Virgin Coconut Oil as Functional Oil
Yashi Srivastava⁎; Anil D. Semwal†; Gopal K. Sharma† ⁎ Central University of Punjab, Punjab, India
† Defence Food Research Laboratory, Mysore, India
Abstract
Virgin coconut oil (VCO) is the freshly obtained mature kernel of the coconut, by mechanical or natural means, with or without the use of heat and without undergoing chemical refining. When compared to copra coconut oil (CCO), marginal differences exist with respect to iodine value, saponification value, refractive index, fatty acid profile, specific gravity, and moisture content. VCO has many health benefits, such as preventing the oxidation of low density lipoprotein lipid increasing the antioxidant enzymes. Additionally, total polyphenol, antioxidant activity, tocopherol, phytosterol, monoglycerides, and diglyceride content in VCO samples are different from CCO samples. In vivo studies on Wistar albino rats prove that VCO samples are better in reducing hypercholesterimia and diabetes. VCO was found to be good frying oil in terms of stability and acceptability after 8 h of frying of soaked Bengal gram dhal. Blends of VCO were found to be stable for up to 12 months of storage in various flexible and rigid packaging systems, at varying temperatures. After VCO extraction, the resulting residual material obtained, termed as virgin coconut meal (VCM), has been used to make different traditional Indian sweets (ladoo & burfi) as well as baked goods (biscuit & cake).
Keywords
Virgin coconut oil; Coconut milk; Cholesterol; Diglycerides; 1-Monoglycerides; Polyphenols
Acknowledgment
The authors are grateful to the National Agricultural Innovation Project (NAIP) and Director, Central Plantation Crops Research Institute (CPCRI), Kasargod, Kerala for providing the training and images of the VCO preparation machine, as well as Director, DFRL, Mysore to conduct the complete study.
1 Introduction
The coconut palm (Cocos nucifera), also referred to as “the tree of life”, belongs to family ‘Arecaceae’. It is one of the most important plants, being used for limitless applications worldwide. It is the only known species in the genus Cocos having a tall trunk, growing up to 30 m tall, with pinnate leaves 4–6 m long, and pinnae 60–90 cm long; old leaves break away cleanly, leaving the trunk smooth. There are several different varieties of coconut palms, including tall and dwarf. The common, termed “tall”, varieties are Ceylon, Indian, Jamaica, Malayan, Java, and Laguna. Malayan, green, orange, and Fijian are some of the “dwarf” varieties. Tall varieties are commonly cultivated for commercial purposes. They are slow to mature and only produce flowers 6–10 years after planting. The life span of tall varieties is about 60–100 years. The coconut palm is ideally suited to a humid tropical coastal climate; it can tolerate poor sandy soils and exposure to saline water. The top three countries in coconut production are Indonesia (3.1 million hectares), the Philippines (2.7 million hectares) and India (1.5 million hectares). These countries generate three quarters of the total world production (in nut equivalent) of about 64 billion nuts (Mridula, 2011). The Philippines is the leading exporter of virgin coconut oil (VCO) in the world market. Thailand, Indonesia, India, Malaysia, Sri Lanka, Vietnam, Fiji, Western and Samoa are also producers and exporters of VCO. Major buyers are the USA, Canada, Europe, Asia and the Pacific, the Middle East, Australia, and South Africa (Muralidharan and Jayashree, 2011; Siriphanich et al., 2011).
Coconut fruit (drupe) are light in weight, buoyant, highly water resistant and consist of a husk (35%), shell (28%), meat (28%) and water (15%). It is the source for producing juice, milk, and oil (Nair, 2011; Agyemang-Yeboah, 2011). Coconut is a homestead crop (i.e., traditional agro forestry systems as base crop) in south East Asia, providing income, employment, nutrition, social needs, and natural beauty to local populations involved in its growth (Anithakumari and Lekha, 2011).
The value added products are tender coconut water, coconut protein isolate, coconut skim milk, coconut flour and virgin coconut oil. Commercial interest for all of these exist in international markets, which can strengthen coconut prices worldwide (Songkro et al., 2010). FAO and APCC (2014) reported that VCO production in the Asian Pacific region had increased up to 5%. There are number of industries involved in processing, marketing, and exporting value added coconut products, including ‘Pure Tropic’, a tender coconut water packed in tetra pack for consumption in the US under the brand name ‘Tendo’. ‘Vita Coco’ is also a producer of tender coconut water marketed under iťs own name. ‘Zico’ is another company owned by US Olympic medalists, producing and marketing energy drinks made of pure tender coconut water. ‘Coyo’ (Australian company) makes coconut milk based ice cream. There are > 500 companies in Sri Lanka, and 1000 in Philippines, which are involved in producing coconut products, only a few exist in India (Bose, 2011).
The presence of medium chain fatty acids and good digestibility warrants the use of coconut in value added products for edible and industrial (for the manufacture of chemical feed stocks, synthetic detergents, soaps, and cosmetics) purposes (Che Man and Marina, 2006). The conventional refining process which has been used to produce coconut oil, deodorization processes carried out at high temperatures and exposure to sun light result in the deactivation of bioactive components (e.g., tocopherols and polyphenols) (Grimwood et al., 1975). Virgin coconut oil (VCO) is a newcomer to the fats and oil market, and is fast becoming as valued as virgin olive oil. The term VCO refers to oil that is obtained from fresh, mature coconut kernels by mechanical or natural means, with or without the use of heat, but specifically without any chemical refining (Villarino et al., 2007). Public awareness of functional food oils has increased and it is expected that in the near future, VCO will gain dramatic growth in the market. The demand for this oil continues to rise, which can be attributed not only to its superior flavor, but to potential health benefits as well.
2 Standards
Codex Alimentarius (2006) and Bawalan and Chapman (2006) have defined ‘Virgin oils’ as vegetable oils suitable for human consumption in their natural state, and may only be purified by washing with water, settling, filtering, and centrifuging. The current codex standard for coconut oil, which is based on commercial refined, bleached, and deodorized coconut oil (RBD CNO), states that edible vegetable oils may be refined by alkali extraction and washing, bleaching, and deodorization, to remove undesirable constituents and prolong shelf life (Codex Alimentarius, 2006). Due to specific needs of coconut producers, the Bawalan and Chapman (2006) developed a standard for VCO. In 2004, an intergovernmental technical panel drafted the interim Philippine National Standard for VCO (PNS/BAFPS 22:2004). Marina et al. (2009) reported that VCO did not differ much from Refined Bleached Deodorized (RBD) coconut oil in its values of iodine (IV), peroxide (PV), saponification (SV) and free fatty acids (FFA), when compared to commercial samples from Malaysia and Indonesia. According to the study, the values obtained are within the specification limits of the Codex standard (2006) for refined coconut oil. The study revealed that medium chain fatty acids ranged from 60% to 63% and VCO from Malaysia had relatively higher contents of lauric acid than Indonesian samples. Currently there are three standards for VCO: the Philippines National Standard designated as PNS/BAFPS 22:2004, the Asian and Pacific Coconut Community (APCC) (Table 1).
Table 1
| Parameters | Codex Alimentarius | APCC | PNS/BAFPS 22:2004 |
|---|---|---|---|
| % Fatty acid composition | |||
| C6:0 | ND-0.7 | 0.4–0.6 | ND-0.7 |
| C8:0 | 4.6–10.0 | 5.0–10.0 | 4.6–10.0 |
| C10:0 | 5.0–8.0 | 4.5–8.0 | 5.0–8.0 |
| C12:0 | 45.1–53.2 | 43.0–53.0 | 45.1–53.2 |
| C14:0 | 16.8–21.0 | 16.0–21.0 | 16.8–21.0 |
| C16:0 | 7.5–10.2 | 7.5–10.0 | 7.5–10.2 |
| C18:0 | 2.0–4.0 | 2.0–4.0 | 2.0–4.0 |
| C18:1 | 5.0–10.0 | 5.0–10.0 | 5.0–10.0 |
| C18:2 | 1.0–2.5 | 1.0–2.5 | 1.0–2.5 |
| C18:3 | ND-0.2 | – | ND-0.2 |
| C20:0 | ND-0.2 | – | – |
| C20:1 | ND-0.2 | < 0.5 | – |
| C20:2-C24:1 | ND | – | ND |
| Iodine value | 6.3–10.6 | 4.1–11.0 | – |
| Free fatty acid | None | < 0.4% | 0.2% |
| Moisture % weight max | – | 0.1–0.5 | 0.2% |
| Matter volatile at 105C, m/m | 0.2% | 0.2% | 0.2% |
| Peroxide value | < 15 meq active oxygen/kg oil | < 3 meq/kg | 3 |
| Microbiological contamination | – | < 10 cfu | – |
3 Methods for the Extraction of VCO and Preparation of VCM
Many previous authors have reported on VOC methods of extraction. Normal coconut oil has been extracted from copra and undergoes refining process, while VCO is produced through coconut milk. As per definition, virgin oils never go through the refining steps; their superior quality is attributed to this difference in processing. The next section will describe common VCO extraction methods from different research articles.
3.1 Wet Extraction
This method excludes the use solvents, which may lower the cost and energy requirements (Villarino et al., 2007). The principle behind this method is to break cream emulsion and liberate oil. In the wet method extraction, coconut milk is subjected to processing under mild temperatures, resulting in VCO which retains more biologically active components (Grimwood et al., 1975). In this process coconut milk is subjected to the following steps: creaming, flocculation or clustering, and coalescence. Its commercial potential is more attractive and it is more eco-friendly than solvent extraction.
3.2 Fermentation
In this method, separation of coconut milk emulsion is done by using pure culture of L. plantarum and L. delbrueekii. The L. plantarum strain IAM 1041 inoculation demonstrated a more rapid breaking of the coconut milk emulsion and the liberation of the oil, compared to L. delbrueckii (Che Man et al., 1997). This was because the L. plantarum strain could multiply faster in coconut milk at 40–50°C under microaerophilic conditions that increased the fermentation process. Coconut milk emulsion can also be separated by adjusting the pH between 3 and 5.6 using bacterial cultures (Chen and Diosady, 2003). Che Man et al. (1996) improved the oil recovery up to 60% using 25% acetic acid at different levels for 10–14 h at room temperature. Other treatments are also employed to break the coconut milk emulsion, such as heat, brine solution, refrigeration, enzymes, and acidification short waves (Seow and Gwee, 1997).
3.3 Enzymatic
A plant cell wall consists of complex carbohydrate molecules such as cellulose, hemicellulose, or mannans. (Christensen, 1991). For destruction of the cell wall and liberation of oil, some degradation enzyme such as cellulose, alpha-amylase, polygalacturonase, or protease, at 1% level, is applied to extract 74% of the oil (Che Man et al., 1996). Cell wall degrading enzymes can be used to extract oil by solublizing the structural cell wall components (mannan, galactomannan, arabinoxylogalactan, and cellulose) of the oil seeds.
3.4 Low Temperature/Centrifuge/Super Critical Carbon Dioxide Extraction Technique
Refrigeration is a common method to prepare VCO by cooling the coconut milk (at 4°C for 2, 4, 6, 8, and 24 h) in order to separate the two phases; upper cream phase and lower watery phase. The cream is further subjected to mild heating (50°C) in an oven for 1, 2, 4, 6, and 24 h to separate VCO (Songkro et al., 2010). Marina (2008) has reported two methods, that is Robledano-Luzuriage and Krauss-Maffei, known to apply a freeze and thaw operation in the extraction of coconut oil. In previous methods, one fresh coconut kernel was pressed to obtain approximately equal amounts of emulsion and residue. The residue was pressed again to obtain more emulsion and residue. The emulsion was centrifuged to obtain cream, skim milk, and some solids or protein. Songkro et al. (2010) applied the same cooling technique (fresh coconut milk to 4°C for 0, 0.5, 1, 1.5, 2, and 4 h) but after cooling, the sample was centrifuged at 9000 rpm at 10°C for 5 min resulting in two separated layers. The oily layer obtained was subjected to mild heating (50°C) for 15 min, and again centrifuged for 5 min at 25°C at similar rpm. This method gives the highest yield of VCO in comparison to refrigeration. Super critical carbon dioxide extraction technique has also been used to produce VCO at pressure range from 13.8 to 34.5 MPa and a constant temperature of 70°C shows a variation in yield is pressure dependent. Only 20% yield was obtained at 13.8 MPa, whereas the extracted yield increased to > 95% as the pressure increased to 20.7 MPa. At 34.5 MPa the yield was almost 100% (Nik Norulaini et al., 2009). Further studies such as dynamic method were used to investigate the solubility of virgin coconut oil (VCO) in SC-CO2 at temperatures and pressures ranging from 313 K to 353 K and 20.7 MPa to 34.5 MPa, respectively. VCO solubility increases with temperature at pressures between 31.0 and 34.5 MPa, while at pressures between 20.7 and 24.1 MPa, VCO solubility decreases with an increase in temperature (Zuknik et al., 2016).
3.5 Extraction of VCO (Process Optimized by Central Plantation Crop Research Institute, Kasargod, Kerela, India)
VCO was extracted by the method described by Villarino et al. (2007), with some modifications. The principal steps involved were production of coconut milk and extraction of oil. Extraction of oil was done using two processes: cold extraction and hot extraction (Tables 2 and 3).
3.5.1 Preparation of Coconut Milk
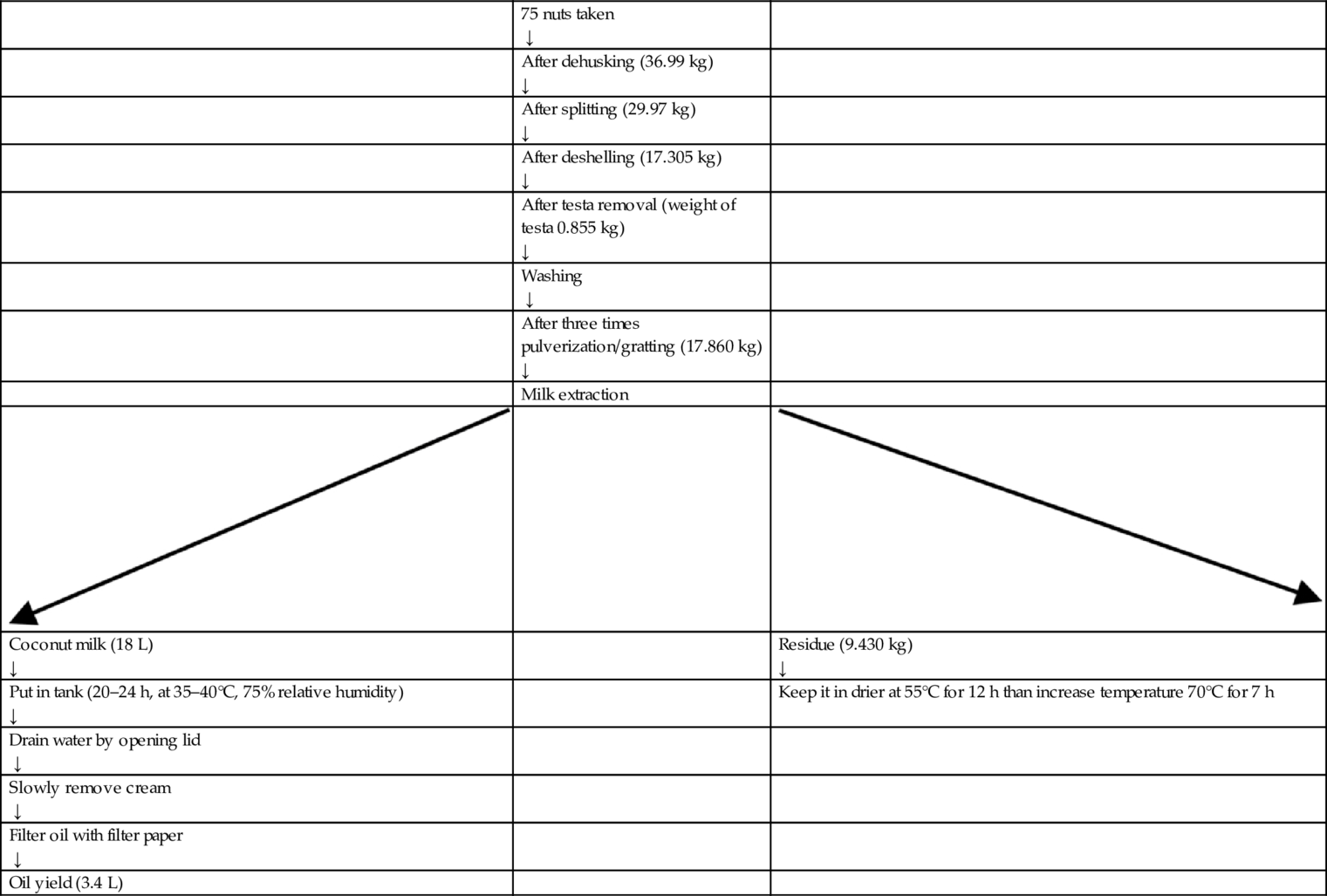
The production of coconut milk involves selection of nuts, dehusking, deshelling, testa removal, washing, grating, and milk extraction. Fully mature 10–11 month old nuts were selected for VCO production (Fig. 1A). As an indicator of maturity, the husk was yellowish to brown in color and made a sloshing sound when shaken. Using a tool specialized to the task, the husk and shell were removed without breaking the coconut kernel (Fig. 1B and C). After breaking the coconut in two halves, the kernel was scooped out (Fig. 1D). The testa of the coconut kernel was removed with a testa remover machine (Fig. 1E). The coconut kernel, minus its testa, was washed and fed in a mechanical grating machine containing rotating blades (Fig. 1F–J). Milk was extracted from the grated coconut meat using a manually operated hydraulic coconut milk press (Fig. 1K). The coconut milk obtained from the first extraction was collected separately, and the residue was subjected to extraction a second and third time. The first, second, and third milk extracts were combined through vigorous stirring for a few minutes.

Preparation of Cold Extracted Virgin Coconut Oil (CEVCO)
In cold extraction method, coconut milk was allowed to stand for 20–24 h. Under favorable conditions (35–40°C, 75% relative humidity), the oil separates from the water and the protein (Fig. 1M). Ambient airborne lactic acid bacteria, which has the capability to break the protein bonds, act on the coconut milk mixture, causing VCO separation. The fermenting container should be made of food-grade transparent plastic. It should be wide mouthed for easy removal of the curd. The extraction container can also be a food grade stainless steel cylindrical tank with a conical bottom, an outlet tap and a sight glass for visual access and assessment of the different layers as the oil separates. Oil can be withdrawn from the outlet tap using levels shown in the sight glass as a guide. Proper operating conditions and sanitary precautions were strictly followed. Four distinct layers could be visible in the container after settling for 20–24 h. The bottom layer was made up of gummy sediment. The next layer was the watery, skim milk, which is no longer fit for human consumption. The next layer was the separated oil for recovery as VCO. The top layer was floating curd. The curd also contained a considerable amount of trapped oil. By carefully separating the distinct layers, the oil can be separated. The separated oil contained some adhering particles of curd and needed filtration. Oil was filtered through sterilized filter paper in a funnel. The procedure to extract CEVCO from 100 nuts is shown in flowchart (Table 2).
Table 2
| 75 nuts taken ↓ | ||
| After dehusking (36.99 kg) ↓ | ||
| After splitting (29.97 kg) ↓ | ||
| After deshelling (17.305 kg) ↓ | ||
| After testa removal (weight of testa 0.855 kg) ↓ | ||
| Washing ↓ | ||
| After three times pulverization/gratting (17.860 kg) ↓ | ||
| Milk extraction | ||
 |  | |
| Coconut milk (18 L) ↓ | Residue (9.430 kg) ↓ | |
| Put in tank (20–24 h, at 35–40°C, 75% relative humidity) ↓ | Keep it in drier at 55°C for 12 h than increase temperature 70°C for 7 h | |
| Drain water by opening lid ↓ | ||
| Slowly remove cream ↓ | ||
| Filter oil with filter paper ↓ | ||
| Oil yield (3.4 L) |
Preparation of Hot Extracted Virgin Coconut Oil (HEVCO)
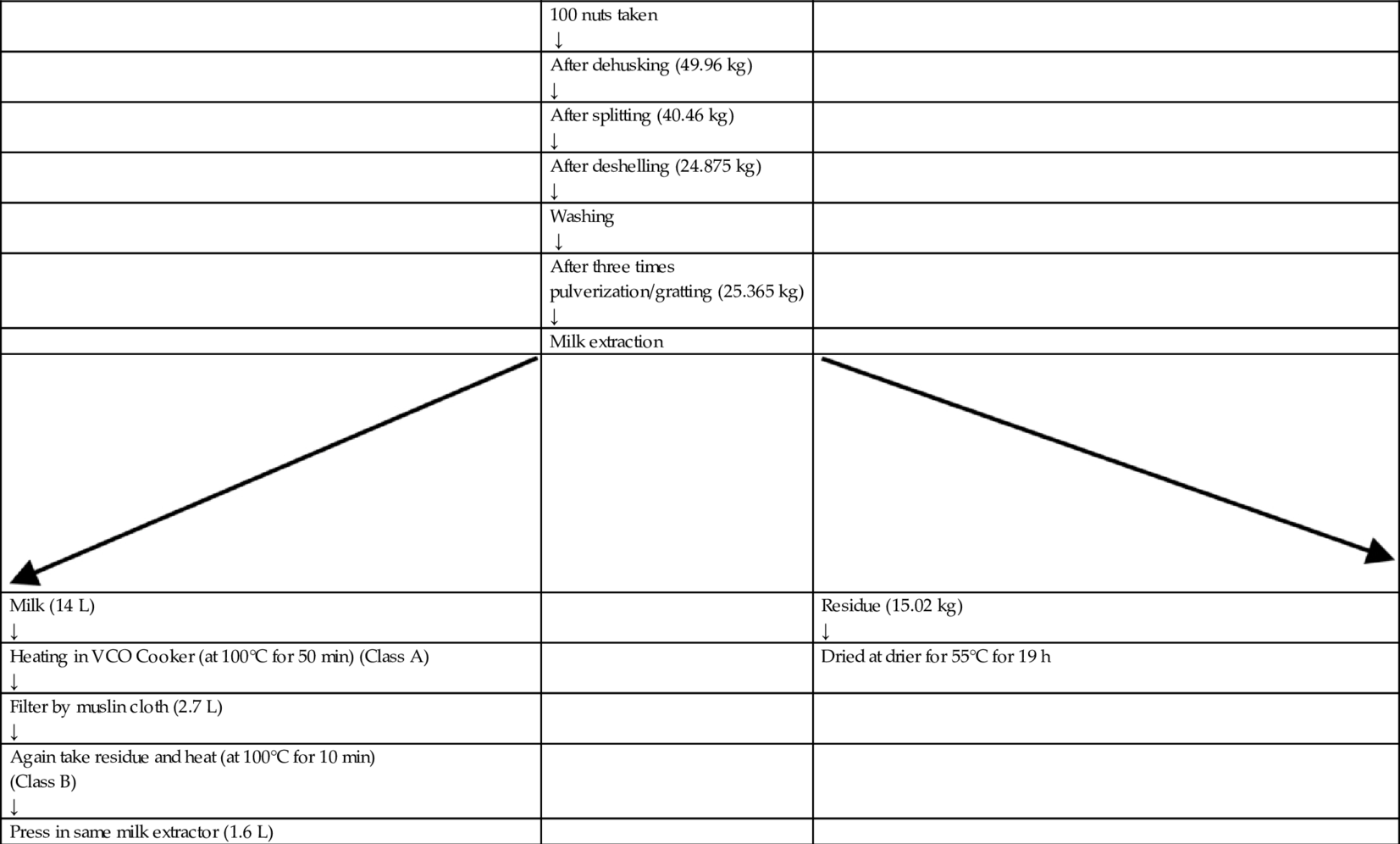
Coconut milk is an emulsion of oil and water that is stabilized by protein. To recover the oil from coconut milk, the protein bond was broken using heat in double walled boiler, known as a VCO cooker, with slow heating to coagulate the protein and release the oil. Separation of class A oil from the proteinaceous residue (kalkam) by straining the mixture through a muslin cloth. The remaining kalkam can be slow heated again to separate remaining oil (Class B) (Fig. 2A–H). The procedure for making HEVCO from 100 nuts is given in Table 3.

Table 3
| 100 nuts taken ↓ | ||
| After dehusking (49.96 kg) ↓ | ||
| After splitting (40.46 kg) ↓ | ||
| After deshelling (24.875 kg) ↓ | ||
| Washing ↓ | ||
| After three times pulverization/gratting (25.365 kg) ↓ | ||
| Milk extraction | ||
 |  | |
| Milk (14 L) ↓ | Residue (15.02 kg) ↓ | |
| Heating in VCO Cooker (at 100°C for 50 min) (Class A) ↓ | Dried at drier for 55°C for 19 h | |
| Filter by muslin cloth (2.7 L) ↓ | ||
| Again take residue and heat (at 100°C for 10 min) (Class B) ↓ | ||
| Press in same milk extractor (1.6 L) |
3.6 Preparation of Virgin Coconut Meal (VCM)
Virgin coconut meal after the extraction of VCO was supplied by Central Plantation Crops Research Institute (CPCRI) of Kasargod, in Kerala, India. The VCM was powdered in an ultra centrifugal mill (Retsch RI, Germany) and passed through 500 μ (35mesh) for use as in ingredient in food products.
4 Physicochemical Properties and Therapeutic Use of VCO
Following the Second World War, negative publicity against the vegetable oil industry created a common misconception that vegetable oil contributes to heart disease. Because of this, knowledge about the benefits coconut oil has been out of the public eye and interest for decades (Hegde, 2009). Coconut oil is rich in saturated medium chain fatty acids, and though the assumption could be made that it is cholesterogenic, animal studies demonstrate this to be flawed thinking, as they were used as hydrogenated coconut oil. There is a remarkable difference between coconut oil and other seed oils, in that it contains skin of the coconut kernel known as ‘coconut testa’ which plays an important role in the phenol content of the oil. Phenollic dependent antioxidant capacities are expected more for VCO than copra oil, because solubility of polar phenolic substances in non polar coconut oil is certainly improved at a high temperature (Kapila et al., 2009). VCO contains phenollic compounds like caffeic acid, p-coumaric acid, and ferulic acid, while syringic acid was not detected in refined bleached deodorized coconut oil (Seneviratne and Dissanayake, 2008). There is a direct relationship between the phenollic content and the antioxidant capacity of plants. Antioxidants may function as free radical scavengers, reducing agents, complexes of pro-oxidant metals and quenchers of the formation of singlet oxygen. Marina et al. (2009) reported contrasting results from the previous research, finding that VCO obtained from fermentation had highest phenolic content than VCO obtained from chilling technique as well as refined bleached deodorized coconut oil. Consequently, VCO obtained from fermentation and chilling technique has higher scavenging activities of 95% and 93% respectively with refined bleached deodorized coconut oil. Oil had a slightly lower percent, that is 81%. VCO itself contains beneficial natural antioxidants, namely tocopherols, which can protect oil against atmospheric oxidation and rancidity (Enig, 2000). VCO has been reported to have antibacterial, antiviral, antinociceptive, and anti-inflammatory properties (Zakaria et al., 2011a, 2011b).
Physico-chemical and sensory properties are the distinguishing features which differentiate refined bleached deodorized coconut oil and VCO. Villarino et al. (2007) determined that descriptive sensory analysis provides important criteria to better discriminate VCO from refined coconut oil, because RBD coconut oil has distinct yellow color, and an imperceptible aroma while VCO was almost colorless and had an acid, cocojam (aroma associated with roasted coconut), latik (aroma of cooked coconut with sweet sensation), nutty, and rancid aromas, in sensory analysis of a trained panelist. VCO contains more unsaponifiable components like vitamin E and polyphenols than refined bleached deodorized coconut oil, resulting in an increased level of antioxidant enzymes and reduced lipid peroxide content (Nevin and Rajamohan, 2006). Dayrit et al. (2008) has differentiated the VCO from RCO by using P31 NMR. He found out that 1-monoglycerides was higher in VCO (0.027%) than RCO (0.019%), and total sterols were more in VCO (0.096%) compared to RCO (0.032%), while diglyceride was lower in VCO (1.55%) than RCO (4.10%). Currently, due its high market value, VCO is prone to adulteration by the addition oils of lesser value. An expeditious and efficient method for detecting adulteration of VCO needs to be developed. Some methods for monitoring adulteration in VCO using FTIR (Rohmana and Che Man, 2010), and a SAW sensor electronic nose have been created to distinguish a pattern of volatile compounds present in tested samples. VCO was differentiated with refined bleached deodorized palm kernel olein using an electronic nose (Marina et al., 2010).
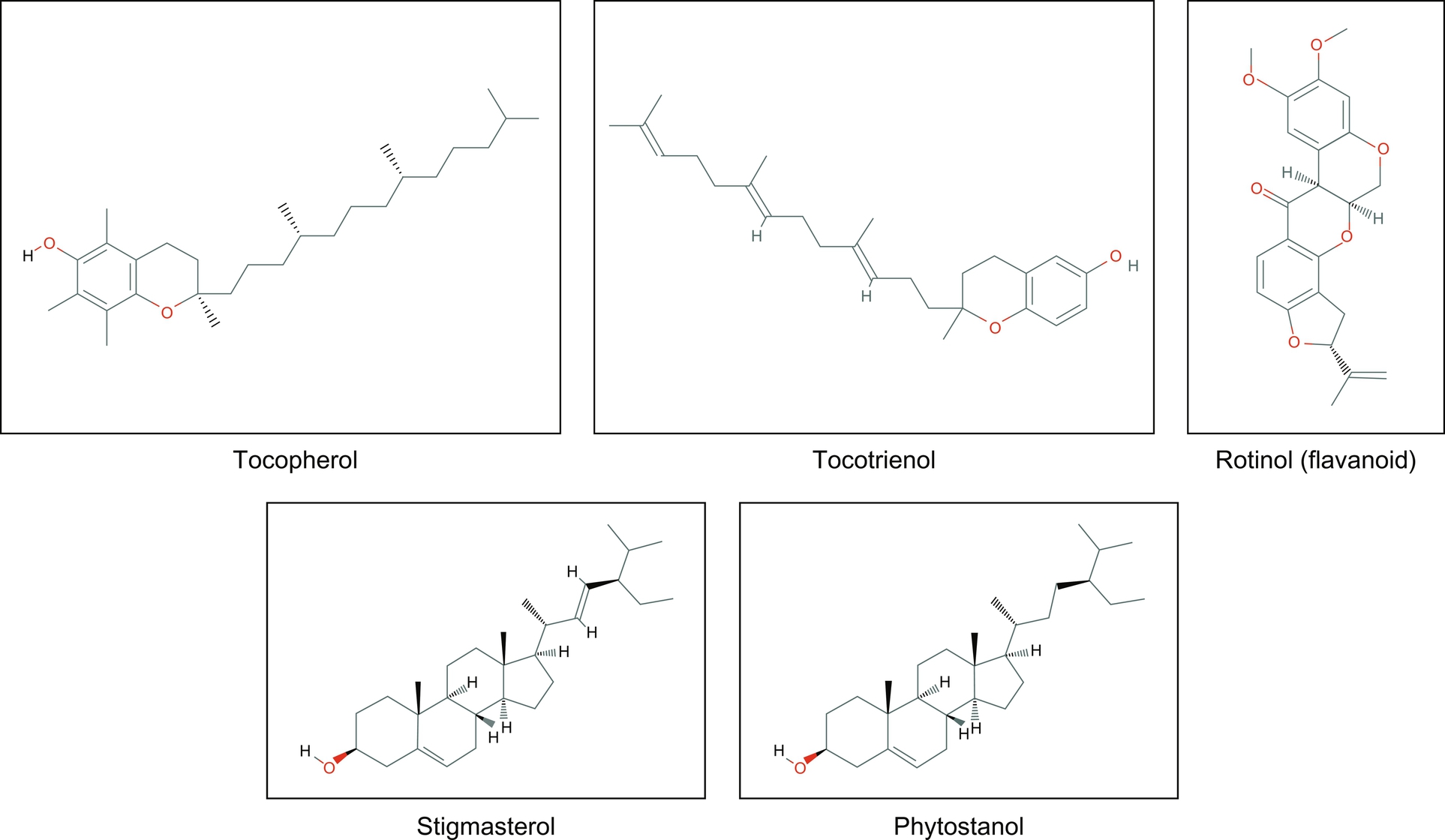
As per earlier studies, VCO with more vitamin E and polyphenols than copra oil, prompting increased levels of antioxidant enzymes in rats fed with diets containing these coconut oils for 45 days (Nevin and Rajamohan, 2006). Some preliminary effects of coconut oil on HIV/AIDS gave very encouraging results with a dramatic reduction in the subjects’ viral load and immune system enhanced, as reflected in the CD4/CD8 count (Dayrit et al., 2000). Coconut oil, being a plant product, is rich in biological active compounds such as tococpherol, tocotrienols, phytostenols, and polyphenols (Fig. 3). VCO is rich in medium chain triglycerides, providing wellness for individuals suffering from short bowel syndromes, childhood epilepsy, aptic fibrosis, bypass surgery recipients, and for premature babies (Babayan and John, 1991). It has potential to meet the needs of people requiring high energy foods in their diets. VCO is not only able to reduce the level of total cholesterol, triglycerides, phospholipids, low-density lipoprotein (LDL) and very low density lipoprotein cholesterol, but can also increase the concentration of high density lipoprotein (HDL), which is good cholesterol (Srivastava et al., 2013; Nevin and Rajamohan, 2004).
5 Value Added Products, Economy, Status, and Government Policies
> 10 Million farming families depend on the coconut crop or ‘orphan crop’ for their livelihood. Even though iťs a major exporter of coconut, India consumes > 50% of its coconut production of 15.84 billion nuts per annum, as raw nuts for culinary and religious purposes. 35% of the production is utilized for conversion to copra, 11% for tender nut, 2% for seed purposes, and a scant 2% is utilized for value added products and industrial purposes (Muralidharan and Jayashree, 2011). Ironically, in the home countries of coconut growth, researchers are often attracted to international funding for support, in spite of its potential to bring positive impact (e.g. economic benefits) to local farm communities (Etherington, 2005). Governmental bodies worldwide are providing assistance to promote more research and development of coconuts, such as ACIAR in Australia, Centre de Cooperation Internationale en Recherche Agronomique pour le Development (CIRAD, formerly IRIIO) in France, and the Coconut Development Board in India, to name a few. ACIAR has provided 4.5 million of funding for 11 research projects. The main target for ACIAR projects are crop improvement, crop protection, and an increase to the socioeconomics of coconut production. The Coconut Development board (CDB) is able to extend help for export to international markets, having been designated ‘Export Promotion Council’ for coconut products (other than coir and coir products) by the government of India. CDB has launched a Technology Mission on Coconut (TMOC) program, under which domestic and NRI entrepreneurs, cooperatives, and producer companies undertaking coconut based projects are eligible for financial assistance. Coconut is a smallholder’s crop, comprising just 2% of agriculture and associated sectors in India. The average productivity of coconut in the country is 8303 nuts per hectare, that is 48 nuts per palm per year. The government of India has established the Ministry of Food Processing Industries for the promotion of food processing in the country. APEDA is also supports the advertising, publicity, and export of Indian agriculture products. The Small Farmers Agri Business Consortium (SFAC) is extending 10% venture capital funding for Farmers Producer Organizations (FPO). 25% of the capital subsidy is available from the Coconut Development Board for producing various coconut products. The lack of entrepreneurial spirit could be a reason for India’s small coconut production (Bose, 2011). The incentives made under the Technology Mission on Coconut (TMOC) program has increased the efforts in the coconut sector for developing value added products and increasing product use. Under the program, 164 coconut processors with infrastructure facilities worth 152.43 crores for processing 1216 million nuts per year have been established. 9 tender coconut packing processors, having capacity to process 36 million nuts per year, and 13 activated carbon processors with a capacity to produce 30,000MT per annum, have been established. These efforts have helped India to excel in the world coconut market, along with Sri Lanka and the Philippines (Sebastian, 2011).
Various oil cakes, or press cakes, have been used to feed poultry, fish, and swine on an industrial scale. Being rich in protein, some of these have also been considered ideal for food supplementation. However, with increasing emphasis on cost reduction of industrial processes, and value addition to agro-industrial residues, oil cakes could be an ideal source of proteinaceous nutrients, and as support matrix for various biotechnological applications. Several oil cakes, edible oil cakes in particular, offer potential benefits when utilized as a substrate for bioprocesses. These have been used for fermentative production of enzymes, antibiotics, and mushrooms, to name a few. Biotechnological applications of oil cakes also include their usages for vitamins and antioxidant production (Ramachandran et al., 2007). Recently, the Defense Food Research Laboratory (Mysore, India) prepared traditional Indian sweets like ladoo and burfi using VCM as a base with 15% or 20% VCM incorporation, having a four month shelf life (Srivastava et al., 2010, 2011). VCM based bakery products like biscuits, cake, and bread have been prepared with a six month shelf life.
6 Other Commercial Utilization of VCO
The lauric acid contained in VCO has the ability to convert into monolaurin which can destroy the lipid membrane of bacteria. Hence, this property of VCO was determined to act as a natural food preservative via bacterial destruction. It was reported that the submersion of chicken meat in VCO can decrease moisture content and bacterial colony count while simultaneously increasing the protein content and shelf life of the meat. The submersion of chicken meat for 2 h in VCO has improved the shelf life of the chicken meat stored at room temperature (Salam et al., 2009).
Global warming causes immune suppression, photo aging, and skin carcinogenesis because these common problems are associated with UV radiation. Looking for a solution, public attention has focused on the use of natural ingredients, such as vitamins, minerals, and botanical extracts to protect the skin diseases. It has been assumed that VCO exhibits protective qualities against UVB induced erythema and pigmentation (Merlin et al., 2008). VCO is mainly used for cosmetic and aromatherapy (considered as a complimentary therapy). The oil utilized for aromatherapy, such as massage oil, is made with a carrier oil. Among commercially available carrier oils, VCO has been found to be the best (Lis, 2006). VCO has been blended with lemon, eucalyptus, and lavender oil to produce effective and economical massage oils (Songkro et al., 2010). In addition to being an effective carrier oil, VCO is able to form nano-emulsion with water, the key ingredient of the cosmetic creams. Nano emulsion (mini emulsions or fine disperse emulsions or submicron emulsions) is an attractive system for many industrial applications, due to its purity and simplicity. Nano-emulsion (oil-in-water [o/w] or water-in-oil) is transparent or translucent colloidal dispersion, usually in the 20–500 nm size range (Usn et al., 2004). It is formed by the dispersion of one liquid phase into the second liquid phase to form a droplet (Fernandez et al., 2004). Whitening cream of VCO and emulium kappa (emulsifier) is made as a nano emulsion (Al-Edresi and Baie, 2009). Songkro et al. (2010) has blended VCO with lemon, eucalyptus, and lavender oil to produce massage oil.
Generally, medium chain fatty acids are utilized in flavor industries because they are more polar, hydrophilic, and can dissolve a variety of polar substances which are insoluble in conventional fats and oils. VCO is considered good frying oil as it has relatively high oxidative stability after 8 h of soaked Bengal gram dhal frying. The oxidative stability of VCO was indicated by peroxide value, FFA, TBA, TC, and anisidine value. The color and viscosity of VCO was strongly affected by increased formation of dimmers, trimers, polymers, epoxides, alcohols, and hydrocarbons. The increment in frying temperature and time leads to polyphenol oxidation, and decrease in antioxidant activity. This can be deduced from the present study that VCO can become a good frying medium for commercial world.
In recent years the rise in world petroleum prices devalued national currencies. Research has proved that coconut oil is a viable source for natural biofuel. It can substitute diesel and act as blend substitute for straight kerosene. The relatively low cost of nuts and labour could make production of coconut oil for fuel an workable economic proposition.
7 Future Perspective of VCO
In spite of the fact that VCO possesses numerous medicinal applications and health benefits, marketing is greatly hindered by the consistent message from media declaring coconut oil a harmful saturated fat which is best avoided. In developing countries, manufacture of coconut value-added products exists as a cottage industry for local markets. To enhance the widespread circulation of coconut products in rural and urban areas, governments should implement strong supportive policies. There have been major failures by export marketing authorities, and by research institutes, who have only focused on crop technologies, leaving the task of developing marketing strategies untouched. These failures occur amid volatile markets and falling prices of coconut oil on world markets, and has had catastrophic consequences for small farmers and their families. In the development of product diversification and attention to processing technologies, local knowledge and local demand were largely ignored. Australian involvement in coconut R&D is now driven by its mission to help developing countries, particularly those in the Asian Pacific region. VCO is not very popular commercially because many weakness still need to be acknowledged and addressed, such as unimproved coconut palms, lack of appropriate research, untapped international markets for coconut value added products, and failure to adopt cheaper, efficient, effective environmentally friendly coconut processors.
8 Conclusion
In global markets the first appearance of VCO resulted in wide interest and acceptance, not only among scientific communities, but also in the public as a functional food. VCO does not undergo the RBD process, which destroys some of the biologically active components such as phenolic compounds. Clinical trials have demonstrated VCO to have many beneficial properties as it contains a high polyphenols content and antioxidant potential compared to refined coconut oil. Further studies are required to establish effective purity criteria for VCO.