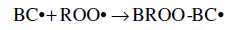 (a)
(a)Food products, especially fruits, vegetables, teas, nuts, legumes, and whole grains, contain a group of naturally occurring compounds named “phytochemicals” or “phytonutrients”, which are biologically active organic substances that impart colors, flavors, aromas, odors, and protection against diseases. These compounds, including phenolics, thiols, carotenoids, ascorbic acid, tocopherols, sulforaphane, indoles, isothiocyanates, and glucosinolates, may help protect human cellular systems from oxidative damage through a variety of mechanisms, and thus lower the risk of chronic diseases in human beings. Since phytochemicals not only possess unique health benefits, but also can be utilized as natural colorants, they are drawing tremendous attention. However, phytochemical stability is affected by many variables, including pH, oxygen, temperature, time of processing, light, water activity (aw), enzymes, structure, self-association, concentration, metallic ions, atmospheric composition, copigments, the presence of antioxidants, and storage conditions, suggesting that these molecules are unstable and highly susceptible to degradation and decomposititon. Anthocyanins are a group of naturally-occurring and water-soluble flavonoids responsible for their diverse color characteristics, including red, blue, and purple colors in fruits, vegetables, and grains as well as food products derived from them. The daily intake of anthocyanins is estimated to be 12.5 mg per capita in the United States (Wu et al., 2006). Anthocyanins cannot only be utilized in the food industry as natural colorants but also possess potential health benefits in prevention of certain chronic diseases. At present, grape skin and red cabbage are the predominant concentrated sources of anthocyanin colorants (Francis, 2000). As food colorants, anthocyanins’ stability is of great concern since they are usually less stable and more sensitive to condition changes such as pH in comparison with synthetic colorants. In recent years, many studies have concentrated on the health benefits of anthocyanins from different perspectives such as biological activities and bioavailability. Anthocyanins have been considered as health promoting compounds due to their antioxidant activity (Satue-Gracia et al., 1997; Yang et al., 2009), anti-inflammatory (Tsuda et al., 2002), anti-cancer (Chen et al., 2006), antiarteriosclerosis (Xia et al., 2006), inhibiting oxidation of low-density lipoprotein and liposomes (Satue-Gracia et al., 1997), hyperlipidemia (Kwon et al., 2007), and hypoglycemic effects (Sasaki et al., 2007).
Anthocyanins are important in the human diet and their compositional characteristics, functionality, and stability need to be better understood. Generally, the color stability of anthocyanins is influenced by numerous parameters such as environmental, processing, and storage conditions, suggesting that these molecules are unstable and highly susceptible to degradation (Markakis, 1982; Skrede et al., 1992; Francis, 2000; Malien-Aubert et al., 2001). For example, they are able to chelate metal ions such as Fe, Cu, Al, and Sn present in the media or packaging, resulting in a change of color. Additionally, the major drawback in the use of anthocyanins as food colorants is their low stability and higher cost. Consequently, it is of great interest to conduct research on the stability and composition of anthocyanins in foods, although some studies on the stability of anthocyanins have been published (Bassa and Francis 1987; Inami et al., 1996; Shi et al., 1992).
Betalains are a class of red and yellow indole-derived, water-soluble nitrogenous pigments found in plants of the Caryophyllales and some higher order of fungi such as the Basidiomycetes (Strack et al., 1993). As natural colorants, betalains can be extracted from red beet (Stintzing and Carle 2004), cactus pears (Stintzing et al., 2001), and Amaranthaceae plants (Cai et al., 2005). In comparison with anthocyanins, betalains retain their appearance over the broad pH range 3–7, but degrade below pH 2 and above pH 9 (Jackman and Smith, 1996). The health effects of betalains could be contributed to anti-inflammatory activities (Gentile et al., 2004), antiradical and antioxidant activities (Stintzing et al., 2005), and inhibition of lipid oxidation and peroxidation (Kanner et al., 2001).
Betalain stability is influenced by a variety of factors, including concentration, structure such as glucosylation and acylation, pH, temperature, aw, O2, N2, light, enzymes, matrix constituents, antioxidants, chelating agents, metals, and storage conditions. For instance, color retention during and after processing in foods containing betalain could be considerably improved by exclusion or removal of undesirable factors such as metal ions, light, oxygen and by addition of food additives such as antioxidants and chelating agents.
Carotenoids are one of the major phytochemicals in nature, which are widely distributed in plants as well as in the animal kingdom. In the past decade the biological functions of carotenoids have drawn considerable interest. For instance, carotenoids have been shown to exert protective effects against cardiovascular and eye diseases, as well as skin and stomach cancers (Canfield et al., 1993). In addition, several carotenoids, such as α- and β-carotene, possess vitamin A activity. Numerous studies have also shown that carotenoids may act as antioxidants through a mechanism of quenching singlet oxygen (Palozza and Krinsky, 1992) or free radicals (Jørjensen and Skibsted, 1993). Obviously, the significance of carotenoids to mankind as neutraceuticals is indisputable.
The structural characteristic of all carotenoids is its polyenoic chain, which is responsible for their physical and chemical properties, and provides this group of natural compounds with their coloring and antioxidant activities, as well as their biological functions (Rascón et al., 2011). The structures break down with attack by free radicals, such as singlet molecular oxygen and other reactive species. The common degradation pathways are isomerization, oxidation, and fragmentation of carotenoid molecules. This degradation can be induced by heat, light, oxygen, acid, transition metal, or interactions with radical species (Boon et al., 2010). Heat, light, and acids promote isomerization of the trans-form of carotenoids to the cis-form. Light, enzymes, pro-oxidant metals, and co-oxidation with unsaturated lipids, on the other hand, induce oxidation. Pyrolysis occurs under intense heat with expulsion of low molecular weight molecules. Generally, all of above can influence the color of foods as well as their nutritional value (Rao and Rao, 2007). A better understanding of the factors that influence the stability of carotenoids will help limit their degradation during processing. Also some attempts can be made to increase the stability of the carotenoids.
Catechins are a group of low molecular weight flavan-3-ols isomers including four major compounds, (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG), and four minor compounds, (+)-catechin (C), (-)-catechin gallate, (-)-gallocatechin, and (-)-gallocatechin gallate, which are present in a variety of foods, such as tea, wine, fruits, and chocolate. Catechins are water soluble, colorless, and astringent. The basic structure of catechins is composed of two benzene rings (A and B rings) and a dihydropyran heterocycle (C ring) with a hydroxyl group on carbon 3. EC has an ortho-dihydroxyl group in the B ring at carbons 3’ and 4’, while EGC has a trihydroxyl group in the B ring at carbons 3’, 4’, and 5’. ECG differs from EC with a gallate moiety esterified at carbon 3 of the C ring, while EGCG has both trihydroxyl group in the B ring at carbons 3’, 4’, and 5’, and a gallate moiety esterified at carbon 3 of the C ring. The concentrations of catechins in foods highly depend on the food sources and vary to a large extent. Tea has the highest level of catechins among all the food sources. Generally EGCG is the most abundant catechin in tea leaves and green tea, oolong tea, and black tea, followed by EGC, ECG, and EC, while GC and C are minor components (Shahidi et al., 2004). Green tea contains 30–42% catechins on a dry basis compared to black tea which contains 3–10% (Arts et al., 2000).
Glucosinolates are a group of plant secondary metabolites present in all families of Brassica, such as rapeseed, cabbage, cauliflower, brussel sprouts, turnip, calabrese/broccoli, Chinese cabbage, radishes, mustard seed, horse radish, and so on. A large body of epidemiological evidence has indicated that the protective effects of Brassica vegetables against cancers of the alimentary tract and lungs may be partly due to their high content of glucosinolates (Jones et al., 2006). Glucosinolates constitute a wide class of natural compounds from which approximately a hundred have been identified to date. They possess a common chemical structure consisting in a β-D-1-thioglucopyranose moiety bearing on the anomeric site an O-sulfated thiohydroximate function, and they only differ by their side chain, which can be aliphatic, aromatic, or heterocyclic (indolic) (van Eylen et al., 2008; Lopez-Berenguer et al., 2007). Glucosinolates are chemically stable until they come into contact with the enzyme myrosinase, which is stored compartmentalized from glucosinolates in plant tissue (Kelly et al., 1998). They become accessible to myrosinase when the plant tissue is disrupted (Rodrigues and Rosa, 1999). The hydrolysis gave rise to an unstable aglycone intermediate, thiohydroxamate-O-sulfonate, which is spontaneously converted to different classes of breakdown products including isothiocyanates, thiocyanates, nitriles, epithionitriles, hydroxynitriles, and oxazolidine-2-thiones (Rungapamestry et al., 2006). One of the principal forms of chemoprotection, however, is thought to arise from isothiocyanates formation, which may influence the process of carcinogenesis partly by inhibiting Phase I and inducing Phase II xenobiotic metabolizing enzyme activity (Song and Thornalley, 2007).
The extent of hydrolysis of glucosinolates and the nature and composition of the breakdown products formed are known to be influenced by various characteristics of the hydrolysis medium. Intrinsic factors such as coexisting myrosinase and its cofactors ascorbic acid, epithiospecifier protein (ESP), ferrous ions as well as extrinsic factors such as pH and temperature can affect the hydrolysis of glucosinolates (Fenwick and Heaney, 1983; van Poppel et al., 1999). Although some of the hydrolysis products are believed to have a health beneficial effect, they can also have an undesirable effect on odor and taste. For instance, bitterness can be caused by gluconapin, sinigrin and 5-vinyloxazolidine-2-thione. Allyl isothiocyanate brings about a pungent and lachrymatory response upon chewing and cutting of Brassica, which is important to consumer acceptance of the health promoting vegetables (Ludikhuyze et al., 2000). Therefore, studying the factors influencing the stability of glucosinolates is highly desirable.
The most interesting and researched isoflavones reported in literature include genistein, daidzein, and glycitein, mainly found in soybeans and soy products, and coumestrol, formonometin, and biochanin A, found in a variety of plants, such as alfalfa, chickpeas, clove seeds, and some pulses (Murphy et al., 1999). Soybeans and soy products are the predominant sources of isoflavones consumed in the human diet worldwide. Genistein, daidzein, and glycitein are the basic chemical structures of aglycons of soy isoflavones. Their derivatives containing a β-glucoside group are genistin, daidzin, and glycitin, respectively, also found in soy beans and soy products. The glucoside conjugates, having either an acetyl or a malonyl β-glucoside, acetyle genistin, acetyl daidzin, acetyl glycitin, malonylgenistin, malonyldaidzin, and malonylglycitin, are also of interest.
Anthocyanins are sensitive to pH. In general, anthocyanins are observed to fade at pH values above 2. However, acylation with hydroxycinnamic acids does not only bring about distinct bathochromic and hyperchromic shifts, but also promotes stability at near neutral pH values, which is explained by intramolecular copigmentation due to the stacking of the hydrophobic acyl moiety and the flavylium nucleus, thus reducing anthocyanin hydrolysis (Dangles et al., 1993). Anthocyanin stability in the pH range 1–12 during a period of 60 days storage at 10 and 23 °C was exmined on the 3-glucoside of cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin (Cabrita et al., 2000). The stability of the six anthocyanidin 3-glucosides changed significantly in the pH 1–12 range. The result revealed that malvidin 3-glucoside with bluish color was rather intense and relatively stable in the alkaline region. The extent of color loss upon a pH increase is translated into hydration constants, which inversely are used to predict the stability at a given pH (Hoshino and Tamura 1999; Stintzing et al., 2002). Bao et al. (2005) characterized anthocyanin and flavonol components from the extracts of four Chinese bayberry varieties, and investigated color stability under different pH values. The study indicated that the anthocyanin was most stable at pH 1.5. The result further exhibited that all color parameters significantly changed above pH 4, and the peaks above pH 4.0 at 515 nm also reduced remarkably, suggesting the pigment was highly unstable above pH 4.0. Cyanidin 3-O-β-D-glucopyranoside (cy3glc), found in its flavylium ion form with intense color, is a typical anthocyanin, and commonly exists in berries including blueberry, cowberry, elderberry, whortleberry, blackcurrant, roselle, and black chokeberry. Petanin is an anthocyanins acylated with aromatic acids present in the family Solanaceae (Price and Wrolstad, 1995). The effect of pH range of 1–9 and storage temperature of 10 and 23 °C for 60 days on color stability of cy3glc a simple anthocyanin and petanin a complex anthocyanin was evaluated (Fossen et al., 1998). It was revealed that, in comparison with cy3glc, petanin had higher color intensity and higher or similar stability throughout the pH range of 1–9. For example, 84% of petanin was retained after 60 days storage at 10 °C at pH 4.0, while the corresponding solution of cy3glc was totally broken down. It was proposed that the use of petanin as food colorant could be possible in slightly alkaline products such as baked goods, milk, and egg.
Betalains are widely considered as food colorings because of their broad pH stability, pH 3–7 (Stintzing and Carle, 2004), which allows their application in low acid foods. Although altering their charge upon pH changes, betalains are not as susceptible to hydrolytic cleavage as are the anthocyanins. The optimum pH of betanin stability is pH 4–6. Betanin solutions were reported to be less stable at pH 2 in comparison with pH 3 (von Elbe et al., 1974). Betaxanthin shows stability at pH 4–7 (Cai et al., 2001), and exhibits maximal stability at pH 5.5, which corresponds the optimum pH of betacyanins (Savolainen and Kuusi 1978). Betaxanthin was proven to show higher stability than betanin at pH 7. pH’s outside of 3–7 readily induce the degradation of betalains. Betanin displays most stability at pH 5.5–5.8 in the presence of oxygen, while reducing pH from 4 to 5 is favorable under anaerobic condition. Anaerobic conditions favor betanin stability at a lower pH (4.0–5.0). In addition, the optimum pH of betanin stability shifts towards 6 at elevated temperature. Acidification induces recondensation of betalamic acid with the amine group of the additional residue, while alkaline conditions result in aldimine bond hydrolysis (Schwartz and von Elbe, 1983). Although betalain degradation mechanisms in acidic condition are not well understood, it was observed that betanidin decomposed into 5, 6-dihydroxyindole-2-carboxylic acid and methylpyridine-2, 6-dicarboxylic acid under alkaline conditions (Wyler and Dreiding, 1962). C15 isomerization of betanin and betanidin into isobetanin and isobetanidin, respectively, was found at low pH values (Wyler and Dreiding 1984). Vulgaxanthin I was reported to be more easily oxidized and be less stable than betanin at acidic pH (Savolainen and Kuusi 1978).
pH conditions significantly affected betacyanin degradation in purple pitaya juice (Herbach et al., 2006a). It was observed that pH 6 resulted in elevated hydrolytic cleavage of the aldimine bond, while at pH 4, decarboxylation and dehydrogenation were favored. The combination effect of heat and pH on betacyanin stability in Djulis (Chenopodium fromosanum), a native cereal plant in Taiwan, was reported (Tsai et al., 2010). The results indicated that thermal stability of betacyanin was dependent on the pH. Among identified 4 peaks including betanin (47.8%), isobetanin (30.0%), armaranthin (13.6%), and isoamaranthinee (8.6%), betanin and isobetanin contributes over 70% of FRAP reducing power or DPPH scavenging capacity, suggesting that the two compounds are a main source of the antioxidant activities.
There are a few reports about pH and carotenoids stability. Sims et al. (1993) found that acidification of milled carrots to pH 4 or 5 with citric acid could improve juice color. β-carotene is stable to pH changing, which was reported to be stable in foods over the range pH 2–7 (Kearsley and Rodriguez, 1981). One of the drawbacks in processing carrot juice is that the sterilization temperature has to be raised because carrots are mildly acidic (pH 5.5 ~ 6.5) foods. However, this treatment can result in substantial loss of color. To remedy this problem, carrot juices are often acidified before processing so that the sterilization temperature can be lowered. It has been reported that heating carrots in an acetic acid solution can prevent coagulation of the extracted juice during heat sterilization. Luteoxanthin can be formed from violaxanthin under acidic conditions. Neochrome can be attributed to conversion of neoxanthin under acidic conditions too (Chen et al., 2007).
Catechins as a mixture are extremely unstable in neutral or alkaline solutions (pH > 8), whereas in acidic solutions (pH < 4) they are stable (Chen et al., 2001). Their stability at pH 4–8 is pH-dependent, where the lower the pH, the higher the stability. EC is the most stable isomer followed by ECG. EGCG and EGC are equally unstable in alkaline solutions (Su et al., 2003). Noticeable color change of green tea catechins (GTC) from light brown to dark brown occurs after degradation in alkaline solution.
Acid hydrolysis of glucosinolates leads to the corresponding carboxylic acid together with hydroxyl ammonium ion and has been used in the identification of new glucosinolates. Base decomposition of glucosinolates results in the formation of several products. In addition to allyl cyanide, and ammonia, thioglucose is obtained from 2-propenylglucosinolate with aqueous sodium hydroxide (Fenwick et al., 1981). Thioglucose has also been reported as a product of the reaction of 2-propenylglucosinolate with potassium methoxide (Friis et al., 1977). However, basic degradation of 4-hydroxybenzylgluosinolate gives thiocyanate, indol-3-ylmethylglucosinolate produces glucose, sulphate, H2S, thiocyanate, indol-3-ylacetamide (2-(1H-indol-3-yl) acetamide), indol-3-yl methyl cyanide (2-(1H-indol-3-yl) acetic acid), 3-(hydroxymethyl) indole (1-H-indol-3-yl) methanol), 3,3′-methylene diindole(di(1H-indol-3-yl)methane), indole-3-carbaldehyde 2-(1H-indol-3-yl) acetaldehyde, and indole (Schneider and Becker, 1930).
The effect of pH value on stability of glucoraphanin (4-methylsulfinyl-Bu glucosinolate) has been reported. The content of glucoraphanin decreased to less than 0.03 mg/mL when it was kept at the condition of pH value less than 6 for nine days, but the content of glucoraphanin still remained at 0.0806 mg/mL when the extraction pH was 6.6. In addition, the degradation of glucoraphanin was accelerated if stored under acidic condition. Therefore, glucoraphanin should be stored at neutral pH condition (Wang et al., 2009). 4-hydroxybenzyl isothiocyanate was unstable in aqueous media, showing a half-life of 321 min at pH 3.0, decreasing to 6 min at pH 6.5. Alkali pH values decrease the stability of 4-hydroxybenzyl isothiocyanate by promoting the formation of a proposed quinone that hydrolyzes to thiocyanate (Borek and Morra, 2005). On the basis of experimental data obtained using glucobrassicin (GBS) extracted from kohlrabi leaves, a general scheme in which various indole derivatives (i.e. indole-3-carbinol (I3C), indole-3-acetonitrile (IAN), and 3,3′-diindolylmethane (DIM)) is generated, depending on the pH of the reaction (Clarke, 2010). In enzymatic breakdown of GBS, myrosinase action at pH 7 and at room temperature leads to the complete breakdown of GBS after 1 h regardless of the lighting conditions. In daylight and at room temperature, incubation with myrosinase at pH 3 resulted in only a partial degradation of GBS. After 1 h, the proportion of unchanged GBS was 56% and the breakdown was almost complete only after 24 h. The chemical breakdown of GBS was studied using aqueous buffered solutions with pH 2–11. Whatever the pH, no degradation product of GBS was noticed after 2 h. Moreover, in this study, a number of other glucosinolates were found to be stable in the same conditions (Lopez-Berenguer et al., 2007).
It was found that pH had a significant effect on sulforaphane nitrile production. A neutral or alkaline pH resulted in predominately sulforaphane production, whereas an acidic pH (3.5, typical of salad dressings) gave rise to more sulforaphane nitrile (Ludikhuyze et al., 2000). Under certain circumstances the aglycone may yield a nitrile, rather than an isothiocyanate. This tendency is enhanced at acid pH and so may be of particular concern during the preparation and storage of such products as pickled cabbage, coleslaw, and sauerkraut (Chevolleau et al., 1997). Allyl isothiocyanate (AITC), a hydrolysis product of Sinigrin, was known as the principal nematicidal ingredient in B. juncea. Its half-lives were 31, 34, 31, and 26 days in pH 5.00, 6.00, 7.00, and 9.00, respectively (Gmelin and Virtanen, 1961).
At elevated temperatures, malonyldaizin was most stable at pH 2 and less stable at neutral or alkaline pH, but another conjugate form of daidzin, acetyledaidzin, showed best stability at pH 7 and least at pH 10 (Mathias et al., 2006). It is also worth noting that free isoflavones were found in heating daidzin conjugates in 3 M acid condition, but at a low molarity acid condition (0.01 M), no daidzin was detected after heating the conjugates. The same study concluded that the conversions of malonylgenistin at acidic conditions are less significant than those in neutral or alkaline conditions and acetylgenistin was most stable in alkaline conditions, however glycitin was not analyzed due to its minimum contribution (< 5%). Ionization of malonyl carboxyl group of isoflavones under different pH may have effect on their association with soy protein moiety, thus indirectly affecting the stability of isoflavones (Nufer et al., 2009).
Skrede et al. (1992) examined the color and pigments stability of strawberry and blackcurrant syrups which were processed and stored under identical conditions. The study revealed that color stability was dependent on total anthocyanin level rather than qualitative pigment composition, since anthocynin pigments of blackcurrant syrup were more stable than those of unfortified strawberry syrup. Color stability of strawberry syrup fortified with equal anthocyanin levels was similar to blackcurrant syrup. At high concentrations, anthocyanins may self-arrange, resulting in reduced hydrolytic attack (Hoshino and Tamura 1999), which was found to result in color intensification in red raspberry (Melo et al., 2000).
The concentration of betalain plays a crucial role in stabilizing betalain during food processing, because betacyanin stability appears to increase with pigment concentration (Moßhammer et al., 2005).
The stability of anthocyanins is markedly influenced by temperature. The thermal kinetic analysis of anthocyanins has been extensively reported in a wide variety of anthocyanin-rich products (Kirca and Cemeroglu, 2003; Mishra et al., 2008; Zhao et al., 2008; Zhang et al., 2009; Jiménez et al., 2010), including purple corn, blackberry, orange juice, and purple potato peel. The parameters such as activation energy and reaction rate constant were obtained through first order kinetics, where the Arrhenius equation is used for temperature dependence. Most of the studies considered as isothermal treatments were tested at temperatures below 100 °C. Non-isothermal heat treatments more than 100 °C, e.g. extrusion, deep-fat frying, spray drying, and sterilization, are also considered in anthocyanin-rich products, including extruded corn meal with blueberry and grape anthocyanins in breakfast cereals (Camire et al., 2002), sterilized grape pomace (Mishra et al., 2008), vacuum-fried blue potatoes (Da Silva et al., 2008), and spray-dried açai pulp (Tonon et al., 2008).
Anthocyanins are sensitive to temperature. Pigment loss was 32% at 77 °C, 53% at 99 °C, and 87% at 121 °C in concord grapes (Sastry and Tischer, 1953). Kirca and Cemeroglu (2003) reported the degradation kinetics of anthocyanins in blood orange juice. Extrusion of corn meal with grape juice and blueberry concentrates resulted in up to 74% anthocyanin degradation in the extruded cereal upon extruder die temperature reached 130 °C (Camire et al., 2002). Processing strawberry jams resulted in losses of 40–70% of the initial anthocyanin content (García-Viguera et al., 1999). Strawberry jams stored for up to nine weeks at 38 °C caused more anthocyanin losses in comparison with those at 21 °C for nine weeks. De Ancos et al. (2000) found that there was an increase of total anthocyanins in raspberries after frozen storage at −24 °C for one year.
Recently, kinetic parameters of anthocyanin degradation during storage at 20 and 30 °C in different sources of muscadine grape (Cv. Noble) pomaces were examined (Cardona et al., 2009). Color degradation followed a first-order kinetic model. Color degradation was delayed potentially due to the removal of soluble compounds from the stock matrix by Amberlite XAD-4 resins, yielding improvements of 18.6–26.1% at 20 °C and 27.5–38.0% at 30 °C in color stability during storage. Monomeric anthocyanin decomposition and non-enzymatic browning index have been measured in reconstituted blackberry juice heated at high temperature range of 100–180 °C in a hermetically sealed cell (Jiménez et al., 2010). It displayed that anthocyanin degradation at 140 °C was faster than the appearance of non-enzymatic browning products, and as indicated that the reaction rate constant for anthocyanin decomposition (3.5 × 10-3 s-1) was twice that for the non-enzymatic browning index (1.6 × 10-3 s-1). The thermal stability of anthocyanins in two commercial açai species was evaluated, since anthocyanins were the predominant phenolics in both E. oleracea and E. precatoria species, and contributed to approxiamately 90% of the trolox equivalent antioxidant capacity in fruits (Pacheco-Palencia et al., 2009). Specifically, açai pulps were heated at 80 °C for 1, 5, 10, 30, and 60 min, in the presence and absence of oxygen, and compared to a control without heating. There was no significant difference (p < 0.05) in polyphenolic degradation during heating between the presence and absence of oxygen. However, 34 ± 2.3% of anthocyanins in E. oleracea and 10.3 ± 1.1% of anthocyanins in E. precatoria were lost under thermal conditions. Correspondingly, 10–25% in antioxidant capacity was lost. It was reported that extensive anthocyanin degradation occurred due possibly to accelerated chalcone formation with prolonged anthocyanin exposure to high temperatures (Delgado-Vargas et al., 2000). Interestingly, cyanidin-3-rutinoside displayed a higher thermal stability (7.0 ± 0.6% loss following heating at 80 °C for 1 h) than did cyanidin-3-glucoside (up to 72 ± 5.3% loss under identical heating conditions) in both açai species, which was in agreement with previous studies in in E. oleracea juice (Pacheco-Palencia et al., 2007) and blackcurrants (Rubinskiene et al., 2005a).
Three wheat cultivars were used to evaluate the composition and stability of anthocyanins over three crop years (Abdel-Aal et al., 2003). Anthocyanins were extracted with acidified methanol, partially purified, and freeze-dried following methanol removal by evaporation at 40 °C. A four-factor full-factorial experiment was designed to study the effects of temperature (65, 80, and 95 °C), time (0, 1, 2, 3, 4, 5, and 6 h), SO2 (0, 500, 1000, 2000, and 3000 ppm), and pH (1, 3, and 5). It showed that blue wheat anthocyanins were thermally most stable at pH 1. Their degradation was slightly lower at pH 3 as compared to pH 5. Elevating the temperature from 65 to 95 °C increased degradation of blue wheat anthocyanins. Additionally, wheat pigments containing cyanidin-3-O-glucoside were reported to be thermally most stable at pH 1.0. Cyanidin-3-dimalonylglucoside, cyanidin-3-glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside, and their respective malonated counterparts are major anthocyanins present in color corn. The thermal stability of anthocyanins was studied in five purple corn hybrids grown in China (Zhao et al., 2008). The sample was stirred in a solution of 60% (v/v) ethanol acidified with citric acid at 60 °C for 120 min. The ethanol extracts were centrifuged at 9000 rpm and 20 °C for 10 min. The supernatants were evaporated to dryness at 40 °C to finally produce Chinese purple corn extracts (EZPC). Thermodynamic characteristics of the EZPC samples were measured by differential scanning calorimetry, where the degree of conversion of the sample with time and its relationship with temperature were reported. Thermodynamic analysis revealed that the conversion of EZPC followed an Arrhenius relationship. The degree of conversion was 0.1, 8.6, and 73.6% in 5 min at 100, 130, and 150 °C, respectively, indicating that temperature was the most important parameter affecting the stability of EZPC.
Temperature is one of the important parameters in affecting stability of betalain, especially during food processing and storage. Generally, betalains are heat-liable. Elevated temperature expedites pigment degradation. The effect of thermal processing on betalain stability in red beet, purple pitaya juices, and cactus fruit juices was reported by numerous studies (Czapski, 1990; Herbach et al., 2004a, 2004b; Herbach et al., 2006a). During thermal processing, betanin may be broken down by a series of reactions such as isomerization, decarboxylation, and hydrolytic cleavage, leading to a gradual reduction of red colour, and eventually the production of a light brown color (Huang and von Elbe, 1986). Betalain stability was shown to dramatically reduce in temperature range 50–80 °C. First-order reaction kinetics was observed in thermal degradation of betacyanin in betanin solutions, red beet as well as purple pitaya juices (von Elbe et al., 1974; Herbach et al., 2004b).
Thermal degradation products from phyllocactin (malonylbetanin), betanin, and hylocerenin (3-hydroxy-3-methylglutarylbetanin) isolated from purple pitaya juice was observed by Herbach et al. (2005). It was revealed that hydrolytic cleavage was the major breakdown mechanism in betanin, while decarboxylation and dehydrogenation predominated in hylocerenin. Phyllocactin degradation was involved in decarboxylation of the malonic acid moiety, betanin production via demalonylation, and subsequent degradation of betanin. Additionally, heating degradation of betanin in three different systems of water and glycerol, water and ethylene glycol, as well as water and ethanol at temperature range 60–86 °C was investigated by Altamirano et al. (1993). Betanin showed the lowest stability in water and ethanol system, indicating that the first step of the thermal betanin degradation is the nucleophilic attack on the aldimine bond, since ethanol has a high electron density on the oxygen atom. Moreover, the authors (Wybraniec and Mizrahi, 2005) reported a rapid degradation of betacyanins in ethanol, resulting in single and double decarboxylation and further identifying different monodecarboxylation products in ethanolic and aqueous solutions, suggesting the effect on the solvent on decarboxylation mechanism. Furthermore, the structures of mono- and bidecarboxylated betacyanins produced from heating red beet and purple pitaya preparations were elucidated by Wybraniec et al. (2006).
High temperature could cause the decrease of carotenoids in food. Much work has been done to investigate the influence of temperature on the stability of carotenoids (Richardson and Finley, 1985). It was found that canning resulted in the highest destruction of carotenoids, followed by high temperature short time (HTST) heating and acidification (Chen et al., 1996). Fresh sweet potatoes, carrots, and tomatoes contain negligible quantities of cis-β-carotene, whereas the proportion in canned products is approximately 25, 27, and 47%, respectively (Rock, 1997). The cooking of vegetables promotes isomerization of carotenoids from the trans- to the cis-forms. Thermal processing generally causes some loss of lycopene in tomato-based foods. The cis-isomers increase with temperature and processing time (Shi et al., 2008). Heat treatment promotes isomerization of the carotenoids in foods, from trans- to cis-isomeric forms, and the degree of isomerization is directly correlated with the intensity and duration of heat processing (Rock, 1997). It was observed that a 48% loss of total carotenoids for hot-air-dried samples (Tai and Chen, 2000). For each carotenoid, the loss of zeaxanthin was 54%, β-cryptoxanthin 40%, all-trans-β-carotene 48%, and all-trans-lutein 42%. Most significantly, about 95% of lutein 5,6-epoxide was degraded during hot-air-drying. Also, the contents of violeoxanthin and violaxanthin were reduced by 78 and 60%, respectively (Lin and Chen, 2005).
Catechins exhibit remarkable stability with temperature increase at slightly acidic pH (4.9), and isomers have similar heat stability (Arts et al., 2000). However, at neutral or alkaline pH, degradation and isomerization occur during heating and sterilization and cause significant loss of catechins (Kim et al., 2007). When boiled in water at 98 °C for seven hours, 20% loss of GTC from longjing tea was reported, but no GTC content change was observed when boiled in water at 37 °C for the same period of time (Chen et al., 2001).
The stability of glucosinolates to temperature differs widely from individual to individual. The order of thermostability of individual glucosinolates, from lowest to highest kd (degradation rate constant of glucosinolates) value at 80 °C is: glucoiberin < progoitrin ≈ sinigrin < glucoraphanin < gluconapin < glucobrassicin < 4-methoxyglucobrassicin < 4-hydroxyglucobrassicin. At 120 °C, due to the differences in the activation energies the order changes to: gluconapin < progoitrin < glucoraphanin < glucoiberin < 4-hydroxyglucobrassicin ≈ sinigrin < 4-methoxyglucobrassicin < glucobrassicin. The variation at 80 °C between the most and least stable glucosinolate is much higher than at 120 °C (Bones and Rossiter, 2006). It has been observed that level of glucosinolates reduced by more than 60% within 10 min at 100 °C, but there was no enzymatic degradation in the leaf samples at ambient temperature (Mohn et al., 2007). During thermal processing of Brassica vegetables, glucosinolate contents can be reduced because of several mechanisms: enzymatic breakdown, thermal breakdown and leaching into the heating medium. The most important enzyme during the degradation of glucosinolates is myrosinase, which is heat sensitive. It was reported that the activity of broccoli myrosinase was decreased by more than 95% after a 10 min treatment at 70 °C (Ludikhuyze et al., 2000).
Isoflavones from soybeans and soy products are considered to be heat stable, even above 100 °C (Wang et al., 1996; Coward et al., 1998), but can go through interconversions between different forms at mild process and storage temperatures, even at temperatures below 50 °C (Matsuura et al., 1993). Malonylgenistin, malonyldaidzin, and malonylglycitin conjugates are heat labile. Hot aqueous ethanol extraction was found to cause the conversion of malonylglucosides to β-glucosides. Dry heat, such as toasting in producing toasted soy flour, and extrusion heat used in producing textured vegetable protein (TVP), lead to the decarboxylation of malonylglucosides to form acetyleglucosides (Mahungu et al., 1999): While a first order kinetics of isoflavone degradation showed that isoflavones are more susceptible to moist heat than dry heat by 10–100 times (Chien et al., 2005). The de-esterification of malonylglucosides to underivatized β-glucosides was found in baking or frying of TVP at 190 °C and baking of soy flour in cookies, and furthermore, other ingredients in the cookies, such as sugar and butter seemed to accelerate this conversion (Coward et al., 1998). It was shown that extraction, process and cooking temperatures, and storage time play significant roles in the rate of loss of malonylglucosides (Barbosa et al., 2006). When high purity daidzin, genistin, and glycitin were investigated, they exhibited stability at the boiling point of water, but at 135 °C, after 60 min, the degradation of genistin and glycitin was observed while the concentration of daidzin remained unchanged (Xu et al., 2008). This is explained by the structural differences that the aglycone of genistin contains a hydroxyl group at the 5 position and the aglycone of glycitin has a methoxy group at the 6 position, but daidzin doesn’t have any of these groups that could lead to molecular degradation. The stability of three pure compounds at much higher temperatures (160, 15, 200, and 215 °C) were also examined in the same study and a drastic decrease of all three isoflavones was seen. Meanwhile, daidzein, genistein, glycitein, acetyldaidzin, and acetylgenistin were detected. It was proposed that the aglycones were produced by removing glucoside groups and that acetylation of glucosides may have happened during heating. Isoflavones are associated with the hydrophobic interior of globular soy protein and it is suggested that the higher protein content and its native stage may have protective effect on the stability of isoflavones during extraction and processing and that denaturation causes their exposure to thermal degradation (Malaypally et al., 2010).
The influence of domestic cooking on the degradation of anthocyanins and anthocyanidins of blueberries (Vaccinium corymbosum L.) from cultivar Bluecrop was investigated (Queiroz et al., 2009). Ten anthocyanins were separated in methanolic extracts. Of the six anthocyanidins, four (delphinidin, cyanidin, petunidin, and malvidin) were identified in the hydrolysates. The rate of degradation of anthocyanins is time and temperature dependent. Degradation of anthocyanins in whole blueberries cooked in stuffed fish was between 45 and 50%; however, degradation of anthocyanidins was in the range 12–30%, suggesting that cooking can preserve anthocyanidin degradation. Furthermore, thermal blanching above 80 °C is a good way to be effective in deactivating PPO and significantly improving the recovery and stability of anthocyanins in blueberry juice (Rossi et al., 2003).
Anthocyanins in rices give rise to lots of varieties such as red and black rice. The dark purple color of black rice comes from the high content of anthocyanins located in the pericarp layers (Abdel-Aal et al., 2006). The effects of the three cooking methods (i.e. electric rice cooker, pressure cooker, and absorption method using a gas range) in a predominant cultivar of California black rice (Oryza sativa L. japonica var. SBR) on the stability of anthocyanins were examined (Hiemori et al., 2009). The major anthocyanins in black rice are cyanidin-3-glucoside (572.47 µg/g) and peonidin-3-glucoside (29.78 µg/g); while minor ones are three cyanidin-dihexoside isomers and one cyanidin hexoside. Of the three cooking methods, pressure cooking gave rise to the highest loss of total anthocyanins (79.8%), followed by the rice cooker (74.2%) and gas range (65.4%). The results showed that cyanidin-3-glucoside content reduced with concomitant increases in protocatechuic acid across all cooking methods, suggesting that cyanidin-3-glucoside in black rice is degraded predominantly into protocatechuic acid during cooking. Additionally, Xu et al. (2008) observed that 100% of peonidin-3-glucoside was lost in black soybean during thermal processing.
High hydrostatic pressure (HHP) is a promising alternative to traditional thermal processing techniques for retaining food quality. Corrales et al. (2008) investigated the influence of HHP on anthocyanin stability. Cyanidin-3-glucoside decreased 25% after 30 min treatment of 600 MPa at 70 °C, whereas only 5% was lost after 30 min heating at the same temperature and ambient pressure, indicating that pressure can expedite anthocyanin degradation at elevated temperatures. However, the anthocyanin decomposition in a red grape extract solutions was minor after 60 min combined application of 600 MPa and 70 °C (Corrales et al., 2008). Additionally, cyanidin-3-glucoside was observed to be stable in a model solution at 600 MPa and 20 °C for up to 30 min. Furthermore, there was a significant change in anthocyanin content of strawberry and blackberry purées after 15 min treatments at 500–600 MPa at room temperature (Patras et al., 2009). However, fruit juices including blackcurrants (Kouniaki et al., 2004), raspberries (Suthanthangjai et al., 2005), strawberries (Zabetakis et al., 2000), and muscadine grape (Del Pozo-Insfran et al., 2007) displayed higher storage stability of anthocyanins after 15 min exposure to high pressures (500–800 MPa) at room temperature in comparison with the control juices. The effect of processing (thermal pasteurization and HHP), ascorbic acid, and polyphenolic cofactors from rosemary (Rosmarinus officinalis) on color stability in muscadine (Vitis rotundifolia) grape juice was assessed (Talcott et al., 2003). This study exhibited that HHP gave rise to greater loss in anthocyanins than pasteurization, most likely due to action from residual oxidase enzymes. It was proposed that processing to inactivate residual enzymes should be carried out prior to copigmentation in order to prevent degradation of anthocyanins in the presence of ascorbic acid. The effects of combined pressure and temperature treatments on retention and storage stability of anthocyanins in blueberry (Vaccinium myrtillus) juice were elucidated (Buckow et al., 2010). The temperature chosen was 60–121 °C, and the combined temperature–high pressure processing were 40–121 °C under 100–700 MPa. The study demonstrated that at atmospheric pressure, 32% degradation of anthocyanins was recorded after 20 min heating at 100 °C, whereas at 600 MPa, nearly 50% of total anthocyanins were decomposed at 100 °C, indicating that anthocyanins were rapidly degraded with increasing pressure. Additionaly, combination of pressure and temperature application of pasteurized juice resulted in a slightly faster degradation of total anthocyanins during storage compared to heat treatments at atmospheric pressure.
Carotenoids are widely used in the food industry and are often subjected to high temperatures:
Cooking affects carotenoid content, with variable degrees of stability evident among the different compounds. Hydrocarbon (i.e. β-carotene, lycopene) and hydroxylated (i.e. lutein) carotenoids are less susceptible to destruction than epoxides. Generally, the most common household cooking methods, including microwave cooking, steaming, or boiling in a small amount of water, do not drastically alter carotenoid content of vegetables. For example, mild heat treatment of yellow-orange vegetables, such as carrots, sweet potato, and pumpkin, results in a loss of only about 8–10% of the α- and β-carotene, whereas 60% of the total xanthophylls in green vegetables, such as brussel sprouts and kale, are lost with similar cooking methods. Among the xanthophylls, lutein is the most stable and is more resistant to heat than the others, with a reported reduction of 18–25% from microwave cooking of these vegetables (Rock, 1997).
Other processing methods also have different influences on carotenoids retention. For oven products, kneading leads to limited degradation of carotenoids in bread crust and water biscuits (on average, 15 and 12%, respectively), bread leavening had minimal effects (3%), while baking strongly reduced carotenoids in bread crust and water biscuits (29 and 19%, respectively). In pasta the longer kneading extrusion phase leads to major loss (48%), while the drying step does not provoke significant changes (Abdel-Aalet, 2002). The different carotenoid losses of bread are therefore a direct consequence of the different time–temperature processing conditions (Hidalgo et al., 2010). Blanching before continuous processing promotes carotenoid, retention due to the inactivation of peroxidase and lipoxidase activity. These enzymes play a role in indirect oxidation of carotenoids by producing peroxides. What’s more, under enzyme extraction, blanching provides greater penetration of pectinase and cellulase into the cells and enhances the release of pigments (Lavellia et al., 2007). Higher pigment retention can be achieved by reducing the blanching time to 1 min. When vegetables were microwave cooked at 700 W, in most cases the carotenoid contents decreased along with the increase of heating time (Chen and Chen, 1993). As the heating time increased to 8 min, the losses of most pigments reached plateaus. Drying, extreme heat, or extensive cooking time, as occurs with canning at high temperatures for extended periods, give rise to oxidative destruction of the carotenoids. Enzyme-extracted carotenoids displayed higher stability because enzyme-extracted carotenoids remain in their natural state, bound with proteins through covalent bonding or weak interactions. This bonded structure prevents pigment oxidation, whereas solvent extraction dissociates the pigments from the proteins and causes water insolubility and ease of oxidation. Therefore, enzyme-extracted pigments have higher stability, especially compared to carotenoids normally extracted (Cinar, 2005).
Optimum conditions for carotenoids retention during preparation/processing differ from one food to another. But no matter what the processing method chosen, retention of carotenoids decreases with longer processing time, higher processing temperature, and cutting or pureeing of the food. Reducing processing time and temperature, and the time lag between peeling, cutting and pureeing, and processing improves retention significantly. High temperature/short processing time is a good alternative (Dutta et al., 2005).
Generally, processing and preparation cause a decrease of catechin content in foods. Full oxidation process in black tea manufacture brings about degradation of flavonols, resulting in lower content of catechins in black tea than in green tea that goes through a manufacturing process of inactivation of enzymes by heat where little oxidation occurs. Heat processing can also give rise to thermally induced epimerization of epicatechins (EC, ECG, EGC, and EGCG) during green tea production, which produces epicatechin epimers (C, CG, GC, and GCG) that are not originally present in green tea leaves (Chen et al., 2001; Xu et al., 2003). It was found that the levels of green tea epicatechin (GTE) derivatives (EC, ECG, EGC, and EGCG) in canned or bottled tea drinks (16.4–268.3 mg/L) are lower than that in tea traditionally prepared in a cup or a teapot (3–5 g/L), although they exhibited higher levels of epicatechin epimers when treated at 120 °C for 10–60 minutes. Therefore, the stability of catechins during sterilization in manufacturing tea drinks depends largely on the pH, other ingredients such as citric acid and ascorbic acid in the drinks, and temperature. It was also found that brewing tea using tap water resulted in faster epimerization and degradation of catechins than using purified water, and it indicated that ions in tap water and the pH difference were possible explanations for the difference (Wang et al., 2000). A study on microwave technology in replacement of enzyme inactivation and drying processes in green tea production has demonstrated a slightly higher content of catechins (Gulati et al., 2003). Depending on the foods, preparations, and processes, degrees of catechins degradation in fruits and vegetables after processing also vary to a large extent. Compared to fresh sweet cherries, canned cherries have about 63% less total catechins; and peeled apples have 23% less total catechins than whole apples (Arts et al., 2000).
Recently, far-infrared (FIR) irradiation technology was investigated on green tea by-product in an effort to utilize by-products, improve the color of green tea leaf and stem extracts, and potentially increase the antioxidant activities (Lee et al., 2008). In this study, irradiation decreased overall phenolic contents of green tea leaf extracts and increased phenolic contents of green tea stem extract, and it was also observed that catechin content was decreased in both green tea leaf and stem extracts. However, FIR heating applied at different temperatures (80–150 °C) after the drying and rolling stages in green tea processing has been shown to increase the total flavanol content, EGC, and EGCG content in green tea up to 90 °C, then there was a decreasing trend above this temperature (Lee et al., 2006). It was suggested that catechins may be prevented from binding to the leaf matrix by microwave energy; however, it is uncertain if FIR-treated tea leaves perform the same way.
Most Brassica (Brassicaceae, Cruciferae) vegetables are mainly consumed after being cooked which induces inactivation of spoilage and pathogenic microorganisms. Cooking considerably affects their health-promoting compounds such as glucosinolates (van Eylen et al., 2008). There are two cooking methods to choose: cooking at high power with short heating times or cooking at low power with long heating times. Total glucosinolate concentrations were significantly influenced by cooking time. It was shown that the concentration of total glucosinolates was significantly lowered by 12.4 and 17.3% after microwaving cabbage for 315 and 420 s, respectively. The glucosinolates responsible for the significant reduction in total glucosinolates during microwaving over 7 min (420 s) were the alkenyl glucosinolates sinigrin (reduction of 22.3%), and gluconapin (reduction of 18.5%), the indole glucosinolates 4-hydroxyglucobrassicin (reduction of 22.7%), and glucobrassicin (reduction of 12.3%). Interestingly, the alkenyl glucosinolates glucoiberin and progoitrin remained unchanged.
Different cooking methods bring about different rates and extents of myrosinase inactivation. Cooking at high temperatures denatures myrosinase in vegetables, resulting in a lower conversion of glucosinolates to isothiocyanates, which delivers the health benefit after chewing (Song and Thornalley, 2007). At the same time glucosinolates probably were leached into heating medium (van Eylen et al., 2008). It was found that a residual myrosinase activity of cabbage was 4.6-fold higher than the corresponding microwaved sample after being steamed for 420 s and reaching an average temperature of 68 °C. In conventional cooking such as steaming, heating starts at the surface of the food, and heat is slowly transferred to the center by conduction. Conversely, in microwave cooking, microwaves permeate the center of the food by radiation, and the heat generated within the food is transferred toward the surface of the food. In this respect, an equivalent rise in temperature take places more quickly in microwave processing than steaming (Rungapamestry et al., 2006). Thus, microwave cooking could cause more loss of glucosinolate in food than conventional cooking methods. The studies (Oerlemans et al., 2006) showed that the ESP, known as the cofactors of myrosinase, favored nitrile production over isothiocyanates in broccoli under certain conditions, and indicated that heating broccoli may result in a more bioactive product because of inactivion of ESP at temperatures more than 50 °C – which means that we should choose the right cooking method and optimum temperature. It was reported that the indolyl glucosinolates were more sensitive to heat treatment compared to other types of glucosinolates and aliphatic glucosinolates (38 and 8%, respectively) (Schneider and Becker, 1930). Only limited degradation was observed at lower temperatures (< 110 °C) for most glucosinolates. Indole glucosinolates displayed more degradation than aliphatic glucosinolates at lower temperatures (Bones and Rossiter, 2006). Conventional cooking does not affect the aliphatic glucosinolates significantly. The indole glucosinolates, however, decreased to a higher extent (38%). Heat treatment gave rise to substantial decomposition of indole glucosinolates with thiocyanate and indole acetonitriles as products while autolysis gave little indole acetonitriles but high levels of thiocyanate and carbinols.
In the loss of glucosinolates due to tissue fracture produced by shredding ready-to-cook vegetables, involvement of myrosinase-catalysed hydrolysis was suggested (Song and Thornalley, 2007). When vegetables were diced to 5 mm cubes or sliced to 5 mm squares (for leaf material), up to 75% of the glucosinolate content was lost during the subsequent 6 h at ambient temperature. The extent of glucosinolate loss increased with post-shredding time. When vegetables were shredded into large pieces, or coarsely shred, losses of total glucosinolate content were much less (<10%). After chopping and storage of both broccoli and cabbage at room temperature, there were significant reductions in aliphatic glucosinolates (e.g. glucoraphanin), but an increase in some indole glucosinolates. Total glucosinolates and the indole 4-methoxyglucobrassicin, in particular, were also found to be enhanced in un-chopped broccoli heads during storage at 20 °C (Jones et al., 2006). For canned vegetables, the thermal degradation of glucosinolates is thought to be the most important mechanism, because canned vegetables undergo a substantial heat treatment (Bones and Rossiter, 2006). Canning was the most severe heat treatment studied (40 min, 120 °C) and it reduced total glucosinolates by 73%. Thermal degradation has been studied in red cabbage, where cooking reduced both indole (38%) and alkyl (8%) content (Fenwick and Heaney, 1983). Gluconasturtin has been shown to undergo a non-enzymatic, iron-dependent degradation to a simple nitrile. Upon heating the seeds to 120 °C, thermal degradation of this heat-labile glucosinolate increased simple nitrile levels many-fold (Fenwick and Heaney, 1983). For red cabbage, higher temperatures (>110 °C) resulted in significant degradation of all identified glucosinolates (Bones and Rossiter, 2006). The isothiocyanates (ITCs) were stable up to 60 °C and were degraded by more than 90% after a 20 min treatment at 90 °C. It was observed that a mild heat treatment, such as blanching, has little impact on the glucosinolates and high-pressure treatment in combination with mild temperatures could be an alternative to the thermal process in which the health beneficial ITCs, in particular sulforaphane, are still maintained (Matusheski et al., 2001).
Aqueous extraction at a high temperature used in tofu and soymilk production led to almost entire conversion to β-glucosides conjugates. Grinding soy flour and hexane extraction of fat from soy flour have no effect on the glucoside conjugates (Wang et al., 1996). Alkaline extraction during isolated soy protein production was found to be the major step for loss of isoflavones and it was also found to alter the distribution of isoflavone constituents, which exhibited an increase in the aglycones and decrease in the glucosides content. The loss of isoflavone content in traditional tofu making can be as high as 44%. It was well documented in a study that isoflavones were fractionated into the okara and whey during the coagulation step, which caused the significant loss (Jackson et al., 2002). In another study, tofu was heated in water at different temperatures and the loss of total isoflavone content was found due mainly to the daidzein series. In addition, the decrease of the aglycones was strongly temperature dependent, thus, it was suggested that besides leaching from the tofu matrix, thermal degradation of daidzein may have taken place (Grun et al., 2001). Soy milk production not only causes dilution of isoflavones, but also results in thermal degradation during pasteurization of soy milk. Soy milk can be pasteurized at 95 °C for 15 min or UHT processed at 150 °C for 1–2 s. Fermentation process leads to 76% loss of isoflavone content, mainly caused by leaching from the materials during the soaking, de-hulling, and cooking steps (Wang et al., 1996). Despite some reported data, degradation of isoflavones during processing and storage remains an unsolved problem, mainly due to the complexity of conversions between different forms in separate steps or simultaneously (Shimoni, 2004). In addition, malonyl isoflavones stored under UV-Vis light exhibited accelerated degradation and their glucosides were not affected (Rostagno et al., 2005). γ-irradiation at doses of 2, 5, and 50 kGy applied on defatted soy flour had insignificant effect on isoflavone content and its profile (Aguiar et al., 2009).
Enzymes are one of the factors in promoting color fading during fruit and vegetable processing, especially the endogenous enzymes such as glycosidases, peroxidases, and polyphenol oxidases (PPO) released upon tissue maceration. In blueberry, endogeneous PPO oxidizes monophenols and hydroxycinnamic acid derivatives to o-diphenols and o-quinones, which further react with anthocyanins to form brown products (Kader et al., 1998). Also, PPO can oxidize anthocyanins in blueberry during processing (Rossi et al., 2003). Anthocyanidin glucosides are influenced by glucosidases resulting in the formation of the highly labile aglycones, which in turn oxidize easily, ultimately leading to color fading accompanied by browning (Stintzing and Carle, 2004). Due to steric hindrance, the sugar moiety of anthocyanins limits their suitability as a substrate for PPO; however, β-glucosidase can catalyze, and further remove the sugar moiety in anthocyanins, giving rise to the formation of anthocyanidins, which then can be more easily oxidized by PPO (Zhang et al., 2005).
There are several enzymes associated with betalain stability, including peroxidase (Martínez-Parra and Muñoz, 2001), PPO (Escribano et al., 2002), β-glucosidase (Zakharova and Petrova, 2000), and betalain oxidase, which may account for betalain degradation and color losses if not properly inactivated by blanching. Generally, endogenous enzyme activities during processing and storage may contribute to decolorization following decompartmentation. The resulting degradation products through the above enzymes are similar to those of thermal, alkaline, or acid degradation. Shih and Wiley (1981) found that the optimum pH for enzymatic degradation of both betacyanins and betaxanthins was around 3.4. Membrane-bound and cell wall-bound peroxidases in red beet were identified (Wasserman and Guilfoy, 1984). In red beet, peroxidase was stable at pH 5–7 with a greatest activity at pH 6, whereas PPO had optimum activity at pH 7, retaining its stability at pH 5–8. Furthermore, red beet peroxidase was inactivated at temperatures above 70 °C, while PPO displayed thermal stability, losing its activity above 80 °C (Parkin and Im, 1990). Betacyanins appeared to be more readily degradation catalyzed by peroxidases than betaxanthins, while betaxanthins were more prone to chemical oxidation by H2O2, this is supported by the inhibition of betaxanthin oxidation upon the addition of catalase (Wasserman et al., 1984). PPOs isolated from red beet also belong to decolorizing enzymes. Betaxanthin and betacyanin degradation was found to be complete after 1 h, while the maximum PPO activity was observed to be close to the greatest pigment concentration (Shih and Wiley, 1981). Endogenous or exogenous β-glucosidase could catalyze degradation of pigments. However, both malonylated anthocyanins (Zryd and Christinet, 2004) and acylation of betalains were observed to inhibit pigment cleavage by endogenous or exogenous β-glucosidase, leading to enhanced color retention upon enzymation during food processing. In addition, a betalain oxidase in red beet was found to break down betanin into cyclo-Dopa 5-O-β-glucoside, betalamic acid, and 2-hydroxy-2-hydro-betalamic acid (Zakharova et al., 1989).
There are relationships between enzymes and the stability of carotenoids. The major cause of carotenoid destruction during processing and storage of foods is enzymatic oxidation. Enzymatic degradation of carotenoids may be a more serious problem than thermal decomposition in many foods. Also enzymatic activity is the main determinant of carotenoids preservation. As a result of their antioxidant activity, the carotenoids are easily degraded by exposure to hydroperoxides. During processing, naturally occurring enzymes (mainly lipoxygenase) catalyze the hydroperoxidation of polyunsaturated fatty acids, such as linoleic acid, producing conjugate hydroperoxides. Radicals from the intermediate steps of this reaction are responsible for oxidative degradation of carotenoids. Blanching before continuous processing is an efficient way to restrain the activity of enzymes. The effect of blanching on carotenoids is generally believed to be due to the inactivation of peroxidase and lipoxidase activity (Lavellia et al., 2007).
In tea leaves, PPO exists separately from catechins. During tea manufacturing, the rolling process causes the breakage of plant cells, and thus allows enzymatic oxidation of catechins to occur. Both 3’–4’ and –4’–5’-hydroxylated catechins can be affected by PPO, especially for the o-diphenol (Balentine, 1997). Peroxidase is also found in tea, but plays a very limited role in oxidation or fermentation reactions.
In vegetable tissue, glucosinolates are always accompanied by a glucosinolate-hydrolyzing thioglucosidase – myrosinase. In intact plants, the enzyme and the substrate occur in separate tissue compartments. Conversion of glucosinolates into active compounds by myrosinase only takes place after cell disruption such as by mastication or processing. The enzymatic reaction yields glucose and an aglycone, which spontaneously decomposes into a wide range of products depending on the reaction conditions, such as pH, substrate, and the cofactors including ascorbic acid, ESP, and ferrous ions. For example, in the presence of residual ESP activity at these stages (microwaved up to 45 s or steamed up to 210 s) of cooking cabbage, despite possessing the highest myrosinase activity, the yield of allyl isothiocyanate (AITC) from sinigrin was minimal. But with further cooking (microwaved for 120 s or steamed for 420 s) and denaturation of ESP (ESP activity was significantly reduced at a temperature of 50 °C and above) the production of AITC from the hydrolysis of cooked cabbage increased with a proportionate reduction in formation of the cyanoepithioalkane. The high yield of AITC on hydrolysis was due to the denaturation of ESP despite the low myrosinase activity as cooking time extended. It has been suggested that ESP may cause allosteric inhibition of myrosinase and 1-cyano-2,3-epithiopropane (CEP) from sinigrin in cabbage in the presence of residual ESP activity (Rungapamestry et al., 2006). Some of the hydrolysis products have an undesired effect on odor and taste. For instance, bitterness can be caused by gluconapin, sinigrin, and 5-vinyloxazolidine-2-thione. AITC produces a pungent and lachrymatory response upon chewing and cutting of Brassica. Since odor and taste are important to consumers in selecting the health-promoting vegetables, it is of interest to study the activity of myrosinase (Ludikhuyze et al., 2000; Verkerk and Dekker, 2004).
Myrosinase is heat sensitive. Broccoli myrosinase was stable until 45 °C, and its activity was reduced by more than 95% after a 10 min treatment at 70 °C. The stability of myrosinase to temperature differs widely from vegetable to vegetable. Myrosinase in a crude red cabbage extract was stable up to 60 °C, while myrosinase in a crude white cabbage extract was only stable up to 50 °C (Verkerk and Dekker, 2004). During processing, the myrosinase activity and glucosinolate concentrations are dependent on the method and duration of processing (Rungapamestry et al., 2006). The activity of myrosinase in cooked cabbage was significantly influenced by cooking treatment, cooking time, and an interaction between the two factors. Myrosinase in microwaved cabbage showed an initial significant decrease of 27.4% in activity after being cooked for 45 s and an abrupt reduction of 96.7% after cooking for 120 s, as compared to raw cabbage. The activity then remained stable in cabbage cooked for up to 420 s. Myrosinase is very stable under pressure. Moderate pressure (50–250 MPa) only had a limited effect on its activity (van Eylen et al., 2008). Pressure treatment at 700 Mpa for 50 min at 50 °C resulted in approximately 5% of enzyme inactivation, while pressure treatment at 750 MPa for 50 min at 50 °C gave rise to an approximately 20% of enzyme inactivation during the isothermal/isobaric conditions, suggesting that the reaction rate increases with elevating pressure. This implies that a high myrosinase activity can still be retained after pressure treatment. As myrosinase is heat sensitive, blanching will lead to myrosinase inactivation. Pressure blanching can be a good alternative to thermal blanching because undesired quality related enzymes such as lipoxygenase (i.e. blanching indicator) can be inactivated while desired nutrition related enzymes can be maintained (Verkerk and Dekker, 2004). On the other hand, cooking methods that retain some of the endogenous myrosinase activity may also be beneficial by increasing the conversion of glucosinolates to isothiocyanates that have great benefit to human beings during chewing (Song and Thornalley, 2007).
The stability of anthocyanins is structure dependent. The modification of anthocyanin structure has an influence on the stability of the natural colorant throughout the food processing and storage. Furthermore, structure modifications also affect anthocyanin bioavailability, metabolism, and biological properties. Acylation in the anthocyanin molecules confers stability as deacylated pigments were found to be less stable, which was reported in sweet potato (Bassa and Francis, 1987), morning glory (Teh and Francis, 1988), and Tradescanina paccida (Malien-Aubert et al., 2001). Further study showed that acylation in anthocyanins boosts both heat and light stabilities, whereas glucosidation stablizes anthocyanins only in the presence of light (Inami et al., 1996). It was proposed that acylation through intramolecular copigmentation (Garzón and Wrolstad, 2001; Baublis et al., 1994) and the sterical conditions given by the glycosylation pattern (Eiro and Heinonen, 2002) stabilize the anthocyanin molecules. Aromatic residues of the acyl groups stack hydrophobically with the pyrylium ring of the flavylium cation and dramatically reduce the susceptibility of nucleophillic attack of water. In general, 5-glycosylated structures break down more easily than 3-glycosides followed by aliphatic acyl-anthocyanins and aromatic acyl derivatives. It was evident that the stability of pelargonidin 3-glucoside was stronger than that of acylated pelargonidin 3-sophoroside-5-glucoside. The copigmentation increases with the degree of methoxylation and glycosylation of the anthocyanin chromophore. The stability of the chromophore is enhanced by preventing the formation of a pseudobase or chalcone. Pelargonidin 3-glucoside and cyanidin 3-glucoside lost 80 and 75% of their color, respectively, after six months of storage; however, cyanidin 3-(2”-xylosyl-6”-glucosyl)-galactoside had 40% of its color left, suggesting that trisaccharidic anthocyanins retained their color better than the monoglucosidic ones due to the sterically compact structure, which inhibits the copigments from intervening with the anthocyanin chromophore to form intermolecular complexes (Mazza and Brouillard, 1990; Eiro and Heinonen, 2002). Polyacylated anthocyanins including Tradescantia pallida, Ipoema tricolor cv Heavenly Blue, red cabbage, and Zebrina pendula have demonstrated great stability during processing, storage, and pH changes (Teh and Francis, 1988; Dangles et al., 1993). Anthocyanins from Zebrina pendula and Ipomoea tricolor Cav. (cultivar Heavenly Blue) have been reported to be very stable (Asen et al., 1977). The pigments in Zebrina pendula have been identified as tricaffeoylcyanidin-3,7,3’-triglucoside and caffeoylferuloylcyanidin-3,7,3’-triglucoside, which exhibited exceptional stability to pH change, being ascribed to the acyl groups which prevented the formation of a pseudobase or chalcone.
Anthocyanin stabilization may be ascribed to acylation by the nonaromatic malonic acid, which is facilitated by hydrogen bonding between the carboxylate group and the core aglycone (Dangles, 1997). For instance, Saito et al. (1988) reported that it boosted stability of both malonynated and glucuronosylated anthocyanins from flowers in the red daisy (Bellis perennis). Glucuronosylated anthocyanins isolated from red daisy (Bellis perennis) flower petals or obtained by enzymatic in vitro synthesis of red daisy glucuronosyltransferase BpUGT94B1 were used to evaluate the effect of glucuronosylation on the color stability of anthocyanins toward light and heat stress (Osmani et al., 2009). Cyanidin-3-O-2″-O-glucuronosylglucoside displayed enhanced color stability under light in comparison with both cyanidin 3-O-glucoside and cyanidin 3-O-2″-O-diglucoside. However, there was no difference in heat stability observed among monoglucosylated, diglucosylated, and glucuronosylated cyanidin derivatives, whereas the glucuronosylated elderberry extract exhibited increased heat stability. Glucuronosylation of around 50% of total anthocyanins in elderberry extract led to increased color stability in response to both heat and light, suggesting that enzymatic glucuronosylation may be utilized to stabilize natural colorants in industry. Some studies indicated that acylated anthocyanins demonstrate increased resistance to heat, light, and SO2, which may have contributed to glycosidic residues as spacers in folding, assuring the correct positioning of the aromatic rings (Figueiredo et al., 1996). Rommel et al. (1992) reported that acylated anthocyanins are less prone to color changes via endogenous β-glucosidase. Additionally, acylated structures had higher resistance to heat and light degradation (Inami et al., 1996).
Structurally, condensation of betalamic acid with amino compounds or cyclo-Dopa leads to the different stability of betazanthins and betacyanins. Betacyanins were much more stable than betaxanthins at ambient temperature (Sapers and Hornstein, 1979) and heat (Herbach et al., 2004a). The stability of betacyanin could be explained by substitution with aromatic acids because of intramolecular stacking, which is ascribed to the U-shape folding of the molecule in prevention of the aldimine bond from hydrolysis. Among different betacyanins, glycosylated structures are more stable than aglycones, due probably to the higher oxidation-reduction potentials of the former (von Elbe and Attoe, 1985). In addition, the sterical position of the aromatic acid was assumed to affect stability, with 6-O-substitution being more effective than 5-O-substitution (Schliemann and Strack, 1998). The esterification of betacyanins with aliphatic acids was also found to result in enhanced pigment stability (Barrera et al., 1998). The study showed that pigment solutions of Myrtillocactus geometrizans (Martius) Console appear to be more stable than the respective solutions in red beet, due partly to the existence of betanidin 5-O-(6′-O-malonyl)-β-glucoside in Myrtillocactus geometrizans solution. Herbach et al. (2005) reported that phyllocactin and hylocerenin were less susceptible to hydrolytic cleavage than betanin, implying the protection of the aldimine bond by aliphatic acid moieties. Additionally, phyllocactin and hylocerenin appear to be decarboxylated upon thermal treatment. The decarboxylated betacyanins show absorption maxima identical to their precursors, and possess boosted pigment integrity. Therefore, hylocerenin solutions have higher chromatic and tinctorial stability towards thermal degradation than betanin-based solutions, although the half-life of heated betanin was 11-fold higher than that of vulgaxanthin I. Phyllocactin solutions were found to be less stable because of substitution with malonic acid, which is prone both to cleavage of the carboxyl group at the β-position and to deacylation. Moreover, betanidin has 17-fold higher half-life than isobetanidin upon degradation by active oxygen species, which has been ascribed to the glycosylation and the lower oxidation–reduction potential of betanidin when compared to betani (von Elbe and Attoe, 1985).
Copigmentation is a natural phenomenon and occurs in fruit and vegetable-derived products such as juices and wines. It is considered as an interaction in which pigments and other non-colored organic components form molecular associations or complexes, leading to an enhancement in the absorbance and/or a shift in the wavelength of the maximum absorbance of the pigment by being detected both as a hyperchromic effect and as a bathochromic shift (Baranac et al., 1996). As one of the color stabilizing mechanisms, intermolecular copigmentation by the formation of anthocyanins and copigments (mostly polyphenolics) can explain the hyperchromic and bathochromic effects. In principle, it is formed by stacking the copigment molecule on the planar polarizable nuclei of the anthocyanin-colored forms. Therefore, the nucleophilic attack from water at position 2 of the pyrylium nucleus, which results in colorless hemiketal and chalcone forms, is partially prevented. Additionally the copigment molecule interacts with the excited state anthocyanin more strongly than with the ground state anthocyanin, exhibiting bathochromic shift (Alluis et al., 2000). In copigmentation, an anthocyanin chromophore is covalently linked to an organic acid, a simple phenolic acid, an aromatic acyl group, or a flavonoid (Bloor and Falshae, 2000). It would be most efficient if anthocyanin and copigment moieties are covalently linked. For example, the sugar residues of anthocyanins are acylated by phenolic acids. Copigmentation provides brighter, stronger, and more stable colors than those produced by anthocyanin alone. On the other hand, anthocyanins make reactions with other phenolics via weak hydrophobic forces to form loose intermolecular interactions. Intermolecular copigmentation reactions have long been elucidated in wines (Boulton, 2001). Simple anthocyanin molecules copigmentation in fruits, vegetables, and beverages has also been investigated. It was observed that the addition of copigments enhanced anthocyanin color stability during storage (Eiro and Heinonen, 2002). For example, ferulic and caffeic acid addition dramatically boosted the color of pelargonidin 3-glucoside throughout a six month storage period, being 220 and 190% of the original color intensity, respectively, at the end of storage. On the other hand, the addition of gallic, ferulic, and caffeic acids lowered the color stability of the acylated anthocyanin during storage, indicating that these phenolic acids reduce the protective intramolecular mechanism of the acylated anthocyanins. Generally, acylation of the anthocyanindin causes an increase in the relative proportion of the flavylium cation, therefore protecting the red color at higher pH, enhancing the stability of anthocyanin. Also, flavones enhance the color of anthocyanins by stabilizing the quinoidal bases due to intermolecular copigmentation phenomena in the presence of colorless matrix compounds (Mistry et al., 1991). These copigments may also protect the flavylium ion from hydration.
The stability of the anthocyanins is in direct proportion to phenolic concentration (Bakowska et al., 2003), suggesting that copigmentation confers the increase in stability of anthocyanins. Additionally, the copigmentation of caffeic acid enhanced the stability of the grape anthocyanins in a yoghurt system (Gris et al., 2007). Furthermore, polysaccharide copigmentation boosted anthocyanin stability in Hibiscus sabdariffa L. in the solid state (Gradinaru et al., 2003). Eiro and Heinonen (2002) studied intermolecular copigmentation with five anthocyanins (pelargonidin 3-glucoside, cyanidin 3-glucoside, malvidin 3-glucoside, acylated cyanidin trisaccharide, cyanidin trisaccharide) and five phenolic acids (gallic, ferulic, caffeic, rosmarinic, chlorogenic) acting as copigments. A UV-visible spectrophotometer was used to monitor the hyperchromic effect and the bathochromic shift of the pigment–copigment complexes. The stability of the complexes with different molar ratios of the anthocyanin-copigment was examined during a storage period of six months. During the storage period, cyanidin 3-(2″-xylosyl-6″-(coumaroyl-glucosyl))-galactoside exhibited the maximum color stability among five anthocyanins. The strongest copigmentation reactions occurred in malvidin 3-glucoside solutions. Malvidin 3-glucoside lost its color quickly, disappearing after 55 days. The greatest copigments for all anthocyanins were ferulic and rosmarinic acids.
Baublis et al. (1994) investigated stabilities of anthocyanins from Concord grapes, red cabbage, tradescantia, and ajuga by using RP-HPLC analysis. Except tradescantia, the other three pigments exhibited approximately 90% degradation after being stored for 15 days. Further analysis found that copigments such as rutin, chlorogenic acid, and caffeic acid could assist in intermolecular stabilization of anthocyanins in tradescantia. Such increased stability in tradescantia was attributed to B ring substitution of the chromophore, intramolecular copigmentation, and the high degree of acylation, enhancing in stability by inhibiting hydration and fading. The study of colorants’ stability has been conducted in sugar and non-sugar drink models at three pH values (3, 4, and 5) under thermal and light conditions mimicking rapid food aging (Malien-Aubert et al., 2001). It was concluded that colorants rich in acylated anthocyanins (purple carrot, red radish, and red cabbage) show great stability due to intramolecular copigmentation. For colorants without acylated anthocyanins (grape-marc, elderberry, black currant, and chokeberry), intermolecular copigmentation confers a major color protection. Colorants with high amount of flavonols and with the highest copigment–pigment ratio display a remarkable stability. No singinficant color change was found by the addition of sugar. The effect of the copigmentation on the kinetics and thermodynamics of purple potato peel anthocyanins was measured by the shift of the exothermic peaks with the differential scanning calorimetry (DSC) analysis in both liquid and solid states (Zhang et al., 2009). It was observed that citric acid monohydrate and glucose increased the stability of purple potato peel, while ascorbic acid lowered the stability of purple potato peel by the activation–energy evaluation in the liquid state. On the other hand, the copigmentation with the ascorbic acid, citric acid monohydrate, and glucose improved the stability of purple potato peel by the transition–temperature evaluation in the solid state.
An increase in color intensity in strawberry beverages has been reported after addition of phenolic acids serving as copigments (Rein and Heinonen, 2004). Heat stability and copigmentation behavior of purified strawberry anthocyanins (PSA) in the presence of rose petal polyphenolics (RPP), along with strawberry beverage color change during thermal treatment upon the addition of polyphenolic copigments in RPP has been examined (Mollov et al., 2007). The copigments are sinapic acid, chlorogenic acid, and caffeic acid. The results revealed that the addition of polyphenolic copigments extracted from distilled RPP lowers the thermal degradation of anthocyanins, permiting enhanced color stability of the processed strawberries. For instance, after 1 h heating at 85 °C, the non-copigmented and copigmented strawberry anthocyanins retained 71 and 79% of relative concentration, respectively. High concentrations of copigments such as flavonols (quercetin and kaempferol) have been reported (Henning, 1981). Anthocyanin polymerization mediated by flavan 3-ols has been found in strawberry jam, red raspberry jams (García-Viguera et al., 1999), and wines (Rivas-Gonzalo et al., 1995) during storage. Aside from intramolecular stabilization mechanisms, flavonoids as copigments can contribute to anthocyanin stability and color tonation, due mainly to intermolecular interactions by aromatic ring stacking and H-bonding with the anthocyanins (Malien-Aubert et al., 2001; Eiro et al., 2002). A pigment was formed in the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and catechin (Escribano-Bailón et al., 2001). The stability of this pigment in relation to pH, discoloration by SO2, and storage in aqueous solution was examined. The color of the pigment exhibited more stability with regard to bleaching by SO2 than that of malvidin 3-O-glucoside. When the pH was raised from 2.2 to 5.5, the pigment solution became more violet, whereas anthocyanin solutions were almost colorless at pH 4.0, indicating that the anthocyanin moiety of the pigment was protected against water attack, and thus the formation of its quinonoidal forms was favored.
Catechins can react with anthocyanins to form copigments though non-covalent bonds, which results in the red wine color changes during maturation and aging (Mazza et al., 1993; Gonzalez-Manzano et al., 2008). As a copigment, catechins were reported to stabilize anthocyanin and inhibit its degradation during UV treatment (Parisa et al., 2007).
Isoflavones extracted from red clove, including formononetin, biochanin A, and prunetin, were reported to form copigments with anthocyanin in muscadine grape juice and wine and to improve color stability (Talcott et al., 2005).
Dangles et al. (1992) have studied anti-copigmentation. It was shown that cyclodextrins expedited color loss of the anthocyanin solution by forming inclusion complexes with the colorless carbinol pseudobase and the yellowish chalcones. It was proposed that starch-rich foods might also be susceptible to a fading reaction upon processing.
Sulfite has an effect on anthocyanin stability (Berké et al., 1998). It has been shown to lose color, but, in an almost reversible reaction. Addition of SO2 and maintaining a given pH during processing of blue wheat whole meals or of the isolated anthocyanins play a crucial role in stabilizing pigments (Abdel-Aal et al., 2003). The optimal SO2 concentrations were 500–1000 ppm for whole wheat meals and 1000–3000 ppm for isolated anthocyanins.
The addition of sodium hydrogen sulfite has been reported to possess antioxidant activity (Lindsay, 1996). Although the reason for antioxidant activity is complex, the possible reaction of sulfite with carbonyl groups, reducing sugars and disulfide bonds in proteins may result in a protective effect. In addition, the application of sodium hydrogen sulfite prevents the growth of microorganisms such as fungi (Cinar, 2005).
The advantage of using sodium hydrogen sulfite was undoubtful in retaining a high amount of carotenoids; however, using of sulfite salts in foods has been severely regulated because of possible adverse reactions to these compounds by asthmatic patients. Finding a proper substitute may be a solution to this problem in the future.
It has been shown that some kinds of glucosinolates could react with sulphur dioxide. The pungency of mustard, cress, and radish is dependent upon the hydrolysis of glucosinolates and on the nature and amount of the products thus formed. Condiment mustard is prone to oxidation and certain continental type preparations contain SO2 to inhibit both this process and subsequent discoloration of the product. It is possible for the pungent 2-propenyl isothiocyanate to react with SO2 to form 2-propenyl aminothiocarbonyl sulphonate. Not only does this reaction lead to a loss of pungency, but decomposition of the involatile sulphonate to 2-propenyl thiol and related sulphides is linked to the development of a garlic-like off-odor on storage (Austin et al., 1968b). The addition of SO2 to horseradish powder has a detrimental effect on flavor. Preliminary experiments have shown that the reaction of 2-propenyl isothiocyanate with SO2 is much faster than that of 2-phenylethyl isothiocyanate, the other major component of horseradish essence. This presumably leads to the reduction in odor already mentioned (Chevolleau et al., 1997).
In order to reduce the degradation of carotenoids, there are many treatments prior to the dehydration process. One of the common treatments is pre-soaking (Tai and Chen, 2000). Being soaked in 1% sodium hydrogen sulfite solution prior to dehydration can reduce the loss of carotenoids in daylily flowers. Comparing with the daylily flowers, which are dried by hot air directly, the loss of zeaxanthin was reduced by 54%, β-cryptoxanthin 44%, all-trans-lutein 28%, 13-cis-lutein 39%, and all-trans-β-carotene 44%. Obviously, the application of sodium hydrogen sulfite solution could protect carotenoids from undergoing oxidative degradation during hot-air-drying.
Kaack and Austed (1998) investigated the effect of vitamin C on flavonoid degradation in 13 European elderberry cultivars. They revealed that ascorbic acid protected the anthocyanins, but not quercetin, from oxidative degradation. Under condition of high concentration of oxygen, anthocyanin level reduced dramatically during juice processing and storage. However, purging of the elderberry juice with N2 and/or addition of ascorbic acid decreased the oxidative degradation rate of the cyanidin-3-glucoside, cyanidin-3-sambubioside, and quercetin. On the other hand, ascorbic acid impairs anthocyanin stability, although betalains can effectively be stabilized by ascorbic acid (Shenoy, 1993). Nonetheless, anthocyanins after chelating metal ions were reported to be capable of preventing ascorbic acid oxidation by complex formation (Sarma et al., 1997). The addition of ascorbic acid in reduction of color stability of strawberry syrup was reported. Concurrent loss of anthocyanin pigments and ascorbic acid has been observed in different fruit juices such as strawberry and blackcurrant juices (Skrede et al., 1992), as well as cranberry juice (Shrikhande and Francis, 1974), which gave rise to both anthocyanin and ascorbic acid degradation by either H2O2 formed through oxidation or condensation of ascorbic acid directly with anthocyanins (Markakis, 1982). The study from Choi et al. (2002) characterized the change in pigment composition with two different levels of ascorbic acid content in blood orange juice placed in HDPE plastic bottles, pasteurized, and stored at 4.5 °C. The result revealed that ascorbic acid degradation was highly correlated (r > 0.93) to anthocyanin pigment degradation, suggesting a possible interaction between the two compounds in stored blood orange juice.
Açai anthocyanin stability against hydrogen peroxide (0 and 30 mmol/L) over a range of temperatures (10–30 °C) was evaluated, and further compared to other anthocyanin sources including black carrot, red cabbage, red grape, purple sweet potato, and a noncommercial extract from red hibiscus flowers (Hibiscus sabdariffa) (Del Pozo-Insfran et al., 2004). Also, pigment stability in a model beverage system was measured in the presence of ascorbic acid and naturally occurring polyphenolic cofactors. It was found that anthocyanins were the most stable in the presence of hydrogen peroxide in red grape, while açai and other pigments rich in acylated anthocyanins exhibited lower color stability in a temperature-dependent pattern. In the presence of ascorbic acid, acylated anthocyanin sources usually possessed improved color stability. Improvement of color stability in blood orange juice by the addition of tartaric acid, tannic acid, and antioxidant agents was reported (Maccarone et al., 1985). There was a positive correlation between anthocyanin content and juice stability in the conditions of three different storage temperatures with aseptic glass bottles (Bonaventura and Russo, 1993).
Potential interactions of betalains in red beet with their matrix compounds have been examined in detail (Delgado-Vargas et al., 2000). The presence of matrix compounds such as organic acid supplements efficiently boosted betacyanin stabilization. The effective result was reported in pitaya juice heated at pH 4 when adding 1.0% ascorbic acid (Herbach et al., 2006a). Addition with some compounds, especially antioxidants, stabilizes betalain. For instance, the supplementation of ascorbic and isoascorbic acids was found to enhance betalain stability by O2 removal, although different optimum ascorbic acid concentration ranges of 0.003–1.0% were reported (Attoe and von Elbe, 1984). The addition of ascorbic, isoascorbic, and citric acids to purple pitaya juice and purified pigment preparation can stabilize betacyanins (Herbach et al., 2006a). On the contrary, Pasch and von Elbe (1979) revealed that 1000 ppm ascorbic acid as a pro-oxidant decreased half-life time of betanin, being attributed to hydrogen peroxide bleaching effect during ascorbic acid degradation. There exist some discrepancies between ascorbic and isoascorbic acid in enhancement of betalain stability (Barrera et al., 1998; Herbach et al., 2006a), even though isoascorbic acid displayed superior oxygen conversion due to its higher redox potential. For example, ascorbic acid exhibited greater betacyanin retention than isoascorbic acid upon utilization at identical concentrations in purple pitaya. The ineffectiveness of phenolics in betalain stability suggests that betanin oxidation does not involve a free radical chain mechanism (Attoe and von Elbe, 1985). Chen et al. (2007) researched on different drying treatments to the stability of Taiwanese mango. It was reported that the pre-soaking step may prevent the epoxy-containing carotenoids from degradation during the subsequent extraction procedure. They also compare the protective effect to different kinds of carotenoids (violaxanthin, neochrome, neoxanthin, and so on) by soaking in ascorbic acid with soaking in sodium hydrogen. Luteoxanthin can be formed from violaxanthin under acidic conditions. Neochrome can be attributed to conversion of neoxanthin under acidic conditions too. It was showed that different kinds of carotenoids fit to different soaking conditions.
Ascorbic acid has been reported to significantly increase the stability of catechins at a neutral or alkaline pH, maybe due to its antioxidant properties or its ability to lower oxygen concentration dissolved in solutions (Chen et al., 2001), however, it can also act as prooxidant and accelerate the degradation of catechins. In addition to ascorbic acid, other reducing agents, such as dithiothreitol (DTT), tris (2-carboxyethyl) phosphine (TCEP), as well as encapsulation in chitosan–tripolyphosphate nanoparticles, were also found to improve the stability of catechins in an alkaline solution .
In the presence of ascorbic acid, indolylglucosinolates give rise to a variety of products on hydrolysis by myrosinase. A feature of plant myrosinase is its activation by ascorbate. Early work had shown that ascorbate created an allosteric effect on the activity of the enzyme. Subsequently the mechanism of ascorbate activation has been worked out where it was shown that ascorbate acts as a catalytic base (Schneider and Becker, 1930).
It was shown that the content of glucoraphanin was 0.0815 mg/mL when ascorbic acid was added to the extraction, and it remained at 0.0925 mg/mL without this addition. So removing ascorbic acid from extraction upon stored has been suggested (Wang et al., 2009).
Some metal cations including Al3+, Cr3+, Cu2+, Fe2+, Fe3+, and Sn2+ were found to expedite betanin degradation (Czapski, 1990). For instance, metal ions such as Cu+, Cu2+, and Hg2+ combine with betanin to form metal-pigment complexes accompanied by bathochromic and hypochromic shifts. However, the spectra could be reversible by the addition of EDTA (Attoe and von Elbe, 1984), where EDTA inhibits metal-catalyzed betanin degradation by pigment stabilization and metal-pigment complex formation. EDTA improved betanin’s half-life time by 1.5-fold (Pasch and von Elbe, 1979). As a chelating agent, citric acid boosted betacyanin stability (Herbach et al., 2006a), explained by partially neutralizing the electrophilic center of betanin via association with the positively charged amino nitrogen. Interestingly, the addition of EDTA didn’t enhance vulgaxanthin I stability (Savolainen and Kuusi, 1978).
The effect of metal ions on stability of carotenoids was reported. Fe3+, Fe2+, and Al3+ had the negative effect on the stability of carotenoids, whereas Mn2+ had the smaller effect (Yao and Han, 2008). Astaxanthin is one of the few carotenoids containing four oxygen donors. Usually, these oxygen donors can coordinate with heavy metal ions such as Cu(II) and Fe(III). It was found that Cu(II) markedly induces the conversion of trans-astaxanthin to its cis-forms, which mainly consist of 9-cis-astaxanthin and 13-cis-astaxanthin as suggested by UV-visible spectra and HPLC measurements. Increasing either incubation time of Cu(II) and trans-astaxanthin in ethanol or the Cu(II)/astaxanthin ratio gave rise to an increased percentage of cis-isomers derived from trans-astaxanthin. These results provide important information on the effects of dietary factors on the bioavailability and bioactivity of trans-astaxanthin (Zhao et al., 2005).
Catechins can chelate metal ions such as iron and copper, and this may be due to their antioxidant activities by inhibiting transition metal-catalyzed free radical formation. The O-3’4’-dihydroxyl group on the B ring is likely to be the metal binding site (Hider et al., 2001). However, it was suggested that the gallate moiety of the gallocatechins also binds metals (Midori et al., 2001). The same study found that Cu2+ strongly increased the antioxidant activity of EGCG during 2,2′-azobis(2,4-dimethylvaleronitrile) (AMVN)-initiated lipid peroxidation, but Fe2+ adversely affected the antioxidant activity of EGCG (Midori et al., 2001). Although potassium, calcium, magnesium and aluminum are the predominant minerals found in tea (Eden, 1976), there may be weak interaction between catechins and aluminum or magnesium, and catechins do not chelate potassium, calcium (Hider et al., 2001).
(S)-2-Hydroxybut-3-enylglucosinolate of Crambe abyssinica meal has been found to be decomposed by heating in the presence of metal salts including FeSO4, Fe(NO3)3, CuC12, and CuSO4 with Fe2+ and Cu+ being the most active species. N-methylindol-3-methylglucosinolate was self decomposed by Fe2+ (aq) to the nitrile (Zabala et al., 2005). Glucosinolates with a hydroxyl group in the 2 position of the side chain (e.g. 2-hydroxybut-3-enylglucosinolate) have been shown to give thionamides with Fe2+, although an eight-fold excess of Fe2+ is necessary, but it seems unlikely that these compounds would be generated during food processing (Austin et al., 1968a).
The effect of sugar addition on the anthocyanin stability is influenced by anthocyanins’ structure and concentration as well as type of sugar. Anthocyanin stability by linkage of sugar residues could take place by formation of hydrogen bonds between glycosyl groups and the aglycone, which is influenced by the site of glycosylation and the type of sugar added (Delgado-Vargas et al., 2000; Stintzing et al., 2002). At low concentration of sucrose (86 g/L), anthocyanin degradation in blackcurrant, elderberry, and red cabbage extracts was higher in soft drinks compared to buffer systems both at pH 3 (Dyrby et al., 2001). Conversely, the anthocyanins’ stability in strawberry increased after the sucrose concentration increased by 20% (Wrolstad et al., 1990). Additionally, although some anthocyanic extracts displayed lower stability in sugar-added systems, the addition of 20 g/L of sucrose to a pH 3 drink system containing red cabbage and grape extracts did not affect light and thermal stability of the anthocyanins (Duhard et al., 1997). In the study from Queiroz et al. (2009), 200 g of blueberries were mixed with 200 g of sugar to make jams. A 5 g measure of blueberry samples ground to paste with mortar and mixed with water was used to evaluate the degradation of anthocyanins and anthocyanidins at 100 °C. °Brix in jams play an important role in degradation of anthocyanins and anthocyanidins, where 64–76 °Brix caused 20–30% degradation, whereas 80 °Brix led to degradation of 50–60%, suggesting that anthocyanin degradation is realted to °Brix of the samples as well as the rate of sugar degradation resulted from Maillard reaction (Duhard et al., 1997). Anthocyanin thermostability was affected by sugar addition such as fructose and glucose (Rubinskiene et al., 2005b). Additionally, anthocyanidins’ degradation in blueberry jam displayed similar patterns to that mintored for anthocyanin degradation in jam. Jams with high 80 °Brix showed degradation approximately 60%. The degradation rate constant of anthocyanins in an isotonic soft drink system in the dark was 6.0 × 10−2 h−1 for acerola and 7.3 × 10−4 h−1 for açai, respectively, indicating that the addition of sugars (55 g of sucrose, 5.5 g of fructose, 5.5 g of glucose in 1 l) and salts (0.15 g of sodium benzoate, 3.0 g of citric acid, 0.14 g of sodium citrate, 0.5 g of sodium chloride, 0.5 g of potassium chloride, and 0.4 g of potassium phosphate monobasic in 1 l) had a negative effect on the anthocyanin stability (Vera de Rossoa and Mercadante, 2007).
The effect of fluorescent light on the degradation rates of the major cranberry anthocyanins was assayed in model systems in the presence of oxygen in the temperature range 25–55°C (Attoe and Von Elbe, 1981). The study revealed that light degraded most of anthocyanins at 40 °C. Light exhibited a significant effect on anthocyanin degradation in the presence of molecular oxygen. Conversely, light-induced trans-cis-isomerization of coumaric acid substituents in anthocyanins offers a way to stabilize color (George et al., 2001). Acerola is a good source of ascorbic acid, carotenoids, as well as cyanidin-3-α-O-rhamnoside and pelargonidin-3-α-O-rhamnoside. Açai is rich in the anthocyanins cyanidin-3-glucoside and cyanidin-3-rutinoside. The addition of anthocyanic extracts from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) as a colorant and functional ingredient in isotonic soft drinks and in buffer solution was evaluated (Vera de Rossoa and Mercadante, 2007). The study revealed that the degradation of anthocyanins from both tropical fruit sources followed first-order kinetics in all the systems under air, either in the presence or absence of light. Light exerted a significantly negative influence on anthocyanin stability in both açai added systems, isotonic soft drink (p < 0.001) and buffer (p < 0.001). The degradation rate of açai anthocyanins extract in the buffer system was 7.1 times faster under light than in the dark. Additionally, in the presence of light, the anthocyanin degradation was 1.2 times quicker for acerola and 1.6 times quicker for açai in soft drink isotonic systems, as compared to their respective buffer solutions.
Light was found to affect betalain stability (Cai et al., 1998; Herbach et al., 2006a), which can be attributed to betalain absorption of light in the UV and visible range resulting in excitation of electrons of chromophore to a more energetic state, thus bringing about higher reactivity or lowered activation energy of the molecule (Jackman and Smith, 1996). An inverse relationship between betalain stability and light intensity in the range 2200–4400 lux was reported by Attoe and von Elbe (1981). Deterioration of betalain stability influenced by light was observed at temperatures below 25 °C, while there was no impact of light on stability at temperatures above 40 °C (Huang and von Elbe, 1986). Additionally, light-induced degradation was found to be oxygen dependent. On the other hand, the addition of 0.1% isoascorbic acid and 1.0% ascorbic acid, respectively, into red beet and purple pitaya juices was shown to inhibit light-induced betacyanin degradation during juice storage.
β-carotene effectively protects oil against light deterioration by quenching singlet oxygen even at concentration below 20 ppm. But light can make the carotenoids unstable. Carotenoids are known to exist in different geometric forms (cis- and trans-isomers). These forms may be interconverted by light (Rock, 1997). Stability of biomass of two microalgae species was predominantly affected by light, followed by oxygen content, whereas the storage temperature is only important to a lesser extent (Gouveia and Empis, 2003). A similar conclusion has been made by Lin and Chen (2005), that light has a greater influence on isomerization and degradation of cis-isomers of β-carotene than temperature. For lycopene pigments, light effects were more destructive than those of the high temperature (Nachtigall et al., 2009). The sensitivity of carotenoids to non-sensitized direct light is dependent on the wavelength of irradiation. Under fluorescent light, which means the involvement of singlet oxygen was ruled out, the higher the unsaturation, the slower is the rate of carotenoids autoxidation. This reveals that a higher degree of unsaturation offers a greater protection to β-carotene against autoxidation. Also it has been reported that the deterioration of the carotene was probably due to absorption of light in the visible region. The photocatalyzed oxidation of β-carotene is also more severe in ultraviolet than in visible light (Bonnie and Choo, 1999). Exposure to light, especially direct sunlight or ultraviolet light, induces trans-cis photoisomerization and photodestruction of carotenoids. Thus, work on carotenoids must be performed under subdued light; for example, all the extraction procedures were conducted under dimmed light to avoid isomerization or degradation loss of carotenoids (Chen et al., 2007). As compared with pure carotenoids, carotenoid-arabinogalactan complexes exhibit an enhanced stability toward photodegradation (Polyakov et al., 2010). Open columns and vessels containing carotenoids should be wrapped with aluminum foil, and thin-layer chromatography development tanks should be kept in the dark or covered with dark material or aluminum foil. Polycarbonate shields are available for fluorescent lights, which are notorious for emission of high-energy, short-wavelength radiation (Sajilata et al., 2008).
In darkness, I3C was the unique compound formed that accounted for the total radioactivity after 1 h. A very weak proportion of 3,3′-diindolylmethane (DIM) (4%) was observed only after a 24 h incubation period. This indicates that the enzymatic activity of myrosinase is not influenced by the lighting conditions but indole derivatives are more reactive in the presence of light and that condensation must be photochemically initiated (Lopez-Berenguer et al., 2007). It has been reported that the content of glucoraphanin was 0.0548 mg/mL when the extraction was illuminated for nine days. If stored in darkness for nine days, it remained at 0.0775 mg/mL. So glucoraphanin should be stored away from light and packaged (Wang et al., 2009).
Light storage can be more destructive to each carotenoid and vitamin A than dark storage during the storage of carrot juice. The 9-cis isomers were the major carotenoid isomers formed in carrot juice under light storage, while 13-cis was favored under dark storage. In canned tomato juice, because it is in a dark environment and the exposure of juice to atmospheric oxygen is excluded, the degradation of all-trans plus cis forms of lutein preceded slowly. Compared to dark storage, most cis-isomers of β-carotene were at lower levels in canned tomato juice, which can be due to the fact that the canned juice is in a dark environment and the exposure to air is excluded (Xu et al., 2006). Generally, lower storage temperature, lower oxygen pressure, comparatively dry, under dark condition, addition of antioxidant like BHT and so on, could make carotenoids relatively stable in storage process.
The aim of post-harvest treatment is to manipulate metabolism in fruits and vegetables during storage in order to extend shelf life. Ferreres et al. (1996) reported on the stability of the anthocyanin pigments of Spanish red onion (cultivar ‘Morada de Amposta’) stored in perforated films for seven days at 8 °C. A small increase in anthocyanins was found after one day of storage, followed by a decrease after seven days of storage. It was observed that there was a big difference in the stability of the individual anthocyanins. The glucosides were more stable than the corresponding arabinosides. The malonated anthocyanins were more stable than the corresponding non-acylated pigments, suggesting that anthocyanin acylation is one of the major structural factors influencing pigment stability, which is in agreement with previous reports (Mazza and Miniati, 1993). Rodríguez-Saona et al. (1999) have evaluated two acylated pelargonidin-based anthocyanins from red-fleshed potatoes (Solanum tuberosum) and red radishes (Raphanus sativus) and two extraction methods (C-18 resin and juice processing) during 65 weeks of storage at 25 °C and 2 °C in the dark. It was shown that higher stability was obtained in juices with C-18 purified radish anthocyanins (22 week half-life) and lowest stability with potato juice concentrate (ten week half-life). Anthocyanin degradation greatly depended on storage temperature, with degradation kinetics following a quadratic model at 25 °C and a linear model at 2 °C. The addition of 10, 20, and 40% of sucrose by weight to IQF strawberries prior to freezing displayed a protective effect on the anthocyanin degradation after storage of −15 °C for three years (Wrolstad et al., 1990). The sucrose addition also delayed browning and polymeric color formation. Additionally, the effect of thawing on anthocyanin stability was examined by maintaining strawberry samples at 20 °C for 24 h. The thawed samples were then refrozen at −80 °C and powdered for analysis. The result revealed that thawing accelerated the color decomposition rate.
The effect of cultivars (Chandler, Tudla, and Oso Grande) and storage temperature on the color stability of strawberry (Fragaria × ananassa) jam was investigated (García-Viguera et al., 1999). Strawberry fruit and sugar were mixed with pectin and citric acid, manufactured for 15 min at 78 °C under 500 mm Hg vacuum, heated at 92 °C and allowed to cool to 88 °C before filling into glass jars. Jams were stored at 20, 30, or 37 °C in the dark for 200 days. The result indicated that Oso Grande cultivar showed the highest anthocyanin degradation (35.30% cyanidin 3-glucoside, 44.71% pelargonidin 3-glucoside, and 33.02% pelargonidin 3-rutinose) during processing. There were no differences in degradation kinetics of anthocyanins among cultivars at the same temperature. However, differences were found in anthocyanin stability under storage temperatures, being much more stable at the lower temperature, which was in agreement with previous studies (Jackman and Smith, 1996). Patras et al. (2009) studied the effect of storage time and temperature on degradation of anthocyanins in strawberry jam. The data exhibited that lightness value significantly reduced (p < 0.05) over 28 days of storage at 4 and 15 °C. The reaction rate constant for anthocyanins increased from 0.95 × 10−2 day−1 to 1.71 × 10−2 day−1 at 4 and 15 °C. The effect of storage time, temperature, and light on the degradation of monomeric anthocyanin pigments extracted from skins of grape (Vitis vinifera var. Red globe) was evaluated through stepwise regression analysis (Morais et al., 2002). The extract of pigments redissolved in distilled water containing 0.01% HCl were stored in the air at 24, 32, and 40 °C, and analyzed after 1, 3, 6, 8, and 14 days of storage both in light, using a lamp of 1.5 W, and in the dark. It was concluded that the overall decomposition rate of peonidin-3-glucoside and malvidin-3-glucoside was significantly dependent on storage time and temperature. However, light exerted a negligible impact on the decomposition rate.
During the storage of carrot juice, the concentration changes of lutein, α-carotene, β-carotene, and vitamin A in the carrot juice decreased with increasing storage temperature (Chen et al., 1996). Saldana et al. (1976) studied the effect of storage on quality of carrot juice and found that there was no effect on color or β-carotene when carrot juice was stored at 20 °C for nine months. Both isomerization and degradation of β-carotene may proceed simultaneously during the storage of tomato juice. The contents of all-trans plus cis forms of lutein were found to decrease following the increase of storage temperature, implying that degradation may still proceed even in the absence of light. All-trans-lutein was not detected after storage at 4 and 25 °C for five weeks. However, the same phenomenon occurred after storage for four weeks at 35 °C. This result showed that the higher the storage temperature, the faster was the degradation of all-trans-lutein. Similar trends also applied to 9-cis- and 13-cis-lutein, under light the degradation tends to be faster than under dark (Lin and Chen, 2005). Generally, the higher the storage temperature, the greater are the losses of all-trans plus cis- forms of carotenoids and more cis-isomers of carotenoids were formed during storage.
Refrigeration at 4 °C and freezing might be the best preservation processes for maintaining high level of glucosinolates in broccoli (Rodrigues and Rosa, 1999). The frozen vegetables had, however, been blanched by steaming prior to freezing. When stored in a domestic refrigerator (4–8 °C), the contents of individual and total glucosinolates of vegetables decreased during storage for seven days. There were slight changes in the first three days of storage. After storage for seven days the total glucosinolate analyte content was decreased 11–27%. For individual glucosinolates, the losses of glucoiberin, glucoraphanin, and glucoalyssin were higher than of sinigrin, gluconapin, and progoitrin. The loss of the glucoiberin in broccoli was 40–50%, whereas the loss for gluconapin was 5–10% in all vegetables studied. When stored at ambient temperature (12–22 °C), there was no significant decrease in glucosinolate content of the Brassica vegetables studied. But at that time, the vegetables had visibly started to decay. Storage at −85 °C may cause significant loss of glucosinolates due to freeze–thaw fracture of plant cells and accessibility of myrosinase to glucosinolates with subsequent enzymatic conversion of glucosinolates to isothiocyanates during thawing. The loss of individual glucosinolates was 10–53% and the loss of total glucosinolates was 33% – much higher than for storage in a refrigerator at 4–8 °C (Song and Thornalley, 2007).
Lower storage temperature can extend the shelf life of catechins and for ready-to-drink tea beverages. Low temperature (4 °C) and acidic pH (4.0) were found to be the optimal storage conditions for catechin preservation (Bazinet et al., 2010). Addition of butylated hydroxytoluene (BHT) at a level of 0.1% was reported to have a significant effect on longer stability of catechins with over 90% EGCG remaining on day 130 stored at 37 °C (Demeule et al., 2002). BHT in glycerin was also found to improve the t90 (time for 10% degradation to occur) to up to 76 days at 50 °C, which offers a potential for glycerin based vehicles to stabilize EGCG (Proniuk et al., 2002).
At lower storage temperature (10 °C) up to seven days, individual isoflavone concentrations in ethanol extraction solutions remained constant, but at higher temperatures (25 and 40 °C) malonylglucosides degraded to glucosyl forms and aglycones are unchanged (Rostagno et al., 2005). Interestingly, genistein was reported to be able to react with itself or with lysine in the non-enzymatic Maillard browning reaction, thus, leading to loss of isoflavones during storage of soy protein isolates at mild conditions, therefore, a storage condition was suggested at aw less than 0.3 and temperature greater than 4 °C to prevent Maillard reaction (Davies et al., 1998).
A high RH of 98–100% is recommended to maintain post-harvest quality in broccoli (Rodrigues and Rosa, 1999). RH only appears to be a critical factor in glucosinolate retention when post-harvest temperatures rise above approximately 4 °C. For example, glucoraphanin content declined by more than 80% in broccoli heads left at low RH and 20 °C for five days. Similarly, broccoli heads stored in open boxes with low RH at 20 °C showed a 50% decrease in glucoraphanin content during the first three days of storage, whereas heads stored in plastic bags with high RH more than 90% displayed no significant loss at the same temperature. The decrease in glucoraphanin coincided with a marked loss of visual quality (i.e. yellowing), indicating probable loss of membrane integrity and mixing of glucosinolates with myrosinase at low RH (Toivonen and Forney, 2004).
aw plays a crucial role in betanin susceptibility to aldimine bond cleavage because of the water-dependent hydrolytic reaction, a reduced mobility of reactants and limited oxygen solubility. The improvement of betanin stability with lower aw was reported by Kearsley and Katsaborakis (1981), where aw reduction was most effective below 0.63. An increase of around one order of magnitude in betalain degradation rates was observed when aw increased from 0.32 to 0.75 (Cohen and Saguy, 1983). Serris and Biliaderis (2001) revealed that betanin had the greatest degradation at aw of 0.64 in encapsulated beet root pigments, being explained by lowering mobility of the reactants at reduced aw values. Amaranthus pigment powders showed higher stability than the respective aqueous solutions, being ascribed to varying aw values (Cai et al., 1998). Additionally, some stabilizers like pectin, guar gum, and locust bean gum appeared to improve storage stability of red beet solutions by lowering the aw value. Betacyanins’ stability was reported to increase after reduction of aw by spray-drying (Cai and Corke, 2000) and by concentration (Castellar et al., 2006).
The study from Lavellia et al. (2007) gives rise to some practical points about processing and storage conditions required to maintain high carotenoid contents in dehydrated carrots. Partial dehydration of carrots to intermediate moisture levels could be proposed instead of removing water completely, according to the following protocols: (1) reduction of aw values to 0.31–0.54, corresponding to 6–11% of moisture (on wet weight basis) – in this aw range microbial growth is arrested, enzymatic activity and non-enzymatic browning are at minimum, and our data indicate maximum carotenoid stability; and (2) reduction of aw values to 0.54–0.75, corresponding to 11–22% of moisture – In this aw range the microbial growth rate and the enzymatic activity are still at minimum; however, the most effective factors which account for carotenoid stability are still to be investigated. Furthermore, the occurrence of non-enzymatic browning cannot be ruled out. Both criteria should be combined with optimized packaging conditions, which reduce exposure of product to air and light during storage.
There is no direct effect of aw on the degradation of isoflavones in soy protein products, but high aw (above 0.6) may favor the activities of endogenous β-glucosidase, which hydrolyze glucosides to their respective aglycones (Huang et al., 2009).
In the presence of O2, both betanidin and betanin were found to be unstable. The stability of betanin was negatively correlated with oxygen concentration (Czapski, 1990), indicating the involvement of O2 in betanin degradation. The degradation kinetics of betanin influenced by O2 was reported (Attoe and von Elbe, 1984). Conversely, betanin stability was observed to be improved in a N2 environment (Drunkler et al., 2006). It was reported that amaranthin was less stable than betanin under anaerobic conditions (Huang and von Elbe, 1986).
Oxygen was a critical factor in β-carotene degradation. It was also found that oxidation was the major cause of β-carotene destruction. Exclusion of oxygen during storage of powders would extend their shelf life (Wagner and Warthesen, 1995). Carotenoids, even in the crystalline state, are susceptible to oxidation and may be broken down rapidly if samples are stored in the presence of even traces of oxygen. The structures are broken down when attacked by free radicals, such as singlet molecular oxygen and other reactive species. The mechanism of carotenoids oxidative degradation can be described as auto-oxidation:
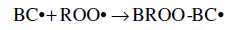 (a)
(a)
 (b)
(b)
 (c)
(c)
When the reaction follows equation (a) then (c), β-carotene is degraded without any free radical being trapped. This is auto oxidation. In contrast, the antioxidant reaction follows equation (a) then (b), with consumption of radicals. Both reactions depend on the oxygen partial pressure (PO2). At high PO2, oxygen addition to the β-carotene-radical adduct is favored, β-carotene-derived peroxyl radicals are formed, and most of the β-carotene are consumed by auto oxidation. However, at low PO2, addition of oxygen to the β-carotene-radical adduct is less favored, and the adduct may trap a second peroxyl radical to produce an antioxidant effect.
In photosensitized oxidation, carotenoids, especially lycopene and β-carotene, are effective quenchers of singlet oxygen (1O2). The quenching of 1O2 by β-carotene can be the physical quenching of: (1) excited sensitizer molecules or (2) singlet oxygen. Physical quenching proceeds by energy transfer from 1O, to the carotenoids molecule. A similar process can occur between a carotenoid and an excited sensitizer. If truly catalytic, the carotenoid should remain intact. However, usually a chemical reaction sets in, destroying the carotenoid molecules (Bonnie and Choo, 1999).
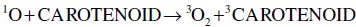 (d)
(d)
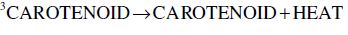 (e)
(e)
Pérez and Mínguez (2000) indicated that a more convenient strategy to prevent carotenoids degradation in oily food additives such as paprika oleoresins should be to minimize contact of food with oxygen. This could be achieved by applying vacuum, use of novel packaging materials, or by encapsulation techniques, thus avoiding oxygen-mediated auto-oxidation reactions.
Storage under regular atmosphere revealed that the keeping quality for all four glucosinolates is between four and seven days, while storage under 1.5 kPa O2 and 15 kPa CO2 showed a keeping quality of at least 14 days, indicating that to preserve the nutritional quality of broccoli, modified atmosphere packing (MAP) is a viable option (Rangkadilok et al., 2002). Controlled atmosphere (CA) storage is very effective in maintaining broccoli quality, and can double post-harvest life (Rodrigues and Rosa, 1999). Ideal atmospheres to maintain quality were 1–2% O2; 5–10% CO2 when temperatures were kept between 0 and 5 °C (Schouten et al., 2009). Care needs to be taken that O2 does not drop below 1% as this can cause the development of off-odors (Cantwell and Suslow, 1999). ‘Marathon’ broccoli heads stored for 25 days at 4 °C, under a CA atmosphere of 1.5% O2, 6% CO2 contained significantly higher glucoraphanin levels than heads stored in air at the same temperature.
Glucoraphanin and glucoiberin contents reflected the rises in total glucosinolates. Transferring heads to air after storage had no effect on glucosinolate content. The reported increase in glucoraphanin under air is puzzling, as it contradicts much of the storage work showing a decline in glucosinolates in broccoli heads held in air at 4 and 20 °C (Forney et al., 1991). As it is often difficult to maintain low temperatures throughout the broccoli distribution and marketing phase and in fluctuating temperatures, MAP can help extend shelf life. Optimum broccoli quality was obtained when atmospheres within MAP reached 1–2% O2 and 5–10% CO2. At 20 °C, however, broccoli in air lost 50% of its glucoraphanin in seven days. In contrast, under MAP there was no significant decrease in glucoraphanin over ten days.
Catechins are effective scavengers and more superior than vitamin C and E with respect to some active oxygen radicals, but they have less prominent effect on hydroxyl free radicals. It was shown that the rate of catechin oxidation increases with pH and concentration of oxygen (Chen et al., 2001; Mochizuki et al., 2002). Another study indicated that as the number of phenolic hydroxyl groups increases, the more rapid they can scavenge peroxyl radicals, thus, EGCG can scavenge peroxyl radicals more quickly than EC and EGC; and, furthermore, the pyrogallol structure in the B ring plays an important role in rapid scavenging ability of catechins, therefore, EGC can scavenge peroxyl radicals more quickly than EC (Kondo et al., 2001). The oxidation mechanism was proposed to undergo sequential steps, involving the loss of hydrogen atoms, generation of semiquinone radical intermediate (reversible), and formation of quinine oxidized product (irreversible).
Phytochemicals are plant-derived secondary compounds that offer functions to improve the overall appearance in foods, and may exert physiological effects beyond nutrition promoting human health and well-being. In order to maintain phytochemicals’ functionality and better physiological and biochemical activities, new technologies and measurements need to be taken to minimize phytochemicals’ degradation. For instance, anthocyanins as colorants commercially have been limited due to their lack of stability and difficult purification. Carotenoids are the natural yellow-orange color range pigments present in a selection of foods. However, they are poorly soluble in water. Betalain sources such as betaxanthin may substitute carotenoids’ utilization in the range of yellow-orange as food colorants. Anthocyanins are considered as the most widely applied natural colors. Nevertheless, the instability of anthocyanins at pH values above 3 makes betacyanins the natural color of selection to offer red-purple color utilized in low acid foods. Additionally, intensified research is needed to fill the gaps of intraspecific variability, horticultural, and technological measures targeting at optimizing phytochemicals’ quality and quantity. Moreover, phytochemical loss can be minimized during processing and storage by selecting the respective temperature and pH regimes as well as minimizing oxygen and light access.
It is becoming evident that beneficial aspects of phytochemicals supporting human defense mechanisms are of increasing interest. However, the biological effects of phytochemicals are far from being clear. For instance, there is little information published with respect to the physiological role of betalains in mammals. Less data were found to be associated with changes of health benefits such as antioxidant capacity of phytochemicals after being subjected to heat, light, pH, and processing treatments. It remains to be clarified if the whole structures or rather their degraded compounds are responsible for the reported bioactivities. The relationship between phytochemicals’ stability changes and health benefit would be the next urgent topic. Future studies including pharmacokinetics, bioavailability, and tissue distribution of ingested compounds, and interference with signal transduction pathways need to be conducted. Structural transformations with altering pH, charge changes, copigmentation, and the formation of degradation products will also need to be considered to assess the benefit of phytochemicals.
Abdel-Aal, E. –S. M., and Hucl, P. (2003) Composition and stability of anthocyanins in blue-grained wheat. Journal of Agricultural and Food Chemistry, 51, 2174–2180.
Abdel-Aal, E. –S, Young, J.S.M, and Rabalski, I. (2006) Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. Journal of Agricultural and Food Chemistry, 54, 4696–4704.
Abdel-Aal, E.-S.M., Young, J.C., Wood, P.J., Rabalski, I., Hucl, P., Falk, D. et al. (2002) Einkorn: A potential candidate for developing high lutein wheat. Cereal Chemistry, 79, 455–457.
Aguiar, C.L., Baptista, A.S., Walder, J.M.M., Tsai S.M., Carra-O-Panizzi, M.C., and Kitajima, E.W. (2009) Changes in isoflavone profiles of soybean treated with gamma irradiation. International Journal of Food Science and Nutrition, 60, 387–394.
Alluis, B., Perol, N., Elhajji, H., and Dangles, O. (2000) Water-soluble flavonol (3-hydroxy-2-phenyl-4H-1-benzopyran-4-one) derivatives: Chemical synthesis, colouring, and antioxidant properties. Helvetica Chimica Acta, 83, 428–443.
Altamirano, R.C., Drdá k, M., Simon, P., Rajniakova, A., Karovicová, J. and Preclík, L. (1993) Thermal degradation of betanine in various water alcohol model systems. Food Chemistry, 46, 73–75.
Arts, I.C.W., Putte, B., and Hollman, P.C.H. (2000) Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. Journal of Agricultural and Food Chemistry, 48, 1746–1751.
Asen, S., Stewart, R.N., and Norris, K.H. (1977) Anthocyanin and pH involved in the color of Heavenly Blue morning glory. Phytochemistry, 16, 1118.
Attoe, E.L. and von Elbe, J.H. (1981) Photochemial Degradation of Betanine and Selected Anthocyanins. Journal of Food Sciences, 46, 1934–1937.
Attoe, E.L. and von Elbe, J.H. (1984) Oxygen involvement in betanin degradation. Z Lebensm Unters Forsch, 179, 232–236.
Attoe, E.L. and von Elbe, J.H. (1985) Oxygen involvement in betanine degradation: effect of antioxidants. Journal of Agricultural and Food Chemistry, 50, 106–110.
Austin, F.L., Gent, C.A., and Wolff, I.A. (1968a) Degradation of natural thioglucosides with ferrous salts. Journal of Agricultural and Food Chemistry, 16, 752–755.
Austin, F.L., Gent, C.A., and Wolff, I.A. (1968b) Enantiomeric 3-hydroxy-pent-4- enethionamides from thioglucosides of Crambe and Brassica seeds by action of ferrous salts. Canadian Journal of Chemistry, 46, 1507–1512.
Bakowska, A., Kucharska, A.Z., and Oszmianski, J. (2003) The effects of heating, UV irradiation, and storage on stability of the anthocyanin-polyphenol copigment complex. Food Chemistry, 81, 349–355.
Balentine, D.A. (1997) Tea. In: Kirk-Othmer Encyclopedia of Chemical Technology 4th ed. New York: John Wiley & Sons, Inc.
Bao, J.S., Cai, Y.Z., Sun, M., Wang, G.Y., and Corke, H. (2005) Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. Journal of Agricultural and Food Chemistry, 53, 2327–2332.
Baranac, J.M., Petranović, N.A., and Dimitrić-Marković, J.M. (1996) Spectrophotometric study of anthocyan copigmentation reactions. Journal of Agricultural and Food Chemistry, 44, 1333–1336.
Barbosa, A.C.L., Lajolo, F.M., and Genovese, M.I. (2006) Influence of temperature, pH and ionic strength on the production of isoflavone-rich soy protein isolates. Food Chemistry, 98, 757–766.
Barrera, F.A., Reynoso, C.R., and Gonzáles de Mejía, E. (1998) Estabilidad de las betala´ınas extra ´ıdas del garambullo (Myrtillocactus geometrizans). Food Science Technology International, 4, 115–120.
Bassa, I.A. and Francis, F.J. (1997) Stability of anthocyanins from sweet potatoes in a model beverage. Journal of Food Science, 52, 1753–1755.
Baublis, A., Spomer, A., and Berber-Jiménez, M.D. (1994) Anthocyanin pigments: comparison of extract stability. Journal of Food Science, 59, 1219–1221.
Bazinet, L., Araya-Farias, M., Doyen, A., Trudel, D., and Têtu, B. (2010) Effect of process unit operations and long-term storage on catechin contents in EGCG-enriched tea drink. Food Research International, 43, 1692–1701.
Berké, B., Chéze, C., Vercauteren, J., and Deffieux, G. (1998) Bisulfite addition to anthocyanins: revisited structures of colourless adducts. Tetrahedron Letters, 39, 5771–5774.
Bloor, S.J. and Falshaw, R. (2000) Covalently linked anthocyanin-flavonol pigments from blue Agapanthus flowers. Phytochemistry, 53, 575–579.
Bonaventura, S. and Russo, C. (1993) Refrigeration of blood oranges destined for transformation. Fruit Processing, 10, 284–289.
Bones, A.M. and Rossiter, J.T. (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry, 67, 1053–1067.
Bonnie, T.Y. and Choo, Y.M. (1999) Oxidation and thermal degradation of carotenoids. Journal of Oil Palm Research, 2, 62–78.
Boon, C.S., McClements, D.J., Weiss, J., and Decker, E.A. (2010) Factors influencing the chemical stability of carotenoids in foods. Critical Reviews in Food Science and Nutrition, 515–532.
Borek, V. and Morra, M.J. (2005) Ionic thiocyanate (scn-) production from 4-hydroxybenzyl glucosinolate contained in sinapis alba seed meal. Journal of Agricultural and Food Chemistry, 53, 8650–8654.
Boulton, R. (2001) The copigmentation of anthocyanins and its role in the color of red wine: a critical review. American Journal of Enology and Viticulture, 52, 67–87.
Buckow, R., Kastell, A., Terefe, N.S., and Versteeg, C. (2010) Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. Journal of Agricultural and Food Chemistry, 58, 10076–10084.
Cabrita, L., Fossen, T., and Andersen, M. (2000) Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chemistry, 68, 101–107.
Cai, Y. and Corke, H. (2000) Production and properties of spray-dried Amaranthus betacyanin pigments. Journal of Food Science, 65, 1248–1252.
Cai, Y., Sun, M., and Corke, H. (1998) Colorant properties and stability of Amaranthus betacyanin pigments. Journal of Agricultural and Food Chemistry, 46, 4491–4495.
Cai, Y., Sun, M., and Corke, H. (2001) Identification and Distribution of Simple and Acylated Betacycanins in the Amaranthaceae. Journal of Agricultural and Food Chemistry, 49, 1971–1978.
Cai, Y. and Corke, H. (2001) Effect of postharvest treatments on Amaranthus betacyanin degradation evaluated by visible/near-infrared spectroscopy. Journal of Food Science, 66, 1112–1118.
Cai, Y., Sun, M., and Corke, H. (2005) Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends in Food Science Technology, 16, 370–376.
Camire, M.E., Chaovanalikit, A., Dougherty, M.P., and Briggs, J. (2002) Blueberry and grape anthocyanins as breakfast cereal colorants. Journal of Food Science, 67, 438–441.
Canfield, L.M., Krinski, N.I., and James, A.O. (eds) (1993) Carotenoids in Human Health, New York, Academy of Science.
Cantwell, M. and Suslow, T. (1999) Broccoli: recommendations for maintainin postharvest quality. http://www.postharvest.ucdavis.edu/produce/producefacts/ veg/broccoli.shtml.
Cardona, J.A., Lee, J-H., and Talcott, S.T. (2009) Color and Polyphenolic Stability in Extracts Produced from Muscadine Grape (Vitis rotundifolia) Pomace. Journal of Agricultural and Food Chemistry, 57, 8421–8425.
Castellar, M.R., Obón, J.M., and Fernández-Lo´pez, J.A. (2006) The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia stricta fruits. Journal of the Science of Food and Agriculture, 86, 122–128.
Chen, B.H. and Chen, Y.Y. (1993) Stability of chlorophylls and carotenoids in sweet potato leaves during microwave cooking. Journal of Agricultural and Food Chemistry, 41, 1315–1320.
Chen, H.E., Peng, H.Y., and Chen, B.H. (1996) Stability of carotenoids and vitamin A during storage of carrot juice. Food Chemistry, 57, 497–503.
Chen, J.P., Tai, C.Y., and Chen, B.H. (2007) Effects of different drying treatments on the stability of carotenoids in Taiwanese mango (Mangifera indica L.). Food Chemistry, 100, 1005–1010.
Chen, P.N., Kuo, W.H., Chiang, C.L., Chiou, H.L. et al. (2006) Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chemico–Biological Interacttions, 163, 218–229.
Chen, Z., Zhu, Q.Y., Tsang, D., and Huang, Y. (2001) Degradation of Green Tea Catechins in Tea Drinks. Journal of Agricultural and Food Chemistry, 49, 477–482.
Chevolleau, S., Gasc, N., Rollin, P., and Tulliez, J. (1997) Enzymatic, chemical, and thermal breakdown of 3H-labeled glucobrassicin, the parent indole glucosinolate. Journal of Agricultural and Food Chemistry, 45, 4290–4296.
Chien, J.T., Hsieh, H.C., Kao, T.H., and Chen, B.H. (2005) Kinetic model for studying the conversion and degradation of isoflavones during heating. Food Chemistry, 91, 425–434.
Choi, M.H., Kima, G.H., and Lee, H.S. (2002) Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Research International, 35, 753–759.
Cinar, I. (2005) Stability studies on the enzyme extracted sweet potato carotenoproteins. Food Chemistry, 89, 397–401.
Clarke, D.B. (2010) Glucosinolates, structures and analysis in food. Food Analytical Methods, 2, 310–325.
Cohen, E. and Saguy, I. (1983) Effect of water activity and moisture content on the stability of beet powder pigments. Journal of Food Science, 48, 703–707.
Corrales, M., Lindauer, R., Butz, P., and Tauscher, B. (2008) Effect of heat/pressure on cyanidin-3-glucoside ethanol model solutions. Journal of Physics: Conference Series, 121, 142003.
Coward, L., Smith, M., Kirk, M., and Barnes, S. (1998) Chemical modification of isoflavones in soyfoods during cooking and processing. American Journal of Clinical Nutrition, 68, 1486S–1491S.
Czapski, J. (1990) Heat stability of betacyanins in redbeet juiceandin betanine solutions. Z Lebensm Unters Forsch, 191, 275–278.
Da Silva, P.F. and Moreira, R.G. (2008) Vacuum frying of high-quality fruit and vegetable-based snacks. LWT–Food Science Technolnology, 41, 1758–1767.
Dangles, O., Saito, N., and Brouillard, R. (1993) Anthocyanin intramolecular copigment effect. Phytochemistry, 34, 119–124.
Dangles, O., Stoeckel, C., Wigand, M.C., and Brouillard, R. (1992) Two very distinct types of anthocyanin complexation: copigmentation and inclusion. Tetrahedron Letters, 33, 5227–5230.
Davies, C.G.A., Netto, F.M., Glassenap, N., Gallaher, C.M., Labuza, T.P., and Gallaher, D.D. (1998) Indication of the maillard reaction during storage of protein isolates. Journal of Agricultural and Food Chemistry, 46, 2485–2489.
De Ancos, B., Ibanez, E., Reglero, G. et al. (2000) Frozen storage effects on anthocyanins and volatile compounds of raspberry fruit. Journal of Agricultural and Food Chemistry, 48, 873–879.
Del Pozo-Insfran, D., Del Follo-Martinez, A., Talcott, S.T., and Brenes, C.H. (2007) Stability of copigmented anthocyanins and ascorbic acid in muscadine grape juice processed by high hydrostatic pressure. Journal of Food Science, 72, S247–S253.
Delgado-Vargas, F., Jimenez, A.R., and Paredes-Lopez, O. (2000) Natural pigments: carotenoids, anthocyanins, and betalains: characteristics, biosynthesis, processing, and stability. Critical Reviews in Food Science Nutrition, 40, 173–289.
Del Pozo-Insfran, D., Brenes, C.H., and Talcott, S.T. (2004) Phytochemical Composition and Pigment Stability of Açai (Euterpe oleracea Mart.). Journal of Agricultural and Food Chemistry, 52, 1539–1545.
Demeule, M., et al. (2002) Green tea catechins as novel antitumor and antiangiogenic compounds. Current Medicinal Chemistry, 2, 441–463.
Drunkler, D.A., Fett, R., and Bordignon-Luiz, M.T. (2006) Avaliacão da estabilidade de betalaı´nas em extrato de beterraba (Beta vulgaris L.) com α-, β-, e, γ-ciclodextrinas. Boletim Centro de Pesquisa de Processamento de Alimentos, 24, 259–276.
Dutta, D., Chaudhuri, U.R., and Chakraborty, R. (2005) Structure, health benefits, antioxidant property and processing and storage of carotenoids. African Journal of Biotechnology, 4, 1510–1520.
Duhard, V., Garnier, J.C., and Megard, D. (1997) Comparison of the stability of selected anthocyanins colorants in drink model systems. Agro Food Industry Hi-Tech, 8, 28–34.
Dyrby, M., Westergaard, N., and Stapelfeldt, H. (2001) Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chemistry, 72, 431–437.
Eden, T. (1976) The chemistry of tea leaf and of its manufacture . In: T. Eden, Tea (3rd edition) London: Longman Group Limited.
Eiro, M.J. and Heinonen, M. (2002) Anthocyanin color behavior and stability during storage: effect of intermolecular copigmentation. Journal of Agricultural and Food Chemistry, 50, 7461–7466.
Escribano-Bailón, T., Ä lvarez-García, M., Rivas-Gonzalo, J.C., Heredia, F.J., and Santos-Buelga, C. (2001) Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-o-glucoside and (+)-catechin. Journal of Agricultural and Food Chemistry, 49, 1213–1217.
Escribano, J., Gandía-Herrero, F., Caballero, N., and Pedreno, M.A. (2002) Subcellular localization and isoenzyme pattern of peroxidase and polyphenol oxidase in beet root (Beta vulgaris L.). Journal of Agricultural and Food Chemistry, 50, 6123–6129.
Fenwick G.R. and Heaney R.K. (1983) Glucosinolates and their breakdown products in cruciferous crops, foods and feeding stuffs. Food Chemistry, 11, 249–271.
Fenwick, G.R., Heaney, R.K., Gmelin, R., Rakow, D., and Thies, W. (1981) Glucosinalbin in Brassica napus – a re-evaluation. Zeitschrift fuer Pflanzenzuechtung, 87, 254–259.
Ferreres, F., Gil, M.I., and Tomas-Barberan, F.A. (1996) Anthocyanins and flavonoids from shredded red onion and changes during storage in perforated films. Food Research International, 29, 389–395.
Figueiredo, P., Elhabiri, M., Toki, K., Saito, N., Dangles, O., and Brouillard, R. (1996) New aspects of anthocyanin complexation. Intramolecular copigmentation as a means for colour loss. Phytochemistry, 41, 301–308.
Forney, C.F., Mattheis, J.P., and Austin, R.K. (1991) Volatile compounds produced by broccoli under anaerobic conditions. Journal of Agriculrural and Food Chemistry, 39, 2257–2259.
Fossen, T., Cabrita, L., and Andersen, Ø.M. (1998) Color and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chemistry, 63, 435–440.
Francis, F.J. (2000) Anthocyanins and betalains: composition and applications. Cereal Foods World, 45, 208–213.
Friis, P., Larsen, P.O., and Olsen, C.E. (1977) Base-catalyzed Neber-typ rearrangement of glucosinolates [1-(b-D-glucosylthio)-N-(sulfonatooxy)alkylideneamines]. Journal of the Chemistry Society, Perki Trans I: Org Bioorg Chem (1972–1999), 1977, 661–665.
García-Viguera, C., Zafrilla, P., Romero, F., and Abellán, P. et al. (1999) Color stability of strawberry jam as affected by cultivar and storage temperature. Journal if Food Science, 64, 243–247.
Garzón, G.A. and Wrolstad, R.E. (2001) The stability of pelargonidinbased anthocyanins at varying water activity. Food Chemistry, 75, 185–196.
Gentile, C., Tesoriere, L., Allegra, M., Livrea, M.A., and D’Alessio, P. (2004) Antioxidant betalains fromcactus pear (Opuntia ficus-indica) inhibits endothelial ICAM-1expression. Annals of the New York Academy of Science, 1028, 481–486.
George, F., Figueiredo, P., Toki, K., Tatsuzawa, F., Saito, N., and Brouillard, R. (2001) Influence of trans-cis-isomerization of coumaric acid substituents on colour variance and stabilisation in anthocyanins. Phytochemistry, 57, 791–795.
Gmelin, R. and Virtanen, A.I. (1961) Glucobrassicin der precursor von SCN-, 3-indolylacetonitril and ascorbigen in Brassica oleracea species. Annals of the New York Academy of Science, 107, 1–25.
Gonzalez-Manzano, S., Mateus, N., de Freitas, V., and Santos-Buelga, C. (2008) Influence of the Degree of Polymerisation in the Ability of Catechins to Act as Anthocyanin Copigments. European Food Research and Technology, 227, 83–92.
Gouveia, L. and Empis, J. (2003) Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: effect of storage conditions. Innovative Food Science and Emerging Technologies, 4, 227–233.
Gradinaru, G., Biliaderis, C.G., Kallithraka, S., Kefalas, P., and Garcia-Viguera, C. (2003) Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in solid state: effects of copigmentation and glass transition. Food Chemistry, 83, 423–436.
Gris, E.F., Ferreira, E.A., Falcao, L.D., and Bordignon-Luiz, M.T. (2007) Caffeic acid copigmentation of anthocyanins from Cabernet Sauvignon grape extracts in model systems. Food Chemistry, 100, 1289–1296.
Grun, I.U. et al. (2001) Changes in the profile of genistein, daidzein, and their conjugates during thermal processing of tofu. Journal of Agricultural and Food Chemistry, 49, 2839–2843.
Gulati, A., Rawat, R., Singh, B., and Ravindranath, S.D. (2003) Application of Microwave Energy in the Manufacture of Enhanced-Quality Green Tea. Journal of Agricultural and Food Chemistry, 51, 4764–4768.
Henning, W. (1981) Flavonolglycoside der Erdbeered (Fragaria x ananassa Duch.), Himbeeren (Rubus idaeus L.) und Brombeeren (Rubus fruticosus L.). Z. Lebensm Unters Forsch., 173, 180–187.
Herbach, K.M., Stintzing, F.C., and Carle, R. (2004a) Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. Journal of Food Science, 69, C491–C498.
Herbach, K.M., Stintzing, F.C., and Carle, R. (2004b) Thermal degradation of betacyanins in juices from purple pitaya (Hylocereus polyrhizus [Weber] Britton and Rose) monitored by high-performance liquid chromatography-tandemmassspectrometric analyses. European Food Research and Technology, 219, 377–385.
Herbach, K.M., Stintzing, F.C., and Carle, R. (2005) Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Comm Mass Spectrom., 19, 2603–2616.
Herbach, K.M., Stintzing, F.C., and Carle, R. (2006a) Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. Journal of Agricultural and Food Chemistry, 54, 390–398.
Herbach, K.M., Rohe, M., Stintzing, F.C., and Carle, R. (2006b) Structural and chromatic stability of purplepitaya (Hylocereus polyrhizus [Weber] Britton and Rose) betacyanins as affected by the juice matrix and selected additives. Food Research International, 39, 667–677.
Hidalgo, A., Brandolini, A., and Pompei, C. (2010) Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chemistry, 121, 746–751.
Hider, R.C., Liu, Z.D., and Khodr, H.H. (2001) Metal chelation of polyphenols. Methods in Enzymology, 335, 190–203.
Hiemori, M., Koh, E., and Mitchell, A.E. (2009) Influence of Cooking on Anthocyanins in Black Rice (Oryza sativa L. japonica var. SBR). Journal of Agricultural and Food Chemistry, 57, 1908–1914.
Hoshino, T. and Tamura, H. (1999) Anthocyanidin glycosides: color variation and color stability. In: R. Ikan (ed.) Naturally Occurring Glycosides, New York/Weinheim: John Wiley & Sons, pp. 43–82.
Huang, A.S. and von Elbe, J.H. (1986) Stability comparison of two betacyanine pigments-Amaranthine and betanine. Journal of Food Science, 51, 670–674.
Huang, R., Chou, C. Stability of Isoflavone Isomers in Steamed Black Soybeans and Black Soybean Koji Stored under Different Conditions. Journal of Agricultural and Food Chemistry, 57, 1927–1932.
Inami, O., Tamura, I., Kikuzaki, H., and Nakatani, N. (1996) Stability of anthocyanins of Sambucus canadensis and Sambucus nigra. Journal of Agricultural and Food Chemistry, 44, 3090–3096.
Jackman, R.L. and Smith, J.L. (1996) Anthocyanins and betalains. In: G.F. Hendry, and J.D. Houghton (eds) Natural Food Colorants, London: Blackie Academic and Professional, pp. 244–309.
Jackson, C.-J.C. et al. (2002) Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochemistry, 37, 1117–1123.
Jiménez, N., Bohuon, P., Lima, J., Dornier, M., Vaillant, F., and Perez, A.M. (2010) Kinetics of anthocyanin degradation and browning in reconstituted blackberry juice treated at high temperatures (100–180 °C). Journal of Agricultural and Food Chemistry, 58, 2314–2322.
Jones, R. B., Faragher, J, B., Winkler, S. A review of the influence of postharvest treatments on quality and glucosinolate content in broccoli (Brassica oleracea var. italica) heads. Postharv Biol Technol., 2006, 41, 1–8.
Jørjensen, K. and Skibsted, L.H. (1993) Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm.-Unters.-Forsch., 196, 423–429.
Kaack, K. and Austed, T. (1998) Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods and Human Nutrition, 52, 187–198.
Kader, F., Haluk, J.-P., Nicolas, J.-P., and Metche, M. (1998) Degradation of cyanidin 3–glucoside by blueberry polyphenol oxidase: kinetic studies and mechanisms. Journal of Agricultural and Food Chemistry, 46, 3060–3065.
Kanner, J., Harel, S., and Granit, R. (2001) Betalains – A new class of dietary cationized antioxidants. Journal of Agricultural and Food Chemistry, 49, 5178–5185.
Kearsley M.W. and Rodriguez N. (1981) The stability and use of natural colours in foods: anthocyanin, p-carotene and riboflavin. Journal of Food Technology, 16, 421–431.
Kelly, P.J., Bones, A., and Rossiter, J.T. (1998) Sub-cellular immunolocalization of the glucosinolate sinigrin in seedlings of Brassica juncea. Planta, 206, 370–377.
Kim, E.S. et al. (2007) Impact of heating on chemical compositions of green tea liquor. Food Chemistry, 103, 1263–1267.
Kirca, A. and Cemeroglu, B. (2003) Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chemistry, 81, 583–587.
Kondo, K., Kurihara, M., and Fukuhara, K. (2001) Mechanism of Antioxidant Effect of Catechins. Methods of Enzymology, 335, 203–217.
Kouniaki, S., Kajda, P., and Zabetakis, I. (2004) The effect of high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants (Ribes nigrum). Flavour Fragrance Journal, 19, 281–286.
Kwon, S.H., Ahn, I.S., Kim, S.O., Kong, C.S., Chung, H.Y., Do, M.S., and Park, K.Y. (2007) Anti-obesity and hypolipidemic effects of black soybean anthocyanins. Journal of Medicinal Food, 10, 552–556.
Lavellia, V., Zanonib, B., and Zaniboni, A. (2007) Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chemistry, 104, 1705–1711.
Lee, S., Kim, S., Jeong, S., and Park, J. (2006) Effect of far-infrared irradiation on green tea. Journal of agricultural and Food Chemistry, 54, 399–403.
Lee, S.C., Jeong, S.M., Lee, J.M., Jang, A., Kim, D.H., and Jo, C. (2008) Effect of irradiation on total phenol and catechins contents and radical scavenging activity of green tea leaf and stem extract. Journal of Food Biochemistry, 32, 782–794.
Lin, C.H. and Chen, B.H. (2005) Stability of carotenoids in tomato juice during storage. Food Chemistry, 90, 837–846.
Lindsay, R.C. (1996) Food additives. In: O.R. Fennema (ed.) Food Chemistry Marcel Dekker: New York, pp. 768–823.
Lopez-Berenguer, C., Carvajal, M., and Moreno, D.A., Garcia-Viguera, C. (2007) Effects of microwave cooking conditions on bioactive compounds present in broccoli inflorescences. Journal of Agricultural and Food Chemistry, 55, 10001–10007.
Ludikhuyze, L., Rodrigo, L., and Hendrickx, M. (2000) The activity of myrosinase from broccoli (Brassica oleracea L. cv. Italica): Influence of intrinsic and extrinsic factors. Journal of Food Protection, 63, 400–403.
Maccarone, E., Maccarrone, A., and Rapisarda, P. (1985) Stabilization of anthocyanins of blood orange fruit juice. Journal of Food Science, 50, 901–904.
Mahungu, S.M., Diaz–Mercado, S., Li, J., Schwenk, M., Singletary, K., and Faller, J. (1999) Stability of Isoflavones during Extrusion Processing of Corn/Soy Mixture. Journal of Agricultural and Food Chemistry, 47, 279–284.
Malaypally, S.P. and Ismail, B. (2010) Effect of protein content and denaturation on the extractability and stability of isoflavones in different soy systems. Journal of Agricultural and Food Chemistry, 58, 8958–8965.
Malien-Aubert, C., Dangles, O., and Amiot, M.J. (2001) Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. Journal of Agricultural and Food Chemistry, 49, 170–176.
Markakis, P. (1982) Stability of anthocyanins in foods. In: P. Markakis (ed.) Anthocyaninsas Food Colors, London: Academic Press Inc., pp. 163–180.
Martínez-Parra, J. and Muñoz, R. (2001) Characterization of betacyanin oxidation catalyzed by a peroxidase from Beta vulgaris L. roots. Journal of Agricultural and Food Chemistry, 49, 4064–4068.
Mathias, K., Ismail, B., Corvalan, C.M., andv Hayes, K.D. (2006) Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. Journal of Agricultural and Food Chemistry, 54, 7495–7502.
Matsuura, M. and Obata, A. (1993) β-glucosidases from soybeans hydrolyze daidzin and genistin. Journal of Food Science, 58, 144–147.
Matusheski, N.V., Wallig, M.A., Juvik, J.A., Klein, B.P., Kushad, M.M., and Jeffery, E. (2001) H. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. Journal of Agricultural and Food Chemistry, 49, 1867–1872.
Mazza, G. and Brouillard, R. (1990) The mechanism of co-pigmentation of anthocyanins in aqueous solutions. Phytochemistry, 29, 1097–1102.
Mazza, G. and Miniati, E. (1993) Anthocyanins in Fruits, Vegetables and Gains, Boca Raton, Florida: CRC Press.
Melo, M.J., Moncada, M.C., and Pina, F. (2001) On the red colour of raspberry (Rubus idaeus). Tetrahedron Leters, 41, 1987–1991.
Midori, K., Tamiyoshi, S., Kinuyo, N., and Masaaki, T. (2001) Effects of pH and Metal Ions on Antioxidative Activities of Catechins. Bioscience, Biotechnology and Biochemistry, 65, 126–132.
Mishra, D.K., Dolan, K.D., and Yang, L. (2008) Confidence intervals for modeling anthocyanin retention in grape pomace during nonisothermal heating. Journal of Food Science, 73, E9–E15.
Mistry, T.V., Cai, Y., Lilley, T.H., and Haslam, E. (1991) Polyphenol interactions. Part 5: anthocyanin co-pigmentation. Journal of the Chemistry Society Perkin Transactions, 2, 1287–1296.
Moßhammer, M.R., Stintzing, F.C., and Carle, R. (2005) Development of a process for the production of a betalain-based colouring foodstuff from cactus pear. Innovative Food Science and Emerging Technologies, 6, 221–231.
Mochizuki, M., Yamazaki, S., Kano, K., and Ikeda, T. (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochimica et Biophysica Acta, 1569, 35–44.
Mohn, T., Cutting, B., Ernst, B., and Hamburger, M. (2007) Extraction and analysis of intact glucosinolates-A validated pressurized liquid extraction/liquid chromatography-mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. Journal of Chromatology A, 1166, 142–151.
Mollov, P., Mihalev, K., Shikov, V., Yoncheva, N., and Karagyozov, V. (2007) Colour stability improvement of strawberry beverage by fortification with polyphenolic copigments naturally occurring in rose petals. Innovative Food Science and Emerging Technolologies, 8, 318–321.
Morais, H., Ramos, C., Forgács, E., Cserháti, T., and Oliviera, J. (2002) Influence of storage conditions on the stability of monomeric anthocyanins studied by reversed-phase high-performance liquid chromatography. Journal of Chromatology B, 770, 297–301.
Murphy, P.A. et al. (1999) Isoflavones in retail and institutional soy foods. Journal of Agricultural and Food Chemistry, 47, 2697–2704.
Nachtigall, A.M., Da Silva, A.G., Stringheta, P.C., Silva, P.I., and Bertoldi, M.C. (2009) Correlation between spectrophotometric and colorimetric methods for the determination of photosensitivity and thermosensitivity of tomato carotenoids, Boletim do Centro de Pesquisa e Processamento de Alimentos, 27, 11–18.
Nufer, K.R., Ismail, B., and Hayes, K.D. (2009) The Effects of Processing and Extraction Conditions on Content, Profile, and Stability of Isoflavones in a Soymilk System. Journal of Agricultural and Food Chemistry, 57, 1213–1218.
Oerlemans, K., Barrett, D.M., Suades, C.B., Verkerk, R., and Dekker, M. (2006) Thermal degradation of glucosinolates in red cabbage. Food Chemistry, 95, 19–29.
Osmani, S.A., Hansen, E.H., Malien-Aubert, C.L., Olsen, C-E., Bak, S., and Møller, B.L. (2009) Effect of Glucuronosylation on Anthocyanin Color Stability. Journal of Agricultural and Food Chemistry, 57, 3149–3155.
Pacheco-Palencia, L.A., Duncan, C.E., and Talcott, S.E. (2009) Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chemistry, 115, 1199–1205.
Pacheco-Palencia, L., Hawken, P., and Talcott, S. (2007) Juice matrix composition and ascorbic acid fortification effects on the phytochemical, antioxidant, and pigment stability of açai (Euterpe oleracea Mart.). Food Chemistry, 105, 28–35.
Palozza, P. and Krinsky, N.I. (1992) Antioxidant effects of carotenoids in vivo and in vitro: An overview. Methods Enzymology, 213, 403–420.
Parisa, S., Reza, H., Elham, G., and Rashid, J. (2007) Effect of Heating, UV irradiation and pH on the Stability of the Anthocyanin Copigment Complex. Pakistan Journal of Biological Science, 267–272.
Parkin, K.L. and Im, J-S. (1990) Chemical and physical changes in beet (Beta vulgaris L.) root tissue during simulated processing—Relevance to the “black ring” defect in canned beets. Journal of Food Science, 55, 1039–1041.
Pasch, J.H. and von Elbe, J.H. (1979)_Betanine stability in buffered solutions containing organic acids, metal cations, antioxidants, or sequestrants. Journal of Food Science, 44, 72–74.
Patras, A., Brunton, N.P., Da Pieve, S., and Butler, F. (2009) Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innovative Food Science and Emerging Technologies, 10, 308–313.
Pérez, A., Jarén, M.,and Mínguez, M.I. (2000) Effect of high-temperature degradative processes on ketocarotenoids present in paprika oleoresins. Journal of Agricultural and Food Chemistry, 48, 2966–2971.
Polyakov, N.E., Leshina, T.V., Meteleva, E.S., Dushkin, A.V., Konovalova, T.A., and Lowell, D. (2010) Enhancement of the photocatalytic activity of tio2 nanoparticles by water-soluble complexes of carotenoids. Journal of Physical Chemistry, 114, 14200–14204.
Price, C.L. and Wrolstad, R.E. (1995) Anthocyanin pigments of Royal Okanogan Huckleberry Juice. Journal of Food Science, 60, 369–374.
Proniuk, S., Liederer, B.M., and Blanchard, J. (2002) Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer prevention. Journal of Pharmceutical Science, 91, 111–116.
Queiroz, F., Oliveira, C., Pinho, O., (2009) Ferreira, I.M.P.L.V.O. Degradation of anthocyanins and anthocyanidins in blueberry jams/stuffed fish. Journal of Agricultural and Food Chemistry, 57, 10712–10717.
Rangkadilok, N., Tomkins, B., Nicolas, M.E., Premier, R.R., Bennett, R.N., Eagling, D.R., and Taylor, P.W.J. (2002) The effect of post-harvest and packaging treatments on glucoraphanin concentration in broccoli (Brassica oleracea var. italica). Journal of Agricultrural and Food Chemistry, 50, 7386–7391.
Rao, A.V. and Rao L. G. (2007) Carotenoids and human health. Pharmacology Research, 55, 207–216.
Rascón, M.P., Beristain, C.I., García, H.S., and Salgado, M.A. (2011) Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and Soy protein isolate as wall materials. LWT–- Food Science Technology, 44, 549–557.
Rein, M.J. and Heinonen, M. (2004) Stability and enhancement of berry juice color. Journal of Agricultural and Food Chemistry, 52, 3106–3114.
Richardson, T. and Finley, J.W. (1985) Chemical changes in natural food pigments. In: T. Richardson and J.W. Finley (eds) Chemical Chances in Food during Processing, London: Springer Verlag, p. 431.
Rivas-Gonzalo, J.C., Bravo-Haro, S., and Santos-Buelga, C. (1995) Detection of compounds formed through the reaction of malvidin 3- monoglucoside and catechin in the presence of acetaldehyde. Journal of Agricultural and Food Chemistry, 43, 1444–1449.
Rock, C.L. (1997) Carotenoids: biology and treatment. Pharmacology and Therapeutics, 75, 185–197.
Rodrigues, A.S. and Rosa, E.A.S. (1999) Effect of post-harvest treatments on the level of glucosinolates in broccoli. Journal of Science and Food Agriculture, 79, 1028–1032.
Rodríguez-Saona, L.E., Giusti, M.M., and Wrolstad, R.E. (1999) Color and Pigment Stability of Red Radish and Red-Fleshed Potato Anthocyanins in Juice Model Systems. Journal of Food Science, 64, 451–456.
Rommel, A., Wrolstad, R.E., and Heatherbell, D.A. (1992) Blackberry juice and wine: processing and storage effects on anthocyanin composition, color and appearance. Journal of Food Science, 57, 385–391.
Rossi, M., Giussani, E., Morelli, R., Lo Scalzo, R., Nani, R.C., and Torreggiani, D. (2003) Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Research International, 36, 999–1005.
Rostagno, M.A., Palma, M., and Barroso, C.G. (2005) Short-term stability of soy isoflavones extracts: Sample conservation aspects. Food Chemistry, 93, 557–564.
Rubinskiene, M., Jasutiene, I., Venskutonis, P., and Viskelis, P. (2005a) HPLC determination of the composition and stability of blackcurrant anthocyanins. Journal of Chromatology Sciemce, 43, 478–482.
Rubinskiene, M., Viskelis, P., Jasutiene, I., Viskeliene, R., and Bobinas, C. (2005b) Impact of various factors on the composition and stability of black currant anthocyanins. Food Research International, 38, 867–871.
Rungapamestry, V., Duncan, A.J., Fuller, Z., and Ratcliffe, B. (2006) Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea Var. capitata) cooked for different durations. Journal of Agricultrural and Food Chemistry, 54, 7628–7634.
Saito, N., Toki, K., Honda, T., and Kawase, K. (1998) Cyanidin 3-malonylglucuronylglucoside in Bellis and cyanidin 3-malonylglucoside in Dendranthema. Phytochemistry, 27, 2963–2966.
Sajilata, M.G., Singhal R.S., and Kamat, M.Y. (2008) The carotenoid pigment zeaxanthin-a review. Comprehensive Reviews in Food Science and Food Safety, 7, 29–49.
Saldana, G., Stephens, T.S., Lime, and B.J. (1976) Carrot beverages and lots of padding. Journal of Food Science, 41, 1243–1244.
Sapers, G.M. and Hornstein, J.S. (1979) Varietal differences in colorant properties and stability of red beet pigments. Journal of Food Science, 44, 1245–1248.
Sarma, A.D., Sreelakshmi, Y., and Sharma, R. (1997) Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry, 45, 671–674.
Sasaki, R., Nishimura, N., Hoshino, H., Isa, Y., Kadowaki, M., Ichi, T. et al. (2007) Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to down regulation of retinol binding protein 4 expression in diabetic mice. Biochemical Pharmacology, 74, 1619–1627.
Sastry, L. and Tischer, R.G. (1953) Behavior of the anthocyanin pigments in concord grapes during heat processing and storage. Food Technology, 6, 82.
Satue-Gracia, M., Heinonen, I.M. and Frankel, E.N. (1997) Anthocyanins as antioxidants on human low-density lipoprotein and lecithinliposome systems. Journal of Agricultural and Food Chemistry, 45, 3362–3367.
Savolainen, K. and Kuusi, T. (1978) The stability properties of golden beet and red beet pigments: influence of pH, temperature, and some stabilizers. Z Lebensm Unters Forsch, 166, 19–22.
Schliemann, W. and Strack, D. (1998) Intramolecular stabilization of acylated betacyanins. Phytochemistry, 49, 585–588.
Schneider, W. and Becker, M.A. (1930) Case of Walden inversion in glucoside cleavage. Naturwissenschaften, 18, 133.
Schouten, R.E., Zhang, X.B., Verkerk, R., Verschoor, J.A., Otma, E.C. et al. (2009) Modeling the level of the major glucosinolates in broccoli as affected by controlled atmosphere and temperature. Postharvest Biology and Technology, 53, 1–10.
Schwartz, S.J. and von Elbe, J.H. (1983) Identification of betanin degradation products. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 176, 448–453.
Serris, G.S. and Biliaderis, C.G. (2001) Degradation kinetics of beetroot pigment encapsulated in polymeric matrices. Journal of Science and Food Agriculture, 81, 691–700.
Shahidi, F. and Naczk, M. (2004) Phenolics in Food and Neutraceuticals. Boca Raton, Florida: CRC Press.
Shenoy, V.R. (1993) Anthocyanins – prospective food colours. Current Science, 64, 575–579.
Shrikhande, A.J. and Francis, F.J. (1974) Effect of flavonols on ascorbic acid and anthocyanin stability in model system. Journal of Food Science, 39, 904–906.
Shi, J. and Sophia, J. (2008) Stability of lycopene during food processing and storage. Agriculture and Agri-Food Canada, Lycopene, 17–36.
Shi, Z.L., Lin, M. and Francis, F.J. (1992) Anthocyanins of Tradescantia pallida potential food colorants. Journal of Food Science, 57, 761–765.
Shih, C.C. and Wiley, R.C. (1981) Betacyanine and betaxanthine decolorizing enzymes in the beet (Beta vulgaris L.) root. Journal of Food Science, 47, 164–172.
Shimoni, E. (2004) Stability and shelf life of bioactive compounds during food processing and storage: soy isoflavones. Journal of Food Science, 69, R160–R166.
Sims, C.A., Balaban, M.O., and Mathews R.F. (1993) Optimization of carrot juice color and cloud stability. Journal of Food Science, 58, 1129–1131.
Skrede, G., Wrolstad, R.E., Lea, P., and Enersen, G. (1992) Color stability of strawberry and blackcurrant syrups. Journal of Food Science, 57, 172–177.
Song, L.J. and Thornalley, P.J. (2007) Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chemistry and Toxicology, 45, 216–224.
Stintzing, F.C. and Carle, R. (2004) Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends in Food Science and Technology, 15, 19–38.
Stintzing, F.C., Herbach, K.M., Moßhammer, M.R., Carle, R., et al. (2005) Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia ssp.) clones. Journal of Agricultural Food Chemistry, 53, 442–451.
Stintzing, F.C., Schieber, A., and Carle, R. (2001) Phytochemical and nutritional significance of cactus pear. European Food Research and Technology, 212, 396–407.
Stintzing, F.C., Stintzing, A.S., Carle, R., Frei, B., and Wrolstad, R.E. (2002) Color and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agricultural and Food Chemistry, 50, 6172–6181.
Strack, D., Steglich, W., and Wray, V. (1993) Betalains. In: P.M. Dey and J. Harborne (eds) Methods in Plant Biochemistry. London: Academic Press Limited, pp: 421–450.
Su, Y.L., Leung, L.K., Huang, Y., and Chen, Z. (2003) Stability of tea theaflavins and catechins. Food Chemistry, 83, 189–195.
Suthanthangjai, W., Kajda, P., and Zabetakis, I. (2005) The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus idaeus). Food Chemistry, 90, 193–197.
Tai, C.Y. and Chen B.H. (2000) Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. Journal of Agricultural and Food Chemistry, 48, 5962–5968.
Talcott, S.T., Brenes, C.H., Pires, D.M., and Del Pozo-Insfran, D. (2003) Phytochemical stability and color retention of copigmented and processed muscadine grape juice. Journal of Agricultural and Food Chemistry, 51, 957–963.
Teh, L.S. and Francis, F.J. (1988) Stability of anthocyanins from Zebrina pendula and Ipomoea tricolor in a model beverage. Journal of Food Science, 53, 1580–1581.
Toivonen, P.M.A. and Forney, C. (2004) Broccoli. In: The Commercial Storage of Fruits, Vegetables and Florist and Nursery Stock. USDA, ARS Agriculture Handbook #66.
Tonon, R.V., Brabet, C., and Hubinger, M.D. (2008) Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering, 88, 411–418.
Tsai, P-J., Sheu, C-H., Wu, P-H., and Sun, Y-F. (2010) Thermal and pH stability of betacyanin pigment of djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. Journal of Agricultural and Food Chemistry, 58, 1020–1025.
Tsuda, T., Horio, F., Osawa, and T. Cyanidin 3-O-â-glucoside suppresses nitric oxide production during a zymosan treatment in rats. Journal of Nutritional Science and Vitaminology, 48, 305–310.
van Eylen, D., Oey, I., Hendrickx, M., and van Loey, A. (2008) Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. Journal of Food Engineering, 89, 178–186.
van Poppel, G., Verhoeven, D.T.H., Verhagen, H., and Goldbohm, R.A. (1999) Brassica vegetables and cancer preventions epidemiology and mechanisms. Advances in Experimental Medicine and Biology, 472, 159–68.
Vera de Rossoa, V. and Mercadante, A.Z. (2007) Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innovative Food Science and Emerging Technologies, 8, 347–352.
Verkerk, R. and Dekker, M. (2004) Glucosinolates and myrosinase activity in red cabbage Brassica oleracea L. Var. Capitata f. rubra DC. after various microwave treatments. Journal of Agricultural and Food Chemistry, 52, 7318–7323.
von Elbe, J.H. and Attoe, E.L. (1985) Oxygen involvement in betanine degradation-measurement of active oxygen species and oxidation reduction potentials. Food Chemistry, 16, 49–67.
von Elbe, J.H., Maing, I.Y., and Amundson, C.H. (1974) Colour stability of betanin. Journal of Food Science, 39, 334–337.
Wagner, L.A. and Warthesen, J.J. (1995) Stability of spray-dried encapsulated carrot carotenes. Journal of Food Science, 60, 1048–1053.
Wang, H. and Helliwell, K. (2000) Epimerisation of catechins in green tea infusions. Food Chemistry, 70, 337–344.
Wang H. and Murphy P.A. (1996) Mass Balance Study of Isoflavones during Soybean Processing. Journal of Agricultural and Food Chemistry, 44, 2377–2383.
Wang, X.Y., Zhou, R., and Jiang, L.J. (2000) Study on the stability of glucoraphanin extracted from broccoli. Shipin Keji, 34, 250–252, 257.
Wasserman, B.P., Eiberger, L.L., and Guilfoy, M.P. (1984) Effect of hydrogen peroxide and phenolic compounds on horseradish peroxidase-catalysed decolorization of betalain pigments. Journal of Food Science, 49, 536–538.
Wasserman, B.P. and Guilfoy, M.P. (1984) Solubilization of the red beet cell wall betanin decolorizing enzyme. Journal of Food Science, 49, 1075–1077.
Wrolstad, R.E., Skrede, G., Lea, P., and Enersen, G. (1990) Influence of sugar on anthocyanin pigment stability in frozen strawberries. Journal of Food Science, 55, 1064–1065, 1072.
Wu, X., Beecher, G.R., Holden, J.M., Haytowitz, D.B., Gebhardt, S.E., and Prior, R.L. (2006) Concentration of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry, 54, 4069–4075.
Wybraniec, S. and Mizrahi, Y. (2005) Generation of decarboxylated and dehydrogenated betacyanins in thermally treated purified fruit extract from purple pitaya (Hylocereus polyrhizus) monitored by LC-MS/MS. Journal of Agricultural and Food Chemistry, 53, 6704–6712.
Wybraniec, S., Nowak-Wydra, B., and Mizrahi, Y. (2006) 1H and 13CNMR spectroscopic structural elucidation of new decarboxylated betacyanins. Tetrahedron Letters, 47, 1725–1728.
Wyler, H. and Dreiding, A.S. (1962) Constitution of the beet pigment betanin. IV. Degradation products of betanidin. Helv Chim Acta, 45, 638–640.
Wyler, H. and Dreiding, A.S. (1984) Deuteration of betanidin and indicaxanthin. (E/Z)-Stereoisomerism in betalaines. Helv Chim Acta, 67, 1793–1800.
Xia, X., Ling, W., Ma, J., Xia, M. et al. (2006) An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. Journal of Nutrition, 136, 2220–2225.
Xu, B. and Chang, S.K.C. (2008) Total phenolics, phenolics acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. Journal of Agricultural and Food Chemistry, 56, 7165–7175.
Xu, J.Z., Leung, L.K., Huang, Y., and Chen, Z.Y. (2003) Epimerisation of tea polyphenols in tea drinks. Journal of Food Science and Agriculture, 83, 1617–1621.
Xu, X.M., Chen, X.M., and Jin, Z.Y. (2006) Stability of astaxanthin from Xanthophyllomyces dendrorhous during storage. Shipin Yu Shengwu Jishu Xuebao, 25, 29–36.
Yang, J., Martinson, T.E., and Liu, R.H. (2009) Phytochemical profiles and antioxidant activities of wine grapes. Food Chemistry, 116, 332–339.
Yao, L. and Han, J.R. (2008) Effects of environmental factors on the stability of carotenoid in sclerotia of Penicillium sp. PT95. Shanxi Nongye Daxue Xuebao, 28, 73–76.
Zabala, M.T., Grant, M., Bones, A.M., Bennett, R., Lim, Y.S., Kissen, R., and Rossiter, J.T. (2005) Characterisation of recombinant epithiospecifier protein and its over-expression in Arabidopsis thaliana. Phytochemistry, 66, 859–867.
Zabetakis, I., Leclerc, D., and Kajda, P. (2000) The effect of high hydrostatic pressure on the strawberry anthocyanins. Journal of Agricultural and Food Chemistry, 48, 2749–2754.
Zakharova, N.S., Petrova, T.A., and Bokuchava, M.A. (1989) Betalain oxidase and betalain pigments in table beet seedlings. Soviet Hournal of Plant Physiology, 36, 273–277.
Zakharova, N.S. and Petrova, T.A. (2000) β-Glucosidases from leaves and roots of common beet, Beta vulgaris. Applied Biochemistry and Microbiology, 36, 458–461.
Zhang, C., Ma, Y., hao, X.Y., and Mu, J. (2009) Influence of copigmentation on stability of anthocyanins from purple potato peel in both liquid state and solid state. Journal of Agricultural and Food Chemistry, 57, 9503–9508.
Zhang, Z., Pang, X., Xuewu, D., Ji, Z., and Jiang, Y. (2005) Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chemistry, 90, 47–52.
Zhao, L.Y., Chen, F., Zhao, G.H., Wang, Z.F., Liao, X.J., and Hu, X.S. (2005) Isomerization of trans-astaxanthin induced by copper(II) ion in ethanol. Journal of Agricultural and Food Chemistry, 53, 9620–9623.
Zhao, X.Y., Corrales, M., Zhang, C., Hu, X. S., Ma, Y., and Tauscher, B. (2008) Composition and thermal stability of anthocyanins from Chinese purple corn (Zea mays L.). Journal of Agricultural and Food Chemistry, 56, 10761–10766.
Zryd, J-P. and Christinet, L. (2004) Betalains. In: K.M. Davies (ed.) Plant Pigments and their Manipulation, Boca Raton, Florida: CRC Press, Pp. 185–247.