells, M cells, and enteroendocrine cells and are developed from common stem cells located near the base of the intestinal crypts. Maturation of the intestinal mucosa and GALT is initiated by microbial colonization. Central to sensing the colonizers of the intestine is the expression of wide range of germline-encoded pattern recognition receptors (PRRs) by intestinal epithelial cells (IECs) and residential immune cells in the gut. PRRs, such as toll-like receptors (TLRs) and C-type lectin receptors (CLRs), are found on the cell surface or in endosome, and cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) of the intestinal mucosa can recognize the pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) which are expressed by the constituents of resident microbiota and pathogens. However, the mechanisms behind the differential recognition between resident microbiota and pathogens via PRRs-MAMPs interaction are not well understood (Maynard et al., 2012; Koboziev et al., 2014; Daliri and Lee, 2015; Jakobsson et al., 2015).
In the adaptive immune system, T (CD4 +) cells are activated and are differentiated into Th1 or Th2 cells, depending on the interactomes. The Th1 cells produce proinflammatory cytokines like IFNγ, TNFα, and IL-2 while Th2 cells produce IL-4, IL-5, IL-6, and IL-13, respectively. The Th1 cells thus stimulate phagocytosis and eliminate pathogenic microbes while Th2 cells produced IL-4 that induces B lymphocytes to produce antibodies (IgA, IgG) to neutralize and eliminate pathogens and pathogenic epitopes. In this regard, transcription factors STAT (signal transducer and activator of transcription)-1, STAT-4, and T-bet, along with IL-12, IFNγ, are responsible for Th1 response while STAT-6, GATA-3 transcription factors, and IL-4, IL-5, and IL-13 are associated with Th2 cells response (Delcenserie et al., 2008; Lescheid, 2014). Another T cell subset (Th17) may be developed which produces proinflammatory cytokines, IL-17. However, it remains critical to sustain Th1/Th2 balance. The resultant direction rules the disease pathology. In chronic inflammation, it is Th1 cells that take the lead, though regulatory T cells (T reg) may intervene in the immune response with the liberation of high levels of IL-10 and moderate levels of transforming growth factor (TGFβ) (Haller et al., 2000; Delcenserie et al., 2008; Lescheid, 2014; Daliri and Lee, 2015). The GALT is a critical regulator in differentiation of pathogenic microbes while preserving a tolerance toward residential microbiota and food antigens. Several microbial metabolites and structural components also participate in this regulation.
4 Gut-Probiotics Interaction
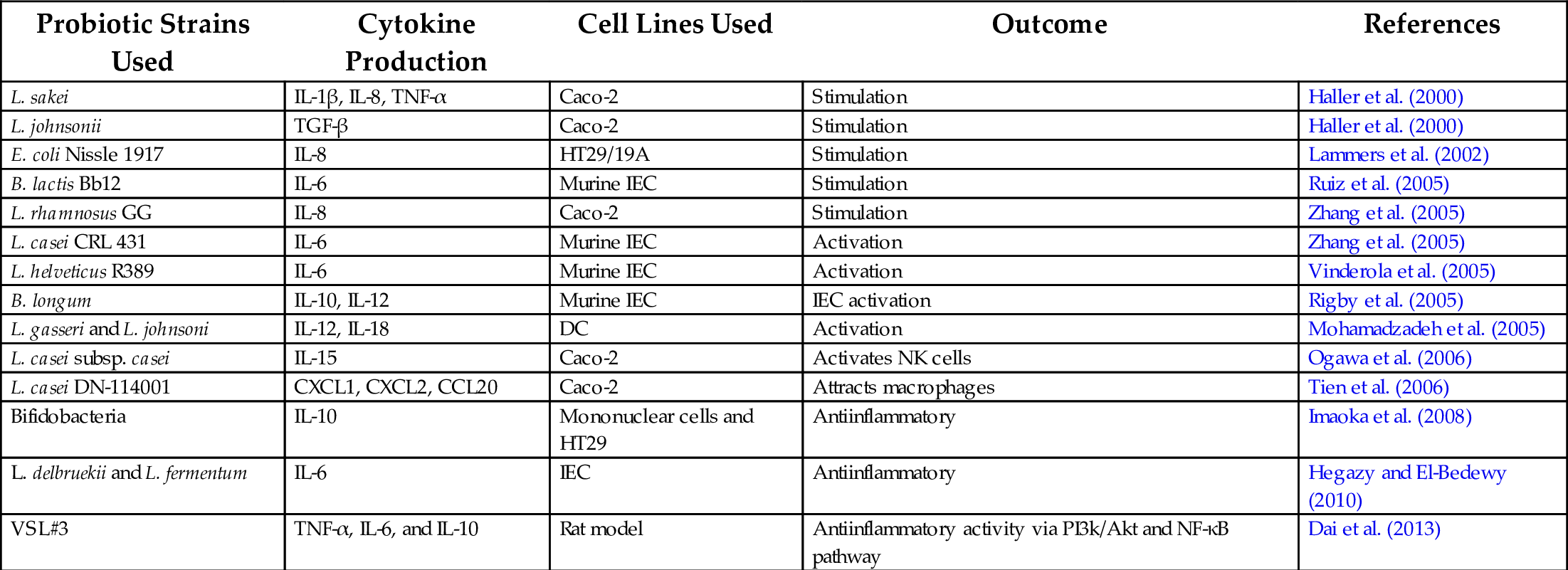
The gut-probiotic interaction is of prime importance in laying a foundation for good health. During the neonatal period, interaction with early microbial colonizers and growing intestinal epithelial cells (IECs) enable the development of mature GALT. With time, GALT develops tolerance toward gut microbiota at the early phase of life (Delcenserie et al., 2008). It appears that microbiota undergo clonal selection and achieve endurance, possibly functioning like an organ. In many cell line-based studies, it was shown that strain dependent probiotic-IEC interactions induce to produce several cytokines that can modulate the in vitro expression of pro- and antiinflammatory cytokines (Haller et al., 2000; Ruiz et al., 2005; Tien et al., 2006; Ogawa et al., 2006; Lescheid, 2014; Kang and Im, 2015). Probiotics, such as Lactobacillus sakei, modulates production of IL-1β, IL-8, TNF-α by Caco-2 cells (Haller et al., 2000). Bifidobacterium lactis Bb12 induces IEC to produce IL-6 proinflammatory cytokine, a growth factor of B lymphocytes, and supports production of platelets (Ruiz et al., 2005). In a separate study, Vinderola et al. (2005) showed that coincubation of primary IEC with probiotic Lactobacillus helveticus R389 and Lactobacillus casei CRL 431 in mice also stimulated IL-6 expression. Zhang et al. (2005) also demonstrated TNF-α-induced IL-8 production in Caco-2 cells using live and dead Lactobacillus rhamnosus GG (Adams, 2010) (Table 1).
Table 1
| Probiotic Strains Used | Cytokine Production | Cell Lines Used | Outcome | References |
|---|---|---|---|---|
| L. sakei | IL-1β, IL-8, TNF-α | Caco-2 | Stimulation | Haller et al. (2000) |
| L. johnsonii | TGF-β | Caco-2 | Stimulation | Haller et al. (2000) |
| E. coli Nissle 1917 | IL-8 | HT29/19A | Stimulation | Lammers et al. (2002) |
| B. lactis Bb12 | IL-6 | Murine IEC | Stimulation | Ruiz et al. (2005) |
| L. rhamnosus GG | IL-8 | Caco-2 | Stimulation | Zhang et al. (2005) |
| L. casei CRL 431 | IL-6 | Murine IEC | Activation | Zhang et al. (2005) |
| L. helveticus R389 | IL-6 | Murine IEC | Activation | Vinderola et al. (2005) |
| B. longum | IL-10, IL-12 | Murine IEC | IEC activation | Rigby et al. (2005) |
| L. gasseri and L. johnsoni | IL-12, IL-18 | DC | Activation | Mohamadzadeh et al. (2005) |
| L. casei subsp. casei | IL-15 | Caco-2 | Activates NK cells | Ogawa et al. (2006) |
| L. casei DN-114001 | CXCL1, CXCL2, CCL20 | Caco-2 | Attracts macrophages | Tien et al. (2006) |
| Bifidobacteria | IL-10 | Mononuclear cells and HT29 | Antiinflammatory | Imaoka et al. (2008) |
| L. delbruekii and L. fermentum | IL-6 | IEC | Antiinflammatory | Hegazy and El-Bedewy (2010) |
| VSL#3 | TNF-α, IL-6, and IL-10 | Rat model | Antiinflammatory activity via PI3k/Akt and NF-κB pathway | Dai et al. (2013) |
Besides stimulation in cytokines and chemokines production, many probiotic strains can influence phagocytosis (Arunachalam et al., 2000), enhance NK cell activity (Ogawa et al., 2006), stimulate IgA production (Park et al., 2002), suppress lymphocyte proliferation (Sturm et al., 2005), induce apoptosis (Carol et al., 2006) and cell-mediated immunity (de Waard et al., 2003). Arunachalam et al. (2000) demonstrated the increased phagocytosis activity in PBMC (peripheral blood mononuclear cells) on consumption of B. lactis HN019, and, more recently, by using probiotic Enterococcus faecium AL41 (Dvorožňáková et al., 2016).
The important roles thus played by the probiotics are of being immune-modulators. As per reports, probiotics that play a beneficial role to the host are antiinflammatory. To demonstrate this property and other mechanisms, in vitro studies are carried out using human intestinal cells (HT-29 and Caco-2). However, an ex-vivo screening method has also been reported to identify the antiinflammatory property of probiotic strain in question (Kwon et al., 2010). Mohamadzadeh et al. (2005) showed that some probiotic strains induce DCs to express high levels of IL-12 and IL-18 while Rigby et al. (2005) demonstrated that Bifidobacterium longum can induce murine colonic DCs to produce IL-10 and IL-12. In practice, immune cells from mesenteric lymph nodes are collected and cocultured with probiotic strains for 72 h, followed by the simultaneous measurement of IL-10 and IL-12. A promising antiinflammatory strain shows induction of a high level of IL-10 and low level of IL-12 expression. Some strains are also proinflammatory and express IL-12, IL-1β, and TNF-α (Table 1). The candidate probiotic strains are antiinflammatory and induce tolerance signaling. In one study, a mixture of five probiotic strains (IRT5: Bifidobacterium bifidum, L. casei, Lactobacillus acidophilus, Lactobacillus reuteri, and Streptococcus thermophilus) could induce the enhanced upregulation of induced regulatory T cell (iTregs, CD4 + Foxp3 +) populations in immune cells (dendritic cells) of mesenteric lymph nodes more than any combination of 1, 2, or 3 strains (Kwon et al., 2010). It is evident that subtle balance is regulated between Th1 and Th2 cells which governs the resultant immunological response.
5 Dysbiosis is the Cause of Inflammation at Gut
Dysbiosis is the state of alterations in microbial flora in the gut, directly causing several particular inflammatory diseases. The gut is the locus of intense activity allochthonus microbial flora and it can be changed with the influence of several internal and external factors (Carding et al., 2015). Influences such as infection (Kamada et al., 2013), antibiotic use (Dethlefsen et al., 2008), diet (de Filippo et al., 2010), disease condition-obese (Ley et al., 2006; Armougom et al., 2009), and age (Kalliomäki et al., 2008) are the driving factors, directly and indirectly causing dysbiosis and disease. It is an experimentally established fact that food born viral pathogens in murine modeling altered the gut microflora and induced both local and systemic inflammation (Kamada et al., 2013). Antibiotic use has a direct relation to the alteration of gut microbiota and is the major cause of dysbiosis (Hawrelak and Myers, 2004). It causes the overgrowth of existing microflora, C. difficile, causing antibiotic-associated diarrhea (Boccardo et al., 2004) as well as reduced SCFA production resulting in electrolyte imbalance and diarrhea (Bengmark, 1996). Diet determines the state of microbiota in a human host. Western diet with high fat and low fiber induces increased growth of gram-negative bacteria with resultant increase concentration of LPS (Cani et al., 2007); which in turn interact with TLR-4 and initiate the inflammatory cascade (Wright et al., 1990). Diet, either animal or plant-rich, influences the gut flora. Experimental data show the abundance of bile-tolerant, amino acid metabolizing flora like Alistips, Bilophila, and Bacteroides among volunteers with animal-rich diet with decreased levels of members of polysaccharides metabolizing Firmicutes (Roseburia, Eubacterium rectale, and Ruminococcus bromii) (David et al., 2014). Diet rich in polyphenols may alter the composition of gut flora. Red wine polyphenol consumption caused to increase bacterial members such as Enterococcus, Prevotella, Bifidobacterium, Bacteriodes, and E. rectale in healthy volunteers. Many studies have been conducted to find a relationship between several autoimmune disorders and dysbiosis using germ-free mice (GF) (Carding et al., 2015). Turnbaugh et al. (2006) showed that colonic flora from obese mice colonized in GF mice caused an increase in body weight and fat in comparison to flora colonized from lean mice. Ingested food after digestion leaves many undigested organic products, along with digestive enzymes, damaged epithelial cells and mucus, and thus becomes the source of energy for colonic flora on fermentation. This in turn produces a gamut of metabolites which become determinants in restoring health and/or disease. Alterations of colonic flora (from obese and lean mice) produce different metabolites and results pathophysiology (Bermon et al., 2015). A large number of metabolites are produced. Among them, SCFA, long chain fatty acids (LCFA), branched chain fatty acids (BCFA), CLA, conjugated linolenic acid (CLNA), amines, vitamins, phenolic compounds, flavonoids, and with metabolites xenobiotic properties are very important (Hawrelak and Myers, 2004; Festi et al., 2014; Carding et al., 2015). Increased amount of SCFA (propionate) production was found evident in obesity, due to alteration of gut microbiota (Schwiertz et al., 2010). Diet intake influences the composition of gut flora and health conditions. Azoxymethane-induced mice undergo dysbiosis and develop colon cancer. However, intervention of our probiotic P. pentosaceus GS4 reinstates the colonic flora and enhances the metabolite CLA production to mitigate carcinogenesis (Dubey et al., 2015, 2016). Alteration in gut microbiota composition was evident among Type2 diabetic subjects where member bacteria, Bacteriodes and Prevotella, were in higher amounts than Firmicutes and Clostridia (Larsen et al., 2010).
6 Dysbiosis and Inflammatory Diseases
Currently a large number of diseases are associated with dysbiosis. Alteration in microbial composition sets the foundation of pathophysiological changes in humans. The condition which governs the abundance of Firmicutes (Escherichia coli) and Bacteriodes (Bacterioides fragilis) also rules the availability of abundant lipopolysaccharides (LPS) and other cellular-structural fragments like lipid A, peptidoglycans (PG), which are part of PAMPs. Abundance of gram negative bacteria and LPS showed a link to a high fat diet, high rate of bile acid secretion resulting damage to the gut barrier, leakage and LPS-mediated endotoxicity followed by inflammation of the gut (Cani et al., 2008). The hypothesis was validated experimentally in a mouse model inducing type 2 diabetes and obesity (Hakansson and Molin, 2011). The interaction of LPS with colonic macrophages (CD14) and TLR 4 triggers the release of proinflammatory cytokines like IL-6, IL-1, and TNF-α. Additionally, cellular fragments of gram positive bacteria like lipoteichoic acid (LTA) can bind to CD14 mediated TLR2, and induce IEC to release proinflammatory cytokines but reduced IL-12 and IFNγ (Hermann et al., 2002).
Inflammation at the gut refers to diseases like IBD which includes UC and CD. It is characterized by chronic relapsing inflammation afflicting the intestinal mucosa (Hawrelak and Myers, 2004). UC is mostly restricted to the colon and rectum while CD may happen in any part of the alimentary canal; however severity is observed in the terminal ileum. Both are different diseases and have separate presentations and pathophysiology. These two constitute IBD, which is majorly a result of dysbiosis (Mukhopadhyay et al., 2012) and breach of the intestinal barrier (Maloy and Powrie, 2011). In particular, transmembranous tight junction proteins, claudin 2, is upregulated while claudin 5 and claudin 8 are downregulated, resulting in breach of gut epithelia. Along with claudins, dysregulations of defensins are also involved in the process of dysbiosis (Peterson et al., 2014). Alteration in microbiota like decrease in members of Firmicutes and corresponding increase in Bacteroidetes and Enterobacterioceae are evidence of IBD (Hansen et al., 2010). In a separate study, it was shown that T-bet deficient transgenic mice had an altered microbiota. Transcription factor T-bet deficiency causes the development of colitogenic mice and fails to develop Th1. Interestingly, microbiota of these mice also induced inflammation in recipient wild mice, proving that dysbiosis is directly linked with inflammation at gut (Garrett et al., 2007).
Besides, IBS (irritable bowel syndrome) (Gophna et al., 2006), GERD (gastroesophageal reflux disease) (Frank et al., 2007), celiac disease (Nadal et al., 2007), SIBO (small intestinal bacterial overgrowth), C. difficile-associated disease (Lozupone et al., 2012), atopy and asthma (de los Angeles et al., 2007; Wagner et al., 2008; Mikami et al., 2009), autism, schizophrenia (Lyte, 2014), autoimmune disorders (Vaahtovuo et al., 2008; Roesch et al., 2009), and colon cancer (Scanlan et al., 2008; Dubey and Ghosh, 2013; Dubey et al., 2016) are also in association with dysbiosis. Dysbiosis leads to an increased risk of neoplastic transformation which is found to be directly related to chronic inflammation of the gut and the development of colon cancer (Greer and O’Keefe, 2011). It can be metaphorically described as “holes in a pot of water”. Aside from these, several other lifestyle diseases are directly or indirectly involved in developing obesity: T1D, T2D, hypertension, and cardiovascular diseases respectively (Backhed et al., 2007; Olefsky and Glass, 2010; Cani and Delzenne, 2011). The imbalance in the constitution of flora leads to leaky gut formation due to disruption of the gut barrier, damage in tight junctions and inflow of foreign antigens, proteins (non self) and formation of antibodies (Hänsch, 2012). Many foreign proteins are homologous to self-proteins (molecular mimicry) of the thyroid and pancreas, which result attacks on its own protein due to molecular mimicry by formed antibodies, a reaction that leads to autoimmune diseases such as Hashimoto’s thyroiditis and T1D (type1diabetes) (Wen et al., 2008).
7 What Makes Probiotic Special for Reducing Inflammation in the Gut?
Probiotics dispense beneficial effects to host to reinstate the intestinal disturbances stemming from changes in gut flora (dysbiosis) and the release of various metabolites. The potential for this is strain specific. In general, it is not only the live strains, but also the dead cellular components of probiotic strains which modulate the health (Adams, 2010). Several surface active compounds like surface layer protein (SLP), lipoteichoic acid (LTA), lipopolysaccharide (LPS), heat killed antigen (HKA)(Adams, 2010); molecular metabolites like bacteriocin, vitamins, short chain fatty acids (SCFA), long chain fatty acids (LCFAs)(Mishra et al., 2016), production of antihistamine (Dev et al., 2008), γ-aminobutyric acid (GABA) (Selhub et al., 2014), citrullination (Chirivi et al., 2013; Daliri and Lee, 2015); biohydrogenation property like to produce conjugated linoleic acid (CLA) (Dubey et al., 2012) or conjugated linolenic acid (CLNA) with properties like antioxidant (Gowri and Ghosh, 2013), cholesterol assimilation (Daliri and Lee, 2015); production of β-galactosidase; immunomodulation (Bermon et al., 2015); pathogen suppression (Adams, 2010), antagonism (competition for food and space) and many other components, give strains probiotic potential (Fig. 2). Probiotics either heat-killed Enterococcus faecalis FK-23 or caused dead Bifidobacteria-induced immune response in animal studies (Adams, 2010). EC-12, a commercially available dietary probiotic formula composed of heat-killed E. faecalis could stimulate GALT and combat vancomycin-resistant enterococci in chick models (Sakai et al., 2006). Likewise, live and dead cells of gram-positive probiotic strains (L. casei, L. helveticus) were able to stimulate IL-6 production on intervention in murine intestinal epithelial cells (Vinderola et al., 2005). It has also been demonstrated that heat-killed, whole cell lysate, and SLP preparations of the probiotic strain GS4 stimulated the adaptive immune system and produced likely antibodies (Ghosh and Dubey, 2014). In a separate study, Matsuguchi et al. (2003) showed that a preparation of six-heat killed Lactobacillus strains were stimulated to secret proinflammatory TNF-α in mouse spleenocytes with markedly different stimulations among strains (Matsuguchi et al., 2003). Growth of fish cell line (SAF-1) cultivated in the presence of cytoplasmic extracts of probiotic strains (L.delbrueckii subsp. lactis) was inhibited, indicating induction of apoptosis (Salinas et al., 2008). Viable cytoplasmic extracts or/and nonviable probiotic strains were demonstrated to induce immune response and maintain homeostasis.
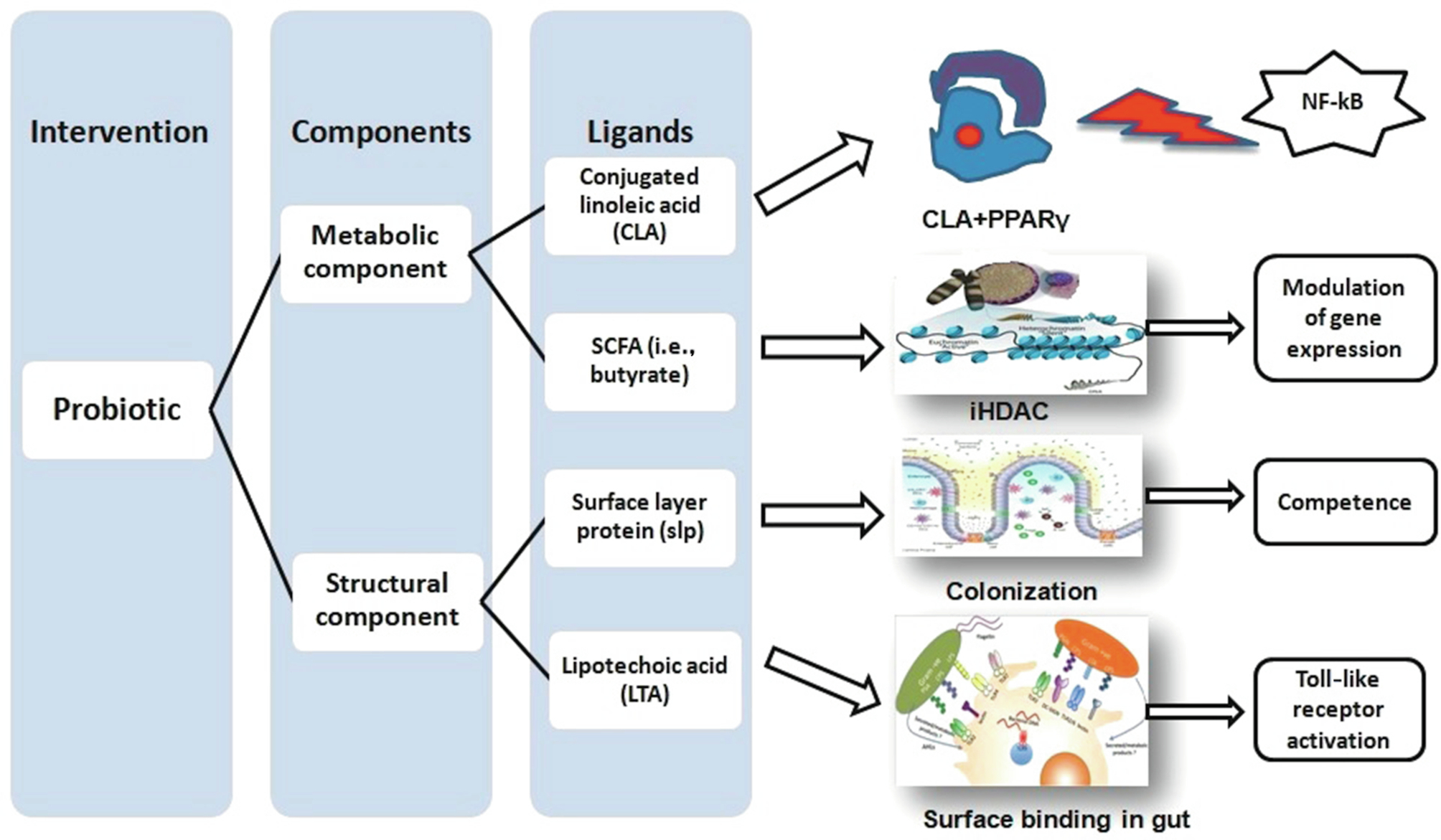
8 How Do Probiotics Regulate Inflammation?
Probiotics regulate inflammation in a host by several mechanisms following different pathways. The primary goal of probiotics is to enhance epithelial barrier function (Madsen, 2012). Some probiotic strains can stimulate intestinal epithelial cell (IEC) signaling pathways such as (1) inhibition of NF-kB activity, (2) alteration of MAPK and ERK pathways, (3) activation of PI3K and Akt, and (4) PPARγ dependent pathway, respectively. Some probiotic strains participate in proteasome function (Thomas and Versalovic, 2010), others in expression of cytoskeleton anchoring proteins (Anderson et al., 2010), some can produce cytoprotective heat shock proteins to fortify gut barrier function, and other strains initiate TLRs-PAMPs interactions to influence activation of DCs and Th1 (Daliri and Lee, 2015).
SCFA is the result of probiotic metabolism of resistant starch and undigested polysaccharides in the distal intestine which humans have difficulty digesting, in order to harvest energy for colonic cell health. SCFA butyrate is very important as it has antiinflammatory and anti-cancer activity (Greer and O’Keefe, 2011). Additionally, it inhibits angiogenesis, induces apoptosis, regulates transcriptional upregulation to detoxify enzymes, and activates glutathione-S-transferase (Scharlau et al., 2009). SCFA (propionate and acetate) are natural ligands for GPR (G-protein-coupled receptor) 41 and GPR43, respectively, which are widely expressed in the distal intestine, colon, and adipocytes (Xiong et al., 2004; Maslowski et al., 2009; Ang and Ding, 2016). These signaling molecules bind to respective receptors and regulate inflammatory pathways by decreasing the inflammatory response. SCFA also participates in antiinflammatory mechanisms by modulating intracellular levels of calcium in neutrophils; inducing immune cells in inhibiting the expression of adhesion molecules, and chemokine production which in turn suppressed the recruitment of macrophages and neutrophils (Bermon et al., 2015). Again, SCFA (butyrate) acts as an inhibitor of histone deacetylases (HDI) and therefore can affect gene expression, arrest growth, induce antiinflammation and apoptosis (Carding et al., 2015).
PSA (polysaccharide A) is elicited by some probiotic strains of human origin, like B. fragilis, and has the potential to induce IL-10 expression from regulatory Foxp3 + CD4 + T cells (van Baarlen et al., 2013). The strain GS4 (P. pentosaceus) has the ability to biohydrogenate linoleic acid (LA) to CLA. Besides substrate LA, it can utilize sesame oil to produce CLA (Dubey et al., 2012, 2015). Furthermore, the strain was able to modulate the dysbiosis afflicted in mouse models due to azoxy-methane induction, and participated in reinstating the gut microbiota (Dubey et al., 2015). Experimental evidence showed that GS4 could also mitigate the inflammation, regulate transcriptional up-regulation to detoxify enzymes and induce apoptosis (Dubey et al., 2015, 2016). CLA was determined to be a potential probiotic compound for controlling inflammation of the gut. CLA, it being a natural ligand for PPARγ, arrests the NF-kB pathway and thus stops or modulates inflammation (unpublished data). Bassaganya-Riera et al. (2012) demonstrated that probiotic bacteria with CLA producing potential suppressed DSS-induced colitis targeting macrophage PPARγ.
Probiotic strain L. salivarius LS33 has peptidoglycan that was determined to be protective against colitis in mouse models via NLR-peptidoglycan interaction (Fernandez et al., 2011). Strain specific LTA and cell wall bound LTA are also part of MAMPs which mediated immune response via MAMPs-PRRs interaction (van Baarlen et al., 2013). Surface layer proteins (SLP) of probiotic strains offer an additive property for inhibition of pathogenic strains for adherence to enterocytes and nutritional translocation. The SLP-minus strains of L. helveticus fb213, L. acidophilus fb116, L. acidophilus fb214 showed a decreased degree of cell adherence to HT29 (Meng et al., 2014). The P. pentosaceus GS4 strain also possesses SLP of 98 kDa and shows adherence ability to HCT 116. Removal of SLP shows much reduced level of adherence (Dubey and Ghosh, 2011; Ghosh and Dubey, 2014). It therefore has important role to play in the cell biology and immunology (unpublished data). Similarly, strain specific potential like pilin, of L. rhamnosus GG (LGG), binds well with mucosa and modulated IL-8 induction in Caco2 cells. The strain has potential to elicit protein molecules, like p75 and p40, which has demonstrated protection against colitis in mouse model (van Baarlen et al., 2013).
Probiotics regulate the immunity and inflammatory genes in the gut (Plaza-Diaz et al., 2014). Probiotics and commensals interact with immune cells in the gut, including IEC, M cells, dendritic cells (DC), macrophages, T and B cells, respectively. Experimental evidence proves that strain specific probiotic has potential to reduce the inflammation of the gut. In particular, the influence of probiotics leads to antiinflammatory response in cultured cell lines (HT29, HCT116, Caco-2) by the gene expression of mucin, TLRs, NF-kB, interleukins, and caspases. Surface active antigens also induced downregulation of proinflammatory and upregulation of antiinflammatory genes in vitro experimental conditions (Plaza-Diaz et al., 2014). Protective and beneficial roles played by probiotics, with the modulation of immunity and inflammatory gene expression, have been extensively reviewed elsewhere (van Baarlen et al., 2013; Plaza-Diaz et al., 2014). Beyond targeted metabolites, surface active compounds and genes, recent research reveals many more untapped metabolites of commensals and probiotics are also participating to impact the health status of a host (Pirhaji et al., 2016).
9 Use of Probiotics and Consequences
9.1 Inflammatory Diseases
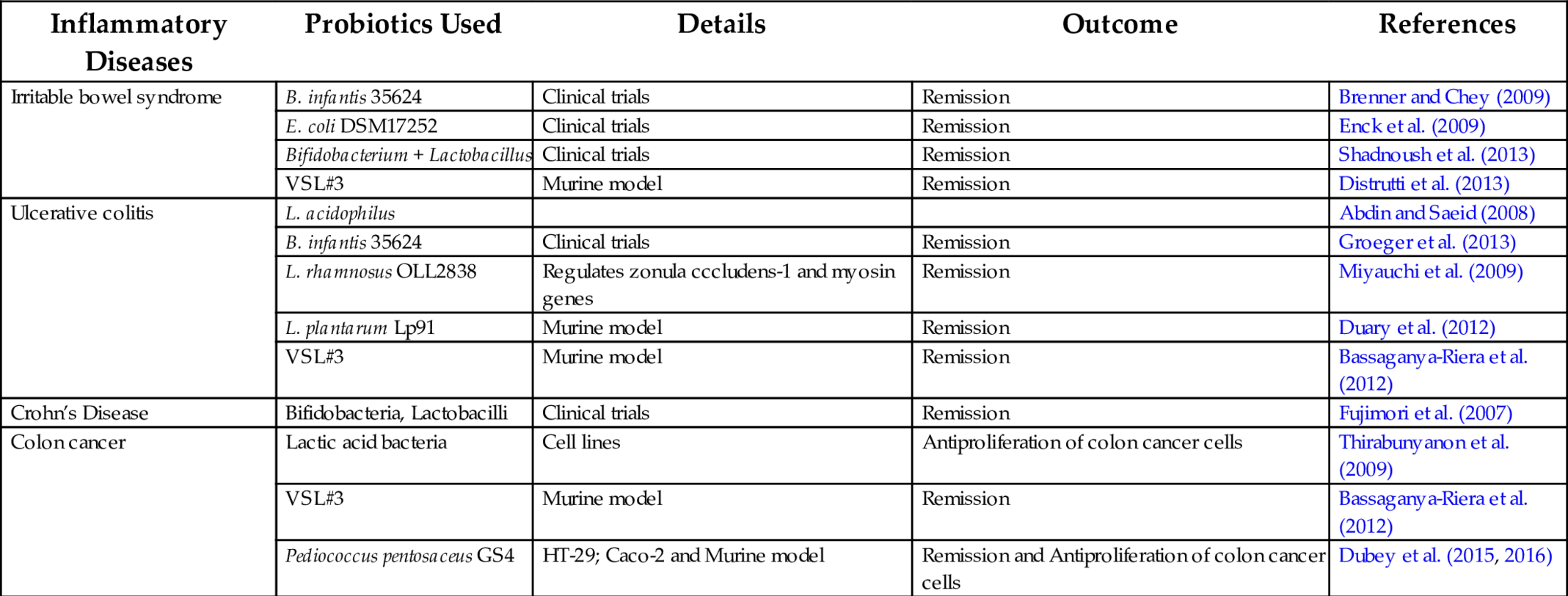
Irritable bowel syndrome: Irritable Bowel Syndrome (IBS) is the most common functional gastrointestinal disorder with a reported prevalence in the general population between 6% and 46% (Wikipedia, https://en.wikipedia.org/wiki/Irritable_bowel_syndrome#Epidemiology). IBS is characterized by a collection of functional gastrointestinal symptoms such abdominal pain, defecatory frequency, and/or constipation. The etiology of IBS is still not clear and numerous factors are involved in the damage to the mucosa, including microorganisms, psychological factors, and dietary habits (Chey et al., 2015). In addition, the gut-associated immune system is upregulated as evidenced by increased inflammatory cytokines such as IL-1, IL-6, and IL-10 (Strober and Fuss, 2011). The upregulated gastrointestinal associated immune tissue is known to stimulate discharge of enterochromaffin cells and other cells, which release serotonin and/or histamine resulting in GI symptoms. The type of colonizing microflora may play an important role in regulating immunity (Swidsinski et al., 2005). IBS patients host an intestinal microflora containing few Lactobacilli and a lowered Bifidobacteria fecal concentration. In normal conditions, an immunologic tolerance is maintained with respect to the commensal enteric bacteria, which prevents intestinal inflammation. This controlled homeostatic response is lost in susceptible individuals who then develop a chronic aggressive cellular immune response at the intestinal level. Oral administration of LGG in patients with Crohn’s disease resulted in the promotion of intestinal IgA immune response, as well as reduction in the pain and severity in many inpatients (Lescheid, 2014). The positive role of probiotics in IBS therapy has been established, but the beneficial effect of bacterial supplementation as an adjunct to treatment is an emerging trend (Swidsinski et al., 2005; Wang et al., 2007) (Table 2).
Table 2
| Inflammatory Diseases | Probiotics Used | Details | Outcome | References |
|---|---|---|---|---|
| Irritable bowel syndrome | B. infantis 35624 | Clinical trials | Remission | Brenner and Chey (2009) |
| E. coli DSM17252 | Clinical trials | Remission | Enck et al. (2009) | |
| Bifidobacterium + Lactobacillus | Clinical trials | Remission | Shadnoush et al. (2013) | |
| VSL#3 | Murine model | Remission | Distrutti et al. (2013) | |
| Ulcerative colitis | L. acidophilus | Abdin and Saeid (2008) | ||
| B. infantis 35624 | Clinical trials | Remission | Groeger et al. (2013) | |
| L. rhamnosus OLL2838 | Regulates zonula cccludens-1 and myosin genes | Remission | Miyauchi et al. (2009) | |
| L. plantarum Lp91 | Murine model | Remission | Duary et al. (2012) | |
| VSL#3 | Murine model | Remission | Bassaganya-Riera et al. (2012) | |
| Crohn’s Disease | Bifidobacteria, Lactobacilli | Clinical trials | Remission | Fujimori et al. (2007) |
| Colon cancer | Lactic acid bacteria | Cell lines | Antiproliferation of colon cancer cells | Thirabunyanon et al. (2009) |
| VSL#3 | Murine model | Remission | Bassaganya-Riera et al. (2012) | |
| Pediococcus pentosaceus GS4 | HT-29; Caco-2 and Murine model | Remission and Antiproliferation of colon cancer cells | Dubey et al. (2015, 2016) |
Irritable bowel diseases (IBD): Probiotics used to treat patients suffering from IBD (LP9-Brenner and Chey, 2009). However, only two probiotic formulae, VSL#3 and E. coli strain Nissle 1917, are in use with support from experimental evidence through multiple clinical trials (Hormannsperger and Haller, 2010). VSL#3 is a mixture of eight probiotic strains (L. acidophilus, Lactobacillus bulgaricus, L. casei, Lactobacillus plantarum, Bifidobacterium breve, Bifidobacterium infantis, B. longum, and S. thermophilus), however, convincing probiotic potential has yet to be established though a large number experimental and clinical studies (Hormannsperger and Haller, 2010). E. coli Nissle 1917 showed defense against EHEC O157 infection (Zyrek et al., 2007) and reduced DSS-induced colitis in gnotobiotic mice (Ukena et al., 2007). However, no proper mechanism was demonstrated in either case. Involvement of cell wall component, LPS-TLR2, was explained to demonstrate apoptosis in IECs with the liberation of heat shock proteins (hsp 25 and hsp 70) (Hormannsperger and Haller, 2010) (Table 2).
Diabetes and obesity: A large number of research based reports indicate a distinct link between diabetes and obesity (Festi et al., 2014). These pathogenecities are the outcome of metabolic disorder due to dysbiosis (Turnbaugh et al., 2006). Fat enriched diet with increased lipoproteins like chylomicron, low density lipoprotein (LDL), very low density lipoprotein (VLDL), and intermediate densitylipoprotein (IDL) modifies the intestinal microbiota and cause metabolic disorders which initiate inflammation, insulin resistance and type II diabetes (Amar et al., 2011). These lipoproteins enable transport of cholesterol and triglycerides within the blood stream. Absorption of cholesterol from the intestine has been reduced by improving the intestinal microflora via administration of probiotics (Cani et al., 2007; Huey-Shi et al., 2009; Round and Mazmanian, 2009; Larsen et al., 2010). Amar et al. (2011) illustrated in vivo animal modeling and showed that TNFα continuously released in the adipose tissue during obesity, to activate protein kinase C and to increase the phosphorylation of the insulin receptor substrate on serine residue such as ser-307, leading to inactivation insulin signaling molecule and hence, to insulin resistance. Efficacy of probiotics in reducing serum cholesterol levels demonstrated in vivo models which subsequently improved insulin resistance.
Colon cancer: Colorectal cancer is one of the leading causes of cancer morbidity and mortality in many countries (Dubey and Ghosh, 2012) and it is thought that chronic inflammation (Dubey and Ghosh, 2013) is caused by an interaction between dietary factors and genetic predisposition (Klein et al., 2013; Festi et al., 2014). Epidemiological studies have shown that consumption of fermented milk products containing probiotic bacteria help to reduce the risk of cancer at a number of sites. L. acidophilus, L. casei Shirota strain and LGG have been shown to have inhibitory properties on chemically induced tumors in animals (Festi et al., 2014) (Table 2). There is some evidence that probiotic P. pentosaceus GS4 can interfere with various stages of the azoxymethane induced colon cancer process in experimental mouse models, such as degenerative effects on secondary organs like liver, kidney, and intestine (Dubey et al., 2015),with the prevention of DNA damage in the colon by live bacteria, suppression of preneoplastic changes in the colon and suppression of colon tumors in animals (Dubey et al., 2016). Preliminary studies on the effect of probiotic consumption in the control of cancer show promise.
Rheumatoid arthritis: Probiotics are now in use to control rheumatoid arthritis. Remission of symptoms of RA has been demonstrated in mouse models using Lactobacillus salivarius, possibly resulting in the decrease of IL-10, TNF-α and an increase in TGF-β levels (Sheil et al., 2004). In a randomized double-blind, placebo controlled clinical trial, Alipour et al. (2014) showed L. casei to be effective as a supplement in the treatment of 22 patients with rheumatoid arthritis who received daily 1 capsule containing 108 of colony forming units for 8 weeks. This treatment brought down high sensitivity C-reactive protein, tender and swollen joints and inflammatory cytokines among treated patients. A similar experiment was carried out by de los Angeles et al. (2011) using L. rhamnosus GR-1 and L. reureri RC-14 to treat 15 RA patients. Patients received a capsule daily for 3 months. Results revealed the impressive effect of probiotic use in the alleviation of RA. The health assessment scores were more improved than the 14 patients who received a placebo. However, there is little unanimity in the use of probiotics in the treatment of RA and although they are regarded as safe; may pose a threat to the health of immunocompromised patients. A review of various studies reveals that Lactobacillus GG is the most suitable and safe probiotic as it has undergone the highest number of extensive safety evaluation experiments, both in vitro and in vivo (Snydman, 2008).
HIV: Results of the “Probio-HIV” clinical trial published in PLOS One (d’Ettorre et al., 2015) reveals the fact that probiotic intervention reduces inflammatory consequences by improving GI tract immunity among HIV-afflicted individuals. Patients received combined antiretroviral therapy with probiotic supplements. Probiotic intervention clearly demonstrated a statistically significant reduction in the activation of CD4 + T lymphocytes with a reduction in the expression of marker proteins such as lipopolysaccharide binding protein and high sensitivity-C reactive protein (d’Ettorre et al., 2015).
Mental health: Some probiotic strains (L. helveticus and B. longum) have the potential to produce serotonin and influence human mental health (Maynard et al., 2012; Lyte, 2014). Resistance to insulin leads to T2D, and also causes an increased risk of depression epidemiologically (Nicolau and Masmiquel, 2013). Psychological stress may increase the permeability of the gut barrier (Selhub et al., 2014). A clinical trial demonstrated that oral probiotic intervention reduced anxiety, mental stress, and improved positive attitude (Bested et al., 2013).
Besides the implementation of probiotics in certain diseases to mitigate the inflammation, the used of probiotic functionalities may be credited with the following activities beneficial to health:
9.2 Suppression of Histamine Signaling
Some probiotics have the ability to suppress histamine signaling, and are therefore important in the treatment of allergic diseases (Dev et al., 2008). Different strains of Bifidobacterium spp. (B. infantis and B. longum) showed efficacy in the treatment of nasal allergy in induced animal models. The experiment demonstrated the suppression of allergy biomarkers (histamine H1 receptor, histidine decarboxylase (HDC) mRNA expression, HDC activity, and histamine content) with the dosage of 40 mg/rat for a period of 4 weeks.
9.3 Reduction of Appetite and Glucose Uptake
Falcinelli et al. (2015) reported that probiotic treatment reduces appetite and glucose levels to control obesity and T1D. Appetite is controlled by many compounds including hormone leptin, and is produced mainly by adipose tissues. An experiment exposed zebrafish larvae to L. rhamnosus for 8 days. Results demonstrated a modulation in the composition of gut microbiota, with a direct relationship to upregulation of genes related to reduction of glucose uptake and appetite (Falcinelli et al., 2015).
9.4 Repair of Damaged Epithelial Barrier
The most important anti-inflammatory property offered by several probiotics is their potential in maintaining the intactness of the gut-epithelial barrier (Rao and Samak, 2013). A number of studies, including animal models and clinical trials, demonstrated the damaged epithelial repairing roles of probiotics under diseased conditions (Khailova et al., 2013; Sindhu et al., 2014; Dubey et al., 2015). In all these experiments, major probiotic species from the genus Lactobacillus (L. rhamnosus GG, L. plantarum DSM 9834, L. reuteri R2LC, L. paracasei NCC2461), P. pentosaceus GS4, and B. infantis, Saccharomyces boulardii, Saccharomyces cerevisiae UFMG 905, and E.coli Nissle 1917 were used (Lescheid, 2014).
9.5 Antimicrobial Peptides and Antagonism
Similar to restoring of the damaged gut-epithelial barrier, production of antimicrobial peptides (AMP) such as bacteriocins, nicin (Thomas and Versalovic, 2010) helps to foster innate immunity (Lescheid, 2014) to antagonizing pathogenic strains (Gowri and Ghosh, 2010a). Some probiotic strains (VSL#3) induce host epithelial cells to release AMP like β-defensin-2 involving NF-kB, AP-1, and MAPK pathways (Schlee et al., 2008).
10 Conclusion
Disruption in the composition of microbiota has been found to be a key factor in the development of diseases in a host, causing local inflammation. Probiotics, either live or dead, have gained convincing experimental results to modulate the microbial composition of the gut to restore lost flora diversity and to mitigate inflammation and consequences (Lebeer et al., 2010). One of the newly evolved therapeutic approaches against different inflammatory diseases is probiotic micro-flora-mediated therapy. Probiotics are an important component of functional foods. They can modulate gut microflora and maintain homeostasis in the GI tract; and contribute in normalization of intestinal colonization. Probiotics are beneficial whether used as single or with multiple strains however, beneficial effects generally depend on specific strains.
Probiotics collectively demonstrate potential to mitigate or eliminate inflammation in the gut by interacting with IECs, expressing related genes and augmenting GALT. Probiotics, both alive and dead, exert beneficial effects to host. This is an important feature due to the fact that live probiotics faces several pharmacological difficulties. One study shows that removal of LTA from potential probiotics may enhance the antiinflammatory activity (Lebeer et al., 2010). Therefore, genetically modified probiotics (GMP) may be used to control inflammation of the gut in the future. Chronic inflammation causes disturbance to normal behavior and mental health. Besides reducing the inflammation of the gut, probiotics will also offer solutions in managing psychological well-being with the release of chemical neurotransmitters (Lyte, 2014).
The total inflammatory network developed due to dysbiosis is the root cause of several syndromes and diseases. Again, dysbiosis is the result of alterations of microbiome and is influenced by both internal and external conditions. Results obtained from several clinical trials provide confidence for the use of probiotics as a living drug for the alleviation of inflammation in the gut. The potential use of probiotics, with prebiotics, is so expansive that they will become a panacea for many diseases in near future.