Bioactive Peptides—Impact in Cancer Therapy
Edwin E. Martínez Leo; Armando M. Martín Ortega; Maira R. Segura Campos Autonomous University of Yucatan, Yucatán, Mexico
Abstract
Food-derived proteins and peptides play an important role in functional foods because their ability to modulate the metabolism and act as regulatory compounds suggest their potential use as nutraceutical and functional food ingredients to prevent and treat diseases.
Protein hydrolysates have been used to decrease allergies of certain native proteins, provide nutrients and produce peptides with specific biological effects: antihypertensive, anti-inflammatory, hypolipidemic, hypoglycaemic, antioxidant, antimicrobial and antithrombotic. Peptides with antioxidant, immunomodulatory and anticancer activity, are a dietary alternative to conventional approaches (e.g. chemotherapy, radiotherapy) used to prevent and treat cancer.
The multigenic and environmental complexity of cancer leads to the realization that establishing food strategies to decrease disease cancer risk is both difficult and challenging, therefore the study of food as alternatives to conventional cancer treatments offers numerous pathways to advance the development of potential anticancer foods.
Advances through research in cancer treatment are essential to improve the prognosis of patients affected by this disease. Efforts include the development of more effective and less toxic therapies. These include targeted therapies, immunotherapies and vaccines for cancer treatments, and the improvement of those that have existed for decades at the pharmacological and dietary level. The purpose of this chapter is to analyze the principal biopeptides that could have a positive effect on the dietary treatment of cancer and its principal alterations, inflammation and oxidative stress.
Keywords
Anti-inflammatory; Antioxidant; Biopeptides; Cancer; Nutraceutical
1 Cancer: A Worldwide Inflammatory Disease
Carcinogenesis is a process of successive steps occurring at a phenotypic and genetic level, identified histologically as hyperplasia, early adenoma, late adenoma, carcinoma and metastases. Cancer cells have a high rate of mutation. It is hypothesized that “cancer is initiated in a population of rare cells with stem cell properties” (NCI, 2014). All body tissues are derived from stem cells that are located within a specific area of each organ or tissue and are defined by their ability to self-renew and differentiate into the cell types that make up each organ.
Stem cells are prone to the accumulation of multiple mutations that are characteristic of carcinogenesis. Stem cells and tumor cells share important properties: self-renewal capacity, ability to differentiate, telomerase activity, activation of antiapoptotic pathways, increased transmembrane activity and ability to migrate and metastasise (Lee and Muller, 2010).
The genesis of cancer involves certain genetic alterations that allow excessive and unregulated cell proliferation that becomes autonomous. The damage to the genetic material leads to a failure in DNA repair, causing mutations that trigger the expression of genes favoring the development of a cancer phenotype, and altered, uncontrolled cell replication. Any population of cells that make up a tumor are the result of clonal expansion from a single precursor cell that has suffered genetic damage; hence, the tumors are clonal (Mathews, 2015).
Cancer is one of the main causes of death worldwide. In 2012 there were 14 million new cases, with 8.2 million deaths linked to cancer, and it is expected that the number of cases will increase to 22 million over the next two decades (WHO, 2014). Approximately 30% of cancer deaths are due to environmental factors and unhealthy lifestyles, which have become synonymous with a developing, modern, capitalist, globalized and technological society. Certain factors, such as insufficient consumption of fruits and vegetables, lack of physical activity, smoking, alcohol consumption, stress and high-fat mass index, are entirely preventable and modifiable (WHO, 2014). This is the reason that most cancer pathophysiology is focused on the environmental aspect, which leads to disorders in the redox state of the organism and an imbalance in proinflammatory processes linked to the alteration of gene modulation on oncogenes. These factors are potentially reversible, therefore, finding food strategies that decrease disease risk are now being studied, creating opportunities to progress the development of potential anticancer foods.
Cancer is a complex disease that involves environmental and multigenic components in which numerous imbalances in DNA modulation, activation of proto-oncogenes and inhibition of tumor suppressor genes are implicated (Mathews, 2015). Proto-oncogenes are genes directly or indirectly involved in cell proliferation. Their genetic alteration leads to activation of oncogenes. Imbalances in modulation signals of oncogenes lead to altered mechanisms of cell growth and proliferation, which causes the transformation of a normal cell to cancer (NCI, 2014). Mutations of genes that turn into oncogenes can either be inherited, or result from exposure to environmental substances that cause cancer (NCI, 2014). Gene mutation positively influences carcinogenesis due to an increase in their expression, providing an increased cell proliferation capacity. There are > 100 identified oncogenes, such as ERBB2 in breast and ovarian cancer, members of the RAS family in cancers of the lung, pancreas and colon, and members of the MYC lymphoma family (Pylayeva-Gupta et al., 2011).
Tumor suppressor genes regulate replication mechanisms, their mutation causes a loss of expression of regulatory molecules, such as p53 and retinoblastoma protein (RB), and a consequent loss of growth control mechanisms, replication and cell division (Lee and Muller, 2010) that results in cancer progression due to an imbalance between cell proliferation and death (apoptosis).
Epidemiological, pharmacological and genetic evidence substantiate the association between inflammation and oncogene expression, due to changes in the cellular environment (Mantovani et al., 2008). Recent studies associate the effects of chronic inflammation on genetic alterations in cancer due to epigenetic modifications, including DNA and histone methylation, which ultimately leads to silencing of tumor suppressor genes.
2 Chronic Inflammation and Oxidative Stress as Potential Triggers of Cancer
Inflammation is a complex physiological response of an organism to cell injury, which may have biologically originated from bacteria or viruses. The chemicals that cells produce are in a state of imbalance, as in the instance of free radicals (FR), reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Bayarsaihan, 2011). FR formation can be increased in many pathological situations due to the action of stimulants, such as opsonized bacteria, viruses, immunoglobulins and chemotactic peptides, among others; and exposure to various deleterious agents, such as ionizing radiation, ozone, nitrogen dioxide, sulfur dioxide, streptozotocin, doxorubicin (adriamycin), smog, acid rain, pesticides, various radioactive compounds and cigarette smoke (Kisten et al., 2014).
The redox imbalance, generated by an increase in ROS and RNS and/or a decrease in their reduction mechanisms (antioxidant), leads to a chemical cell state called oxidative stress, in which there is an alteration in oxidative-reductive intracellular homoeostasis. There are many natural sources of oxidative stress, for example, exposure to environmental oxidants, toxins, heavy metals, ultraviolet light (UV) and inflammation. High levels of ROS exert a toxic effect on biomolecules, particularly DNA, proteins and lipids; leading to oxidative damage in multiple cell locations, subsequently causing various diseases, including cancer (Reuter et al., 2010).
Normally, 95% of the O2 consumed by human cells is reduced to H2O during the generation of adenosine triphosphate (ATP) in the mitochondrial electron transport chain. However, about 5% is converted to ROS. ROS are products of normal cell metabolism and play a vital role in stimulating signaling pathways in animal and plant cells in response to changes occurring in the intracellular and extracellular environment. Most ROS are generated within the cell, in the mitochondrial respiratory chain, by the partial chemical reduction of the oxygen molecule (O2). ROS species include hydrogen peroxide (H2O2), O2– and OH ∙ (Cayuela, 2012). Under a sustained stress environment, ROS are produced over a long period of time and can damage cell structure and function, inducing mutations and neoplastic transformation.
Cancer is an oxidative stress-induced disease. Neoplastic cells inherently produce a greater amount of ROS than healthy cells. Cancer cells generate ROS and RNS that react with cell membranes by generating reactive aldehydes and lipid peroxidation products, such as malondialdehyde, acrolein, and crotonaldehyde, which react directly with the nitrogenous bases of DNA, generating exocyclic DNA adducts (propane-DNA adducts, ethene-DNA adducts) (Nair et al., 2007). These adducts are mutagenic and producers of base substitutions, generating a potentially carcinogenic environment (Kisten et al., 2014).
The direct effects of ROS, generally attributed to its high concentrations at the site of damage, include breaks in DNA strands, mutations, aberrant DNA crosslinking and mutations in proto-oncogenes and tumor suppressor genes, thus promoting neoplastic transformation. For example, ROS can decrease the expression and enzymatic activity of DNA repair genes and can increase DNA methyltransferase expression, leading to a global genome hypermethylation; resulting in colonic adenomatous polyps (APC), cyclin-dependent kinase 2 (CDK2), susceptible breast cancer-1 gene (BRCA1), RB, and the DNA repair gene, mutL homolog 1 (hMLH1) (Reuter et al., 2010).
Meanwhile, most of the NO− is synthesized by nitric oxide synthase (NOS), usually after receiving an inflammatory or immune stimulation. The calcium-independent inducible isoform (iNOS) increases the amounts of NO formed and is only expressed during the inflammatory process. The iNOS are induced by cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and lipopolysaccharide (LPS). These RNS can induce other reactive species by excessive lipid peroxidation. Proteins and lipids are primary targets for oxidative attack and modification of these molecules can increase the risk of mutagenesis (Saavedra et al., 2010). Moreover, the characteristic tumor microenvironment (TME) in cancer can lead to increased metabolic rate, increased energy demand, and excessive mitochondrial ATP synthesis, which collectively promote the increased production of ROS in the cell, which subsequently leads to infiltration of inflammatory cells into the tumor tissue, contributing to oxidative stress and increasing the inflammatory process (Essick and Sam, 2010).
Several studies describe cancer patients as having higher plasma ROS concentrations compared to healthy individuals, and a decrease in antioxidant responsedirectly related to decreased levels of glutathione peroxidase and superoxide dismutase (SOD) (Khansari et al., 2009; Stachowicz-Stencel et al., 2011).
The association between chronic inflammation, cancer, and oxidative stress is well known (Reuter et al., 2010). The inflammatory process is an inducer of ROS and RNS and proinflammatory cytokine production (TNF-α, IL-1β, IFN-γ) that exacerbates the same generation of oxidants, resulting in cell deregulation, prolonged inflammatory processes and decreased antioxidant capacity, thereby facilitating a mutagenic environment (Khansari et al., 2009). However, during inflammation, important epigenetic changes occur that favor the development of cancer (Barros and Offenbacher, 2009). Necrosis factor kappa-beta (NF-κβ) over expressed in patients with chronic inflammation has demonstrated patterns of altered histone methylation status, leading to modified cell replication and proliferation processes (Covarrubias et al., 2015).
Several studies show that inflammation increases the risk of cancer and may promote tumor progression. In colon cancer, chronic inflammation and colonic damage directly cause DNA alterations. Furthermore, chronic inflammation and loss of protective mucosa increase intestinal permeability to environmental toxins and mutagens which induce mutations in stem cells, leading to cancer (Grivennikov and Karin, 2010). Inflammation may stimulate proliferation of cells that contain oncogenic mutations induced by carcinogens, and can result in the production of ROS and RNS by immune cells, as well as immune stimulation mediated by ROS production in pre-malignant cells, inducing mutagenic enzymes expression and inactivation of damaged DNA (Zeng et al., 2014). As depicted in Fig. 1, there is ample evidence that inflammation has a role in tumor promotion (Grivennikov and Karin, 2010).
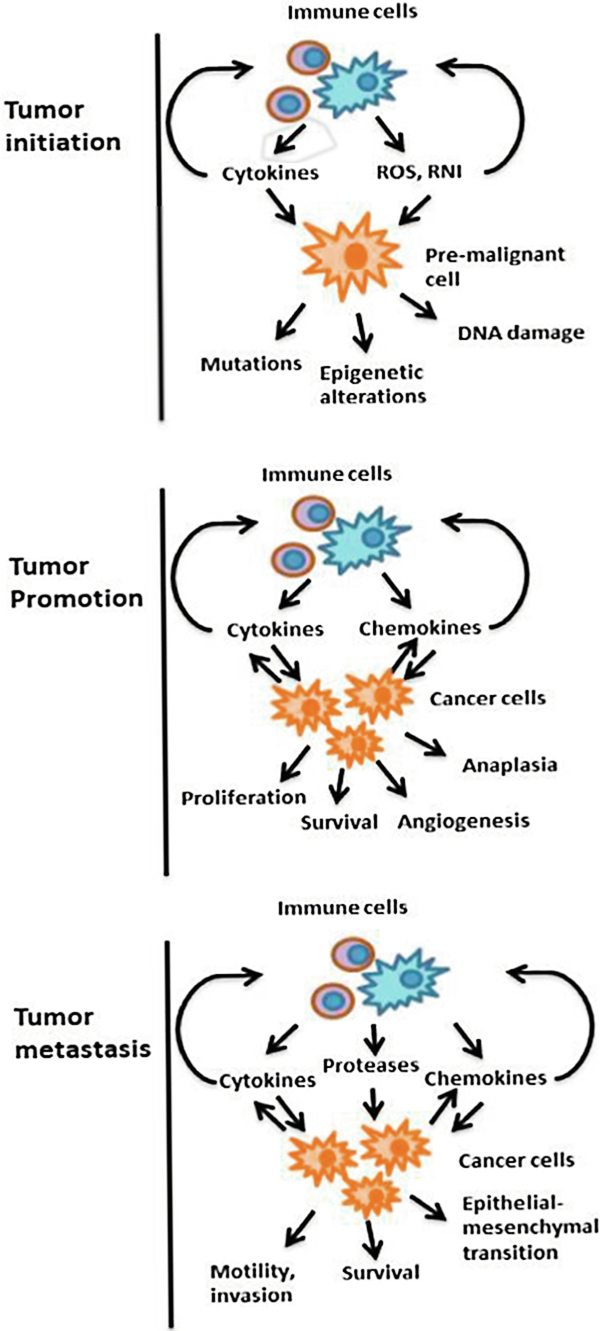
2.1 Inflammation and Metastasis
The majority (90%) of cancer deaths are due to metastasis. Immune cells are present in all tumors due to molecular changes in the invasive front of the tumor, and are involved in various forms of direct and indirect interactions with cell metastasis. Indeed, it has been found that the inflammatory microenvironment influences several key stages of metastasis (Rohan et al., 2014).
The process of epithelial-mesenchymal transition (EMT), which is critical for metastasis to occur, is mediated by various inflammatory cytokines (TGF-β, IL-1, TNF-α and IL-6) and may be a consequence of TNF-κβ activation. Inflammatory signals also regulate the production and activity of various proteases, which degrade the extracellular matrix and facilitate the invasion of cancerous cells. Chemokines can directly stimulate the migration of malignant cells through blood vessels, while cytokines, such as TNF-α, can increase vascular permeability (Laua et al., 2009; Lawrence, 2009). Moreover, cytokines are important for the survival, recruitment, colonization and growth of metastatic cells through the same mechanisms that affect the growth and survival of primary tumors (Chaffer and Weinberg, 2011).
A high body-mass index (BMI) is associated with increased risk of developing cancer because the tissue contains more macrophage infiltration and is enriched in gene expression of macrophages associated with proinflammatory pathways, including IL-6, IL-8, C C chemokine receptor type 5 (CCR5) and peroxisome proliferator-activated receptor (PPAR). Proinflammatory cytokines promote lipolysis and additional free fatty acid production, leading to a positive feedback for chronic inflammation (Ito, 2007).
C chemokine receptor type 5 (CCR5) and peroxisome proliferator-activated receptor (PPAR). Proinflammatory cytokines promote lipolysis and additional free fatty acid production, leading to a positive feedback for chronic inflammation (Ito, 2007).
The recruitment of monocytes leads to their differentiation into macrophages, due to exposure to hypoxic conditions, resulting in tumor-associated macrophages (TAMs) residing in the TME, which has a direct role in the development of angiogenesis, migration and invasion of tumor cells in the metastasis process. Furthermore, TAMs guide a tumor intravasation outside and inside the tumor vasculature in distant organs of metastasis, including lung and bone cells. The role of TAM in metastasis is depicted in Fig. 2 (Williams et al., 2016).
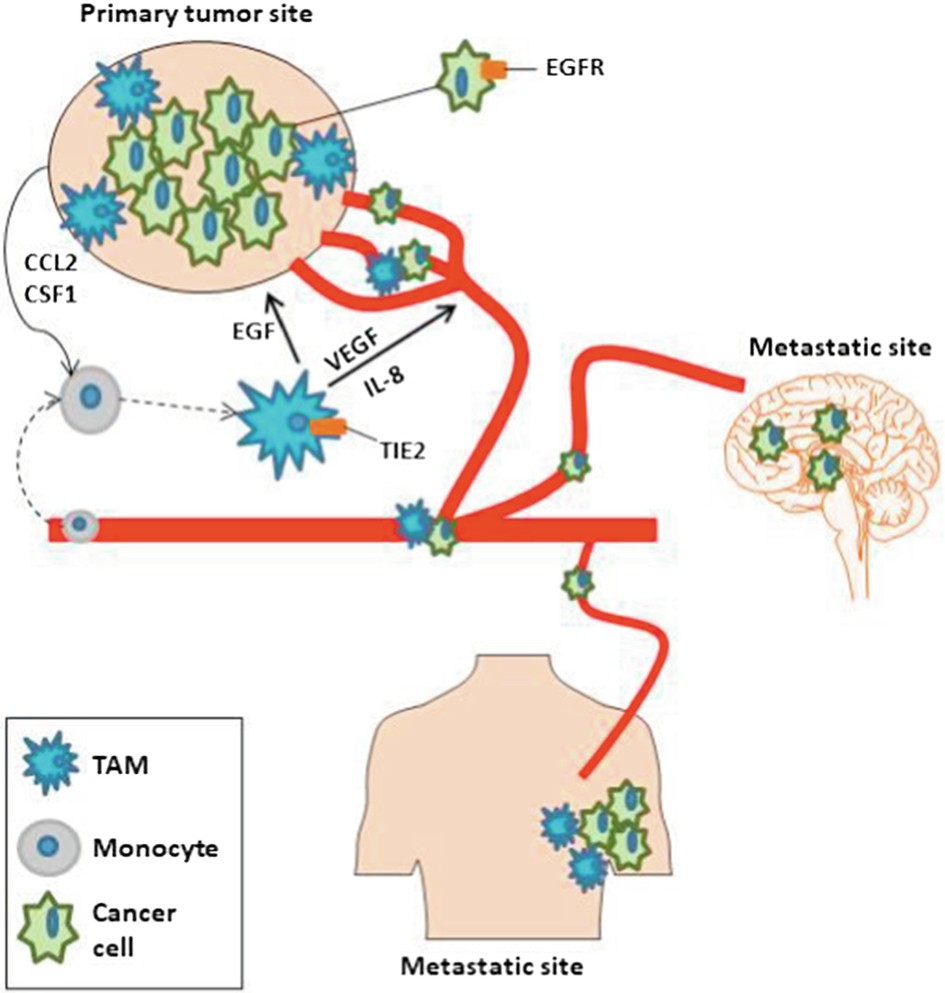
2.2 Inflammation and Tumor Cell Proliferation
Tumor growth (tumor promotion) is the sum total of a malignant cell proliferation versus cell death. Both processes are strongly affected by inflammation and inflammatory cytokines produced by immune cells, which infiltrate the tumor, such as IL-6 and TNF-α, as they can be used as mitogens and survival factors for premalignant cells (Horng, 2015). This tumor growth is mediated by cytokines, which activate oncogenic transcription factors, NF-κβ and signal transducer and activator of transcription 3 (STAT3). The activation of these factors are found in 50% of all cancers, and is required to express a variety of target genes important for tumorigenesis (Valenzuela et al., 2011).
The uncontrolled proliferation of tumor cells requires the regulation of multiple signaling pathways, including signaling cascades involved in survival, proliferation and cell cycle progression. The most important effects of oxidants in these signaling pathways have been observed in mitogen-activated protein (MAP)/activating protein 1 (AP-1) and NF-κβ. Induction of redox-sensitive pathways for cell proliferation is required for cell division, which has substantial energy requirements and produces metabolic energy-generating reactions, which must be buffered to prevent oxidative damage and ultimate cell death (Grivennikov and Karin, 2010).
Chronic inflammation is present in all stages of cancer, and the oxidative-reductive imbalances lead to increased oxidative stress, increased cell damage and a decrease in the body’s response to carcinogens.
3 Functional Food and Dietary Bioactive Compounds
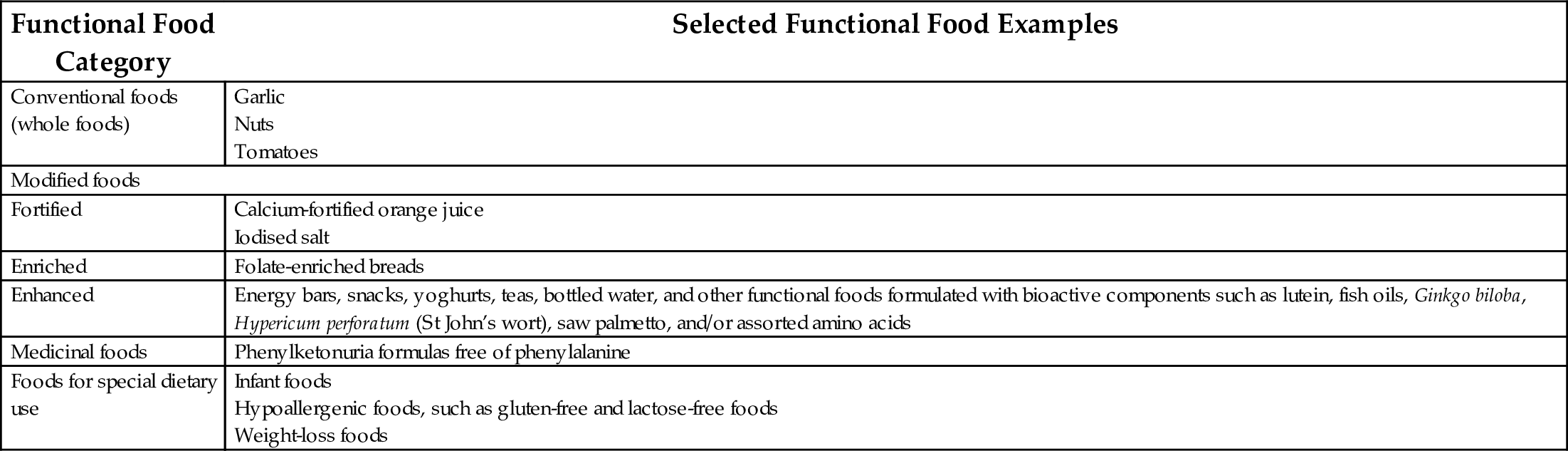
Functional foods are defined as “any food in its natural or processed form that, besides its nutritional components, contains additional components that promote health, physical ability and mental state of a person.” The International Life Sciences Institute (ILSI) establishes that a food is functional if it can satisfactorily demonstrate that it has a beneficial effect on one or more specific functions in the body, improving health and well-being and/or decreasing the risk of disease (Howlett, 2008). According to the American Dietetic Association (ADA), functional foods are “foods that potentially have a beneficial effect on health when consumed as part of a varied diet on a regular basis at effective levels, including fortified, enriched or enhanced foods” (Hasler et al., 2009). Currently, the ADA categorizes functional foods as conventional, modified, medicinal and for special dietary uses, as shown in Table 1.
Table 1
| Functional Food Category | Selected Functional Food Examples |
|---|---|
| Conventional foods (whole foods) | Garlic Nuts Tomatoes |
| Modified foods | |
| Fortified | Calcium-fortified orange juice Iodised salt |
| Enriched | Folate-enriched breads |
| Enhanced | Energy bars, snacks, yoghurts, teas, bottled water, and other functional foods formulated with bioactive components such as lutein, fish oils, Ginkgo biloba, Hypericum perforatum (St John’s wort), saw palmetto, and/or assorted amino acids |
| Medicinal foods | Phenylketonuria formulas free of phenylalanine |
| Foods for special dietary use | Infant foods Hypoallergenic foods, such as gluten-free and lactose-free foods Weight-loss foods |
Source: Hasler, C.M., Brown, A.C., ADA, 2009. Position of the American Dietetic Association: functional foods. J. Am. Diet Assoc. 109, 735–746.
Bioactive compounds are chemicals with potential biological activities and impart functionality to a food beyond its nutritional aspects. Bioactives are usually of plant origin and are products of the secondary metabolism of plants, and are therefore commonly referred to as “phytochemicals” (Keith and Miller, 2013). Phytochemicals have demonstrated antioxidant, anticarcinogenic, antiinflammatory, immunomodulating and antimicrobial properties (Heber, 2004).
3.1 Functional Proteins and Bioactive Peptides
Functional proteins and bioactive peptides are proteins and peptides that can to exert specific biological effects on the body, in addition to their nutritional value as a source of amino acids (Korhonen and Pihlanto, 2003; Rutherfurd-Markwick and Moughan, 2005). Most bioactive peptides are spontaneously generated during in vivo protein digestion. Currently, the production of bioactive peptides from food proteins is performed by in vitro enzymatic hydrolysis, using commercial enzymes, such as alcalase, flavourzyme, pepsin and pancreatin (Segura et al., 2013).
Peptides have been isolated from enzymatic protein hydrolysates of milk, sardines, corn, soy, egg, jelly, and beans, among others (Korhonen and Pihlanto, 2003). The scientific literature shows that bioactive peptides can exert their action both locally (gastrointestinal tract) and systemically, because they can pass through the intestinal epithelium and reach peripheral tissues through the bloodstream (Segura et al., 2010).
Various peptides present antioxidant, antihypertensive, antiinflammatory, hypoglycaemic, immunomodulatory and anticancer activity in vitro and in vivo (Herrera et al., 2014a, b). The novel nutritional concept is the use of food-derived proteins and peptides to enhance a biological function and prevent or decrease the risk of disease, due to a demonstrated effect on the environment of the regulated expression of the human genome (Panchaud et al., 2012).
Biopeptides and several proteins have been proposed to treat dental disease, mineral malabsorption, diarrhea, hypertension, thrombosis and immunodeficiencies. For example, an enteral formula containing TGF-β [Modulen IBD (Nestle, Vevey, Switzerland)] is effective in treating Crohn’s disease, inducing clinical remission and healing mucosa, as a result of its antiinflammatory effect (Hartman et al., 2009; Afzal et al., 2004).
Commercially, bioactive peptides are fundamental constituents of products or ingredients of functional foods, such as the dairy product, Calpis® cultured milk (trademark AMEEL®) marketed in Japan by Calpis Co., which has hypotensive action associated with valyl propyl proline (VPP) and isoleucyl propyl proline (IPP) biopeptides; and the CholesteBlock® drink marketed in Japan by Kyowa Hakko, with hypocholesterolaemic action associated with isolated soy biopeptides (Hartmann and Meisel, 2007).
This review examines the effect of antioxidant and anti-inflammatory peptides that could be used as a alternative dietary cancer treatment.
3.2 Proteins and Bioactive Peptides with Antiinflammatory and Immunomodulatory Activity
Studies of peptides with antiinflammatory activity are limited; however, among the described mechanisms of action, inhibition of the processes that regulate superoxide ion production by neutrophils during the acute phase response in inflammation, is important. This also revealed an important role in the inhibition of leukocyte elastase, which is responsible for the degradation of collagen and proteoglycans and high phagocytosis, for example, in rheumatoid arthritis, emphysema, cystic fibrosis, bronchitis, asthma, acute respiratory distress syndrome and other conditions of chronic inflammation (Drago et al., 2006; Herrera et al., 2014a; Mulero et al., 2011).
Various foods have been proven to decrease the production of certain proinflammatory cytokines important for the development and complication of cancer, such as egg biopeptides (Leea et al., 2009), blueberries (Laua et al., 2009) and the Momordica grosvenori Swingle fruit (Pana et al., 2009), which exhibit a significant decrease in TNF-α, IL-6, IL-1β, IFN-γ, IL-8, IL-17, NOS and cyclooxygenase-2 (COX-2) expression.
Peptides present in egg yolk have antiinflammatory activity in LPS-stimulated RAW 264.7 macrophage cells, modulating the expression of proinflammatory cytokines and promoting antiinflammatory cytokine (Xua et al., 2012). Among the antiinflammatory peptides, those with immunomodulatory activity are of particular interest. The most studied of these are derived from dairy products. This effect seems to be related to the net positive charge of these peptides, which are structurally organized and cause the formation of ion channels in the membrane of bacterial cells, leading to lysis of the plasma membrane and cell death (Bellamy et al., 1992).
Whey has been shown to have antioxidant and immunomodulatory activity (Marshal, 2004). The most accepted mechanism of action associates these activities with a high content of cysteine-rich proteins that contribute to the synthesis and, consequently, increased levels of glutathione, a powerful intracellular antioxidant. Glutathione is also necessary for the activity and proliferation of immune cells, particularly T-cells (Harris et al., 2015).
Clinical studies have shown that administration of whey protein concentrate, in addition to conventional treatments, was useful in the treatment of AIDS, hepatitis B and hepatitis C (Bayford, 2010). In accordance with these findings, administration of such concentrates in AIDS patients is reported to potentially offset the common, glutathione deficiency in these patients (Moreno et al., 2006; Micke et al., 2001). However, results are inconclusive because the duration of treatment and dose of whey used among studies are inconsistent.
The human milk proteins have shown significant immunomodulatory benefits. This is mainly attributed to the casein phosphopeptides and lactoferrin contents, which show resistance to digestion and maintained activity after processes such as pasteurization (Wada and Lönnerdal, 2015).
Studies in animals and cell models suggest that lactoferrin possesses immunomodulatory activity (Siqueiros-Cendón et al., 2014). These studies indicate that lactoferrin may have dual mechanisms of action, the first involves the inhibition of the production of various cytokines, such as TNF-α and IL-1β. The existence of lactoferrin receptors on monocytes, lymphocytes, macrophages, neutrophils and epithelial cells suggest that lactoferrin may directly affect the regulation of cytokine production by regulating signaling pathways mediated by these receptors. The second mechanism of action could be related to inhibition of innate immunity stimulation, by binding to lipid A of bacterial LPSs, as well as oligonucleotides containing unmethylated CpG oligodeoxynucleotides, thereby inhibiting stimulation of Toll-like receptors from macrophages (Onishi, 2011).
Consistent with its immunomodulatory activity, lactoferrin is able to inhibit local inflammatory responses to skin inflammation in humans and modulate the expression of proinflammatory genes in mouse intestine (Wakabayashi et al., 2006), as well as in animal models of skin inflammation, mediated by allergens and intestinal inflammation with a direct action on the levels of proinflammatory cytokines and IL-10, after 24 h of administration (Takakura et al., 2006).
Currently, there are transgenic cows expressing the human lactoferrin gene and, therefore, produce human lactoferrin in the milk (Thomassen et al., 2005). The antimicrobial capacity, antiviral or immunomodulatory are not exclusive to lactoferrin or ovotransferrin, but include peptides resulting from their digestion, such as lactoferricin (LFcina) (Wong et al., 2014) or ovotransferrin A, a 92-amino acid (OTAP-92) peptide, which retains this activity (Ibrahim et al., 2014).
LFcina is a peptide corresponding to the amino-terminal of lactoferrin, which has a bactericidal activity as potent as lactoferrin itself. It has been shown that this peptide also possesses antiviral and immunomodulatory activity. Indeed, in addition to the mechanisms described for immunomodulation lactoferrin, LFcina can inhibit the action of cytokines and released ILs, such as IL-6. It also has the ability to bind DNA, enter the cell, cross the nuclear membrane and act as a transcription factor. Finally, lactoferricin has the ability to potentiate the effect of antiviral and antibacterial agents (Wong et al., 2014).
Lysozyme is another protein known for its immunomodulatory activities, which inactivates many microorganisms by binding to the bacterial wall and breaking the bond between the β-1,4 N-acetylglucosamine and N-acetylmuramic acid. Lysozyme has immunoregulatory functions, which, when combined with immunotherapy, enhances the immune response in immunosuppressed cancer patients (Sava, 1996). It has been suggested that the lysozyme produced by immunomodulation may result from stimulation of the phagocytic function, and peptidoglycan hydrolysis products can act as adjuvants or immunomodulators (Li-Chan and Nakai, 1989).
3.3 Protein and Bioactive Peptides with Anticancer Activity
Several studies have demonstrated, in animal models and in vitro, that whey proteins have anticancer activity. The mechanism of action appears to be related, as previously mentioned, with increase in glutathione synthesis, with consequent stimulation of immunity and antioxidant activity (Xiao et al., 2005). In addition, glutathione is a substrate of two kinds of enzymes: a selenium-dependent glutathione peroxidase and an enzyme belonging to the glutathione transferase family. Both favor elimination of mutagens and carcinogens, which can promote development of cancer (Micke et al., 2001). Glutathione has also been shown to exert iron chelating activity. Iron can act as a mutagen, causing oxidative tissue damage. The anticancer activity of whey proteins has also been associated with inducing the production of somatostatin, a known antiproliferative agent in colon cancer (Wada and Lönnerdal, 2015). However, there are very few human clinical studies to corroborate the results obtained in vitro or in animal models, and the obtained results are ambiguous so further studies are necessary.
The anticancer activity of human lactoferrin has been widely studied. Phase I clinical studies, in which lactoferrin was administered to patients with refractory solid tumors, proved to be nontoxic with a dose tolerance of 1.5–9 g/day (Hayes et al., 2006).
In animal models and in vitro it has been shown that bovine lactoferrin has anticancer activity, inhibiting both tumor growth and metastasis formation. Thus, it has been shown to inhibit carcinogenesis in the colon, esophagus, lung and bladder when administered to rats orally in the post-initial cancer state. Concerning their mechanism of action, intestine bovine lactoferrin enhances the immune response, inducing caspase-1 activity with consequent production of mature IL-18, resulting in potentiation of antitumor activity T- and NK-cells. Consequently, these cells can produce IFN-γ which, together with IL-18, can inhibit angiogenesis. Although the mechanism by which lactoferrin results in the activation of caspase-1 and IL-18 is unknown, it may occur by activating specific receptors within the bowel, epithelial and immune cells. In this regard, it is reported that lactoferrin may induce selective apoptosis of cancer cells by binding to these specific receptors (Iigo et al., 2005; Mahanta et al., 2015).
Lfcina has antitumor activity, with the ability to inhibit the formation of metastases and induce selective apoptosis of cancer cells. Thus, the active Lfcina mitochondrial apoptotic pathway is at least partly related to the generation of ROS mechanisms (Riedl et al., 2015).
3.4 Protein and Bioactive Peptides with Antioxidant Activity
Peptides with antioxidant activity are obtained from various proteins. Protein hydrolysates and peptide fractions can be used as functional ingredients in food systems to reduce oxidative changes during storage. Antioxidant peptides may limit oxidative damage in foods (act as natural antioxidants) and protect cells against in vivo oxidation when ingested (Vioque et al., 2006).
There are many studies describing the antioxidant potential from various peptides in food. These peptides are considered to have the potential to control oxidative processes in the human body (Samaranayaka and Li-Chan, 2011). The possible use of antioxidants during cancer treatment is not conclusive because they might interfere with drug treatment; various studies point to the role of certain antioxidant compounds in preventing cancer.
Most studies related to the intake of antioxidants are epidemiological, and evaluate the decrease in cancer risk attributable to the intake of foods rich in antioxidant compounds (Sharma et al., 2009). Few studies examine the effects subsequent to cancer diagnosis.
Preclinical studies have shown that some antioxidants can protect cells from the damaging effects of chemotherapy and radiotherapy and, therefore, decrease the high toxicity of these treatments. However, this protective effect would also apply to neoplastic cells and may decrease the effectiveness of treatment (Viñas et al., 2012). Peptides can be used to prevent cancer and other diseases by their ability to modulate antioxidant enzymes as an important regulator of proinflammatory processes in the organism. Similarly, various peptides have shown an antioxidant effect in extracellular fluids, which helps balance the redox state of the organism.
Some antioxidant peptides, such as those structurally related to Pro-His-His, may exert a strong synergistic effect with certain other antioxidants, such as phenolic compounds (Saito et al., 2003). In potato, three biopeptides (Phe-Gly-Glu-Arg, Phe-Asp-Arg-Arg and Phe-Gly-Glu-Arg-Arg) have been identified with an inhibitory effect on linoleic acid oxidation at 55.3%, 58.5% and 61.7%, respectively, using a β-carotene bleaching test. Oral administration of these peptides at doses of 100 mg/kg in Wistar rats decreased ethanol-induced damage to the stomach lining by 67.9%, 57.0% and 60.3%, respectively (Kudoa et al., 2009).
In marine organisms, peptides displaying significant antioxidant activity have been found, including the hydrolysates of tilapia surimi (Sun et al., 2013). These peptide fractions exhibit amino acid sequences rich in Trp, Met, Cys and Tyr. Trials in liver cancer cell lines (HepG2) treated with H2O2 showed that one of the three protein fractions exhibited a significant decrease in ROS, compared to controls (Wiriyaphana et al., 2013).
Pinctada fucata-derived peptides demonstrated the ability to restore antioxidant activity in vitro by inducing the activity of antioxidant enzymes such as SOD, catalase (CAT) and glutathione peroxidase (GSH-Px), and decreasing lipid peroxidation (Wua et al., 2013).
Other foods, such as palm oil (Changa et al., 2015), sweet potatoes (Zhang et al., 2014) and egg (Memarpoor-Yazdia et al., 2012), also contain peptides with proven antioxidant activity and, in some studies, fractionated and ultrafiltered protein hydrolysates are reported to show differences in antioxidant activity. In corn gluten, fractions < 10 kDa exhibited higher antioxidant activity (Zhuanga et al., 2013), while in Mucana pruriens (velvet bean) increased in vitro antioxidant activity was most potent for peptides < 1 kDa (Herrera et al., 2014a, b).
Although there are still no studies on the role of antioxidant peptides in cancer patients, their mechanisms of action and potential chemopreventive benefits are expected to be similar to that of other bioactives displaying antioxidant activity. However, unlike other compounds, peptides could favor the modulation of antioxidant enzymes. The development of research in this area opens the possibility to develop new food and innovative treatments for cancer treatment, providing robust scientific evidence of their effectiveness on the physiological status of people with cancer can be proven.
4 Conclusion
Chronic inflammation and oxidative stress are directly involved in the development of chronic diseases, such as cancer. People with cancer have elevated levels of free radicals and proinflammatory cytokines, and low plasma concentrations of antioxidants. Therefore, a diet based on functionality, rather than nutrition alone, will be crucial to improving the physiological state of cancer patients. The use of functional foods offers patients a wider choice of treatment options. Currently, the development of bioactive peptides for cancer are an important ally in its prevention and treatment, and directly relates to their effects on inflammation and oxidative stress. Although more studies are needed to define the effectiveness of dietary peptides in the physiology of cancer, many of their mechanisms of action are already defined and, therefore, these molecules have a potential therapeutic role in this disease.