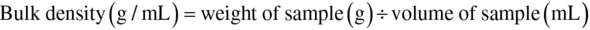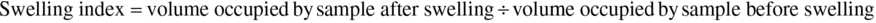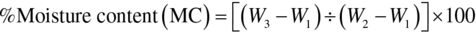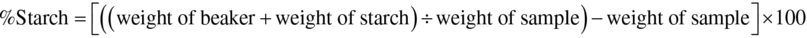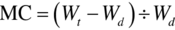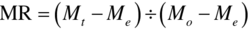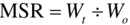Unripe Plantain Flours
Ijeoma A. Olawuni*; Florence O. Uruakpa†; Abimbola Uzoma* ⁎ Federal University of Technology, Owerri, Nigeria
† Missouri State University, Springfield, MO, United States
Abstract
Background and Objectives
Green, or unripe, plantain (Musa spp.) is rich in micronutrients and contains resistant starch which makes the flour a good source of dietary fiber. Reports on the effects of maturation time, and pretreatment on the functional properties and drying kinetics of the flours, are unavailable or very limited. The drying methods directly effect the final quality of the product, influencing rate of moisture loss and extent of exposure to contaminants. The traditional method of drying produces flours with varying moisture content and exposure to dust and other contaminants. This yields plantain flours with varied qualities. It is hypothesized that the use of a solar dryer will yield flour of consistent moisture content and better quality. There is a need to develop specifications for drying plantain pulp in order to obtain high quality flour and reduce wastage. This would add value to its use in food processing. This chapter discusses the impact of drying methods, maturation time, and pretreatments on the functionality of plantain flours.
Methods
Green-horn cultivars of fresh plantains were harvested at different periods of maturity (from the 9th to 13th week) at Federal University of Technology Owerri Farm (FUTO Farm). The different batches were washed, peeled, and cut into slices 1 cm thick. Each batch of sliced plantains was blanched, treated with citric acid or sodium metabisulphite. Samples were dried using a solar dryer, oven, or direct sun. Then samples were ground into flour. Untreated flour served as the control. The drying attributes of samples dried in the solar chamber, oven, and direct sun were investigated. Afterwards, the stability and microbial load of the flours was examined after a 6 month storage period.
Results
Data show that at week 12, water absorption capacity among samples differed (P < .05): control (2.25 mL/g), citric acid-treated (2.78 mL/g), and sodium metabisuphite-treated (2.74 mL/g) flours. Higher pH values were recorded for flours treated with sodium metabisulphite. Maturity time and pretreatment had no effect on the oil absorption capacity, gelling point, and bulk density of the flour samples. Findings show high microbial count was observed in oven dried samples with values of 3.3 × 105–4.0 × 104 CFU/mL for bacteria, and 4.2 × 105–2.6 × 105 CFU/mL for mold. This could be a result of high water activity of oven dried plantain flour. However, there was no salmonella in the flours, making them safe for consumption.
Conclusion and Significance
Thus, plantain flours can be stored for more than 6 months without spoilage. The usefulness of plantain flour could be enhanced by manipulating drying techniques and pretreatments to give desired quality attributes.
Keywords
Flours; Plantain; Drying methods; Pretreatment; Drying characteristics; Maturation time; Functional properties
1 Introduction
The plantain (Musa paradisiacal L) is an important perennial monocotyledonous crop in tropical and subtropical regions worldwide (Swennen, 1990). Plantains, (AAB) as well other cooking bananas (AAB and ABB), East Africa cooking bananas, beer bananas (AAA) and dessert bananas (AAA) belong to genus Musa. They constitute a major source of carbohydrates for millions of people in Africa, the Carribbean, Latin America, Asia, and the Pacific. Due to the perishable nature of the fruits, the rate of plantain postharvest losses varies between countries, according to the organization of production and market chains, and modes of consumption. In many producing countries, there are no data on postharvest losses (N’daAdopo, 1996). The assessment of these postharvest losses is rather complex because both green mature plantains and over ripe fruits are consumed. However, factors likely to depreciate quality and provoke postharvest losses include poor transportation and distribution facilities in the production areas, harvest at maturity close to fruit ripening, and poor storage conditions. In Cameroon, the most evident postharvest losses are at the producer level, in enclave sites during the rainy season (N’daAdopo, 1996). These losses should be less than 35% in developing countries, as previously estimated by FAO (1989).
2 ACTUAL Problem
The drying methods have a direct effect on the final quality of product, influencing the rate of moisture loss and extent of exposure to contaminants. The traditional method of drying is usually associated with varying moisture content and exposure to dust and other contaminants, consequently resulting in plantain flour with variation in quality. The use of a solar drying cabinet may result in the production of flours of a more consistent and higher quality. The degree of brown pigments (mellanoidin compounds) produced during the drying process of the plantain pulp, depend on a number of factors, such as the level of reducing sugar, heat, pH, and temperature. These factors can be controlled in order to reduce browning of the final product. This discoloration issue can be prevented or controlled by the use of pretreatments. Water-soluble sulfide salts commonly used to prevent discoloration are potassium metabisulphite (K2S2O5) and sodium metabisulphite (Na2S2O5). Besides these chemical treatments, blanching may also be employed. Plantain flour comes from different grades of fruit, and no standard quality characteristics exist to determine suitability for human health and nutrition or specific industrial uses. Other factors that may influence the use of plantain flour are maturity index and drying methods. Thus, the effects of these pretreatments and factors on specific properties of dried plantain flour needs to be investigated. Dried plantain flour is predominantly produced by open-air sun drying. This drying method practiced by rural farmers utilizes sun energy, which is environmentally friendly, but plagued with numerous disadvantages (Ekechukwu and Norton, 1999; Hollick, 1999; Togrul and Pehlivan, 2004; Bala et al., 2003). It is probable that using a distributive-type passive solar dryer could produce plantain flour with prolonged shelf-life, while preserving essential micronutrients which give the flour its desired qualities.
3 Justification, Objectives, and Scope of This Study
This chapter aims to (1) determine the maturity indices as the plantain fingers develop; (2) establish the most suitable stage of maturity at which the plantain may be harvested green for high quality shelf-stable flour for nutritional and functional application; (3) understand the flour drying kinetics, using open-air, sun drying, and solar cabinet dryer, with special attention given to predicting drying parameters and the influence of processing variables, such as air temperature and relative humidity; (4) investigate the influence of these parameters and pretreatments (blanching and sulphating) singly and combined on the functional, pasting, and thermal properties of plantain flour and its potentials a food ingredient; and (5) assess the microbial stability of flour samples during 6 months of storage. The specific objective was to compare the quality characteristics of solar and sun-dried plantains chemically treated, and untreated.
Green plantain is rich in micronutrients and contains resistant starch which makes its flour a good source of dietary fiber (Juarez-Garcia et al., 2006 and Mota et al., 2000). The resistant starch in plantain occurs in native starch granules, and its crystallinity makes less susceptible to hydrolysis (Gonzalez-Soto et al., 2007). This property imparts positive effects in the human colon, by alleviating colon diseases such as cancer (Langkilde et al., 2002; Englyst et al., 1992). Plantain is rich in micronutrients and the resistant starch composition is directly related to its maturity (starch content). The different stages of the fruit development present different physicochemical properties. The drying conditions and the method of drying alter the morphology of the starch granules. These parameters affect the quality of the final product. Therefore, it is important to develop specifications for harvesting plantain and drying its pulp to obtain high quality flour. This will reduce annual wastage by adding value to its applications and yielding more income to farmers.
4 Agronomy
Plantains (Musa paradisiacal) are widely used in meals and snacks in Nigerian diets. They a are staple food in tropical countries (Oke et al., 1998). The diversity in the plantain crop results from a variety of cultivars. All modern plantain cultivars have three sets of chromosomes (i.e., they are triploid). Many are hybrids derived from the cross of two wild species, Musa acuminata and Musa balbisiana. The currently accepted scientific name for all such crosses is Musaparadisiaca (TAXON, 2018). Devos et al. (1980) reported more than 70 cultivars in Africa. Over 20 cultivars have been reported in Nigeria, although only a few have commercial importance. Plantains are grown 30oN and South of the equator (Purseglove, 1968). Different types of plantain include:
- • French Plantain: Inflorescence is complete at maturity (many hands consisting of many rather small fruits) followed by inflorescence axis covered with persisting hermaphrodite flowers, and male flowers, the male bud is large and persistent (Ogazi, 1996).
- • French Horn Plantain: Inflorescence is incomplete at maturity (hands consisting of large fingers followed by few hermaphrodite flowers).
- • Horn Plantain: Inflorescence is incomplete, few hands consisting of few but very large fingers, no hermaphrodite flowers, and no male inflorescence.
As reported by Food Network (2018), plantain can be classified scientifically by kingdom, division, class, order, family, genus, and species as follows:
- Kingdom: Plantae
- Division: Magnoliophyta
- Class: Liliopsida
- Order: Zingiberales
- Family: Musaceae
- Genus: Musa
- Species: Musa paradisiaca
In planting plantains, control measures require using wholesome corms, planting in good soil, and covering the fruit well with a plastic sleeve to minimize mechanical damage or injury. Immature fruits tend to ripe earlier than mature fruits, so it is important to control this effect by allowing the plantain to grow up to three quarter full before harvesting (Hoffman and Yang, 1980). It is recommended that plantain be harvested at the right stage of maturity, which is at ¾ full growth. When harvesting, care should be taken to prevent the plantain from falling to the ground. Once the stem is removed from the tree for distribution and transit, rot and spotting fungi can become active and they can initiate decomposition. Therefore, careful handling during the harvest is an essential part of fruit disease control. Fruits that are over mature tend to ripen in transit, and are more susceptible to rotting and peel blemishes. Ethylene produced by ripe fruit can trigger adjacent green fruits to ripen (Chan, 1983). The ripening process in plantain is summarized into eight stages as follows:
- • Green
- • Green with trace of yellow
- • More green than yellow
- • More yellow than green
- • Only a green tip on pedicel remaining
- • All yellow
- • Yellow flecked with brown
- • Large brown areas
5 Economic and Social Impact
Bananas and plantains are grown in more than 120 countries, in backyards or in mixed cropping systems by small holders, and occasionally in monoculture. The total production is about 64 million tons with 23% of AAB plantains, 16.5% of cooking bananas and 18% of highland cooking bananas and beer bananas. The most prevalent combination of mixed cropping is cultivating plantains with coffee and cocoa. Tables 1 and 2 present data on the production, consumption, and food energy of plantains/cooking bananas in some producing countries.
Table 1
| Countries | Yearly Production (× 1000 Tons) | Consumption (Kg/Person/Year) | Plantain Calories (Calories/Person/Day) |
|---|---|---|---|
| Africa | 600 | 67 | 111 |
| Burundi | 1000 | 81 | 195 |
| Cameroon | 1000 | 81 | 194 |
| Cote d’Ivoire | 1530 | 49 | 112 |
| Congo | 1800 | – | – |
| Nigeria | 6700 | 150 | 348 |
| Uganda | 2150 | 91 | 222 |
| Latin America | 2463 | 82 | 197 |
| Colombia | 960 | – | – |
| Ecuador | 600 | 70 | 135 |
| Venezuela | 580 | – | – |
| Peru | 105 | – | – |
| Asia | 1150 | 66 | 115 |
| Philippines | – | 65 | 137 |
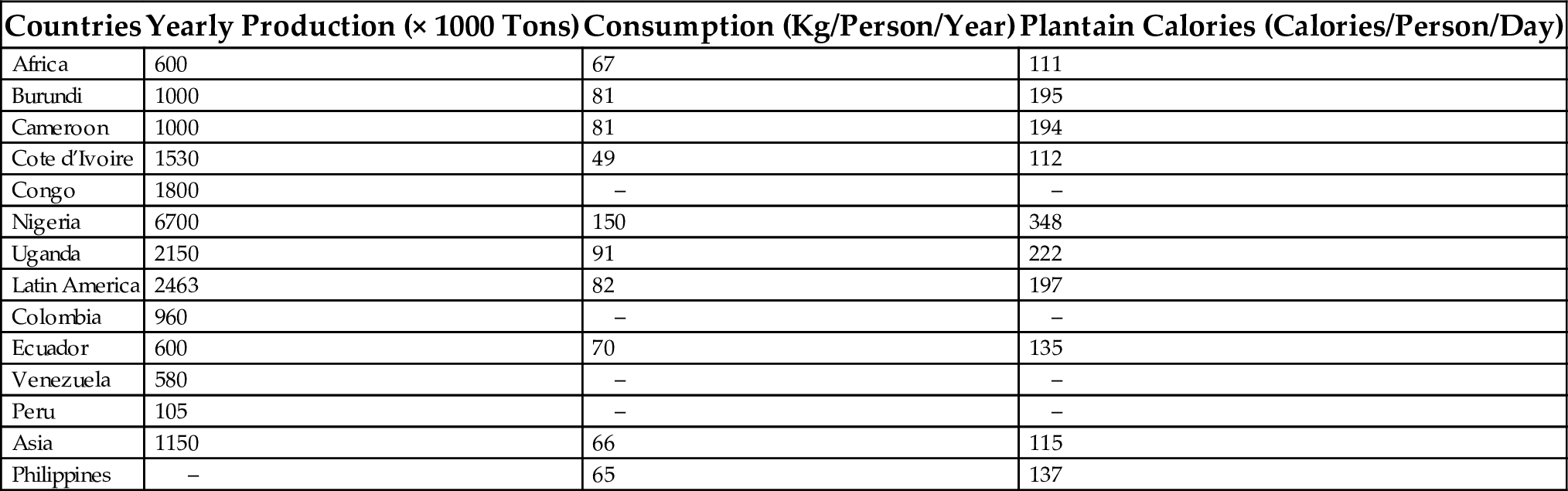
Courtesy FAO, 1989. Preventing of post-harvest food losses fruits, vegetable and root crops; and training manual. FAO Code 17(J11), 1–3—Data available on FAO public domain http://www.fao.org/inpho.
Table 2
| Countries | AFE From Plantain and Other Cooking Bananas (Kcal/Inhabitant/Day) |
|---|---|
| Uganda | 436 |
| Gabon | 432 |
| Rwanda | 422 |
| Cote d’Ivoire | 189 |
| Cameroon | 173 |
| Ghana | 172 |
| Colombia | 169 |
| Dominica Republic | 142 |
| Guinea | 140 |
| Ecuador | 119 |
Courtesy Treche, I.P., 1997. Plantain: Post Harvest Operations—Data available on FAO public domain. Retrieved 20 August 2017, http://www.fao.org/in-action/inpho/en/.
According to Treche (1997), 69.4% of plantains and other cooking bananas are used for human consumption, while 8% are used for animal feed. Postharvest losses and transformed quantities in the world are 11.5% and 11%, respectively. In most cases plantains are locally consumed. Additionally, plantains show great adaptation to urban consumption and exportation to specific markets. This will vary among countries because of prevailing eating habits. A list of various methods of preparation and consumption follows.
- • Ripe or unripe plantain pulp cooked in water or steam
- • Pastry from unripe plantain cooked in water and pounded in a mortar
- • Ripe pastry prepared from plantain flour and boiling water
- • Ripe or unripe plantain pulp roasted on charcoal fire
- • Unripe plantain pulp cooked with water, meat, or fish, palm oil, salt, and various spices
- • Slices of unripe or ripe plantain pulp fried (by deep-fat frying) in palm oil or vegetable oil.
6 Primary Products
6.1 Boiled Plantain
The fingers of ripe or unripe plantain are peeled and cooked in boiling water or steam for 20–50 min, depending on the cultivar and ripeness of the fruit. Plantains prepared this way are consumed with various sauces and accompanying dishes. Mature green fruits are peeled and cooked in water mixed with palm oil, goat or cow meat, salt, and different spices (condre in Cameroon). It is a traditional dish for people of West Cameroon for weddings, funerals, and other traditional ceremonies. The pulp of unripe plantains can be made into porridge by cutting the peeled fruit into pieces and cooking them with water, salt, palm or groundnut oil, groundnut paste, ground tomatoes, pepper, and seasonings, and fresh or smoked fish or meat.
6.2 Plantain Pastry
Unripe plantain pulp, after cooking in water or steam, is pounded in a wooden mortar, transforming it into a homogenous pliable pastry. The addition of a few pieces of cooked cassava can be kneaded in, to improve the elasticity and cohesiveness of the dough. This food called, “ntubo” in Cameroon, “foufou” in Cote d’Ivoire, “fufu” in Nigeria and Ghana, is always eaten with a sauce which is usually rich in proteins. It is a staple food in certain regions of these countries. Plantain pastry is sometimes served surrounded with green leafy vegetables, or mixed with beans.
6.3 Roasted Plantain
The entire pulp of unripe or partly ripe plantains is roasted on heated charcoal. About 15 min is adequate to cook 2–4 fingers of plantain, depending on the tastes of the consumer. Roasted plantain can be procured on the roadside, where it is prepared by women and consumed warm, with other delicacies such as roasted plums, avocado, roasted fish, or meat kebab.
6.4 Fried Plantain
Ripe or unripe plantain are peeled and cut into slices, and fried in palm oil or other vegetable oil, for 4–5 min at 160–180°C. This can be served with roasted fish, chicken or meat kebabs, depending on what is available. Fried ripe plantain or “aloko” in Cote d’Ivoire, “red-red” in Ghana, and “dodo” in Nigeria, is a meal relished by many. Fruits of certain cooking bananas, such as topala, pelipita, kalapua, also produce good quality fried plantains.
6.5 Plantain Fritters
The fruit of overripe plantains are pounded and mixed with a small quantity of maize, or other local cereal flour, with a proportion of about ¼ the fruit weight, to form a homogenous pastry. Salt is also added to this mixture. The fritters, obtained by deep-frying small balls of the dough in palm oil (160–180°C for 4–5 min), are eaten hot or warm, by themselves, or accompanied by foods such as sauce, spices, or fried beans. Overripe fruits of dessert or cooking bananas can also be used.
6.6 Plantain Chips
Plantain chips are the most popular plantain products in Nigeria. They are prepared by frying round slices of unripe or partially ripened plantains in vegetable oil. Best quality plantain chips have been obtained in Cameroon by frying round slices of fruit (2 mm thick) in refined palm oil between 160°C and 170°C for 2–3 min (Benion, 1980). These generally absorb less frying oil than chips from cooking bananas and dessert bananas.
7 Secondary and Derived Products
Jams, marmalades, juice, vinegar, beer, and alcohol can be made from ripe plantain fruits. Plantain and banana pulp and peel have been used in combination to make wine (Berry, 1992), while the peels and trunk are utilized for various agricultural purposes (Ketiku, 1973; Ankrah, 1974). In some towns and villages in Nigeria (e.g., Ile-Ife), a nonalcoholic drink called “Sekete” is prepared by women. To make this drink, ripe plantain fruits are peeled and soaked in water for 2–3 days, then the mixture is filtered to obtain a drink which is bottled and sold locally. Ogazi (1998) reported the production of beer from over ripe plantain fruit with an alcoholic content of 5%, v/v and specific gravity between 0.998 and 1.0034.
8 Production of Plantain Flour
Unripe plantain is traditionally processed into flour in Nigeria (Ukhum and Ukpebor, 1991) and in other West and Central African countries (Fig. 1). This traditional technology is equally present in Amazonian, Bolivia. The preparation method consists of manually peeling the fruit, then cutting the fruit into small pieces and air drying them for few days. The flour is mixed with boiling water to prepare an elastic pastry, called “amala” or “fufu” in Nigeria and “fou-fou” in Cameroon, which is eaten with various sauces and soups (Ngalani, 1989). The color of the flour obtained is light or dark brown, due to enzymatic browning activity. Some improvement to this traditional process is done by blanching the plantain fruit at 80°C for 5 min and cutting them into pieces, or by soaking the cut pieces for about 3 min in sodium metabisulfite solution 41 g/L + 3 g citric acid. After the soaking treatment, the fruit is drained and dried in a drying oven at 65°C for 48 h, or in the sun for some days to yield a flour with an off-white color (Ngalani, 1989). Plantain flour containing 10% of residual humidity that is hermetically sealed in plastic bags, can be kept for many months without quality deterioration.
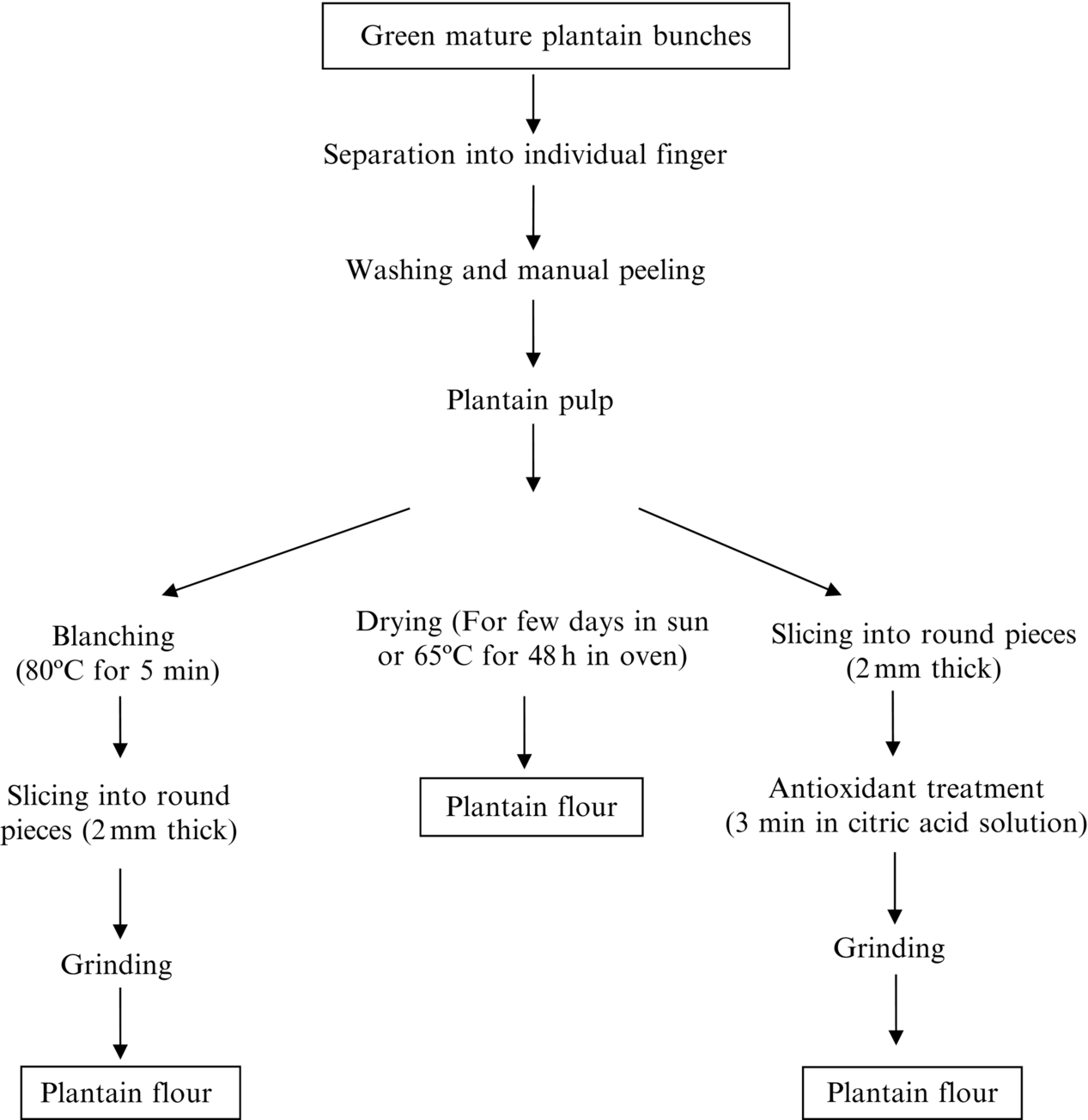
Dehydration is one of the oldest methods of food preservation (Adams, 2004) and converting plantain into flour could contribute to reduced losses and would make flour available year round. Air drying, alone or in combination with sun drying, is used for preserving unripe plantain (Demirel andTurhan, 2003). Besides helping in preservation, drying adds value to plantain. Instant plantain flours can be created from ripe and unripe fruits, by cooking and subsequent oven dehydration at 76°C and at 88–92°C respectively (Ukhum and Ukpebor, 1991). The products have commercial potential, when used alone or with ingredients in other foods, such as food for infants, desserts, soups, and sauces. Gwanfobge et al. (1988) demonstrated use of plantain flour at an industrial level, with full or low starch content in order to maintain the texture of various foods which are usually subjected to freezing and thawing before being prepared and/or consumed. The dietary fiber, resistant starch, proteins, and mineral contents of commercially produced cookies is increased when wheat flour is substituted with 7% unripe plantain flour (Pacheco-Delahaye et al., 2008). Solar-drying, drum drying, and microwave radiation are suitable drying methods to produce plantain flour.
A study has shown that flour made from ripe plantain (stage 4–5 ripeness) has been used to make bread, cookies, and instant flour (Ngalani and Crouzet, 1995). Furthermore, extruded cookies have been made with a mixture of flour (33.2%), plantain flour (17%), groundnut cake (25%), sucrose (20%), coconut (4%), and sodium chloride (0.8%). Soyamusa, a baby food from plantain flour (60%), full fat soybean flour (32%), and sucrose (8%) fortified with 0.15% of multivitamins, and 0.85% calcium carbonate, has been made and used in Nigeria (Ogazi, 1996). Recent work carried out in Cameroon has defined simple recipes for fritters and cakes made of plantain flour that gained wide acceptance and were well-liked by consumers. The cakes were made by baking a homogenous batter in the oven at 150°C for about 50 min. Batter for the cakes was made from 100 g plantain flour, 100 g fresh semiskimmed milk, 75 g sugar, 50 g butter, 5.5 g baking powder, 3 g of ground lemon peel, and one egg. The qualities of the ingredients in the batter can be modified and adapted according to consumers’ preferences.
9 Nutritional Content of Plantain
The plantain has a high carbohydrate content, the bulk of which is starch (83%) (Platt, 1985). Similarly, Pacheco-Delahaye et al. (2008) reported that starch is the main component (84%) of unripe plantain flour. Juarez-Garcia et al. (2006) reported 83% total starch in plantain flour, where 66% of the total starch is available, while 18% is resistant starch. According to Ketiku (1973), the protein hydrolysis of plantain fruit identified 16 amino acids, which should increase during ripening. The highest amino acid concentration observed was comprised of arginine, aspartate, and glutamine. Plantains supply about 3 g protein/100 g; thus, plantain alone cannot meet the daily human 70 g protein requirement. However, when eaten in adequate quantities along with other foods, it can supply ample quantities of energy and some protein. The fat content of plantain is very low so it does not contribute much to the energy content of the fruit. Ketiku (1973) reported the fat content of unripe fruit as 1% and ripe fruit as 2%. Although the total lipid content remains fairly unchanged during ripening, Ogazi (1996) reported that the composition of fatty acids, especially within the phospholipids fractions, has been observed to change with an increase in the unsaturation (a reduction of linoleic acid and an increase in linolenic acid). Plantain offers a substantial amount of ascorbic acid, carotene, thiamine, and minerals. The ash content of the unripe and ripe plantain fruit is about 2%, w/w of the total dry weight. A high level of dietary fiber has been reported by Singn and Heldman (2001), who found 6%–16% total fiber. Gwanfogbe et al. (1988) reported a dietary fiber level of 8%.
10 Requirement for Export and Quality Assurance
The quality of plantain bunches or fruit, is judged by important criteria at all stages in the market chain, irrespective of the cultivars (N’daAdopo, 1996). Different standards are applied by individuals in the distribution network to assess plantain quality:
- • Bunches with well filled fingers and sufficiently round fruits at the time of harvest
- • Fresh fruits without cracks
- • Fruits without mechanical damage
- • Well defined orange rose fruit
- • Fruits without pest or fungal attacks
Besides market trends, other factors that affect the price of mature green plantain bunches include bunch quality at harvest (size, weight, level of finger filling, and color of the fruit) and its freshness under transportation and storage conditions.
11 Storage of Plantain
Fresh unripe plantain is perishable and requires a cool temperature of 57°F to keep them from over ripening. Fresh unripe plantain can be stored in controlled atmospheric conditions, which require a sealed space where levels of atmospheric gases can be monitored and adjusted (Karikari et al., 1983). Plastic tents can be used to create plantain storage featuring controlled atmosphere. When this is done, the first fruit that produces ethylene causes other fruits to ripen, therefore, it is recommended that fruits of the same maturity be bagged together. According to Woodroof (1986), a simpler and cheaper method is to seal fruit in polythene bags. Plantains sealed in polythene bag remain green for a longer period than those stored in perforated polythene bags. As the fruits respire, the atmospheric condition in the bag changes with a decrease in O2 and increase in CO2. Respiration is inhibited because of the reduced O2, which prolongs the shelf life (FAO, 1978). However, these problems can be overcome to some extent by sulphiting the fruit before drying. This inactivates the enzyme oxidizers. The fruit can then be dried and stored in moisture-proof containers (FAO, 1978). Plantain powders can store satisfactorily at room temperature in hermetically sealed containers up to 1 year. Plantains chips tend to have a poor storage life, and are highly susceptible to becoming soft and rancid. However, chips treated with antioxidants have been stored satisfactorily at room temperature in hermetically sealed containers for up to 6 months with no development of rancidity. Karel et al. (1975) showed that mechanical damage was also an important factor in reducing storage life.
12 Selected Experimental Study
12.1 Production of Unripe Plantain Flours
Suckers of fresh mature plantain fingers were planted and harvested at the Federal University of Technology Owerri (FUTO) farm in Nigeria. Harvesting was done at different periods of anthesis, ranging from week 9 to 13. Unripe plantains at different stages of maturity were cleaned, peeled, and sliced to 1cm thickness using a slicer. Table 3 shows the pretreatments and instructions used in the preparation of plantain flours. The slices were dried using a solar dryer or sun drying. The dried samples were milled into flour and screened through a 100 μm sieve, yielding flour with 0.01 mm particle size. The flour was packaged in hermetic glass containers and stored at room temperature for analysis and use.
Table 3
| Pretreatment Solutions | ID Code | Pretreatment Directions |
|---|---|---|
| Control | CN | – |
| Blanched | BL | 3 min in boiling H2O |
| Citric acid | CA | 3 min in 1% CA |
| Sodium metabisulphite | NaM | 5% Na2S2O5 (5 min) |
| Blanched + sodium metabisulphite | BLNaM | BL + 5% Na2S2O5 (5 min) |
| Blanched + citric acid | BLCA | BL + 1% CA (5 min) |
13 Characterization of Plantain Flour
13.1 Bulk Density
Bulk density of flour was determined using the gravimetric method of Okaka and Potter (1979). Exactly 10 g of flour was put in a calibrated measuring cylinder. The bottom of the cylinder was tapped repeatedly on a firm pad on a laboratory bench until a constant volume was observed. Once constant volume was achieved, the volume was measured and recorded. The density was obtained by weight of the flour divided by volume:
13.2 Swelling Index
The swelling index (SI) was determined as the ratio of swollen volume to ordinary volume of a unit weight of flour using a modified method by Abbey and Ibeh (1988). One gram of the flour was weighed into a clean dry measuring cylinder. The volume occupied by the sample was recorded before 5 mL of distilled water was added to the sample. This was left to stand undisturbed for 1 h, and the volume was recorded. The swelling index of the samples was given by:
Analysis was conducted in triplicates.
13.3 Water and Oil Absorption Capacities
A method described by Carcea-Bencini (1986) was used to measure the water and oil absorption capacities. One gram of flour was weighed into six clean dry centrifuge tubes. The tubes were labelled separately for oil and water. Then 10 mL of distilled water was added to three tubes while10 mL of oil was added to the other three. All tubes were then stirred manually. The mixture was allowed to stand for 30 min at room temperature, then centrifuged for 30 min at 1500 rpm. The supernatant was decanted and the volume in the measuring cylinder was noted and converted to weight (in grams) by multiplying by the density of oil (0.902 g/mL) and water (1 g/mL). The oil and water absorption capacities were expressed as grams of oil or water absorbed per gram of flour sample.
13.4 Wettability
Wettability describes the capacity of the particles to absorb water on their surface, thus initializing reconstitution. The gap between the surface of 300 mL water in the beaker and the tip of an inverted test tube was measured to be 10 cm. Then 1g of the flour sample was released into the water, and the time it took for the last sample to get wet was recorded.
13.5 pH (Hydrogen Ion Concentration)
Plantain juice was obtained by blending 30 g of plantain with 90 mL of distilled water. Exactly 30 g of the juice was weighed. The pH electrode was washed with distilled water. The electrode was placed in the filtrate. The electrode was allowed to stabilize for a few minutes. The pH value of the filtrate was taken according to the method of AACC (2000).
13.6 Gelling Point
Exactly 5 g of sample was suspended in a beaker containing 20 mL of water and heated while being continuously stirred. The temperature at which the suspension gelled was recorded as the gelatinization temperature.
13.7 Moisture Content
Moisture content was determined by standard official methods of analysis (AOAC, 1984). This involved drying to a constant weight at 100–105°C and calculating moisture as the loss in weight of the dried sample. An empty moisture Petri dish was dried in an oven for 15 min and allowed to cool in desiccator containing calcium chloride for 20 min and weighed. Then 2 g of sample was weighed into Petri dish and placed in oven at 100–105°C for 3 h. It was brought out cooled in a desiccator and re-weighed. The process was repeated until a constant weight was obtained and the percentage moisture content was calculated as loss in weight of the original sample.
where W1 = weight of empty Petri dish; W2 = weight of empty Petri dish + sample (before drying); W3 = weight of Petri dish + constant weight dried sample.
13.8 Total Starch
Starch content of the plantain flour (dry basis) was determined using starch a isolation method as described by Oyewole and Afolami (2001). Exactly 100 g of flour was weighed and blended into slurry with water using Binatone blender. The slurry was filtered through a 150 μm sieve with enough water to wash out the starch. The filtrate was allowed to settle overnight in a preweighed 500 mL beaker under refrigeration temperature (4°C) to prevent fermentation. The water was decanted and the starch was placed in an oven to dry at 50°C to a constant weight. The starch was calculated as:
13.9 Thermal Analysis
Starch gelatinization temperature and enthalpy were determined by differential scanning calorimetry (DSC). For the rheological visco-amylograph (RVA) data of plantain flour samples, 3 g of sample was mixed with 25 g of water prior to a standard heating and cooling procedure, which was programmed as follows: 0–1 min, 50°C, up to 11–13 min, 50°C. For the DSC, 10% of samples were prepared on hermetic aluminum pans and subjected to a heating rate of 10°C/min starting from 30°C to 120°C. Based on the curve, the gelatinization temperature and enthalpy of denaturation (∆ H) were obtained (Paredes Lópes et al., 1994). This was carried out on solar, sun, and oven dried samples without treatments.
13.10 Drying Characteristics
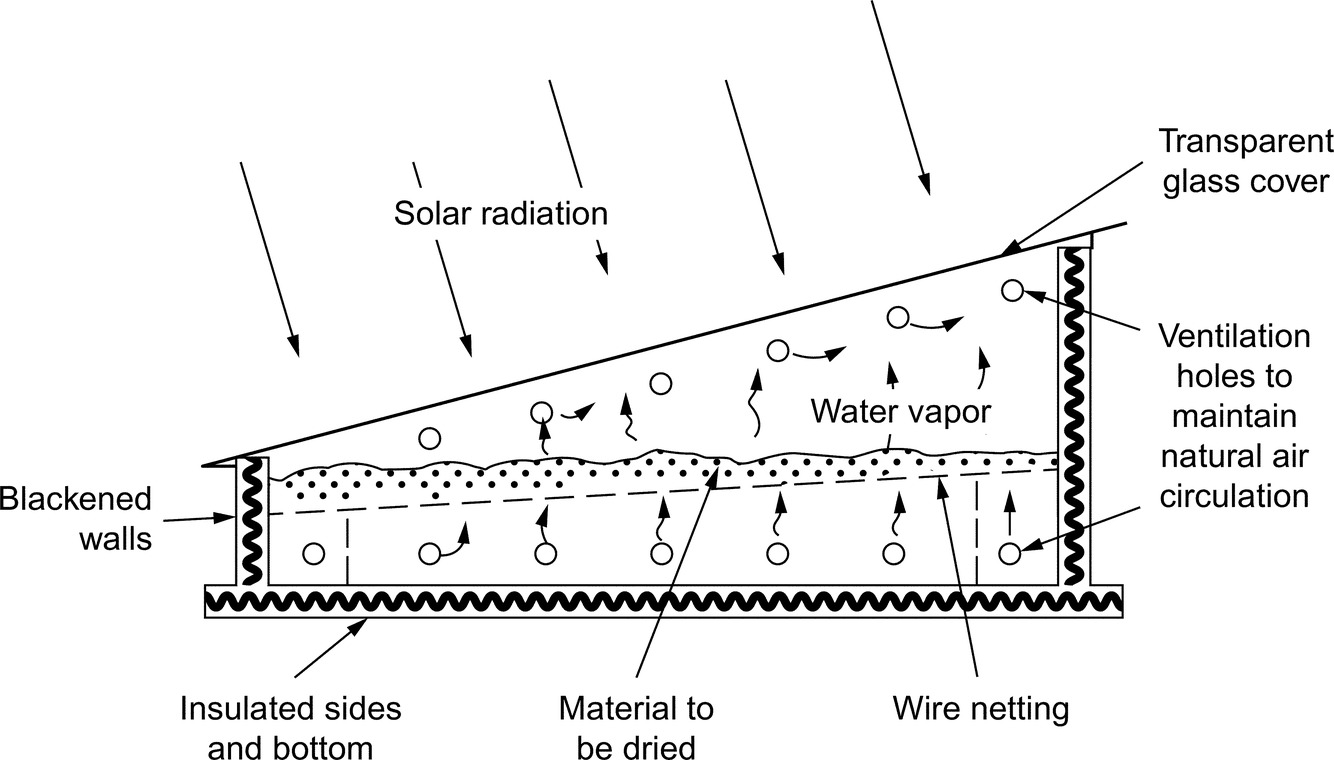
The drying characteristics of unripe plantain slices dried using a locally fabricated solar food dryer, were compared with those of plantain dried using traditional sun drying. Unripe plantain was washed, peeled, and cut into 1 cm slices. Slices were then placed in small trays for weighing and drying. Samples of exactly 250 g of sliced plantain were weighed (OHAUS triple beam balance 700/800 series) for drying. The samples were dried for 8 h (9 a.m.–5 p.m.) on each experimental day. The ambient temperature, the collector temperature and the temperature of drying chambers were monitored on an hourly basis during the drying process. The drying process was terminated when there was no detectable difference in mass loss between successive mass measurements. In this chapter, the parameters used in the drying activities of the plantain slices include, moisture content (MC), moisture ratio (MR), and mass shrinkage ratio (MSR) (Figs. 2 and 3).

13.11 Moisture Content
In this chapter, the instantaneous moisture content (MC) was defined on dry basis using the method described by Ekechukwu and Norton (1999):
where Wt = mass of the product being dried at time, t; Wd = mass of dried product.
13.12 Moisture Ratio
This is a dimensionless parameter comparing the moisture content of a crop product dried at a time, t, to the initial moisture content (both relative to equilibrium moisture content; EMC) (Ozbek and Dadali, 2007; Shivhare et al., 2000).
The formula is (Doymaz, 2007; Falade et al., 2007):
where Me = equilibrium moisture content; Mo = initial moisture content; Mt = final moisture content at time, t.
13.13 Mass Shrinkage Ratio
This is a measure of the structural change occurring in the product as a result of moisture loss, and is defined Midilli et al. (2002) as:
where Wo = the initial mass of the product and Wt = the mass of the product at time, t.
14 Microbiological Analyses
14.1 Sample Preparation
Samples were processed for analyses by the methods of Lyne and Collins (1990). Ten grams of each sample for microbiological evaluation were aseptically transferred into 90 mL of 0.1% sterile peptone water, shaken thoroughly and appropriate dilutions (up to 106) were prepared for microbiological studies using the pour plate technique. Poured plates without samples inoculated served as control.
14.2 Yeast and Molds
Yeasts were enumerated on Yeast Extract Agar (YEA) while moulds were isolated on Potato Dextrose Agar (PDA). Fungal plates were incubated at 30°C for up to 72 h.
14.3 Total Viable Counts
Total counts of viable bacteria (aerobic mesophiles) were made on Plate Count Agar (PCA, Oxoid, Hampshire, United Kingdom). Plates for bacteria isolation were incubated at 37°C for 48 h.
14.4 Salmonellae
A 101 dilution of each sample was enriched in tetrathionate broth (Difco), incubated at 37°C for 6 h before inoculation on Salmonella-Shigella agar (Oxoid) for isolation of salmonellae. Incubation was at 37°C for 48 h. Colonies with black spots were recognized as Salmonella and confirmed by the production of H2S and citrate utilization, differentiating it from Shigella.
14.5 Staphylococci
Counts of staphylococci were made on Mannitol Salt Agar while Baird-Parker medium, coagulase, and catalase tests were used to differentiate Staphylococcus aureus from the other staphylococci isolated (Harrigan and McCance, 1976).
14.6 Characterization and Identification of Microbial Isolates
After incubation, colonies were randomly picked from fungal plates and identified on the bases of cultural, morphological, and biochemical characteristics (Onions et al., 1981).
For bacterial plates, all colonies from a sector of incubated plates were picked and purified by repeated subculturing, before being examined microscopically for Gram reaction, cell morphology (using 24 h old cultures), motility, pigmentation, and sporulation (Harrigan and McCance, 1976). Biochemical analysis included catalase and oxidase activities, nitrate reduction, patterns of sugar utilization, as well as urea and starch hydrolysis (Harrigan and McCance, 1976). The isolates were identified on the basis of the results obtained from biochemical characterization, by analyzing the results using Bergey’s manual of systematic bacteriology (Sneath et al., 1986).
15 Selected Experimental Study Results
15.1 Influence of Maturity and Pretreatment on the Functional Characteristics of Unripe Plantain Flours
15.1.1 Oil Absorption
The oil absorption capacities of all the pretreated samples (Table 4) were higher than the control for all weeks of fruit maturity, however, there were no significant differences (P < .05). Based on this, water absorption can be enhanced by harvesting at the 9th week of maturity and blanching, after treatment with sodium metabisulphite with a value of 3.3 mL/g. According to Fagbemi (1999), good oil absorption capacity of flour samples suggested that they may be useful in food preparations that involve oil mixing, such as baked goods containing oil as a significant ingredient. The water/fat binding capacity of proteins is an index of its ability to absorb and retain oil, which in turn influences the texture and mouth feel of food products such as ground meat formulations, doughnuts, pancakes, baked goods, and soups.
Table 4
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 2.5868a | 3.1841a | 3.1065a | 2.9305a | 3.2837a |
| CA | 2.8852a | 3.0041a | 2.82a | 2.8646a | 3.1817a |
| BL | 2.8438a | 2.8930a | 2.8156a | 2.9875a | 3.1135a |
| CABL | 2.9481a | 2.9918a | 2.8056a | 2.6952a | 2.6304a |
| NaM | 2.91325a | 3.4292a | 3.0582a | 3.0172a | 3.0241a |
| NaMBL | 3.29785a | 3.1763a | 2.9379a | 2.8401a | 2.8826a |
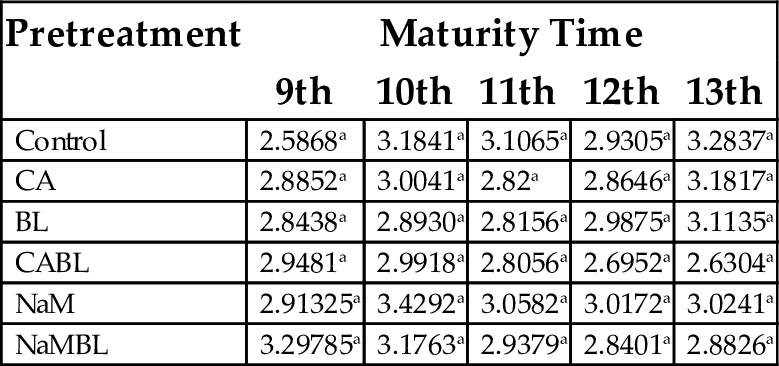
Values with the same superscript in a column are not significantly different (P < .05). CA, citric acid; BL, blanched; CABL, citric acid and blanched; NaM, sodium metabisulphite; NaMBL, sodium metabisulphite and blanched.
15.1.2 Swelling Index
The effect of maturity and pretreatment on the swelling power of flour produced from plantain flours is presented in Table 5. Bainbridge et al. (1996) stated that good quality starch with high starch content and paste viscosity will have a low solubility and high swelling volume and power. The swelling power may be attributed to the low levels of fat in the plantain. This is because high levels of fat lead to formation of amylase-lipid complexes that restrict swelling. It might also be due to a more highly ordered internal arrangement of starch granules, as found in yam with a swelling capacity of 9% (Soni et al., 1985). The swelling capacity ranged from 1.37% (9th week control) to 3.76% (BL at 13th week). This indicated BLA pretreatment was effective in increasing the swelling power of the plantain flour. These values were comparable to those reported by Jimoh and Olatidoye (2009) for soybean fortified yam flour (6.8%–9.6%). Generally, the plantain samples showed a better swelling capacity than cassava, and this could be due to the small particle size of plantain starch, which results in higher digestibility (Ojinnaka et al., 2009). The variation in swelling power could be due to amylase/amylopectin ratio and the characteristics of amylase and amylopectin in terms of molecular weight distribution, degree of branching, length of branches and conformation of the molecules (Hoover and Ratnayake, 2002). This general observation can be attributed to the deteriorative effect of high temperatures on the molecular conformation of the starch present. These are in agreement with observations made by Muyonga et al. (2004).
Table 5
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 1.37a | 1.43a | 1.625a | 1.73c | 1.805b |
| CA | 1.655a | 1.50a | 1.57a | 1.83c | 1.785b |
| BL | 1.855a | 1.855a | 2.915a | 2.155b | 3.76a |
| CABL | 2.445a | 1.975a | 2.895b | 2.155b | 2.46b |
| NaM | 1.415a | 2.00a | 1.535b | 1.595c | 1.885b |
| NaMBL | 1.855a | 2.23a | 2.70b | 2.77a | 2.465b |
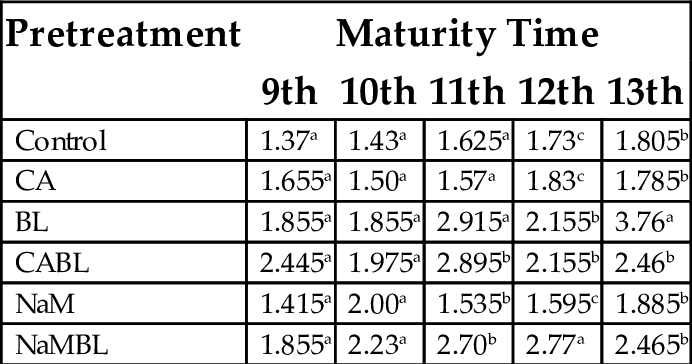
Values with the same superscript in a column are not significantly different (P < .05).
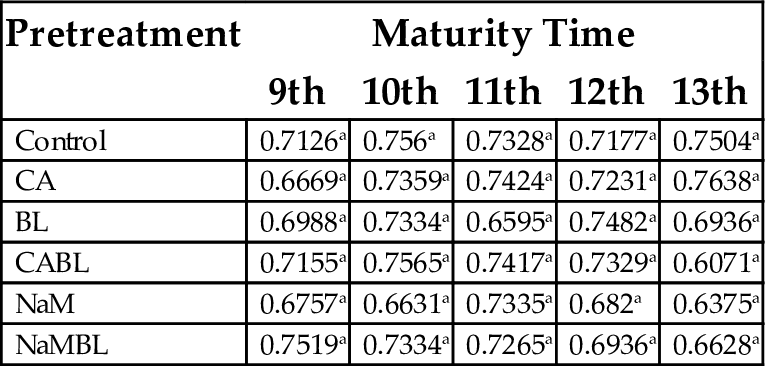
15.1.3 Bulk Density
According to Bhattacharya and Prakash (1994), bulk density of foods increases with an increase in starch content. Okezie and Bello (1988) stated the high bulk density of food material is important in relation to its packaging. However, according to the results listed in Table 6, there were no significant effect (P > .05) of maturity time and pretreatment on the bulk density of plantain flour. Although there was no significant difference (P > .05) between bulk densities of the flours, the highest bulk density (0.76) was recorded for CA at the 13th week of maturity. The general low bulk density of the flours could be an advantage in formulation of baby foods, where a high nutrient density to low bulk density is desired.
Table 6
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 0.7126a | 0.756a | 0.7328a | 0.7177a | 0.7504a |
| CA | 0.6669a | 0.7359a | 0.7424a | 0.7231a | 0.7638a |
| BL | 0.6988a | 0.7334a | 0.6595a | 0.7482a | 0.6936a |
| CABL | 0.7155a | 0.7565a | 0.7417a | 0.7329a | 0.6071a |
| NaM | 0.6757a | 0.6631a | 0.7335a | 0.682a | 0.6375a |
| NaMBL | 0.7519a | 0.7334a | 0.7265a | 0.6936a | 0.6628a |
Values with the same superscript in a column are not significantly different (P < .05).
15.1.4 Gelling Point
Results in Table 7 indicate no significant differences in the gelling point of the flour samples, when compared with the control for all weeks of maturity. Flours made from plantain harvested in the 11th week and treated with citric acid, gave the least value of gelling point of 61°C, while treatment with sodium metabisulphite (NaM) gave the highest value of 76°C.
Table 7
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 68a | 62a | 66.5a | 64a | 60a |
| CA | 68a | 69a | 61a | 64a | 70a |
| BL | 59a | 68a | 60a | 67a | 69a |
| CABL | 64a | 66a | 69a | 65a | 61a |
| NaM | 68a | 76a | 72a | 56a | 64a |
| NaMBL | 65a | 68a | 60a | 68a | 60a |
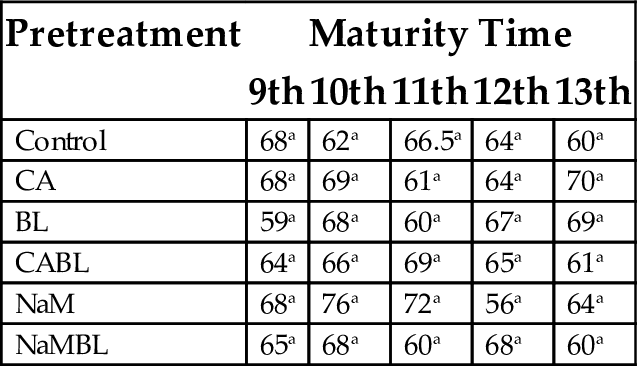
Values with the same superscript in a column are not significantly different (P < .05).
15.1.5 Water Absorption Capacity
Water absorption capacity is an indication that a particular flour would be useful in food preparation, such as baked goods, which require hydration to improve handling characteristics. Giami and Alu (1993) stated that the water binding capacity of 1.25 g/g and previously shown, is an indication of good potential for use in baked goods. Table 8 presents the effect of maturity time and pretreatment on the water binding capacity of flour produced from plantain. The water absorption capacity ranged from 0.7635 mL/g (CABL at 11th week) to 2.7744 mL/g (NaM at 10th week). Lower values could be associated with lower carbohydrate content in flours’ whose complex molecule will demand less water during hydrolysis (Oduro et al., 2006). Higher water binding capacities can also be attributed to low association of starch polymers in the granules (Soni et al., 1985), which might not be the case for the plantain flours. High water absorption and swelling capacities have both economic and culinary advantages. It is evident that NaM pretreatment provides the highest water absorption capacity, irrespective of fruit maturity. The increase in water absorption capacity as a result of NaM pretreatment could suggest possible increase in the level of their incorporation into food formulation, such as dough or batter, in other to improve its handling characteristics and also to maintain freshness of the product.
Table 8
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 2.4548a | 2.2434a | 2.241a | 2.2541b | 2.2019a |
| CA | 2.2044a | 2.3511a | 2.369a | 2.7769a | 2.6526a |
| BL | 1.0282b | 1.0709b | 1.0058b | 1.4700c | 1.13325b |
| CABL | 0.9595b | 1.3151b | 0.7635b | 0.905d | 0.7941b |
| NaM | 2.6289a | 2.7744a | 2.4475a | 2.7357a | 2.2231a |
| NaMBL | 1.2526b | 1.2645b | 0.7898b | 0.8337d | 0.7985b |
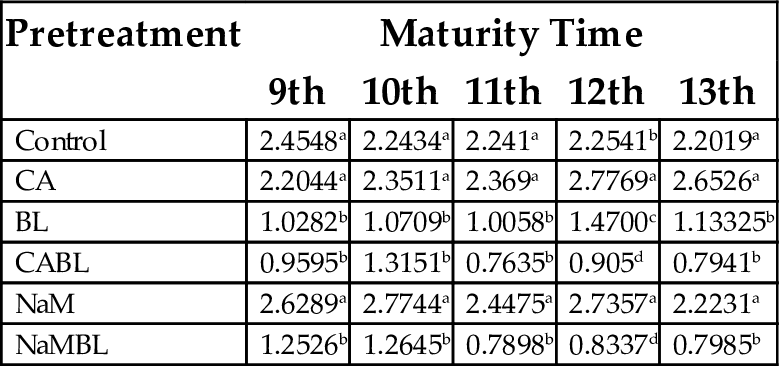
Values with the same superscript in a column are not significantly different (P < .05).
15.1.6 pH (Hydrogen Ion Concentration)
Assessment of pH in plantain is used primarily to estimate consumption quality and hidden attributes. Acids make an important contribution to the postharvest quality of the fruit, as taste is mainly a balance between the sugar and acid contents. Because of this, postharvest assessment of acidity is important in the evaluation of the taste of flours. Analysis of variance for pH of the flours produced from different varieties indicated that pH of the flours was significantly affected (P < .05) in 9th and 13th week of maturity, but not significant in the other weeks (Table 9). This was affected by the time of maturity and the treatments undertaken. The pH values recorded ranged from 5.28 (CABL at 10th week) to 7.63 (NaMBL at 11th week). Emperatriz et al. (2008) reported values in the range of 4.6–6.1 when plantain flours were produced from different dehydration procedures. Dadzie (1998) reported values within the range of 6.0–6.5. Deviations from reported values could be attributed to variations in maturity time and pretreatment, as well as differences in the morphology of the plantain varieties. Irrespective of time of maturity, higher pH values were recorded for all flours treated with sodium metabisulphite and blanched, indicating the significant role of the pretreatments.
Table 9
| Pretreatment | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Control | 6.3bc | 6.35a | 6.585a | 6.075a | 5.765c |
| CA | 6.895ab | 5.945a | 6.855a | 6.74a | 6.78a |
| BL | 5.645bc | 5.6a | 6.26a | 6.11a | 6.875a |
| CABL | 5.425bc | 5.28a | 5.79a | 6.1a | 6.5b |
| NaM | 6.785ab | 6.985a | 6.73a | 7.115a | 7.01a |
| NaMBL | 7.545a | 6.87a | 7.625a | 7.24a | 7.45a |
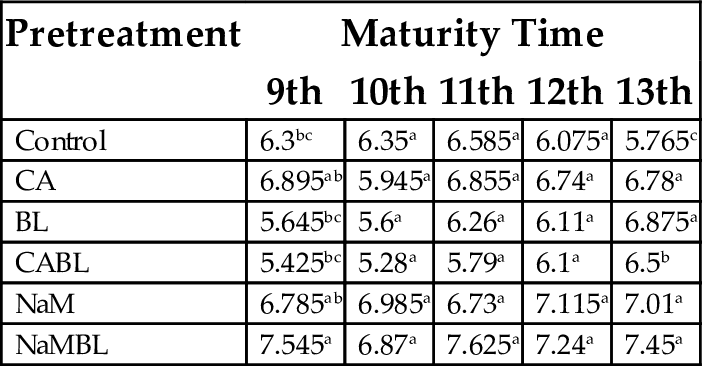
Values with the same superscript in a column are not significantly different (P < .05).
15.1.7 Moisture Content
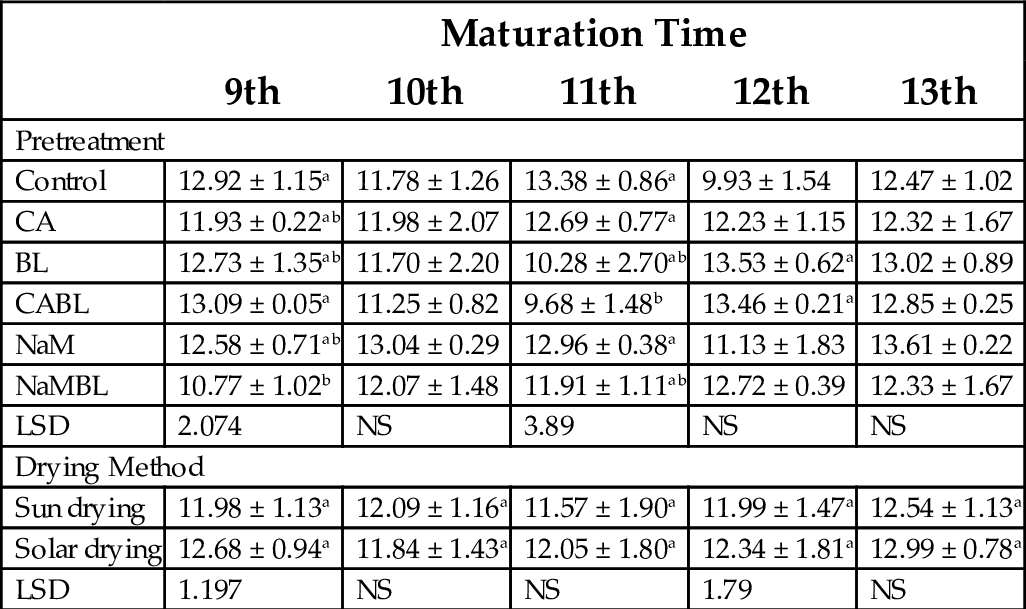
Table 10 summarizes the moisture content of plantain flour obtained by sun drying, and solar drying. The lowest value was 9.68% for flour samples treated with a combination of citric acid and blanching, while the highest was 13.53%, measured in flour treated by blanching alone. These values are acceptable for the established goal, which is to reach a stable shelf life (20% moisture), and corroborates previous reports (Kayisu et al., 1981; Gwanfogbe et al., 1988; Daramola and Osanyinlusi, 2006). Most differences between various treatments at various weeks were not significant.
Table 10
| Maturation Time | |||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Pretreatment | |||||
| Control | 12.92 ± 1.15a | 11.78 ± 1.26 | 13.38 ± 0.86a | 9.93 ± 1.54 | 12.47 ± 1.02 |
| CA | 11.93 ± 0.22ab | 11.98 ± 2.07 | 12.69 ± 0.77a | 12.23 ± 1.15 | 12.32 ± 1.67 |
| BL | 12.73 ± 1.35ab | 11.70 ± 2.20 | 10.28 ± 2.70ab | 13.53 ± 0.62a | 13.02 ± 0.89 |
| CABL | 13.09 ± 0.05a | 11.25 ± 0.82 | 9.68 ± 1.48b | 13.46 ± 0.21a | 12.85 ± 0.25 |
| NaM | 12.58 ± 0.71ab | 13.04 ± 0.29 | 12.96 ± 0.38a | 11.13 ± 1.83 | 13.61 ± 0.22 |
| NaMBL | 10.77 ± 1.02b | 12.07 ± 1.48 | 11.91 ± 1.11ab | 12.72 ± 0.39 | 12.33 ± 1.67 |
| LSD | 2.074 | NS | 3.89 | NS | NS |
| Drying Method | |||||
| Sun drying | 11.98 ± 1.13a | 12.09 ± 1.16a | 11.57 ± 1.90a | 11.99 ± 1.47a | 12.54 ± 1.13a |
| Solar drying | 12.68 ± 0.94a | 11.84 ± 1.43a | 12.05 ± 1.80a | 12.34 ± 1.81a | 12.99 ± 0.78a |
| LSD | 1.197 | NS | NS | 1.79 | NS |
15.1.8 Total Starch Composition
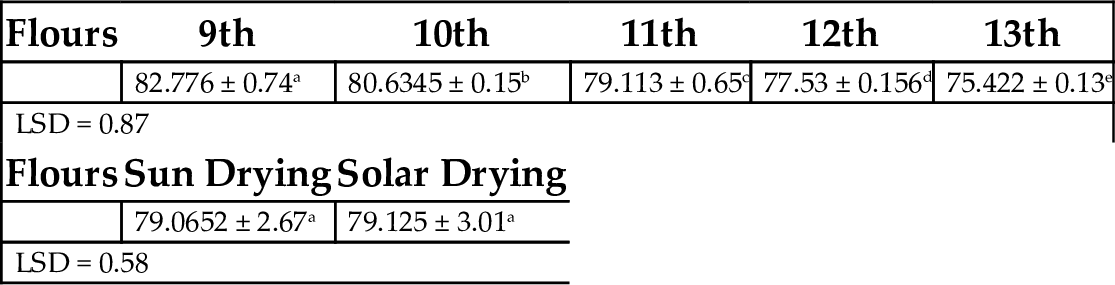
Table 11 shows total starch composition of 75.42%–82.78% for unripe plantain flour harvested from the 9th to 13th week of maturity. This was in agreement with the work of Vieira da Mota et al. (2000), who reported a starch component of 63.50%–74.65% in unripe plantain flour. Consequently, when considering plantain flour as a starchy staple food, there was no significant difference in flours produced by sun drying and solar drying.
Table 11
| Flours | 9th | 10th | 11th | 12th | 13th |
|---|---|---|---|---|---|
| 82.776 ± 0.74a | 80.6345 ± 0.15b | 79.113 ± 0.65c | 77.53 ± 0.156d | 75.422 ± 0.13e | |
| LSD = 0.87 | |||||
| Flours | Sun Drying | Solar Drying | |||
| 79.0652 ± 2.67a | 79.125 ± 3.01a | ||||
| LSD = 0.58 | |||||
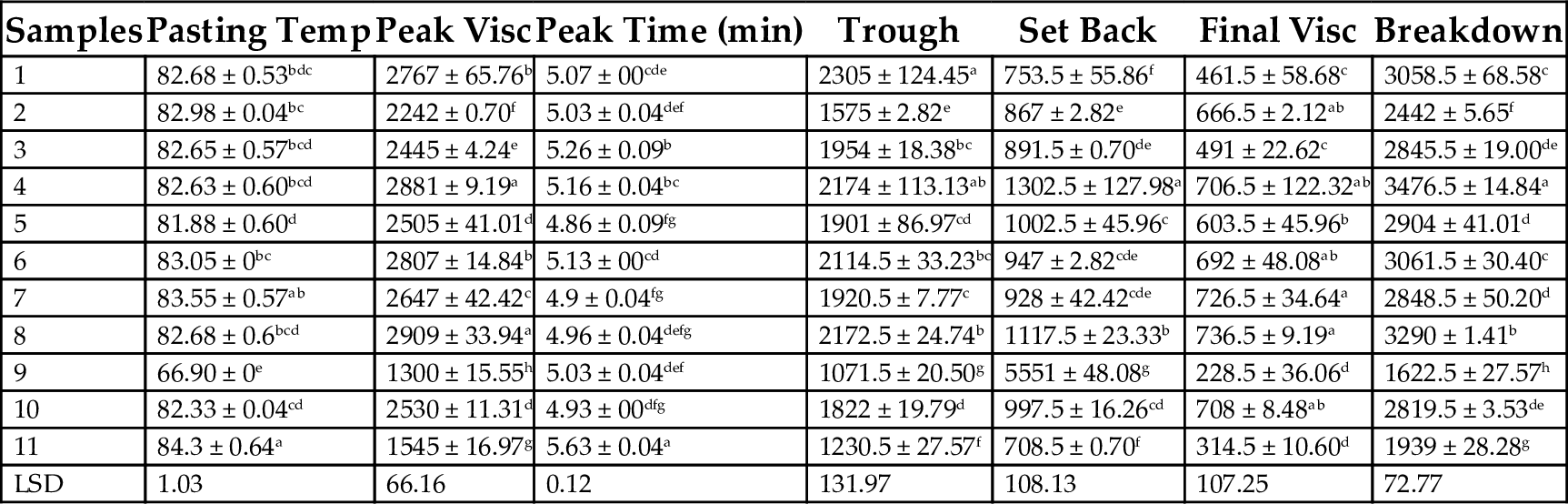
15.1.9 The Effect of Maturity Time and Drying Method on Pasting Properties of Plantain Flour
The pasting temperature varied from 66.9°C to 84.3°C. Sun-dried samples from 12th week mature plantains had the lowest temperature while oven-dried samples had the highest temperature. The lower pasting temperatures for sun-dried plantain compared with oven-dried samples indicates that for sun-dried samples, there is the presence of starch highly resistant to swelling, due to its higher moisture content. This is in agreement with the low swelling power values of sun-dried samples. This is because the pasting temperature is the temperature indicated at the time of maximum peak value of RVA, giving an indication of the gelatinization time during processing (Chinma et al., 2009). Also, it is the temperature at which the first detectable increase in viscosity is measured, an index characterized by the initial change due to the swelling of starch (Emiola and Delarosa, 1981). The peak viscosity varied from 1300–2900 cP where sun-dried samples at 11th week of maturity had the highest value and sun-dried sample with 12th week of maturity had the lowest value. However, oven-dried samples had a low peak viscosity of 1545 cP. The values of the pasting properties for solar-dried samples were generally higher than sun and oven dried samples. The peak viscosity indicated the water-binding capacity of the starch or mixture in a product. This correlates with final product quality and provides an indication of the viscous load likely to be encountered during mixing (Ingbian and Adegoke, 2007). It also indicates the ability of products to swell freely before their physical breakdown. The increase in peak viscosity may be attributed to the long drying time, which allows for more starch debranching to smaller units, indicating starch structural damage. The peak viscosity indicates that low drying temperatures result in higher peak viscosity values. One explanation is that reducing the drying temperature increases the drying time, thus enabling the plantain flour to swell freely before the physical breakdown (Tunde-Akintunde and Ogunlakin, 2011). This results in a reduction in cooking time of plantain flour. Also, the high values of peak viscosity for solar-dried samples indicates the samples could be used for products requiring high gel strength and elasticity. Olanipekun et al. (2009) opined that low viscosity is desirable in weaning foods because it results in increase in nutrient and energy densities without excessive increase in viscosity. This indicates that sun dried or oven dried plantain flour at 12th week of maturity can be utilized in formulation of puddings for weaning foods.
The peak time varied from 4.9–5.6 min, where the solar-dried sample at the 13th week of maturity had the lowest value and the oven dried sample had the highest value. Peak time provides an indication of minimum temperature required to cook flour (Tunde-Akintunde and Ogunlakin, 2011). Peak time values are lower than the peak time of 5.1–5.8 min reported for instant yam-breed fruit composite flour (Adebowale et al., 2008). Thus, the reduction in the cohesion of the paste obtained from oven-dried plantain samples. The trough viscosity value for the plantain samples ranged from 1071–2305 cP, and these values were significantly different at P < .05. Trough viscosity is the maximum viscosity value in the constant temperature phase of the RVA profile, and measures the ability to withstand breakdown during cooling. Thus, plantain flour samples at the 9th week of maturity prepared by sun drying will withstand breakdown during cooling over solar or oven dried samples. The decrease trough viscosity with increase in drying temperature may also be attributed to higher drying temperatures, which caused a decrease in viscosity value.
The setback value which varied from 753.5–1302.5 cP for solar dried samples, 551–1117 cP for sun-dried samples, and 708.5 cP for oven-dried samples, indicates the tendency of starch to retrograde on cooling. This value peaked at the 12th week of maturity for solar dried samples and the 11th week of maturity for sun dried samples. The final viscosity varied between 228.5 and 736.5 cP, where sun dried plantain flour samples at the 11th and 12th week of maturity had the highest and lowest values respectively. According to Adebowale et al. (2008), final viscosity is the change in the viscosity after holding cooked starch at 50°C. Thus, the use of oven drying as an alternative to sun and solar drying should be carried out at lower temperatures if a plantain flour meal with high viscosity is preferred. Breakdown viscosity varied from 1622.5 to 3467.6 cP. Starch samples from mature plantain at the 9th week of maturity have high values, irrespective of the drying method employed. Breakdown viscosity value is an index of stability of starch and a measure of ease with which swollen granules can be disintegrated (Kaur and Singh, 2005). Cohesiveness of paste is attributed to the extent of breakdown of starch molecules during heating and stirring. High values of breakdown viscosity indicate cohesion and subsequently, higher paste stability.
16 Effect of the Maturity Time and Drying Method on the Thermal Properties of Plant Starch
Table 12 shows the effect of drying method and maturity time on the thermal properties of plantain starch. Gelatinization describes the irreversible disruption of molecular order within a starch granule when heated in excess water. Starch denaturation temperature (Td) and enthalpy of denaturation (∆ H) are influenced by starch compositions, molecular structure of amylopectin and granule architecture (Tester, 1997). The Td values obtained for plantain starch ranges from 78.00°C to 83.22°C at the end of gelatinization, with the oven dried starches having the highest Td value. Enthalpy of denaturation varied significantly among the samples, with solar dried starch obtained from the 9th week of maturity having the highest value of 11.81 J/g. The denaturation temperature of plantain starch was comparable to that of corn starch and potato starch (Lai et al., 2000), indicating the same degree of ordered arrangement of amylopectin in the starch granule. The difference in ∆ H reflects melting of amylopectin crystallites. Variations in ∆ H of samples could represent differences in bonding forces between double helices that form amylopectin crystallites, resulting in different alignment of hydrogen bonds within starch molecules (McPherson and Jane, 1999).
Table 12
| Samples | Td (oC) | Mean ∆ H ± SD |
|---|---|---|
| 1 | 79.82 ± 0.24b | 11.81 ± 0.57a |
| 2 | 79.84 ± 019b | 8.54 ± 0.32c |
| 3 | 79.07 ± 1.21b | 9.36 ± 0.49c |
| 4 | 79.78 ± 0.03b | 10.96 ± 0.40ab |
| 5 | 78.66 ± 0.01bc | 8.49 ± 0.57cd |
| 6 | 78.66 ± 0.42bc | 7.91 ± 0.35d |
| 7 | 79.41 ± 0.24b | 11.06 ± 0.53ab |
| 8 | 79.77 ± 0.25b | 10.79 ± 0.89b |
| 9 | 79.00 ± 0.45bc | 0.66 ± 0.03f |
| 10 | 78.00 ± 0.52bc | 8.10 ± 0.19d |
| 11 | 83.22 ± 0.45a | 3.41 ± 0.12e |
| LSD | 1.06 | 0.96 |
Means in a column with the same letter are not significantly different (P ≤ .05). Means of three replicates (viscosity in centipoise).
Note: Samples 1–5, solar-dried plantain flours with 9–13 weeks of maturity respectively; samples 6–10, sun-dried plantain flours with 9–13 weeks of maturity respectively; sample 11, oven-dried plantain flour.
17 Impact of Drying and Maturity Time on the Functional Properties of Plantain Flour
17.1 Oil Absorption
There were no significant differences as result of the drying method except for the 11th week (Table 13). However, the highest value of 3.148 was obtained for solar dried plantain flour with 10th week of maturity, while the least value of 2.71 was obtained for solar dried samples at the 11th week of maturity. According to Fagbemi (1999), good oil absorption capacity of flour samples suggest that they may be useful in food preparations that involve oil mixtures such as bakery products, where oil is an important ingredient. The water/fat binding capacity of proteins is an index of its ability to absorb and retain oil, which in turn influences texture and mouth feel of food products such as ground meat formulations, doughnuts, pancakes, baked goods, and soups.
Table 13
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 2.839a | 3.078a | 3.132a | 2.810a | 3.100a |
| Solar drying | 2.986a | 3.148a | 2.716 | 2.968a | 2.938a |
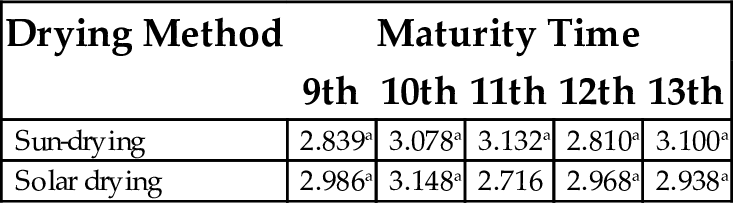
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
17.2 Swelling Index
Swelling index is a quality criterion in some food formulations such as baked products. It is an evidence of noncovalent bonding/interactions between molecules within starch granules as well as a factor of the ratio of amylose and amylopectin (Bate-Smith and Rasper, 1969). As shown in Table 14, swelling index increased with maturity time. Sun drying has been observed to play a role in obtaining starch with high swelling power and desirable organoleptic properties.
Table 14
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 1.66b | 1.735b | 2.1383a | 2.0167a | 2.3933a |
| Solar drying | 1.87a | 1.9283a | 2.275 a | 2.0617a | 2. 3267a |
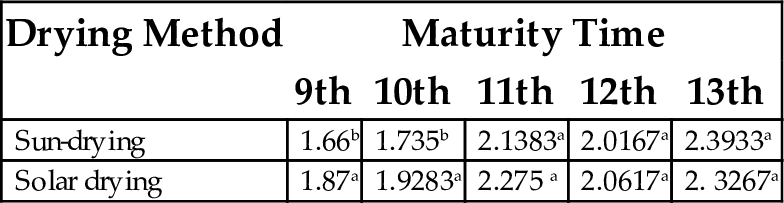
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
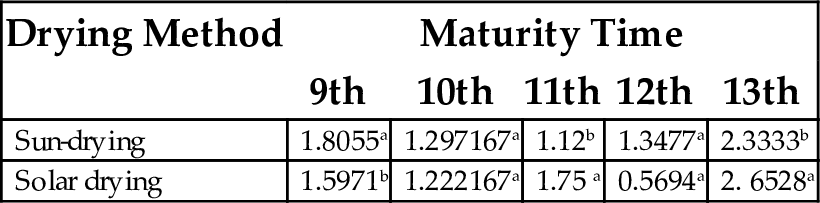
17.3 Wettability
There are significant differences in the wettability factor for flour samples due to the drying method used, except for samples at the 10th week of maturity (Table 15). However, the highest value of 2.65 min was obtained for the 13th week (solar dried plantain flour), while for solar dried flour, the 12th week matured plantain produced the least value (0.57).
Table 15
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 1.8055a | 1.297167a | 1.12b | 1.3477a | 2.3333b |
| Solar drying | 1.5971b | 1.222167a | 1.75 a | 0.5694a | 2. 6528a |
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
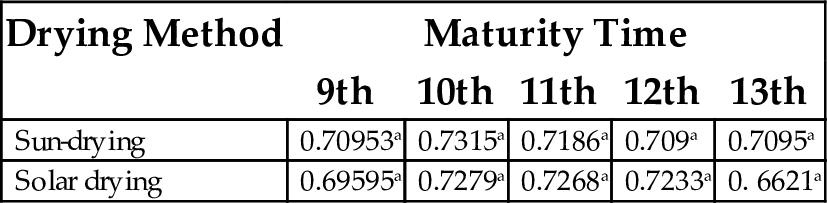
17.4 Bulk Density
The bulk density (g/mL) ranged from 0.6621 to 0.7728 g/mL, with solar-dried flour at 13 weeks of maturity having the least value and solar dried samples at the 10th week maturity had the highest value. There were no significant differences among maturity times irrespective of drying method applied (Table 16).
Table 16
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 0.70953a | 0.7315a | 0.7186a | 0.709a | 0.7095a |
| Solar drying | 0.69595a | 0.7279a | 0.7268a | 0.7233a | 0. 6621a |
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
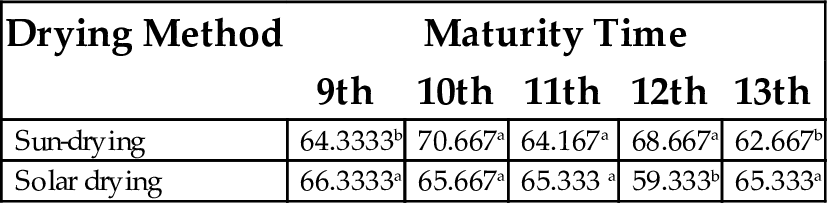
17.5 Gelling Point
The gelling point ranged between 59.333°C and 70.667°C, with sun-dried flour at the 10th week maturity having the highest value and the solar dried sample at 12th week maturity had the least value (Tables 17 and 18). Variations in the gelation point may be attributed to relative ratios of different constituent proteins, carbohydrates and lipids in the food sample (Sathe et al., 1982). The lower the gelling point, the better it serves as a good binder and consistency enhancer in food preparations of semisolid beverages, and the better the gelling ability of the flour (Adeyemi and Umar, 1994).
Table 17
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 64.3333b | 70.667a | 64.167a | 68.667a | 62.667b |
| Solar drying | 66.3333a | 65.667a | 65.333 a | 59.333b | 65.333a |
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
Table 18
| Samples | Pasting Temp | Peak Visc | Peak Time (min) | Trough | Set Back | Final Visc | Breakdown |
|---|---|---|---|---|---|---|---|
| 1 | 82.68 ± 0.53bdc | 2767 ± 65.76b | 5.07 ± 00cde | 2305 ± 124.45a | 753.5 ± 55.86f | 461.5 ± 58.68c | 3058.5 ± 68.58c |
| 2 | 82.98 ± 0.04bc | 2242 ± 0.70f | 5.03 ± 0.04def | 1575 ± 2.82e | 867 ± 2.82e | 666.5 ± 2.12ab | 2442 ± 5.65f |
| 3 | 82.65 ± 0.57bcd | 2445 ± 4.24e | 5.26 ± 0.09b | 1954 ± 18.38bc | 891.5 ± 0.70de | 491 ± 22.62c | 2845.5 ± 19.00de |
| 4 | 82.63 ± 0.60bcd | 2881 ± 9.19a | 5.16 ± 0.04bc | 2174 ± 113.13ab | 1302.5 ± 127.98a | 706.5 ± 122.32ab | 3476.5 ± 14.84a |
| 5 | 81.88 ± 0.60d | 2505 ± 41.01d | 4.86 ± 0.09fg | 1901 ± 86.97cd | 1002.5 ± 45.96c | 603.5 ± 45.96b | 2904 ± 41.01d |
| 6 | 83.05 ± 0bc | 2807 ± 14.84b | 5.13 ± 00cd | 2114.5 ± 33.23bc | 947 ± 2.82cde | 692 ± 48.08ab | 3061.5 ± 30.40c |
| 7 | 83.55 ± 0.57ab | 2647 ± 42.42c | 4.9 ± 0.04fg | 1920.5 ± 7.77c | 928 ± 42.42cde | 726.5 ± 34.64a | 2848.5 ± 50.20d |
| 8 | 82.68 ± 0.6bcd | 2909 ± 33.94a | 4.96 ± 0.04defg | 2172.5 ± 24.74b | 1117.5 ± 23.33b | 736.5 ± 9.19a | 3290 ± 1.41b |
| 9 | 66.90 ± 0e | 1300 ± 15.55h | 5.03 ± 0.04def | 1071.5 ± 20.50g | 5551 ± 48.08g | 228.5 ± 36.06d | 1622.5 ± 27.57h |
| 10 | 82.33 ± 0.04cd | 2530 ± 11.31d | 4.93 ± 00dfg | 1822 ± 19.79d | 997.5 ± 16.26cd | 708 ± 8.48ab | 2819.5 ± 3.53de |
| 11 | 84.3 ± 0.64a | 1545 ± 16.97g | 5.63 ± 0.04a | 1230.5 ± 27.57f | 708.5 ± 0.70f | 314.5 ± 10.60d | 1939 ± 28.28g |
| LSD | 1.03 | 66.16 | 0.12 | 131.97 | 108.13 | 107.25 | 72.77 |
Means in a column with the same letter are not significantly different (P ≤ .05). Means of three replicates (viscosity in centipoise).
Note: Samples 1–5, solar dried plantain flours with 9–13 weeks of maturity respectively; samples 6–10, sun dried plantain flours with 9–13 weeks of maturity respectively; sample 11, oven dried plantain.
17.6 Water Absorption
The water absorption capacity ranged between 1.568 and 1.804 mL/g. The sun dried sample at the 10th week maturity had the highest value, while solar-dried flour at the same maturity time gave the least value (Table 19). The water absorption capacity is important in formulation of ready-to-eat food and high water absorption capacity may assure product cohesiveness (Houson and Ayenor, 2002). The difference depends on the amount and nature of hydrophilic constituents (Ayele and Nip, 1994).
Table 19
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 1.7047b | 2.0346a | 1.6373a | 1.7971b | 1.6493a |
| Solar drying | 1.8047a | 1.6385b | 1.5683 a | 1.8614a | 1.6185a |
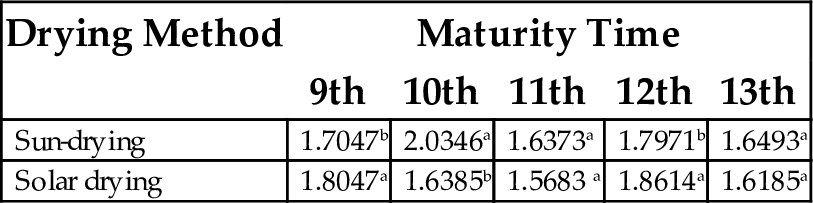
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
17.7 pH (Hydrogen Ion Concentration)
Table 20 shows a significant difference (P < .05) in pH values among flour samples, irrespective of time of maturity. Similarly, there was significant difference among drying methods used. Solar-dried flour at the 13th week of maturity gave the highest pH value of 6.92, while sun dried flour at the 10th week of maturity gave the least pH value of 6.06.
Table 20
| Drying Method | Maturity Time | ||||
|---|---|---|---|---|---|
| 9th | 10th | 11th | 12th | 13th | |
| Sun-drying | 6.36b | 6.06b | 6.51b | 6.40b | 6.53b |
| Solar drying | 6.51a | 6.28a | 6.78 a | 6.73a | 6.93a |
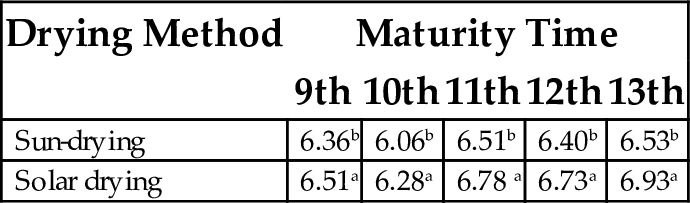
Each value represents mean of three replicates. Mean values having same superscript within column are not significantly different (P < .05).
Table 21 shows the maximum and minimum coefficient of determination (R2) for drying models fitted to moisture ratio data of 66 plantain samples. All models assessed were observed to be appropriate, to varying degrees, for the plantain samples investigated. However, Verma, two-term and page models were found to be the best three models for correlating drying kinetic data of plantain pellets when closeness of the maximum and minimum R2 values for the models were considered. The values of R2 and RMSE for Verma, two-term and page models are shown here.
Table 21
| Drying Model | R2 | RMSE | ||
|---|---|---|---|---|
| Max | Min | Max | Min | |
| Verma | 0.9989 | 0.9721 | 0.0024 | 0.0008 |
| Two-term | 0.9964 | 0.9702 | 0.0029 | 0.0008 |
| Page | 0.9930 | 0.9360 | 0.0062 | 0.0009 |
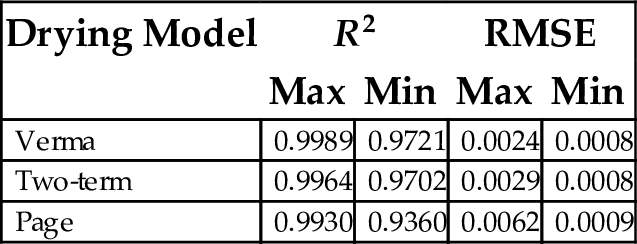
From Table 21, the Verma model was the most appropriate when coefficient of determination (R2), sum square of error (SSE) and root mean square of error (RMSE) were used as the criteria for assessment. It is known that the nearer R2 is to 1.00, SSE and RMSE are to zero, the better the predictive ability of the model.
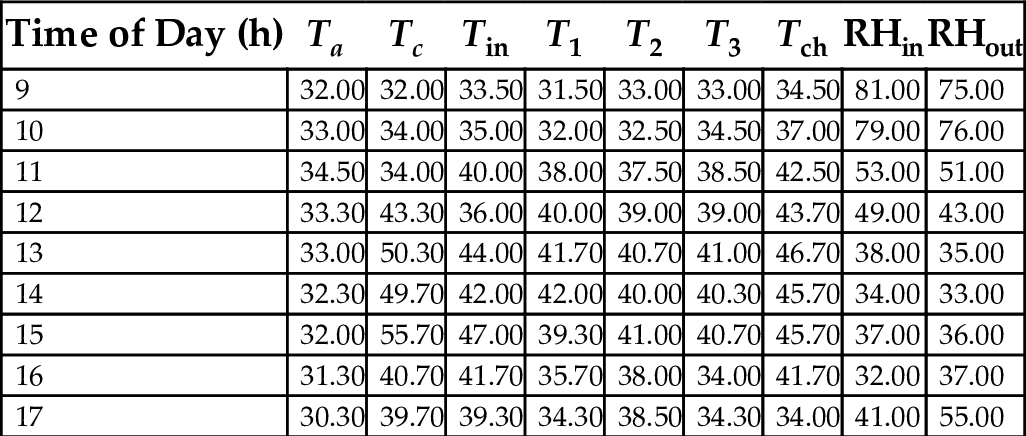
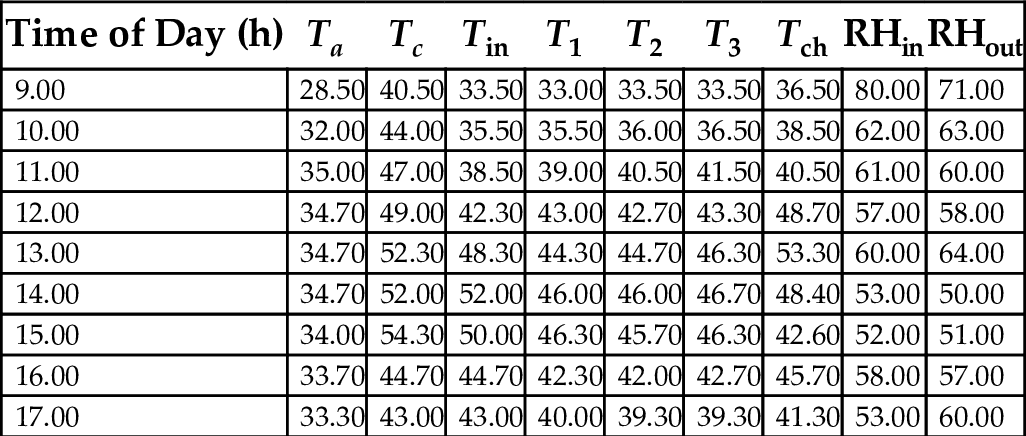
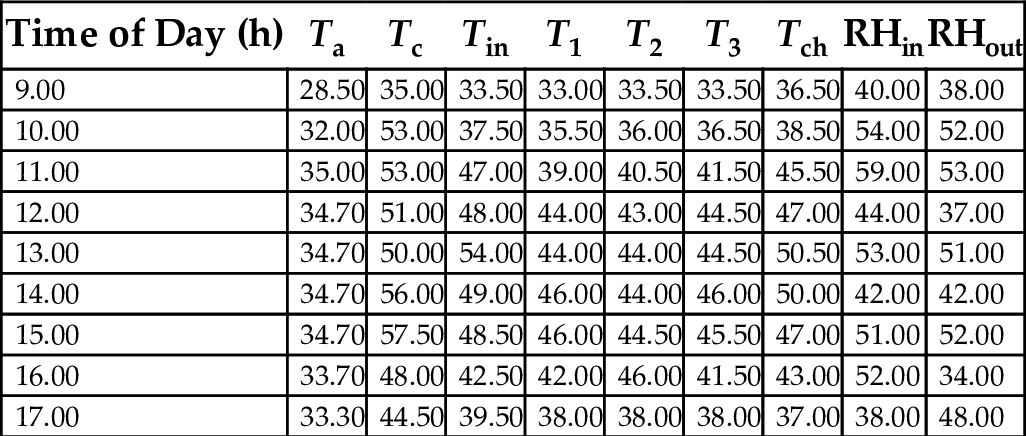
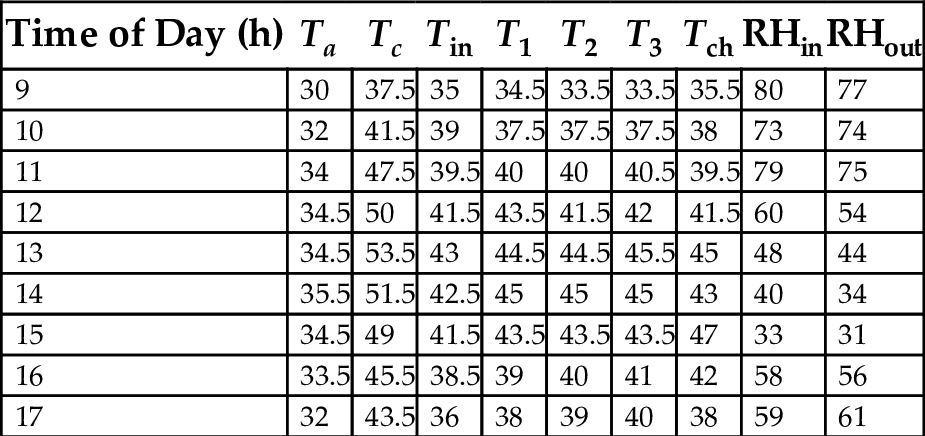
17.8 Mean Hourly Variation of the Temperatures and Relative Humidity of a Three-Chamber Solar Dryer
Tables 22–26 showed hourly variation of temperature and relative humidity of the three-chamber solar food dryer measured during the drying period from Jan. 18, 2012 to Feb. 27, 2012. The minimum temperature was 28.50°C and the maximum was 57.00°C. Relative Humidity was 32% (min) and 81% (max). This temperature range was considered suitable for drying vegetables, fruits, and root crops because bacteria are effectively killed and enzymes in activated within this temperature range, while the water soluble vitamins are largely retained in the dried products (Reza et al., 2009).
Table 22
| Time (h) | Ta | Tc | Tin | T1 | T2 | T3 | Tch | RHin | RHout |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 28.67 | 34.00 | 32.67 | 32.00 | 33.67 | 33.67 | 34.33 | 40.00 | 42.00 |
| 10 | 29.00 | 36.00 | 34.00 | 35.67 | 35.00 | 35.67 | 35.67 | 42.00 | 40.00 |
| 11 | 32.67 | 40.00 | 37.67 | 37.33 | 38.00 | 38.67 | 39.33 | 45.00 | 42.00 |
| 12 | 33.67 | 41.00 | 40.67 | 39.00 | 39.33 | 40.00 | 41.67 | 40.00 | 42.00 |
| 13 | 34.67 | 45.00 | 42.33 | 42.33 | 40.67 | 41.00 | 45.00 | 47.00 | 41.00 |
| 14 | 35.00 | 52.00 | 43.67 | 48.67 | 47.33 | 28.00 | 48.67 | 46.00 | 47.00 |
| 15 | 35.33 | 53.33 | 44.00 | 48.67 | 48.00 | 48.00 | 50.00 | 40.00 | 41.00 |
| 16 | 32.00 | 48.67 | 40.00 | 44.33 | 43.67 | 44.33 | 43.00 | 41.00 | 46.00 |
| 17 | 30.67 | 45.67 | 36.00 | 40.33 | 40.67 | 40.33 | 40.67 | 40.00 | 50.00 |
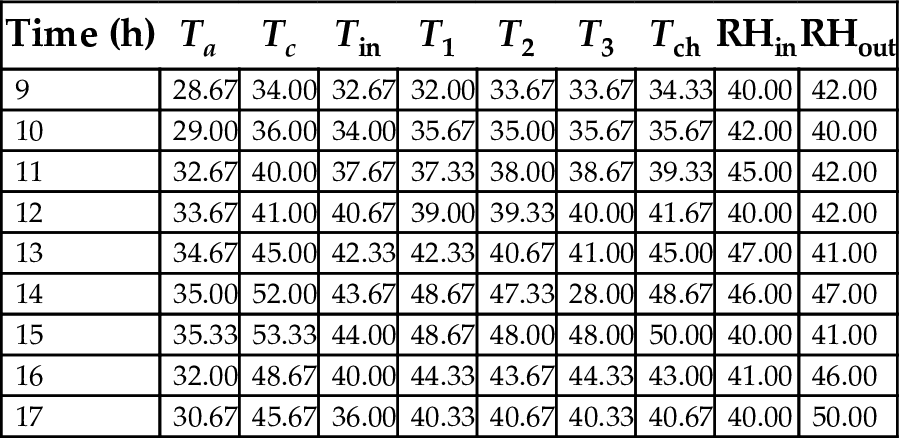
Ta, ambient temperature; Tc, collector temperature; Tin, inlet chamber temperature; T1, T2, T3, drying chambers temperatures; Tch, chimney temperature; RHin, RHou, relative humidity in and out.
Table 23
| Time of Day (h) | Ta | Tc | Tin | T1 | T2 | T3 | Tch | RHin | RHout |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 32.00 | 32.00 | 33.50 | 31.50 | 33.00 | 33.00 | 34.50 | 81.00 | 75.00 |
| 10 | 33.00 | 34.00 | 35.00 | 32.00 | 32.50 | 34.50 | 37.00 | 79.00 | 76.00 |
| 11 | 34.50 | 34.00 | 40.00 | 38.00 | 37.50 | 38.50 | 42.50 | 53.00 | 51.00 |
| 12 | 33.30 | 43.30 | 36.00 | 40.00 | 39.00 | 39.00 | 43.70 | 49.00 | 43.00 |
| 13 | 33.00 | 50.30 | 44.00 | 41.70 | 40.70 | 41.00 | 46.70 | 38.00 | 35.00 |
| 14 | 32.30 | 49.70 | 42.00 | 42.00 | 40.00 | 40.30 | 45.70 | 34.00 | 33.00 |
| 15 | 32.00 | 55.70 | 47.00 | 39.30 | 41.00 | 40.70 | 45.70 | 37.00 | 36.00 |
| 16 | 31.30 | 40.70 | 41.70 | 35.70 | 38.00 | 34.00 | 41.70 | 32.00 | 37.00 |
| 17 | 30.30 | 39.70 | 39.30 | 34.30 | 38.50 | 34.30 | 34.00 | 41.00 | 55.00 |
Ta, ambient temperature; Tc, collector temperature; Tin, inlet chamber temperature; T1, T2, T3, drying chambers temperatures; Tch, chimney temperature; RHin, RHout, relative humidity in and out.
Table 24
| Time of Day (h) | Ta | Tc | Tin | T1 | T2 | T3 | Tch | RHin | RHout |
|---|---|---|---|---|---|---|---|---|---|
| 9.00 | 28.50 | 40.50 | 33.50 | 33.00 | 33.50 | 33.50 | 36.50 | 80.00 | 71.00 |
| 10.00 | 32.00 | 44.00 | 35.50 | 35.50 | 36.00 | 36.50 | 38.50 | 62.00 | 63.00 |
| 11.00 | 35.00 | 47.00 | 38.50 | 39.00 | 40.50 | 41.50 | 40.50 | 61.00 | 60.00 |
| 12.00 | 34.70 | 49.00 | 42.30 | 43.00 | 42.70 | 43.30 | 48.70 | 57.00 | 58.00 |
| 13.00 | 34.70 | 52.30 | 48.30 | 44.30 | 44.70 | 46.30 | 53.30 | 60.00 | 64.00 |
| 14.00 | 34.70 | 52.00 | 52.00 | 46.00 | 46.00 | 46.70 | 48.40 | 53.00 | 50.00 |
| 15.00 | 34.00 | 54.30 | 50.00 | 46.30 | 45.70 | 46.30 | 42.60 | 52.00 | 51.00 |
| 16.00 | 33.70 | 44.70 | 44.70 | 42.30 | 42.00 | 42.70 | 45.70 | 58.00 | 57.00 |
| 17.00 | 33.30 | 43.00 | 43.00 | 40.00 | 39.30 | 39.30 | 41.30 | 53.00 | 60.00 |
Ta, ambient temperature; Tc, collector temperature; Tin, inlet chamber temperature; T1, T2, T3, drying chambers temperatures; Tch, chimney temperature; RH in, RHout, relative humidity in and out.
Table 25
| Time of Day (h) | Ta | Tc | Tin | T1 | T2 | T3 | Tch | RHin | RHout |
|---|---|---|---|---|---|---|---|---|---|
| 9.00 | 28.50 | 35.00 | 33.50 | 33.00 | 33.50 | 33.50 | 36.50 | 40.00 | 38.00 |
| 10.00 | 32.00 | 53.00 | 37.50 | 35.50 | 36.00 | 36.50 | 38.50 | 54.00 | 52.00 |
| 11.00 | 35.00 | 53.00 | 47.00 | 39.00 | 40.50 | 41.50 | 45.50 | 59.00 | 53.00 |
| 12.00 | 34.70 | 51.00 | 48.00 | 44.00 | 43.00 | 44.50 | 47.00 | 44.00 | 37.00 |
| 13.00 | 34.70 | 50.00 | 54.00 | 44.00 | 44.00 | 44.50 | 50.50 | 53.00 | 51.00 |
| 14.00 | 34.70 | 56.00 | 49.00 | 46.00 | 44.00 | 46.00 | 50.00 | 42.00 | 42.00 |
| 15.00 | 34.70 | 57.50 | 48.50 | 46.00 | 44.50 | 45.50 | 47.00 | 51.00 | 52.00 |
| 16.00 | 33.70 | 48.00 | 42.50 | 42.00 | 46.00 | 41.50 | 43.00 | 52.00 | 34.00 |
| 17.00 | 33.30 | 44.50 | 39.50 | 38.00 | 38.00 | 38.00 | 37.00 | 38.00 | 48.00 |
Ta, ambient temperature; Tc, collector temperature; Tin, inlet chamber temperature; T1, T2, T3, drying chambers temperatures; Tch, chimney temperature; RHin, RHout, relative humidity in and out.
Table 26
| Time of Day (h) | Ta | Tc | Tin | T1 | T2 | T3 | Tch | RHin | RHout |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 30 | 37.5 | 35 | 34.5 | 33.5 | 33.5 | 35.5 | 80 | 77 |
| 10 | 32 | 41.5 | 39 | 37.5 | 37.5 | 37.5 | 38 | 73 | 74 |
| 11 | 34 | 47.5 | 39.5 | 40 | 40 | 40.5 | 39.5 | 79 | 75 |
| 12 | 34.5 | 50 | 41.5 | 43.5 | 41.5 | 42 | 41.5 | 60 | 54 |
| 13 | 34.5 | 53.5 | 43 | 44.5 | 44.5 | 45.5 | 45 | 48 | 44 |
| 14 | 35.5 | 51.5 | 42.5 | 45 | 45 | 45 | 43 | 40 | 34 |
| 15 | 34.5 | 49 | 41.5 | 43.5 | 43.5 | 43.5 | 47 | 33 | 31 |
| 16 | 33.5 | 45.5 | 38.5 | 39 | 40 | 41 | 42 | 58 | 56 |
| 17 | 32 | 43.5 | 36 | 38 | 39 | 40 | 38 | 59 | 61 |
Ta, ambient temperature; Tc, collector temperature; Tin, inlet chamber temperature; T1, T2, T3, drying chambers temperatures; Tch, chimney temperature; RHin, RHout, relative humidity in and out.
The results of the microbiological analysis of plantain flour samples are shown in the previous graphs. In this study, high bacterial and mold counts were observed in oven dried samples, with values ranging from 3.3 × 105 to 4.0 × 104 CFU/mL, and 4.2 × 105 to 2.6 × 105 CFU/mL for bacteria and mold respectively. This could be as result of high water activity of oven dried plantain samples. According to James et al. (2005), when conditions of water activity (aw) favor growth, bacteria Bacillus, and molds of several genera are usually the only ones that develop. Many aerobic spore formers are capable of producing amylase, which enables them to utilize flour and related products as sources of energy, provided sufficient moisture is present to allow growth to occur. With less moisture, mold growth occurs and may be seen as typical mycelial growth and spore formation. However, sun dried samples showed lowest bacterial and mold counts when compared to solar and oven dried samples, irrespective of the pretreatment given. Neither yeast nor E. coli was detected in all plantain flours tested. Additionally, no Samonella spp. was detected in all samples except for solar dried plantain samples with maturity time of 9–10 weeks, having counts ranging from 5.0 × 105 to 2.1 × 106 CFU/mL. Salmonella spp. cause conditions such as typhoid, paratyphoid, and food poisoning. Generally, Salmonella spp. should not be detectable in 25 g of foodstuff (Mossel et al., 1995). The counts of Staphylococcus aureus range between 1 × 104 and 1 × 105 CFU/mL for some plantain samples, though some had zero level detection, as shown in the graph.
18 Conclusion
The results obtained confirmed the feasibility of producing starch flour with moisture contents at adequate levels for stable shelf life. The flours have considerable content of starch, as well as different functional behaviors which make them of interest as ingredients of food production. This study has shown higher maturity time and treatment with sodium metabisulphite increased oil absorption capacity and pH of plantain flours, while treatment with citric acid increased water absorption capacity as the weeks of maturity increased. Swelling index increased with increasing maturity time. Blanching also increased the swelling index of plantain flours. Low density plantain flour could be obtained by citric acid treatment before blanching, for a 13th week mature plantain. The usefulness of plantain flour could be enhanced by monitoring plantain maturity time, and applying the best pretreatment, as required. Drying air temperature was found to be the main factor to influence drying kinetics of unripe plantain. Time required for drying unripe plantain was considerably decreased with increments in drying air temperature. The Verma experimental model was best when compared to others, by producing the highest R2, and it can be used sufficiently to describe drying characteristics of unripe plantain.
19 Recommendation
Color is an important physical characteristic in flour quality. It was not monitored in this study, therefore, further studies are recommended in this regard, as well as to determining the maturity state at which unripe plantains can be most suitable for consumption by diabetics.