Probiotics as an Adjunct to Conventional Treatment in Vulvovaginitis: Past, Present, and Future
Princy L. Palatty⁎; Poornima R. Bhat⁎; Ramakrishna P. Jekrabettu⁎; Thomas George⁎; Sueallen D’souza⁎; Soniya Abraham⁎; Mohammed Adnan⁎; Michael Pais⁎; Taresh Naik⁎; Devika Gunasheela†; Manjeshwar S. Baliga⁎ ⁎ Father Muller Medical College, Mangalore, India
† Gunasheela Infertility Hospital, Bangalore, India
Abstract
The reproductive health of a woman is vital not only for her general health, but also for that of her partner and child. In this regard, the vaginal microbiota, which is made up of predominantly Lactobacillus community, plays an important role in the maintenance of a woman’s health. When this vaginal microbiotic ecosystem is disrupted or decreased and replaced by pathogenic microbes, it sequentially and progressively leads to vaginosis, which, in severe cases, may lead to obstetrical complications such as urinary tract infections, the enhancement of sexually transmitted infections, fertility issues, chorioamnionitis, and preterm delivery. Recent reports indicate that exogenous strains of probiotics to be useful in reestablishing a normal healthy vaginal flora and through judicious selection and delivery of probiotic strains to mitigate and eliminate the vaginosis. This review addresses the recent developments in the field and as well as evident gaps in present understanding and knowledge. Through meticulous research, these gaps can be bridged for better scientific understanding and human/patient benefit.
Keywords
Vulvovaginitis; Probiotics; White discharge per vagina; Vaginal pruritis; Clindamycin
1 Introduction
Women’s health consists of physical, psychological, and social challenges which are considerably different from those of men of the same age group. There are various factors affecting women’s health through out their lives such as genetic susceptibility to illness and disease, varying hormone levels, environmental exposures, physiological variations with time, gender specific social, and other conditions (Senie, 2015). Of all ailments bothering women, the urogenital health is one of the most neglected aspects in traditional communities. This innocuous disease affects more than one billion women worldwide annually and many patients suffering from these ailments have scant resources or knowledge for maintaining their health. In addition to this, urogenital health may have effects on child birth and quality of life. The VV has a negative impact on woman’s personal confidence and self-worth by causing psychosexual problems like dysparenunia, a sense of shame, and unworthiness in informing either doctors or the partner (Mastromarino et al., 2013). In lieu of these observations, reproductive tract infections have become a major concern in public health worldwide and studies aim to remedy them (Reid et al., 2001a,b; WHO, 2003).
2 Anatomy of Female Genital System
The female genital system can be divided into External Genital Organs and Internal Genital Organs (Dutta and Konar, 2014). The external genitalia is also known as vulva or pudendum, and is externally visible. They consist of mons veneris (mons pubis), labia minora and majora, hymen, clitoris, vestibule, urethra, Skene glands, greater vestibular (Bartholin) glands, vestibular bulbs, and conventional perineum (Dutta and Konar, 2014). Internal genitalia include vagina, cervix, uterus, fallopian tubes, and ovaries. They are placed internally in the body and special instruments are needed for examination (Dutta and Konar, 2014).
2.1 External Genitalia
Mons Pubis: It is a pad of subcutaneous adipose connective tissue which is anterio superior to pubic symphysis. This area will be covered by hair in adults (Dutta and Konar, 2014).
Labia majora: Vulva on either side is bound by two longitudinal folds or elevations composed of subcutaneous adipose tissue and fat called Labia majora. The size is highly variable in individuals. On the surface they are made up of skin consisting of squamous epithelium, hair follicles, sebaceous glands, and sweat glands. Beneath the skin they contain adipose tissue and connective tissue with rich venous plexus. They are homologous to the scrotum in males. They begin anteriorly from the mons and meet medially in front of the anus to form Posterior Commisure. The inner side of labia majora lacks hair follicles (Dutta and Konar, 2014).
Labia minora: On the either side within the labia majora, two folds of thick skin, devoid of hair follicles are present called labia minora or nymphae. They are made up of skin, connective tissues, sebaceous glands, erectile muscle fibers, nerve endings, and blood vessels. They are exposed only when the labia majora are separated, though this is not the case with parous women. In the upper part, the clitoris is enclosed by the labia minora in front and behind to form the prepuce and frenulum, respectively. The labia minora’s lower part fuses to the skin to form the fourchette which is a fold of skin in the midline. The fossa navicularis is present between the fourchette and vaginal orifice. The labia minora are similar to the ventral aspect of the penis in males (Dutta and Konar, 2014; Miranda, 2015).
Clitoris: It is a highly erectile organ located below the anterior joining of labia minora. It is homologous to the penis in males. It is different from penis in that the urethra in females is completely separate from clitoris (Dutta and Konar, 2014).
Vestibule: It is a triangular space bounded between clitoris, fourchette, and labia minora. It consists of four orifices.
Urethral meatus: It is made up of membranous connective tissue, connecting bladder and vestibule. It is located in the midline about 1 cm anterior to the vaginal orifice and 1–1.5 cm below the pubic arch. It gives rise to paraurethral or Skene’s glands, opening bilaterally (Dutta and Konar, 2014; Miranda, 2015).
Skene glands: They secrete lubrication to urethra, opening bilaterally into the posterior wall of urethral opening or vestibule directly.
Vaginal orifice: It is a posteriorly located space in the vestibule, differing in size. In nulliparous women it may be bounded by labia minora on either side, and covered by hymen. In parous women it may be exposed (Dutta and Konar, 2014; Berek, 2007; Miranda, 2015).
Hymen: Vaginal orifice is covered by a septum of mucus membrane called hymen, which varies in shape greatly. It will be usually cresentric or circular in virgins and is ruptured during sexual intercourse. During child birth it gets extremely lacerated to form cicaterized nodules which are of different sizes, forming carunculae myrtiformis on either side of the vaginal orifice (Dutta and Konar, 2014).
Bartholins glands: The greater vestibular or Bartholin’s gland are responsible for lubricating the vagina by their alkaline secretion during sexual excitement. They are located on the posterior part of vestibular bulb in the superficial perineal pouch and open on either side of the posterior part of the vestibule above the hymen (Dutta and Konar, 2014).
Vestibular bulbs: Beneath the mucous membrane of the vestibule, elongated erectile tissues are located bilaterally called vestibular bulbs. They are incorporated into the bulbocavernosus muscle on either side of the vaginal orifice in front of the bartholins gland (Dutta and Konar, 2014).
Perineum: It forms the pelvis floor and is formed by muscles and fascia (Berek, 2007).
2.2 Internal Genitalia
(A) Vagina: It is a hollow fibromuscular tube connecting the internal uterine cavity with the exterior vulva. It is located within the pelvis posterior, extending to the bladder and anterior to rectum. In a dorsal lithotomy position, the axis of the vagina is almost pointing toward the sacrum, whereas in an upright standing position it is horizontal. It forms an excretory channel for uterine secretions, namely menstrual blood. It serves as organ for copulation and as the birth canal during labor. In an erect position, the canal is directed upward and backward forming 45 degree angle with the horizontal plain. The long axis lies almost parallel to the pelvic inlet and at a 90 degree angle to uterus. The diameter of the canal is approximately 2.5 cm, being narrowest at the introitus and widest at the upper part; it has good distensible properties (Dutta and Konar, 2014; Berek, 2007; Miranda, 2015).
The vagina has four walls: anterior, posterior, and two lateral walls. The anterior and posterior walls are widely spaced, whereas the lateral walls are stiffer, especially in the center, thus forming an “H-”shaped structure in the transverse section. The space between the vaginal walls and impending uterine cervix forms fornices. The vaginal walls become blended with cervix, and the two are inseparable after the fornices. There are four fornix, one anterior, one posterior, and two lateral fornices. The anterior fornix is narrowest, the upper third is related to the bladder and the lower two-thirds relate to the urethra. The posterior fornices are deepest, related to the pouch of Douglas in the upper third, rectum in middle, and the anal canal and perineal body in the lower third. The lateral fornices are related to pelvic cellular tissue, ureter, and uterine artery in the upper third; levator ani in the middle; and bulbocavernosus muscle, vestibular bulbs, and bartholins glands in the lower third (Soccol et al., 2010; Senok et al., 2009; Dovnik et al., 2015; Mastromarino et al., 2013; Dutta and Konar, 2014). On the microscopic level, the vagina is composed of three layers (Dutta and Konar, 2014; Berek, 2007; Miranda, 2015; Magowan et al., 2009).
(a) Mucosa: Non stratified keratinised sqamous epithelium without glands. The mucosa has a pattern of transverse ridges called rugae. Lubrication mainly occurs by secretion of the bartholins gland and cervix by transudation. Mucosa is hormone sensitive. Estrogen leads to proliferation and maturation of cells. Mixed bacterial flora, with a predominance of lactobacilli, colonize the vaginal mucosa. Normal pH is slightly acidic 3.5–4.
(b) Muscularis: It consists of connective and smooth muscles packed loosely. They have two layers: the outer longitudinal muscle layer and inner circular muscle layer.
(c) Adventitia: It is an endopelvic fascia which gets attached to muscularis.
(B) Uterus: It is a hollow pyriform fibromuscular organ divided into the upper uterine body or corpus, middle isthmus, and lower cervix. It lies between the bladder and rectum, in the pelvic cavity.
(a) Uterine body: It consists of a globe-shaped fundus lying above the opening of the fallopian tubes, the body proper which is a triangular area and the cornua of uterus which lies superio lateral to the body and is attached to the uterine tube, round ligament, and the ligament of the ovary. Two lateral tube-like extensions of the uterus, one on either side, are called fallopian tubes; they terminate into the body with finger like projections called fimbria. The ovaries are located nearby, on either side of the fimbria.
(b) Isthmus: This is a constricted portion between the body and cervix. It is connected to anatomical internal os above and histological internal os below.
(c) Cervix: It is the lowest or bottommost portion of uterus, separating the body of uterus above, from the vagina below. It is cylindrical in shape and measures 2.5 cm in length and diameter. It extends from histological internal os to external os opening into the vagina. It consists of vaginal and supra vaginal parts. In nulliparous women, the external os looks conical whereas in parous women, it has a slit forming anterior and posterior lip. The body of the cervix is made up of outer serous perimetrium, middle thick bundles of musclular layers and connective tissue called myometrium, and an inner mucosal layer called endometrium. Microscopically, mucosa contains endocervical glands. The transformation zone between vagina and cervix called the portio vaginalis is sensitive to hormonal effects and is constantly irritated due to hormones, infections, and trauma. As a result, the portio vaginalis is more prone to in situ carcinoma. Mucosa of the cervix has endocervical glands producing limited watery secretions. These secretions are alkaline, with a pH of 7.8, and highly hormone sensitive. Under the influence of estrogen, they provide nutrition and facilitate the ascent of sperm, whereas under influence of progesterone, they prevent their entry. Cervical mucus forms the bulk of vaginal discharge, sometimes resulting in mucus plugs which block the cervical canal. They have bacteriolytic property.
3 Normal Flora of the Vagina
Microbiological flora of female genital tract is complex, dynamic, and not well understood. Efforts are being made to isolate the microbiome of the genital tract for many years (Lamont et al., 2011). The normal flora content of the female genital tract is dependent on various factors such as age, hormones, and pH in the genital tract of the host, to name a few (Davis, 1996). In newborns, the genital mucosa is sterile; they acquire commensals which are varied flora of nonpathogenic organisms from skin, genitalia, and the intestine. Then, under the influence of maternal estrogen, there will be glycogen deposition in vaginal cells and various bacilli may be seen (predominantly Lactobacilli or Deoderlin’) for the first month, similar to adult commensal flora. After one month of life, glycogen gets depleted and pH goes up to 7 until menarche. Vaginal flora at prepuberty age is dominated by diphtheroids, Staphylococcus epidermidis, streptococci, and Escherichia. coli and large varieties of other aerobic and anaerobic organisms. After menarche, under the influence of estrogen, cells get glycogen deposition and Lactobacillus becomes the predominant species yet again (Todar, 2015), along with, and in lesser numbers, other varied microbiota including Staphylococcus, Ureaplasma, Corynebacterium, Streptococcus, Peptostreptococcus, Gardenerella, Bacteroides, Mycoplasma, Enterococcus, Escherichia, Veillonella, Bifidobacterium, and Candida, to name a few. These normal flora are of immense help in maintaining normal vaginal health and prevention of disease. These resident flora commonly found at a given place normally are called as commensals. Transient organisms include those nonpathogens or potential pathogens which are present temporarily in the mucosa. Until normal resident flora is intact, transient flora is harmless; however when normal commensals are disturbed, transient flora can flourish, proliferate, and lead to disease (Kirmani, 1988). The flora can also be influenced by various other factors like local factors (temperature, moisture), immunity, heat and personal factors like time in the menstrual cycle, pregnancy, infections, methods of birth control, frequency of sex, number of sexual partners, as well as various habits and practices such as douching and previous use of antibiotics (Linhares et al., 2010). It is a highly complex and finely balanced ecosystem (Zhou et al., 2004).
4 Vulvovaginitis
Vulvovaginitis includes a spectrum of diseases resulting in vaginal or vulvar symptoms such as increased vaginal discharge, odor, itching, burning, and discomfort. Vulvovaginitis may be attributed to vaginal and vulvar tissue infection or inflammation and change in normal flora (Sobel, 2015a; Hainer and Gibson, 2011). In practice, vulvitis, vaginitis, and vulvovaginitis are terms used interchangeably to refer to lower genital tract infections in women (Joishy et al., 2005). The fact that it is the most common reason for women to seek physician advice in the United States indicates that its incidence is not affected by the socioeconomic demographics (Hainer and Gibson, 2011). The CDC estimates that in the USA, 40–45% vulvovaginitis cases are bacterial vaginosis, 20%–25% cases are vulvovaginal candidiasis and 15%–20% cases are trichomoniasis (CDC, 2006). In India, the incidence of bacterial vaginosis is 45%, vulvovaginal candidiasis 31%, trichomoniasis 2%, gonorrhea 3%, nonspecific urogenital causes 5%, and other causes 14% (Puri et al., 2003).
From an etiological perspective, infectious sources are the most common cause of vulvovaginitis followed by noninfectious sources. The incidence of noninfective vaginosis is less common and caused by myriad abiotic factors (enlisted in Table 1). The infective vaginosis is proved to be caused by pathogenic microbes and is classified as bacterial vaginosis when caused by bacteria like Prevotella sp., Mobiluncus sp., Group B streptococcus infection, Gardnerella vaginalis, Ureaplasma, Mycoplasma, and numerous others (Jahic et al., 2013). The fungal VV is commonly caused by Candida albicans, but could also be caused by the non C. albicans like Candida glabrata and others, though this is rare. The protozoal VV is caused by infection with Trichomonas vaginalis (TV) (CDC, 2010). Additionally, reports also indicate that 50% of all infective vaginosis are caused by bacteria, 50% cases by fungi and parasites and that polymicrobial vaginitis is also common (MHFW, 2007).
Table 1
| Type of VV | Etiology of VV |
|---|---|
| Bacterial vaginosis | Gardenerella Vaginalis, Mycoplasma hominis, anaerobic bacteria like Prevotella and Mobiluncus |
| Vulvovaginal candidiasis | Candida albicans, Candida krusei, Candida glabrata |
| Trichomoniasis | Trichomonas vaginalis |
| Atrophic vaginitis | Estrogen deficiency |
| Erosive lichen planus | Unknown |
| Irritant or allergic contact dermatitis | Contact irritation or allergic reaction |
From a pathogenesis perspective, reports indicate that the vaginal pH plays an important role in maintaining health of the vagina. Under the influence of estrogen, vaginal cells will have glycogen deposits from puberty to menopause. Normal vaginal flora is a complexly dynamic and balanced ecosystem which maintains the normal pH in vagina by converting glycogen into lactic acid. Thus acidic pH is maintained and the environment becomes unfavorable to pathogenic growth. Any of these etiological factors can disturb the estrogen levels, affect the vaginal pH, change normal vaginal flora, and disrupt the balance among the organisms. As a result pH changes and the environment becomes unfavorable to normal flora and favorable for pathogens to flourish. After menopause, atrophic changes may be seen in the vagina which can lead to vaginal dryness and other symptoms. Thus various physiological, physical, personal, medical, and other factors may have a role in disease and its progression (CDC, 2006). Patients with vulvovaginitis present with the following symptoms (Faro, 1993; Shivadas, 2010; Soper, 2015):
- (a) Changes in volume, odor, color, consistency of vaginal discharge
- (b) Itching in vulvar region or pruritis
- (c) Burning sensation in vagina
- (d) Irritation in vagina
- (e) Redness or erythema
- (f) Dysuria
- (g) Dyspareunia or painful sexual intercourse
- (h) Spotting/bleeding
- I Signs found on clinical examination
- (a) Redness of introitus or erythema
- (b) Excoriation of genital area
- (c) Vaginal discharge
- (d) Cervical erosions, if associated with cervicitis
- (e) Adenexal motion tenderness
Vulvovaginal candidiasis is often marked with intense inflammation including itching and soreness and thick white curdy odorless discharge. Vulval itching could be one of the associated symptoms in candidiasis. These symptoms could be more experienced during the premenstrual period (Sobel, 2015a). Bacterial vaginosis is associated with thin gray or yellow discharge, a musty or fishy odor (Puri et al., 2003), minimal inflammation, and comparatively less irritation. Generally speaking, bacterial vaginosis is never associated with vulvar itching (Anderson et al., 2004; Sobel, 2015a). Trichomoniasis is characterized by profuse thin malodorous, yellow to green discharge and may be associated with a burning sensation, intense itching, dysuria, and dyspareunia. These symptoms could be noted more during or after menstrual periods (Sobel, 2015a).
Vaginal dryness and dyspareunia may be signs of atrophic vaginitis (Shivadas, 2010). Some of the considerations for clinical examinations are the presence of cheesy discharge, itching, signs of inflammation like vulvar or vaginal oedema, excoriations, and fissures means an increased likelihood of candidiasis. Lack of fishy odor makes diagnosis of bacterial vaginosis unlikely. Lack of dyspareunia makes diagnosis of trichomoniasis unlikely (Anderson et al., 2004). These considerations may aid in diagnosing the probable cause, however none of these features of patient history or clinical examination may be conclusive in establishing a definitive diagnosis (Hainer and Gibson, 2011). Many times symptoms will be recurrent, especially in vulvovaginal candidiasis which is known for recurrence. It is estimated that > 50% of women above 25 years of age will have vulvovaginal candidiasis a some point in their lifetimes, and 5% of them will develop recurrent vulvovaginal candidiasis, characterized by having at least > 4 episodes of vulvovaginal candidiasis in 1 year, or > 3 episodes of vulvovaginitis candidiasis unrelated to antibiotic use in 1 year (Ringdahl, 2000).
Abnormal vaginal discharge (in terms of quantity, odor, color) is usually indicative of vaginal infections but, in a few cases, could indicate mucopurulent cervicitis, which could be caused by sexually transmitted infections such as Neisseria gonorrhoeae or Chlamydia trachomatis. Some of the clues leading to diagnosis of Chlamydial cervicitis are (a) age < 24 years, (b) sexual intercourse with new partner in past 2 months, (c) mucopurulent cervicitis, (d) cervical bleeding by swabbing the endocervical mucosa, (e) no contraceptives use (Egan and Lipsky, 2000). If more than two following signs are present in women with white discharge, the possibility of C. cervicitis could be considered. In such situations, cultures for Chlamydia species and N. gonorrhoeae should be done. Possibility of cervicitis in abnormal vaginal discharge could be considered, however vaginal discharge is a poor predictor of cervical infections/cervicitis (WHO, 2003). If abnormal vaginal discharge is associated with other symptoms like abdominal pain, fever, menometrorrhagia, dyspareunia, dysuria, nausea, vomiting, bleeding, and uterine tenderness on pelvic examination a with abnormal vaginal discharge, then the possibility of pelvic inflammatory diseases could be considered and relevant investigations should be done accordingly (WHO, 2003).
Patients suffering from BV are at an increased risk for the acquisition of the some STI’s like HIV, N. gonorrhoeae, C. trachomatis, HSV-2, complications after gynecological surgery, complications of pregnancy, and increased recurrence of BV. Similarly, TV has association with increased transmission of HIV. In pregnancy TV can be associated with preterm rupture of membranes and preterm delivery (CDC, 2010). Complications of VVC are rare but can still cause chorioaminionitis in pregnancy, vulvar vestibulitis, and have a persistent disease or common recurrence. The clinical symptoms and signs associated with different vaginosis are listed in Table 2.
Table 2
| Type | Discharge | Itching | Pain | Vagina | Vulva |
|---|---|---|---|---|---|
| Bacterial Vaginosis | Fishy odor, thin, white or gray colored, homogeneous discharge | May not be the primary complaint | May not be the primary complaint | Vaginal inflammation may be absent | Vulva may not be affected |
| Vulvovaginal Candidiasis | Odorless, cheesy, curdy, white, thick discharge | Itching is commonly associated | Burning sensation, dyspareunia, dysuria are commonly associated | Redness, fissures, cracks, signs of inflammation are common | Vulval excoriations are common |
| Trichomoniasis | Yellow to green froathy purulent discharge | May not be presenting symptom | Dyspareunia, dysuria and vaginal soreness are common | Signs of inflammation with strawberry like inflamed vagina | Vestibular redness may be present |
| Atrophic vaginitis | Yellow or white- gray, odorless thin discharge | Not commonly seen | Vaginal dryness, painful sexual intercourse may be present | Vaginal redness and easily traumatized | Thin and dry vestibule, loose labia majora with thin subcutaneous fat. Labia minora thin and friable. |
| Erosive lichen planus | Yellow or gray | Excessive itching may be present | Pain, dyspareunia postcoital bleeding may be present | Redness with friable epithelium | Erosions and plaques are common distinctive from others |
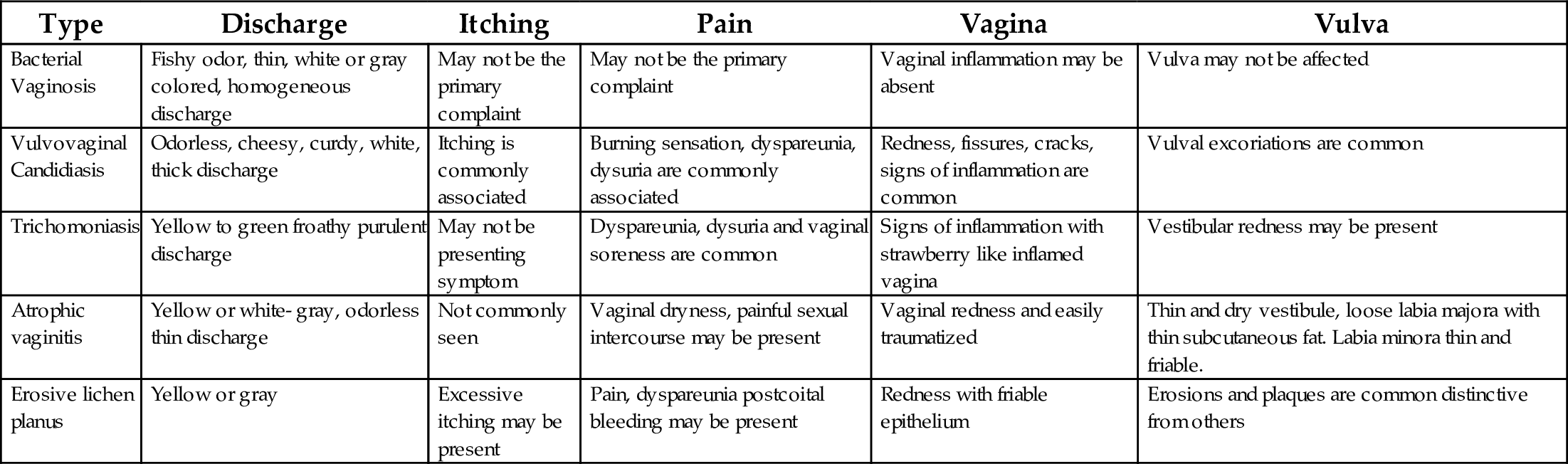
Table 3
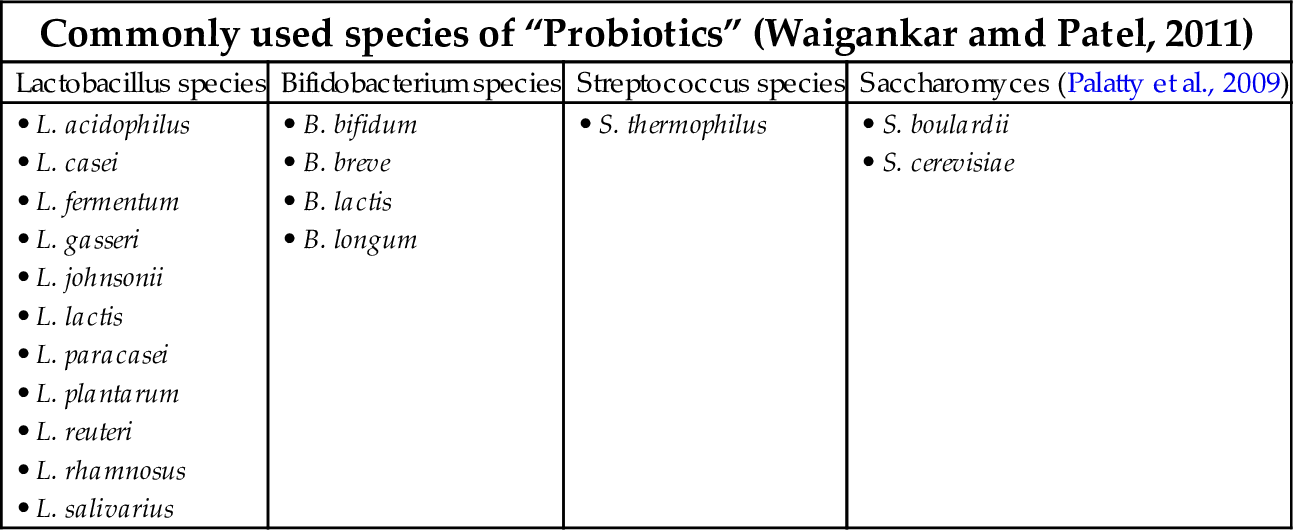
| Commonly used species of “Probiotics” (Waigankar amd Patel, 2011) | |||
|---|---|---|---|
| Lactobacillus species | Bifidobacterium species | Streptococcus species | Saccharomyces (Palatty et al., 2009) |
| • L. acidophilus • L. casei • L. fermentum • L. gasseri • L. johnsonii • L. lactis • L. paracasei • L. plantarum • L. reuteri • L. rhamnosus • L. salivarius | • B. bifidum • B. breve • B. lactis • B. longum | • S. thermophilus | • S. boulardii • S. cerevisiae |
5 Characteristic Features of Different Types of Vulvovaginitis
5.1 Bacterial Vaginosis
It is a polymicrobial clinical syndrome due to an imbalance between hydrogen peroxide producing lactobacilli species and anerobic organisms in the vagina by pathogenic organisms such as G. vaginalis, Mobiluncus species, Mycoplasma hominis, and Peptostreptococcus species and various other anerobes (Egan and Lipsky, 2000). It is estimated that BV is the most common cause of vulvovaginitis; one-third to one-quarter of affected women remain asymptomatic (Egan and Lipsky, 2000). High numbers of cases of BV are reported to sexually transmitted disease clinics but the role of sexual transmission of BV is still unclear. It also afflicts women who are not sexually active. Studies done to investigate the role of treating male partners for reducing recurrence have no benefits (CDC, 2010). Nonetheless, women with BV have an increased risk for sexually transmitted diseases like N. gonorrhoeae, C. trachomatis, HIV, HSV-2, etc. (CDC, 2010). Pregnancy, multiple sexual partners, douching, and use of an IUD pose significant risk for BV. BV in pregnancy can predispose the mother for premature rupture of membranes, preterm labor, and complications in pregnancy (Egan and Lipsky, 2000). BV can be recurrent and may require repeated treatments. The persistence of BV could also predispose the afflicted to complications after gynecological surgeries (Owen and Clenney, 2004). BV could be diagnosed with various clinical criteria like Amsel’s criteria and gram staining of vaginal discharge. Gram staining is considered the gold standard for diagnosing BV by identifying concentration of gram negative rod-like organisms characteristic of BV (CDC, 2010). Nugents scoring and Spiegel diagnostic criteria for identifying causative organisms in gram staining could be used in diagnosis of BV (Egan and Lipsky, 2000). The important criteria for clinical diagnosis consist of four points.
- 1. Patient should have homogeneous, thin, white vaginal discharge that smoothly coats the vaginal walls
- 2. Presence of clue cells on microscopic examination
- 3. pH of vaginal fluid > 4.5
- 4. A fishy or amine odor of vaginal discharge before or after addition of 10% KOH (positive whiff test)
Three out of four criteria must be met to establish accurate clinical diagnosis of bacterial vaginosis in 90% of affected women. Among the four previously listed diagnostic criteria, the presence of “clue cells’ is the most significant (Egan and Lipsky, 2000). Other tests include the DNA probe test for G. vaginalis. The prolineaminopeptidase card test and OSOM BV blue test are available. Polymerase chain reaction (PCR) for G. vaginalis could also be used for to achieve the highest certainty. Culture is not indicated for low specificity and pap smear has no clinical utility in diagnosis of BV.
5.2 Vulvovaginal Candidiasis
Vulvovaginal candidiasis is caused by C. albicans in 80%–90% of patients. Recently there has been an increased occurrence of C. glabrata and C. tropicalis, probably due to overuse of over the counter antifungal drugs (Horowitz et al., 1992). It is estimated that about 75% of women will experience VVC some time in their life and about 5% of them have recurrent VVC (Egan and Lipsky, 2000). More than 50% of asymptomatic women have candida as a part of normal vaginal flora, hence it is very difficult to establish candida as a definitive cause. Risk factors include use of contraceptive pills, intrauterine devices, spermicide, diaphragm, immunocompromise, diabetes, pregnancy, taking antibiotics, practicing oral sex, and first intercourse at a young age, to name a few. However, candidiasis is not known to be sexually transmitted, nor is it related to the number of sexual partners (Egan and Lipsky, 2000).
Complications of VVC are rare, though it can cause chorioamnionitis in pregnancy, vulvar vestibulitis, and be a persistent and recurrent disease. The latter is the most common. Recurrence is defined as more than four episodes in a 12-month period, and it is not clearly understood whether persistence of infection, precipitating factors, intestinal reservoir, or sexual transmission is responsible for the return of disease (Sobel, 1992; Egan and Lipsky, 2000). Non albican candida species like C. glabrata and others, which are not easily identified on microscopy, are known to cause recurrent vulvovaginal candidiasis (RVVC). VVC could also be classified as uncomplicated and complicated VVC, and includes recurrent VVC and severe VVC (CDC, 2010).
Clinical symptoms of white, curdy, thick, odorless vaginal discharge, pruritis vulvae, vaginal irritation, dysuria, and signs of vaginal inflammation like fissures, excoriation, redness, vulvar edema, and normal vaginal pH could help in clinical evaluation. Demonstration of hyphae, pseudohyphae, budding yeast cells, in saline/KOH wet mount slide and gram staining could help in diagnosis of candidiasis. When patients are symptomatic but hyphae in wet mount cannot be seen, culture in Nickerson’s medium or Sabouraud’s dextrose agar should be considered. If the patient is asymptomatic but the culture is positive, treatment should not be started. In RVVC, unusual species could be expected (Sobel, 2015c).
5.3 Trichomoniasis
Trichomoniasis is caused by protozoan T. vaginalis (Owen and Clenney, 2004). It accounts for about 10%–25% of incidence of vaginitis and is known to be the third most common cause of cases diagnosed. Use of intrauterine contraceptives, tobacco smoking, and multiple sexual partners are said to be the risk factors for trichomoniasis. Female patients with trichomoniasis present with profuse, thin, green to yellow, purulent malodorous vaginal discharge, dysuria, and increased vaginal pH. It could be transmitted sexually (Sobel, 2015b) and male partners of infected women can be asymptomatic or may be having nongonococcal uretheritis (Cudmore et al., 2004). Trichomoniasis has an increased association with other sexually transmitted diseases and can increase the transmission of human immunodeficiency virus (Owen and Clenney, 2004; CDC, 2010; Egan and Lipsky, 2000). Around 20%–50% women could be asymptomatic with persistent trichomonas infection (Sherrard et al., 2011). In pregnancy, trichomoniasis may be associated with preterm rupture of membranes and preterm deliveries (Egan and Lipsky, 2000; Cudmore et al., 2004). Trichomoniasis is not known to infect oral and rectal mucosa. It is diagnosed primarily via saline wet mount preparation where motile trichomonads could confirm the presence of trichomoniasis. This test, which has a sensitivity of 60%–70%, should be done immediately for best results. In symptomatic, microscopy negative cases, culture, and microscopy, which has sensitivity of 98% and almost 100% specificity, could be tried for T. vaginalis. Other tests like DNA probe test, rapid card test (OSOM trichomonas rapid test), Affirm vpIII nucleic acid probe test and PCR, could also be done to diagnose trichomoniasis (APHL, 2013). Pap smear has low sensitivity in diagnosis of T. vaginalis (CDC, 2010). In infected male partners, the wet mount test has low sensitivity, so culture and PCR testing would be more appropriate and helpful (CDC, 2010).
5.4 Treatment of VV
The treatment of VV is specific and depends on the organism associated (Sobel, 2015a). Antimicrobials like nitroimidazoles, lincosamides, macrolides, floroquinolones, cephalosporins, and antifungal drugs are the most commonly used group of drugs in treatment of vulvovaginitis and associated cervicitis (WHO, 2003). Among these drugs nitroimidazoles are effective for both bacterial vaginosis and trichomoniasis (Amit et al., 2013). Lincosamides, such as clindamycin, are other group of drugs shown to be effective in bacterial vaginosis. Most of the topical and oral antifungals available are effective in VVC. Recurrence of infections are common in vulvovaginitis. Concomitant treatment of sexual partners is indicated in few infections like trichomoniasis, gonorrhea, and chlamydia infections (CDC, 2010). In conventional practice, VV, especially bacterial VV, is treated with the standard regimens of metronidazole or clindamycin. Fungal VV is treated with antifungals like fluconazole and clotrimazole. Protozoal VV is treated with metronidazole or its congeners. Treatment could be administered orally or intravaginally (CDC, 2010). The subsequent sections addresses the pharmacology, usefulness, and side effects of antibacterials and antifungals used in treatment of VV.
6 Antibacterial Drugs
Nitroimidazoles are a group of antimicrobials with established actions against anaerobic and certain protozoal organisms. They are effective against a variety of anaerobic and protozoal organisms, inhibiting their growth and survival. These drugs are the first choice for certain genitourinary anaerobic infections like bacterial vaginosis, and protozoal infections like trichomoniasis (Amit et al., 2013). Metronidazole forms the prototype of this group, used extensively in treatment of bacterial vaginosis and trichomoniasis. 5-Nitroimidazoles enter the bacterial or protozoal cell as prodrug by passive diffusion. Inside the bacterial cytoplasm or specific organelle in protozoa where there is lack of drug resistant cells, prodrug is activated by intracellular reduction. In this process, there is a transfer of electron to the nitro group of the drug, forming short lived nitroso free radicals. These radicals, being cytotoxic, can interact with DNA molecules, leading to extreme injury of the DNA. They cause inhibition of DNA synthesis, oxidative damage to DNA leading to breakage of strands, degradation, and finally causing cell death (Löfmark et al., 2010). These are selectively toxic to microorganisms that are anerobic or microaerophilic and hypoxic or anoxic cells (Kapoor et al., 2003). With respect to toxicity, studies have shown that high doses metronidazole can cause neurotoxic features in humans. Long-term high dosing of metronidazole is carcinogenic in rodents; in bacteria screening methods it was found to be mutagenic (Brunton et al., 2011). Additionally, studies have also revealed that metronidazole is safe in pregnancy irrespective of trimester, but it is generally not advised in the first trimester (Martindale, 2015). Some of the newer versions of the drugs in this family include tinidazole, secnidazole, ornidazole, and satranidazole.
Lincosamide: A class of drug that includes lincomycin and clindamycin is very important in the treatment of VV. Of the two, clindamycin is most commonly used and is a congener of lincomycin. It is an effective alternative for use in treatment of bacterial vaginosis (CDC, 2010). Clindamycin enters the cell and binds reversibly to 50 S ribosomal subunit of the sensitive micro organism, thereby inhibiting the protein synthesis in its early stages. Clindamycin is mainly bacteriostatic, but at high concentrations it may attain bactericidal properties against sensitive strains of organisms. Its mechanism is similar to that of macrolides: chloramphenicol. All these drugs have site of action in close vicinity on 50 S subunit of the ribosomes (Brunton et al., 2011; Martindale, 2015). Clindamycin is sensitive to most of the Pneumococci, Streptococcus pyogenes, Viridans streptococci, methicillin sensitive strains of Staphylococcus aureus; anaerobic bacteria like Bacteroides fragilis, Bacteroides melaninogenicus, Fusobacterium, Peptostreptococcus, Peptococcus, Clostridium perfringens, Actinomyces israelii, Nocardia asteroides; typical bacteria like Chlamydia, Pneumocystis jiroveci, and T. gondii (Brunton et al., 2011).
The adverse effects associated with clindamycin include diarrhea, known as pseudomembraneous enterocolitis (Martindale, 2015). Vaginal use can rarely cause diarrhea. It is estimated that 2%–20% cases of clindamycin are associated with diarrhea. If untreated, complications could be fatal. Other minor gastrointestinal side effects like gastritis, nausea, vomiting, abdominal cramps, and unpleasant taste are frequent (Brunton et al., 2011). Hypersensitivity reactions ranging from urticaria, rashes, fatal Steven Johsonson’s syndrome, and vesiculobullous exofoliative dermatitis have been reported (Martindale, 2015). Transient leucopenia, agranulocytosis, thrombocytopenia, eosinophilia, musculoskeletal joint inflammation, and liver abnormalities including jaundice and rise in liver enzymes have been reported. Clindamycin can cause renal dysfunction, though occurrences are rare (Martindale, 2015). Injection site reactions like thrombophlebitis on intravenous use and sterile abscess from intramuscular use are reported. Cardiopulmonary arrest can result from too rapid iv infusion. Benzyl alcohol containing clindamycin preparation should be avoided in neonates, as they are known to cause neonatal gasping syndrome (Martindale, 2015). Topical use of clindamycin results in local irritation, dry skin, and contact dermatitis as side effects. Intravaginal use may be associated with local irritation and a burning sensation.
Macrolides: are bacterostatic protein synthesis inhibitor antimicrobials. They consist of erythromycin and its semisynthetic derivatives like clarithromycin and azithromycin. Further semisynthetic derivatives of erythromycin with activity against macrolide resistant strains are called ketolides, which includes telithromycin. They are mainly sensitive against gram positive bacteria like Streptococci, Pneumococci, Staphylococci, Corynebacterium, Mycoplasma, Leigonella, C. trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, and a few gram negative bacteria. Azithromycin is highly sensitive against Chlamydia species, Legionella, and N. gonorrhoeae. They act by binding to 50 S ribosomal subunits of bacteria and prevent protein synthesis. They prevent translocation of t-RNA from acceptor site to donor site, resulting in a halt of protein synthesis (Brunton et al., 2011).
Tetracyclines: These are congeners of polycyclic naphthacenecarboxamide. They include chlortetracycline, oxytetracycline, demeclocycline, methacycline, doxycycline, minocycline, and glycyclines, including tigecycline. Antibiotics in this group are called broad spectrum antibiotics since they are active against aerobic and anaerobic gram positive, gram negative bacteria, Ricketessia and Chlamydia. Tetracyclines enter bacterial cells either by passive diffusion porins or by active pumping. Inside the cell they bind to the 30S ribosomal subunit during protein synthesis and prevent access of amino aceyl t-RNA to the acceptor site on mRNA-ribosomal complex, thus preventing protein synthesis and death of the organism. The adverse effects associated with use of tetracycline include gastrointestinal disturbances including nausea, vomiting, epigastric distress, and pseudomembranous enterocolitis is also possible. Photosensitivity is reported with demeclocycline, doxycycline, and others. Renal toxicity including azotemia, nephrogenic diabetes insipidus, and Fanconi syndrome has been reported with tetracyclines. Hepatotoxicity is a known adverse drug reaction (ADR) with tetracyclines. Tetracyclines are known to increase susceptibility to liver damage during pregnancy. Discoloration of teeth in children is due to enamel discoloration. Others side effects include thrombophlebitis raised intracranial pressure, vestibular toxicity, hypersensitivity reactions, hematological abnormalities including leukocytosis, thrombocytopenic purpura, eosinophilia, fever, and asthma. Tetracyclin is not recommended during pregnancy (Brunton et al., 2011).
Beta lactam antibiotics: This group of antimicrobials includes penicillins, cephalosporins, and carbapenems. Lactam antibiotics interfere with bacterial cell wall synthesis. The bacterial cell wall is made of a rigid peptidoglycan complex consisting of glycan chains cross linked with peptide chains. This cross linking is done by an enzyme called transpeptidases. Lactam antibiotics inhibit the transpeptidases enzyme, thereby leading to formation of a deficient cell wall which undergoes lysis and death. This peptidoglycan cell wall is unique to bacteria compared to human cells. Lactams specifically act only on bacterial cell wall, and do not affect the cell walls of the patient. Due to high rate of resistance to Neisseria, they are no longer considered as therapy for gonorrhea infections, unless sensitivity of the drug has been proven as effective in a particular geographical area. However cephalosporins are sensitive and are considered as a first line of defense in the treatment of gonorrhea (Brunton et al., 2011). Cephalosporins are similar to penicillins in mechanism of action. The adverse effects associated include pain at the site of injection, diarrhea, hypersensitivity reactions, nephrotoxicity, bleeding (some cephalosporins cause hypoprothrombinemia), neutropenia, and thrombocytopenia (Brunton et al., 2011).
7 Antifungal Drugs
7.1 Imidazoles and Triazoles
The azole antifungals include the imidazole and triazole, with a similar mechanism of action and sensitivity pattern to different fungi. They include topical and oral antifungal drugs. Clotrimazole, miconazole, ketoconazole, econazole, butoconazole, oxiconazole, sertaconazole, and sulconazole are imidazoles; terconazole, itraconazole, fluconazole, voriconazole, posaconazole, and isavuconazole are triazoles. Triazoles are newer drugs, less toxic and more effective than the imidazoles. This group of the antifungals inhibits the enzyme 14 demethylase a CYP 450-dependent microsomal enzyme, which converts lanosterol to ergosterol, which is required for the fungal cell wall synthesis. This results in impaired biosynthesis of ergosterol and accumulation of 14 methyl sterol, which then consequentially inhibits the growth of microorganisms by impairing certain membrane bound enzyme systems and alters the permeability of the sensitive fungi.
Ketoconazole is the first orally active azole available for systemic use featuring corticosteroid suppression properties. In addition to fungal steroid inhibition, it also blocks human gonadal and adrenal steroid synthesis (Harvey et al., 2008). It has been replaced with other newer drugs that trigger fewer ADRs and a wider spectrum of activity. Itraconazole is another drug with similar mechanism of action like ketoconazole, and is available for oral use. The major benefit of Itraconazole is that, unlike ketoconazole, it lacks the endocrinological side effects. The adverse effects include nausea, vomiting, taste changes, diarrhea, abdominal cramps, anorexia, hepatotoxicity, hypertriglyceridemia, hypokalemia, rashes, congestive heart failure, adrenal insufficiency, pedal edema, hypertension, rhabdomyolysis, and anaphylactic reactions (Brunton et al., 2011; Harvey et al., 2008).
Fluconazole: It is a fluorinated bistriazole with a broad spectrum of activity. The mechanism of action is the same as other congeners. Unlike ketoconazole, it lacks endocrine adverse effects. It can penetrate both normal and inflamed meninges and attain good concentration in CSF, which make it a good candidate for therapy in treating fungal infections of the brain. It also attains good concentration in bone marrow and can be used in treating fungal infections of bone marrow transplant recipients. The adverse effects associated with its use include nausea, vomiting, headache, abdominal pain, diarrhea, skin rashes, hepatic failure, Steven Johson’s Syndrome, reversible alopecia, and teratogenic effects such as skeletal and cardiac abnormalities if taken during pregnancy.
7.2 Imidazole and Triazoles for Topical Use
Clotrimazole: It is an antifungal imidazole used for topical application in superficial/mucocutaneous candidiasis, dermatophytosis, and pityriasis versicularis. It is metabolized in the liver and excreted in feces and urine. Initially clotrimazole was also given orally at dose of 200 mg/day but is no longer in use because many newer better drugs are available. The mechanism of action and sensitivity of the drug is the same as that of other azole drugs. It is useful in curing dermatophyte infections in 60%–70% cases, 80% in cutaneous candidiasis, > 80% in VVC, and almost 100% in immunocompromised hosts having oral and pharyngeal candidiasis using troches.
The adverse effects include reddening of the skin, stinging sensation, itching, urticaria, edema, vesication, and desquamation on topical application to the skin. On vaginal application, ~ 1.6% users experience mild burning sensation of the vagina, skin rashes, lower abdominal cramps, and increased frequency of urination. The sexual partner of woman using vaginal clotrimazole may experience irritation of penis (Brunton et al., 2011). Intravaginal clotrimazole may damage condoms or diaphragms made up of latex rubber therefore contraceptive failure can occur (Martindale, 2015). If clotrimazole is taken orally, patients can experience mild gastrointestinal and neurological adverse effects. It also has a porphyrogenic effect in vaginal use therefore alternative therapies should be considered in vulnerable patients (Martindale, 2015).
7.3 Drugs Used in Resistant Infections
Amphotericin B: is a mixture of derivatives of fungi Streptomyces, belonging to the polyene macrolide group. It can cause serious adverse effects and is used primarily in resistant life threatening systemic fungal infections like mucormycosis and candidemia. The amphotericin B molecule binds to fungal ergosterol, disrupting the integrity of cell wall by creating pores or channels. These channels can cause electrolytes (like intracellular K+) to leak from the fungi and cause cell death. Amphotericin B specifically binds to ergosterol which is characteristic in fungi and some protozoa. Ergosterol is absent in bacteria, animals, and humans, as a result it acts specifically on fungi. It can act synergistically with flucytosine. It is sensitive to most of the fungi, particularly Aspergillus and Candida. The adverse effects associated with its use include anaphylaxis, fever with chills, renal impairment like azotemia, hypokalemia, hyponatremia, excess magnesium loss, hypotension, anemia, thrombophlebitis, thrombocytopenia, hepatic dysfunction, and varied adverse neurological effects. It is a drug with low therapeutic index.
Prognosis of most of these antimicrobial regimens in vulvovaginal infections is good; however recurrence, failure of therapy, and development of antimicrobial resistance are also possible. Antimicrobial resistance is encountered quite frequently with the therapy of VV. It is said to be on the rise with different drugs, in different types of VV. Long-term therapy in recurrent infections and oft-repeated therapy with antecedent adverse effects preclude its use. The recurrence rate of BV is around 57% at 12 months. Around 5%–15% patients with VVC would have recurrence > 4 times in 12 months leading to recurrent VVC. Around 17% of the patients with TV would have reinfection within 3 months. These infections may be seen even during pregnancy and in nursing mothers, where some of these drugs could be contraindicated. Furthermore, many instances of vulvovaginitis afflict patients with other sexually transmitted infections where combinations of regimens should be used, causing increased chances of drug-drug interactions and adverse events (WHO, 2003). The treatment of this common disease presents many difficulties and the use of probiotics is being researched and has become the matter of interest for more successful solutions with fewer adverse side effects.
7.4 Probiotics in Vulvovaginitis
Probiotics by definition are termed as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” as defined by WHO (Soccol et al., 2010). Substances such as nondigestible food components increase growth and activity of probiotics and are called “prebiotics.” Prebiotics and probiotics could be called “synbiotics.” Probiotics belong to Lactic Acid Bacillus strains (LAB), and consist of numerous substrains (Ehrström et al., 2010). Probiotics are gram positive, nonspore forming, facultative, or anaerobic bacilli (Shalev, 2002). Probiotics are derived from milk and dairy products like yogurt and curd. The historical use of probiotics in promoting human health dates back to ancient times and is mentioned in some of the older texts of Hinduism and early Christianity (Soccol et al., 2010).
At the beginning of 20th century Illya Ilyich Metchnikoff, the Nobel Prize winner in medicine 1908 at Pasteur institute, was able to link the benefits of bacteria in yogurt with human health. Later Tissier reported that breastfed infants had microorganisms producing bifidofactor in the gut, which may have role in maintaining flora in intestine and protecting against infections. This work was followed by numerous studies of probiotics and by the end of the century, researchers were able to link probiotics with the metabolic, trophic, and protective effects. Metabolic effects included digestions of nondigestible dietary fats, endogenous mucus, savings of energy, production of vitamin K, and absorption of mineral ions. Trophic effects included control of epithelial cell proliferation, homeostasis, and regulation of the immune system. Protective functions include effects against pathogens and barrier functions.
Lactobacilli are distributed throughout the gastrointestinal and genital tract in the human body, forming part of normal healthy flora. Lactobacilli, especially Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus iners, are the most commonly found microbes in the vagina of healthy women of reproductive age (Falagas et al., 2006). It is now clear that disruption of the dynamic equilibrium of vaginal microbiota may lead to excessive colonization of pathogenic organisms causing VV. Modulation of these bacteria would lead to overgrowth of pathogenic bacteria, therefore reestablishing the normal flora colonization with the supplementation of probiotics would counter the pathogenesis and prevent the development of vaginitis (Ehrström et al., 2010). For years probiotics have been used as food supplements with excellent safety profiles (Senok et al., 2009; Dovnik et al., 2015).
Probiotics are said to confer various health benefits such as maintaining the innate immune system of the gut, prevention of antibiotic induced diarrhea, alleviation of constipation, traveler’s diarrhea, inflammatory bowel syndrome, reduction of hypercholestremia, protection against colon and bladder cancer, osteoporosis, allergic rhinitis, and weight loss. However few benefits have been clinically evaluated (Soccol et al., 2010). The bacterial strains commonly used as probiotics are Lactobacillus, Streptococcus, and Bifidobacterium; other organisms are used therapeutically, including Enterococci and Yeasts (Soccol et al., 2010). Many in vitro studies and clinical studies were conducted to assess the benefits of probiotics in vulvovaginitis. These studies are summarized in Tables 3–5. Probiotics are found to be effective in the treatment of urogenital tract infections, especially vulvovaginitis caused by BV and VVC, by their varied mechanisms of action.
Table 4
| Intervention | Disease Studied | Results | References |
|---|---|---|---|
| Comparison of yogurt containing L. acidophilus 1 × 108 CFU OD × 2 months vs pasteurized yogurt | BV and RVVC | 60% vs 25% cure rate | Shalev (2002) |
| LR GR1 and LF RC 14 suspended in skimmed milk given once daily for 14 days | BV, RVVC and UTI | They have potential to restore urogenital flora in 7 days | Reid et al. (2001a,b) |
| Oral capsules containing 108 CFU of L. rhamnosus GR-1 plus L. fermentum RC-14 or L. rhamnosus, each day for 28 d | BV | Normal vaginal flora was restored | Reid et al. (2004) |
| LRGR1 109 + LRRC14 109 capsule orally twice daily for 30 days vs placebo after initial remission with metronidazole | BV | Resolution of BV 88% vs 40% after 30 days | Anukam et al. (2006) |
| Oral capsule containing 109 CFU of L. rhamnosus GR-1 + L. fermentum for 60 days | BV | Probiotics colonized the vagina properly and the Nugent score normalized after the treatment | Reid et al. (2004) |
| Oral capsules containing 2.5 × 109 CFU of L. rhamnosus GR-1 and L. reuteri RC-14, 14 days vs placebo | BV | Significant reduction of Nugent score in probiotic group | Petricevic et al. (2008) |
| Vaginal tablets containing 106 CFU of L. acidophilus and estriol 0.03 mg, once daily or twice daily for 6 days | BV | Microbiological cure (Nugent criteria) and clinical cure were observed on days 15 and 28 after intervention | Parent et al. (1996) |
| Vaginal tampons containing 108 CFU of L. gasseri, L. casei var. rhamnosus, and L. fermentum, 5 tampons during menstruation | BV | Microbiological cure was observed based on Nugent score and Amsel criteria | Eriksson et al. (2005) |
| Vaginal tablet containing 107 CFU of L. acidophilus, 0.03 mg of estriol and 600 mg of lactose, daily for 6 d | BV | Vaginal flora was enhanced significantly by the probiotic administration in combination with low-dose estriol | Ozkinay et al. (2005) |
| Vaginal tablets containing 109 CFU of L. brevis, L. salivarius subsp. salicinius, and L. plantarum, for 7 d | BV | All of the patients in the probiotic group were free of BV, showing a normal or intermediate vaginal flora | Hemalatha et al. (2012) |
| Vaginal capsules containing between 108 and 1010 CFU of L. gasseri LN40, L. fermentum LN99, L. casei subsp. rhamnosus LN113, and P. acidilactici LN23, for 5 days, after conventional treatment of bacterial vaginosis | BV and VVC | LN had a good colonization rate in the vagina of patients with BV and women receiving LN | Ehrström et al. (2010) |
| Vaginal capsule containing 108 CFU of L. rhamnosus, L. acidophilus, and S. thermophiles, 21 days, for 7 days on 7 days off, and 7 days on | BV prophylaxis | Reduction in recurrence | Ya et al. (2010) |
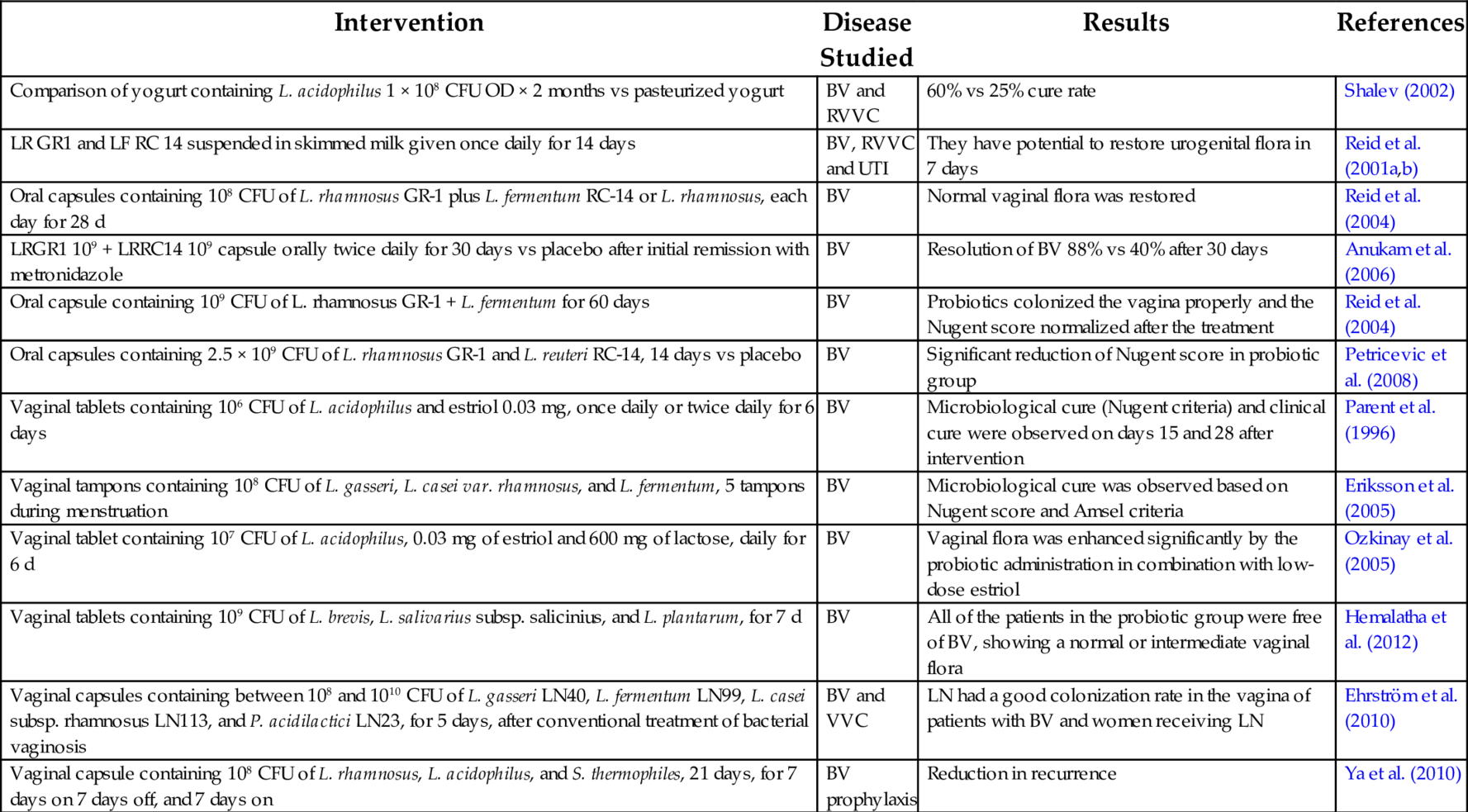
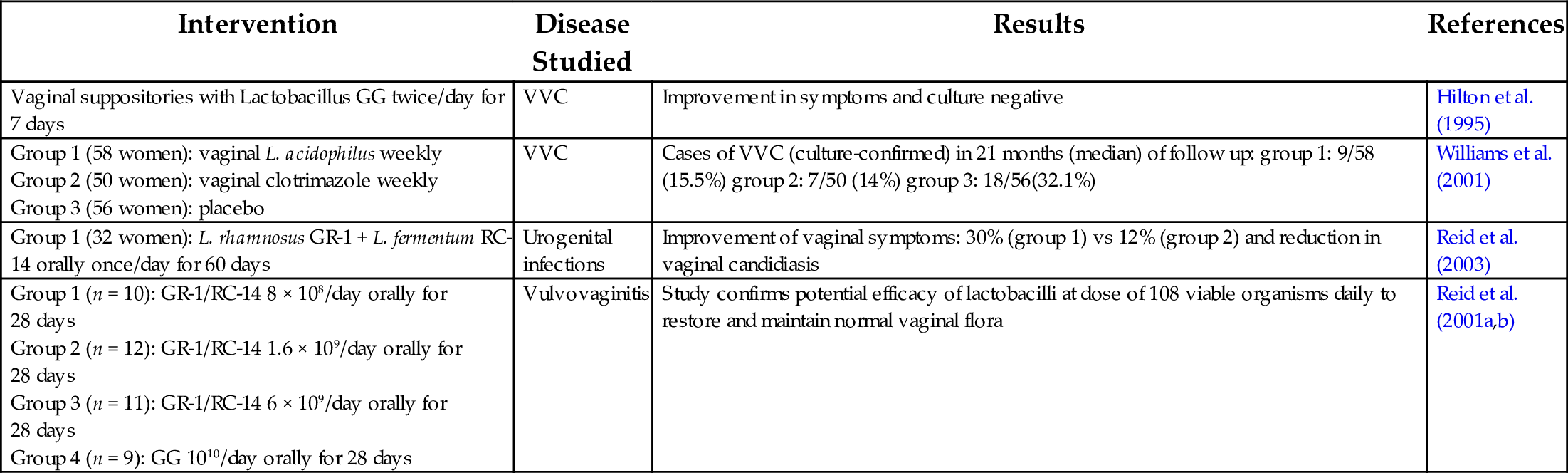
Table 5
| Intervention | Disease Studied | Results | References |
|---|---|---|---|
| Vaginal suppositories with Lactobacillus GG twice/day for 7 days | VVC | Improvement in symptoms and culture negative | Hilton et al. (1995) |
| Group 1 (58 women): vaginal L. acidophilus weekly Group 2 (50 women): vaginal clotrimazole weekly Group 3 (56 women): placebo | VVC | Cases of VVC (culture-confirmed) in 21 months (median) of follow up: group 1: 9/58 (15.5%) group 2: 7/50 (14%) group 3: 18/56(32.1%) | Williams et al. (2001) |
| Group 1 (32 women): L. rhamnosus GR-1 + L. fermentum RC-14 orally once/day for 60 days | Urogenital infections | Improvement of vaginal symptoms: 30% (group 1) vs 12% (group 2) and reduction in vaginal candidiasis | Reid et al. (2003) |
| Group 1 (n = 10): GR-1/RC-14 8 × 108/day orally for 28 days Group 2 (n = 12): GR-1/RC-14 1.6 × 109/day orally for 28 days Group 3 (n = 11): GR-1/RC-14 6 × 109/day orally for 28 days Group 4 (n = 9): GG 1010/day orally for 28 days | Vulvovaginitis | Study confirms potential efficacy of lactobacilli at dose of 108 viable organisms daily to restore and maintain normal vaginal flora | Reid et al. (2001a,b) |
7.5 Mechanism of Action of Probiotics
Different mechanisms have been postulated to explain the mechanism of probiotics in treatment of vulvovaginitis. The mechanism of action of probiotics is a debated issue and may be strain or species specific. Often multiple mechanisms are attributed to a single strain or species, however no individual strain would be expected to have all possible mechanisms (Soccol et al., 2010). A few of the known mechanisms are listed here:
- 1. Probiotics replace the normal microbiota of the vagina and prevent colonization of pathogenic organisms by competing for food and other requirements.
- 2. Probiotics can metabolize the glucose and produce lactic acid, acetic acid, and propionic acid, which causes a rise in vaginal pH, preventing pH-sensitive pathogenic organisms from thriving or survive in an unfavorable environment (Shalev, 2002).
- 3. A few strains like of lactobacillus, like Lactobacillus delbrueckii, Lactobacillus fermentum RC 14, are known to produce hydrogen peroxide which can counteract the pathogens. Lactobacillus rhamnosus GR-1 is known to resist the killing of normal flora by spermicidal nonoxynol-9 (Reid and Hammond, 2005).
- 4. Probiotics can secrete some antimicrobial products, like bifido factor and a bacterosin like substance called pentosin TV35b, which can fight against pathogens (Senok et al., 2009).
- 5. Some biosurfactants are known to be produced by probiotics, such as Surlactin produced by Lactobacillus acidophilus RC-14 (Falagas et al., 2006).
- 6. Some strains can produce collagen-binding proteins which can inhibit pathogen adhesion to vaginal epithelium (Waigankar and Patel, 2011).
- 7. Probiotics can coaggregate the pathogens and kill them or prevent them from spreading infections (Cribby et al., 2008).
- 8. Immunomodulation through the toll-like receptor 9 is possibly responsible for antiinflammatory activity of probiotics (Waigankar and Patel, 2011).
- 9. Immune regulation at various levels is also possibly responsible for disease prevention. They are known to increase production of antiinflammatory cytokines, decrease the production of proinflammatory cytokines, stimulate dendritic cells, thereby modulating Th1- or Th2-mediated immunity regulation (Suchetha et al., 2015).
- 10. Reduction of 70kDA heat shock protein production and decrease in vaginal mannose-binding lectin concentration, reduces the capacity of microbial killing (Cribby et al., 2008).
- 11. They may play a role in mucous membrane integrity and mucin production (Suchetha et al., 2015).
Probiotics could be considered as a single organism or combinations of multiple strains: “probiotic cocktails” (Palatty et al., 2009). The various strains of probiotics used are listed in Table 3. The salient features of some important strains of probiotics are listed here.
7.6 L. acidophilus
It is a heterofermentative organism, lives in acidic medium and ferments lactose into lactic acid, ethanol, carbon dioxide, and acetic acid, which can prevent growth of pathogens. It assists in production of vitamin B complex vitamins, bile conjugation, and regulation of amino acids by separation from bile. They can assist in improving gastrointestinal functions in diarrhea, promote immunity, prevent yeast infections of the genitourinary tract, and lower cholestrol levels (Palatty et al., 2009).
L. rhamnosus GR-1: Adheres strongly to uroepithelium and prevents adhesion of pathogens in the vagina. They could be found in the vagina after oral administration of the strains.
L. fermentum RC-14: Produce biosurfactants, produce a large amount of hydrogen peroxide and prevent adhesion of pathogens to genital epithelium. They could be found in the vagina after oral administration of the strains (Mastromarino et al., 2013).
Lactobacillus brevis, Lactobacillus salivarius FV-2, Lactobacillus plantarum FV-9: These have all the previously mentioned features. Additionally, they reduce vaginal inflammatory cytokines, like IL 1, 6, and have inhibitory action on HSV-2 in cell cultures (Mastromarino et al., 2013).
8 Clinical Studies With Probiotics in Women’s Health
Probiotics in pregnancy: Currently no data suggests that the probiotics are unsafe in pregnancy and breastfeeding. Two studies evaluated probiotics during the first trimester reported that there were no malformations of the fetus. One meta-analysis and eight randomized control trials regarding probiotic use in pregnancy during third trimester (32–36 weeks) have not reported any increase in fetal anomalies. These studies were done for lactobacillus and Bifidobacterium. It is unlikely that probiotics could affect the pregnancy and fetal development (Elias et al., 2011).
Probiotics and breastfeeding: Probiotics are not expected to be secreted in breastmilk. There were no reports of increased adverse effects in infants of lactating women on probiotics. Studies were done only in Lactobacillus and Bifidobacterium strains; therefore other strains need further evaluation. Probiotics appear to be safe in lactating mothers (Elias et al., 2011).
Probiotics in BV: Many in vitro and clinical studies have been done to evaluate the efficacy of the probiotics, either orally or intravaginally, alone or as an adjunct to antimicrobial therapy in the treatment of the BV (Cribby et al., 2008). Many studies have reported beneficial effects of probiotics in treatment of BV and prevention of associated STIs (Ehrström et al., 2010; Cribby et al., 2008). However a Cochrane review conducted in 2009 evaluating role of probiotics in BV, had inconclusive results due to variation in methodologies, parameters used to assess, different strains of probiotics used, and the varied adjunct therapy used in different trails. They opined that oral metronidazole and oral probiotic combination apparently had beneficial effects compared to different regimens they assessed in BV. They suggest that further standardized, well-designed randomized control trials, with a larger sample size of patients, would be required for conclusive report to develop recommendations (Senok et al., 2009). A few studies have also been done to assess the efficacy of probiotics in BV during pregnancy resulting in positive reports (Facchinetti et al., 2013). Nevertheless, BV can pose as a great challenge in the therapy, being the most common condition in women, associated with morbidities in certain populations, including greater incidence of post-operative infections, adverse pregnancy outcome, disease recurrence, development of antimicrobial resistance, and increased susceptibility to STI. The use of probiotics for BV therapy has an excellent safety profile; it should be considered a good alternative to existing treatments.
Probiotics in VVC: VVC, due to its high prevalence and high rates of recurrence, presents a significant challenge in treatment. Complicated VVC including RVVC has limited therapeutic options. Many in vitro and clinical studies have reported beneficial effects of probiotics in the treatment of VVC (Xie et al., 2013). In a review done by Falagas et al. (2006), it was reported that either oral or local probiotic will be an effective option in treatment of VVC; however some studies had failed to support these findings so the results remain inconclusive. They reported that methodological shortcomings in various studies conducted had resulted in inconclusive assessment of probiotics in VVC. They opine that a few strains of probiotics, like L. acidophilus, L. rhamnosus GR-1, L. fermentum RC-14, are show potential and could be used to prevent recurrence in RVVC. However, they agree that better designed, standardized, uniform, large sample sized clinical trials with specified strains of probiotics and adjunctive therapy would be the needed, in order to develop a definitive view.
Probiotics in Trichomoniasis: There is not much evidence to support the effects of probiotics in TV. The subject needs further research and evaluation.
Probiotics in Cervicitis: Probiotics could be helpful in preventing gonococcal and chlamydia infections. The subject needs further research and evaluation.
Comparison of oral vs intravaginal probiotics in vulvovaginitis:
Probiotics are available for oral consumption as tablets, capsules, powder for suspension and intravaginal application as pessary, vaginal tablet, and cream. Intravaginal probiotics appear to be a more obvious solution for successful colonization and local effect in the vagina (Ehrström et al., 2010). However, many studies have claimed that oral probiotic supplementation can also produce similar rates of lactobacilli colonization in the vagina (Reid et al., 2001a, b). Oral ingestion of probiotics could be well accepted by practically most women. There are many barriers for the orally supplemented probiotics to achieve concentration in vagina. The roadmap for orally supplemented probiotics is as follows: they have to sustain viability in the gastric acid pH, pass through the bile, survive in the gut, pass through the rectum, and finally colonize the vagina and uroepithelium (Reid et al., 2001a, b). Few studies have reported that oral supplementation of probiotics of some strains, like L. rhamnosus GR-1, L. fermentum RC-14, are effective equivalents and ascend from the rectum and effectively colonize in the vagina (Reid et al., 2001a, b). There is a conflict in using probiotics in vulvovaginitis in that no clear consensus exists regarding the preferred route of administration in vulvovaginitis (Senok et al., 2009; Dovnik et al., 2015; Falagas et al., 2006).
9 Adverse Effects
Probiotics are generally considered safe, without any adverse effects. However, there have been a few incidents where probiotic strains have been isolated in infectious wounds. Approximately 0.2% positive blood culture for lactobacillemia have been reported in Finland during a 5-year period from 1995 to 2000. Infectious endocarditis, fungemia, and abscess of the liver have been associated with probiotics. Interestingly, in only a few cases probiotics consumption had positive isolates from infectious sites. Continued vigilance in identifying, typing, and cataloguing all probiotic bacteria associated with bacteremia would be helpful to ascertain the possible of cause (Borriello et al., 2003). Such adverse effects are seen more in patients who are immunocompromised or have serious underlying diseases (Falagas et al., 2006). A case of metabolic acidosis has been reported after oral consumption of L. acidophilus (Ku et al., 2006). Probiotics such as Streptococci and Enterococci use may give rise to theoretical concerns regarding adverse effects due to the fact that they are pathogens themselves. Pancreatitis has been reported in a study where six different strains were installed directly into the intestine (Cribby et al., 2008). Properties of individual probiotics are specific to strain and species; therefore adverse effects of individual probiotics should not be overgeneralized (Martindale, 2015). Probiotics have to be used with caution in patients who are immunocompromised, preterm neonates, and special populations (Martindale, 2015).
10 Conclusions
Studies in the recent past have shown that probiotics are promising in women’s reproductive health and the scientific evidence for their use in specific clinical scenarios is strong. In this regard, the most important aspect that needs to be considered, with the advent of increasing cases of antibiotic-resistant pathogenic microorganisms, is the use of probiotics for the treatment of VV in a natural and nontoxic treatment modality. Probiotics are being shown to be help the host overcome infection, prevent infections of the reproductive tract, restore and maintain a healthy vaginal ecosystem, and thereby improve female health in relation to reproductive health. The other important aspect is that probiotics are a cost-effective in the treatment of VV and will be of great use with a potential for wide application. Further studies are required to ascertain their benefits in different disease states and in different age groups in various parts of the world. From a mechanistic perspective, although much remains to be learned about the use of appropriate probiotic strains, it is evident that benefits can be strain dependent. In addition to this, studies are also required to understand the underlying mechanistic issues and interactions of different probiotic organisms.