Nutraceutical and Medicinal Importance of Seabuckthorn (Hippophae sp.)
Prakash C. Sharma; Meenu Kalkal Guru Gobind Singh Indraprastha University, New Delhi, India
Abstract
Seabuckthorn (Hippophae sp.), a hardy deciduous shrub of family Elaeagnaceae, grows wild at higher altitudes of Asia and Europe, and is also cultivated in different countries including China, Russia, and India. Since ancient times, seabuckthorn has been known for its immense nutraceutical, medicinal, and ecological value. Seabuckthorn berries are a rich source of phytochemicals like vitamins (especially vitamin C and E), flavonoids, carotenoids, sugars, amino acids, organic acids, fatty acids, and mineral elements. Many research studies have reported diverse medicinal properties of seabuckthorn preparations, namely antimicrobial, antiulcerogenic, antioxidative, anticarcinogenic, radioprotective, hepatoprotective, antihypertensive, antiinflammatory, and immunomodulatory properties. These medicinal properties of seabuckthorn are attributed to the presence of important bioactive compounds in different parts of the seabuckthorn plant, mainly in berries, leaves, and seeds. A large number of seabuckthorn products have recently acquired significant attention and gains in the health, cosmetics, and food industry. The present review covers nutraceutical and medicinal applications of seabuckthorn.
Keywords
Seabuckthorn; Hippophae; Nutraceuticals; Medicinal plant; Bioactives; Phytochemicals
Acknowledgment
Meenu Kalkal thankfully acknowledges Indraprastha Research Fellowship from Guru Gobind Singh Indraprastha University, New Delhi 110078, India.
1 Introduction
Changes in human life style and food habits have generated the major challenge of “lifestyle diseases” in recent times. These days, “eating healthy food” has become a common catchphrase among educated consumers. Traditional medicine approaches have a long history of contributing toward to health maintenance, disease prevention, and treatment, particularly for infectious and chronic diseases. In ancient times, Hippocrates (460–377 BC), popularly known as “the father of modern medicine,” advocated the healing effects of food, recommending their effective role as medicine to treat and prevent diseases. He quoted “let food be your medicine and medicine be your food.” New developments in this area have emphasized the use of food products with both medicinal and nutritional value.
Nutraceuticals is a broad term coined by Stephen DeFelice in 1989, founding chairman of the Foundation of Innovation Medicine (United States). It is a combination of two words, “nutrition” and “pharmaceutical.” The term originally described any product derived from food sources with extra health benefits in addition to the basic nutritional value. Therefore, nutraceuticals may be considered as a food, or part of a food, that provides health benefits toward prevention and treatment of diseases. These include isolated nutrients, dietary supplements, genetically engineered “designer” foods, and herbal products. Nutraceuticals can be classified on the basis of their natural sources: from plants, animals, or microorganisms. Plant based nutraceuticals are gaining importance around the globe as they are safe, more effective and affordable than other therapeutic agents. In addition, plants produce a diverse range of bioactive molecules, making them a rich source of nutraceuticals. Recently, much interest has been generated in a number of ancient crops, including seabuckthorn, as a source of many nutraceuticals and therapeutical foods or supplements.
Seabuckthorn (Hippophae sp.) is a cold tolerant, deciduous shrub with chromosome number of 2n = 24 (Rousi, 1971). The genus name Hippophae was derived from two Latin words, “hippo” meaning “horse” and “phaos” meaning “to shine.” Traditionally, in ancient Greece, leaves and young branches of the seabuckthorn plant were used as animal feed, especially for horses, to support weight gain and make their coats shiny (Rongsen, 1992). The plant is highly adaptable to extreme and varying environmental conditions including drought, salinity, high altitude, alkalinity, and extreme temperature (Li and Schroeder, 1996). In addition to wide adaptability, the plant has also acquired global importance by virtue of its immense nutraceutical and medicinal value.
1.1 Taxonomical Classification
Seabuckthorn (Hippophae L.) has been included in the family Elaeagnaceae, order Elaeagnales, super order Celastraneae, subclass Rosidae, class Magnoliopsida, and division Magnoliophyta. Classification of this genus is still controversial. In 1753, Carl Linnaeus recorded this plant for the first time and established the genus Hippophae, including species H. rhamnoides Linn. Subsequently, various other species and their subspecies were reported and named by different taxonomists (Table 1). The different people have used morphological descriptors like phyllotaxy, position of scales, characteristics of bark, fruit, and seed. Later, molecular data have also been employed for this purpose. Servettaz (1908) recognized one species H. rhamnoides with three subspecies namely rhamnoides, salicifolia, and tibetana. Later, on the basis of morphological traits, Rousi (1971) divided Hippophae into three species: H. rhamnoides, H. salicifolia, and H. tibetana. In 1978, Liu and He added a fourth species, H. neurocarpa. Lian (1988) presented Hippophae rhamnoides subsp. gyantsensis as a new independent species. Lian et al. (1995) introduced a new species, H. goniacarpa, and two subspecies, subsp. litangensis and subsp. stellatopilosa. Lian and Chen (2002) upgraded subsp. litangensis to species level. Swenson and Bartish (2002) reviewed the taxonomic status of the genus Hippophae and argued in favor of seven species, of which H. rhamnoides is the most variable with eight subspecies. At present, this is the most accepted classification and nomenclature for genus Hippophae. However, minor modifications are still undergoing as Lian et al. (2003) reported, another subsp. wolongensis of H. rhamnoides. Nevertheless, Hippophae rhamnoides is the most important and widespread species, followed by Hippophae salicifolia and Hippophae tibetana in Eurasian region.
Table 1
| Linnaeus (1753) | Servettaz (1908) | Rousi (1971) | Liu and He (1978) | Lian (1988) | Lian et al. (1995) | Lian and Chen (2002) |
|---|---|---|---|---|---|---|
| Genus: Hippophae Species: rhamnoides | Species: H. rhamnoides Subspecies: rhamnoides Subspecies: tibetana Subspecies: salicifolia | Species: H. salicifolia Species: H. tibetana Species: H. rhamnoides Subspecies: carpatica Subspecies: caucasica Subspecies: fluviatilis Subspecies: gyantsensis Subspecies: mongolica Subspecies: rhamnoides Subspecies: sinensis Subspecies: turkestanica Subspecies: yunnanensis | Species: H. salicifolia Species: H. tibetana Species: H. rhamnoides Subspecies: carpatica Subspecies: caucasica Subspecies: fluviatilis Subspecies: gyantsensis Subspecies: mongolica Subspecies: rhamnoides Subspecies: sinensis Subspecies: turkestanica Subspecies: yunnanensis Species: H. neurocarpa | Species: H. salcifolia Species: H. tibetana Species: H. rhamnoides Subspecies: carpatica Subspecies: caucasica Subspecies: fluviatilis Subspecies: mongolica Subspecies: rhamnoides Subspecies: sinensis Subspecies: turkestanica Subspecies: yunnanensis Species: H. neurocarpa Species: H. gyantsensis | Species: H. salcifolia Species: H. tibetana Species: H. rhamnoides Subspecies: carpatica Subspecies: caucasica Subspecies: fluviatilis Subspecies: mongolica Subspecies: rhamnoides Subspecies: sinensis Subspecies: turkestanica Subspecies: yunnanensis Species: H. neurocarpa Subspecies: stellatopilosa Species: H. gyantsensis Species: H. goniacarpa Subspecies: litangensis | Species: H. salcifolia Species: H. tibetana Species: H. rhamnoides Subspecies: carpatica Subspecies: caucasica Subspecies: fluviatilis Subspecies: mongolica Subspecies: rhamnoides Subspecies: sinensis Subspecies: turkestanica Subspecies: yunnanensis Species: H. neurocarpa Subspecies: stellatopilosa Species: H. gyantsensis Species: H. goniacarpa Species: H. litangensis |

1.2 Geographical Distribution
Seabuckthorn grows in diverse areas of harsh environmental conditions, ranging from seacoasts and riverbeds, to cold deserts. It is found growing naturally at higher altitudes of temperate zones of Asia and Europe (Rousi, 1971). It is a temperate plant of the Eurasian continent with wide spread distribution across 38 countries, namely Afghanistan, Azerbaijan, Belarus, Bhutan, Britain, Bulgaria, Canada, China, Czech Republic, Denmark, Estonia, Finland, France, Germany, India, Iran, Italy, Kyrgyzstan, Kazakhstan, Latvia, Lithuania, Moldova, Mongolia, Nepal, Netherlands, Hungary, Norway, Pakistan, Poland, Portugal, Romania, Russia, Slovakia, Sweden, Switzerland, Turkey, Ukraine, and Uzbekistan (Rajchal, 2009).
Distribution of seabuckthorn taxa across Europe and Asia is shown in Fig. 1. Attitudinally, it ranges from seashore of the Baltic Sea in Europe to the regions of Mount Everest in Asia, up to an altitude of approximately 7000 m, suggesting high adaptability of the plant to various ecological conditions. Geographically, it is distributed between 27°–69°N latitude and 7°W–122°E longitude (Rousi, 1971).

1.3 Plant Morphology
Seabuckthorn shows huge variation in morphological features, depending on the species and microclimatic variations. There is variation in height from approximately 1 m of Hippophae tibetana, to approximately 15 m of Hippophae salicifolia. Seabuckthorn bears hard thorns originating from the stem. Thorniness varies from 1 to 5 thorns/cm2 of stem and the degree of thorniness differs among species. H. rhamnoides plants are generally dioecious, however, Sen et al. (2014) reported rare deviation from dioecious sex to either monoecious or polygamomonoecious (i.e., trimonoecious or trioecious). Flowers are very small in size and yellowish in color. Fruit (berry) vary in both shape (round, oval to long) and color (yellow to orange-red) (Fig. 2). Berries bear a single ovoid to elliptical seed of 2.8–4.2 mm in size, having dark brown skin (Suryakumar and Gupta, 2011). The leaves are usually small, 3–8 cm long, and 0.4–1 cm wide, alternate, linear, lanceolate, and covered with silvery stellate scales which reflect sunshine and prevent moisture loss (Rongsen, 1992). The root system is well established, consisting of primary, secondary, and tertiary roots covered with root hairs which are seen prominently in the apical region. About 80% of its feeding roots are in the topsoil; this feature helps in preventing soil erosion (Rongsen, 1992). Roots are characterized by the presence of root nodules containing a nitrogen fixing actinomycetes, Frankia (Clawson et al., 1998).

2 Harvesting
Ripe seabuckthorn berries give off a musky odor in field. Mature berries are harvested manually or mechanically. Harvesting is a difficult and time consuming process as berries are very soft, small, and extremely delicate. Moreover, thorniness of the plant also makes harvesting difficult. Considering the difficulties faced in manual harvest of the berries, mechanical harvesters are becoming a necessity of the seabuckthorn industry. Mechanical harvesting can be classified as being direct or indirect (Fu et al., 2014). Direct harvesting relies on direct contact of the device with the berries; for example, vacuum suction harvesters remove berries by generating negative pressure using a vacuum pump. Indirect harvesting relies mainly on vibration harvesters and cutting harvesters, which remove berries without touching them physically. Vibration harvesters involve shaking the stems or branches while cutting harvesters involve cutting of branches (Kalia et al., 2011). Shaking the plants causes severe bark damage, so even after successful harvesting of berries, plant will still need to be cut down. Therefore, the cutting harvest method is a more realistic option with a major drawback, a lower yield in the following year. More efforts are required to develop improved harvesting equipment for higher yields at lower costs with less damage to the plant and berries. Another suggested harvest possibility is hormonal treatment causing easy fruit detachment from the branches. Development of thornless varieties will also certainly improve harvesting.
3 Postharvest Processing
Different processing methods have been developed to separate useful components and develop different products from the berries, leaves, and bark of seabuckthorn (Fig. 3).

3.1 Extraction
Extraction is the initial process toward separation of bioactive compounds that can be subsequently used as nutraceuticals, food ingredients, and in cosmetic products. General methods of extraction used for medicinal plants are maceration, infusion, digestion, decoction, percolation, soxlet (hot continuous extraction), aqueous alcoholic extraction, counter current extraction, ultrasonic extraction, supercritical fluid extraction, and phytonics. Solvent extraction is the most commonly used method for extract preparation in seabuckthorn. Solvents such as methanol, ethanol, acetone, hexane, petroleum ether, and their combinations have been used most often at laboratory scale for extraction. However, the presence of solvent residues in the extract limits its use for nutraceutical applications (Ranjith et al., 2006). Therefore, supercritical-CO2 extraction is gaining popularity, yielding superior quality solvent-free extract.
3.2 Analytical Techniques for Extract Analysis
Plant extract usually contains a mixture of various types of bioactive compounds whose separation, identification, and quantification is a big challenge. A number of chromatographic techniques, such as paper chromatography (PC), thin layer chromatography (TLC), gas chromatography (GC), counter-current chromatography (CCC), high performance liquid chromatography (HPLC), high performance liquid chromatography-mass spectrometry (HPLC-MS), gas chromatography-mass spectrometry (GC-MS), and capillary electrophoresis (CE), have been exploited for the study of phytochemicals present in seabuckthorn extracts (Guliyev et al., 2004). The conventional methods such as paper chromatography and thin layer chromatography are not presently in much use. Counter current chromatography based on liquid-liquid chromatography is an ideal technique for preparative isolation of natural products, however, rarely used in seabuckthorn processing. High speed-CCC has been used for the preparative isolation of some flavonoid derivatives and protocatechuic acid from seabuckthorn juice concentrates (Gutzeit et al., 2007). GC, HPLC, and CE remain the most widely used techniques for the analysis of various compounds in seabuckthorn (Table 2). Nuclear magnetic resonance (NMR) has also been widely used for qualitative and quantitative study of metabolites present in tissue, organ, or whole plant extracts (Rosch et al., 2004; Li et al., 2013; Kortesniemi et al., 2014).
Table 2
| Technique | Plant Material | Compounds Analyzed | References |
|---|---|---|---|
| GC | Berry oil (pulp and seed) | Fatty acids | Dulf (2012) |
| Pulp oil | Fatty acids | Kaminskas et al. (2006) | |
| Seed oil | Phytosterols | Li et al. (2007) | |
| Berries and leaves | FA | Pop et al. (2014) | |
| Berries | Volatile compounds | Socaci et al. (2013) | |
| Leaves | Volatile compounds | Tian et al. (2004) | |
| Berries | Inositols and methyl-inositols | Yang et al. (2011) | |
| HPLC | Berries | Flavonoids | Chen et al. (2007) |
| Berries | Flavonol aglycones (myricetin, quercetin, and kaempferol) and flavonol glycosides | Hakkinen and Auriola (1998) | |
| Berries at different maturation stages | Kaempherol, quercetin, and l-ascorbic acid | Jeppson and Gao (2008) | |
| Berries | Carotenol fatty acid esters | Pintea et al. (2005) | |
| Berries and leaves | Phenolic acids | Arimboor et al. (2008) | |
| Berry juice | Phenolic compounds | Rosch et al. (2004) | |
| Berries, seeds, leaves, and pulp | Phenolic compounds | Sharma et al. (2007) | |
| Leaves | Catechin, rutin, quercetin, kaempferol, and isorhamnetin | Zu et al. (2006) | |
| Berries and leaves | Free carotenoids and carotenoid esters | Pop et al. (2014) | |
| CE | Berries and seeds | Epicatechin, catechin, rutin, kaempferol, and quercetin | Chu et al. (2003) |
| Berries | Trans-resveratrol, catechin, l-ascorbic acid, myricetin, and quercetin | Gorbatsova et al. (2007) |

4 Biochemical Profiling of Seabuckthorn
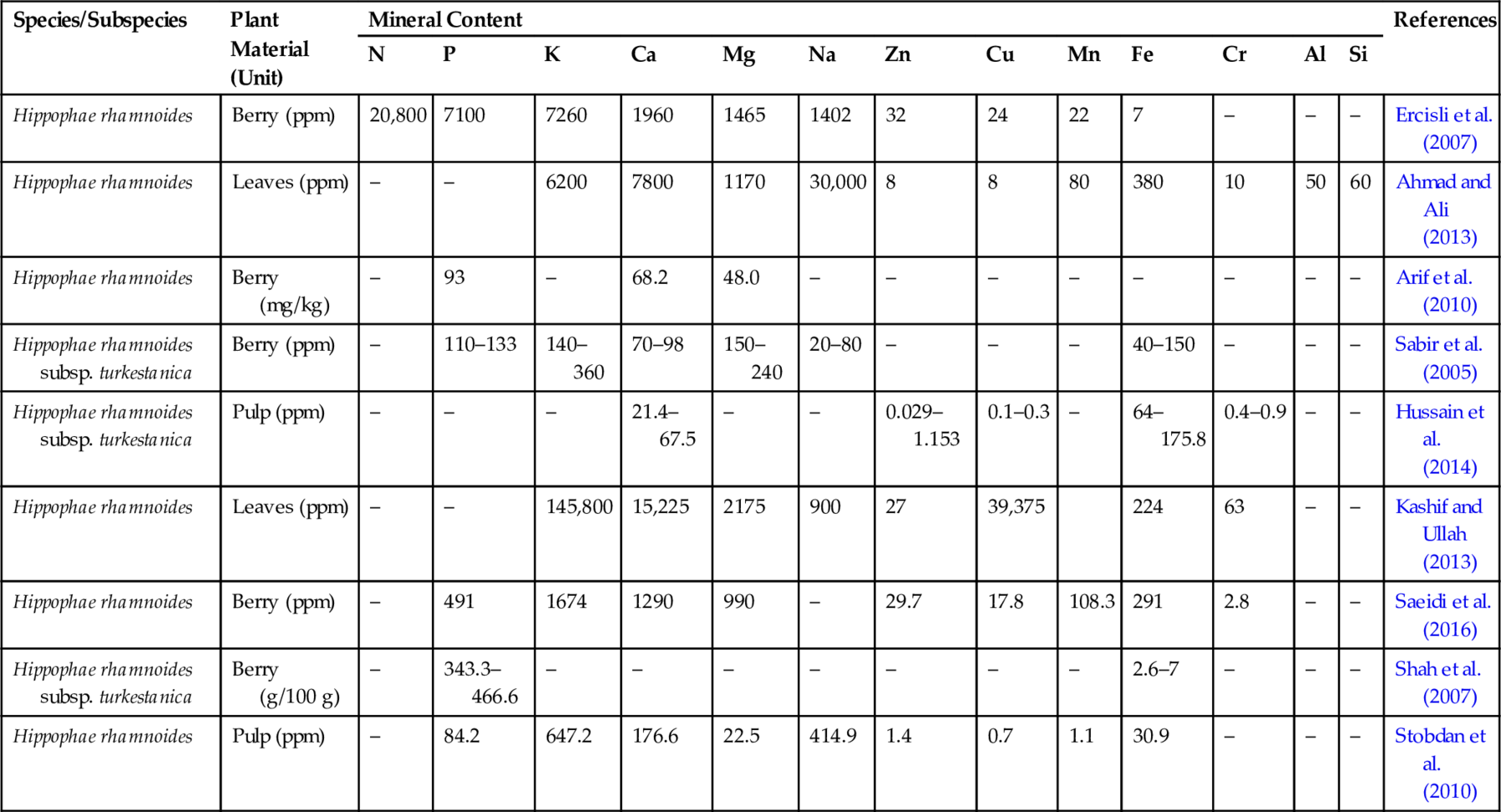
Seabuckthorn has attracted the attention of researchers, scientists, and environmentalists around the world for its immense nutritional and medicinal value. More than 190 bioactive compounds have been reported from different parts of the plant (Bal et al., 2011). It is also known as a “multipurpose-wonder plant” or “golden bush” because every part of the plant, such as fruit (berry), leaf, seed, stem, root, and thorn has some nutraceutical or medicinal value (Li and Schroeder, 1996). Various phytochemicals like carbohydrates, vitamins, fatty acids, amino acids, organic acids, flavonoids, carotenoids, tocopherols, and mineral elements are found in the different parts of the plant. Seabuckthorn berries are reported to contain a very high content of vitamin C (a natural antioxidant) in addition to vitamin E and K (Table 3). In addition, the plant also contains other vitamins, such as vitamin A and vitamin B (Dhyani et al., 2010; Stobdan et al., 2010). However, a large variation in vitamin content exists and may be attributed to various factors like geographical location, climate, genome, harvesting time, extraction method, and method of processing. Seabuckthorn juice is rich in amino acids, including essential amino acids. Bal et al. (2011) have tabulated the amino acid content of various amino acids in seabuckthorn juice. However, there is a huge unexplainable variation between the content of the two reports cited (Chen, 1988; Zhang et al., 1989). Glucose and fructose are the main sugar components found in the berries accounting for 90% of the sugar component (Bal et al., 2011) (Table 4). Low amounts of ethyl glucose and sucrose have also been reported. Berry juice also contains sugar alcohols like quebrachitol, methyl-myo-inositol, and myo-inositol (Yang et al., 2011; Zheng et al., 2012) (Table 4). Organic acids present in seabuckthorn berries are predominantly malic acid and quinic acid, together making around 90% of all fruit acids (Bal et al., 2011). Other organic acids reported are citric, tartaric, and oxalic acid (Beveridge et al., 2002) (Table 5). A large variation has been reported in the content of organic acids, which may be related to different factors as stated above. Different parts, including leaves, seeds, and berries, contain different mineral elements (Table 6). These mineral elements play a key role in many metabolic processes, their content in the berries varies with fruit maturity and content of minerals in soil.
Table 3
| Species/Subspecies | Vitamin C | References | |
|---|---|---|---|
| Berries (mg/100 g) | Berry Juice (mg/100 mL) | ||
| Hippophae rhamnoides subsp. rhamnoides | 413.5–436.2 | 386.8–391.1 | Gutzeit et al. (2008) |
| Hippophae rhamnoides | 191–295.6 | – | Shah et al. (2007) |
| Hippophae rhamnoides | – | 19–121 | Ercisli et al. (2007) |
| Hippophae rhamnoides | – | 29–176 | Tiitinen et al. (2005) |
| Hippophae rhamnoides | – | 29–128 | Tiitinen et al. (2006) |
| Hippophae rhamnoides subsp. sinensis | – | 420–1320 | Kallio et al. (2002) |
| Hippophae rhamnoides subsp. rhamnoides | – | 90–210 | |
| Hippophae rhamnoides subsp. mongolica | – | 10–110 | |
| Hippophae rhamnoides | 28–201 | – | Yao et al. (2009) |
| Hippophae rhamnoides subsp. sinensis | 263–399 | – | Arif et al. (2010) |
| Hippophae rhamnoides subsp. sinensis | – | 250–1660 | Zheng et al. (2011) |
| Hippophae rhamnoides subsp. mongolica | – | 50–140 | Zheng et al. (2012) |
| Hippophae rhamnoides subsp. turkestanica | 250–333 | – | Sabir et al. (2005) |
| Hippophae rhamnoides subsp. turkestanica | 100–250 | – | Sabir et al. (2003) |
| Hippophae rhamnoides | 28–310 | – | Rousi and Aulin (1977) |
| Hippophae rhamnoides | 56–3909 | – | Korekar et al. (2014) |
| Hippophae rhamnoides | 168.3–184 | – | Arimboor et al. (2006) |
| Hippophae rhamnoides | 275 | – | Stobdan et al. (2010) |
| Species/Subspecies | Vitamin K | References | |
| Seed Oil (mg/100 g) | Pulp Oil (mg/100 g) | ||
| Hippophae rhamnoides | 109.8–230 | 54–59 mg/100 g | Zeb (2004) |
| Species/Subspecies | Vitamin E | References | |
| Berries (mg/100 g) | |||
| Hippophae rhamnoides | 3.4 | Stobdan et al. (2010) | |

Table 4
| Species/Subspecies | Plant Material (Unit) | Sugar | References | ||
|---|---|---|---|---|---|
| Glucose | Fructose | ||||
| Hippophae rhamnoides subsp. mongolica | Juice (g/100 mL) | 1.75–3.50 | 0.34–2.62 | Zheng et al. (2012) | |
| Hippophae rhamnoides subsp. sinensis | 1.6–12.5 | 1.6–11.7 | Yang et al. (2011) | ||
| Hippophae rhamnoides subsp. rhamnoides | 0.5–2.9 | 0.1–0.6 | |||
| Hippophae rhamnoides subsp. mongolica | 3.5–7.2 | 0.9–4.3 | |||
| Hippophae rhamnoides | 1.5–4.2 | 0.2–3.5 | Tiitinen et al. (2006) | ||
| Hippophae rhamnoides subsp. sinensis | 2.32 ± 0.04 | 2.26 ± 0.07 | Tang et al. (2001) | ||
| Hippophae rhamnoides subsp. rhamnoides | 0.27–0.33 | 0.08–0.10 | |||
| Hippophae rhamnoides | Berry (g/100 g) | 0.26–2.10 | 0.14–0.54 | Raffo et al. (2004) | |
| Species/Subspecies | Plant Material (Unit) | Sugar Alcohols | References | ||
| Quebrachitol | Methyl-Myo-inositol | Myo-inositol | |||
| Hippophae rhamnoides subsp. sinensis | Juice (mg/100 mL) | 411.6–924.2 | 17–99.7 | 0–55.9 | Yang et al. (2011) |
| Hippophae rhamnoides subsp. rhamnoides | 190.9–511.2 | 0–28.7 | 0–15.1 | ||
| Hippophae rhamnoides subsp. mongolica | 180.7–275.9 | 3.5–29.7 | 15.9–22.9 | ||
| Hippophae rhamnoides subsp. mongolica | 150–240 | 10–30 | 10–40 | Zheng et al. (2012) | |
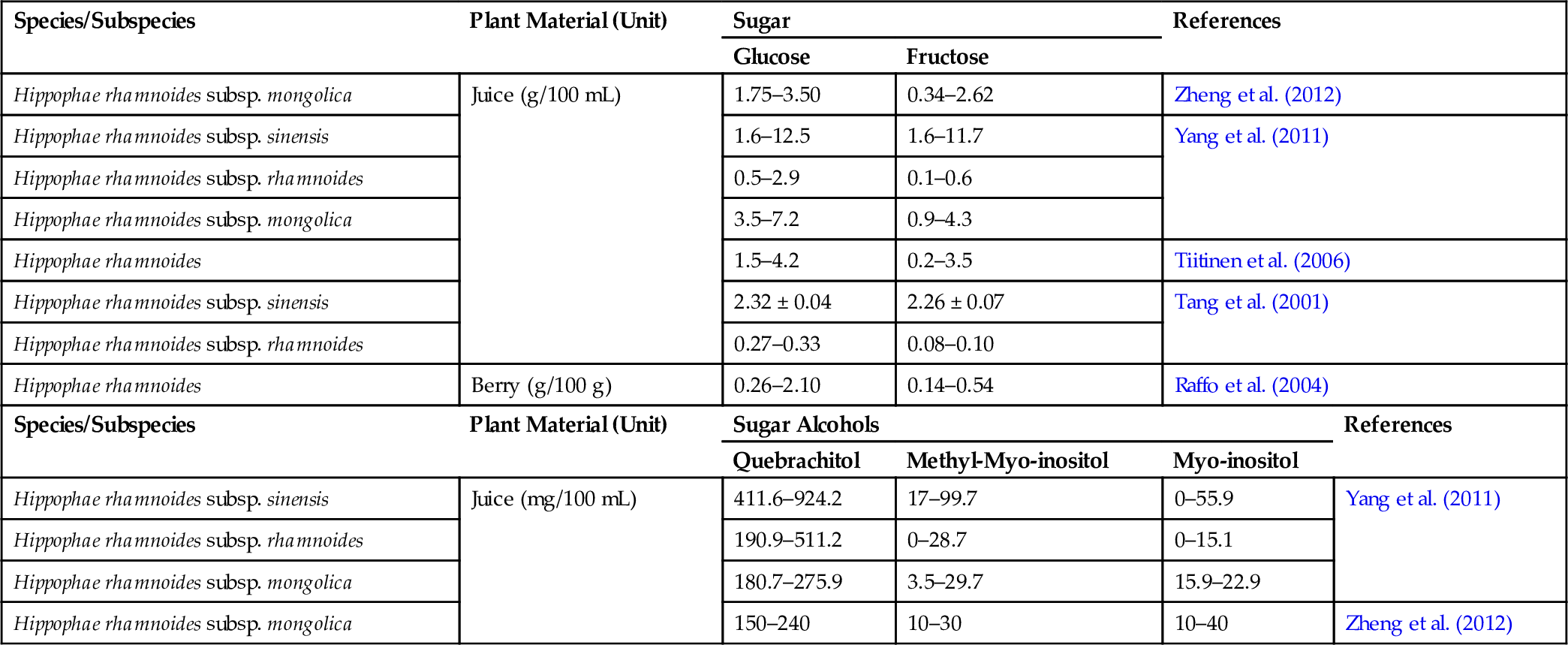
Table 5
| Species/Subspecies | Plant Material (Unit) | Quinic Acid | Malic Acid | Citric Acid | References |
|---|---|---|---|---|---|
| Hippophae rhamnoides subsp. mongolica | Juice (g/100 mL) | 1.23–2.05 | 0.84–3.28 | 0.02–0.05 | Zheng et al. (2012) |
| Hippophae rhamnoides subsp. sinensis | 0.7–7.5 | 2.3–9.2 | ND | Yang et al. (2011) | |
| Hippophae rhamnoides subsp. rhamnoides | 0.7–2.1 | 2.9–4.7 | ND | ||
| Hippophae rhamnoides subsp. mongolica | 1.3–2.6 | 0.8–2.7 | ND | ||
| Hippophae rhamnoides | 2.30 | 1.39 | 0.19 | Beveridge et al. (2002) | |
| Hippophae rhamnoides | 0.9–2.5 | 1.9–3.6 | < 0.05 | Tiitinen et al. (2006) | |
| Hippophae rhamnoides | Berries (g/100 g) | 0.81–2.82 | 1.94–4.66 | 0.09–0.16 | Raffo et al. (2004) |

Table 6
| Species/Subspecies | Plant Material (Unit) | Mineral Content | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | Zn | Cu | Mn | Fe | Cr | Al | Si | |||
| Hippophae rhamnoides | Berry (ppm) | 20,800 | 7100 | 7260 | 1960 | 1465 | 1402 | 32 | 24 | 22 | 7 | – | – | – | Ercisli et al. (2007) |
| Hippophae rhamnoides | Leaves (ppm) | – | – | 6200 | 7800 | 1170 | 30,000 | 8 | 8 | 80 | 380 | 10 | 50 | 60 | Ahmad and Ali (2013) |
| Hippophae rhamnoides | Berry (mg/kg) | – | 93 | – | 68.2 | 48.0 | – | – | – | – | – | – | – | – | Arif et al. (2010) |
| Hippophae rhamnoides subsp. turkestanica | Berry (ppm) | – | 110–133 | 140–360 | 70–98 | 150–240 | 20–80 | – | – | – | 40–150 | – | – | – | Sabir et al. (2005) |
| Hippophae rhamnoides subsp. turkestanica | Pulp (ppm) | – | – | – | 21.4–67.5 | – | – | 0.029–1.153 | 0.1–0.3 | – | 64–175.8 | 0.4–0.9 | – | – | Hussain et al. (2014) |
| Hippophae rhamnoides | Leaves (ppm) | – | – | 145,800 | 15,225 | 2175 | 900 | 27 | 39,375 | 224 | 63 | – | – | Kashif and Ullah (2013) | |
| Hippophae rhamnoides | Berry (ppm) | – | 491 | 1674 | 1290 | 990 | – | 29.7 | 17.8 | 108.3 | 291 | 2.8 | – | – | Saeidi et al. (2016) |
| Hippophae rhamnoides subsp. turkestanica | Berry (g/100 g) | – | 343.3–466.6 | – | – | – | – | – | – | – | 2.6–7 | – | – | – | Shah et al. (2007) |
| Hippophae rhamnoides | Pulp (ppm) | – | 84.2 | 647.2 | 176.6 | 22.5 | 414.9 | 1.4 | 0.7 | 1.1 | 30.9 | – | – | – | Stobdan et al. (2010) |
4.1 Berries
Seabuckthorn berries are highly nutritious and the most important part of the plant. Generally, berries are composed of pulp (68%), seed (23%), and peel (7.75%). They are a rich source of many bioactive compounds that vary with species, origin, maturity, fruit size, fruit color, geographic location, and method of extraction. Berries, being highly acidic, are sour in taste and have a unique aroma suitable for flavoring food products. Berries are reported to contain carbohydrates (glucose and fructose), vitamins (C, E, and K), essential fatty acids, amino acids, organic acids, flavonoids (isorhamnetin, quercetin, and kaempferol), tocopherols, carotenoids, and mineral elements (Bal et al., 2011). Besides nutrition, berries provide preventive effects against cardiovascular diseases, mucosal injuries, skin problems, cancer, and also support immune system function (Rajchal, 2009). Flavonoids, especially isorhamnetin, have been reported to have cardioprotective, hepatoprotective, antitumor, and anticancer activities (Teng et al., 2006; Maheshwari et al., 2011; Li et al., 2014, 2015). Carotenoids (especially zeaxanthin and beta-cryptoxanthin esters) isolated from seabuckthorn berries have been used as food additives, cosmetic ingredients, and nutraceuticals (Suryakumar and Gupta, 2011).
4.1.1 Berry Pulp
Pulp is the major constituent of the berry, and an important source of seabuckthorn oil. In addition to palmitic (16:0) acid, pulp oil contains high levels of rare and monounsaturated palmitoleic acid (16:1n-7), a major constituent of skin fat. Pulp oil has attracted an increasing interest due to its role in the treatment of atopic dermatitis (Yang et al., 1999) and protective ability against UV light, and therefore provides raw material for many pharmaceutical and cosmetic industries (Suryakumar and Gupta, 2011). Moreover, high content of tocopherols, carotenoids, 16:1 fatty acid and other bioactive molecules, makes pulp oil a valuable health supplement (Ranjith et al., 2006)
4.1.2 Berry Juice
Seabuckthorn berry juice is yellow in color and acidic in pH. Juice can be extracted from the fruits by pressing and has a freezing point of − 22°C, allowing it to remain liquid even at subzero temperatures (Bal et al., 2011). The juice is highly rich in vitamin C and carotenes, in addition to suspended solids. It is highly nutritious, containing vitamin C, carotenoids, amino acids, and mineral elements (Beveridge et al., 1999). Seabuckthorn berry juice was used in ancient times in various effective pharmacological formulations for easy expectoration, promoting blood circulation, removing blood stasis, and invigorating spleen function (Zeb, 2004).
4.2 Leaves
The seabuckthorn leaves are known for their high content of nutrients and bioactive compounds, especially phenolics, carotenoids, free and esterified sterols, triterpenols, and isoprenols (Suryakumar and Gupta, 2011). Catechin, rutin, quercetin, kaempferol, and isorhamnetin are the major flavonoids reportedly found in seabuckthorn leaf extract (Zu et al., 2006). Each of these flavonoids has important health benefits for the human body. Numerous food products have been made from the dried leaves of seabuckthorn, such as tea and tea powders. Tea made from seabuckthorn leaves is an important source of vitamins, antioxidants, amino acids, fatty acids, and minerals, and is beneficial for normalizing blood pressure, lowering cholesterol, preventing and controlling blood vessel diseases, and boosting immunity. Bioactive oil from leaves and branches has been found useful in formulations of various ointments for treating various skin diseases and UV radiation induced injury.
4.3 Bark
The seabuckthorn bark is widely used in Mongolian folk medicine for the treatment of a wide range of gastrointestinal symptoms (Xu et al., 2007). In India, bark of Hippophae salicifolia has been used for healing burn wounds (Dhyani et al., 2010) and as an effective blood purifier (Ali and Kaul, 2012). Serotonin isolated from bark extract also possesses antitumor activity by providing protection from radioactive radiation (Gol’dberg et al., 2007). Xu et al. (2006) extracted proanthocyanidins from seabuckthorn bark extract which have been reported to possess various health promoting activities.
4.4 Seed and Seed Oil
The seed of the seabuckthorn is dark brown in color, 2.8–4.2 mm in size, ovoid to elliptical in shape, and is known to contain a variety of medicinally important compounds (Li and Schroeder, 1996; Suryakumar and Gupta, 2011). It is composed mainly of carbohydrates, lipids, and proteins. The seeds yield 10%–20% oil and this proportion never exceeds that of pulp oil (Zeb, 2004). Seed oil is comprised mostly of unsaturated fatty acids, α-linolenic acid (C18:3n-3) and linoleic acid (C18:2n-6), at 20%–30% and 30%–40% range, respectively (Yang and Kallio, 2002). Other constituents of the seed oil include α, β, γ, and δ-tocopherols, tocotrienols, phytosterols, and carotenoids. (Cenkowski et al., 2006). Seabuckthorn seed oil is reported to have a therapeutic role in atopic dermatitis, gastric ulcers, cardiovascular diseases, and treating burn wounds (Yang et al., 1999; Johansson et al., 2000; Eccleston et al., 2002; Basu et al., 2007; Upadhyay et al., 2009; Xing et al., 2012). Seed oil has been approved for clinical use in hospitals in Russia and is well documented in ancient literature of Asia and Europe for medicinal use. Seabuckthorn oil contains unique fatty acid composition (Table 7) and has desirable physical properties such as low viscosity, high transmittance, and unique thermal characteristics which make it appropriate for potential use as functional food, and also for non food applications, like in cosmetics (Beveridge et al., 1999).
Table 7
| Species/Subspecies | Berry Oil | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C15:0 | C16:0 | C16:1n-9 | C16:1n-7 | C17:0 | C18:0 | C18:1 | C18:1n-9 | C18:1n-7 | C18:2n-6 | C18:3n-3 | C20:1 | C20:1n-9 | ||
| H. rhamnoides subsp. carpatica | 0.22–0.61 | 0.03–0.05 | 20.80–37.33 | 0.02–0.14 | 9.63–24.64 | 0.02–0.03 | 0.82–2.86 | – | 22.29–45.90 | 4.55–6.78 | 3.05–10.87 | 1.86–4.17 | 0.12–0.23 | 0.02–0.15 | Dulf (2012) |
| H. rhamnoides subsp. sinensis | – | – | 14.1–29.2 | – | 9.7–28.8 | – | 1–2 | 14.4–24.8 | 5.1–8.3 | 12.5–33.9 | 6.5–18.1 | – | – | Yang and Kallio (2001) | |
| H. rhamnoides subsp. rhamnoides | – | – | 18.5–28.1 | – | 21.4–31 | – | 1–1.4 | 13.4–18.9 | 6.6–8.5 | 10.2–19.7 | 6.2–12.1 | – | – | Yang and Kallio (2001) | |
| H. rhamnoides | – | – | 19.9–35.8 | – | 21.8–51 | 1–1.8 | 14.3–36.7 | – | 0–6.5 | – | – | – | Ercisli et al. (2007) | ||
| Species/Subspecies | Pulp Oil | References | |||||||||||||
| C14:0 | C15:0 | C16:0 | C16:1n-9 | C16:1n-7 | C17:0 | C18:0 | C18:1 | C18:1n-9 | C18:1n-7 | C18:2n-6 | C18:3n-3 | C20:1 | C20:1n-9 | ||
| H. rhamnoides subsp. carpatica | 0.23–0.59 | 0.03–0.04 | 23.17–39.22 | 0.01–0.16 | 11.05–26.70 | 0.02–0.03 | 0.61–2.53 | 20.81–53.08 | 5.34–6.85 | 2.25–4.57 | 0.54–3.14 | 0.07–0.24 | 0.03–0.20 | Dulf (2012) | |
| H. rhamnoides subsp. sinensis | – | – | 18.9–32 | – | 12.1–32.5 | – | 0.9–1.9 | 13.1–25.3 | 6.2–9.9 | 6–27 | 3.8–13.7 | – | – | Yang and Kallio (2001) | |
| H. rhamnoides subsp. rhamnoides | – | – | 20.4–31.5 | – | 27.1–39 | – | 0.6–1.1 | 13.3–19.3 | 8.3–10.7 | 6.3–16.5 | 1.1–6.8 | – | – | Yang and Kallio (2001) | |
| H. rhamnoides subsp. rhamnoides | – | – | 34.3–35.5 | – | 35.0–38.5 | – | 1.1–1.2 | 3.2–3.6 | – | 12.4–13.0 | 1.1–1.5 | – | – | Cenkowski et al. (2006) | |
| H. rhamnoides | 0.3–0.9 | – | 28.3–35.8 | – | 45.6–51.8 | – | 0.1–1.0 | 9.7–16.8- | – | – | – | – | – | Ranjith et al. (2006) | |
| H. salicifolia | 0.1–0.3 | – | 28–29 | – | 32.9–41 | – | 0.1–0.7 | 12.6–17.6 | – | – | – | – | – | Ranjith et al. (2006) | |
| H. tibetana | 0.8–1.1 | – | 25.7–28.5 | – | 32.1–36 | – | 0.4–0.5 | 22–26 | – | – | – | – | – | Ranjith et al. (2006) | |
| Species/Subspecies | Seed Oil | References | |||||||||||||
| C14:0 | C15:0 | C16:0 | C16:1n-9 | C16:1n-7 | C17:0 | C18:0 | C18:1 | C18:1n-9 | C18:1n-7 | C18:2n-6 | C18:3n-3 | C20:1 | C20:1n-9 | ||
| H. rhamnoides subsp. carpatica | 0.09–0.24 | 0.11–0.30 | 7.14–12.44 | – | 0.16–0.53 | 0.03–0.05 | 2.91–3.84 | – | 13.57–20.09 | 1.27–2.29 | 33.72–42.35 | 28.13–32.60 | 0.21–0.49 | 0.06–0.16 | Dulf (2012) |
| H. rhamnoides subsp. sinensis | – | – | 7.7–9.6 | – | – | – | 2.1–3.3 | – | 12.9–23.2 | 1.9–2.5 | 38.2–43.6 | 20.2–36.3 | – | – | Yang and Kallio (2001) |
| H. rhamnoides subsp. rhamnoides | – | – | 6.7–8.2 | – | – | – | 2.6–4.1 | – | 14.5–20 | 1.8–3.7 | 37–43 | 25.4–36 | – | – | Yang and Kallio (2001) |
| H. rhamnoides subsp. rhamnoides | – | – | 6.7–7.2 | – | – | – | 2.4–2.6 | – | 13.0–13.6 | – | 35.3–35.9 | 37.4–38.5 | – | – | Cenkowski et al. (2006) |

5 Traditional Applications of Seabuckthorn
Historically, in Chinese and Tibetan medicine systems, seabuckthorn berries have been used widely for different preparations, as evident by reports in both ancient and recent literature. It is said that the army of Mughal conqueror, Genghis Khan, marched with inexhaustible and enormous energy due to regular consumption of seabuckthorn berries. As previously stated, different parts of the seabuckthorn plant have been used in traditional medicines, especially in Chinese, Tibetan, Mongolian, and middle Asian practice (Guliyev et al., 2004) for the treatment and prevention of various diseases. In historical records, Chinese people during the Tang dynasty were the first to use seabuckthorn as medicine 1200 years ago. More recently, in 1977, it was listed in the Chinese Pharmacopeia, considering its importance in expelling phlegm, arresting coughs, promoting digestion, removing food stagnancy, and promoting blood flow to remove blood stasis (Burke et al., 2005). Numerous pharmacological effects and related medicinal products have been recorded in Yue Wang Yao Zhen from the Tang dynasty, and in Sibu Yidian, an ancient Tibetan medical book (Mingyu et al., 1991). In Tibetan medicine, seabuckthorn has been used since ancient times in various clinical implications, like lowering fever, diminishing inflammation, counteracting toxicity, and abscesses, treating cough and colds, keeping warm, easing respiration, clearing sputum, relieving constipation, and treating tumors, especially of the stomach and the esophagus. (Guliyev et al., 2004). In Mongolia, extracts from leaves and branches of the plant are used medicinally to treat colitis and enterocolitis in humans and animals (Guliyev et al., 2004). In Russia, the oil from seeds and fruits is used treat chronic dermatoses, eczema, psoriasis, thrombosis, lupus erythematosus, burns, frostbite, and cervical erosion.
6 Nutraceuticals in Seabuckthorn
Human societies, through their traditional and indigenous knowledge and use of wild plants, realized long ago that food, medicines, and health are interrelated. Use of raw materials from medicinal plants in different drug formulations has regained popularity in recent times. Traditionally, parts of the seabuckthorn are consumed raw, roasted, fried, cooked, boiled, or processed, to make different food products. Both traditional and modernized communities of different settlements in Central Himalaya use the fruit and other parts of the plant for treating headache, severe cold, cough, and throat infections (Dhyani et al., 2010). Seabuckthorn berries, owing to their unique flavor, are being processed for a variety of products such as squash, syrup, jam, jellies, savory chutney (local jelly), pickles, candies, pies, alcoholic and nonalcoholic beverages, and for flavoring dairy products (Bal et al., 2011, Dhyani et al., 2010). The high content of vitamin C in berries, a natural antioxidant, provides many health promoting effects. Seabuckthorn fruit and seed oil is used as a source for ingredients in several commercially available nutraceuticals and cosmetic products, due to its unique fatty acid composition. Seabuckthorn leaves also possess remarkable nutraceutical quality, especially due to their high amount of phenolics.
7 Pharmacological Effects of Seabuckthorn
Review of the available literature on seabuckthorn reveals that the majority of the in vitro and in vivo pharmacological studies have been carried out using crude extracts from different plant parts like leaves, bark, fruit pulp, and seeds. These studies validate some of the traditional uses of seabuckthorn and also report new applications. Although chemical composition of different seabuckthorn materials has been extensively studied, leading to the isolation and identification of wide range of valuable compounds, only a limited number of studies have focused on the characterization of specific bioactive compounds effective in treatment and prevention of diseases. A review of the various pharmacological properties of seabuckthorn is presented in the following text.
7.1 Anticancer and Antitumor Activity
The role of Hippophae in prevention and control of cancer is primarily based on studies involving laboratory animals and different cancer cell lines. Antiproliferative effects of ethanol extract from leaves of Hippophae rhamnoides L. on human acute myeloid leukemia cells (KG-1a, HL60, and U937) showed activation of the S-phase checkpoint leading to the deceleration of cell cycle and apoptosis induction (Zhamanbaeva et al., 2014). Ethanol extract from Hippophae rhamnoides branches was evaluated for its antitumor effect in mice skin using two stage carcinogenesis test including 7,12-dimethylbenz[a]anthracene as an initiator and 12-O-tetradecanoylphorbol-13-acetate (TPA) as a promoter, and was found to inhibit TPA induced tumor promotion (Yasukawa et al., 2009). Hydroalchoholic extract from dried berries of Hippophae rhamnoides was found to reduce carcinogen-induced for stomach and skin papillomagenesis in mice. Increased activities of phase II (GST and DTD) and antioxidant enzymes (SOD, CAT, GPX, and GR), as well as upregulation of DNA-binding activity of IRF-1 transcription factor, a known antioncogenic transcription factor causing growth suppression and apoptosis induction, might be implicated in the anticancer effects (Padmavathi et al., 2005). Grey et al. (2010) studied the antiproliferative effects of ethyl acetate and ethanol:water (1:1) extracts from berries with varying composition on Caco-2 (colon) and Hep G2 (liver) cancer cell lines. The ethyl acetate extract was found to possess the strongest inhibitory effect on Caco-2 cells and ethanol: water extract on Hep G2 cells. Additionally, photochemical analysis showed that ethyl extract contained high levels of urosolic acid with low amounts of phenols, while ethanol:water extract had high amounts of phenols and proanthocyanidin.
In a recent study, the anticancer activity of methanol extract of Hippophae salicifolia (MEHS) bark was studied in a dose dependent manner (Chakraborty et al., 2015). After treatment with MEHS at doses of 50 and 100 mg/kg body weight, a significant decrease in tumor volume, tumor weight, viable cell count and increase in life span by 34% and 43%, respectively, was observed using Ehrlich Ascites Carcinoma (EAC) cells on Swiss albino mice. The hematological, biochemical, and liver tissue antioxidant parameters were also significantly restored to the normal level after treatment with MEHS. A water soluble Hippophae rhamnoides antifatique polysaccharide (HRWP-A) was isolated from ethanol fruit extract, and studied for inhibiting the Lewis Lung Carcinoma (LLC) growth in tumor bearing mice. Results suggested that HRWP-A mediated antitumor effects may be due to its effect on increasing the proliferation of lymphocytes augmenting the phagocytosis and cytotoxicity of macrophages. Furthermore, NO and TNF-α levels were promoted along with an increase in NK cell activity and CTL cytotoxicity in LLC tumor-bearing mice (Wang et al., 2015). Isorhamnetin, a flavonol aglycone isolated from Hippophae rhamnoides L., was investigated through flow cytometry for its antitumor activity in human hepatocellular carcinoma cells (BEL-7402) (Teng et al., 2006). Another study on isorhamnetin stated its role in the inhibition of colorectal cancer (CRC), by suppressing the PI3K-Akt-mTOR pathway, and thereby suggesting that this compound may act as potent anticancer agent (Li et al., 2014, 2015). Antiproliferative activity of isorhamnetin against lung cancer was also studied in vitro on A549 lung cancer cells and in vivo on C57BL/6 mice transplanted with Lewis lung carcinoma cells (Li et al., 2015). The findings of the previously described studies demand that research should be extended to human subjects, as it would be helpful in developing anticarcinogens which could probably strengthen immunity and prevent the side effects of chemotherapeutic agents.
7.2 Antiinflammatory Activity
A large number of in vitro and animal studies have been conducted to evaluate the effect of seabuckthorn extract in controlling inflammation. Epigallocatechin and urosolic acid isolated from ethanol extract of seabuckthorn branches exhibited marked antiinflammatory activity (Yasukawa et al., 2009). Seabuckthorn leaf extract showed antiinflammatory potential in dose dependent manner as studied in adjuvant induced arthritis rat models by suppressing lymphocyte proliferation (Ganju et al., 2005). Inhibitory effect of Hippophae rhamnoides leaf extract was demonstrated in lipopolysaccharide (LPS) induced nitric oxide production, in the murine macrophage cell line (Padwad et al., 2006). Leaf extract of seabuckthorn protected 60Co γ-irradiated mice from inflammation by modifying HMGB1 (High Mobility Group Box 1 protein) regulated inflammatory pathway (Tiwari and Bala, 2011). In a double-blind, randomized, placebo- controlled trial of 90 days with human subjects, seabuckthorn berries caused a reductive effect on C-reactive protein (Larmo et al., 2008).
7.3 Antimicrobial Activity
The antibacterial and antifungal activity of crude leaf and seed extract, and seed oil of Hippophae salicifolia, was studied against selected bacterial and fungal cultures. Of these, only seed extract exhibited significant antimicrobial activity (Gupta et al., 2012). Aqueous and hydroalcoholic leaf extracts have shown growth inhibitory effects against Bacillus cereus, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis (Upadhyay et al., 2010). Jain et al. (2008) reported significant antidengue activity of seabuckthorn leaf extract as shown in type-2 dengue virus infected blood-derived human macrophages, with a decrease and an increase in TNF-α and IFN-γ, respectively. The methanol extract of seabuckthorn leaves evaluated against three bacterial pathogens, Escherichia coli, Arthrobacter protophormial, and Micrococcus luteus, showed maximum zone of inhibition for E. coli at a concentration of 25 mg/mL (Gill et al., 2012). Additionally, seed oil of Hippophae sp. was also evaluated for its antimicrobial property against E. coli (Kaushal and Sharma, 2011). The methanol extract of seabuckthorn berries revealed antibacterial effect against Staphylococcus aureus, Bacillus subtilis, Streptococcus pneumoniae, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumonia (Chaman et al., 2011). Hiporamin, a purified tannin fraction from seabuckthorn, was found to possess a wide range of antiviral activity against influenza virus strains A and B, herpes simplex type 1, adenovirus type 2, HIV-1, and a mild antibacterial activity against gram-positive and negative microorganisms (Shipulina et al., 2006). Also, methanol extract from the roots and stem of Hippophae rhamnoides was found to exhibit better antimicrobial activity than other antimicrobial agents, namely (+) catechin, ketoconazole, and mycostantin (Jeong et al., 2010). The aqueous extract of Hippophae rhamnoides seeds was also found to possess antibacterial activity with the MIC value of 750 and 1000 ppm against Listeria monocytogenes and Yersinia enterocolitica (Chauhan et al., 2007). Seabuckthorn berry juice was also evaluated for its antibacterial activity against Streptococcus mitis, Streptococcus mutans, Streptococcus sanguinis, Streptococcus gordonii, Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa (Widen et al., 2015). Crude ethanol extract obtained by pressurized liquid extraction (PLE) was tested for its antimicrobial activity against food-borne and clinical microorganisms (Michel et al., 2012). Verma et al. (2011) evaluated antibacterial activity of methanol extract from seabuckthorn leaves at different concentrations (0.5%, 2%, 3%, 4%, and 5%), against 160 microbial isolates obtained from clinical skin and wound infection cases of different animal species, and observed that extract at 5% concentration showed an inhibitory effect almost 50% as compared to standard drugs.
7.4 Hepatoprotective Ability
The use of plant based natural remedies has a long history for the treatment of liver diseases. A variety of plants having hepatoprotective ability have been evaluated for their efficacy against liver diseases induced by various agents. Many studies have been conducted to evaluate the effect of seabuckthorn seed oil and leaf extract for hepatoprotective activity in CCl4-induced liver damaged rats. The phenolic rich fraction (PRF) of seabuckthorn leaf extract containing phenolic constituents, such as gallic acid, myricetin, quercetin, kaempferol, and isorhamnetin, as determined by reverse-phase high performance liquid chromatography (RP-HPLC) was found to be protective against histopathological changes produced by CCl4 like hepatocytic necrosis, fatty changes, and vacuolation. (Maheshwari et al., 2011). Oral administration of seabuckthorn seed oil for 8 weeks at a dose of 0.26, 1.30, and 2.60 mg/kg body weight significantly reduced the elevated levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), triglyceride (TG), cholesterol in serum, and malondialdehyde (MDA) in the liver of CCl4 treated male ICR mice (Hsu et al., 2009); a minimum dose of 0.26 mg/kg exhibited the maximum hepatoprotective effects. Pretreatment with seabuckthorn leaf extract at a dose of 100 and 200 mg/kg body weight significantly protected the Sprague-Dawley male albino rats from CCl4-induced liver injury, by significantly restricting the CCl4-induced increase of GOT, GPT, ALP, and bilirubin, and also enhanced GSH and decreased MDA levels, with better maintained protein levels in the serum. A comparative study of efficacy of seed oil and sarcocarp oil of Hippophae rhamnoides on rats was carried out for CCl4 induced liver injury, and found seed oil to be more effective in controlling the increase of ALT in serum and the decrease of SOD (Liu et al., 2006). A clinical study on liver fibrosis reported the effect of seabuckthorn extract in reducing the serum levels of laminin (LN), hyaluronic acid (HA), collagen types III, and IV, total bile acid (TBA), signifying that it may restrain the synthesis of collagen and other components of extracellular matrix (Gao et al., 2003). Moreover, oil from seabuckthorn berries was found to have potent hepatoprotective activity, reducing the expression of COX2, Bcl-2, and p53 proteins, overexpressed in alfatoxin B1 (AFB1) treated chicken livers (Solcan et al., 2013).
7.5 Radioprotective Effect
Widespread use of radiations in various occupations leads to many deleterious effects on the individuals exposed. Therefore, there is an continual need of developing radioprotective agents, which are safe and nontoxic for human health. Seabuckthorn has been screened for radioprotective ability, considering its high antioxidant potential, immunomodulatory ability, and ability of DNA modulation. RH-3, a berry extract, was found effective in a dose dependent manner in inhibiting radiation induced single and double strand breaks in DNA, formation of endonuclease IV detectable radiation induced clusters, and preventing cellular and mitochondrial free radical generation, inhibiting radiation induced apoptosis and cytotoxicity (Sureshbabu et al., 2008; Agrawala and Adhikari, 2009). Similarly, Shukla et al. (2006) found berry extract (REC-1001) effective in reducing DNA-damage, both genomic and mitochondrial, with the maximum protective effects observed at highest dose of 250 μg/mL, and suggested that polyphenols/flavonoids present in the extract might be responsible for free radical scavenging and DNA protection. Herbal preparations from seabuckthorn berries and leaves were reported to render > 80% survival at lethal doses (10 Gy) in mice. A single intra-peritoneal, prophylactic dose of leaf extract (SBL-1) was reported to have radioprotective ability in 90% of the whole body gamma irradiated (10 Gy) mice, possibly by antioxidant action and promotion of error free post irradiation repair pathways (Tiwari et al., 2009). A dose of 30 mg/kg body weight of berry extract (RH-3) in mice facilitated 82% survival by inhibiting Fenton reaction and radiation mediated generation of hydroxyl radicals, superoxide anion mediated Nitroblue tetrazolium (NBT) reduction and FeSO4 mediated lipid peroxidation in liver (Goel et al., 2002). Furthermore, administration of RH-3 extract, 30 min prior to 10 Gy whole body gamma radiation, at 25–35 mg/kg body weight dose reported > 80% survival in mice (Agrawala and Goel, 2002). Pretreatment administration of alcohol extract (RH-3) also resulted in increased survival of crypts in the jejunum and villi cellularity, and decrease in the frequency of apoptosis comparative to the irradiated control (Goel et al., 2003a). This extract induced a strong compaction of chromatin in reversible and irreversible manner, as evidenced from the lack of tail and appearance of intensely stained circular bodies in comet assay (Kumar et al., 2002). Another investigation (Goel et al., 2003b) related reversible and irreversible chromatin compaction with the magnitude of RH-3 induced DNA-protein cross-links. Later, Goel et al. (2006), using testicular system, suggested that RH-3 treatment protected spermatogenesis in gamma irradiated mice by enhancing the spermatogonial proliferation and stem cell survival, and reducing sperm abnormalities. This radioprotective ability can be attributed to the presence of polyphenolic flavonoids and tannins in the extract, and radical scavenging activity.
7.6 Antiatherogenic and Cardioprotective Activity
Seabuckthorn is a rich source of antioxidants, unsaturated fatty acids, and flavonoids which can improve the function of cardiovascular system (Eccleston et al., 2002; Suryakumar and Gupta, 2011). The administration of supercritical CO2 extracted seabuckthorn seed oil has been shown to possess antiatherogenic and cardioprotective activity, as demonstrated by altering relevant parameters using rabbit as an animal model (Basu et al., 2007). Similarly, cardioprotective effect of seabuckthorn pulp oil has been recorded in ischemia-reperfusion (IR) induced model of myocardial infarction in rats (Suchal et al., 2014). Administration of seed oil alleviates myocardial damage in ISO-induced cardiac injury in rats by maintaining hemodynamic, biochemical, histopathological, and ultrastructural perturbations, due to its free radical scavenging and antioxidant activities (Malik et al., 2011).
Consumption of seabuckthorn berries significantly increased fasting plasma concentration of quercetin and isorhamnetin, however, there was no effect on serum triacylglycerol concentrations, HDL and LDL cholesterol levels (Larmo et al., 2009). Supplementing normal diets in healthy human subjects with seabuckthorn juice caused a moderate decrease in the susceptibility of LDL to oxidation, and an increase in plasma HDL-C and triacylglycerol by 20% and 17%, respectively (Eccleston et al., 2002). Polyphenol extracts from seabuckthorn (PESB) demonstrated protective potential against myocardial ischemia reperfusion injury (MIRI) by inhibiting autophagy, which plays a critical role in MIRI in rats (Tang et al., 2016). Total flavones of Hippophae rhamnoides (TFH) prevented in vivo thrombogenesis, probably due to the inhibition of platelet aggregation (Cheng et al., 2003). The protective effect of flavonoids of seabuckthorn (FSBT) was studied on oxidized low-density lipoprotein (ox-LDL) induced injury in endothelial cell line. Pretreatment with FSBT prohibited cell death and secretion disorders by preventing ox-LDL triggered superoxide production, suppressing superoxide dismutase activity and regulating eNOS and LOX-1 expression (Bao and Lou, 2006). Luo et al. (2015) examined the ability of isorhamnetin to inhibit oxidized low-density lipoprotein (ox-LDL)-induced cell apoptosis in THP-1-derived macrophages, and also evaluated antiatherosclerosis effect in high fat diet fed ApoE −/− mice. The hypolipidemic and hypoglycemic effects of total flavonoids from seed residues (FSH) of Hippophae rhamnoides were reported in high fat diet fed mouse model (Wang et al., 2011), as evident by a significant decrease in total cholesterol level in blood serum and liver. Moreover, triglyceride concentration in liver and density of lipoprotein-cholesterol (LDL-C) were also lowered. The results were further supported by transmission electron microscope findings. The rise in serum glucose was significantly suppressed by FSH treatment, while improving impaired glucose tolerance.
7.7 Antiulcerogenic Effect
A formulation made from seabuckthorn berries and pulp was used for the treatment and prevention of glandular gastric ulcer in horses (Huff et al., 2012). The antiulcerogenic effect of a hexane extract (HRe-1) of Hippophae rhamnoides berries was reported on indomethacin- and stress-induced ulcer model (Suleyman et al., 2001). The authors suggested that the hexane soluble component of seabuckthorn berries, including vitamin E and carotenoids, have cumulative antioxidative effects which contribute to the prevention of mucosal injury. The protective effect of a mixed extract from Hippophae rhamnoides and Hypericum perforatum was demonstrated in indomethacin-induced gastric injury by measuring glutathione (GSH), malondialdehyde (MDA), and DNA injury products (Turan et al., 2013). Xing et al. (2002) reported significant reduction in ulcer formation in water-immersion and reserpine-induced models in rats, by supercritical-CO2 extracted seabuckthorn seed and pulp oil at the dose of 7 mL/kg/day. Moreover, administration of a lower dose of 3.5 mL/kg/day significantly reduced the index of pylorus ligation-induced gastric ulcer, and accelerated the healing process of acetic acid-induced gastric ulcer. Subsequently, the effect of seabuckthorn pulp oil was also studied with respect to gastric secretion, gastric emptying, and analgesic activity in rats (Xing et al., 2012). The results of this study demonstrated the efficacy of seabuckthorn in treating stomach discomfort and gastric ulcers. Seabuckthorn procyanidins (SBPC) also plays an important role in the healing of acetic acid-induced gastric lesions, possibly by the acceleration of the mucosal repair (Wang and Chen, 2007). Hippophae rhamnoides extract has also been shown to prevent methotrexate-indomethacin combination induced damage in gastric tissues of rats as studied biochemically and histopathologically (Yilmaz et al., 2014). Dogra et al. (2013) considered seabuckthorn oil as the best therapeutic agent for dexamethasone-induced gastric ulcerations and erosions (GUEs) followed by famotidine, lansoprazole, misoprostol, and sucralfate in dogs.
Helicobacter pylori infection is associated with chronic inflammation, accumulation of reactive oxygen species, oxidative damage to gastric mucosa and increased risk of gastric ulcers. Ethanol extract of seabuckthorn leaves inhibited the growth of H. pylori strains in vitro (Li et al., 2005).
7.8 Effect on Platelet Aggregation
Total flavones of Hippophae rhamnoides (TFH) at a concentration of 0.3–3.0 μg/mL in a dose dependent manner prevented collagen induced platelet aggregation (Cheng et al., 2003). Johansson et al. (2000) also observed a platelet aggregation inhibitive effect of supercritical-CO2 extracted seabuckthorn oil in healthy normolipidemic men. Administration of seabuckthorn pulp and seed oil caused a decrease in the rate of adenosine-5′-diphosphate-induced platelet aggregation, however, no effect was seen on plasma lipids, fatty acid composition of plasma and platelet phospholipids, or on platelet aggregation induced by arachidonic acid. Seabuckthorn berry juice (300 mL/day for 8 weeks) showed a modest decrease in platelet aggregation induced by any of the aggregation agonists tested, namely ADP, collagen, and arachidonic acid (Eccleston et al., 2002).
7.9 Hypoglycemic Effect
Extracts from different parts of seabuckthorn were evaluated for their hypoglycemic effect in streptozotocin (STZ) induced diabetic rats. Aqueous extract of seabuckthorn seed residues (ASSR) significantly lowered the body weight, serum glucose, total cholesterol and low-density lipoprotein cholesterol levels in STZ induced type 2 diabetic rats, and also significantly increased insulin sensitivity index (Zhang et al., 2010). Sharma et al. (2011) reported that administration of commercially available seabuckthorn pulp produced a significant reduction in the levels of blood glucose and thiobarbituric acid reactive substances (TBARS). Reduced glutathione (GSH) was brought back to near normal levels by administration of seabuckthorn pulp, and degenerative changes of pancreatic beta cells in STZ-diabetic rats were also minimized. Kim et al. (2010a) reported the reducing effect of seabuckthorn leaf powder supplemented diet on blood glucose and cholesterol levels in mice. Seabuckthorn seed protein (SSP) showed a significant hypoglycemic and antiinflammatory effect as evidenced by the lower body weight, fasting blood glucose levels, serum lipids, inflammatory factors and insulin levels, and lipid contents of SSP treated diabetic mice (Yuan et al., 2016). Flavonoids from the seed residue (FSH) and fruit residue (FFH) of Hippophae rhamnoides significantly decreased the levels of blood glucose and lipids in normal mice. The effect on glycometabolism may be related to the control of glyconeogenesis (Cao et al., 2003). Pure seabuckthorn juice and l-quebrachitol enriched seabuckthorn juice were evaluated for their effect on type-2 diabetic db/db mice model. Pure seabuckthorn juice reduced the feed intake, weight gain, random blood glucose levels, expression of insulin receptor β in the liver, significantly improved glucose tolerance, and the integrity of pancreatic tissue. While seabuckthorn juice enriched with l-quebrachitol increased fasting insulin level in plasma, it showed similar effects on random blood glucose levels, glucose tolerance, and the pancreatic tissue, as observed for pure seabuckthorn juice (Xue et al., 2015). Methanol extract of seabuckthorn leaves exhibited antidiabetic activity as measured by alpha-glucosidase inhibitor assay (Bhardwaj et al., 2015).
7.10 Antiaging Potential and Skin Whitening
Skin aging is a dermatologic change that progresses with age or when exposed to ultraviolet radiation (UVR). The exposure to UVR is the main cause of oxidative stress in the skin, and therefore is an important risk factor for the development of skin problems, for example, wrinkles, lesions, and skin cancer. Study on UV-radiation induced skin aging in hairless mice suggested that seabuckthorn fruit blend has protective potential against skin aging by regulating the moisture content, MMP expression levels and SOD activity (Hwang et al., 2012). Acne severity has a connection to the amount of sebum produced. Akhtar et al. (2010) reported that w/o emulsion of hydroalcoholic fruit extract from Hippophae rhamnoides decreases sebum secretion. Similarly, H. rhmanoides fruit extract (w/o emulsion) applied to skin over a period of 8 weeks was found suitable for improvement, and quantitative monitoring of stratum corneum water content and reducing transepidermal water loss in people with dry skin (Khan et al., 2011). The water-in-oil emulsion made from methanol extract of Hippophae rhamnoides was also found effective for the treatment of grade I and grade II acne vulgaris studied over a period of 12 weeks. Moreover, this formulation was found to improve barrier function in human subjects as analyzed by Tewameter and Corneometer (Khan and Akhtar, 2014).
Melasma/hyperpigmentation and solar damage of the skin remains a difficult problem to treat. Various types of whitening agents are used to treat hyperpigmentation. A trend has been observed recently to use plant extracts as skin whitening agents. Khan et al. (2013) reported formulations containing plant extracts of Hippophae rhamnoides and Cassia fistula as an effective alternative treatment of melasma, as evaluated biometrologically by measuring pigment density using a mexameter.
7.11 Eye diseases
Aqueous extract of seabuckthorn leaves was evaluated for its therapeutic role in oxidative stress induced cataract using isolated goat lenses (Dubey et al., 2016). The results showed best anticataract activity of extract at a dose of 1000 μg/mL, by significantly restoring the decreased levels of malonaldehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH). Total flavones from Hippophae rhamnoides demonstrated their protective effects against light-induced retinal degeneration by increasing the antioxidant defense mechanisms, suppressing pro-inflammatory and angiogenic cytokines, and inhibiting retinal cell apoptosis (Wang et al., 2015). Another study has documented the retinoprotective effect of seabuckthorn seed proanthocyanidins against visible light induced retinal degeneration in rabbits via antioxidant, antiinflammatory, and antiapoptotic mechanisms (Wang et al., 2016).
7.12 Immunomodulatory
Seabuckthorn possesses significant immunomodulatory properties, as studied in vitro using rat spleenocytes, macrophages, human peripheral blood mononuclear cells (PBMCs) and other cell lines, and in vivo using chick, mice, and rat models. Alcoholic leaf and fruit extracts of seabuckthorn were evaluated for their ability to arrest the chromium-induced inhibition of lymphocyte proliferation (Geetha et al., 2002). Geetha et al. (2005) reported the leaf extract (100 μg/mL) stimulated IL-2 and γ-IFN production, even in the absence of concanavalin A (ConA), and also inhibited chromium-induced decline in IL-2 and γ-IFN production, but it did not change IL-4 production, suggesting its significant immunomodulatory activity, specifically by activating the cell-mediated immune response. The ethanol solution of seabuckthorn flavones was found to stimulate production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and increased expression of phosphorylated-IκB, NF-κB, and phosphorylated-p38 in PBMCs, with significantly suppressed expression of CD25 (IL-2R) in a mouse macrophage cell line, suggesting its beneficial immunomodulatory effect against bacterial infection (Mishra et al., 2008). Ramasamy et al. (2010) reported immunoprotective effect of seabuckthorn berries alone and in combination with glucomannan against T-2 toxin induced immunosuppression.
7.13 Neuroprotective Ability
Hydroalcoholic extract of Hippophae rhamnoides was evaluated for its neuroprotective ability using human neuroblastoma cell line-IMR32 against hydrogen peroxide (H2O2) induced cytotoxicity. The findings of the study confirmed neuroprotective effects by enhancing cell viability (Shivapriya et al., 2015). Hippophae rhamnoides juice showed protection against the lead acetate-induced deficits in learning and memory, and changes in neurobiochemical parameters (Xu et al., 2005).
7.14 Antiobesity
Obesity is one of the most widespread metabolic disorders in the present day society. Recently, natural and alternative antiobesity agents are being employed for the management of obesity. Seabuckthorn leaf tea is reported to have significant antivisceral obesity and antioxidant effects, mediated by the regulation of lipid and antioxidant metabolism in high-fat diet obese mice (Lee et al., 2011). Ethanol extract of seabuckthorn leaves possesses antiobesity and hypoglycemic effects, as evaluated on C57BL/6J mice by effectively preventing body weight gain and fat accumulation in the liver. Adipose tissue mass, hepatic lipid profile, and serum leptin levels were also reduced significantly (Cha et al., 2012; Pichiah et al., 2012). Similarly, seabuckthorn leaf juice rich in polyphenols was given to mice fed a high-fat diet, causing the enhanced gene expression of fatty acid beta-oxidation enzymes in the liver, which increased the lipid metabolism and inhibited pancreatic lipase activity, thereby reducing lipid transportation to the body and increasing fecal lipid excretion, ultimately inhibiting body fat deposition (Nishi et al., 2007).
7.15 Antioxidant and Cytoprotective Ability
Oxidative damage is the major cause of many clinical results, and most damage caused to a biological systems is due to the generation of free radicals. Antioxidant effects of various seabuckthorn extracts have been studied in vitro, using different assays and in vivo using mice models. Narayanan et al. (2005) reported alcoholic leaf extract of seabuckthorn at 200 μg/mL dose significantly inhibited cytotoxicity, production of reactive oxygen species (ROS), and maintained antioxidant levels similar to that of control cells. Additionally, the mitochondrial integrity was restored, along with prevention of Hypoxia induced DNA damage. Aqueous and hydroalcoholic extracts of Hippophae rhamnoides L. showed cytoprotective activity against hydrogen peroxide and hypoxanthine- xanthine oxidase induced damage to BHK-21 cell line (Maheshwari et al., 2011). Seabuckthorn seed oil inhibited the CCl4 induced toxicity, as evident from a significant increase in the activities of antioxidant related enzymes, such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and reduction of malondialdehyde, indicating its potential as a natural antioxidant agent (Ting et al., 2011). Seabuckthorn flavones at 100 μg/mL dose significantly inhibited tert-butyl hydroperoxide induced cytotoxicity and free radical production, and also restored the antioxidant status similar to that of control cells (Geetha et al., 2009). Alcoholic extracts of leaf and fruit of seabuckthorn at 500 μg/mL dose had a significant cytoprotective effect against sodium nitroprusside induced oxidative stress in murine macrophages (Geetha et al., 2002). Podder et al. (2013) investigated the cytoprotective effects of methanol extract of seabuckthorn against paraquat (PQ)-induced toxicity via antioxidant mechanisms in A549 cells. Lim et al. (2013) studied the antioxidant and cytoprotective effect of seabuckthorn fruit extract (SFE) against cellular oxidative stress in mouse embryonic fibroblast cells, and attributed to its effective radical scavenging activity, as well as altered cell cycle regulation, which prevent apoptotic cell death induced by cellular oxidative stress. The alcoholic leaf and fruit extracts of seabuckthorn at concentration of 500 μg/mL inhibited free radical formation, apoptosis and restored the antioxidant status and mitochondrial transmembrane potential (Geetha et al., 2002). Kim et al. (2010a,b) evaluated antioxidant and α-glucosidase inhibitory effects from the butanol fractions of seabuckthorn leaves and reported the highest α-glucosidase inhibitory effect (86%) at 5 μg/mL dose. Another study examined the inhibitory effect of 80% acetone extract from branch bark of seabuckthorn on nitric oxide (NO) production in RAW 264.7 cells (Yang et al., 2007). Beneficial effects of hexane extract from seabuckthorn berries was demonstrated in nicotine-induced oxidative stress in rats (Suleyman et al., 2002). Olas et al. (2016) demonstrated the antioxidant and antiplatelet activity of phenolic fraction from Hippophae rhamnoides fruits in human blood platelets and plasma. Zeb and Ullah (2015) reported protective ability of seabuckthorn seed oil against oxidative stress caused by thermally oxidized lipids, by reducing their toxic effects.
7.16 Healing Property
Seabuckthorn based preparations have been widely used in the healing of internal and external wounds, scrapes, ulcers, and mucosal injuries. Detailed studies by Upadhyay et al. (2009, 2011) have demonstrated wound healing activity of supercritical CO2-extracted seed oil and aqueous leaf extract of seabuckthorn in rats. Wound healing processes were evidenced by significant increase in wound contraction, level of hydroxyproline, hexosamine, total proteins, and upregulated expression of collagen type-III DNA, in comparison to control, and reference control treated with silver sulfadiazine (SS) ointment. Histological examinations and matrix metalloproteinases (MMP-2, 9) expression further confirmed the healing efficacy of SBT leaf extract. Moreover, it was observed that seabuckthorn treatment leads to increase in endogenous antioxidants (enzymatic and nonenzymatic) and decrease in lipid peroxide levels in wound granulation tissues (Gupta et al., 2005, 2006; Upadhyay et al., 2009, 2011). Gupta et al. (2008) demonstrated that multi plant leaf extract of Hippophae rhamnoides, Aloe vera, and Curcuma longa (1:7:1) possess significant wound healing efficacy in both normal and diabetic wounds.
7.17 Antistress and Adaptogenic Ability
The aqueous leaf extracts of Hippophae rhamnoides, and Hippophe salicifolia were evaluated for their adaptogenic ability against high altitude multiple stress (cold-hypoxia-restraint) using rats as animal models. Pretreatment with aqueous extract of Hippophae salicifolia significantly attenuated reactive oxygen species (ROS) production, protein oxidation, and lipid peroxidation in C-H-R stressed rats, and also had demonstrated a role in maintaining antioxidant status as similar to control rats (Rathor et al., 2015). The maximum adaptogenic and antistress activity was observed at 100 mg/kg body weight dose of extract, when administered 30 min before exposure to C-H-R stress (Saggu et al., 2007). Furthermore, while investigating mechanism of adaptogenic activity, Saggu and Kumar (2007) suggested that seabuckthorn leaf extract treatment caused shifting of anaerobic metabolism to aerobic during C-H-R exposure and post stress recovery. Purushothaman et al. (2011) reported seabuckthorn leaf extract curtails hypoxia-induced transvascular permeability by decreased water content and fluorescein leakage in the lungs, and decreased albumin and protein content in the bronchoalveolar lavage fluid (BALF). A comparative study on adaptogenic potential of Hippophae salicifolia, Hippophae rhamnoides subsp. mongolica, and Hippophae rhamnoides subsp. turkestanica carried out in rats exposed to Cold-hypoxia-restraint (C-H-R) stress suggested that Hippophae salicifolia and Hippophae rhamnoides subsp. turkestanica have comparable adaptogenic potential, however, it was much higher than that of Hippophae rhamnoides subsp. mongolica (Sharma et al., 2015).
The literature pertaining to the medicinal properties os seabuckthorn indicates that most of the studies are limited to the laboratory animal models such as rat, rabbit, chick, mice, or cell lines. The findings may provide a platform for further clinical research using human subjects. Therefore, more clinical studies are needed to understand beneficial effects of seabuckthorn on human health. Although various seabuckthorn products are available in market without any major side effects, there is still a need of more research regarding safety and efficacy of these products.
8 Commercial Products of Seabuckthorn
As outlined in the previous text, both comprehensive contemporary research and ancient literature have documented the tremendous utility of the seabuckthorn plant for human health. As a result, recent demand for seabuckthorn products has grown phenomenally across the globe. Seabuckthorn products in the market range over different categories, like food supplements, food products and nutraceuticals to cosmoceuticals. Development of seabuckthorn products depends largely on the quality of raw material, post harvest processing conditions and processing technology used. In china, seabuckthorn cultivation spreads over 300,000 ha of land, and 150 processing factories are currently engages in the production of various products. Products in the market range from food products to cosmetics, including tea, juice, juice powder, squash, oil softgels, wine, nutritional oil capsules, puree, jam, seed oil, fruit oil, pigments, sunscreens, skin care creams, beauty creams, body lotions, shampoos, cleansing bars, serum, soothing salves, body wash, and lip balm. A variety of seabuckthorn based formulations have also been developed and are available in the form of liquid, powder, plaster, paste, pills, liniments, and aerosols. The are used for treating burns, gastric ulcers, chilblains, scales, oral mucositis, rectal mucositis, cervical erosion, radiation damage, and skin ulcers. Some of the important seabuckthorn products available in the market are given in Table 8. Considering the present trend of growth and popularity, it is justifiable to expect seabuckthorn products may soon comprise many of the human health products on the market worldwide.
Table 8
| Manufacturer Name | Product Categories |
|---|---|
| SBT Seabuckthorn | Tea, naturally healing oils, nutritional capsules, cleansing bars, skin creams, shampoos, soothing salves |
| Seabuck Wonders | Berry and seed oil, seed oil softgels, Omega-7 complete softgels, facial cream, body lotion, facial cleanser, deep hydrating serum |
| SIBU (sea berry therapy) | Oil softgels, seed and fruit oil, cleansing bars, facial cleanser, facial toner, facial serum, facial cream, age defying eye cream |
| F & D Nature Food Inc. | Oil and seed soft capsules, wine, tea, juice, juice powder |
| HillBerry | Natural wellness drink |
| Natural Bath and Body Products (P) Ltd. | Antiwrinkle creams and serums, rejuvenating and exfoliating face care, daily facial, lips and eye care hair care, natural facial mask |
| Shanxi wutaishan seabuckthorn products Co. Ltd. | Seed and fruit oil, oil capsules |
| Sanddorn | Classical juice and liqueur, fruit spreads, jelly babies and natural cosmetics |
| MONT echo | Seed oil, fruit oil and floral waters |
| Badger | Sunscreens, lip balms, moisturizers, muscle rubs, and other personal care products |
| Summerbee products | Creams and oils |
| Nvigorate | Puree, syrup, jam, tea, splash |
| Vaasan Aito Saippua Oy | Soap, shampoo, and bodywash |
| The healing arc Inc. | Creams, oil, lip balm, and juices |
a Information presented in the table is collected from different web resources.
9 Conclusion
The nutraceutical and medicinal value of seabuckthorn as reported in ancient texts and existing as part of the traditional knowledge base has been well validated through modern scientific investigations. The different parts of the plant and their extracts have been shown to possess various medicinal properties useful in the prevention and treatment of a number of human diseases and metabolic disorders. The presence of major bioactive compounds in seabuckthorn, like flavonoids, vitamins, sugar alcohols, fatty acids, and minerals, make it a valuable plant resource for the development of various nutraceuticals and functional foods. However, there is a need for further clinical research, particularly involving human subjects, for the identification and development of seabuckthorn based drug formulations. The important radioprotective ability of seabuckthorn products also needs further investigations, considering the present day environmental situation. Additionally, efforts should be made to identify and isolate individual active components from the crude extracts having defined health benefits. Hopefully, more global efforts will be initiated to increase seabuckthorn cultivation and breeding of superior farmer friendly and industry suitable cultivars.