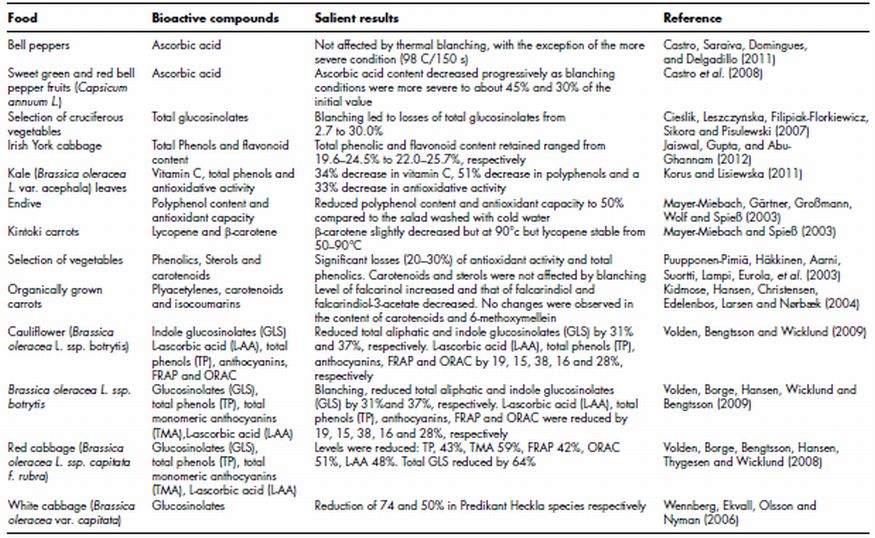
Table 11.1 Effect of blanching on levels of phytochemicals in plant foods
Whilst a wealth of new technologies (and these are reviewed elsewhere in this book) are available that can be used to render a food safe or improve organoleptic properties, the application of heat is still the most common form of processing applied to all foods. From an industrial perspective most manufacturers of plant foods employ thermal processing in some form before their foods appear on supermarket shelves. As outlined in Chapters 3 and 4 the importance of phytochemicals from plant foods has long been recognised and therefore a wealth of information exists as to how thermal processing can affect these important components. However, to keep a pace with consumer and industrial trends thermal processing techniques are continuously evolving. Therefore there is a need to keep abreast with how recent advances affect the phytochemical content of plant foods. A principle objective therefore of the present chapter is to review and critically evaluate contemporary work in this area with view to providing plant food manufacturers and researchers with a state of the art view of the area. As alluded to in many of the chapters of the ‘the handbook’ many thousands of phytochemicals have been identified and to give an overview of how thermal processing affects them all is beyond the remit of a single book chapter. Therefore I have adopted the approach of selecting five phytochemical groups as case studies these are (1) Polyphenols and anthocyanins, (2) Carotenoids, (3) Glucosinolates/ Isothiocyanates, (4) Polyacetylenes and (5) Ascorbic acid. The rationale for the selection of these groups in based on diversity of chemical and physical properties, emerging significance and depth and volume of knowledge presently available. The proceeding chapter is divided into five sections based on the nature of the thermal strategy adopted. The order of the sections is based on severity of thermal challenge starting with the least severe (blanching) and finishing with most severe (frying).
Blanching, especially of vegetables, is an essential step for maintaining the quality of the final products as it inactivates enzymes that would otherwise lead to visual and/or organoleptic deterioration of the final product. Blanching is typically carried out prior to subsequent thermal processing by immersing the vegetable in water at 90–100°C for a relatively short period of time. In general it is used in industrial process where there is a lag between processing steps that could lead to losses in quality due to enzymatic activity. Many authors have investigated the effect of blanching on the phytochemical content of plant foods and Table 11.1 lists some recent examples of studies in this area. Whilst blanching is generally considered to be a mild thermal treatment, in many cases investigators have reported that it leads to significant losses in levels of phytochemical groups. This is especially true for ascorbic acid which is generally considered to be the most thermally labile of phytonutrients present in plant foods. For example, losses of this compounds from 55% in a selection of cruciferous vegetables (Cieślik, Leszczyńska, Filipiak-Florkiewicz, Sikora and Pisulewski, 2007) to 34% in kale has been reported (Korus and Lisiewska, 2011). As outlined, losses of this nature are not surprising given the thermally labile nature of the compound and the fact that it is reasonably hydrophilic, therefore losses via leaching into the surrounding water would be expected. The severity of the blanching conditions can also affect the stability of ascorbic acid. Castro et al. (2008) reported that ascorbic acid content decreased progressively to about 45 and 30% of the initial value as the severity of blanching conditions increased from 70–98°C and 1–2.5 min. Most authors have not investigated thermal degradation products of ascorbic acid, however it is likely that products such as furfural, 2-furoic acid, 3-hydroxy-2-pyrone (Yuan and Chen, 1998) that have been reported for model solutions are formed.
Whilst glucosinolates are not themselves considered to impart health promoting properties, in most cases investigators have measured the levels of these compounds in blanched plant foods rather than their biologically active degradation products isothiocyanates. The assumption therefore is that there is a direct relationship between glucosinolate content and isothiocyante levels, which may not be the case as thermal inactivation of myrosinase, the enzyme responsible for conversion of glucosinolates to isthiocyanates, may also have occurred. Nevertheless numerous reports have indicated that glucosinolates are susceptible to losses when subjected to blanching. In two related studies Volden et al. (2008, 2009) reported losses of glucosinolates of up to 37% following blanching. Significantly in both studies the authors reported that blanching had a greater influence on glucosinolate content than the other thermal treatments examined, which included boiling and steaming. Few authors have studied the thermal degradation of glucosinolates in plant foods, however Oerlemans et al. (2006) investigated the relative thermal stability of indole and aliphatic glucosinolates in red cabbage in which myrosinase had been inactivated to eliminates its role in the degradation process. The authors concluded that following blanching at 90°C for 3 min both indole and aliphatic glucosinolates had similar predicted degradation rates. Polyphenols are also susceptible to loss following blanching either via thermal degradation or leaching. In fact in some cases higher losses of polyphenols than ascorbic acid have been reported. For example, Mayer-Miebach et al. (2003) reported that polyphenol losses of up to 50% occurred when endive was blanched at low temperatures (50–55°C, 5–10 min). As is the case for ascorbic acid more severe blanching conditions result in greater losses of polyphenols with Jaiswal et al. (2012) reporting that total phenolic and flavonoid content retention ranged from 19.6–24.5% to 22.0–25.7% in Irish York cabbage. In this case the authors did not speculate as to the thermal fate of the lost phenolics, however they did suggest that leaching was the major route to loss. Polyphenols in most cases are mildly polar and therefore will be solubilised when immersed in hot water. Interestingly however Jaiswal et al. (2012) reported that at high temperatures an increase of 7–12% in the levels of polyphenols was observed when they are expressed on a dry weight basis. This phenomenon has been reported for other phytochemicals and most authors have attributed it to a loss of soluble solids into the leaching water without a corresponding of loss of the phytochemical into the water resulting in a net increase in levels of the compound when expressed on a dry weight basis. However in most cases this has been shown to occur for hydrophobic molecules such as carotenoids and not a largely polar entity such as a polyphenol. In fact Kidmose et al. (2004) reported that levels of the hydrophobic polyacetylene falcarinol increased in blanched organically grown carrots compared to their fresh counterparts even when expressed on a fresh weight basis. However other polyacetylenes measured (Falcarindiol and falcarindiol-3-actetate) were reported to decrease significantly in blanched samples. Similar to polyacetylenes, carotenoids are hydrophobic molecules and this combined with a degree of heat stability means that most studies have concluded that carotenoids are either unaffected by blanching (Kidmose, Hansen, Christensen, Edelenbos, Larsen and Nørbæk, 2004) or decrease slightly (Mayer-Miebach and Spieß, 2003).
Table 11.1 Effect of blanching on levels of phytochemicals in plant foods

In recognition of the deleterious effect blanching can have on phytochemical content some authors have investigated the potential of other enzyme inactivation routes with a view to increasing retention of these compounds during this crucial step. Rawson et al. (2011) reported that replacing water immersion based blanching with ultrasound pre-treatment could significantly improve the retention of polyacetylenes in freeze and hot air dried carrot disks. Other alternatives to water immersion based blanching such as superheated steam and hot water spray (Sotome, Takenaka, Koseki, Ogasawara, Nadachi, Okadome, et al., 2009) are also available and could help minimise leaching based losses of phytochemicals in plant foods.
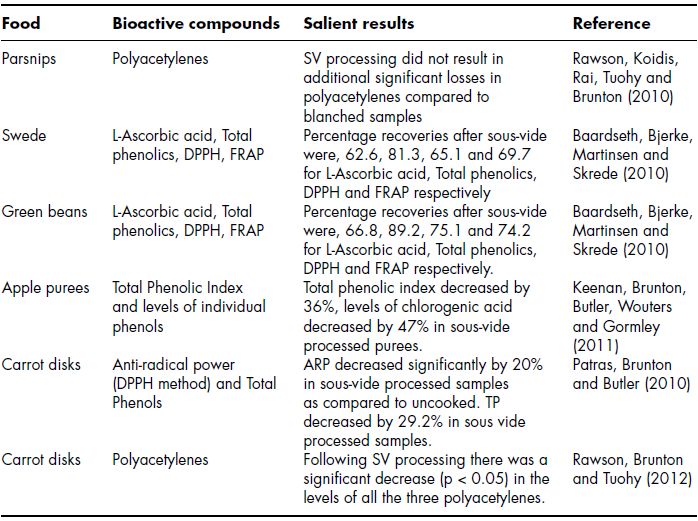
Sous vide processing, which involves thermal treatment of foods in vacuumised packs at temperatures of 90°C, is often considered a minimal processing strategy, however it has the ability to impart an appreciable shelf of up to 25 days at 4°C to a plant food. In many cases sous vide processing can deliver these shelve lives whilst having less of an effect on quality attributes such as colour, nutritional quality and flavour. In theory sous vide should have many advantages with respect to retention of phytochemicals over other methods because (1) the influence of leaching into the surrounding water is eliminated as foodstuffs are not directly in contact with water and (2) oxidatively labile compounds should be protected as foods are heated and stored under a vacuum . However, examination of Table 11.2 reveals that to date sous vide cooking has not delivered on its considerable potential for retention of phytochemicals in plant foods. Table 11.2 lists seven recently conducted studies on the effect of sous vide processing on a number of phytochemical groups and in only one case the technique was reported to have no effect on phytochemical content (Rawson, Koidis, Rai, Tuohy and Brunton, 2010). Even in the study where no effect was observed sous vide gave no additional degradation of the phytochemical studied (polyacetylenes) following blanching. In all other cases substantial reduction in levels of phytochemicals were observed. In the most severe case sous vide processing resulted in a 47% reduction in chlorogenic acid in sous vide processed apple purees (Keenan, Brunton, Butler, Wouters and Gormley, 2011). Sous vide processing has also been shown to result in significant reduction in antioxidant capacity, total phenolic content and levels of ascorbic acid in green beans and swede (Baardseth, Bjerke, Martinsen and Skrede, 2010) and carrot disks (Patras, Brunton and Butler, 2010). The reason why the use of sous vide processing has not increased retention of phytochemicals in plant foods remains unclear and in common with other thermal strategies most studies to date have concentrated on merely quantifying the effect of the method on phytochemical content rather than developing and understanding the underlying causes of the effects observed. More detailed investigation are therefore necessary which concentrate on degradation routes, hence providing recommendations for preserving phytochemicals in plant foods subjected to sous vide processing.
Table 11.2 Effect of sous vide processing on levels of phytochemicals in plant foods
Although in theory pasteurisation can apply to a food in any form, it usually refers to the application of heat to reduce viable pathogens in liquids and for the purposes of this chapter only pasteurisation of liquids will be considered. Pasteurisation is usually carried out at temperatures below boiling (although this depends on the food to which it is applied) and the purpose is to increase shelf life without having an adverse effect on the eating quality of the food. Table 11.3 lists a sample of recent studies concerned with the effect pasteurisation has on the phytochemical properties of mostly plant based beverages. A wide variety of responses to pasteurisation have been reported ranging from no change to significant decreases. It should be noted also that processing of plant based beverages usually involves other unit processes apart from pasteurisation, which can also affect phytochemical content.
Table 11.3 Effect of pasteurisaton on levels of phytochemicals in plant foods
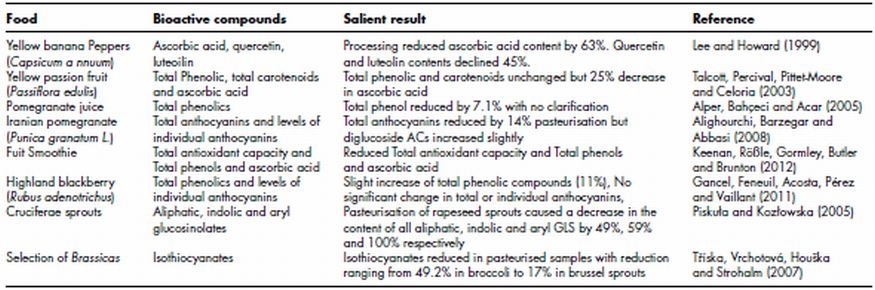
In common with other thermal strategies pasteurisation usually results in a decrease in ascorbic acid content. Table 11.3 lists a number of studies where decreases ranging from 25% (Lee and Howard, 1999) to 63% (Talcott, Brenes, Pires and Del Pozo-Insfran, 2003) have been reported. In the case of pasteurisation of beverages reductions of this nature are purely a reflection of the heat and oxidative lability of the compound as the beverage is not in direct contact with the heating medium and therefore no leaching can occur. Fruit juices are the most popular item in the plant based beverage category and some of these products are well recognised sources of ascorbic acid. Therefore losses of this compound as a result of pasteurisation could undermine the marketability of the product. Despite this no effective strategy seems to be available to limit losses as a response to pasteurisation.
As is often the case with polyphenols and anthocyanins in plant foods such a variety of responses to pasteurisation have been reported that it is difficult to come to a definite conclusion on the subject. For example, Alighourchi et al. (2008) reported that pasteurisation reduced total anthocyanins by 14% in an Iranian pomegranate juice. Alper et al. (2005) also reported that phenolic content was reduced by 7.1% in a pomegranate juice. Keenan et al. (2012) reported that pasteurisation of a fruit smoothie reduced total antioxidant capacity and total phenols. In contrast, Gancel et al. (2011) reported that there was a slight increase in total phenolic compounds (11%) and no significant change in total or individual anthocyanins. The major route to enzymatic degradation of polyphenols is of course via the action of polyphenol oxidases (PPO) and maceration of whole fruits and vegetables will place cell content in contact with this extracellular enzyme. Therefore some of the degradation reported may be due to degradation of polyphenols by PPO prior to pasteurisation. In fact Keenan et al. (2012) reported that PPO activity in a fresh fruit smoothie increased significantly in the first 10 h after preparation.
The effect of pasteurisation on levels of glucosinolates appears to be more straight forward, with most authors reporting that pasteurisation reduced levels of this phytochemical group (Piskuła and Kozłowska, 2005). Similar to polyphenol oxidase maceration of foods during the preparation of plant food beverages places the enzyme responsible for breakdown of glucosinolates in contact with its substrate, thus resulting in a reduction in glucosinolate content. However it is this breakdown product itself that is active against Phase I and Phase II enzymes. Taking glucoraphin as an example, the active breakdown product is sluphoraphane but depending on the action of epithiospecifier protein (ESP) either the active isothiocyanate sulfurophane is formed or the less active sulfurophane nitrile. When conditions are favourable for ESP activity more of the nitrile is formed. The principle is important here when discussing pasteurisation as the temperatures required to deliver this heat treatment are close to those required to inactivate ESP. Therefore some authors have shown that heat treatments at temperatures 60–70°C for 5–10 min favour formation of sulfurophane but not the nitrile (Matusheski, Juvik and Jeffery, 2004). At temperatures above 100°C (for 5–15 min) no isothiocyantes are formed as myrosinase itself is inactivated. The question therefore arises as to the heat stability of isothiocyantes themselves as many are volatile and thus susceptible to loss by evaporation. It would appear that isothiocyanate stability is mostly a function of the matrix in which it is found. For example Rose et al. (2000) reported that methylthioalkyl isothiocyanates from watercress were not present in aqueous extracts due to their volatility. However Ji et al. (2005) found that the methylthioalkyl isothiocyanate phenylethyl isothiocyanate (PEITC) was stable in aqueous buffers at pH 7.4. Thus it is possible that juices made from cruciferous vegetables could contain significant amounts of isothiocyanates providing they were made within 24 h and were refrigerated. In fact it has been reported that while isothiocyanates were reduced by levels of 17–49% in a range of cruciferous vegetables they were present in the pasteurised samples (Tříska, Vrchotová, Houška, and Strohalm, 2007).
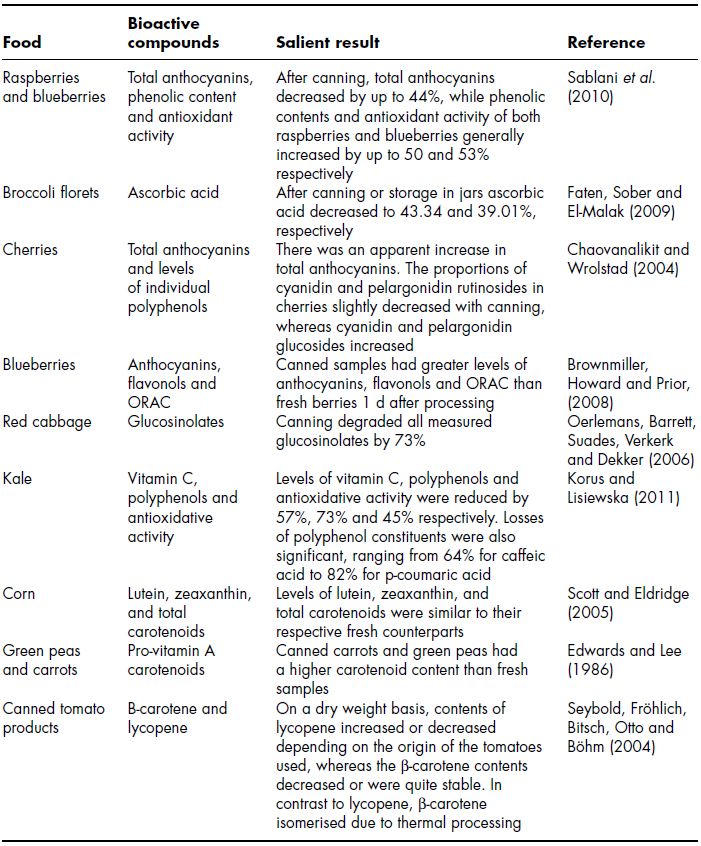
The objective of sterilisation is to render a foodstuff safe for long-term storage at ambient temperatures. Whilst consumers are demanding more fresh-like products, especially for plant foods, a significant proportion of foods are still processed to sterilisation temperatures (121°C) and then stored in brines or syrups in jars or aluminium cans. Table 11.4 lists a selection of recent studies on the effect of sterilisation on the content of a selection of phytochemicals in plant foods. Given that sterilisation could be regarded as the most severe of the heat treatments reviewed in this chapter readers may suspect that it is the most deleterious to phytochemical content. However a brief examination of Table 11.4 reveals that a range of responses have been reported ranging from severe reduction to increases to isomerisation. A particularly diverse range of responses have been reported for polyphenols and anthocyanins. Korus and Lisiewska (2011) reported that antioxidative activity was reduced in canned kale by 57, 73 and 45% respectively and that losses of polyphenol constituents were also significant, ranging from 64% for caffeic acid to 82% for p-coumaric acid. In contrast Sablani et al. (2010) reported that phenolic contents and antioxidant activity of both raspberries and blueberries generally increased by up to 50 and 53% respectively in organically grown berries. Chaovanalikit and Wrolstad (2004) also reported that there was an apparent increase in total anthocyanins in particular cyanidin and pelargonidin glucosides in canned cherries. The loss of polyphenols following a severe heat treatment such as canning is easy to rationalise given the probability for heat induced degradation and leaching, however increases in polyphenol content are less easy to understand. A number of explanations have been put forward for this phenomenon including (1) increased extraction efficiency after canning, (2) complete inactivation of PPO and (3) depolymerisation of high-molecular-weight phenolics. Explanation 2 seems the least likely explanation as it would not necessarily result in increased levels of polyphehols. However no experimental evidence has been offered for hypotheses 1 and 3 and therefore a satisfactory explanation is still not available. A variety of responses for carotenoids to sterilisation have been reported, however it is probably fair to say that in general carotenoids are reasonably resistant to sterilisation and in some cases increases have been reported (Edwards and Lee, 1986; Seybold, Fröhlich, Bitsch, Otto and Böhm, 2004). However, perhaps the most remarkable finding with regard to the effect of severe heat treatments such as sterilisation on carotenoids is that it can actually increase bio-availability. This is because heating generally favours the formation of cis-carotenoid isomers (Shi and Le Maguer, 2000; Shi, Maguer, Kakuda, Liptay and Niekamp, 1999), which are more bio-available because cis-isomers are more soluble in bile acid micelles and may be preferentially incorporated into chylomicrons (Boileau, Merchen, Wasson, Atkinson and Erdman Jr, 1999). Perhaps because glucosinolate containing vegetables are infrequently subjected to canning, few studies have examined the effect of sterilisation on glucosinolate content. However, Oerlemans et al. (2006) concluded that canning reduced glucosinolate levels in red cabbage by 73%. An earlier investigation showed a decline in available glucosinolates in canned cabbage as compared to fresh and frozen cabbage (Dekker and Verkerk, 2003).
Table 11.4 Effect of sterilisation on levels of phytochemicals in plant foods
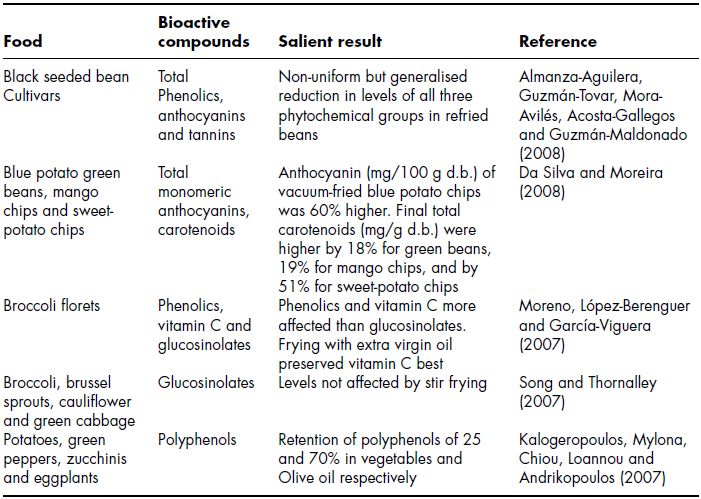
Plant foods (excluding starchy foods such as potatoes) are infrequently subjected to frying and therefore the effect of this thermal practice on phytochemicals is less well studied than other thermal processing methods. Table 11.5 summarises the limited number of recent studies with regard to the effect of frying on levels of phytochemicals in plant foods. During frying, a complex series of various chemical reactions takes place, such as thermoxidation, hydrolysis, polymerisation and fission (Fritsch, 1981). Whilst the temperature of the heat medium (oil) is much higher than in other thermal processing techniques foods are generally only subjected to this process for a relatively short period of time. It would appear however that despite this frying of plant foods causes dramatic decreases in phytochemical content. Some authors have examined the ability of modified frying techniques such as vacuum frying to reduce phytochemical loss during frying. Da Silva and Moreira (2008) compared the ability of vacuum frying to retain anthocyanins and total phenolos in a range of plant foods. Whilst the authors concluded that vacuum increased frying retention of these compounds, when compared to conventional frying retention was still very low (≥50% in most cases) in green beans, potatoes, mango and sweet potato. Other authors have also reported retention levels of this order for polyphenols in refried black beans (Almanza-Aguilera, Guzmán-Tovar, Mora-Avilés, Acosta-Gallegos and Guzmán-Maldonado, 2008) and zucchinis (courgette) and egg plants (aubergine) (Kalogeropoulos, Mylona, Chiou, Ioannou and Andrikopoulos, 2007). The influence of frying oil on polyphenol content in plant foods has also been investigated with Kalogeropoulos et al. (2007) reporting that frying in olive oil resulted in 50% greater retention of polyphenols than frying in vegetable oil. Moreno et al. (2007) also reported that frying in olive oil retained vitamin C better than frying in vegetable oil in broccoli florets. When plant food are fried, phenolic anti-oxidants are lost by steam distillation (Fritsch, 1981) and, furthermore, are consumed by reacting with lipid free radicals, originally formed by the action of oxygen on unsaturated fatty acids, to form relatively stable products which interrupt the propagation stage of oxidative chain reactions. During pan-frying the anti-oxidant loss is expected to occur to a greater extent as a result of higher surface-to-volume ratio, higher temperatures and contact with atmospheric oxygen, as the potatoes remain partly uncovered by oil. Indeed Song and Thornalley (2007) reported that glucosinolates were unaffected by stir frying in broccoli, brussel sprouts, cauliflower and green cabbage.
Table 11.5 Effect of frying on levels of phytochemicals in plant foods
Thermal processing encompasses a suite of techniques but is currently the most commonly employed method for domestic and industrial processing of plant foods. Thermal processing techniques have been shown to elicit a range of responses on phytochemical content in plant foods. It is dangerous therefore to make generalisations and recommendations as to thermal methods suitable for retention of phytochemicals as to some extent the effect is dependent on the chemical identity of the phytochemical and the matrix in which it is contained. For example, the severity of the heat process is not always reflected in the effect it has on phytochemical content. Blanching is a relatively mild thermal process, however in most cases it results in a reduction in phytochemical content. At the other end of the scale sterilisation has been shown to increase the bioavailability of carotenoids by inducing isomerisation. As stated elsewhere in the book, ascorbic acid is the most heat labile of the phytochemicals commonly encountered and in general any thermal processing results in a reduction of this compound. For the other phytochemical groups reviewed here it is not possible to come to a uniform conclusion as all have been shown to increase, decrease or be unaffected by thermal processing. A number of factors that can either decrease or increase phytochemical content appear to determine the final phytochemical content in a plant food. These include the severity of the heat process, the thermal stability of the phytochemical, the solubility of the phytochemical in the surrounding medium, the binding of the phytochemical in the food matrix and the oxidative lability of the phytochemical. Thermal processing techniques are available that can insulate plant foods from some of these effects but not all. There is scope therefore in cases where phytochemical content is severely affected by thermal processing for the use of non-thermal processing in series or in combination with conventional thermal processing. Whilst some authors have reported on the degradation pathways resulting in reductions in phytochemical content to date, most studies have concentrated on quantifying the response of the phytochemical to processing. There is therefore a need to thoroughly investigate these pathways with view to understanding the chemical and enzymatic pathways involved.
Alighourchi, H., Barzegar, M., and Abbasi, S. (2008) Anthocyanins characterization of 15 Iranian pomegranate (Punica granatum L.) varieties and their variation after cold storage and pasteurization. European Food Research and Technology, 227(3), 881–887.
Almanza-Aguilera, E., Guzmán-Tovar, I., Mora-Avilés, A., Acosta-Gallegos, J., and Guzmán-Maldonado, S. (2008) Phytochemical content of black seeded bean cultivars after cooking and frying. Annual Report-Bean Improvement Cooperative, 51, 104.
Alper, N., Bahçeci, K. S., and Acar, J. (2005) Influence of processing and pasteurization on color values and total phenolic compounds of pomegranate juice. Journal of Food Processing and Preservation, 29(5-6), 357–368.
Baardseth, P., Bjerke, F., Martinsen, B. K., and Skrede, G. (2010) Vitamin C, total phenolics and antioxidative activity in tip-cut green beans (Phaseolus vulgaris) and swede rods (Brassica napus var. napobrassica) processed by methods used in catering. Journal of the Science of Food and Agriculture, 90(7), 1245–1255.
Boileau, A. C., Merchen, N. R., Wasson, K., Atkinson, C. A., and Erdman Jr, J. W. (1999) Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. The Journal of Nutrition, 129(6), 1176–1181.
Brownmiller, C., Howard, L. and Prior, R. (2008) Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. Journal of Food Science, 73(5), H72–H79.
Castro, S. M., Saraiva, J. A., Lopes-da-Silva, J. A., Delgadillo, I., Loey, A. V., Smout, C., and Hendrickx, M. (2008) Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chemistry, 107(4), 1436–1449.
Castro, S. M., Saraiva, J. A., Domingues, F. M. J. and Delgadillo, I. (2011) Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.). LWT-Food Science and Technology, 44(2), 363–369.
Chaovanalikit, A., and Wrolstad, R. (2004) Anthocyanin and polyphenolic composition of fresh and processed cherries. Journal of Food Science, 69(1), FCT73–FCT83.
Cieślik, E., Leszczyńska, T., Filipiak-Florkiewicz, A., Sikora, E., and Pisulewski, P. M. (2007) Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chemistry, 105(3), 976–981.
Da Silva, P. F., and Moreira, R. G. (2008) Vacuum frying of high-quality fruit and vegetable-based snacks. LWT – Food Science and Technology, 41(10), 1758–1767.
Dekker, M., and Verkerk, R. (2003) Dealing with variability in food production chains: a tool to enhance the sensitivity of epidemiological studies on phytochemicals. European Journal of Nutrition, 42(1), 67–72.
Edwards, C., and Lee, C. (1986) Measurement of provitamin A carotenoids in fresh and canned carrots and green peas. Journal of Food Science, 51(2), 534–535.
Faten, B., Sober, S. and El-Malak, G. (2009) Effect of some preservation processes on the phytochemical compounds with antioxidant activities of broccoli. Arab Universities Journal of Agricultural Sciences, 17(2), 351–359.
Fritsch, C. (1981) Measurements of frying fat deterioration: A brief review. Journal of the American Oil Chemists’ Society, 58(3), 272–274.
Gancel, A.-L., Feneuil, A., Acosta, O., Pérez, A. M., and Vaillant, F. (2011) Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Research International, 44(7), 2243–2251.
Jaiswal, A. K., Gupta, S., and Abu-Ghannam, N. (2012) Kinetic evaluation of colour, texture, polyphenols and antioxidant capacity of Irish York cabbage after blanching treatment. Food Chemistry, 131(1), 63–72.
Ji, Y., Kuo, Y. and Morris, M. E. (2005) Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharmaceutical research, 22(10), 1658–1666.
Kalogeropoulos, N., Mylona, A., Chiou, A., Ioannou, M. S., and Andrikopoulos, N. K. (2007) Retention and distribution of natural antioxidants (α-tocopherol, polyphenols and terpenic acids) after shallow frying of vegetables in virgin olive oil. LWT – Food Science and Technology, 40(6), 1008–1017.
Keenan, D. F., Brunton, N., Butler, F., Wouters, R., and Gormley, R. (2011) Evaluation of thermal and high hydrostatic pressure processed apple purees enriched with prebiotic inclusions. Innovative Food Science andamp; Emerging Technologies, 12(3), 261–268.
Keenan, D. F., Rößle, C., Gormley, R., Butler, F., and Brunton, N. P. (2012) Effect of high hydrostatic pressure and thermal processing on the nutritional quality and enzyme activity of fruit smoothies. LWT – Food Science and Technology, 45(1), 50–57.
Kidmose, U., Hansen, S. L., Christensen, L. P., Edelenbos, M., Larsen, E., and Nørbæk, R. (2004) Effects of Genotype, Root Size, Storage, and Processing on Bioactive Compounds in Organically Grown Carrots (Daucus carota L.). Journal of Food Science, 69(9), S388–S394.
Korus, A., and Lisiewska, Z. (2011) Effect of preliminary processing and method of preservation on the content of selected antioxidative compounds in kale (Brassica oleracea L. var. acephala) leaves. Food Chemistry, 129(1), 149–154.
Lee, Y., and Howard, L. (1999) Firmness and Phytochemical Losses in Pasteurized Yellow Banana Peppers (Capsicum a nnuum) As Affected by Calcium Chloride and Storage. Journal of agricultural and food Chemistry, 47(2), 700–703.
Matusheski, N. V., Juvik, J. A., and Jeffery, E. H. (2004) Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry, 65(9), 1273–1281.
Mayer-Miebach, E., Gärtner, U., Großmann, B., Wolf, W., and Spieß, W. E. L. (2003) Influence of low temperature blanching on the content of valuable substances and sensory properties in ready-to-use salads. Journal of Food Engineering, 56(2–3), 215–217.
Mayer-Miebach, E., and Spieß, W. E. L. (2003) Influence of cold storage and blanching on the carotenoid content of Kintoki carrots. Journal of Food Engineering, 56(2–3), 211–213.
Moreno, D. A., López-Berenguer, C., and García-Viguera, C. (2007) Effects of Stir-Fry Cooking with Different Edible Oils on the Phytochemical Composition of Broccoli. Journal of Food Science, 72(1), S064–S068.
Oerlemans, K., Barrett, D. M., Suades, C. B., Verkerk, R., and Dekker, M. (2006). Thermal degradation of glucosinolates in red cabbage. Food Chemistry, 95(1), 19–29.
Patras, A., Brunton, N. P., and Butler, F. (2010) Effect of water immersion and sous-vide processing on antioxidant activity, phenolic, carotenoid content and color of carrot disks. Journal of Food Processing and Preservation, 34(6), 1009–1023.
Piskuła, M., and Kozłowska, H. (2005) Biologically active compounds in Cruciferae sprouts and their changes after thermal treatment. Polish Journal of Food and Nutrition Sciences, 14(4), 375–380.
Puupponen, P. R., Hakkinen, S. T., Aarni, M., Suortti, T., Lampi, A. M., Eurola, M., Piironen, V., Nuutila, A. M. and Oksman, C. K. M. (2003) Blanching and long-term freezing affect various bioactive compounds of vegetables in different ways. Journal of the Science of Food and Agriculture, 83(14), 1389–1402.
Rawson, A., Koidis, A., Rai, D. K., Tuohy, M., and Brunton, N. (2010) Influence of Sous Vide and Water Immersion Processing on Polyacetylene Content and Instrumental Color of Parsnip (Pastinaca sativa) Disks. Journal of Agricultural and Food Chemistry, 14, 58(13), 7740–7747.
Rawson, A., Tiwari, B. K., Tuohy, M. G., O’Donnell, C. P. and Brunton, N. (2011) Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrasonics Sonochemistry, 18(5), 1172–1179.
Rawson, A., Tuohy, M.G. and Brunton, N.P. (2012) An investigation of the effects of thermal and non-thermal processing methods on polyacetylenes from Apiaceae. Thesis submitted to NUIG Galway. PP 1–222.
Rose, P., Faulkner, K., Williamson, G. and Mithen, R. (2000) 7-Methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis, 21(11), 1983–1988.
Sablani, S. S., Andrews, P. K., Davies, N. M., Walters, T., Saez, H., Syamaladevi, R. M., and Mohekar, P. R. (2010) Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. Journal of the Science of Food and Agriculture, 90(5), 769–778.
Scott, C. E. and Eldridge, A. L. (2005) Comparison of carotenoid content in fresh, frozen and canned corn. Journal of Food Composition and Analysis, 18(6), 551–559.
Seybold, C., Fröhlich, K., Bitsch, R., Otto, K., and Böhm, V. (2004) Changes in Contents of Carotenoids and Vitamin E during Tomato Processing. Journal of Agricultural and Food Chemistry, 52(23), 7005–7010.
Shi, J., and Le Maguer, M. (2000) Lycopene in tomatoes: chemical and physical properties affected by food processing. Critical Reviews in Food Science and Nutrition, 40(1), 1–42.
Shi, J., Maguer, M. L., Kakuda, Y., Liptay, A., and Niekamp, F. (1999) Lycopene degradation and isomerization in tomato dehydration. Food Research International, 32(1), 15–21.
Song, L., and Thornalley, P. J. (2007) Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food and Chemical Toxicology, 45(2), 216–224.
Sotome, I., Takenaka, M., Koseki, S., Ogasawara, Y., Nadachi, Y., Okadome, H., and Isobe, S. (2009) Blanching of potato with superheated steam and hot water spray. LWT – Food Science and Technology, 42(6), 1035–1040.
Talcott, S. T., Brenes, C. H., Pires, D. M., and Del Pozo-Insfran, D. (2003) Phytochemical stability and color retention of copigmented and processed muscadine grape juice. Journal of Agricultural and Food Chemistry, 51(4), 957–963.
Tříska, J., Vrchotová, N., Houška, M., and Strohalm, J. (2007) Comparison of total isothiocyanates content in vegetable juices during high pressure treatment, pasteurization and freezing. High Pressure Research, 27(1), 147–149.
Volden, J., Bengtsson, G. B. and Wicklund, T. (2009) Glucosinolates, l-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chemistry, 112(4), 967–976.
Volden, J., Borge, G. I. A., Hansen, M., Wicklund, T. and Bengtsson, G. B. (2009) Processing (blanching, boiling, steaming) effects on the content of glucosinolates and antioxidant-related parameters in cauliflower (Brassica oleracea L. ssp. botrytis). LWT-Food Science and Technology, 42(1), 63–73.
Wennberg, M., Ekvall, J., Olsson, K., and Nyman, M. (2006) Changes in carbohydrate and glucosinolate composition in white cabbage (Brassica oleracea var. capitata) during blanching and treatment with acetic acid. Food Chemistry, 95(2), 226–236.
Yuan, J.-P., and Chen, F. (1998) Degradation of Ascorbic Acid in Aqueous Solution. Journal of Agricultural and Food Chemistry, 46(12), 5078–5082.