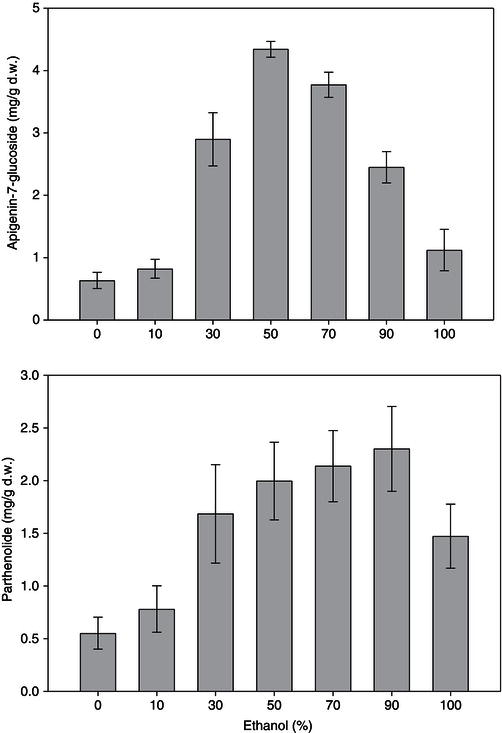
Figure 17.1 Effect of content of ethanol (%) in water on extraction of apigenin-7- glucoside from chamomile flowers and parthenolide from feverfew leaves.
Phytochemicals are a diverse group of plant derived chemicals which have received much attention in recent years due to their many health benefits including antioxidant, anticarcinogenic and anti-inflammatory activity (Dillard and German, 2000; Schreiner and Huyskens-Keil, 2006). They can be classified into sub-groups according to their chemical structure, which include terpenoids (e.g. carotenoids), phytosterols, polyphenols (e.g. tannins, flavonoids, phenolic acids) and glucosinolates (Chapter 4).
Phytochemicals make up less than 10% of the plant matrix (Harjo, Wibowo and NG, 2004) therefore to prepare phytochemical rich foods they may first need to be extracted from the plant matrix. It is important to note that the phytochemical content of plants used may vary depending on the species or organ (e.g. roots, leaves, flowers, fruits), therefore extraction conditions used may also vary. Some phytochemicals are limited to specific taxonomic groups, for example, glucosinates are specific to cruciferous vegetable crops, while others (e.g. polyphenols) are present in a wide range of plants ( Schreiner and Huyskens-Keil, 2006).
Firstly, this chapter will focus on the principles of conventional methods used to extract phytochemicals from plants with a view to incorporating them into foods and beverages, including the various extraction methods used, the factors affecting extraction of these bioactive compounds, and limitations to the use of these methods. The final section of this chapter will give an account of conventional extraction techniques used to extract phytochemicals from various plant species and plant organs, from roots to fruits, reported in the literature.
The aim of extraction is to maximise the yield of compounds of interest, while minimising the extraction of undesirable compounds. Traditionally, fresh plant material was used for the extraction of plants. However, nowadays this is not as common, as it requires very rapid post-harvest processing to avoid degradation of the plant. The extraction of phytochemicals from plants is now mostly done using dried plant as the starting material in order to inhibit the metabolic processes which can cause degradation of the active compounds, therefore extending the shelf life of the plant material.
Traditionally, phytochemicals have been extracted from plants using solid-liquid extraction techniques. This section will first cover conventional methods used for the extraction of phytochemicals from plants and it will then focus on the factors that influence the quality of the resulting extract.
Solid-liquid extraction methods used for the extraction of phytochemicals from plants include maceration, infusion and Soxhlet extraction. These extraction processes involve firstly the diffusion of the solvent into the plants cells, solubilisation of the phytochemical compounds within the plant matrix and finally diffusion of the phytochemical-rich solvent out of the plant cells. This section will also cover the extraction of essential oils from plants using steam and hydrodistillation.
Macerations are produced by steeping the plant material in a liquid, which is generally an organic solvent, at room temperature. For this extraction process the plant material is soaked in the solvent in a closed container. The solution can be stirred to increase the rate of extraction of the phytochemicals from the plant material. After extraction is complete the plant material is separated from the solvent by filtration. The plant material can then undergo another extraction step by adding fresh solvent to the material and letting it soak. This step can be repeated several times to ensure complete extraction of phytochemicals from the plant material, however it is a very time and solvent consuming process. Maceration can take from hours to days for a single extraction, and can take weeks for repeated maceration of the plant material (Seidel, 2006). Although it is time consuming it is a useful extraction method for heat labile compounds as it is carried out at room temperature.
Infusion is a similar process to maceration but the extraction is carried out at a set temperature (normally higher than room temperature and up to 100 ° C) for a set period of time (from minutes to hours) and water is generally used as the extraction solvent. As for maceration, after extraction is complete the mixture is filtered. Traditionally, infusions were made by using boiling water as the extracting solvent, for example, making a cup of tea. Following immersion in boiling water the plant material was left to steep and finally filtered to remove the plant material from the extract.
Soxhlet extraction has been used for many years in the extraction of phytochemicals from plants, and is often used as a reference for evaluating other solid-liquid extraction methods or new non-conventional extraction methods (Wang and Weller, 2006). In a Soxhlet extraction system the plant material is put in a thimble-holder, which has perforated sides and bottom so liquid can fall through. There is a collection flask below the thimble and a reflux condenser above it. Heat is applied to the flask containing solvent; the solvent evaporates and travels to the condenser. Condensed solvent then falls into the thimble containing the plant material, when it reaches a certain level it is unloaded back into the solvent flask. The solute is separated from the solvent by distillation, as the solute is left in the flask and fresh solvent passes into the plant material. This procedure is repeated until complete extraction of plant material is achieved (Wang and Weller, 2006). Solvent and particle size will need to be selected depending on the phytochemicals which need to be extracted; this will be discussed in more detail in section 17.2.1.4.
Steam and hydrodistillation are extraction techniques used to extract water-insoluble volatile constituents from various matrices, including the extraction of essential oils from plants and are widely used in the perfume industry for extraction of essential oils. For steam distillation the steam is percolated through the plant material. The steam dissolves the essential oil in the plant material and then enters a condenser. The mixture of condensed water and oil is collected and finally separated by decanting. For hydrodistillation the only difference is that the plant material is submerged in the water, which is then heated until it boils. The extraction conditions can be optimised by modifying the distillation time and temperature. The conditions may also need to be modified depending on the material being extracted, for example, for the extraction of tough material (roots or bark) glycerol may be added to the water to assist extraction (Seidel, 2006). However, for many medicinal plants the conditions used to extract essential oil are well defined in the European Pharmacopeia (2004).
The efficiency of the solid-liquid extraction methods are affected by factors such as solvent type, ratio of solvent to plant material, temperature, time and structure of the matrix (e.g. particle size, plant organ).
As previously mentioned, the extraction of phytochemicals is dependent on the dissolution of each compound in the plant material matrix and their diffusion into the external solvent (Shi, Nawaz, Pohorly, Mittal, Kakuda and Jiang, 2005), therefore the choice of extraction solvent is one of the most important matters to consider for solid-liquid extraction. The factors that need to be considered when choosing the solvent or solvent system for extraction of phytochemicals are safety of the solvent and potential for formation or extraction of undesirable compounds and finally solubility of the target compounds (Seidel, 2006).
In recent years, organic solvents (e.g. methanol) have been used to extract phytochemicals from plant material (Naczk and Shahidi, 2004). These extraction procedures were efficient and resulted in high yields of phytochemicals, but the solvents may be harmful to human health if ingested and therefore would not be desirable for inclusion in a food or beverage. To produce phytochemical rich extracts for incorporation into foods and beverages it is necessary to use food grade solvents (e.g. water, ethanol or mixtures of these).
Water is a polar solvent that has been used for many years to extract phytochemicals from plant materials, for example, infusions of medicinal herbs or teas have been used traditionally to treat many conditions including inflammation. Ethanol may also be used as an extraction solvent, because even if it is found in the final extract it is safe for human consumption. However, under EU legislation if a food contains more than 1.2% ethanol, no health claims on the efficacy of the resulting extract can be made (European Parliament and Council of Europe, 2006). Therefore, if the extraction solvent contains ethanol it must be removed before inclusion of the extract into a functional food or beverage. It should be noted that if any other organic solvents are used for the extraction of phytochemicals all solvent residues must be totally removed from the extracts before they can be incorporated into foods or beverages. Depending on the polarity of the compounds to be extracted mixtures of ethanol and water may need to be used, so the water can extract the more polar compounds and the ethanol the more hydrophobic compounds. An example of the extraction of various bioactives of with mixtures of ethanol and water is shown in Figure 17.1. It is clear that the maximum level of the bioactive marker from chamomile flowers (apigenin-7-glucoside) is extracted at an ethanol content of 50%, while the maximum bioactive marker is extracted from feverfew (parthenolide) at 90% ethanol.
Finally, the pH of the extraction solvent can be changed to selectively extract or improve the extraction of certain plant bioactives. For example, anthocyanins are unstable at neutral or alkaline pH and as a result acidic aqueous solvents are often used for the extraction of these compounds (Mateus and de Freitas, 2009).
Many studies have investigated the effect of temperature on the extraction of polyphenolics from plant material (Joubert, 1990; Price and Spitzer, 1994; Labbe, Tremblay and Bazinet, 2006; Lim and Murtijaya, 2007). In general, a higher extraction temperature causes an increase in the rate of diffusion of the soluble plant phytochemicals into the extraction solvent, thereby reducing extraction time. An increase in temperature can cause an increase in the concentration of some phytochemicals, which is possibly due to an increase in the solubility of many of these bioactive compounds, or to the breakdown of cellular constituents resulting in the release of the phytochemicals (Lim and Murtijaya, 2007). In addition an increase in the extraction temperature may also inhibit enzymatic activities thus resulting in an increase in the yield of the bioactive compounds. Marete, Jacquier and O’Riordan (2009) reported that extraction temperature of 70 ° C and above resulted in a significant increase of total phenols from feverfew due to inactivation of polyphenol oxidase. The temperature used for extraction will be limited depending on the extraction solvent chosen as they all have different boiling points, for example, the boiling point of acetone is 56–57 ° C whereas the boiling point of water is 100 ° C.
The time given to the extraction of phytochemicals from plant material by a food manufacturer may be a compromise between complete extraction of these components and having an extraction process which is both time and cost effective. The time it takes for extraction of phytochemicals will vary depending on the plant species to be extracted, the particle size of the material and the plant organ. For example, the extraction of phytochemicals from leafy material will be faster than the extraction from harder material such as roots or bark (Whitehead, 2005). To produce extracts high in desirable and low in undesirable compounds, the extraction kinetics of both the wanted and unwanted compounds may need to be studied.
Figure 17.1 Effect of content of ethanol (%) in water on extraction of apigenin-7- glucoside from chamomile flowers and parthenolide from feverfew leaves.

Plant material can undergo grinding or milling before extraction to reduce the particle size. The smaller the particle size of the material the shorter the path that the solvent has to travel, which decreases the time for maximum phytochemical content to be extracted (Shi et al., 2005). Also, grinding or milling the plant material to reduce the particle size damages the plant cells which can also lead to increased extraction of phytochemical compounds. For example, Fonseca, Rushing, Thomas, Riley and Rajapakse (2006) explained the suitability of using finely ground samples for the maximum extraction yield of parthenolide in feverfew, which may be localised in trichomes, in small oil glands or may be bound in other tissues. The disadvantage of grinding or milling the plant before extraction is that plant material of small particle size may block filters quicker than bigger particles and this could possibly result in wastage of the extract and extended extraction times (Whitehead, 2005).
In general, the disadvantages of using conventional extraction techniques that are commonly cited in the literature include long extraction times, requirement of large quantities of solvent and the degradation of heat labile phytochemicals by using high temperatures for extraction. The disadvantages of conventional methods as a result of solvent and temperature will be discussed in more detail.
As mentioned earlier, for conventional extraction methods solvent choice is very important. The extraction of phytochemicals with a view to incorporating these compounds into foods means that the solvent choice is limited as it must be food grade. The small selection of solvents available for use may mean that some bioactive compounds may not be soluble in the solvent system chosen. Furthermore, unlike extracts made from water, those made by the extraction of phytochemicals from plants with any organic solvent must also undergo an evaporation step to remove the solvent. It is possible that this evaporation step may result in the formation of undesirable compounds or degradation of the bioactive(s) of interest. This evaporation step may also be costly, as companies have to ensure for health and safety reasons that the solvent is completely removed from the end product. Dried extracts can easily be added into foods, however a liquid extract (e.g. infusions) is preferred for the majority of functional beverages as it is the easiest to mix into the end product (Whitehead, 2005). Lastly, some conventional methods use large amounts of organic solvents during extraction (e.g. maceration) which is neither environmentally friendly nor cost effective.
Many phytochemicals are heat stable and extraction at high temperature has no adverse effects; however there are some phytochemicals which are heat labile. A recent study showed that in acai fruit extracts heat had no effect on its phenolic content, which included flavone glycosides, flavonol derivatives and phenolic acids, but resulted in a significant reduction in the anthocyanin content possibly due to accelerated chalcone formation on exposure to high temperatures (Pacheco-Palencia, Duncan and Talcott, 2009). The effect of heat on anthocyanins degradation is well documented in literature (Harbourne, Jacquier, Morgan and Lyng, 2008; Patras, Brunton, O’Donnell and Tiwari, 2010) therefore high temperatures for long extraction times would not be suitable for extraction of plants containing these compounds. Another phytochemical which has been shown to be heat labile is parthenolide in feverfew extracts (Marete, Jacquier and O’Riordan, 2011). Steam distillation can also result in degradation of bioactive volatiles during the extraction of essential oils. For example, chamomile essential oil extracted by steam distillation had a low content of matricine as it degraded to its breakdown product chamazulene, whereas during supercritical fluid extraction (SFE) there were higher levels of matricine and very little chamazulene present in the essential oil (Kotnik, Skerget and Knez, 2007). Before choosing extraction conditions the bioactive of interest needs to be assessed for heat stability. If the bioactive is heat sensitive it may need to be extracted at lower temperatures for a longer time period or alternatively a non conventional method, which does not use high temperatures, could be used.
As already mentioned extraction conditions can vary greatly depending on the part of the plant used for extraction. Therefore, in this section the extraction of phytochemicals using conventional techniques from various plant species and from different plant organs from roots to fruits will be reviewed.
Roots are the underground part of the plant and include vegetables such as carrots, potatoes and sweet potatoes. Purple sweet potatoes are a good source of anthocyanins and the extraction of these compounds has been studied as they show potential as natural food colorants and also have many health benefits (Fan, Han, Gu and Chen, 2008). Response surface methodology was used to optimise the extraction of fresh pulverised purple sweet potato using acid-ethanol (1.5M HCl) at various extraction temperatures (40–80 ° C), times (60–120 min) and solvent to solid ratios (15:1–35:1) (Fan et al., 2008). The factors that had the most significant effect on the extraction of the anthocyanins from sweet potato were temperature and solvent to solid ratio. The optimum conditions for extraction were extraction temperature of 80 ° C for 60 min at a solvent to solid ratio of 32:1 to yield 1.58 mg/g d.w. of purple sweet potato anthocyanins.
Black carrot is another root vegetable which is also a potential source of anthocyanins that can be used as a functional ingredient, especially as it contains acylated cyanidin derivatives which demonstrate superior heat and pH stability in comparison with other anthocyanins (Turker and Erdogdu, 2006). The effect of extraction temperature (25–50 ° C) and pH (2–4) on the diffusion coefficient of anthocyanins from black carrot slices were studied. The diffusion coefficient increased with an increase in temperature, due to an increase in the solubility of the anthocyanins. For example, in the extract at pH 2 the diffusion coefficient increased from 3.73 to 7.37 m2/s at 25 and 50 ° C, respectively (Turker et al., 2006). However, as anthocyanins are heat labile the extraction temperature cannot be increased indefinitely or it may result in degradation. The diffusion coefficient also increased with a decrease in pH; at an extraction temperature of 37.5 ° C the diffusion coefficient increased from 0.25 to 5.00 m2/s as the pH decreased from 4 to 2. Overall, extraction of black carrot anthocyanins at high temperatures and low pH results in a more time effective process.
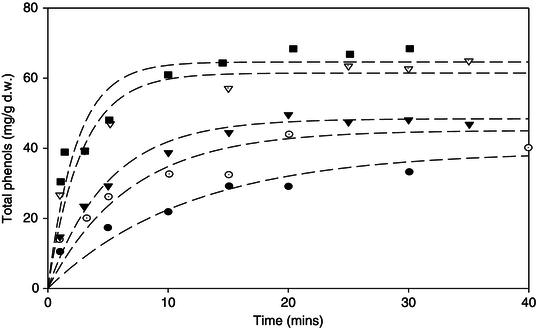
In recent years the extraction of the leaves and stems of many plants has been studied with a view to using the extracts as functional ingredients for incorporation into foods (Harbourne, Marete, Jacquier and O’Riordan, 2011) including meadowsweet (Harbourne, Jacquier and O’Riordan, 2009) and feverfew (Marete et al., 2009). Meadowsweet contains tannins, phenolic acids, flavonoids and salicylates (Blumental, Goldberg and Brinckmann, 2000). Flavonoids have been extracted from meadowsweet leaves using hot aqueous ethanol (70%) (Krasnov, Raldugin, Shilova and Avdeeva, 2006) and also using methanol in a Soxhlet apparatus (Papp et al., 2004), however this was with a view of analysing the compounds present and not for incorporation into foods. Traditionally meadowsweet has been extracted by infusion or maceration (Mills and Bone, 2000). The aqueous extraction kinetics of phenolic compounds from meadowsweet (Harbourne et al., 2009) has recently been examined with a view to maximising the total phenolic content and minimising the content of tannins, which impart a bitter and astringent taste to the extract, and was found to follow a pseudo-first order kinetic model (Figure 17.2). The concentration of phenolic compounds, which included salicylic acid and quercetin (bioactives thought to be responsible for meadowsweet’s health benefits), rose rapidly initially before reaching an equilibrium concentration (Figure 17.2). The rate constant (k) increased from 0.09 to 0.44 min−1 as the temperature increased from 60 to 100 ° C. In addition, increasing the temperature from 60 to 90 ° C resulted in an increase in the total phenols from 39 ± 2 to 61 ± 2 mg/g d.w, however increasing the temperature to 100 ° C showed no further increase (Figure 17.2). Increasing the temperature of the extraction solvent did not have a significant effect on the proportion of tannins and non-tannins extracted. Interestingly, the non-tannin fraction was extracted faster than both the tannins and total phenols from meadowsweet. Therefore, the extraction of tannins is likely to be the rate limiting step in the extraction of total phenols from meadowsweet. The optimum temperature for the extraction of phenolic compounds (including salicylic acid and quercetin) without having any adverse effects on the tannin concentration of meadowsweet was ≥ 90 ° C for 15 min (Harbourne et al., 2009).
Figure 17.2 Extraction kinetics of total phenols from meadowsweet at temperatures 60( ), 70(
), 70( ), 80(
), 80( ), 90(
), 90( ) & 100(
) & 100( ) ° C.
) ° C.
The pH of the extraction solvent can be changed to improve the extraction of some bioactives from the plant matrix. However, it tends to be different depending on the plant species and bioactives of interest and therefore should be optimised specifically for each plant. For example, the maximum total phenols extracted from meadowsweet increased from 43±2 to 57±2 mg/g d.w. with an increase in pH from 3.9 to 6.4, however increasing the pH had no significant effect on the salicylic acid or quercetin content in the extracts (Harbourne et al., 2009). Unlike meadowsweet, the extraction of total catechins from green tea leaves did not significantly change when the green tea was extracted at pH 4, 5 or 6 but when the extraction pH was increased to 7 the total catechin content decreased significantly (Kim, Park, Lee and Han, 1999). Also, Spiro and Price (1987) studied the effect of pH on theaflavins in black tea; in this case increasing the pH to 6.8 had no significant effect on the theaflavin content but decreasing the pH caused an increase in theaflavin content. Likewise, Liang and Xu (2001) found that more acidic conditions favoured the extraction of polyphenols in black tea, while increasing the pH from 4.9 to 9.45 caused a decrease in some of the tea phenols, including thearubigins, theaflavins and catechins.
The flowers of many plants also have a high content of phytochemicals. Chamomile (Matricaria chamomilla L.) flowers are a popular ingredient in many foods and beverages due to their many health benefits. They have a high content of phenolic compounds, including flavonoids such as flavone glycosides (e.g. apigenin-7-glucoside), flavonols (e.g. quercetin glycosides, luteolin glucosides) and caffeic and ferulic acid derivatives. Chamomile also contains an essential oil, the main components of it being α -bisabolol and chamazulene (which is responsible for its blue colour) (Harbourne et al., 2011). The essential oil of chamomile can be extracted using steam or hydrodistillation according to the pharmacopeia standards (4 h distillation period).
Traditionally, chamomile flowers were extracted by infusion or maceration. The effect of extraction temperature and time on the aqueous extraction of total phenols and apigenin- 7-glucoside from whole chamomile flowers was optimised (Harbourne, Jacquier and O’Riordan, 2009a). The extraction of phenolic compounds from chamomile flowers followed a pseudo-first order kinetic model. As the extraction temperature increased from 57 to 100 ° C the rate of extraction ( k) of total phenols increased from 0.028 ± 0.004 to 0.31 ± 0.03 min −1. Also, the total phenolic content increased from 14.6 to 24.5 mg/g d.w with an increase in temperature from 57 to 100 ° C, while the apigenin-7-glucoside content reached a maximum at 90 ° C (0.29 mg/g d.w.). It should be noted that between 90 and 100 ° C there was a significant increase in turbidity of the extract possibly due to tissue degradation of the chamomile flowers during boiling. Therefore, aqueous extraction at 90 ° C × 20 min were the optimum conditions for preparing an extract with a high phenolic content and low turbidity which would be ideal for incorporation into foods or beverages.
The content of ethanol (%) in water on extraction of polyphenols from whole chamomile flowers after steeping at room temperature for 24 h has been examined in our laboratory. An ethanol content of 50% yielded extracts with the maximum apigenin-7-glucoside (Figure 17.1) and total phenol content (unpublished results).
Apple pomace is a by-product from apple juice and apple cider and grape pomace is a by-product from wine making, which have been investigated as a source of phenolic compounds due to their abundance. Extraction of phytochemicals from waste products, such as apple pomace and grape pomace, has received much interest in recent years due to the interest in using natural and low cost sources of phytochemicals for incorporation into foods or beverages.
Apple pomace consists of the peel, core, seed, calyx, stem and soft tissue. It contains many polyphenols including chlorogenic acid, catechins, procyanididns and quercetin glycosides (Lu and Foo, 1997; Cam and Aaby, 2010). Mostly, the extraction of these compounds from apple pomace has been done using organic solvents such as methanol (Cam and Aaby, 2010), however recently the extraction of polyphenols using food grade solvents has also been investigated. Response surface methodology was used to optimise the extraction of apple pomace phenolics with water (Cam and Aaby, 2010; Wijngaard and Brunton, 2010), ethanol and acetone (Wijngaard and Brunton, 2010). In both studies the apple pomace was freeze-dried and milled to a fine powder before extraction. Cam and Aaby (2010) studied the effect of temperature, extraction time and solvent to solid ratio on the extraction of total phenols and 5-Hydroxymethylfurfural (HMF), high levels of which are undesirable in foods and beverages, from apple pomace. Aqueous extraction at 100 ° C for 37 min at a solvent to solid ratio of 100 mL/g were the optimum to yield extracts rich in phenolic compounds (8.3 mg/g d.w.) with limited quantity of HMF (42 mg/L). At times greater than 37 min the total phenol concentration increased but so did the HMF content and at solvent to solid ratios above 100 ml/g there was no further increase in total phenolics. To maximise the extraction of polyphenols from apple pomace using acetone and ethanol, the extraction time, temperature and content of water in the solvent was optimised to give the highest antioxidant activity (Wijngaard and Brunton, 2010). The conditions using ethanol as the solvent were 56% ethanol at 80 ° C for 31 min, which resulted in extracts with an antioxidant value of 4.44 mg Trolox/g d.w. and a total phenolic content of 10.92 mg/g d.w. Optimum conditions using acetone as the extraction solvent were 65% acetone at 25 ° C for 60 min, which yielded an antioxidant value of 5.29 mg Trolox/g d.w. and total phenol content of 14.15 mg/g d.w. Depending on the solvent used the content of individual polyphenols changed. For example, the content of procyanidins, catechin, epicatechin and caffeoylquinic acids were higher in extracts made using water as the solvent, while quercetin glycosides were much higher when acetone was used as the extraction solvent (Cam and Aaby, 2010). Therefore, as mentioned earlier, depending on the compounds of interest the extraction solvent can be modified. Although in contrast to using water all traces of organic solvent used to extract polyphenols must be removed prior to incorporation in foods.
Grape pomace consists of pressed skins and seeds of grapes. They are rich in phenolic compounds including anthocyanins, catechins, cinnamic acids and proanthocyanidins. Anthocyanin-rich extracts from grape pomace are widely used as natural food colorants in many foods and beverages including yogurts, soft drinks and confectionary (Mateus and de Freitas, 2009). The extraction of phenolic compounds from dried grape pomace has been studied using ethanol, water and mixtures of these solvents (Pinelo, Rubilar, Jerez, Sineiro and Nunez, 2005; Spigno, Toramelli and De Faveri, 2007; Lapornik, Prosek and Wondra, 2005). Spigno et al. (2007) studied the extraction of phenolics from dried and milled grape pomace using ethanol at two temperatures (45 and 60 ° C) over a time period of 1–24 h. In general, the extraction of all phenolic compounds increased with an increase in extraction temperature and time. However, after extraction for 20 h at 60 ° C there was a reduction in total phenols, particularly for anthocyanins and tannins due to thermal degradation or polymerisation. As this process may be used for industrial applications, cost and time were considered and as a result extraction conditions of 60 ° C for 5 h were selected even though they did not yield the highest phenolic content. Using these conditions the grape pomace was extracted using mixtures of ethanol and water (10–60%). There was an increase in the yield of total phenols with an increase in water content in the extraction solvent (ethanol) from 10% (24.5 mg/g) to 30% (41.3 mg/g); however above this there was no further significant increase. In another study the effect of using water or aqueous ethanol (70%) on the extraction of anthocyanins from grape pomace was compared, and it was found that the ethanol extracts contained seven times more anthocyanins than those extracted with water (Lapornik et al., 2005). The extraction kinetics of dried and milled grape pomace phenolics have been studied in aqueous ethanol (60%) at 60 ° C over a period of 5 h. Similar to chamomile flowers and meadowsweet leaves the extraction of grape pomace phenolics followed a first order kinetic model. The equilibrium concentration of phenolics was extracted after 120 min (Amendola, Faveri and Spigno, 2010).
By varying the factors affecting extraction, including solvent, temperature, particle size, solvent to solid ratio and time, a wide range of phytochemicals can be extracted using conventional methods. However, the extraction parameters must be optimised depending on the bioactives of interest, the plant species and the plant organ, as the extraction conditions can vary greatly.
Amendola, D., De Faveri, D.M. and Spigno, G. (2010) Grape marc phenolics: Extraction kinetics, quality and stability of extracts . Journal of Food Engineering, 97 , 384–392.
Blumental, M., Goldberg, A. and Brinckmann, J. (2000) Herbal Medicine: Expanded Commission E Monographs. Massachusetts: Integrative Medicine Communications.
Cam, M. and Aaby, K. (2010) Optimization of extraction of apple pomace phenolics with water by response surface methodology . Journal of Agriculture and Food Chemistry, 58 , 9103–9111.
Dillard, C.J. and German, J.B. (2000) Phytochemicals: Nutraceuticals and human health . Journal of the Science of Food and Agriculture, 80 , 1744–1756.
European Parliament and Council of Europe (2006) Directive 2006/1924/EC, relating to the nutrition and health claims made on foods. Official Journal, L 404. Luxembourg: Office for Official Publications of the European Communities.
European Pharmacopoeia (2004) Directorate for the quality of medicines , Council of Europe: Strasbourg.
Fan, G., Han, Y., Gu, Z. and Chen, D. (2008) Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM) . LWT – Food Science and Technology, 41 ,155– 160.
Fonseca, J.M., Rushing, J.W., Thomas, R.L., Riley, M.B. and Rajapakse, N.C. (2006) Post-production stability of parthenolide in Feverfew ( Tanacetum parthenium) . Journal of Herbs, Spices and Medicinal Plants, 12 , 139–152.
Harbourne, N., Jacquier, J.C., Morgan D. and Lyng, J. (2008) Determination of the degradation kinetics of anthocyanins in a model juice system using isothermal and non-isothermal methods , Food Chemistry, 111 , 204–208.
Harbourne, N., Jacquier, J.C. and O’Riordan, D. (2009) Optimisation of the aqueous conditions of phenols from meadowsweet ( Filipendula ulmaria) for incorporation into beverages . Food Chemistry, 116 , 722–727.
Harbourne, N., Jacquier, J.C. and O’Riordan, D. (2009a) Optimisation of the extraction and processing conditions of chamomile ( Matricaria chamomilla L.) for incorporation into a beverage . Food Chemistry, 115 , 15–19.
Harbourne, N., Marete, E., Jacquier, J.C. and O’Riordan, D. (2011) Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages – A review. Food Research International, doi:10.1016/j.foodres.2011.03.009.
Harjo, B., Wibowo, C. and Ng, K.M. (2004) Development of natural product manufacturing processes: Phytochemicals . Chemical Engineering Research and Design, 82 , 1010–1028.
Joubert, E. (1990) Effect of batch extraction conditions on extraction of polyphenols from rooibos tea (Aspalathus-Linearis) . International Journal of Food Science and Technology, 25 (3), 339–343.
Kim, S.H., Park, J.D., Lee, L.S. and Han, D.S. (1999) Effect of pH on green tea extraction . Korean Journal of Food Science and Technology, 31 (4), 1024–1028.
Kotnik, P., Skerget, M. and Knez, Z. (2007) Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale up . Journal of Supercritical Fluids, 43 , 192–198.
Krasnov, E.A., Raldugin, V.A., Shilova, I.V. and Avdeeva, E.Y. (2006) Phenolic compounds from Filipendula ulmaria . Chemistry of Natural Compounds, 42 (2), 148–151.
Labbe, D., Tremblay, A. and Bazinet, L. (2006) Effect of brewing temperature and duration on green tea catechin solubilisation: basis for production of EGC and EGCG-enriched fractions . Separation and Purification Technology, 49 (1), 1–9.
Lapornik, B., Prosek, M. and Wondra, A.G. (2005) Comparison of extracts prepared from plant by-products using different solvents and extraction time . Journal of Food Engineering, 71 , 214–222.
Liang, Y.R. and Xu, Y.R. (2001) Effect of pH on cream particle formation and solids extraction yield of black tea . Food Chemistry, 74 (2), 155–160.
Lim, Y.Y. and Murtijaya, J. (2007) Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods . LWT – Food Science and Technology, 40 (9), 1664–1669.
Lu, Y. and Foo, L.Y. (1997) Identification and quantification of major polyphenols in apple pomace , Food Chemistry, 59 , 187–194.
Marete, E., Jacquier, J.C. and O’ Riordan, D. (2009) Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew ( Tanacetum parthenium) extracts . Food Chemistry, 117 , 226–231.
Marete, E., Jacquier, J.C. and O’Riordan, D. (2011) Effect of processing temperature on the stability of parthenolide in acidified feverfew infusions. Food Research International, doi:10.1016/j.foodres.2011.03.042.
Mateus, N. and de Freitas, V. (2009) Anthocyanins as food colorants. In: K. Gould, K. Davies and C. Winefield (eds.) Anthocyanins: Biosynthesis, Functions, and Applications, New York: Springer Verlag, pp. 283–304.
Mills, S. and Bone, K. (2000) Principles and Practice of Phytotherapy:Modern Herbal Medicine, London: Churchill Livingstone.
Naczk, M. and Shahidi, F. (2004) Extraction and analysis of phenolics in food . Journal of Chromatography A, 1054 , 95–111.
Pacheco-Palencia, L.A., Duncan, C.E. and Talcott, S.T. (2009) Phytochemical composition and thermal stability of two commercial acai species, Euterpe oleracea and Euterpe precatoria . Food Chemistry, 115 , 1199–1205.
Papp, I., Apati, P., Andrasek, V., Blazovics, A., Balazs, A., Kursinszki, L., Kite, G.C., Houghton, P.J., and Kery, A. (2004) LC-MS analysis of antioxidant plant phenoloids . Chromatographia, 60 , S93–S100.
Patras, A., Brunton, N., O’Donnell, C. and Tiwari, B. (2010) Effect of thermal processing on anthocyanin stability in foods: mechanisms and kinetics of degradation . Trends in Food Science and Technology, 21 , 3–11.
Pinelo, M., Rubilar, M., Jerez, M., Sineiro, J. and Nunez, M.J. (2005) Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace . Journal of Agricultural and Food Chemistry, 53 , 2111–2117.
Price, W.E. and Spitzer, J.C. (1994) The kinetics of extraction of individual flavanols and caffeine from a Japanese green tea (Sen Cha Uji Tsuyu) as a function of temperature . Food Chemistry, 50 , 19–23.
Seidel, V. (2006) Initial and bulk extraction. In: S. D. Sarker, Z. Latif, A. I. Gray (eds.) Natural Products Isolation, New Jersey: Humana Press Inc., pp. 27–46.
Schreiner, M. and Huyskens-Keil, S. (2006) Phytochemicals in Fruit and Vegetables: Health Promotion and Postharvest Elicitors . Critical Reviews in Plant Sciences, 25 , 267–278.
Shi, J., Nawaz, H., Pohorly, J., Mittal, G., Kakuda, Y. and Jiang, Y. (2005) Extraction of polyphenols from plant material for functional foods - Engineering and Technology . Food Reviews International, 21 , 139–166.
Spigno, G., Toramelli, L. and De Faveri, D.M. (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics . Journal of Food Engineering, 81 , 200–208.
Spiro, M. and Price, W.E. (1987) Kinetics and equilibria of tea infusion .6. The effects of salts and of pH on the concentrations and partition constants of theaflavins and caffeine in kapchorua pekoe fannings . Food Chemistry, 24 , 51–61.
Turker, N. and Erdogdu, F. (2006) Effects of pH and temperature of extraction medium on effective diffusion coefficient of anthocyanin pigments of black carrot . Journal of Food Engineering, 76 , 579–583.
Wang, L. and Weller, C.L. (2006) Recent advances in extraction of nutraceuticals from plants . Trends in Food Science and Technology, 17 , 300–312.
Whitehead, J. (2005) Functional drinks containing herbal extracts. In: P.R. Ashurst (ed.) Chemistry and Technology of Soft Drinks and Fruit Juices, Oxford: Blackwell Publishing Ltd., pp. 300–335.
Wijngaard, H.H. and Brunton, N. (2010) The optimisation of solid-liquid extraction of antioxidants from apple pomace by response surface methodology . Journal of Food Engineering, 96 , 134–140.