Abbreviations: CHD: coronary heart disease, DPPH: 1,1-diphenyl-2-picrylhydrazyl, FRAP: ferric reducing antioxidant power, FW: fresh weight, HDL: high density lipoproteins, GAE: gallic acid equivalents, LDL: low density lipoprotein, MT: metric tones, Se: selenium, TE: trolox equivalents.
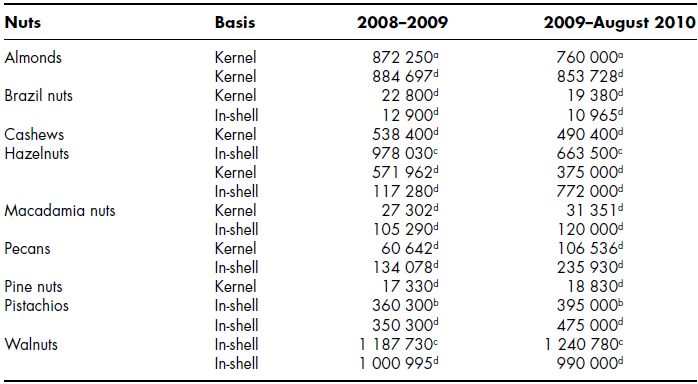
Almonds (Prunus amygdalus), Brazil nuts (Bertholletia excelsa), cashews (Anacardium occidentale L.), chestnuts (Castanea sativa), hazelnuts (Corylus avellana), macadamia nuts (Macadamia integrifolia), pecans (Carya illinoinensis ), pine nuts (Pinus pinea), pistachios (Pistacia vera) and walnuts (Juglans regia L.) are important tree nuts consumed all over the world. Amongst these tree nuts, almonds, hazelnuts, walnuts and pistachios are the most common. Almonds and chestnuts belong to the family of Rosaceae and Fagaceae, respectively, while cashew nuts and pistachios belong to the Anacardiaceae family. Hazel nuts belong to the Betulaceae or Birch family whereas walnuts and pecans belong to the family of Juglandaceae. Almonds are one of the most popular tree nuts in terms of world production followed by hazelnuts, cashews, walnuts and pistachios. The global production of different tree nuts during 2008–2009 and 2009–2010 is shown in Table 7.1 (USDA, 2009, 2010; INC, 2009).
Global production of almond, hazelnut and cashew nut kernels during 2008–2009 was between 872 250–884 697 MT, 571 962 MT and 538 400 MT, respectively. The production of these nuts showed a slight decline during 2009–2010. The production of pine nuts, macadamia nuts and pecan kernels was around 17 330 MT, 27 302 MT and 60 642 MT, respectively. The production of these nuts showed a slight increase during 2009–2010. Walnut in-shell production during 2008–2009 was around 1 000 995–1 117 730 MT. USA, Spain, Syria, Italy, Iran and Morocco are the major almond producing countries in the world (FAO, 2009). Turkey and Italy are the principal producers of hazelnuts (FAO, 2009). Walnuts are widely distributed all over the world, and China, USA, Iran, Turkey and Ukraine are the main walnut producing countries (FAO, 2009). Iran, the USA, Turkey, Syria and China are the main pistachio producing countries (FAO, 2009). Brazil nuts are the largest of the commonly consumed nuts from the giant Brazil nut tree, which is a native of South America. Bolivia, Brazil, Peru, Colombia and Venezuela are the main producer of Brazil nut. Vietnam, India, Nigeria, Côte d’Ivoire and Brazil are the main cashew nut producing countries (FAO, 2009). Spain, Italy, China, Portugal and Turkey are the principal producing countries of pine kernels. Coconut is also a tree nut, however, whether it should be considered as such is a matter of some controversy. Tree nuts are rich sources of various nutrients and phytochemi cals with many potential health benefits. The nutrients and phytochemicals content of different nuts vary with varieties and environment. These phytochemicals possess many functions and reduce the risk of certain types of cancer, coronary heart disease (CHD), atherosclerosis, osteoporosis, type-2 diabetes and some neurodegenerative diseases associated with oxidative stress (Surh et al ., 2003; Kelly et al ., 2006; Tapsell et al ., 2004; Jiang et al ., 2003). Tree nuts are consumed mainly as snacks and are also used as ingredients in a variety of food products. Tree nut oils are also used in many skin moisturizers and cosmetic products (Madhaven, 2001). Chestnuts are tree nuts but are rich in starch and have different nutrients profile in comparison to other common nuts. Consumers generally consider peanuts ( Arachis hypogea ) also as nuts but they are actually botanically legumes. In this chapter, the information about composition, phytochemicals and health benefits of all the common tree nuts except chestnuts and coconut is presented.
Table 7.1 World tree nuts production 2008–2010 (MT)
Sources: a data from USDA Foreign Agricultural Service/USDA Office of Global Analysis, August 2009; b data from USDA Foreign Agricultural Service/USDA Office of Global Analysis, February 2010; c data from USDA Foreign Agricultural Service/USDA Office of Global Analysis, October 2009; d data from International Nut and Dried Fruit Council Foundation (INC), XXVIII World Nut and Dried Fruit Congress Newsletter, Monaco, 29–31 May 2009.

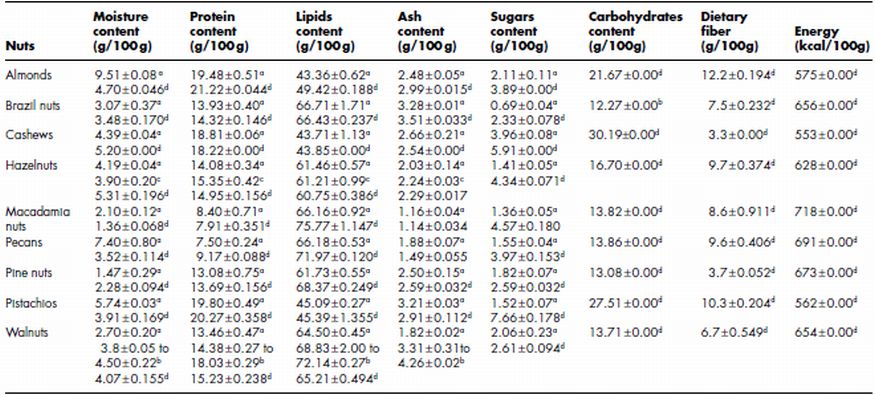
The composition of different tree nuts is compared in Table 7.2. Protein content varies between 7.5 and 21.2%. Almonds, pistachios and cashew nuts contain higher protein content and lower lipids content as compared to walnuts, hazelnuts, Brazil nuts and pine nuts. Tree nuts are rich sources of lipids and some of these have lipids as high as 75%. Tsantili et al. (2010) reported protein and fat content varied from 18.99 to 21.87% and 49.79 to 56.75%, respectively, in different pistachio nut varieties. Ash content that represents the inorganic matter varies from 1.14% to 4.26%; pecan and macadamia nuts have lower ash content as compared to other tree nuts. Carbohydrates content vary from 12.27 to 30.19%, the lowest in Brazil nuts and the highest in cashew nuts. Almonds, Brazil nuts, hazelnuts, macadamias, pecans, pine nuts and pistachios have low starch content (0.25–1.67 g/100 g) while cashews have higher starch content of 23.49 g/100 g (USDA, 2010). Cashew and pine nuts have lower dietary fibre content (3.3–3.7%) as compared to other three nuts. Almonds, pistachios, walnuts, hazelnuts and Brazil nuts have dietary fibre of 6.7–12.2 g/100 g (Table 7.2). Cashews, almonds and pistachios provide lower energy in comparison to other nuts due to their lower lipids content. The difference in chemical constituents of a particular tree nut has been reported to vary with variety and environmental conditions. The majority of tree nut oils contain about 70% unsaturated fatty acids which make them susceptible to oxidative rancidity. Tree nut lipids vary in fatty acid composition, however, the majority are rich in monounsaturated fatty acids (oleic acid, 18:1) and have much lower amounts of polyunsaturated fatty acids (i.e. linoleic acid, 18:2). Cashew nuts (21.12 g/100 g), macadamia nuts (18.18 g/100 g), Brazil nuts (25.35 g/100 g) and pine nuts (24.1 g/100 g) were reported to have more total saturated fatty acids than almonds (9.09 g/100 g), hazelnuts (9.11 g/100 g), pecan (8.35 g/100 g), pistachios (14.24 g/100 g) and walnuts (11.76 g/100 g) by Venkatachalam and Sathe (2006). Polyunsaturated fatty acids were observed to be the main group of fatty acids in walnut oil, ranging from 70.7 to 74.8%; monounsaturated fatty acids ranged from 15.8 to 19.6% and saturated fatty acids ranged from 8.9 to 10.1% (Amaral et al ., 2003). Venkatachalam and Sathe (2006) reported monounsaturated fatty acid content of nut seed oils and found the highest for hazelnuts (83.1 g/100 g), followed by macadamia nuts (77.43 g/100 g), pecan nuts (66.73 g/100 g), cashew nuts (61.68 g/100 g), almonds (61.6 g/100 g), pistachio nuts (51.47 g/100 g), Brazil nuts (29.04 g/100 g) and walnuts (15.28 g/100 g). Tsantili et al . (2010) reported that oleic acid content ranged from 51.6 to 67.86%, linoleic acid (18:2) content from 11.56 to 27.03%, palmitic acid (16:0) from 8.54 to 10.24% and linolenic acid (18:3) from 0.34 to 0.5% in oil from different pistachio varieties. Arranz et al . (2009) reported linoleic acid content of 63.19, 21.39, 7.13 and 33.04%, respectively, in oil from walnuts, almonds, hazelnuts and pistachios. Ryan et al . (2006) reported linoleic acid content of 42.8, 50.31, 45.41, 30.27 and 20.8%, respectively, for oil extracted from Brazil nuts, pecan nuts, pine nuts, pistachio nuts and cashew nuts. These authors reported higher stearic acid (18:0) content in oil extracted from Brazil nuts (11.77%) as compared to oil from pecan nuts (1.8%), pine nuts (4.48%), pistachio nuts (0.86%) and cashew nuts (8.70%). Oleic acid (18:1) was observed to be higher for oil from pistachio and cashew nuts as compared to oil from Brazil nuts (29.09%), pecan nuts (40.63%) and pine nuts (39.55%). Walnuts are a good source of omega-3 fatty acid and have the highest amount of this fatty acid group amongst the different tree nuts. Walnut oil is the only tree nut oil that contains an appreciable amount of α -linolenic acid. Tree nuts are cultivated for use as oil crops in many parts of the world. In the Middle East and Asia, tree nuts are important sources of energy, essential dietary nutrients and phytochemicals (Bonvehi et al., 2000). Brazil nuts are a good source of nutrients, including protein, fibre, selenium (Se), magnesium, phosphorus and thiamine. Pine nuts (8.8 mg/100 g), hazelnuts (6.17 mg/100 g), pecan nuts (4.5 g/100 g) and macadamia nuts (4.13 mg/100 g) are rich in bone-building manganese (USDA, 2010). These nuts also contain niacin, vitamin E, vitamin B6, calcium, iron, potassium, zinc and copper. Alasalvar et al. (2009) reported that the manganese content varied from 2.17 to 19.0 mg/100 g in hazelnut varieties.
Table 7.2 Proximate composition of different nuts (edible portion)
Sources: a data from Venkatachalam and Sathe (2006); b data from Pereira et al . (2008); c data from Alasalvar et al. (2003); ddata from USDA National Nutrient Database for Standard Reference, Release 23 (2010) (assessed on 20 June, 2011).
The composition of nuts with and without seed coat also differed significantly. Endosperm of the majority of tree nuts contains about 70% unsaturated fats, which make them susceptible to rancidity. Brazil nuts have protein, which is a rich source of methionine (Antunes and Markakis, 1977). Nuts are a rich source of arginine that was observed to range from 9.15 g/100 g of protein in pistachios to 15.41 g/100 g of protein in pine nuts (Venkatachalam and Sathe, 2006). Walnuts are good sources of both antioxidants and n-3 fatty acids, with particularly high amounts of α-linolenic acid (6.3 g/100 g), whereas other nuts such as almonds, pecans, and pistachios possess much smaller amounts (0.4–0.7 g/100 g). Almonds are especially high in vitamin E and magnesium. Smeds et al. (2007) reported that certain tree nuts (almond, cashew and walnut) contain the polyphenolics, lignans, in amounts comparable to certain rice types (346–486 µg of lignans/100 g edible portion), but lower than the cereals like rye (10377 µg of lignan/100 g) and wheat (7548 µg of lignan/100 g). Almonds, cashews and walnuts contain between 344 and 912 µg of lignan/100 g edible portion, cashew nut was reported to be the most abundant tree nut source of lignan (912 µg/100 g) (Smeds et al., 2007).
The oxalate content of nuts was reported to vary widely and it was suggested by Ritter and Savage (2007) that people who have a tendency to form kidney stones consume certain nuts in moderate levels. These authors extracted gastric soluble and intestinal soluble oxalates from the nuts using an in vitro assay, which involved incubations of the food samples for 2 h at 37 °C in gastric and intestinal juice. Pistachio nuts (roasted) contained relatively low levels of gastric soluble oxalate (67 mg/100 g FW). Almonds and Brazil nuts were observed to contain high levels of gastric soluble oxalate (538.5 and 492.0 mg/100 g FW, respectively). The intestinal soluble oxalate is the fraction that absorbed in the small intestine. Pecan nuts and pistachios (roasted) contained relatively low levels of intestinal soluble oxalate (155 and 76 mg/100 g FW, respectively) as compared to almonds, Brazil nuts, cashew nuts and pine nuts (222, 304, 216 and 581 mg/100 g FW, respectively). Pinenuts contained the highest levels of intestinal soluble oxalate (581 mg/100 g FW), while roasted pistachio nuts were observed to contain low level (77 mg /100 g FW).
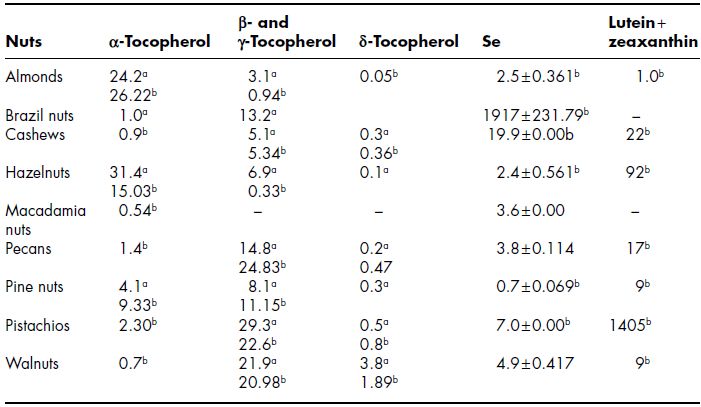
Nuts are a good source of phytochemicals, including phenolics, flavonoids, isoflavones, terpenes, organosulfuric compounds and vitamin E (Bravo, 1998; Kris-Etherton et al., 2002). The majority of nuts have low concentrations of carotenoids, and are not an excellent source of dietary carotenoids. The β-carotene and lutein content were found to be 0.21 and 2.32 mg/100 g (dry weight), respectively, in pistachios (Kornsteiner et al., 2006). Tocopherol content, lutein, zeaxanthin and Se content of different tree nuts is shown in Table 7.3. Almonds and hazelnuts are excellent sources of α-tocopherol (vitamin E). Cashews, Brazil nuts, macadamias, pecans, walnuts and pistachios are poor sources of vitamin E. Higher amount of lutein plus zeaxanthin (1405 mcg) for pistachio nuts as compared to other nuts was reported (Table 7.3). Proanthocyanidins were reported to be present in the majority but not in all nuts, with concentrations of 501 mg/100 g in hazelnuts, 494 mg/100 g in pecans, 237 mg/100 g in pistachios, 184 mg/100 g in almonds, 67 mg/100 g in walnuts, 16 mg/100 g in peanuts, and 9 mg/100 g in cashews (Gu et al., 2004). Brazil nuts are rich food sources of Se (1917 mcg/100 g). Cashews, almonds, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts were reported to have Se content 0.7–19.9 mcg (Table 7.3).
Table 7.3 Tocopherol, selenium and lutein + zeaxantin content of different tree nuts
Sources: aData from Kornsteiner et al. (2006), Data expressed as mg/g oil; bdata from USDA National Nutrient Database for Standard Reference, Release 23 (2010) (assessed on 20 June, 2011), data expressed as (mcg).
Tocopherol, squalene and phytosterol content of oil from different tree nuts is shown in Table 7.4. Almond oil has the highest α-tocopherol content followed by that of hazelnut, pine nut and macademia nut (Yang et al., 2009). Squalene content was reported to be the highest for Brazil nuts (1377 µg/g oil), followed by hazelnuts (186 µg/g oil) and macademia nuts (185 µg/g oil) by Ryan et al. (2006) and Maguire et al. (2004). Ryan et al. (2006) reported that Brazil nut has higher squalene content (1377.8 mg/g) as compared to pine (39.5 mg/g), cashew (89.4 mg/g), pistachio (91.4 mg/g), and pecan (151.7 mg/g). Tree nuts are a good source of phytosterols and amongst the various phytosterols determined in tree nuts, β-sitosterol was reported to be present in the highest amount (Phillips et al., 2005).
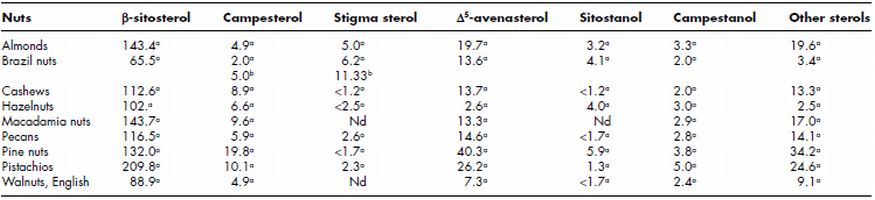
Pistachios nuts contain the higher total phytosterols (β-sitosterol, Campesterol, Stigma sterol, Δ 5-avenasterol, Sitostanol, Campestanol and other sterols) content of 279 mg/100 g. While almonds, macadamia, pine nuts, hazelnuts, pecans, walnuts (English) and Brazil nuts had total phytosterol content of 199, 187, 236, 121, 157, 113 and 95 mg/100 g, respectively (Table 7.5). Campestrol was observed to be higher in pine nuts, pistachios, macadamias and cashews as compared to almonds, Brazil nuts, pecans, hazelnuts and walnuts. Pine nuts and pistachio nuts have higher Δ 5-avenasterol content in comparison to other nuts. Thompson et al. (2006) analysed phytoestrogen content of 121 food samples including seven major tree nuts (almond, cashew, chestnut, hazelnut, pecan, pistachio and walnut). It was reported that tree nuts have four each of isoflavones (formononetin, daidzein, genistein and glycitein) and lignans (matairesinol, lariciresinol, pinoresinol and secoisolariciresinol); and one coumestan (coumestrol). Amongst the tree nuts studied, pistachio was the richest source of total isoflavones (176.9 µg/100 g on an as is basis), total lignans (198.9 µg/100 g), and total phytoestrogens (382.5 µg/100 g). Hazelnut contained higher total isoflavones (30.2 µg/100 g), primarily genistein, as compared to pistachio and walnut and had the sixth highest total lignans (77.1 µg/100 g), primarily secoisolariciresinol, and total phyoestrogens (107.5 µg/100 g).
Table 7.4 Tocopherol, squalene and phytosterol content of oil extracted from different tree nuts
Sources: a data from Yang et al. (2009); bdata from Ryan et al. (2006); cdata from Maguire et al. (2004).
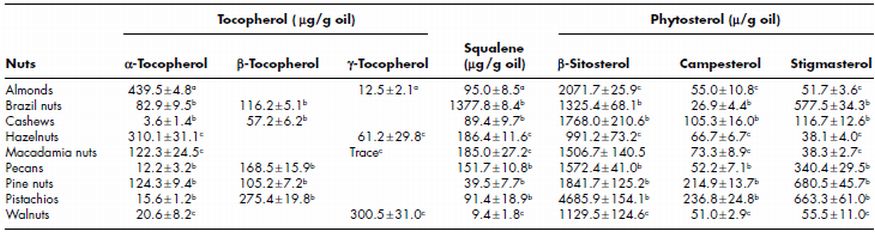
Table 7.5 Phytosterol composition of different tree nuts (mg/100 g)
Sources: a data from Phillips et al. (2005); b data from da Costa et al. (2010).
Nuts are a good source of phenolics (tannins, ellagic acid and curcumin) and flavonoids such as luteolin, quercetin, myricetin, kaempferol and resveratrol (Bravo, 1998; Kris-Etherton et al., 2002). Almonds contain an abundance of flavonoids, including catechins, flavonols and flavonones in their aglycone and glycoside forms (Sang et al., 2002). Pistachio nuts also have several flavonoids and were reported to be rich in resveratrol (Lou et al., 2001), while cashew nuts contain an abundance of alkylphenols (Trevisan et al., 2006). Resveratrol content of 115 µg/100 g for pistachio nuts was reported by Tokusoglu et al. (2005). Walnuts contain a wide variety of phenolics, tocopherols and nonflavonoids such as ellagitannins (Anderson et al., 2001). Hazelnuts contained different phenolic acids such as gallic acid, caffeic acid, p-coumaric acid, ferulic acid and sinapic acid in both free and esterified forms (Shahidi et al., 2007).
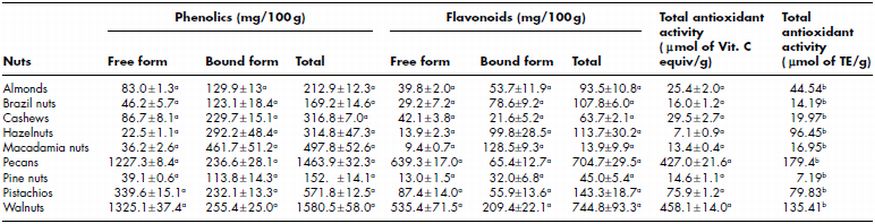
Tree nuts are externally covered with a thin layer of skin known as testa (seed coat). The testa contributes a bitter/astringent taste to nuts and reduces the consumer acceptability. Therefore, testa is removed from the majority of nuts before marketing or using in different food products. Testa constitutes about 1–3% of total weight of cashews. Testa is a rich source of hydrolysable tannins with polymeric proanthocyanidins as major polyphenols (Mathew and Parpia, 1970). Extracts of whole almond seed, brown skin, shell and green shell cover (hull) possess potent free radical-scavenging capacities (Amarowicz, Troszynska and Shahidi, 2005; Jahanban et al ., 2009; Moure, Pazos, Medina, Dominguez and Parajo, 2007; Pinelo, Rubilar, Sineiro and Nunez, 2004; Siriwardhana, Amarowicz and Shahidi, 2006; Siriwardhana and Shahidi, 2002; Wijeratne et al ., 2006). These activities may be related to the presence of flavonoids and other phenolic compounds in nuts. Almond hulls are a rich source of three triterpenoids (about 1% of the hulls), betulinic, urosolic and oleanolic acids (Takeoka et al ., 2000), as well as flavonol glycosides and phenolic acids (Sang et al ., 2002). Sang et al . (2002) isolated catechin, protocatechuic acid, vanillic acid, p-hydroxybenzoic acid and naringenin glucoside, as well as galactoside, glucoside and rhamnoglucoside of 3b-O-methylquercetin and rhamnoglucoside of kaempferol from almond hulls. As a result Almond hulls, which are mainly used in livestock feed, have been suggested as a potential source of antioxidants (Siriwardhana et al ., 2006; Shahidi, Zhong, Wijeratne and Ho, 2009). Pecans and walnuts were reported to have higher total antioxidant activity of 179.4 and 135.4 µ mol of TE/g, respectively as compared to hazelnuts (96.45 µ mol of TE/g), pistachio nuts (79.83 µ mol of TE/g), almonds (44.54 µ mol of TE/g), cashews (19.97 µ mol of TE/g), macadamias (16.95 µ mol of TE/g), brazil nuts (14.19 µ mol of TE/g) and pine nuts (7.19 µ mol of TE/g) by Wu et al . (2004). Antioxidant activity of hazelnuts, walnuts and pistachios with and without seed coat (testa) was compared by Arcan and Yemenicio ğ lu (2009). Yang et al . (2009) evaluated tree nuts for total phenolic and flavonoid contents, antioxidant and antiproliferative activities. Walnuts had the higher total phenolic and flavonoid contents (1580.5 ± 58.0 mg/100 g and 744.8 ± 93.3 mg/100 g, respectively), followed by pecan nuts (1463.9 ± 32.3 mg/100 g and 704.7 ± 29.5 mg/100 g, respectively), pistachios (571.8 ± 12.5 mg/100 g and 143.3 ± 18.7 mg/100 g, respectively) and macadamia nuts (497.8 ± 52.6 mg/100 g and 137.9 ± 9.9 mg/100 g, respectively). Almonds, Brazil nuts, cashews, hazelnuts and pine nuts showed total phenolics and flavonoids content between 152.9 ± 14.1–316.4 ± 7.0 and 45.0 ± 5.4–107.8 ± 6.0, respectively. Walnuts also had the highest total antioxidant activity (458.1 µ mol of vitamin C equiv/g). Both soluble phenolic and flavonoid contents were observed to be positively correlated with total antioxidant activity.
It was reported that the removal of seed coat considerably reduced the total antioxidant activity of hazelnuts, walnuts and pistachios. The removal of seed coat was observed to reduce the total antioxidant activity of hazelnuts, walnuts and pistachios to the extent of 36, 90 and 55%, respectively (Arcan and Yemenicioglu, 2009). These authors reported the antioxidant activity in a one-serving portion (one-serving portion = 42 g) of fresh or dry walnuts equivalent to that of a two-serving portion of black tea (one-serving portion = 200 ml) and 1.2–1.7-serving portions of green and Earl Grey tea (one-serving portion = 200 ml). Ethanolic extract of cashew nut testa was reported to exhibit a significant level of antioxidant activity, which was attributed to its phenolic composition (Kamath and Rajini, 2007). Cashew nut testa has been found to have higher levels of (+)-catechin and (-)-epicatechin as compared to those reported for green tea and chocolate (Trox et al., 2011). Cashew nuts with testa possess significantly higher amounts of carotenoids and tocopherols when compared to testa-free kernels. The presence of such potentially bioactive compounds in the testa-containing cashew nut kernels was suggested as an interesting economical source of natural antioxidants for use in food and nutraceutical industries (Trox et al., 2011). Tomaino et al. (2010) reported higher antioxidant activity of pistachio skin as compared to seed and this has been attributed to gallic acid, catechin, cyanidin-3-O-galactoside, eriodictyol-7-O-glucoside and epicatechin together with other unidentified compounds. They observed gallic acid, catechin, cyanidin-3-O-galactoside, eriodictyol-7-O-glucoside and epicatechin content of 1453.31, 377.45, 5865.12, 365.68 and 104.8 mg/g (fresh weight), respectively, in pistachio skin. Whereas pistachio seed was observed to have gallic acid, catechin and eriodictyol-7-O-glucoside content of 12.66, 2.41 and 31.91 mg/g (fresh weight), respectively. They reported antioxidant activity of 1.65 and 116.32 measured as mg of GAE/g (fresh weight), respectively in pistachio seeds and skins.
Free and bound phenolics and flavonoids distribution vary amongst different nuts (Table 7.6). Yang et al. (2009) reported that walnuts contain the highest soluble-free phenolic content (1325 mg/100 g), followed by pecans (1227 mg/100 g), pistachios (339 mg/100 g), cashews (86.7 mg/100 g), almonds (83 mg/100 g), Brazil nuts (46 mg/100 g), pine nuts (39 mg/100 g), and macadamia nuts (36 mg/100 g). Hazelnuts had the lowest free phenolic content of 22.5 mg/100 g. Macadamia nuts had the highest bound phenolics (462 mg/100 g) followed by peanuts (237 mg/100 g), hazelnuts (292 mg/100 g), walnuts (255 mg/100 g), pecans (293 mg/100 g), pistachios (232 mg/100 g), cashews (230 mg/100 g), almonds (130 mg/100 g), Brazil nuts (123 mg/100 g) and pine nuts (114 mg/100 g). The contribution of bound fraction was insignificant compared to the soluble phenolic fraction of cashew nuts and testa. High temperature (130 °C for 33 min) treated cashew nuts and testa showed a higher phenolic content and antioxidant activity than low temperature (70 °C for 6 h) treated samples (Chandrasekara and Shahidi, 2011). DPPH radical scavenging activity of soluble phenolics extracts of raw cashew nut kernels and testa was 3.17 and 179.3 (mg of GAE/g of defatted meal), respectively; while bound phenolics extract showed 0.13 and 81.16 (mg of GAE/g of defatted meal), respectively for kernel and testa. The DPPH radical scavenging activity of soluble phenolic extracts of kernel and testa significantly increased with increasing roasting temperature, whereas bound extracts generally showed a decrease. The soluble extracts of testa treated at high temperature had a higher DPPH radical scavenging activity than that of low temperature treated testa. Mathew and Parpia (1970) reported the presence of catechin and epicatechin as predominant polyphenolics in cashew nut testa. High temperature treated testa had a higher flavonoid content to that in the raw testa. This increase has been attributed to the liberation and isomerization of such compounds during heat treatment of cashew nuts and testa. Locatelli et al. (2010) reported that roasting at 180 °C for 20 min brought about higher total phenol content of the soluble extract than roasting at the same temperature for 10 min of hazelnut skin. Amaral et al. (2006) studied the effects of roasting of hazel nuts at different temperatures (125–200 °C for 5–30 min)) on phytosterols and observed a modest decrease in the total levels of the beneficial phytosterols (maximum of 14.4%) and vitamin E (maximum of 10.0%) compounds during roasting. A negligible increase of the potentially harmful trans fatty acids was also observed. Bolling et al. (2010) reported that processing and storage change the polyphenol and antioxidant activity of almond skin. They reported that dry roasted (135 °C for 14 min) almonds had 26% less total phenols and 34% less ferric reducing antioxidant power (FRAP) than raw. Storage of almonds at 4 °C and 23 °C for 15 months resulted in gradual increase in flavonoids and phenolic acids, up to 177 and 200%, respectively. However, FRAP and total phenols were found to increase to 200 and 190% of initial values after 15 months. Thus, roasting decreased total phenols and FRAP of almond skin but not flavonoids and phenolic acids, whilst storage for up to 15 months doubled flavonoids and phenolic acids.
Table 7.6 Total phenolics contents, flavonoids contents and antioxidant activity of different tree nuts
Sources: adata fromYang et al. (2009); bdata from Wu et al. (2004).
Bleaching of pistachio shells is done to improve the appearance by increasing the whiteness. This practice is not permitted and actually illegal in many countries. The effects of bleaching (0.1–50% hydrogen peroxide) on phenolic levels and antioxidative capacities in raw and roasted nuts were reported by Seeram et al. (2006). Bleaching decreased total anthocyanin levels and antioxidative capacity of raw and roasted nuts. Raw nuts preserved phenolic levels and antioxidant capacity better than roasted nuts, suggesting contributing effects of other substances and/or matrix effects that are destroyed by the roasting process.
Many epidemiologic and clinical studies have associated frequent consumption of nuts with reduced risk of CHD (Kelly and Sabaté, 2006; Fraser et al., 1992; Hu et al., 1998, 1999; de Lorgeril et al., 2001; Sabaté et al., 2001) and various types of cancer (Jenab et al., 2004; González and Salas-Salvadó, 2006). Nuts are a good source of dietary fibre, which was reported to be higher than legumes, whole grain bread, fruits and vegetables (Salas-Salvado et al., 2006). A lower risk of type-2 diabetes with higher intakes of dietary fibre and lower glycemic loads has already been reported (Chandalia et al., 2000; Luscombe et al., 1999). Brazil nut has higher levels of phytonutrients and its consumption has been associated with many health benefits, mainly including cholesterol-lowering effects, antioxidant activity and antiproliferative effects. Brazil nuts are considered to be the best source of Se from plant-based foods, which is needed for proper thyroid and immune function. Brazil nut has good antioxidant activity and this has been attributed to its high Se content. It is an essential cofactor for glutathione peroxidase, which prevents lipid peroxidation and cell damage (Patrick, 2004). The role of selenium is a chemopreventive agent for a variety of cancers (Patrick, 2004). Selenium and vitamin E work synergistically. Selenium prevents free radical production by reducing peroxide concentrations in the cell whereas vitamin E neutralizes the free radicals when produced (Patrick, 2004).
Tree nuts and their oils are known to contain several bioactive and health-promoting substances. Epidemiological evidence has indicated that the consumption of tree nuts may exert several cardioprotective effects, which were speculated to arise from their lipid component that includes unsaturated fatty acids, phytosterols and tocols (Hu and Stampfer, 1999). Studies have also shown that dietary consumption of tree nut oils may exert even more beneficial effects than consumption of whole tree nuts, possibly due to the replacement of dietary carbohydrates with unsaturated lipids and/or other components present in the oil extracts (Hu and Stampfer, 1999). However, traditionally tree nuts were not considered as very healthy because of their high lipid content. Walnuts are receiving increasing interest as a healthy foodstuff because their regular consumption has been reported to decrease the risk of CHD (Blomhoff et al., 2006; Davis et al., 2007). The health benefits of walnuts are usually attributed to their chemical composition, being good sources of essential fatty acids and tocopherols (Amaral et al., 2003, 2005). Linoleic acid is the major fatty acid in walnuts, followed by oleic, linolenic, palmitic and stearic (Amaral et al., 2003; Ruggeri et al., 1998; Savage et al., 1999); its high content of poly unsaturated fatty acids, it has been suggested, can reduce the risk of heart disease by decreasing total and LDL-cholesterol and increasing HDL-cholesterol (Davis et al., 2007; Tapsell et al., 2004). In addition, walnuts have other components that may be beneficial for health including plant protein, dietary fibre, melatonin (Reiter et al., 2005), plant sterols (Amaral et al., 2003), folate, tannins and polyphenols (Anderson et al., 2001; Li et al., 2006). The chemical constituents, particularly the oil content and the fatty acid and tocopherols have been found to vary significantly among different walnut cultivars and environmental conditions (Amaral et al., 2005). Nut consumption lowered the risk of CHD, which was partly explained by the cholesterol-lowering effect. The favourable fatty acid composition and lipid lowering effect of nuts have been demonstrated in experimental studies with almonds (Hyson et al., 2002), macadamia nuts (Curb et al., 2000), pecans (Morgan and Clayshulte, 2000), pistachios (Edwards et al., 1999) and walnuts (Ros, 2000). Walnuts are good sources of both antioxidants and n-3 fatty acids, in particular high amounts of α-linolenic acid (6.3 g/100 g), whereas other nuts such as almonds, pecans and pistachios possess much smaller amounts (0.4–0.7 g/100 g). Brazil nuts are particularly rich in the antioxidant compound Se, while pecans are rich in bone-building manganese. Ryan et al. (2006) reported that Brazil nuts are a good source of squalene (1377.8 mg/100 g), which is a straight-chain terpenoid hydrocarbon and is a precursor of steroids and also plays an important role in the synthesis of cholesterol and vitamin D in the human body. It has been reported that squalene significantly decreases total cholesterol, LDL cholesterol and triacylglycerols levels in hypercholesterolemic patients (Miettinen et al., 1994; Chan et al., 1996). Tree nuts are a good source of phytosterols, which interferes with cholesterol absorption and results in reduction of serum LDL cholesterol levels (Thompson et al., 2005). Epidemiologic and experimental studies have suggested that the phytosterols may offer protection from colon, breast and prostate cancers (Award and Fink, 2000, 2001).
Alasalvar, C., Amaral. J. S. and Satir, G., and Shahidi, F. (2009) Lipid characteristics and essential minerals of native Turkish hazelnut varieties ( Corylus avellana L.). Food Chemistry, 113, 919–925.
Alasalvar, C., Karamac, M., Amarowicz, R. and Shahidi, F. (2006) Antioxidant and antiradical activities in extracts of hazelnut kernel ( Corylus avellana L.) and hazelnut green leafy cover. Journal of Agricultural and Food Chemistry, 54, 4826–4832.
Alasalvar, C., Shahidi, F., Liyanapathirana, C.M. and Ohshima, T. (2003) Turkish Tombul Hazelnut ( Corylus avellana L.). 1. Compositional Characteristics . Journal of Agricultural and Food Chemistry , 51 , 3790–3796.
Amaral, J.S., Alves, MR., Seabra, R.M. and Oliveira, B.P.P. (2005) Vitamin E composition of walnuts ( Juglans regia L.): A 3-year comparative study of different cultivars. Journal of Agricultural and Food Chemistry, 53, 5467–5472.
Amaral, J.S., Casal, S., Pereira, J.A., Seabra, R.M. and Oliveira, B.P.P. (2003) Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut ( Juglans regia L.) cultivars grown in Portugal. Journal of Agricultural and Food Chemistry, 51, 7698–7702.
Amaral, J.S., Casal, S., Seabra, S.M. and Oliveira, B.P. (2006). Effects of roasting on hazelnut lipids. Journal of Agricultural and Food Chemistry, 54, 1315–1321.
Amarowicz, R., Troszyñska, A. and Shahidi, F. (2005) Antioxidant activity of almond seed extract and its fractions. Journal of Food Lipids, 12, 344–358.
Anderson, K.J., Teuber, S.S., Gobeille, A., Cremin, P., Waterhouse, A.L. and Steinberg, F.M. (2001) Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation , Journal of Nutrition , 131 , 2837–2842.
Antunes, A.J. and Markakis, P. (1977) Protein Supplementation of Navy Beans with Brazil Nuts. Journal of Agricultural and Food Chemistry, 25, 1096–1098.
Arcan, I. and Yemenicioğlu, A. (2009) Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat. Journal of Food Composition and Analysis, 22, 184–188.
Arranz, S., Cert, R., Pérez-Jiménez, J., Cert, A. and Saura-Calixto, F. (2008) Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chemistry, 110, 985–990.
Awad, A.B. and Fink, C.S. (2000) Phytosterols as anticancer dietary components: Evidence and mechanism of action, Journal of Nutrition, 130, 2127–2130.
Awad, A.B., Downie, A., Fink, C.S. and Kim, U. (2000) Dietary phytosterols inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Research, 20, 821–824.
Awad, A.B., Williams, H. and Fink, C.S. (2001) Phytosterols reduce in vitro metastic ability of MDA-MB-231 human breast cancer cells, Nutrition and Cancer, 40, 157–164.
Blomhoff, R., Carlsen, M.H., Anderson, L.F. and Jacobs, D.R. Jr. (2006) Health benefits of nuts: Potential role of antioxidants, British Journal of Nutrition, 96 (Suppl. 2), 52S–60S.
Bolling, B.W., Blumberg, J.B. and Oliver Chen, C.Y. (2010) The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chemistry, 123, 1040–1047.
Bonvehi, J.S., Coll, F.V. and Rius, I.A. (2000) Liquid chromatographic determination of tocopherols and tocotrienols in vegetable oils, formulated preparations, and biscuits. The Journal of AOAC International, 83, 627–634.
Bravo, L. (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews, 56, 317–33.
Chan, P., Tomlinson, B., Lee, C. B. and Lee, Y. S. (1996) Effectiveness and safety of low-dose pravastatin and squalene, alone and in combination, in elderly patients with hypercholesterolemia. The Journal of Clinical Pharmacology, 36, 422–427.
Chandalia, M., Garg, A., Lutjohann, D., von Bergmann, K., Grundy, S.M. and Brinkley, L.J. (2000) Beneficial effects of high dietary fibre intake in patients with type 2 diabetes mellitus. The New England Journal of Medicine, 342, 1392–1398.
Chandrasekara, N. and Shahidi, F. (2011) Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa . Journal of Agricultural and Food Chemistry , 59 , 5006–5014.
Curb, J.D., Wergowske, G., Dobbs, J.C., Abbott, R.D. and Huang, B. (2000) Serum lipid effects of a highmonounsaturated fat diet based on macadamia nuts. Archives of Internal Medicine, 160, 1154–1158.
da Costa, P.A., Ballus, C.A., Teixeira-Filho, J. and Godoy, H.T. (2010) Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Research International, 43, 1603–1606.
Davis, L., Stonehouse, W., Loots, D.T., Mukuddem-Petersen, J., van der Westhuizen, F.H., Hanekom, S.M. and Jerling, J.C. (2007) The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. European Journal of Nutrition, 46, 155–164.
De Lorgeril, M., Salen, P., Laporte, F., Boucher, F. and De Leiris, J. (2001) Potential use of nuts for the prevention and treatment of coronary heart disease: From natural to functional foods. Nutrition, Metabolism and Cardiovascular Diseases, 11, 362–371.
Edwards, K., Kwaw, I., Matud, J. and Kurtz, I. (1999) Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia, Journal of the American College of Nutrition, 18, 229–232.
FAO (2009). http://faostat.fao.org .
Fraser, G.E., Sabaté, J., Beeson, W.L. and Strahan, T.M. (1992) A possible protective effect of nut consumption on risk of coronary heart disease. The adventist health study. Archives of Internal Medicine, 152, 1416–1424.
Gola, U., Nohr, D. and Biesalski, H.K. (2011) Catechin and epicatechin in testa and their association with bioactive compounds in kernels of cashew nut ( Anacardium occidentale L.). Food Chemistry, 128, 1094–1099.
Goli, A.H., Barzegar, M. and Sahari, M A. (2005) Antioxidant activity and total phenolic compounds of pistachio ( Pistachia vera ) hull extracts. Food Chemistry, 92, 521–525.
González, C.A. and Salas-Salvadó, J. (2006) The potential of nuts in the prevention of cancer. British Journal of Nutrition, 96, 87–94.
Gu, L., Kelm, M.A., Hammerstone, J.F., Beecher, G., Holden, J., Haytowitz, D., Gebhardt, S. and Prior, R.L. (2004) Concentrations of proanthocyanidins in common foods and estimations of normal consumption. Journal of Nutrition, 134, 613–617.
Hu, F.B. and Stampfer, M.J. (1999) Nut consumption and risk of coronary heart disease: A review of epidemiologic evidence. Current Atherosclerosis Reports, 3, 204–209.
Hu, F.B., Stampfer, M.J., Manson, J.E., Rimm, E.B., Colditz, G.A., Rosner, B.A., Speizer, F.E., Hennekens, C.H. and Willett, W.C. (1998) Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. British Medical Journal, 317, 1341–1345.
Hyson, D.A., Schneeman, B.O. and Davis, P.A. (2002) Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. Journal of Nutrition, 132, 703–707.
International Nut and Dried Fruit Council Foundation, XXVIII World Nut and Dried Fruit Congress Newsletter, Monaco, 29–31 May, 2009.
Jarvi, A.E., Karlstrom, B.E., Granfeldt, Y.E., Bjorck, I.E., Asp, N.G. and Vessby, B.O. (1999) Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care, 22, 10–18.
Jenab, M., Ferrari, P., Slimani, N., et al. (2004) Association of nut and seed intake with colorectal cancer risk in the European prospective investigation into cancer and nutrition, Cancer Epidemiol . Biomarkers Prevention, 13, 1595–1603.
Jiang, Q. and Ames, B.N. (2003) γ -Tocopherol, but not α -tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB Journal, 17, 816–822.
Jiang, R., Manson, J.E., Stampfer, M.J., Liu, S., Willett, W.C. and Hu, F.B. (2002) Nut and peanut butter consumption and risk of type 2 diabetes in women. Journal of the American Medical Association, 288, 2554–2560.
Kamath, V. and Rajini, P.S. (2007) The efficiency of cashew-nut ( Anacardium occidentale L.) skin extract as a free radical scavenger. Food Chemistry, 103, 428–433.
Kelly Jr, J.H. and Sabaté, J. (2006) Nuts and coronary heart disease: An epidemiological perspective. British Journal of Nutrition, 96, 61S–67S.
Kendall, C.W., Marchie, A., Parker, T.L., Augustin, L.S., Ellis, P.R., Lapsley, K.G., Ternus, M. and Jenkins, D.J. (2003) Effect of nut consumption on postprandial starch digestion-a dose response study. Annals of Nutrition and Metabolism, 47, 636.
Kornsteiner, M., Wagner, K.H. and Elmadfa, I. (2006) Tocopherols and total phenolics in 10 different nut types. Food Chemistry, 98, 381–387.
Kris-Etherton, P.M., Lefevre, M., Beecher, G.R., Gross, M.D., Keen, C.L. and Etherton, T.D. (2004) Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and antiinflammatory effects of flavonoids on atherosclerosis. Annual Reviews on Nutrition, 24, 511–538.
Li, L., Tsao, R., Yang, R., Liu, C.M., Zhu, H.H. and Young, J.C. (2006) Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut ( Juglans regia L.). Journal of Agricultural and Food Chemistry, 54, 8033–8040.
Locatelli, M., Travaglia, F., Coisson, J.D., Martelli, A., Stevigny, C. and Arlorio, M. (2010) Total antioxidant activity of hazelnut skin (Nocciola piemonte PGI): impact of different roasting conditions. Food Chemistry, 119, 1647–1655.
Luscombe, N.D., Noakes, M. and Clifton, P.M. (1999). Diets high and low in glycemic index versus high monounsaturated fat diets: Effects on glucose and lipid metabolism in NIDDM. European Journal of Clinical Nutrition, 53, 473–478.
Madhaven, N. (2001) Final report on the safety assessment of Corylus avellana (Hazel) seed oil, Corylus americana (Hazel) seed oil, Corylus avellana (Hazel) seed extract, Corylus americana (Hazel) seed extract, Corylus avellana (Hazel) leaf extract, Corylus americana (Hazel) leaf extract, and Corylus rostrata (Hazel) leaf extract . International Journal of Toxicology , 20 , 15–20.
Maguire, L.S., O’Sullivan, S.M., Galvin, K., O’Connor, T.P. and O’Brien, N.M. (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. International Journal of Food Science and Nutrition, 55, 171–178.
Mathew, A.G. and Parpia, H.A.B. (1970) Polyphenols of cashew nut kernel testa. Journal of Food Science, 35, 140–143.
Mattes, R.D. (2008) The energetics of nut consumption, Asia Pacific Journal of Nutrition, 17, 337–339.
Miettinen, T.A., and Vanhanen, H. (1994) Serum concentration and metabolism of cholesterol during rapeseed oil and squalene feeding. American Journal of Clinical Nutrition, 59, 356–363.
Morgan, W.A. and Clayshulte, B.J. (2000) Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. Journal of the American Dietetic Association, 100, 312–318.
Moure, A., Pazos, M., Medina, I., Dominguez, H. and Parajo, J.C. (2007) Antioxidant activity of extracts produced by solvent extraction of almond shells acid hydrolysates, Food Chemistry, 101, 193–201.
Patrick, L. (2004) Selenium biochemistry and cancer: A review of the literature. Alternative Medicine Review, 9, 239–258.
Pereira, J.A., Oliveira, I., Sousa, A., Ferreira, I., Bento, A. and Estevinho, L. (2008) Bioactive properties and chemical composition of six walnut ( Juglans regia L.) cultivars. Food and Chemical Toxicology, 46, 2103–2111.
Phillips, K.M., Ruggio, D.M. and Ashraf-Khorassani, M. (2005) Phytosterol composition of nuts and seeds commonly consumed in the United States. Journal of Agricultural and Food Chemistry, 53, 9436–9445.
Reiter, R.J., Manchester, L.C. and Tan, D.X. (2005) Melatonin in walnuts: Infl uence on levels of melatonin and total antioxidant capacity of blood. Journal of Nutrition, 21, 920–924.
Ritter, M.M.C. and Savage, G.P. (2007) Soluble and insoluble oxalate content of nuts. Journal of Food Composition and Analysis, 20, 169–174.
Ros, E. (2000) Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Annals of Internal Medicine, 132, 538–546.
Ruggeri, S., Cappelloni, M., Gambelli, L. and Carnovale, E. (1998) Chemical composition and nutritive value of nuts grown in Italy. Italian Journal of Food Science, 10, 243–252.
Ryan, E., Galvin, K., O’Connor, T.P., Maguire, A.R. and O’Brien, N.M. (2006) Fatty acid profile, tocopherol, squalene and phytosterol content of Brazil, pecan, pine, pistachio and cashew nuts. International Journal of Food Sciences and Nutrition, 57, 219–228.
Sabaté, J., Radak, T. and Brown Jr, J. (2001) The role of nuts in cardiovascular disease prevention. In Wildman, R.E.C. (ed.) Handbook of Nutraceuticals and Functional Foods, CRC Press, Boca Raton, FL, pp. 477–495.
Salas-Salvado, J., Bullo, M., Perez-Heras, A. and Ros, E. (2006) Dietary fibre, nuts and cardiovascular diseases. British Journal of Nutrition, 96 (Suppl. 2), 45S–51S.
Salmeron, J., Ascherio, A., Rimm, E.B., Colditz, G.A., Spiegelman, D., Jenkins, D.J., Stampfer, M.J., Wing, A.L. and Willett, W.C. (1997) Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care, 20, 545–550.
Salmeron, J., Manson, J.E., Stampfer, M.J., Colditz, G.A., Wing, A.L. and Willett, W.C. (1997) Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. Journal of the American Medical Association, 277, 472–477.
Sang, S., Lapsley, K., Jeong, W.S., Lachance, P.A., Ho, C.T. and Rosen, R.T. (2002) Antioxidative phenolic compounds isolated from almond skins ( Prunus amygdalus Batsch). Journal of Agricultural and Food Chemistry, 50, 2459–2463.
Savage, G.P., Dutta, P.C. and Mcneil, D.L. (1999) Fatty acid and tocopherol contents and oxidative stability of walnut oils. Journal of the American Oil Chemists’ Society, 76, 1059–1063.
Seeram, N.P., Zhang, Y., Henning, S.M., Lee, R., Niu, Y., Lin, G. and Heber, D. (2006) Pistachio skin phenolics are destroyed by bleaching resulting in reduced antioxidative capacities. Journal of Agricultural and Food Chemistry, 54, 7036–7040.
Shahidi, F., Alasalvar, F. and Liyana-Pathirana, C.M. (2007) Antioxidant phytochemicals in hazelnut kernel ( Corylus avellana L.) and hazelnut byproducts. Journal of Agricultural and Food Chemistry, 55, 1212–1220.
Shahidi, F., Zhong, Y., Wijeratne, S.S.K. and Ho, C.T. (2009) Almond and almond products: Nutraceutical components and health effects. In C. Alasalvar and F. Shahidi (eds) Tree Nuts: Nutraceuticals, Phytochemicals, and Health Effects, CRC Press, Boca Raton, FL, pp. 127–141.
Siriwardhana, S.S.K.W. and Shahidi, F. (2002) Antiradical activity of extracts of almond and its by-products. Journal of the American Oil Chemists Society, 79, 903–908.
Smeds, A.I., Eklund, P.C., Sjoholm, R E., Willfor, S.M., Nishibe, S., Deyama, T. and Holmbom, B.R. (2007) Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. Journal of Agricultural and Food Chemistry, 55, 1337–1346.
Surh, Y.J. (2003) Cancer chemoprevention with dietary phytochemicals . Nature Review Cancer , 3 , 768–780.
Takeoka, G.R. and Dao, L.T. (2003) Antioxidant constituents of almond [ Prunus dulcis (Mill.) D.A. Webb] hulls. Journal of Agricultural and Food Chemistry, 51, 496–501.
Tapsell, L.C., Gillen, L.J., Patch, C.S., Batterham, M., Owen, A., Bare, M. and Kennedy, M. (2004) Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care, 27, 2777–2783.
Thompson, G.R. and Grundy, S.M. (2005) History and development of plant sterol and sterol esters for cholesterol-lowering purposes. The American Journal of Cardiology, 96, 3S–9S.
Thompson, L.U., Boucher, B.A., Liu, Z., Cotterchio, M. and Kreiger, N. (2006) Phytoestrogen content of foods consumption in Canada, including isoflavones, lignans, and coumestan. Nutrition and Cancer, 54, 184–201.
Tokusoglu, O., Unal, M.K. and Yemis, F. (2005) Determination of the phytoalexin resveratrol (3,5,4’-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography-mass spectrometry (GC-MS). Journal of Agricultural and Food Chemistry, 53, 5003–5009.
Tomaino, A., Martorana, M., Arcoraci, T., Monteleone, D., Giovinazzo, C. and Saija, A. (2010) Antioxidant activity and phenolic profile of pistachio ( Pistacia vera L., variety Bronte) seeds and skins. Biochimie, 92, 1115–1122.
Trevisan, M.T.S., Pfundstein, B., Haubner, R., Würtele, G., Spiegelhalder, B., Bartsch, H. and Owen, R.W. (2006) Characterization of alkyl phenols in cashew ( Anacardium occidentale ) products and assay of their antioxidant capacity. Food and Chemical Toxicology, 44, 188–197.
Trox, J., Vadivel, V., Vetter, W., Stuetz, W., Kammerer, D.R., Carle, R., Scherbaum, V., Tsantili, E., Takidelli, C., Christopoulosa, M.V., Lambrineab, E., Rouskasc, D. and Roussosa, P.A. (2010) Physical, compositional and sensory differences in nuts among pistachio ( Pistachia vera L.) varieties. Scientia Horticulturae, 125, 562–568.
USDA (2009) Foreign Agricultural Service/USDA Office of Global Analysis, August, October.
USDA (2010) Foreign Agricultural Service/USDA Office of Global Analysis, February.
USDA (2010) National Nutrient Database for Standard Reference, Agricultural Research Service, U.S. Department of Agriculture, Washington, DC, Release 23, (assessed on 20 June, 2011)
Venkatachalam, M. and Sathe, S.K. (2006) Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry, 54, 4705–4714.
Wijeratne, S.S.K., Abou-Zaid, M.M. and Shahidi, F. (2006) Antioxidant polyphenols in almond and its co products. Journal of Agricultural and Food Chemistry, 54, 312–318.
Wijeratne, S.S.K., Amarowicz, R. and Shahidi, F. (2006) Antioxidant activity of almonds and their by-products in food model systems. Journal of the American Oil Chemists’ Society, 83, 223–230.
Wu, X., Beecher, G.R., Holden, J. M., Haytowitz, D.B., Gebhardt, S.E. and Prior, R.L. (2004) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. Journal of Agricultural and Food Chemistry, 52, 4026–4037.
Yang, J., Liu, R.H. and Halim, L. (2009) Antioxidant and antiproliferative activities of common edible nut seeds. LWT - Food Science and Technology, 42, 1–8.