14 Stability of phytochemicals during grain processing
Laura Alvarez-Jubete1 and Uma Tiwari2
1 Food Science Department, School of Food Science and Environmental Health, Dublin Institute of Technology, Dublin, Ireland
2 School of Biosystems Engineering, University College Dublin, Dublin, Ireland
14.1 Introduction
Epidemiological studies have shown that whole grain consumption is associated with reduced risk of chronic diseases including cardiovascular disease and cancer (Seal, 2006; Slavin, 2004). Whole grains are a rich source of many nutrients and bioactive phytochemicals. In addition to being high in dietary fibre, resistant starch and oligosaccharides, they also contain a wide array of protective compounds such as phenolic compounds, tocopherols, tocotrienols, carotenoids, plant sterols and lignans (Slavin, 2004). Furthermore, special emphasis has been placed on the potential synergistic effect with recent evidence suggesting that the complex mixture of phytochemicals present in whole grains may be more beneficial than the addition of the individual isolated components (Liu, 2004; Liu, 2007).
Since whole grains are mostly commonly processed one way or another before consumption, it is necessary to evaluate the impact of processing on the nutritional value of grains to properly assess their importance as healthful foods (Slavin, Jacobs and Marquart, 2001). While evidence from animal models and human studies support the role of processing in enhancing the nutritive value of grains, mainly by increasing nutrient bioavailability, processing is often regarded as a negative element in nutrition, decreasing the content of important nutrients and phytochemicals (Slavin et al., 2001). Thus, it is important to identify those grain processing technologies that facilitate grain consumption and improve nutrient bioavailability while providing maximum retention of nutrients and phytochemicals.
There is a higher concentration in nutrients and phytochemicals in the outer bran and germ of the grain compared to the endosperm (Liu, 2007). Thus, milling of grains to separate the bran and the germ from the starchy endosperm to produce refined white flour causes a significant reduction in the content of nutrients and phytochemicals. This process has by far the greatest impact on the phytochemical content of grains. Also, heat processing such as baking can negatively affect the content of organic compounds such as vitamin E, carotenoids and polyphenol compounds (Alvarez-Jubete, Holse, Hansen, Arendt and Gallagher, 2009; Alvarez-Jubete, Wijngaard, Arendt and Gallagher, 2010; Leenhardt et al., 2006; Vogrincic, Timoracka, Melichacova, Vollmannova and Kreft, 2010). On the other hand, thermal processing of cereals, such as baking, can also result in the synthesis of substances with antioxidant properties, such as some Maillard reaction products in bread crust (Lindenmeier and Hofmann, 2004; Michalska, Amigo-Benavent, Zielinski and del Castillo, 2008).
This chapter presents a review of the available literature on commonly used grain processing techniques and their implications in relation with the stability and degradation of some very important grain phytochemicals. In particular, special attention will be paid to the importance of optimising process parameters to prevent or minimise losses of phytochemicals as well as adequately choosing the most adequate substrate (e.g. type of grain, grain species or variety, matrix ingredients, etc.) for each particular grain processing technique.
14.2 Germination
Germination has been used traditionally to modify the functional and nutritive properties of cereals. For instance, barley malting is a widely known controlled germination process used to produce malt for brewing purposes and food applications (Kaukovirta-Norja, Wilhelmson and Poutanen, 2004). In addition to causing a softening of the cereal kernel, germination also typically results in an increase in nutrient content and availability, and a decrease in the levels of antinutritive compounds (Kaukovirta-Norja et al., 2004). Germination of a grain starts with soaking of the grain in water which in turn leads to the resumption of metabolic activity by the grain or seed. In particular, many enzymes are synthesised to degrade macromolecules, thus leading to changes in structure as well as synthesis of compounds, some of them with potential bioactivity (Kaukovirta-Norja et al., 2004). Several important grain phytochemicals including phytates, sterols, phytoestrogens, phenolic compounds as well as antioxidative properties have been studied during the sprouting process of a variety of grains.
A great body of the information available to date on the effect of germination on grain phytochemicals is focused on the study of polyhenol compounds, and also total phenol content and total antioxidant capacity. In general, phenolic compounds have been shown to increase with germination in a number of studies. Alvarez-Jubete, Wijngaard, Arendt and Gallagher (2010) showed that sprouting resulted in an increase in the polyphenol content of the pseudocereal grains amaranth, quinoa and buckwheat. According to the authors, kaempferol and quercetin glycosides in quinoa sprouts reached 56.0 and 66.6 µmol/100 g dry weight basis compared with 36.7 and 43.4 µmol/100 g dry weight basis in quinoa grains. In the case of buckwheat, the main increases due to sprouting were reported in the levels of catechin, 3-coumaric acid and luteolin and apigenin glycosides. The increase in polyphenol content upon sprouting of the pseudocereal seeds may be attributed to the many metabolic changes that take place upon sprouting of seeds, mainly due to the activation of endogenous enzymes (Chavan and Kadam, 1989). It is also likely that germination may increase the extractability of polyphenol compounds, by releasing bound polyphenols therefore making them extractable in solvents such as methanol. Kim, Kim and Park (2004) also found that in buckwheat grains the content of two quercetin glycosides, rutin and quercetin, and that of two other unknown compounds, particularly increased as sprouting day progressed, whereas the content of chlorogenic acid was found to increase only moderately. In a subsequent study, S. Kim, Zaidul, Suzuki, Mukasa, Hashimoto, Takigawa, et al. (2008) compared the phenolic composition of common (Fagopyrum esculentum Moench) and tartary buckwheat (Fagopyrum tataricum Gaertn.) sprouts. The main phenolic compounds determined in the sprouts included chlorogenic acid, four C-glycosylflavones (orientin, isoorientin vitexin, isovitexin), rutin and quercetin. A significant increase upon germination in the quantities of phenolic compounds was noted for both buckwheat species. The main difference reported between the two buckwheat species was in the level of the important bioactive rutin. According to the authors, rutin contents in tartary buckwheat grains and sprouts were much higher than those present in common buckwheat. When comparing common buckwheat and tartary buckwheat sprouts for their levels of anthocyanins, Kim, Maeda, Sarker, Takigawa, Matsuura-Endo, Yamauchi, et al. (2007) also found significant differences in the types and amounts of anthocyanins present. In the same study, the authors highly recommended the use of a new variety/line of tartary buckwheat called Hokkai T10 for the production of sprouts rich in dietary anthocyanins, with their associated health benefits including improved cardiovascular function.
Avenanthramides, a type of phenolic compounds found only in oats, have also been shown to increase significantly upon germination (Kaukovirta-Norja et al., 2004). In their review, Kaukovirta-Norja, Wilhelmson and Poutanen (2004) also described how the content in avenanthramides of oat sprouts can be modified depending on the variety used. In particular, they highlighted the high content in avenanthramides in hull-less oat varieties, which they considered as indicative of the importance of these compounds in plant protection. In contrast, phytoestrogens such as lignans, an important group of phenolic compounds with reported beneficial biological effects, has not been shown to be influenced by germination of oats (Kaukovirta-Norja et al., 2004). Liukkonen, Katina, Wilhelmsson, Myllymaki, Lampi, Kariluoto, et al. (2003), however, noted a slight increase in the amounts of lignans for rye grain following germination. In the same study, the levels of alk(en)ylresorcinols in rye were not shown to be significantly affected by germination.
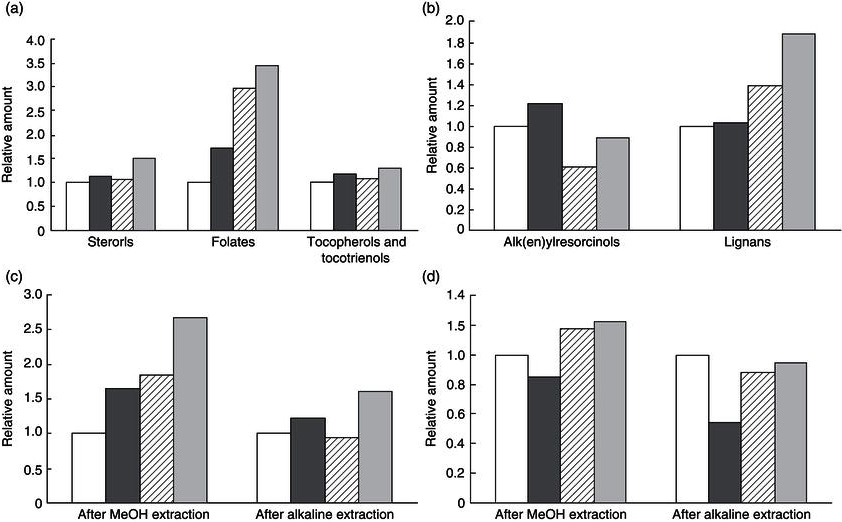
Germination also increases the levels of total phenol content and antioxidant capacity of grains. This is to be expected since germination increases the levels of phenolic compounds in grains which have demonstrated antioxidant capacity in vitro. Alvarez-Jubete, Wijngaard, Arendt and Gallagher (2010) studied the effect of germination on the antioxidative properties of the pseudocereals amaranth, quinoa and buckwheat. The authors reported that total phenol content doubled following sprouting, and quadrupled in the case of amaranth. Buckwheat sprouts showed the highest total phenol content (670.2 mg gallic acid equivalents/100 g dry weight basis), followed by quinoa (147.2 mg gallic acid equivalents/100 g dry weight basis) and amaranth (82.2 mg gallic acid equivalents/100 g dry weight basis). Accordingly, antioxidant capacity (measured by the radical DPPH scavenging capacity assay and the ferric ion reducing antioxidant power (FRAP) assay) was also reported to increase following sprouting, although interestingly, the difference was not found to be significant. Similarly, antioxidant capacity was highest in buckwheat sprouted seeds compared with amaranth and quinoa sprouts (p < 0.01). In addition to pseudocereals, oat sprouts and rye grain have also been investigated for their antioxidant capacity. In their review, Kaukovirta-Norja, Wilhelmson and Poutanen (2004) reported that studies in the area show an increase in antioxidant capacity and total phenol content of oats with germination. Also, a good correlation between antioxidant capacity and total phenol content was found, suggesting that a significant part of the antioxidant capacity may be due to phenolic compounds. In the case of rye grain, Liukkonen, et al. (2003) reported that the antioxidant capacity (DPPH radical scavenging capacity) of germinated rye grains remained practically similar to that of the native rye grains although the total phenol content of methanolic extracts of rye grain (easily extractable or free phenolics) increased notably during germination. The effect of six days germination on the bioactive compounds of rye, as previously published by Liukkonen et al. (2003), is presented in Figure 14.1.
The effect of germination on other phytochemicals in rye grain such as vitamin E compounds, folates and sterols has been shown to differ depending on the type of compound under study (Liukkonen et al., 2003) (Figure 14.1). Similarly to the results obtained in the case of polyphenol compounds, the folate content of rye grains increased significantly upon germination. Moreover, folate levels were at least tripled when germination was conducted at 15 or 25 °C. On the other hand tocopherol and tocotrienol content, as well as sterol levels, in rye grains were not found to be modified significantly by germination of rye during six days at 5, 10 or 25 °C as can be seen in Figure 14.1. In addition, the effect of germination on grain phytochemicals seems to depend not only on the particular phytochemical compound, but also on the type of grain under study. For instance, as already discussed, no effect of germination was observed on the levels of sterols in rye grain. Yet, in the case of oats, sterols were found to increase by up to 20% following germination (Kaukovirta-Norja et al., 2004). Sterols are minor lipids in plants with important biological functions and increasing their levels in foods might be of interest from a nutritional point of view. In addition, these sterols in oat sprouts were shown to be heat-stable during a drying process at different temperatures (Kaukovirta-Norja et al., 2004), which suggests that they may resist subsequent food processing conditions.
Furthermore, the germination process can be optimised to minimise losses of important compounds. An interesting example is that one of β-glucan. β-glucans are types of phytochemicals known to be negatively affected by germination as they are generally broken down during germination. However, the germination process can be optimised in terms of temperature and duration time to minimise losses of high molecular weight β-glucans in grains such as oats (Wilhelmson, Oksman-Caldentey, Laitila, Suortti, Kaukovirta-Norja and Poutanen, 2001). Since the decrease in the molecular weight of β-glucan is very slow initially during germination, a short germination schedule (72 hour, 15 °C) may be employed to produce germinated oat with higher content of retained β-glucan (55–60%) (Wilhelmson et al., 2001).
In summary, germination offers the possibility of modifying the texture and flavour of grains at the same time as modifying their content and/or availability in key phytochemicals such as folates and phenolic compounds. Most widely known germination processes are directed towards obtaining malt for brewing process, however, germinated grains can also be consumed directly, without the need of further processing, due to their characteristic softer structure (Kaukovirta-Norja et al., 2004). In addition, sprouted grains can also be used as attractive novel ingredients in the development of new food products due to their flavour, texture and nutritive properties. For instance, germinated grains can be included in a variety of cereal-based formulations such as extrusion and baking formulations for the development of new products that are appealing to the consumer, palatable and that contain increased amounts of bioactive compounds (Kaukovirta-Norja et al., 2004).
14.3 Milling
Milling of grains generally results in the removal of the bran and germ layers which are rich in fibre and phytochemical compounds, thus causing significant losses in the form of by-products such as hulls and polish waste (Tiwari and Cummins, 2009b). The milling process depends on the nature of the grain and it includes several unit operations such as cleaning, grading, tempering or conditioning, hull removal (de-hulling), pearling etc., followed by milling, to obtain various milling fractions. Research studies show that the majority of phytochemicals are concentrated in the bran and germ fractions of the grain (Adom, Sorrells and Liu, 2003; Anton, Ross, Beta, Fulcher and Arntfield, 2008; Oomah, Cardador-Martinez and Loarca-Pina, 2005; Peterson, 1995; Siebenhandl et al., 2007). Nutritional and clinical studies indicate that whole grains offer distinct advantages over refined flour due to the presence of various bioactive compounds. Similar to any other processing technique milling processing can have both desirable and undesirable effects on the bioactive compounds present in the system. Table 14.1 shows the different milling fractions of several cereals and legumes along with their phytochemical content.
Carotenoids are an important type of grain phytochemical which are located mainly in the outer layers of the grain. As a result, the bran/germ fractions of whole wheat flour contain more carotenoids, i.e. more lutein (164.1–191.7 µg/100 g), zeaxanthin (19.36–26.15 µg/100 g) and β-cryptoxanthin (8.91–10.03 µg/100 g), than the endosperm fractions (Adom, Sorrells and Liu, 2005). Also, the levels of carotenoids in flour can then be modified by the degree of milling, which is defined as the extent to which the bran layers are removed during milling. For instance, increasing the degree of milling in five different rice grain varieties resulted in decreased levels of carotenoid compounds (Lamberts and Delcour, 2008a). In particular, removal of the outer layer by approximately 5% reduces β-carotene and lutein levels by more than 50 and 20% (except for the variety Loto) respectively. Zeaxanthin levels also decreased with increasing degree of milling. Bran layer removal (DOM > 9%) further decreased the levels of carotenoids and resulted in lutein, β-carotene, and zeaxanthin levels, lower than 20 ng/g (with the exception of the Loto variety).
Tocols (tocopherols and tocotrienols) are mainly concentrated in the outer aleurone, sub-aleurone and germ of cereal grains such as barley, wheat, corn and oats (Tiwari et al., 2009b). Thus, similar to carotenoids, tocols are also affected by milling, especially during pearling or de-hulled milling (Butsat and Siriamornpun, 2010; Panfili, Fratianni, Di Criscio and Marconi, 2008). In a study by Wang, Xue, Newman and Newman (1993), the authors observed that a pearling fraction consisting of 20% of the original rye kernel weight had the highest concentrations of α-tocotrienol, α-tocopherol and total tocols compared to whole hull-less barley grain. Similar results can be found upon pearling of oats and wheat (Borrelli, De Leonardis, Platani and Troccoli, 2008; Peterson, 1994). Also, the tocol level in the final product depends on the milling methodology employed. For instance, Butsat and Siriamornpun (2010) employed different methodologies to mill rice and found significant differences in the tocol composition of the bran/germ fractions obtained using the different methodologies. In addition to the milling methodology employed, the degree of milling also plays a major role in influencing the tocol content of grains. As is to be expected, increasing the milling time decreased tocol levels in rice (Chen and Bergman, 2005).
In comparison with carotenoids and tocols, the fate of phenolic compounds during the milling process has been widely reported in the literature. In a study by Glitso and Knudsen (1999), the presence of phenolic compounds in several milling fractions of rye was analysed. Phenolic acids were reported to be concentrated mainly in the pericarp/testa (743 mg/100 g dm), with levels decreasing markedly in aleurone (201 mg/100 g dm) and endosperm fractions (19 mg/100 g dm). Similarly, Heinio, Liukkonen, Myllymaki, Pihlava, Adlercreutz, Heinonen et al. (2008) noted that total phenolic acid content in different rye milling fractions was highest in the bran fraction. In another study, Liyana-Pathirana and Shahidi (2007) evaluated the effect of milling on the total phenolic content and antioxidant capacity of two wheat cultivars namely Triticum turgidum and Triticum aestivum. Several fractions were produced: bran, shorts, flour and semolina. Among the milling fractions obtained, the lowest phenolic content was measured in the semolina fraction (9 ~ 140 µFAE/g) whereas the highest level (~2858 µFAE/g) was detected in the bran portion, thus indicating that bran (outer layers) of the grain contain higher level of phenolic compounds compared to refined flour (endosperm). Similarly, Adom, Sorrells and Liu (2005) reported that approximately 83% of the total phenolic content of wheat is located in the bran and germ. In buckwheat milling fractions, both free and bound phenolic levels were found to be nearly 30-fold higher in the outermost flour fraction of buckwheat compared to the innermost fraction (Hung and Morita, 2008). Similar observations have been reported for oat and barley milling fractions (Gray et al., 2000; Madhujith, Izydorczyk and Shahidi, 2006). Regarding legume grains, several authors have reported that the phenolic content is also affected by the milling process. In a study by Cardador-Martinez, Loarca-Pina and Oomah (2002), the total phenolic content of different dry bean (Phaseolus vulgaris) milling fractions including hull, whole and de-hulled beans was evaluated. The authors observed that the hull fraction of the bean exhibited a 37-fold greater phenolic content compared to whole bean flour or de-hulled beans. Similarly, Oomah, Cardador-Martinez and Loarca-Pina (2005) reported a higher concentration of phenolic compounds in hull compared to other milling fractions of common bean (Phaseolus vulgaris).
Table 14.1 Phytochemical content of milling fractions
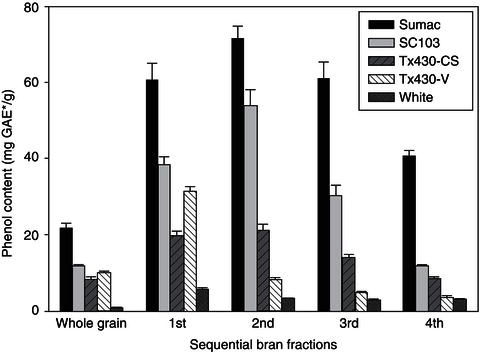
It is worth mentioning that the phenolic content of milling fractions of cereals and pulses also varies significantly depending on the milling methodology and other processing conditions (Table 14.1). For instance, during abrasive milling of grain, grains are decorticated (e.g. bran is removed by successive milling), which may lead to a reduction in the phenolic content (Awika, McDonough and Rooney, 2005; Cardador-Martinez et al., 2002; Fares, Platani, Baiano and Menga, 2010; Oomah, Caspar, Malcolmson and Bellido, 2011). During decortication of sorghum grains, Awika, McDonough and Rooney (2005) reported that the phenol concentration increased during removal of the first or second layer of bran in some cultivars. However, on repeated removal of layers, total phenolic content was reduced significantly as shown in Figure 14.2. On the other hand, de-branning of rice cultivars showed that the first bran fraction contained significantly higher level of total phenolic acids (487.6 mg ferulic acid equivalent/kg) compared to that of second, third and fourth rice bran milling fractions (327.1, 355.4 and 257.4 mg ferulic acid equivalent/kg, respectively) (Abdul-Hamid, Sulaiman, Osman and Saari, 2007).
With regards to individual phenolic compounds, ferulic acid is the most common phenolic acid present in the cell wall of cereal grains. Since this fraction is removed during the de-hulling process, the ferulic acid content of milled wheat fractions is about 50–70-fold higher in bran/germ fractions compared to endosperm fractions (Adom et al., 2005). Also in rye bran, a rich source of phenolic acids, the concentration of ferulic acid and its dehydrodimers is approximately 10–20 times higher in the bran compared to the endosperm (Andreasen, Christensen, Meyer and Hansen, 2000). In the case of barley, the predominant phenolic acid present is p-coumaric as opposed to ferulic acid as in wheat and rye (Siebenhandl et al., 2007).
Flavonoids are another group of important phenolic compounds found only in certain grains and pseudocereals. As previously described for other phenolic compounds, flavonoids are also present in the bran/germ fractions of grains (Adom et al., 2005; Hung et al., 2008; Oomah and Mazza, 1996). In the case of buckwheat, a known rich source of flavonoids, the concentration of flavonoids in buckwheat hull (~74 mg/100 g) is higher in comparison to buckwheat whole grain (18.8 mg/100 g) (Dietrych-Szostak and Oleszek, 1999). Similarly, the bran/germ fraction of wheat contributes 79% of the total flavonoid content of whole grain (Adom et al., 2005). In their study, Adom, Sorrells and Liu (2005) noted that flavonoid content of bran/germ fractions of a number of wheat varieties varied in the range 740–940 µmol of catechin equivalents/100 g and it was 10–15-fold higher compared with the flavonoid content of the respective endosperm fractions (60–80 µmol of catechin equiv/100 g of flour). Kong and Lee (2010) also noted that total flavonoid content in two black rice cultivars was lower in endosperm fractions in comparison with bran fractions.
Anthocyanins are another group of phenolic compounds which are also important pigments in cereals and pulses. These compounds are also concentrated in the outer layers of the grain. The level of anthocyanins in Blue wheat bran has been measured to be 46 mg/100 g, whereas whole wheat meal contains only about 16 mg/100 g (Abdel-Aal and Hucl, 1999). Coloured grains such as black sorghum are known to have significantly more anthocyanin pigments in comparison to other non-coloured sorghums. Thus, the bran of black sorghum is a good source of anthocyanins (4.0–9.8 mg luteolinidin equivalents/g) (Awika et al., 2005). Similarly, Kong and Lee (2010) reported that high levels of anthocyanins are found in bran milling fractions of black rice compared to refined flour (endosperm) fractions.
In summary, these studies show that during the milling process, the outer fractions of grains rich in phytochemicals such as carotenoids, tocols, phenolic acids and flavonoids, are removed. Thus, to avail of the potential health benefits associated with these health beneficial compounds, it is recommended to consume whole grains over highly refined white flours. On the other hand, the degree of milling and/or methodology employed may also be optimised depending on the grain and the phytochemical compounds of interest for maximum retention on the resultant fractions for human consumption.
14.4 Fermentation
Cereal fermentation is one of the oldest biotechnological processes used for the production of both beer and bread (Poutanen, Flander and Katina, 2009). During cereal fermentation, grains are hydrated at room temperature and both endogenous and added enzymes as well as micro-organisms including yeast and lactic acid bacteria start to modify the grain constituents (Katina et al., 2007). In bread production, fermentation is used to produce leavened dough by the leavening agent, which converts the fermentable sugars present in the dough into ethanol and CO2. The most commonly used leavening agent for industrial production of white bread is baker’s yeast, also called Saccharomyces cerevisiae. However, the use of a sourdough starter as in traditional baking such as rye bread making is being increasingly recognised (Poutanen et al., 2009). In a sourdough starter a lactic acid bacteria exists in symbiotic combination with yeasts, resulting in the production of lactic and acetic acids during fermentation, which in turn results in the modification of many important quality and nutritional parameters in comparison to yeast-based breads.
In particular, sourdough fermentation has been used traditionally to improve the flavour and structure of rye and wheat breads (Katina, Arendt, Liukkonen, Autio, Flander and Poutanen, 2005). Thus, a very important characteristic of sourdough fermentation is that it facilitates consumption of whole grains, by improving the texture and palatability of whole grain products, such as rye breads, without removing the bran and germ layers which are rich in important nutrients and phytochemicals (Katina et al., 2005). In addition, sourdough fermentation has the potential to improve important nutritional properties of grains including improved mineral bioavailability and lower glycemic index. Also, sourdough fermentation can increase or decrease levels of several bioactive compounds depending on the nature of the compound and the type of sourdough process (Katina et al., 2005). The information available on the impact of sourdough fermentation on bioactive compounds is however limited.
Liukkonen et al. (2003) studied the effect of sourdough baking on the levels of several bioactive compounds. They showed that sourdough fermentation resulted in more than double the levels of folates and total phenol content of methanol extracts. It is important to note that the observed increase in the levels of total phenols of the methanolic extracts is most likely due to an increase in the levels of free phenols, as the level of total phenol in the alkaline extracts was not modified upon fermentation. Processing can result in a release of polyphenols bound to insoluble residues which can then be extracted with solvents such as methanol (Bonoli, Verardo, Marconi and Caboni, 2004; Waldron, Parr, Ng and Ralph, 1996). The amounts of tocopherols and tocotrienols were significantly reduced, possibly due to oxidation by atmospheric oxygen. On the other hand, the levels of sterols, alk(en)ylresorcinols, lignans, phenolic acids and total phenol content of alkaline extracts changed only slightly. Sourdough fermentation also increased the antioxidant capacity (measured as DPPH radical scavenging activity) of methanol extracts, most likely due to the increased levels of easily extractable phenolic compounds following fermentation.
An increase in the amounts of folates during the fermentation phase of rye and wheat has also been reported (Kariluoto, Vahteristo, Salovaara, Katina, Liukkonen and Piironen, 2004). Interestingly, it was also found that the leavening agent was an important factor affecting the process, and that baker’s yeast contributed markedly to the final folate content by synthesising folates during fermentation (Kariluoto et al., 2004). Moreover, the synthesis of folate by yeast during fermentation can increase the final folate content by up to three-fold whereas the effect of sourdough bacteria may be negligible (Kariluoto, Liukkonen, Myllymaki, Vahteristo, Kaukovirta-Norja and Piironen, 2006).
The effect of combining fermentation with germination of rye to determine whether if these two processes have synergistic effects on bioactive compounds has also been evaluated (Katina et al., 2007). Katina et al. (2007) showed that both pre-processing of rye before fermentation (germination) and type of fermentation, had marked effects on the levels of potentially bioactive compounds of rye grain. The effect of the most effective sourdough fermentation (with S. cerevisiae) and germination on the levels of bioactive compounds in rye is presented in Figure 14.3 (Katina et al., 2007). Yeast fermentation or mixed fermentation (yeast is present as well as lactic acid bacteria) was reported to be the major factor determining the increased levels of folates, sterols, lignans, free ferulic acids and for preserving alk(en)yl-resorcinols during fermentation of both native and germinated rye. This was attributed to the considerably higher pH of yeast fermentations (pH 4.5–6.0), which may be optimum for the activity of cell wall degrading enzymes derived from the grain itself or from indigenous microbes. Also, the further increase in the levels of bioactive compounds following the use of germinated grain as raw material for fermentation was explained on the basis that germinated grain may not only provide higher amounts of fermentable sources, such as sugars and nitrogen sources, but may also provide additional cell wall degrading enzymes, synthesised during germination as well as enzyme-active microbes, which can remain active during further fermentation steps (Katina et al., 2007). Therefore, by optimising a number of bioprocessing methods, the natural bioactivity of wholemeal rye can be further improved. The resultant product can then be used as an ingredient in breads, breakfast cereals and snack foods to improve their nutritional quality (Katina et al., 2007).
Conversely, fermentation has also been shown to have an adverse effect on the content and molecular weight of β-glucans present in barley and oat flours. Degutyte-Fomins, Sontag-Strohm and Salovaara (2002) reported that fermentation of oat bran using rye sourdough starter increased the solubility and degradation of β-glucans, effects that were attributed to the activity of β-glucanase. Lambo, Oste and Nyman (2005) when studying the ability of different lactic acid bacteria to influence the content, viscosity and molecular weight of β-glucans in barley and oat concentrates found that total fibre concentrations for all samples and maximum viscosity for oat samples was decreased after fermentation. Interestingly, the authors also reported that molecular weights were not significantly affected in this study. These results may suggest that the level of acidity obtained upon sourdough fermentation, as well as the chemical composition and enzyme activity of sourdough preferment, may have a marked effect on important characteristics of β-glucans (Tiwari and Cummins, 2009a). Yeast fermentation has also been shown to reduce molecular weight of β-glucans. Andersson, Armo, Grangeon, Fredriksson, Andersson and Aman (2004) studied the effect of factors such as fermentation time on the properties of (1 → 3, 1 → 4)-β-glucan in barley and/or composite wheat flour. The average molecular weight distribution was found to decrease with increasing fermentation time, thus suggesting that (1 → 3, 1 → 4)-β-glucan was degraded by endogenous β-glucanases in the barley and/or composite wheat flour. It was thus concluded that to retain high molecular weight (1 → 3, 1 → 4)-β-glucan, fermentation time should be kept as short as possible.
In summary, sourdough fermentation has been used traditionally to improve the flavour and structure of whole grain rye and wheat breads. Also, sourdough fermentation has the potential to improve important nutritional properties of grains including mineral bioavailability and glycemic index. As described in this section, sourdough fermentation can increase or decrease levels of several bioactive compounds depending on the nature of the compound and the type of sourdough process. Some of the phytochemicals most affected by sourdough fermentation include folates, phenolic compounds (improved extractability) and β-glucans. Yeast fermentation has also been shown to significantly increase the levels of folates and decrease the molecular weight of β-glucans.
14.5 Baking
Bread making is one of the most common and ancient techniques used to process grains and their respective flours into food products for human consumption. Bread, and other related bakery products, is thus a staple in many countries across the world. Bread and bakery products therefore represent a significant portion of our daily food intake. In addition to being an excellent source of energy due to its high starch content, bread can also provide a great variety of compounds with a great potential to beneficially affect human health, such as fibre, minerals, vitamins and also bioactive compounds such as tocopherols, carotenoids, polyphenols and phytosterols. The nutritional quality of the final baked bread will ultimately depend on a number of factors. In particular, the nutritional quality of the flour or flour mix used will largely determine the final nutrient profile of the baked product. For instance, the use of whole meal flours over white or more refined flours will most likely result in breads and bakery products with a higher content of fibre, minerals, vitamins and phytochemicals. In addition, several specific parameters of the baking process such as mixing time, kneading time, fermentation time, baking time and baking temperature, which can affect the degradation of many of the heat and oxygen sensitive bioactive compounds present in the system, will also have an influence on the levels of phytochemicals present in the final baked product.
In the case of folates, the effect of baking on these compounds has partly been covered in section 14.4 on fermentation. As previously commented, fermentation results in a marked increase in the levels of folates in both rye and wheat grains (Kariluoto et al., 2004; Liukkonen et al., 2003). Baking, on the other hand, has been shown to result in a decrease in the levels of folate compounds. In particular, folate losses of approximately 25% have been reported following baking. It is important to note though that these losses were nonetheless compensated by the observed synthesis during fermentation (Kariluoto et al., 2004).
The effect of baking on β-glucans has also been partly covered in section 14.4. As already stated, fermentation was found to induce an adverse effect on the content and molecular weight of β-glucans present in flours such as barley and oats. With regards to mixing, increasing mixing time has also been shown to decrease the average molecular weight distribution of (1 → 3, 1 → 4)-β-glucans (Andersson et al., 2004). Trogh, Courtin, Andersson, Aman, Sorensen and Delcour (2004) also reported that (1 → 3, 1 → 4)-β-D-glucan was degraded during proofing possibly due to the activity of endogenous β-glucanases. They also found that molecular weight decreased further during baking, but not as rapidly as during fermentation, probably due to heat induced inactivation of β-glucanases. In a study by Flander, Salmenkallio-Marttila, Suortti and Autio (2007), the proportion of very high molecular weight β-glucan in whole meal oat bread was found to decrease during the baking process whereas the proportion of lower molecular weight β-glucan was increased. As in previous studies, the degradation of β-glucan in oat bread was attributed to the β-glucanase activity of wheat flour. These data indicate that it is important to preferentially use flours with low (1 → 3, 1 → 4)-β-D-glucan hydrolysing activities and/or to reduce processing time to obtain soluble (1 → 3, 1 → 4)-β-D-glucans of high molecular weight and viscosity that have the capacity to exert beneficial physiological effects.
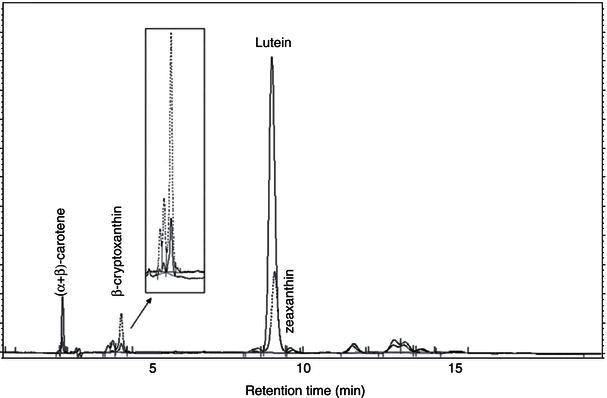
Carotenoids are antioxidant compounds which are in turn themselves susceptible to oxidation and degradation. Evaluating their stability during bread making is therefore vital to assess the potential role of baked cereal foods as sources of these important bioactive compounds. Certain parameters of the bread making process have been shown to significantly affect carotenoids stability. Of the three major steps in the bread making process, highest losses in carotenoid content have been reported to occur during dough making, followed by baking, while negligible losses have been recorded during fermentation (Leenhardt et al., 2006). One factor that is known to play a major role in carotenoid degradation during kneading is the presence of lipoxygenase (LOX), and strong positive correlations have been found between carotenoid losses and LOX activity in different wheat varieties (Leenhardt et al., 2006). For instance, carotenoid losses during kneading have been found to be significantly higher in bread wheat samples (26 and 23% for bread and water biscuit kneading, respectively) compared with einkorn wheat (9 and 6% for bread and water biscuit kneading, respectively). This effect may be attributed to a higher LOX activity in wheat bread compared to einkorn (Hidalgo, Brandolini and Pompei, 2010). Therefore, high-carotenoid wheat genotypes which also express very low LOX activities, such as einkorn wheat, may be employed for the production of high-carotenoid bread. As already mentioned, leavening or fermentation results in minimal reduction in carotenoid content. This result has been attributed to the protective effect of baker’s yeast, the leavening agent used in bread making (Hidalgo et al., 2010). Yeast consumes oxygen during dough fermentation, thereby reducing oxygen availability within the dough system and preventing LOX-mediated carotenoid degradation (Leenhardt et al., 2006). Baking, on the other hand, may strongly reduce carotenoids in bread crust, although this effect may be minimal in the case of bread crumb. According to a study by Hidalgo, Brandolini and Pompei (2010), total carotenoids degradation from flour to final product was of 28% in water biscuits, 24% in bread crumb and 55% in bread crust for bread wheat; and 32, 20 and 43%, respectively for einkorn wheat. Figure 14.4 presents a chromatogram of einkorn wheat flour and bread crust that shows apparent reductions in the levels of carotenoids following baking. Since carotenoids are exceptionally sensitive to heat, the different carotenoid losses between bread crust and crumb can be explained on the basis of the different time–temperature processing conditions of each of the respective systems (Hidalgo et al., 2010). In summary, a careful optimisation of the most crucial stages of bread making affecting the content of carotenoids can result in the production of breads with a higher carotenoid content, which may be desirable for nutrition, and also to improve the organoleptic profile of the baked product. In particular, a reduction in kneading time and intensity may decrease carotenoids loss by limiting oxygen incorporation and thus limiting degradation of carotenoids during bread making. Also, the use of grain genotypes with high content in carotenoids which also express low lipoxygenase activity is recommended.
Similarly to carotenoid compounds, vitamin E compounds are antioxidant compounds which are in turn themselves susceptible to oxidation and degradation. Factors such as light, oxygenation and heat all normally present during bread making can thus accelerate vitamin E destruction (Leenhardt et al., 2006). The degradation of tocols during bread making can therefore be significantly modulated by modifying different parameters of the bread making process such as kneading time (Leenhardt et al., 2006). However, contrary to carotenoids, tocols do not seem to be degraded by LOX-catalysed oxidation. Despite large varietal differences for LOX activity (whole grain flours of einkorn, durum wheat and bread wheat), no varietal differences were reported for vitamin E activity losses during kneading (Leenhardt et al., 2006). Also, no significant differences in vitamin E losses after bread making have been recorded between three wheat species (einkorn, durum wheat and bread wheat), with loss values in all cases below 30%. Wennermark and Jagerstad (1992) had also previously reported vitamin E losses following wheat bread making of 20–40% depending on the bread making method. It was concluded that tocol losses during bread making could thus be attributed to direct oxygenation during dough making and heat destruction during baking (Leenhardt et al., 2006).
Several other factors such as the initial total content of tocols, initial tocol profile and occurrence of antioxidant compounds other than tocols (such as flavonoids) in the initial dough systems have also been shown to significantly affect the final tocol content of the baked bread. The stability of tocols in gluten-free bread systems has also been evaluated. In a study by Alvarez-Jubete, Holse, Hansen, Arendt and Gallagher (2009), the tocopherol content of amaranth, quinoa and buckwheat as affected by baking was examined. The vitamin E losses for gluten-free control, amaranth and buckwheat breads were ≈ 30%, whereas in quinoa bread losses were 13.6%. Also prepared in this study were 100% pseudobreads (100% Q and 100% B breads) and vitamin E losses were lower compared with the respective 50% counterparts (50% Q and 50% B breads). Lowest losses amongst all of the breads studied were recorded for the 100% quinoa bread (7.5%). The authors found a significant negative correlation (R2 = −0.84) between the initial vitamin E content of breads (calculated as if no loss had occurred) and the degree of loss. Thus indicating that, the higher the initial content of vitamin E, the lower the degree of loss (%) obtained. In addition, significant negative correlations were also observed between the degree of vitamin E loss (%) and α-, and γ-tocopherol initial content (R2 = −0.79 and –0.84, respectively), whereas no correlations were obtained for the other tocol compounds. The authors concluded that the observed variation in vitamin E recovery for the different types of breads studied could be partly explained on the basis of differences in the initial α- and γ-tocopherol content, thus suggesting that, in addition to α-tocopherol, γ-tocopherol activity as an antioxidant is also important in these systems. According to Alvarez-Jubete, Holse, Hansen, Arendt and Gallagher (2009), another likely factor responsible for some of the variation observed in the degree of loss among the different grain samples was the presence of compounds with antioxidant capacity other than tocols, such as polyphenol compounds. The presence of flavonoid compounds in quinoa and buckwheat grains, mainly kaempferol and quercetin glycosides in quinoa, and quercetin glycosides and catechins in buckwheat have been reported previously (Alvarez-Jubete et al., 2010; Dietrych-Szostak et al., 1999; Dini, Tenore and Dini, 2004; Watanabe, 1998). Flavonoid compounds spare liposoluble antioxidants such as vitamin E from oxidation when present in the same sample matrix (Scalbert, Manach, Morand, Remesy and Jimenez, 2005).
The effect of rye flour extraction rate on the tocopherol and tocotrienol content of rye breads made by a classical sourdough fermentation method has also been investigated in a recent study by Michalska, Ceglinska, Amarowicz, Piskula, Szawara-Nowak and Zielinski (2007). In this study, the total tocopherol content (α-T, β-T, γ-T, and δ-T) in rye breads varied from 3.42 to 1.19 µg/g dry matter depending on the extraction rate of the flour. Similarly, total tocotrienol content varied from 3.38 to 0.38 µg/g dry matter. The main tocopherol in rye breads was α-T with α-T3 and β-T3 as the main tocotrienols. As expected, the highest levels of tocopherols and tocotrienols were found in bread with a flour extraction rate of 100%. The authors of this study also noted a considerable loss of these bioactive compounds during the baking process, with the content in flours being approximately three-fold higher compared to the respective baked breads. In addition, the total tocol content of the rye breads was also compared to commercial wheat bread roll. The authors found that only the rye bread made with a flour extraction rate of 100% had a similar content of tocopherols and tocotrienols compared to wheat bread whereas the rest of the rye breads (95, 90 and 70% extraction rate) showed lower contents of these compounds. The authors attributed this result to the distinctive bread making process involved in the preparation of the rye breads. The longer fermentation time associated with the sourdough method in which the rye flour is mixed with water and allowed to ferment is most likely the factor responsible for the greater loss of these sensitive bioactive compounds in the sourdough rye breads compared to commercial wheat bread. These results are in agreement with a previous study by Liukkonen et al. (2003), where the authors also reported a significant loss in vitamin E compounds during rye sourdough bread making. Similarly to Michalska, Ceglinska, Amarowicz, Piskula, Szawara-Nowak and Zielinski (2007), Liukkonen et al. (2003) also identified sourdough fermentation as the main step for the degradation of tocopherols and tocotrienols in rye sourdough baking.
A great amount of the research conducted on the effect of baking on grain phytochemicals has focused on the study of phenolic compounds. Grains are known to be moderate sources of polyphenol compounds such as phenolic acids and flavonoids (Manach, Scalbert, Morand, Remesy and Jimenez, 2004). Polyphenol compounds have attracted much attention over the past decade as their intake has been associated with decreased risk of diseases related to oxidative stress such as cancer and cardiovascular disease (Scalbert et al., 2005). However, polyphenol compounds, and in particular flavonoid compounds, have been shown in a number of studies to be heat sensitive (Dietrych-Szostak et al., 1999; Im, Huff and Hsieh, 2003; Kreft, Fabjan and Yasumoto, 2006), and may thus be negatively affected during thermal processing such as baking. The extent of polyphenol loss will be mainly determined by the type of substrate and extraction rate, and also by the processing conditions of the baking process.
For instance, rye flour extraction rate has been shown to affect the phenolic acid profile of rye breads made by sourdough fermentation (Michalska et al., 2007). The main phenolic acid compounds present in rye breads were caffeic, ferulic, p-coumaric and sinapic acids, with ferulic and sinapic acids as the predominant compounds. As expected, rye bread based on lower extraction rate flours showed the lowest contents of phenolic acids (Michalska et al., 2007). Also, the stability of phenolic acids and flavonoid compounds during the bread making process has been assessed in grains such as rye, and also in certain pseudocereals. The change in the content and composition of phenolic acids and ferulic acid dehydrodimers during the rye bread making process was evaluated in a study by Boskov Hansen, Andreasen, Nielsen, Larsen, Bach Knudsen, Meyer et al. (2002). The most predominant phenolic compounds found were ferulic acid and ferulic acid dehydrodimers. The total amount of free and ester-bound phenolic acids and ferulic acid dehydrodimers was slightly lower in the processed samples compared to that in whole meal flour (1575 µg/g and 1472 µg/g in the whole meal and bread crumb respectively). Interestingly, it was reported that only dough mixing resulted in a significant decrease in the content of ferulic acid whereas no significant changes in any of the phenolic compounds present were recorded during dough mixing, dough proofing or baking (Boskov Hansen et al., 2002). Liukkonen et al. (2003) also studied the effect of sourdough baking on the levels of phenolic acids in rye with similar results. The fermentation phase changed very slightly the levels of lignans, alkenylresorcinols and phenolic acids. Also, during baking changes in these compounds were found to be neglible. Therefore, the phenolic acid content in the final baked bread was the same, or slightly higher, than that measured in whole meal rye flour (Liukkonen et al., 2003).
The stability of phenolic acids and flavonoid compounds in the pseudocereals amaranth, quinoa and buckwheat grains during bread making has also been evaluated (Alvarez-Jubete et al., 2010). Polyphenol content was generally found to be reduced in the bread samples when compared to the original grains. In particular, quercetin and kaempferol glycosides content in 100% quinoa breads was 17.1 and 19.2 µmol/100 g, compared with 43.4 and 36.7 µmol/100 g in quinoa seeds. In the case of buckwheat, quercetin glycosides content decreased significantly with bread making, resulting in an increase in quercetin content through hydrolysis. Also, the phenolic acids present in quinoa and buckwheat grains were degraded during baking and their content decreased significantly from grain to bread. However, despite the negative impact of baking on the polyphenol content of pseudocereals, the breads made using quinoa and buckwheat flour still contained flavonoids in significant quantities.
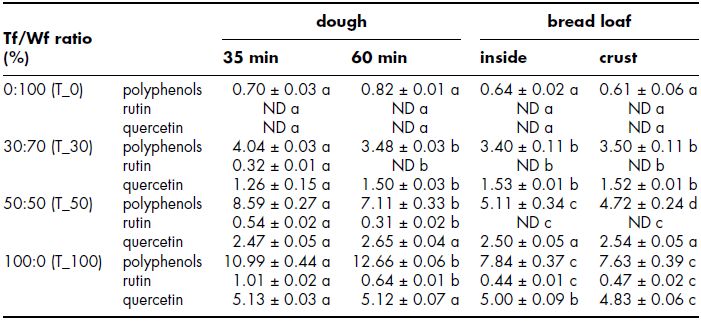
Similarly, Vogrincic, Timoracka, Melichacova, Vollmannova and Kreft (2010) studied the impact of bread making and baking procedure on rutin and quercetin content of tartary buckwheat (Fagopyrum tataricum) bread and breads made of mixtures of tartary buckwheat and wheat flour. The total phenol, rutin and quercetin levels in dough and bread loaves prepared using different levels of buckwheat and wheat flours are summarised in Table 14.2. As expected, rutin and quercetin concentrations increased with increasing percentage of tartary buckwheat flour as these compounds were not present in wheat flour. In agreement with the results by Alvarez-Jubete, Wijngaard, Arendt and Gallagher (2010), rutin content was reported to decrease during the bread making process, whereas quercetin content increased as a result of rutin hydrolysis. In particular, 85% of rutin was transformed into quercetin during dough mixing. The sole addition of water and yeast seem to have facilitated the degradation of rutin into quercetin, possibly by the rutin degrading enzymes naturally present in the flour. The concentration of rutin continued to decrease during proofing and its concentration in dough after 60 min of rising was significantly lower compared to that measured at 35 min of rising. Quercetin content, on the other hand, remained more stable during proofing and no significant changes were observed, except for the dough containing 30% tartary buckwheat flour which experienced a significant increase in quercetin content after 60 min in comparison to 35 min. During baking rutin continued to decrease and, as a result, rutin was only detectable in bread made of 100% tartary buckwheat flour. Similar to what happened during proofing, quercetin content remained constant during baking. Finally, no significant differences were detected in both rutin and quercetin concentrations between crust and crumb of the bread (Vogrincic et al., 2010).
Table 14.2 Total phenol, rutin and quercetin in dough and bread loaves using different levels of tartary buckwheat and wheat flours. Reprinted with permission from Journal of Agricultural and Food Chemistry, 58(8). Vogrincic et al. (2010). Degradation of Rutin and Polyphenols during the preparation of Tartary Buckwheat Bread, 4883–4887. Copyright American Chemical Society.
As the in vitro antioxidant capacity of a sample is derived from its content in antioxidant compounds, baked products formulated with flours rich in antioxidant compounds such as phenolic compounds will consequently be characterised by high in vitro antioxidant capacities. In addition, the use of high extraction rate flours will also generally result in higher antioxidant capacity compared to those products formulated with lower extraction rate flours. Also, thermal processing techniques that can cause a degradation of antioxidant compounds, such as baking, will most likely result in final baked products with decreased antioxidant capacity. The effect of flour extraction rate on the antioxidative properties of traditional rye bread was studied by Michalska, Ceglinska, Amarowicz, Piskula, Szawara-Nowak and Zielinski (2007). As expected, those breads formulated using rye flours with extraction rates of 100–90% were found to have the highest total phenol content when compared to bread made using flour of 70% extraction rate. Moreover, the content of total phenols was about two- to three-fold higher when compared to standard wheat roll. When examined for their free radical scavenging activity against ABTS·+ cation radical, 80% methanol extracts of rye breads formulated on flours with extraction rates of 100–90% had the highest scavenging activity in comparison to the free radical scavenging activity of trolox. In particular, trolox equivalent antioxidant capacity (TEAC) of rye bread formulated on flour with an extraction rate of 70% was approximately 40% lower when compared to that of whole meal rye bread. Also, TEAC for methanol extracts of rye bread were almost three-fold higher when compared to TEAC of wheat roll. When the DPPH scavenging capacity of the rye breads was studied, it was found that breads formulated on flours with extraction rates ranging 100–90% showed about 25% higher radical scavenging activity when compared to bread formulated on flour with a extraction rate of 70%. The lowest radical DPPH scavenging activity was noted for wheat roll which was decreased by approximately 60% when compared to whole meal rye bread.
Liukkonen et al. (2003) studied the effect of sourdough baking on the total phenolic content and antioxidant activity (measured as DPPH radical scavenging capacity) of rye. The sourdough fermentation phase more than doubled the amount of total phenol content measured in methanolic extracts (easily extractable phenolic compounds), most likely due to release of bound phenolics following fermentation. However, this effect was slightly diluted with the addition of fresh ‘unfermented’ whole meal flour to the sourdough. Accordingly, an increase in antioxidant activity was also detected following the sourdough fermentation phase, probably due to the increase in the amount of total phenol content upon fermentation. Baking resulted in a slight increase in the total phenol content of alkaline-extractable phenolic compounds (bound phenolic compounds) whereas a slight decrease was observed in the methanolic fraction. Thus, in comparison to rye whole meal flour, rye sourdough breads had similar total phenol content, which indicates that these compounds are stable during rye sourdough baking. Also, antioxidant capacity of the rye breads was similar to that of the whole meal rye flour.
Another grain that has been extensively studied because of its antioxidative properties is the pseudocereal buckwheat. The antioxidative properties of buckwheat are derived mainly from its high content in phenolic acids and flavonoid compounds. The effect of bread making on the antioxidant properties of methanolic extracts from buckwheat, as well as amaranth and quinoa, were evaluated in a study by Alvarez-Jubete, Wijngaard, Arendt and Gallagher (2010). It was found that gluten-free breads containing 50% of pseudocereal flour had significantly higher total phenol content and antioxidant capacity compared to a gluten-free control based on rice flour and potato starch. Highest values were found in breads containing buckwheat. In addition, when breads were made of 100% quinoa or 100% buckwheat flour, total phenol content and higher antioxidant capacity increased significantly in comparison to those breads containing only 50% of pseudocereal flour. Regarding stability of these properties during processing, following a comparison of the measured total phenol content in breads with the expected values (calculated using the approximation that the pseudocereal flour is the only ingredient contributing to total phenol content in bread) the authors concluded that some degradation may have occurred. This effect was reported to be particularly pronounced in the case of buckwheat, where total phenol content reduction from buckwheat seeds to buckwheat bread was 323–64.5 mg Gallic acid equivalents/100 g dry weight basis. Degradation of antioxidant compounds during quinoa bread making appears to have occurred also, however, to a smaller extent. Despite the loss of total phenol content and antioxidant activity following bread making, all of the breads containing pseudocereals showed significantly higher antioxidant capacity when compared with the gluten-free control. Vogrincic, Timoracka, Melichacova, Vollmannova and Kreft (2010) also evaluated the impact of bread making on the antioxidant activity of tartary buckwheat (Fagopyrum tataricum) with similar results. Several doughs and breads were produced containing 0, 30, 50 and 100% tartary buckwheat flour respectively, with wheat flour as a composite. As expected, for both dough and breads, total phenol content was found to rise when the percentage of tartary buckwheat flour used increased. Also, total phenol content was found to be reduced by the bread making process. In general, it was found that baking was the bread making step that caused a greater effect on the total phenol content, whereas proofing had a slight effect, increasing the level in some cases and decreasing it in others. Interestingly, total phenol content in crust and crumb did not differ greatly. Antioxidant activity also increased in buckwheat dough and breads with a growing percentage of tartary buckwheat flour used. In particular, antioxidant activities of breads containing 0, 30, 50 and 100% of tartary buckwheat flour were approximately 4, 35, 55 and 85%, respectively. It was also found that antioxidant activity remained stable or decreased slightly during the bread making process in those systems containing buckwheat. In the case of the 100% wheat bread, DPPH scavenging activity was found to slightly increase during the bread making process, possibly due to the formation of maillard reaction products.
Maillard reaction products are formed during baking predominantly on bread crust as a result of a reaction between proteins and carbonyl groups of reducing sugars or other food components. Maillard reaction products have been typically considered as having a detrimental effect on health. However, a number of studies have demonstrated that some of these compounds also possess antioxidant properties and may thus affect the final antioxidative properties of baked products, especially in the crust.
Michalska, Ceglinska, Amarowicz, Piskula, Szawara-Nowak and Zielinski (2007) evaluated the effect of bread making on the formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. They showed that changes due to Maillard reaction affected bread crust principally. They also demonstrated that advanced Maillard reaction products (MRPs) resulted in good scavengers of peroxyl and ABTS radicals whereas early MRPs seemed to be correlated with antioxidant activity. They thus concluded that baking favoured the formation of some antioxidant compounds. Previously, Lindenmeier and Hofmann (2004) had also reported on the influence of baking conditions and precursor supplementation on the amounts of the antioxidant pronyl-L-lysine in bakery products. Although this antioxidant was not present in untreated flour, high amounts of pronyl-L-lysine were detected in bread crust, whereas only low amounts were present in the crumb. Interestingly, the amounts of pronyl-L lysine were found to be greatly affected by important parameters of the baking process such as baking time and temperature. In particular, increasing the baking time from 70 to 210 min or increasing the baking temperature from 220 to 260 °C led to a five- or three-fold increase, respectively, in the level of this antioxidant in the crust. Also, the type of ingredients used in the formulation was found to have a major influence on the synthesis of pronyl-L-lysine. For instance, substituting 5% of the flour with lysine-rich protein casein or with 10% of glucose increased the levels of the antioxidant by more than 200%. Finally, following quantitative analyses of commercial bread samples collected from German bakeries they also found that that the decrease of the pH value associated with sourdough fermentation resulted in the production of high amounts of pronyl-L-lysine in baking products. In summary, the authors concluded that the amounts of the antioxidant and chemopreventive compound pronyl-L-lysine in bakery products can be strongly influenced by adjusting both baking parameters and formulation.
In summary, it is possible to formulate baked products with a significant content in phytochemical compounds. However, due to the labile nature of some of these compounds, processing such as baking can lead to a reduction on the concentration of these bioactive compounds. β-glucans, carotenoids, tocols and flavonoids have been shown to be significantly reduced following baking. Thus, it is important that the initial level of phytochemical compounds in the flours is sufficiently high so that the content in the final baked product after processing remains adequate. To this end, the use of flours with a high extraction rate is recommended. In addition, the optimisation of the different baking steps, such as mixing and fermentation times, as well as opting for the most adequate grain variety, may also result in a final baked product with high levels of phytochemicals.
14.6 Roasting
Extensive heat treatment has been shown to cause degradation of heat-labile compounds such as flavonoids. As with most other heat processing techniques, the extent of degradation of phytochemical compounds will mostly depend on the intensity of the process, mainly length and temperature, as well as on the substrate under study.
Roasting has been employed in research studies to evaluate the effect of high temperatures on properties of grains such as total antioxidant capacity and total phenol content. Sensoy, Rosen, Ho and Karwe (2006) evaluated the effect of roasting on total phenolic content and antioxidant capacity of buckwheat. They reported that roasting white or dark buckwheat flour at 200 °C for 10 min did not affect the total phenol content, as measured using the Folin-Ciocalteu assay. On the other hand, a slight decrease in antioxidant capacity, measured using the DPPH radical scavenging assay, was recorded. The effect of roasting temperature and length on buckwheat total phenol content, total flavonoid content and antioxidant capacity was also studied by Zhang, Chen, Li, Pei and Liang (2010). The content of total flavonoids was found to decrease significantly (p < 0.05) with the increase of the roasting temperature from 80 °C to 120 °C and the roasting time from 20 min to 40 min. However, for total phenolics the only significant differences were found when the intensity of the roasting treatment was highest, 120 °C for 40 min, indicating that flavonoid compounds are more instable during intense heat treatment. In particular, when the highest intensity treatment was applied, 120 °C for 40 min, total flavonoids and total phenolics content were noted to decrease by 33 and 9% respectively in comparison with untreated tartary buckwheat flour. The raw tartary buckwheat extracts had high antioxidant properties with scavenging rates of 93.13% on hydroxyl radicals, 92.64% on superoxide radicals and the inhibitory rate of 34.28% on lipid peroxidation. However, antioxidant capacity decreased significantly (p < 0.05) upon roasting. The trends observed for the decrease in antioxidant activities were in accordance with those observed for total flavonoids and total phenolics (r = 0.8401 and 0.9909, respectively). Since a higher correlation was obtained between antioxidant activity and total phenol content, the authors concluded that phenolics were the main antioxidant compounds in tartary buckwheat. In the same study, the authors also evaluated the effect of microwave roasting (700 W for 10 min). Similar results were obtained, with total flavonoids being decreased to a higher extent than total phenolics. Also, antioxidant activity based on the scavenging of hydroxyl and superoxide anion radicals, and inhibition of liposome peroxidation, were decreased after microwave roasting of tartary buckwheat flour. The authors also suggested that the less severe reduction in total phenolics compared to total flavonoids upon thermal treatment could be attributed to the formation of Maillard reaction product. These Maillard reaction products may have reacted with Fiolin-Ciocalteau reagent masking the real decrease in total phenolics.
In another study, microwave oven roasting conditions were optimised to obtain barley grains with high antioxidant activity, measured as the ability to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical and total phenol content according to Folin-Ciocalteu assay (Omwamba and Hu, 2010). Three processing factors were optimised in this study, temperature, time and amount of grain. All three factors under study were found to influence antioxidant activity both individually and interactively. The optimum condition for obtaining roasted barley with high antioxidant activity was found to be at 600 W microwave power, 8.5 min roasting time, and 61.5 g or two layers of grains.
Since heat treatments affect negatively certain phytochemicals, such as phenolic compounds, it is important to optimise thermal processing parameters to prevent or minimise losses of these compounds and to deliver a final food product with an adequate content in phytochemical compounds.
14.7 Extrusion cooking
Extrusion cooking is a very important food processing technology for cereal foods. It is used for the production of breakfast cereals, ready-to-eat foods, baby foods, snack foods, texturised vegetable protein, pet foods, dried soups and dry beverage mixes. In addition to improving digestibility, extrusion cooking of food grains also improves bioavailability of nutrients in comparison to conventional cooking. As discussed in Chapter 6, the presence of phytochemicals in grains is important because of their associated potential health benefits. In addition, phytochemicals in grains may help to prevent lipid oxidation, improving shelf life and consumer acceptance of extruded snacks, by acting as free radical terminators, chelators of metal catalysts, and singlet oxygen quenchers. For example, Viscidi, Dougherty, Briggs and Camire (2004) observed that the addition of ferulic acid and benzoin at levels of 1.0 g/kg or higher generally resulted in delayed onset of oxidation in oat based extrudates.
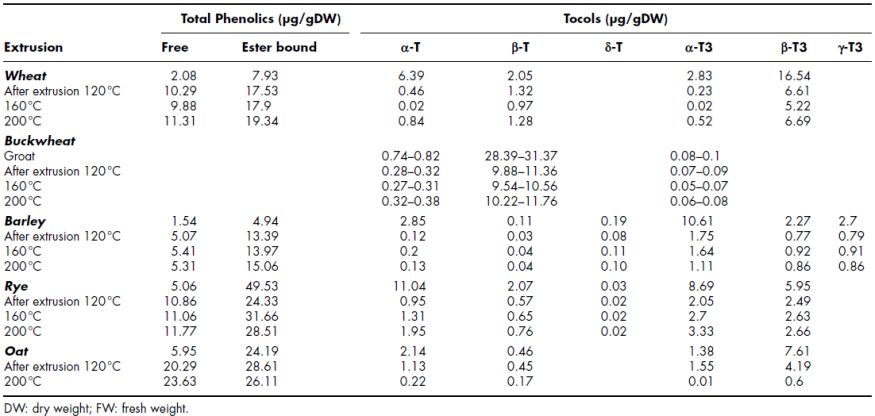
An important quantity of bioactive compounds is lost during extrusion processing as these compounds are sensitive towards a number of processing variables. Critical extrusion process variables such as temperature, screw speed and moisture content may induce desirable modifications, improving palatability and technological properties of extruded products. However, these conditions may also have positive or negative influence on the bioactive compounds of the extrudates. Several studies have shown that extrusion processing significantly reduces measurable bioactive compounds in food products. Table 14.3 summarises some of the data available on the effect of extrusion on the phytochemical content of some cereals and pseudocereals.
Vitamin E compounds (tocopherols and tocotrienols) are known to be affected by the extrusion cooking process (Tiwari et al., 2009b). It has been hypothesised that the stability of tocols may be negatively affected by high temperature during extrusion cooking (Shin, Godber, Martin and Wells, 1997). Zielinski, Kozlowska and Lewczuk (2001) reported a decrease of 30% in tocopherol and tocotrienols in cereals including oat, barley, wheat, rye and buckwheat following extrusion.
The fate of phenolic compounds during the extrusion process of legume flours has also been assessed. Anton, Fulcher and Arntfield (2009) evaluated the replacement of corn flour in corn-based extruded snacks with several common bean (Phaseolus vulgaris) flours at different levels to produce extruded puffed snacks. Total phenol content was found to decrease significantly (up to 10 and 70% for navy bean and small red bean, respectively) when the extrusion cooking temperature was set at 160 °C. In a study using legume flour exclusively, Abd El-Hady and Habiba (2003) evaluated the effect of extrusion process variables such as barrel temperature (140–180 °C) and feed moisture (18 and 22%) on total phenol content of whole meal of peas, chickpeas, faba and kidney beans. They noted a significant decrease in total phenol content of extruded products, effect which was mainly attributed to the individual effects of both temperature and moisture as no interaction effect was noted for feed moisture and barrel temperature.
Table 14.3 The effect of extrusion on the phytochemical content of some cereals
Source: Zieliński et al. (2001); Zieliński et al. (2006)
In cereal grains, Zielinski, Kozlowska and Lewczuk (Zielinski et al., 2001) reported a significant loss of phenolic acids in wheat, barley, rye and oat grains following extrusion cooking at temperatures of 120–200 °C. In a subsequent study, H. Zielinski, Michalska, Piskula and Kozlowska (2006) examined the effect of processing temperature on the extrusion cooking of buckwheat groats and reported a significant decrease in the total phenolic compounds from 4.08 mg/g dry matter (groats) to 1.17, 0.83 and 1.41 mg/g dm for extrudates at 120, 160 and 200 °C, respectively. However, a significant increase in some free/bound phenolic acids such as syringic, ferulic and coumaric acids during extrusion of buckwheat (Fagopyrum esculentum) was also noted in the same study (Table 14.3). The increase in these phenolic compounds was attributed to an increased release of these bioactive compounds from the matrix following extrusion (Zielinski et al., 2006). Yagci and Gogus (2009) also showed an increase of about three-fold in total phenolic content during extrusion cooking of rice-based snacks.
The effect of extrusion cooking on the total phenolic content of grains has also been shown to be cultivar specific according to Korus, Gumul and Czechowska (2007). In their study, Korus, Gumul and Czechowska (2007) studied the effect of extrusion processing on several polyphenol compounds such as myrecetin, quercetin, kaempferol, cyanidin, chlorogenic acid, caffeic acid, ferulic acid and p-coumaric acid. An increase of 14% in the amount of phenolics in dark-red bean extrudates compared to raw flour was noted, whereas in black-brown and cream coloured beans a decrease of 19 and 21%, respectively, was observed. The authors noted that the overall increase observed in dark-red beans during extrusion was mainly brought about by increases in quercetin (by 84%) and ferulic acid (by 40%) along with significant decreases in chlorogenic and caffeic acids by 33 and 9% respectively.
Extrusion cooking of grains has also been reported to have positive or negative effects on anthocyanin content depending on processing conditions and feed characteristics. Significant losses have been reported for anthocyanins during extrusion cooking, losses which have been mainly attributed to the high temperature employed in the process. White, Howard and Prior (2010) investigated the changes in the anthocyanin, flavonol and procyanidin contents of cranberry pomace/corn starch blends during extrusion cooking. They observed significant losses (46–64%) in the anthocyanin content of the extrudates. Furthermore, these losses were found to increase with barrel temperature. Anthocyanin losses of low magnitudes (10%) have also been reported for extrusion cooking of blueberry, cranberry, raspberry, grape powders and corn blends (Camire, Dougherty and Briggs, 2007). Camire, Chaovanalikit, Dougherty and Briggs (2002) observed that, despite losses during extrusion, a sufficient amount of the colorants remained after extrusion, and that a purple colour corn meal extruded product could be obtained following extrusion of corn with blueberry and grape. Notwithstanding the reported decrease in anthocyanins following extrusion, a considerable increase in biologically important monomer and dimer forms of procyanidin has also been reported (Khanal, Howard and Prior, 2009). Khanal, Howard and Prior (2009) observed a considerable increase in monomer and dimer contents, most probably due to the conversion of some higher-level oligomers and polymers into lower oligomer during extrusion. In the same study, the monomer content of grape pomace extruded at barrel temperature of 170 °C and screw speed of 200 rpm increased by approximately 120%, whereas total anthocyanin content was found to decrease significantly (18–53%). Thus, the challenge for the food processor remains in the optimisation of appropriate methodology to reduce the loss of phytochemicals such as vitamin E and phenolic compounds during extrusion cooking.
14.8 Parboiling
Rice parboiling is a hydrothermal treatment consisting of soaking, heating and drying. Parboiling changes both physicochemical and organoleptic properties of the rice grain. It reduces rice stickiness, increases hardness and darkens the colour (Lamberts, Rombouts, Brijs, Gebruers and Delcour, 2008b). According to the literature, colour changes during parboiling can be due to the migration of husk and/or bran pigments, enzymatic browning and non-enzymatic browning of the Maillard type (Lamberts et al., 2008b). Research on the effect of parboiling on the phytochemical content of rice has been mainly dedicated to the study of carotenoid stability and migration. The carotenoid content and composition of raw and parboiled brown and milled rice was studied by Lamberts et al. (2008a). Analyses of the colour of rice flour samples with different extraction rates demonstrated that yellow and red pigments are concentrated in the bran and outer endosperm. As a result, all pigments were removed for degrees of milling of 15% or higher. The colour-determining components present in the fractions of different rice cultivars were identified as the carotenoids β-carotene, lutein and zeaxanthin, with β-carotene and lutein being the predominant compounds. Parboiling brown rice was found to reduce carotenoid levels to trace levels, thus suggesting that compounds other than carotenoids are responsible for the colour of milled parboiled rice. Thus, the decreased brightness and increased red and yellow colour intensities of parboiled rice were attributed by the authors to Maillard reactions and/or physicochemical changes of different rice components occurring during parboiling.
14.9 Conclusions
In summary, it is possible to develop cereal-based foods rich in phytochemical compounds. However, due to the labile nature of some of these compounds, processes such as baking and extrusion can result in a significant decrease in compounds such as β-glucans, carotenoids, tocols and flavonoids. On the other hand, germination and fermentation are cereal processing techniques that may result in increased levels of key phytochemicals such as folates and phenolic compounds. Milling significantly reduces the levels of phytochemicals in grains, as these compounds are mainly concentrated on the bran/germ fractions. Therefore, the use of flours with a high extraction rate is recommended. In addition, optimisation of the different processing steps, such as germination, mixing and fermentation times, as well as opting for the most adequate grain variety, may also result in a final cereal-based product with high levels of phytochemicals.
References
Abd El-Hady, E.A. and Habiba, R.A. (2003) Efffect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. Lebensmittel-Wissenschaft Und-Technologie-Food Science and Technology, 36(3), 285–293.
Abdel-Aal, E.S.M., and Hucl, P. (1999) A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chemistry, 76(3), 350–354.
Abdul-Hamid, A., Sulaiman, R.R.R., Osman, A. and Saari, N. (2007) Preliminary study of the chemical composition of rice milling fractions stabilized by microwave heating. Journal of Food Composition and Analysis, 20(7), 627–637.
Adom, K.K., Sorrells, M.E., and Liu, R.H. (2003) Phytochemical profiles and antioxidant activity of wheat varieties. Journal of Agricultural and Food Chemistry, 51(26), 7825–7834.
Adom, K.K., Sorrells, M.E., and Liu, R.H. (2005) Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. Journal of Agricultural and Food Chemistry, 53(6), 2297–2306.
Alvarez-Jubete, L., Holse, M., Hansen, A., Arendt, E.K. and Gallagher, E. (2009) Impact of Baking on Vitamin E Content of Pseudocereals Amaranth, Quinoa, and Buckwheat. Cereal Chemistry, 86(5), 511–515.
Alvarez-Jubete, L., Wijngaard, H., Arendt, E.K. and Gallagher, E. (2010) Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chemistry, 119(2), 770–778.
Andersson, A.A.M., Armo, E., Grangeon, E., Fredriksson, H., Andersson, R. and Aman, P. (2004) Molecular weight and structure units of (1 - > 3, 1 - > 4)-beta-glucans in dough and bread made from hull-less barley milling fractions. Journal of Cereal Science, 40(3), 195–204.
Andreasen, M.F., Christensen, L.P., Meyer, A.S., and Hansen, A. (2000). Ferulic acid dehydrodimers in rye (Secale cereale L.). Journal of Cereal Science, 31(3), 303–307.
Anton, A.A., Fulcher, R.G., and Arntfield, S.D. (2009). Physical and nutritional impact of fortification of corn starch-based extruded snacks with common bean (Phaseolus vulgaris L.) flour: Effects of bean addition and extrusion cooking. Food Chemistry, 113(4), 989–996.
Anton, A.A., Ross, K.A., Beta, T., Fulcher, R.G., and Arntfield, S.D. (2008). Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). Lwt-Food Science and Technology, 41(5), 771–778.
Awika, J.M., McDonough, C.M., and Rooney, L.W. (2005). Decorticating sorghum to concentrate healthy phytochemicals. Journal of Agricultural and Food Chemistry, 53(16), 6230–6234.
Bonoli, M., Verardo, V., Marconi, E., and Caboni, M.F. (2004). Phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. Journal of Agricultural and Food Chemistry, 52(16), 5195–5200.
Borrelli, G.M., De Leonardis, A.M., Platani, C., and Troccoli, A. (2008). Distribution along durum wheat kernel of the components involved in semolina colour. Journal of Cereal Science, 48(2), 494–502.
Boskov Hansen, H., Andreasen, M.F., Nielsen, M.M., Larsen, L.M., Bach Knudsen, K.E., Meyer, A.S., Christensen, L.P. and Hansen, A. (2002) Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. European Food Research and Technology, 214, 33–42.
Butsat, S. and Siriamornpun, S. (2010) Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chemistry, 119(2), 606–613.
Camire, M.E., Chaovanalikit, A., Dougherty, M.P., and Briggs, J. (2002) Blueberry and grape anthocyanins as breakfast cereal colorants. Journal of Food Science, 67(1), 438–441.
Camire, M.E., Dougherty, M.P. and Briggs, J.L. (2007) Functionality of fruit powders in extruded corn breakfast cereals. Food Chemistry, 101(2), 765–770.
Cardador-Martinez, A., Loarca-Pina, G. and Oomah, B.D. (2002) Antioxidant activity in common beans (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry, 50(24), 6975–6980.
Chavan, J.K. and Kadam, S.S. (1989) Nutritional improvement of cereals by sprouting. Critical Reviews in Food Science and Nutrition, 28(5), 401–437.
Chen, M.H. and Bergman, C.J. (2005) Influence of kernel maturity, milling degree, and milling quality on rice bran phytochemical concentrations. Cereal Chemistry, 82(1), 4–8.
Degutyte-Fomins, L., Sontag-Strohm, T. and Salovaara, H. (2002) Oat bran fermentation by rye sourdough. Cereal Chemistry, 79(3), 345–348.
Dietrych-Szostak, D. and Oleszek, W. (1999) Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum Moench) grain. Journal of Agricultural and Food Chemistry, 47(10), 4384–4387.
Dini, I., Tenore, G.C., and Dini, A. (2004). Phenolic constituents of Kancolla seeds. Food Chemistry, 84(2), 163–168.
Fares, C., Platani, C., Baiano, A. and Menga, V. (2010) Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chemistry, 119(3), 1023–1029.
Flander, L., Salmenkallio-Marttila, M., Suortti, T. and Autio, K. (2007) Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT-Food Science and Technology, 40(5), 860–870.
Glitso, L.V. and Knudsen, K.E.B. (1999) Milling of whole grain rye to obtain fractions with different dietary fibre characteristics. Journal of Cereal Science, 29(1), 89–97.
Gray, D.A., Auerbach, R.H., Hill, S., Wang, R., Campbell, G.M., Webb, C. and South, J.B. (2000) Enrichment of oat antioxidant activity by dry milling and sieving. Journal of Cereal Science, 32(1), 89–98.
Heinio, R.L., Liukkonen, K.H., Myllymaki, O., Pihlava, J.M., Adlercreutz, H., Heinonen, S.M. and Poutanen, K. (2008) Quantities of phenolic compounds and their impacts on the perceived flavour attributes of rye grain. Journal of Cereal Science, 47(3), 566–575.
Hidalgo, A., Brandolini, A. and Pompei, C. (2010) Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chemistry, 121(3), 746–751.
Hung, P.V., and Morita, N. (2008) Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chemistry, 109(2), 325–331.
Im, J., Huff, H.E. and Hsieh, F. (2003) Effects of Processing Conditions on the Physical and Chemical Properties of Bucwheat Grit Cakes. Journal of Agricultural and Food Chemistry, 51, 659–666.
Kariluoto, S., Liukkonen, K.H., Myllymaki, O., Vahteristo, L., Kaukovirta-Norja, A. and Piironen, V. (2006) Effect of germination and thermal treatments on folates in rye. Journal of Agricultural and Food Chemistry, 54(25), 9522–9528.
Kariluoto, S., Vahteristo, L., Salovaara, H., Katina, K., Liukkonen, K.H., and Piironen, V. (2004) Effect of baking method and fermentation on folate content of rye and wheat breads. Cereal Chemistry, 81(1), 134–139.
Katina, K., Arendt, E., Liukkonen, K.H., Autio, K., Flander, L. and Poutanen, K. (2005) Potential of sourdough for healthier cereal products. Trends in Food Science and Technology, 16(1–3), 104–112.
Katina, K., Liukkonen, K.H., Kaukovirta-Norja, A., Adlercreutz, H., Heinonen, S.M., Lampi, A.M., Pihlava, J.M. and Poutanen, K. (2007) Fermentation-induced changes in the nutritional value of native or germinated rye. Journal of Cereal Science, 46(3), 348–355.
Kaukovirta-Norja, A., Wilhelmson, A. and Poutanen, K. (2004) Germination: a means to improve the functionality of oat. Agricultural and Food Science, 13(1–2), 100–112.
Khanal, R.C., Howard, L.R. and Prior, R.L. (2009) Procyanidin Content of Grape Seed and Pomace, and Total Anthocyanin Content of Grape Pomace as Affected by Extrusion Processing. Journal of Food Science, 74(6), H174–H182.
Kim, S., Zaidul, I.S.M., Suzuki, T., Mukasa, Y., Hashimoto, N., Takigawa, S., Noda, T., Matsuura-Endo, C. and Yamauchi, H. (2008) Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chemistry, 110(4), 814–820.
Kim, S.J., Maeda, T., Sarker, M.Z.I., Takigawa, S., Matsuura-Endo, C., Yamauchi, H., Mukasa, Y., Saito, K., Hashimoto, N., Noda, T., Saito, T. and Suzuki, T. (2007) Identification of anthocyanins in the sprouts of buckwheat. Journal of Agricultural and Food Chemistry, 55(15), 6314–6318.
Kim, S.L., Kim, S.K. and Park, C.H. (2004) Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Research International, 37(4), 319–327.
Kong, S. and Lee, J. (2010) Antioxidants in milling fractions of black rice cultivars. Food Chemistry, 120(1), 278–281.
Korus, J., Gumul, D. and Czechowska, K. (2007) Effect of extrusion on the phenolic composition and antioxidant activity of dry beans of Phaseolus vulgaris L. Food Technology and Biotechnology, 45(2), 139–146.
Kreft, I., Fabjan, N. and Yasumoto, K. (2006) Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chemistry, 98(3), 508–512.
Lamberts, L. and Delcour, J.A. (2008a) Carotenoids in Raw and Parboiled Brown and Milled Rice. Journal of Agricultural and Food Chemistry, 56(24), 11914–11919.
Lamberts, L., Rombouts, I., Brijs, K., Gebruers, K. and Delcour, J A. (2008b) Impact of parboiling conditions on Maillard precursors and indicators in long-grain rice cultivars. Food Chemistry, 110(4), 916–922.
Lambo, A.M., Oste, R. and Nyman, M. (2005) Dietary fibre in fermented oat and barley beta-glucan rich concentrates. Food Chemistry, 89(2), 283–293.
Leenhardt, F., Lyan, B., Rock, E., Boussard, A., Potus, J., Chanliaud, E. and Remesy, C. (2006) Wheat lipoxygenase activity induces greater loss of carotenoids than vitamin E during breadmaking. Journal of Agricultural and Food Chemistry, 54(5), 1710–1715.
Lindenmeier, M. and Hofmann, T. (2004) Influence of baking conditions and precursor supplementation on the amounts of the antioxidant pronyl-L-lysine in bakery products. Journal of Agricultural and Food Chemistry, 52(2), 350–354.
Liu, R.H. (2004) Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr., 134(12), 3479S–3485.
Liu, R.H. (2007) Whole grain phytochemicals and health. Journal of Cereal Science, 46(3), 207–219.
Liukkonen, K.-H., Katina, K., Wilhelmsson, A., Myllymaki, O., Lampi, A.-M., Kariluoto, S., Piironen, V., Heinonen, S.-M., Nurmi, T., Adlercreutz, H., Peltoketo, A., Pihlava, J.-M., Hietaniemi, V. and Poutanen, K. (2003) Process-induced changes on bioactive compounds in whole grain rye. Proceedings of the Nutrition Society, 62(01), 117–122.
Liyana-Pathirana, C.M. and Shahidi, F. (2007) The antioxidant potential of milling fractions from breadwheat and durum. Journal of Cereal Science, 45(3), 238–247.
Madhujith, T., Izydorczyk, M. and Shahidi, F. (2006) Antioxidant properties of pearled barley fractions. Journal of Agricultural and Food Chemistry, 54(9), 3283–3289.
Manach, C., Scalbert, A., Morand, C., Remesy, C. and Jimenez, L. (2004) Polyphenols: food sources and bioavailability. American Journal of Clinical Nutrition, 79(5), 727–747.
Michalska, A., Amigo-Benavent, M., Zielinski, H. and del Castillo, M.D. (2008) Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. Journal of Cereal Science, 48(1), 123–132.
Michalska, A., Ceglinska, A., Amarowicz, R., Piskula, M.K., Szawara-Nowak, D. and Zielinski, H. (2007) Antioxidant contents and antioxidative properties of traditional rye breads. Journal of Agricultural and Food Chemistry, 55(3), 734–740.
Omwamba, M. and Hu, Q.H. (2010) Antioxidant Activity in Barley (Hordeum Vulgare L.) Grains Roasted in a Microwave Oven under Conditions Optimized Using Response Surface Methodology. Journal of Food Science, 75(1), C66–C73.
Oomah, B.D., Cardador-Martinez, A. and Loarca-Pina, G. (2005) Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). Journal of the Science of Food and Agriculture, 85(6), 935–942.
Oomah, B.D., Caspar, F., Malcolmson, L.J. and Bellido, A.S. (2011) Phenolics and antioxidant activity of lentil and pea hulls. Food Research International, 44(1), 436–441.
Oomah, B.D. and Mazza, G. (1996) Flavonoids and antioxidative activities in buckwheat. Journal of Agricultural and Food Chemistry, 44(7), 1746–1750.
Panfili, G., Fratianni, A., Di Criscio, T. and Marconi, E. (2008) Tocol and beta-glucan levels in barley varieties and in pearling by-products. Food Chemistry, 107(1), 84–91.
Peterson, D.M. (1994) Barley tocols – effects of milling, malting, and mashing. Cereal Chemistry, 71(1), 42–44.
Peterson, D.M. (1995) Oat tocols – concentration and stability in oat products and distribution within the kernel. Cereal Chemistry, 72(1), 21–24.
Poutanen, K., Flander, L. and Katina, K. (2009) Sourdough and cereal fermentation in a nutritional perspective. Food Microbiology, 26, 693–699.
Scalbert, A., Manach, C., Morand, C., Remesy, C. and Jimenez, L. (2005) Dietary Polyphenols and the Prevention of Diseases. Critical Reviews in Food Science and Nutrition, 45(4), 287–306.
Seal, C.J. (2006) Whole grains and CVD risk. Proceedings of the Nutrition Society, 65(1), 24–34.
Sensoy, I., Rosen, R.T., Ho, C.T. and Karwe, M.V. (2006) Effect of processing on buckwheat phenolics and antioxidant activity. Food Chemistry, 99(2), 388–393.
Shin, T.S., Godber, J.S., Martin, D.E. and Wells, J.H. (1997) Hydrolytic stability and changes in E vitamers and oryzanol of extruded rice bran during storage. Journal of Food Science, 62(4), 704–and.
Siebenhandl, S., Grausgruber, H., Pellegrini, N., Del Rio, D., Fogliano, V., Pernice, R. and Berghofer, E. (2007) Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. Journal of Agricultural and Food Chemistry, 55(21), 8541–8547.
Slavin, J. (2004) Whole grains and human health. Nutrition Research Reviews, 17(1), 99–110.
Slavin, J.L., Jacobs, D. and Marquart, L. (2001) Grain processing and nutrition. Critical Reviews in Biotechnology, 21(1), 49–66.
Tiwari, U. and Cummins, E. (2009a) Factors Influencing beta-Glucan Levels and Molecular Weight in Cereal-Based Products. Cereal Chemistry, 86(3), 290–301.
Tiwari, U. and Cummins, E. (2009b) Nutritional importance and effect of processing on tocols in cereals. Trends in Food Science and Technology, 20(11–12), 511–520.
Trogh, I., Courtin, C.M., Andersson, A.A.M., Aman, P., Sorensen, J.F. and Delcour, J.A. (2004) The combined use of hull-less barley flour and xylanase as a strategy for wheat/hull-less barley flour breads with increased arabinoxylan and (1- > 3,1- > 4)-beta-D-glucan levels. Journal of Cereal Science, 40(3), 257–267.
Viscidi, K.A., Dougherty, M.P., Briggs, J. and Camire, M.E. (2004) Complex phenolic compounds reduce lipid oxidation in extruded oat cereals. Lebensmittel-Wissenschaft Und-Technologie-Food Science and Technology, 37(7), 789–796.
Vogrincic, M., Timoracka, M., Melichacova, S., Vollmannova, A. and Kreft, I. (2010) Degradation of Rutin and Polyphenols during the Preparation of Tartary Buckwheat Bread. Journal of Agricultural and Food Chemistry, 58(8), 4883–4887.
Waldron, K.W., Parr, A.J., Ng, A. and Ralph, J. (1996) Cell wall esterified phenolic dimers: Identification and quantification by reverse phase high performance liquid chromatography and diode array detection. Phytochemical Analysis, 7(6), 305–312.
Wang, L., Xue, Q., Newman, R.K. and Newman, C.W. (1993) Enrichment of tocopherols, tocotrienols, and oil in barley fractions by milling and pearling. Cereal Chemistry, 70(5), 499–501.
Watanabe, M. (1998) Catechins as antioxidants from buckwheat (Fagopyrum esculentum Moench) groats. Journal of Agricultural and Food Chemistry, 46(3), 839–845.
Wennermark, B. and Jagerstad, M. (1992) Breadmaking and storage of various wheat fractions affect Vitamin E. Journal of Food Science, 57(5), 1205–1209.
White, B.L., Howard, L.R. and Prior, R.L. (2010) Polyphenolic Composition and Antioxidant Capacity of Extruded Cranberry Pomace. Journal of Agricultural and Food Chemistry, 58(7), 4037–4042.
Wilhelmson, A., Oksman-Caldentey, K.M., Laitila, A., Suortti, T., Kaukovirta-Norja, A. and Poutanen, K. (2001) Development of a germination process for producing high beta-glucan, whole grain food ingredients from oat. Cereal Chemistry, 78(6), 715–720.
Yagci, S. and Gogus, F. (2009) Effect of Incorporation of Various Food By-products on Some Nutritional Properties of Rice-based Extruded Foods. Food Science and Technology International, 15(6), 571–581.
Zhang, M., Chen, H., Li, J., Pei, Y. and Liang, Y. (2010) Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT – Food Science and Technology, 43(1), 181–185.
Zielinski, H., Kozlowska, H. and Lewczuk, B. (2001) Bioactive compounds in the cereal grains before and after hydrothermal processing. Innovative Food Science andamp; Emerging Technologies, 2(3), 159–169.
Zielinski, H., Michalska, A., Piskula, M.K. and Kozlowska, H. (2006) Antioxidants in thermally treated buckwheat groats. Molecular Nutrition and Food Research, 50(9), 824–832.