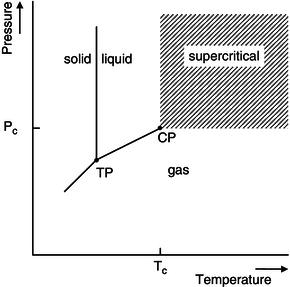
Figure 18.1 Pressure–temperature diagram for a pure component.
Traditionally organic solvents are used to extract phytochemicals from plant materials. Hexane is generally used in oil extraction (Wakelyn and Wan, 2001), while polyphenols can be extracted with various solvents including methanol and ethylacetate (Shi et al., 2005). Sage and rosemary extracts have been extracted with hexane, benzene, methanol, ethyl ether, chloroform, ethylene dichloride and dioxane (Chang et al., 1977). Although solvents are generally removed by ultrafiltration or evaporation (Wakelyn and Wan, 2001), there is a higher risk that unsafe solvents are still present in the final product than when less harmful solvents are used, such as ethanol or CO2.
In addition, interest in more sustainable and non-toxic routes of phytochemical extraction has increased. It has become important to enhance the naturalness of food ingredients from a customer-oriented point of view. In addition, the negative impact on the environment can be reduced by using more environmentally friendly extraction methods. Conventional solid-liquid extraction techniques such as Soxhlet extraction and maceration, are time consuming and use high amounts of solvents (Wang and Weller, 2006). This has highlighted the necessity for more sustainable techniques.
In most cases organic solvents show better solubilities for phytochemicals than environmentally safe solvents such as water and CO2 due to their chemical characteristics. There are various ways of avoiding organic solvents, which are generally not environmentally friendly and seen as unnatural. An additional challenge of extracting phytochemicals from food materials in a sustainable way is the fact that phytochemicals usually are embedded within the plant matrix. Solid-liquid extraction of plants is therefore not as straightforward as general chemical extractions. Besides parameters, such as diffusion coefficients, solvent choice, temperature and concentration difference, the matrix itself plays a large role. Phytochemicals are usually embedded within the plant cellular matrix, and are not readily available. By applying a ‘pretreatment’ to the sample, the matrix can become more accessible to the solvent. Enzymes can be added as described in Kim et al. (2005) and Pinelo et al. (2008) in order to breakdown plant cell walls. Pulsed electric fields can be applied, which entails poration of cell membranes by a field of electrical pulses (Soliva-Fortuny et al., 2009) or ultrasound waves, which can enhance extraction by the effects of bubble cavitation (Luque-García and Luque de Castro, 2003). Another approach is to use other pressure and temperature conditions with environmentally safe and food-grade solvents, such as water, CO2 and ethanol. Hereby the properties of these solvents can be adapted, for example the dielectric constant will alter and therefore solubility parameters will change, which can lead to enhanced extractability of phytochemicals. Pressurised fluids, which are discussed in section 18.2, are an example of this approach.
As the name suggests, pressurised solvents apply pressure to a solvent system, which affects the target molecule’s specificity and speed. By applying certain pressure and temperature conditions, the physicochemical properties of the solvents, including density, diffusivity, viscosity and dielectric constant, can be controlled. By using high pressures and temperatures the extraction of phytochemicals is generally enhanced and the environmentally friendly solvents such as water can obtain similar physicochemical properties as organic solvents. Another advantage may be that through the high pressures used, the cellular matrix is more penetrable.
The two techniques that fall under this category are:
The application of supercritical fluid extraction in industry has been proved in the late sixties, by Zosel and his patent on caffeine removal from coffee with SC-CO2 (Zosel, 1981). SC-CO2 is an alternative process to coffee decaffeination by extraction with ethylacetate, dichloromethane or benzene. Another example of an industrial application is the production of hop oils. More recently SC-CO2 has also been considered as a technique to extract phytochemicals, mainly from by-product. By-products are an inexpensive source of phytochemicals that in many cases is underutilised. PLE is a newer technique and has mainly been used in analytical chemistry as a sample preparation system. Both SC-CO2 and PLE are perceived as environmentally friendly and sustainable technologies, since both consume relatively less organic solvents and can show higher extraction efficiencies than conventional techniques.
Supercritical fluid extractions are taking place above the critical temperature and critical pressure of the applied solvent. The critical temperature is the highest temperature at which an increase in pressure can convert a gas to a liquid phase and the critical pressure is the highest pressure at which a liquid can be converted into a gas by an increase in temperature. If a solvent system is set in a temperature higher than the critical temperature and a pressure higher than the critical pressure, the solvent will be in the so-called ‘supercritical region’ as is shown in Figure 18.1 (Taylor, 1996). In the supercritical region, the physico-chemical properties of the solvent can be advantageous. Supercritical fluids possess a relatively high density (more comparable to liquids) and a relatively low viscosity (more comparable to gases) (Lang and Wai, 2001). Although other solvents, such as nitrous oxide, ammonia and propane can be used, the main medium applied is CO2. A typical setup of a SC-CO2 system is shown in Figure 18.2. The main reason to use SC-CO2 in extraction of phytochemicals is that CO2 has advantageous physical characteristics with its relatively low critical points (McHugh and Krukonis, 1994). CO2 has a critical temperature of 31.3 °C, which is low enough to retain thermo-labile phytochemicals and a critical pressure of 7.3 MPa (=73 bars). In addition, CO2 is environmentally safe, inexpensive, can easily be obtained at a high purity and is food-grade: when used to process foods it does not need to be declared on the food label (Brunner, 2005; Herrero et al., 2010). Another advantage is that by manipulating pressure and temperature, SC-CO2 can be very selective. This makes the application very useful for plant matrices, in which the targeted phytochemicals are in general present at low concentrations and have complex compositions (Lang et al., 2001). Finally, by using SC-CO2, the phytochemical can be easily separated by depressurising, which can eliminate the process of solvent evaporation and phytochemical concentration. These processes are in general very costly and time-consuming (Brunner, 2005).
Various studies and books have described the mass transfer process during SC-CO2 extraction. During solid-liquid extraction the following steps usually take place: (1) entering of the solvent into the solid plant matrix, (2) solubilisation/breakdown of components, (3) transport of solute to the exterior of the plant matrix and (4) migration of the solute from the plant surface layer into the bulk solution (external diffusion) (Aguilera and Stanley, 1999). The mass transfer process can be limited by different parts of the extraction process. When the transport of the solute through the matrix or its pores is limiting, mass transfer is controlled by intra-particle diffusion. The properties of the matrix and solute, and not the flow rate play a large role when this is the case. On the other hand, when the process is controlled by external diffusion, the flow rate has a large effect and enhances mass transfer rates when the flow rate is increased. If the limiting step is to solubilise the components, it is a thermodynamic restraint, but solvent rate will still have an effect (Anekpankul et al., 2007). When developing extraction methods for phytochemical ingredients these steps need to be taken into consideration. When SC-CO2 is used the same principles apply and therefore various studies have been carried out to model mass transfer of phytochemicals into SC-CO2. For example, the SC-CO2 extraction of the triterpenoid nimbin from neem seeds has been modelled successfully by determining the intraparticle diffusion coefficient and the external mass transfer parameter (Mongkholkhajornsilp et al., 2005). To determine if effects are external or internal flow rates can be varied and characteristics of the matrix, such as porosity, can be changed. Determining thermodynamic properties and solubility parameters of solutes in SC-CO2 is another approach researchers have taken. The properties can be described by various models, such as Peng-Robinson equation or group contribution methods (Fornari et al., 2005; Murga et al., 2002).
Figure 18.1 Pressure–temperature diagram for a pure component.

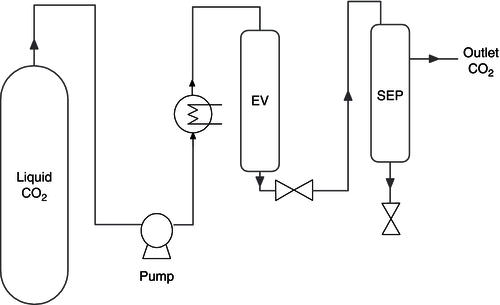
Figure 18.2 A possible setup of a SC-CO2 system.
The sample is added to the extraction vessel (EV). Liquid CO2 is pumped and heated while the backpressure is regulated. The SC-CO2 is pumped through the extraction vessel and the targeted solute will be solubilised. By releasing the pressure the solutes will come out of solution in the separation vessel (SEP) and can be collected.
Sovová (2005) used the concept of broken and intact cells to model the mass transfer of natural products with supercritical fluids. The author divided the extraction into two phases, the first phase controlled by phase equilibrium and the second by internal diffusion. Four types of extraction curves were defined based on composition of solid and fluid phases. Reverchon (1996) used models with different particle shapes when extracting essential oils from sage with SC-CO2. The particle shape of the ground material, proved to be an important factor when fitting the results. Using a slab as a particle shape resulted in better models than when using a more conventional sphere.
The importance of the matrix effect was also emphasised by Björkland et al. (1998). They analysed solubility of solutes such as clevidipine and various oils in SC-CO2 when applied to different matrices, for example filter paper and stainless steel beads. The various matrices showed a large effect on the extraction of the solute into SC-CO2. Especially cellulose based materials inhibited extraction. Most investigators add modifiers in order to increase the polarity of the SC-CO2 system, but adding modifiers has another advantageous effect, namely that interactions between analyte and matrix can be broken. For example, adding 4% methanol to a filter paper matrix in order to extract added clevidipine with SC-CO2 enhanced the recovery of clevidipine 15 times (Björklund et al., 1998). Another way to release the solute from the matrix is to increase the temperature, which can also help in breaking the solute–matrix bond (Langenfeld et al., 1995). An additional important parameter is the particle size of the matrix: the smaller the particle size, the higher the extraction rate, up to a certain point. A smaller particle has a relatively large surface area, which would enhance the extraction. But when the particles are too small, channelling can start to take place and the extraction rate will decrease again (Reverchon and De Marco, 2006). For example, lycopene was extracted from tomato waste by SC-CO2. When a particle size of 0.080 mm was used the extraction rate decreased and the matrix was inhomogenous after extraction, which pointed at the fact that channelling had occurred in the matrix (Sabio et al., 2003).
Another approach is to use empirical models in order to optimise extraction conditions, such as response surface methodology (RSM). Empirical models are based on various conditions such as temperature and pressure and have no need for other facts, such as density change.
Various groups of bioactive compounds can be extracted by SC-CO2. For example, alkaloids, such as caffeine, are soluble in SC-CO2, especially at high densities. Decaffeination of coffee is probably the best known example of SC-CO2, and is largely applied on industrial scale. More recently many studies have focussed on decaffeination of tea (Içen and Gürü, 2009; Kim et al., 2008; Park et al., 2007a; Park et al., 2007b). Since caffeine is not a phytochemical it will not be discussed further here.
Carotenoids are a group of phytochemicals that has often been extracted by SC-CO2. To extract carotenoids a modifier can be added, but this is not essential. For example, in order to extract trans-lycopene from tomato skin, no addition of modifier was necessary (Kassama et al., 2008). Saldaña et al. (2006) did not use modifiers either to study the solubility of β-carotene with SC-CO2. Solubilising free β-carotene was carried out by the quartz crystal microbalance technique and β-carotene that was present in the carrot matrix was extracted by a dynamic SC-CO2 extraction. Although carrots were freeze-dried and ground to a particle size distribution of 0.5–1.0 mm, the solubility of β-carotene from the carrot matrix was five to ten times lower than when free β-carotene was extracted. The authors concluded that the cellular structure of carrots and the presence of carbohydrates to which the carotenoids can be bound interfered with solubilising β-carotene in SC-CO2 (Saldaña et al., 2006). When necessary, a modifier that is often used is ethanol, which can enhance the polarity of SC-CO2 and also help to desorb the solute from the plant matrix (Sanal et al., 2004). Vega et al. (1996) extracted β-carotene from carrot press cake. They optimised the extraction by response surface methodology (RSM). They reported that an ethanol concentration of 10% was optimal due to enhanced solubility of β-carotene in the CO2 at this condition. Another modifier that can be used for extraction of carotenoids from carrots is canola oil. By adding 5% canola oil as a co-solvent, the extraction of α- and β-carotene was enhanced twice, while the extraction of lutein was enhanced four times (Sun and Temelli, 2006). Using vegetable oils as modifier is an interesting application since no evaporation process is needed as when ethanol is added. In order to extract lycopene from tomato, the addition of sunflower seed, peanut, almond and hazelnut oil were tested. Only the addition of hazelnut oil had an enhancing effect, possibly due to the lower acidity. The recovery of lycopene was 60% when hazelnut oil was added in 10%, a pressure of 450 bars was used, a temperature of 65–70 °C and a flow rate of 18–20 kg CO2/h and an average particle size of 1 mm (Vasapollo et al., 2004). An even higher recovery of 75% lycopene was reported when a combination of olive oil and ethanol has been used to enhance the extraction from tomato skins. The maximum recovery of lycopene of 75% was reached when 10% ethanol and 10% olive oil were added to the SC-CO2 system. In this report it was also mentioned that addition of any of the modifiers ethanol, olive oil and water increased lycopene extraction with SC-CO2 (Shi et al., 2009).
In addition to tomato, fruits such as pitanga fruit and watermelon have been extracted with SC-CO2 in order to extract carotenoids (Filho et al., 2008; Vaughn Katherine et al., 2008). But the most extracted fruit is the tomato and predominantly by-products are used. Various studies exist, which are focussed on the optimisation of conditions to maximise the recovery of carotenoids with SC-CO2. Especially pressure (density), temperature, CO2 flow rate, modifier percentage, moisture content and particle size have been tested and reported to affect the extraction. The main phytochemicals recovered from tomato by-product are lycopene and β-carotene, but γ-carotene, lutein, chryptoxanthines and lycoxanthines were also extracted (Vági et al., 2007).
To supercritically extract maximum amounts of lycopene and β-carotene from tomato by-products a minimum pressure of 30 MPa is required. In addition, relatively high temperatures are generally optimal. When a temperature of 80 °C and a pressure of 30 MPa was applied, 88% of the present lycopene was extracted from skins and seeds and 80% of the total present β-carotene (Sabio et al., 2003). In other studies it was noted that the chemical form of the solute mattered. To maximally extract trans-lycopene from tomato skins a temperature of 60 °C instead of 80 °C was beneficial (Nobre et al., 2009). These results were confirmed by Kassama et al. (2008). With regard to flow rate, a lower flow rate of 0.59 g CO2/min was preferred over a flow rate of 1.14 g CO2/min. It is possible that channelling was taking place at the higher flow rate (Nobre et al., 2009). These results agreed with results from Rozzi et al. (2002), which showed that an increase in flow rate decreased the extraction of lycopene from tomato seeds and skins. Various studies show that very wet samples are not suitable for extraction. Vasopollo et al. (2004) showed that drying was needed to obtain quantifiable amounts of lycopene from sun-dried tomatoes, which had an initial moisture content of ca. 60%. A similar effect was detected when wet tomato pomace (82% moisture) was extracted and no traceable amounts of lycopene were recovered. In addition, drying to 58 and 23% moisture did not have a large effect. It was only at a moisture percentage of 5% that the extraction was sufficient (Nobre et al., 2009). In other fruit pomaces, in this case apricot bagasse, the moisture content was also a major factor when extracting β-carotene with SC-CO2. When the moisture content of the freeze-dried apricot pomace was reduced from 14 to 10%, the extraction yield of β-carotene was increased five-fold (Sanal et al., 2004).
Oils can be easily extracted with SC-CO2. It is recommended to use pressures higher than 60 MPa and temperatures may vary from 40 to 80 °C. In general, pressures higher than 300 MPa are not needed and modifiers do not need to be applied. It is out of the scope of this book chapter to discuss the possibilities of oil extraction by SC-CO2. Therefore the authors would like to refer to extensive reviews that are present in the literature on oil and lipid extraction with supercritical fluids (Herrero et al., 2010; Sahena et al., 2009; Temelli, 2009). The only thing that should be noted is that terpenes, which are a group of apolar phytochemicals, are often extracted within the oil fraction. Terpenes are usually present in high amounts in essential oils obtained from citrus peels. Because they are highly reactive they can cause off-flavours in oils. Therefore citrus peels are usually deterpenated before use and SC-CO2 is an appropriate method to accomplish this (Diaz et al., 2005; Jeong et al., 2004).
Rosemary phytochemicals were extracted with SC-CO2 without modifier. SC-CO2 extracted carnosic acid 136% better than in the conventional extraction, which was an acetonic extraction with ultrasound (Tena et al., 1997). Bioactive extracts were produced from sweet cherries under various conditions with SC-CO2. When ethanol was used as a modifier at 10%, the extracts show the highest antioxidant and anticarcinogenic activity. In the optimal extract sakuranetin and sakuranin were the polyphenols responsible for the major antioxidant activity, while perillyl alcohol was the major compound contributing to anti-carcinogenic activity. To obtain sufficiently concentrated extracts with antiproliferative activity a pre-treatment with SC-CO2 was recommended (Serra et al., 2010).
Phenolics have also been extracted from guava seeds. When the seeds were extracted with SC-CO2, ethanol was found to be a better modifier than ethyl acetate. Best results in extracting phenolics were obtained when ethanol was added at 10% and a pressure of 30 MPa and a temperature of 50 °C was applied (Castro-Vargas et al., 2010). SC-CO2 extraction of polyphenols from cocoa seeds was optimised by RSM, showing that a high level of ethanol added to sample in the extractor (200% m/m) was optimal for an extraction at 40 °C. In this way 40% of total present polyphenols could be extracted when compared with traditional organic solvent extraction (Sarmento et al., 2008). Another source of polyphenols that is often used is soy and its isoflavones. When extracting isoflavones from soybean pressed cake, the highest amount of isoflavones was extracted by SC-CO2 using a pressure of 350 bars and 60 °C. By adding manually 16% (m/m) of 70% ethanol/water solution as modifier ((3279/5010)*100 µg/g =) 65% of total present isoflavones were collected. They analysed 12 different isoflavones. It was noted that malonyl glucosides and glucosides were optimally extracted at 350 bars and 60 °C, while acetyl glucosides and aglycones were best extracted at 350 bars and 80 °C (Kao et al., 2008). Others, such as Rostagno et al. (2002) only recovered 40% of total isoflavones present in soy flour with SC-CO2 when compared with a traditional Soxhlet extraction. These authors only measured three isoflavones of which two were aglycones. Optimal conditions determined were 50 °C and 360 bars and 10 mol% of methanol. In other studies methanol was also used as a modifier to extract isoflavones from defatted soybean meal. A maximum of 87.3% isoflavones could be extracted when 80% methanol was added at 7.8 mass%, a pressure of 500 bars was applied, a temperature of 40 °C and a flow rate of 9.80 kg/h (Zuo et al., 2008). Also extraction with SC-CO2 of soy isoflavones daidzein, genistein, glycitein and their glycosides was compared. Ethanol was required as a modifier to extract the isoflavones with SC-CO2. By adding ten times more ethanol, daidzin could be extracted even 1000 times better. Daidzin was easier to extract than daidzein, which wasn’t expected based on the KOW values. By using thermodynamic modelling, it was concluded that ethanol facilitates cluster formation of CO2 molecules, which enhances isoflavone solubility (Nakada et al., 2009).
Catechins have also been extracted with SC-CO2 from green tea leaves. By using 95% (v/v) ethanol as co-solvent at a mass percentage of 4.6%, Park et al. (2007a) managed to extract 82% of the present epigallo-catechin, 71% of epicatechin-gallate, 70% of epigalloctechin-gallate and 50% of epicatechin. The conditions they used were 300 bars and 80 °C. Catechin, epicatechin and gallic acid have also been extracted from grape seed concentrates. Some phenols, such as protocatechuic acid aldehyde, could be extracted with yields higher than 90%, but it was mentioned that at the moment SC-CO2 could probably not compete with traditional grape seed extraction (Murga et al., 2000). The solubility of catechins in SC-CO2 has been modelled by using Peng-Robinson equation of state and the Chrastil model. These models can assist in developing methods to extract polyphenols from food matrices (Murga et al., 2002).
Pressurised liquid extraction is another technique that has potential to be used in the extraction of phytochemicals. It is a technique that makes use of pressurised fluids. Other names for the technique are accelerated solvent extraction, pressurised solvent extraction and subcritical solvent extraction. Any solvent that is normally applied in extractions can be used. Most applications are in the development of analytical methods and the extraction of many groups of phytochemicals has been optimised using organic solvents: capsaicinoids from peppers (Barbero et al., 2006), polyphenols from apples (Alonso-Salces et al., 2001), carotenoids from microalgae (Rodríguez-Meizoso et al., 2008) and polyacetylenes from carrots (Pferschy-Wenzig et al., 2009). If natural and sustainable processing is a requirement, usually ethanol and/or water are used. When extractions are carried out with 100% water, the technique is also called superheated water extraction (SWE), subcritical water extraction, pressurised low polarity water extraction or pressurised hot water extraction (Pronyk and Mazza, 2009). Also in PLE it is important how the phytochemicals are embedded in the matrix, depending on their physicochemical properties and the properties of the matrix itself (Runnqvist et al., 2010). PLE is a technique in which pressure is applied during extraction, which allows the use of temperatures above the boiling point of the solvent. Extracting at elevated temperatures has various advantages on mass transfer and surface equilibria. In general, a higher temperature increases mass transfer by a higher capacity of the solvent to solubilise the phytochemical, and a decrease in viscosity of the solvent. Higher temperatures can also have a beneficial effect on releasing solutes from the matrix as described earlier in the paragraph on supercritical fluid extraction. A higher accessibility of the matrix has also been mentioned due to the applied pressure (Richter et al., 1996), but scientific proof is still ambiguous.
PLE requires smaller amounts of solvent use than traditional extraction and a shorter extraction time. Therefore PLE is generally perceived as a green and sustainable extraction technique (Mendiola et al., 2007). Over recent years many applications have been mentioned and PLE is gaining popularity. Especially since the publication of a patent on extracting polyphenolic compounds from fruits and vegetables with subcritical water (King and Grabiel, 2007), PLE procedures have been further developed. Still most applications are analytical and have used laboratory systems.
During PLE, various conditions are important, such as particle size, temperature and solid to solvent ratio (Luthria and Natarajan, 2010; Mukhopadhyay et al., 2010). Pressure is important in order to be able to reach the required temperature and maintain the water (or other solvent) in the liquid form and not to produce steam. Steam has a much lower dielectric constant, and gas-like diffusion rates and viscosity properties (Smith, 2002). On the other hand, unlike SC-CO2 extractions, changes in pressure do not have large effects on PLE (Mendiola et al., 2007). This is one of the main differences between SC-CO2 and PLE.
As in any extraction the choice of solvent is important as well. In addition, like in SC-CO2, modifiers can play a large role in breaking solute–matrix interactions (Björklund et al., 1998). The literature discussed here will focus on the use of food-grade and more sustainable solvents, such as ethanol and water.
Phenols have been extracted from parsley with PLE. The smallest particle size used (< 0.425 mm) resulted in an optimal extraction of phenols. The solid to liquid ratio also affected phenol extraction. Temperature had a small effect on the total level of phenols extracted, but a large effect on the profile of phenols extracted from parsley. When heated from 40 to 160 °C, apiin and acetyl-apiin were increased, but the amount of malonyl apiin decreased. This indicated that malonyl-apiin is unstable and is partially converted to apiin and acetyl-apiin (Luthria, 2008). Other herbs have been successfully extracted with PLE as well. Phytochemicals from rosemary were extracted successfully with similar results as with SC-CO2. The optimal temperature was dependent on the phytochemical compound, especially its polarity. For example, rosamanol, which is the most polar phytochemical compound present in rosemary, was best extracted at 25 °C (so not at subcritical conditions), while carnosic acid, which is an apolar compound, was best extracted at 200 °C with subcritical water (Ibáñez et al., 2002). The dielectric constant of water changes with increased temperatures. At room temperature and atmospheric pressure water has a high polarity with an ε of 80 (Kim and Mazza, 2006), while at a temperature of 220 °C, water has a lower polarity with an ε of 30. In comparison, methanol has an ε of 33 at room temperature (Smith, 2002). Therefore apolar compounds, such as carnosic acid are better extracted at higher temperatures. When American skullcap, another herb, was extracted, SWE was also a good alternative. The level of total flavonoids extracted was similar to conventional extraction (Bergeron et al., 2005). Saponins were extracted by PLE from cockle seeds, another North American herb. The amount extracted was optimal at 80% ethanol and 125 °C. The procedure was affected by extraction solvent and method, optimal results were obtained with whole cockle seeds (Güçlü-Üstündag et al., 2007).
One of the main tested matrices is red grape pomace; both table grape pomace and wine grape pomace have been optimised for their polyphenol extraction with PLE. When ethanol and water combinations were used of 50 or 70% ethanol, similar amounts of anthocyanins were extracted as with a conventional extraction with methanol/water/formic acid (60:37:3) (Monrad et al., 2010a). When the PLE procedure was optimised for the extraction of procyanidins from the same red grape pomace, 115% of procyanidins could be extracted when an ethanol percentage of 50% was used in comparison to a conventional acetone based extraction. Epicatechin and catechin levels were even increased to 205 and 221%, respectively, when 50% ethanol was used as solvent (Monrad et al., 2010b). The increased extraction of flavanols was also noted by García-Marino et al. (2006). They also noted a higher recovery of catechins and procyanidins from grape seeds with superheated water (thus without addition of ethanol), than with a conventional methanolic extraction. When wine grape skins were extracted with superheated water, an increase in antioxidant activity was noted with an increase in temperature. But the level of total phenols and anthocyanins decreased at temperatures higher than 110 °C. Using subcritical water extraction at 110 °C seemed an excellent alternative to traditional extractions, since the level of extracted total phenols and anthocyanins was the same or higher than conventional hot aqueous and methanolic extractions (Ju and Howard, 2003).
Wijngaard and Brunton (2009) measured levels of total flavonols, chlorogenic acid and phloretin glycoside at various ethanol concentrations using PLE. Optimal extraction conditions were estimated with response surface methodology (RSM). It was reported that the level of the phenols was largely affected by ethanol concentration and little by temperature. Temperatures between 75 and 125 °C were recommended. Temperatures higher than 150 °C formed hydroxymethylfurfural and increased browning components, and hence antioxidant activity (Wijngaard and Brunton, 2009). Similar results were reported when flavonoids were extracted from spinach by PLE. They also noted that at temperatures higher than 150 °C, browning components were formed, which correlated well with antioxidant activities as measured by ORAC. A temperature below 150 °C was advised for PLE with aqueous ethanol, and a temperature below 130 °C for subcritical water extractions (Howard and Pandjaitan, 2008).
Soybeans were extracted with PLE to optimise the level of isoflavones. The best solvent composition was 70% ethanol and a temperature of 100 °C. It was found that malonyl glycosides were the most heat labile and were degraded at 100 °C, while acetyl glycosides were broken down at 135 °C (Rostagno et al., 2004). Similar results were reported when de-fatted soybean flakes were extracted with PLE. A percentage of 80% ethanol and a temperature of 110 °C were optimal. At these conditions 95% of isoflavones and 75% of soysaponins could be recovered (Chang and Chang, 2007). Several cereal sources have undergone pressurised liquid extraction. The main aim was to obtain ferulic acid, which is a phenolic acid that is mainly present in bound form in many cereals and a precursor for the production of vanillin, a costly aroma. PLE did not increase the levels of phenolic acids present when wheat or corn bran were extracted, but by using PLE vanillin was formed from the bound ferulic acid. Buranov and Mazza (2009) reported that both aqueous ethanol and SWE promoted the formation of vanillin.
Srinivas et al. (2010) modelled solubility of gallic acid, catechin and protocatechuic acid in subcritical water. All compounds were better solubilised when the temperature was increased. For example, the solubility of catechin hydrate increased from 2 to 576 g/l when the temperature was increased from 25 to 143 °C. Pressure was applied when needed to keep the water in the liquid state. Thermodynamic properties were calculated from the solubility data.
Subcritical water extraction of mannitol was modelled by measuring the effects of temperature, pressure and flow rate. External mass transfer coefficients and equilibrium coefficients were calculated assuming a fixed-bed system (Ghoreishi and Shahrestani, 2009). Kim and Mazza (2007) modelled the mass transfer of free phenolic acids, such as vanilic acid and syringic acid, from flax shives. They determined by using kinetic and thermodynamic models that the extraction process was controlled by both internal diffusion and external elusion. In addition they noted that the internal diffusion process could also be influenced by the flow rate. In another study the solute–subcritical solvent interactions were studied by modelling of Hansen solubility parameters. It was concluded that by using group contribution methods and computerised algorithms, solubility and extraction conditions can be estimated (Srinivas et al., 2009).
As mentioned earlier phytochemicals are often embedded in the plant matrix, which can cause problems when extracting phytochemicals. For example, phenols can be associated with polysaccharides. It is suggested that they are bound by hydrogen bonds between the hydroxyl groups of phenols and the cross-linking oxygen atoms of polysaccharides or that polysaccharides form secondary hydrophobic structures, such as nanotubes in which complex phenols may be encapsulated (Pinelo et al., 2006). Exogenous enzymes can be added, in order to enhance the extraction of phytochemical compounds. The exact effect of enzymes is still somewhat unclear though. The leading theory suggests that the enzymes degrade the cell walls partially, which enhances porosity and pore size and therefore increases extractability of polyphenols. Another theory is that phytochemicals are chemically bound to a cell structure and can be released by adding exogenous enzymes (Landbo and Meyer, 2001). The composition of cell walls depends on the source, but cell walls mainly consist of polysaccaharides, such as cellulose and pectins. Therefore, for extraction purposes, many applications of cellulases and pectinases have been reported.
One popular application is apple peels. Apple peels mainly comprise of hemicellulose, cellulose and pectin. Pectins are known to be able to encapsulate procyanidins, one group of phenols present in apples (Le Bourvellec et al., 2005). By applying exogenous enzymes, which are targeting the polysaccharides, the polysaccharides are partially degraded and dissolved. This also affects the availability of other compounds present, such as polyphenols (Dongowski and Sembries, 2001). Kim et al. (2005) successfully enhanced the extraction of phenolics from apple peels when they added cellulases from Thermobifida fusca. The increase in phenol content went parallel with an increase in degraded polysaccharides. The authors also reported a correlation between the level of phenols and enzyme activity (Kim et al., 2005). The mass transfer of phenols from apple peels by enzyme-assisted extraction has also been described. The mass transfer was modelled by Fick’s law. Adding cellulolytic, pectinolytic and proteolytic enzymes enhanced the diffusion factors in addition to the mass transfer of phenols (Pinelo et al., 2008).
Black currant pomace is another studied matrix, since it is known that many polyphenols in this pomace are bound (Kapasakalidis et al., 2009). Black currant pomace was treated with four different commercial pectinases and one protease. Except for one pectinase, all enzymes enhanced the extraction of phenols. The same pectinase did not enhance the extraction of phenols from red wine pomace. A reduction in particle size also increased the phenol level (Landbo and Meyer, 2001). In a later study black currant pomace was treated with cellulase from Trichoderma and individual phenol release was measured. Enzyme activity tests demonstrated that besides endocellulase activity the mixture also contained cellobiohydrase and β-glucosidase activities, which is often the case in commercial enzyme preparations. The effects of enzyme concentration, hydrolysis time and temperature were investigated. At 50 °C and a hydrolysis time of 1.5 h the anthocyanin content could be increased 60%. It was concluded that enzyme addition can significantly enhance the extraction of phenols, especially anthocyanins, from the black currant matrix (Kapasakalidis et al., 2009). On the other hand, when grape pomace was treated with pectinases and cellulases the level of anthocyanins was not correlated with the degradation of polysaccharides. Also, rutin extraction was not enhanced by enzymatic treatment, but the level of extracted phenolic acids was well correlated with the degradation of polysaccharides, in particular pectin. In addition, the various polyphenol groups reacted differently biochemically to the enzymatic treatment. Anthocyanins were extracted in the early stage of the enzymatic hydrolysis and were degraded after prolongation. Flavonols were hydrolysed to their aglycones, for example rutin was degraded to quercetin by enzymatic treatment. The study proved the difference in the behaviour of various polyphenols to enzymatic treatment, and hence the importance of monitoring the various groups of polyphenols (Arnous and Meyer, 2010). Attempts to upscale the enzyme-assisted extraction of polyphenols from grape pomace to pilot-scale size have been reported, since this stream is an abundant and polyphenol-rich source. Usually polyphenols are extracted with acidified alcohols or sulfited water. To enhance naturalness of the polyphenol ingredients alternative processes such as enzyme assisted extraction can be applied: but first attempts to upscale resulted in very low recoveries of anthocyanins of 8% (Kammerer et al., 2005). In later studies though, the recovery of anthocyanins could be enhanced until an impressive 64%. The described optimised process existed of a pre-extraction in order to inhibit polyphenol degrading enzymes and an enzymatic extraction: water of 80 °C was added to the pomace, the pomace was ground and left for 1 min at 90 °C to inactivate polyphenol oxidases (pasteurisation), the slurry was then milled with a colloid mill, and pressed with a rack and cloth press. The liquid fraction was collected and the solid fraction was further treated with enzymes. Pectinolytic and cellulolytic enzymes were added in a ratio of 2:1, at pH 4, a temperature of 40 °C and an incubation time of 2 h. After the incubation, the slurry was again pasteurised. The resulting extract was pressed again and the collected liquid fractions were added together and spray-dried into a powder. By using this process, 92% phenolic acids, 92% non-anthocyanin flavonoids and 64% anthocyanins could be recovered (Maier et al., 2008).
In addition to apple peels, black currant pomace and grape pomace, the extraction of various bioactive compounds from many other fruits and vegetables have been studied for enzyme-assisted extraction. For instance, terpenes have been extracted from celery seeds (Sowbhagya et al., 2010), carotenoids from orange peel, sweet potato and carrot (Çinar, 2005), capsaicinoids and carotenoids from peppers (Santamaria et al., 2000), flavones from pigeonpea leaves (Fu et al., 2008) and phenolics from citrus peels (Li et al., 2006). In general, cellulases, pectinases and proteases are added. With some exceptions all enzymes increase the yield of polyphenols. The optimisation and improvement in yield depends on the source and should be considered when enzymes are applied. The added cost should be earned back in the higher yields of phytochemicals and considered per individual case.
Plant phytochemicals are usually entrapped in insoluble cell structures, such as vacuoles or lipoprotein bilayers, which offer significant diffusional resistance to the extraction. Furthermore, the ability of some phytochemicals to form hydrogen bounds with bulk constituents of the cell matrix additionally limits the yield of the extraction process. To overcome diffusion limitations a pre-treatment that will allow larger solute-solvent contact area, that is, release of intracellular compounds into the appropriate solvent, can be used. This chapter will mainly focus on two emerging technologies that increase the plant material porosity by cell disruption: ultrasound and pulsed electric fields (PEF).
Ultrasound is a technique in which soundwaves that are higher in frequency than the human hearing (ca. 16 kHz) are applied to a medium. The lowest frequency generally applied is 20 kHz. If the ultrasound is strong enough, bubbles are formed in the liquid. Eventually the formed bubbles cannot take up the energy any longer and will collapse; this implosion is called ‘cavitation’. This collapse generates the energy for chemical reactions by a change in temperature and pressure within the bubble. Extremely high temperatures of 5000 °C and pressures of 1000 bars have been measured. When a solid matrix is present, it is affected by mechanical forces surrounding the bubble (Luque-García and Luque de Castro, 2003). Ultrasound can result in a higher swelling of the plant material that increases extraction (Vinatoru, 2001). The choice of solvent is important, since solvent properties, including vapour pressure, surface tension, viscosity and density play important roles in cavitational activity (Xu et al., 2007). The authors found that 50% ethanol extracted isoflavones from Ohwi roots much better in comparison to 95% ethanol at an ultrasound power of 20 kHz. When the electrical power was increased from 0 to 650 W the extraction rate of isoflavones was increased as well. The electrical power was an important factor when modelling the mass transfer from isoflavones of the Ohwi root (Xu et al., 2007). Isoflavones have also been extracted from soy products by the assistance of ultrasound. Optimal results were obtained after 20 min at 60 °C and by using 50% ethanol. A percentage of 0–20% higher extraction was achieved at a power of 200 W and a frequency of 24 kHz than when extracted by mixing and stirring (Rostagno et al., 2003). Other flavanoids have also been extracted with assistance of ultrasound. Orange peel particles with a surface area of 2.0 cm2 were optimal. Smaller particles decreased extraction rates, since at smaller sizes particles started floating. Sonication power, temperature and ethanol to water ratio were optimised using a central composite design. An optimal flavonone concentration of 70.3 mg naringin and 205.2 mg hesperidin per 100 g fresh peels was reached at a temperature of 40 °C, a 4:1 (v/v) ethanol to water ratio and a sonication power of 150 W. At these conditions sonication led to an increased extraction percentage of approximately 40% (Khan et al., 2010). Mandarin peels have been subjected to the study of ultrasonic extraction of phenolic acids. The optimal conditions of ultrasonic extraction depended on the type of polyphenol. Phenolic acids were best extracted at 20 min, 30 °C and ultrasonic power of 8 W. For ultrasonic extraction of flavanone glycosides an extraction time of 60 min, a temperature of 40° C and an ultrasonic power of 8 W were found to be optimal. Cinnamic acids were more susceptible to degradation than benzoic acids, when an intensive ultrasound procedure was applied. Depending on the type of phenolic extracted, extraction rates could be enhanced maximally by approximately 50% (Ma et al., 2008). Ghafoor et al. (2009) used response surface methodology to optimise ultrasonic extraction of polyphenols from grape seeds by ethanol concentration, temperature and time. The optimal predicted conditions for ethanol extraction by US were 53% ethanol, 56° C and 29 min, which were very close to the final optimal experimental values. The frequency and power were constant at 40 kHz and 250 W (Ghafoor et al., 2009). Catechins, a group of flavanoids, were 20% better extracted from apple pomace, a by-product of the cider industry, when ultrasound was used in comparison to conventional extraction. In addition, the ultrasonic process was upscaled successfully to a volume of 30 l (Virot et al., 2010).
Capsaicinoids have been extracted from peppers and the procedures optimised. The optimal extraction was carried out with 100% methanol and a temperature of 50° C and 10 min. A constant power of 360 W was used (Barbero et al., 2008). In a later study the extraction of capsaicinoids from peppers was done with the more environmental friendly solvent ethanol in order to upscale the process. First lab trials were conducted with a fixed frequency of 35 kHz and a power of 600 W, which showed optimal results of a recovery of 83% at 1:5 solid to liquid ratio, an ethanol percentage of 95% and a temperature of 45 °C. At the 20 l pilot scale a 76% recovery was reached at a frequency of 26 kHz and 1.08 kW. This recovery was 7% lower than of an industrial maceration process at which peppers are soaked overnight and extracted at 78° C for 3 h. Although the recovery of the UAE was slightly lower, industrial potential may exist, because of possible lower operational costs (Boonkird et al., 2008). Ultrasound as an extraction technique has the potential to be upscaled, at low costs. It has already been used for alcohol beverage maceration at volumes of 100–1000 l (Virot et al., 2010).
The first records on the research on the influence of electric current on biological cells date almost as early as the end of the nineteenth century (Töpfl, 2006). Technological application of pulsed electric fields (PEF) on food production was first explored almost 50 years ago, mainly as a non-thermal alternative to pasteurisation. Numerous research groups are working on different PEF applications in food production, but the number of current successful industrial applications is limited at best.
PEF utilises the influence of a strong electrical field on material located between two electrodes, which leads to cell membrane disruption, thus increasing cell permeability. The exact mechanism of this occurrence is still under debate, but the most accepted theory is the electromechanical model developed by Zimmermann et al. (1974). In essence, cells are highly complicated structures that consist of an intracellular space, which is filled with different organelles and is surrounded by a cell membrane. The cell membrane separates the intracellular and extracellular space and is essentially electroneutral (equivalent to a capacitor in electrical circuits), while free charges of opposite polarities are present on both sides of the membrane. This creates a naturally occurring transmembrane potential. When an external electrical field is applied the additional transmembrane potential is formed, which increases attraction between opposite charges on both sides of the membranes and compresses the membrane. If the stress on the membrane is large enough pore formation occurs. Depending on the treatment applied (electric field strength, pulse duration, number of pulses) the pore formation can be reversible or irreversible; in the latter case cells are destroyed. According to Angersbach et al. (2000), the pore formation is reversible only if the formed pores are small in comparison to the membrane area (low intensity treatment), while if the field strength is large enough irreversible breakdown occurs. The critical field strength where electroporation occurs depends on cell diameter and it typically is in the range of 1–2 kV/cm for plant cells (diameter 40–200 µm) and 12–20 kV/cm for microorganisms (diameter 1–10 µm) (Heinz et al., 2002; Soliva-Fortuny et al., 2009). In the case of plant tissues the cell wall perforation by PEF can potentially lead to higher extractability of cell contents. According to (2010) the electroporation of plant cells and extraction of materials from solid foods typically require 0.1–5 kV/cm field strengths and pulses of 10–000 µs. The reason for higher extractability could be two-fold:
The influence of PEF-induced plant tissue perforation (reversible and irreversible) was explored for various applications in the food industry, such as improving extraction yields after cold pressing of different raw materials, enhancement of drying efficiency or increasing the content of secondary metabolites in cells by stressing plant tissues. Several of the findings will be briefly discussed. An extensive review on the research that has been undertaken so far in this field can be found elsewhere in the literature (Soliva-Fortuny et al., 2009; Vorobiev and Lebovka, 2010).
Several authors studied PEF assisted mechanical pressing of apple mash (Bazhal and Vorobiev, 2000; Schilling et al., 2007; Schilling et al., 2008; Wang and Sastry, 2002). The reported improvements of the juice yields were low to relatively high, depending on process conditions employed (particle size, PEF parameters, etc.), which makes it difficult to clearly conclude whether a higher yield was caused by PEF treatment. For instance, particle size and the type of size reduction method (slicing, milling, grinding) can be essential for an improvement in juice yield (Vorobiev and Lebovka, 2010). Next to the improvement of juice yield, several studies monitored the changes in antioxidant activity and phenolic content of pressed juices after PEF treatment. Schilling et al. (2007) have applied field strengths in the range of 1–5 kV/cm (30 pulses) on apple mash, but could not find any significant improvement in phenolic contents. However, in a subsequent study (Schilling et al., 2008), where field strengths of 3 kV/cm were used, although the juice yield was not increased, there was almost the double amount of the main apple juice phenolic compounds chorogenic acid and phloridizin present in the juice where PEF was used as a pre-treatment.
Guderjan and co-authors (Guderjan et al., 2005; Guderjan et al., 2007) have investigated PEF induced recovery of plant oils and additional content of secondary metabolites in maize, olives, soybeans and rape seed. Guderjan et al. (2005) reported that although there was no significant oil yield improvement as a result of PEF treatment, there was almost a 32% higher content of phytosterols in maize germs and around 20% more soy isoflavonoids present in soybeans when low intensity treatment (reversible electroporation) was used (0.6–1.3 kV/cm, 20–50 pulses). On the other hand, when conditions for irreversible electroporation were used (7.3 kV/cm and 120 pulses) these high yields of secondary metabolites could not be observed. In the subsequent study (Guderjan et al., 2007) they investigated the influence of higher intensity PEF treatment (5 kV/cm with 60 pulses and 7 kV/cm with 120 pulses) on rape seed oil production and bioactive compounds content in the oil obtained both with mechanical pressing and solvent extraction. Although the influence of the irreversible electroporation on increased oil yield was negligible, there was a significant increase in antioxidant capacity of extracted hulled and non-hulled rapeseed. The antioxidant capacity in rape seed is mainly attributed to tocopherols and polyphenols.
Several studies were performed on the influence of different PEF parameters on the evolution of different phenolic compounds in wine production (López et al., 2008; López et al., 2009; Puértolas et al., 2010a) and aging (Puértolas et al., 2010b). In the wine making process all authors observed higher amounts of phenolic compounds present in wine samples after PEF treatment in comparison with the control sample. López et al. (2008) employed a field of 5 and 10 kV/cm and observed that total polyphenol content in wine increased with field intensity, while total content and colour intensity of anthocyanins was the highest at 5 kV/cm. A more intense treatment did not improve the afore mentioned characteristics. In a subsequent study (López et al., 2009) the influence of maceration time was explored together with PEF treatment (5 kV/cm) and it was found that irrespective of maceration times all PEF-treated fresh wines had a higher colour intensity and total polyphenol index. This was confirmed by Puértolas et al. (2010a) with additional information that after four months of wine aging in bottles there was 11% more polyphenolic compounds present in PEF treated wine than in the control sample.
Corrales et al. (2008) have investigated the influence of PEF, ultrasonic (US) and high hydrostatic pressure (HHP) on the extraction of anthocyanins from grape by-products (skin, stems and seeds). Although all applied techniques increased anthocyanin extraction compared to the control sample, PEF treatment (3 kV/cm) and US treatment (35 kHz) showed almost 75% higher yields. On the other hand total antioxidant capacity was the highest in PEF treated samples (4.4 times increase compared to the control sample).
In general the amount of information on influence of PEF treatment on recovery and stimulation of production of secondary metabolites is relatively low compared to results on food pasteurisation. Results so far are sometimes conflicting and more research is required to prove definite advantage of PEF as a pre-treatment for enhanced bioactives recovery.
First of all it is important to know with what matrix and what phytochemicals you are dealing. As mentioned before, phenols, for example, can often be bound to polysaccharides that are part of plant cell walls. But phenols have also been found within the cell cytoplasm, in cell vacuoles or near the cell nucleus (Pinelo et al., 2006). When the phenol is located in the vacuole, it will be more easily available than when it is embedded in the cellular matrix. Thermodynamics and solubility conditions are in this case more important than the breakdown of the cell structure. On the other hand, if the targeted polyphenol molecule is embedded in a cellular structure, the cells need to be broken open, which will make the polyphenols more available. In this case the use of exogenous enzymes or ultrasound would be a better choice. Many studies were carried out on the optimisation of extraction conditions of bioactive compounds from plant matrices, but surprisingly little research considers the matrix effects and the location of the bioactive compound. More research should be focussed on these subjects.
Of the techniques discussed, the application of pulsed electric fields still needs proof of a significant advantage as an extraction aid. More research needs to be carried out if installation of PEF actually increases the yield of the extracted bioactive compounds. As already discussed, the papers reported on PEF are ambiguous on the extraction yields and no clear advantage is apparent.
One of the main challenges in the area of novel extraction techniques is to evaluate the ability of the techniques to be scaled up and implemented at an industrial level. Tests need to be carried out to estimate the capital and production costs in order to make the advantage of the process economically viable. Ultrasound as an industrial technique to enhance extraction has a large potential. Since the relevant parameters energy (energy input per volume of treated material in kWh/L) and intensity (actual power output per surface area of the sonotrode in W/cm2) do not depend on the size of the scale, industrial ultrasonic extractions are promising. An example is the business case in which ultrasound that was implemented to enhance the yield of extraction could be paid back in four months (Patist and Bates, 2008). In addition, several suppliers offer large scale ultrasound extractors and it has already been used in the beverage industry (Virot et al., 2010).
On the other hand, the economic benefits of the use of pressurised fluids in order to enhance extraction need to be further evaluated. Implementing pressurised fluid systems entails a high investment cost since the equipment needs to be adapted to handle high pressures. In addition, the energy to apply the necessary heat and pressure may not be economically viable when compared to traditional extraction processes, such as maceration. The estimation of implementation costs may not be straightforward since PLE systems, for example, do not even exist on a large scale to our knowledge. Therefore in the case of PLE, large scale PLE systems first need to be designed. In order to achieve this, modelling of mass transfer processes is a key to gaining more understanding of the PLE process. Besides, modelling the thermodynamic behaviour of the targeted bioactive molecules under pressurised conditions may result in useful information (Pronyk and Mazza, 2009). In the case of SC-CO2, large scale systems exist, but per specific business case it should be evaluated if the investment in the expensive equipment is profitable.
In addition to upscaling, trends towards more continuous systems have been proposed and need to be designed. This applies to pressurised fluids using multiple units with subcritical and supercritical fluids (King and Shrivinas, 2009), but also to ultrasound systems (Patist and Bates, 2008). In addition, combinations of various novel extraction techniques could make the complete process viable.
One area that has not yet been discussed but may be more developed over the coming years is the use of ionic liquids instead of solvents in the food industry. Ionic liquids consist entirely of ions (Tao et al., 2006). They are generally seen as sustainable since they have a negligible vapour pressure and hence cannot be inhaled or emitted into the environment. Although this does not necessarily mean they are to be considered environmentally safe, since they still could pose threats to ecosystems (Thuy Pham et al., 2010). Food grade ionic liquids exist, and are generally derived from α-amino acids and their ester salts (Tao et al., 2005; Tao et al., 2006). One advantage of ionic liquids is that they are tuneable and can be designed for various purposes (Kroon et al., 2008). In the future, food grade ionic liquids may be designed with a specific function that can replace organic solvents as an extraction medium.
In conclusion, novel extraction technologies of phytochemicals is an area in which many developments are taking place at the moment. Some techniques, such as ultrasound show more potential than others, such as PEF. Especially the development of industrial applicable systems that are economically viable will be a challenge for the future.
Aguilera, J.M., and Stanley, D.W. (1999) Microstructure and mass transfer: solid-liquid extraction. In: J.M. Aguilera Microstructural Principles of Food Processing and Engineering, Gaithersburg, Aspen Publishers, pp. 325–373.
Alonso-Salces, R.M., Korta, E., Barranco, A., Berrueta, L.A., Gallo, B. and Vicente, F. (2001) Pressurized liquid extraction for the determination of polyphenols in apple. Journal of Chromatography A, 933(1–2), 37–43.
Anekpankul, T., Goto, M. Sasaki, M., Pavasant, P. and Shotipruk, A. (2007) Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water. Separation and Purification Technology, 55(3), 343–349.
Angersbach, A., Heinz, V. and Knorr, D. (2000) Effects of pulsed electric fields on cell membranes in real food systems. Innovative Food Science and Emerging Technologies, 1(2), 135–149.
Arnous, A., and Meyer, A.S. (2010) Discriminated release of phenolic substances from red wine grape skins (Vitis vinifera L.) by multicomponent enzymes treatment. Biochemical Engineering Journal, 49(1), 68–77.
Barbero, G.F., Liazid, A., Palma, M. and Barroso, C.G. (2008) Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta, 75(5),1332–1337.
Barbero, G.F., Palma, M. and Barroso, C.G. (2006) Pressurized Liquid Extraction of Capsaicinoids from Peppers. Journal of Agricultural and Food Chemistry, 54(9), 3231–3236.
Bazhal, M. and Vorobiev, E. (2000) Electrical treatment of apple cossetes for intensifying juice pressing. Journal of the Science of Food and Agriculture, 80(11), 1668–1674.
Bergeron, C., Gafner, S., Clausen, E. and Carrier, D.J. (2005) Comparison of the chemical composition of extracts from scutellaria lateriflora using accelerated solvent extraction and supercritical fluid extraction versus standard hot water or 70% ethanol extraction. Journal of Agricultural and Food Chemistry, 53(8), 3076–3080.
Björklund, E., Järemo, M., Mathiasson, L., Jönsson, J. and Karlsson, L. (1998) Illustration of important mechanisms controlling mass transfer in supercritical fluid extraction. Analytica Chimica Acta, 368(1–2), 117–128.
Boonkird, S., Phisalaphong, C. and Phisalaphong, M. (2008) Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a lab- and pilot-plant scale. Ultrasonics Sonochemistry, 15(6), 1075–1079.
Brunner, G. (2005) Supercritical fluids: technology and application to food processing. Journal of Food Engineering, 67(1–2), 21–33.
Buranov, A.U. and Mazza, G. (2009) Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chemistry, 115(4), 1542–1548.
Castro-Vargas, H.I., Rodríguez-Varela, L.I., Ferreira, S.R.S. and Parada-Alfonso, F. (2010) Extraction of phenolic fraction frem guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. The Journal of Supercritical Fluids, 51(3), 319–324.
Chang, L.H. and Chang, C.M. (2007) Continuous hot pressurized fluids extraction of isoflavones and soyasaponins from defatted soybean flakes. Journal of the Chinese Institute of Chemical Engineers, 38(3–4), 313–319.
Chang, S.S., Ostric-Matijasevic, B., Hsieh, O. and Huang, C.-L. (1977) Natural antioxidants from rosemary and sage. Journal of Food Science, 42(4),1102–1106.
Çinar, I. (2005) Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochemistry, 40(2), 945–949.
Corrales, M., Toepfl, S., Butz, P., Knorr, D. and Tauscher, B. (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science and Emerging Technologies, 9(1), 85–91.
Diaz, S., Espinosa, S. and Brignole, E.A. (2005) Citrus peel oil deterpenation with supercritical fluids: Optimal process and solvent cycle design. The Journal of Supercritical Fluids, 35(1), 49–61.
Dongowski, G. and Sembries, S. (2001) Effects of Commercial Pectolytic and Cellulolytic Enzyme Preparations on the Apple Cell Wall. Journal of Agricultural and Food Chemistry, 49(9), 4236–4242.
Filho, G.L., De Rosso, V.V., Meireles, M.A., Rosa, P.T.V., Oliveira, A.L., Mercadante, A.Z. and Cabral, F.A. (2008) Supercritical CO2 extraction of carotenoids from pitanga fruits (Eugenia uniflora L.). The Journal of Supercritical Fluids, 46(1), 33–39.
Fornari, T., Chafer, A., Stateva, R.P. and Reglero, G. (2005) A New Development in the Application of the Group Contribution Associating Equation of State To Model Solid Solubilities of Phenolic Compounds in SC-CO2. Industrial and Engineering Chemistry Research, 44(21), 8147–8156.
Fu, Y.J., Liu, W., Zu, Y.C., Tong, M.H., Li, S.M., Yan, M.M., Efferth, T. and Luo, H. (2008) Enzyme assisted extraction of luteolin and apigenin from pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Food Chemistry, 111(2), 508–512.
García-Marino, M., Rivas-Gonzalo, J.C., Ibáñez, E. and García-Moreno, C. (2006) Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Analytica Chimica Acta, 563(1–2), 44–50.
Ghafoor, K., Choi, Y.H., Jeon, J.Y. and Jo, I.H. (2009) Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. Journal of Agricultural and Food Chemistry, 57(11), 4988–4994.
Ghoreishi, S.M. and Shahrestani, R.G. (2009) Subcritical water extraction of mannitol from olive leaves. Journal of Food Engineering, 93(4), 474–481.
Güçlü-Üstündag, O., Balsevich, J. and Mazza, G. (2007) Pressurized low polarity water extraction of saponins from cow cockle seed. Journal of Food Engineering, 80(2), 619–630.
Guderjan, M., Töpfl, S., Angersbach, A. and Knorr, D. (2005) Impact of pulsed electric field treatment on the recovery and quality of plant oils. Journal of Food Engineering, 67(3), 281–287.
Guderjan, M., Elez-MartØnez, P. and Knorr, D. (2007) Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innovative Food Science and Emerging Technologies, 8(1), 55–62.
Heinz, V., Alvarez, I., Angersbach, A. and Knorr, D. (2002) Preservation of liquid foods by high intensity pulsed electric fields-basic concepts for process design. Trends in Food Science and Technology, 12(3–4), 103–111.
Herrero, M., Mendiola, J.A., Cifuentes, A. and Ibáñez, E. (2010) Supercritical fluid extraction: Recent advances and applications. Journal of Chromatography A, 1217(16), 2495–2511.
Howard, L.R. and Pandjaitan, N. (2008) Pressurized Liquid Extraction of Flavonoids from Spinach. Journal of Food Science, 73(3), C151–C157.
Ibáñez, E., Kubátová, A., Señoráns, F.J., Cavero, S., Reglero, G. and Hawthorne, S.B. (2002) Subcritical Water Extraction of Antioxidant Compounds from Rosemary Plants. Journal of Agricultural and Food Chemistry, 51(2), 375–382.
Içen, H. and Gürü, M. (2009) Extraction of caffeine from tea stalk and fiber wastes using supercritical carbon dioxide. The Journal of Supercritical Fluids, 50(3), 225–228.
Jeong, S.M., Kim, S.Y., Kim, D.R., Jo, S.C., Nam, K.C., Ahn, D.U. and Lee, S.C. (2004) Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. Journal of Agricultural and Food Chemistry, 52(11), 3389–3393.
Ju, Z.Y. and Howard, L.R. (2003) Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phenolics from Dried Red Grape Skin. Journal of Agricultural and Food Chemistry, 51(18), 5207–5213.
Kammerer, D.R., Claus, A., Schieber, A. and Carle, R. (2005) A Novel Process for the Recovery of Polyphenols from Grape (Vitis vinifera L.) Pomace. Journal of Food Science, 70(2), C157–C163.
Kao, T.H., Chien, J.T. and Chen, B.H. (2008) Extraction yield of isoflavones from soybean cake as affected by solvent and supercritical carbon dioxide. Food Chemistry, 107(4), 1728–1736.
Kapasakalidis, P.G., Rastall, R.A. and Gordon, M.H. (2009) Effect of a Cellulase Treatment on Extraction of Antioxidant Phenols from Black Currant (Ribes nigrum L.) Pomace. Journal of Agricultural and Food Chemistry, 57(10), 4342–4351.
Kassama, L.S., Shi, J. and Mittal, G.S. (2008) Optimization of supercritical fluid extraction of lycopene from tomato skin with central composite rotatable design model. Separation and Purification Technology, 60(3), 278–284.
Khan, M.K., bert-Vian, M., Fabiano-Tixier, A.S., Dangles, O. and Chemat, F. (2010) Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chemistry, 119(2), 851–858.
Kim, J.W. and Mazza, G. (2006) Optimization of Extraction of Phenolic Compounds from Flax Shives by Pressurized Low-Polarity Water. Journal of Agricultural and Food Chemistry, 54(20), 7575–7584.
Kim, J.W. and Mazza, G. (2007) Mass Transfer during Pressurized Low-Polarity Water Extraction of Phenolics and Carbohydrates from Flax Shives. Industrial and Engineering Chemistry Research, 46(22), 7221–7230.
Kim, W.J., Kim, J.D., Kim, J., Oh, S.G. and Lee, Y.W. (2008) Selective caffeine removal from green tea using supercritical carbon dioxide extraction. Journal of Food Engineering, 89(3), 303–309.
Kim, Y.J., Kim, D.O., Chun, O.K., Shin, D.H., Jung, H., Lee, C.Y. and Wilson, D.B. (2005) Phenolic Extraction from Apple Peel by Cellulases from Thermobifida fusca. Journal of Agricultural and Food Chemistry, 53(24), 9560–9565.
King, J.W. and Grabiel, R. (inventors) (2007) Isolation of polyphenolic compounds from fruits or vegetables utilizing sub-critical water extraction.
King, J.W. and Srinivas, K. (2009) Multiple unit processing using sub- and supercritical fluids. The Journal of Supercritical Fluids, 47(3), 598–610.
Kroon, M.C., Hartmann, D. and Berkhout, A.J. (2008) Toward a Sustainable Chemical Industry: Cyclic Innovation Applied to Ionic Liquid–Based Technology. Industrial and Engineering Chemistry Research, 47(22), 8517–8525.
Landbo, A.K. and Meyer, A.S. (2001) Enzyme–Assisted Extraction of Antioxidative Phenols from Black Currant Juice Press Residues (Ribes nigrum). Journal of Agricultural and Food Chemistry, 49(7), 3169–3177.
Lang, Q. and Wai, C.M. (2001) Supercritical fluid extraction in herbal and natural product studies – a practical review. Talanta, 53(4), 771–782.
Langenfeld, J.J., Hawthorne, S.B., Miller, D.J. and Pawliszyn, J. (1995) Kinetic Study of Supercritical Fluid Extraction of Organic Contaminants from Heterogeneous Environmental Samples with Carbon Dioxide and Elevated Temperatures. Analytical Chemistry, 67(10), 1727–1736.
Le Bourvellec, C., Bouchet, B. and Renard, C.M.G.C. (2005) Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. Biochimica et Biophysica Acta (BBA) – General Subjects, 1725(1), 10–18.
Li, B.B., Smith, B. and Hossain, M. (2006) Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Separation and Purification Technology, 48(2), 189–196.
López, N., Puértolas, E., Condón, S., Álvarez, I. and Raso, J. (2008) Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innovative Food Science and Emerging Technologies, 9(4), 477–482.
López, N., Puértolas, E., Hernández-Orte, P., Álvarez, I. and Raso, J. (2009) Effect of a pulsed electric field treatment on the anthocyanins composition and other quality parameters of Cabernet Sauvignon freshly fermented model wines obtained after different maceration times. LWT – Food Science and Technology, 42(7), 1225–1231.
Luque-García, J.L. and Luque de Castro, M.D. (2003) Ultrasound: a powerful tool for leaching. (TrAC)Trends in Analytical Chemistry, 22(1), 41–47.
Luthria, D.L. and Natarajan, S.S. (2010) Influence of Sample Preparation on the Assay of Isoflavones. Planta Medicina.
Luthria, D.L. (2008) Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chemistry, 107(2), 745–752.
Ma, Y.Q., Ye, QX.Q., Fang, Z.X., Chen, J.C., Xu, G.H. and Liu, D.H. (2008) Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus unshiu Marc.) Peels. Journal of Agricultural and Food Chemistry, 56(14), 5682–5690.
Maier, T., Göppert, A., Kammerer, D., Schieber, A. and Carle, R. (2008) Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. European Food Research and Technology, 227(1), 267–275.
McHugh, M.A. and Krukonis, V.J. (1994) Introduction. In: Taylor, L.T. Supercritical Fluid Extraction, Stoneham, Butterworth-Heinemann, pp. 1–16.
Mendiola, J.A., Herrero, M., Cifuentes, A. and Ibáñez, E. (2007) Use of compressed fluids for sample preparation, Food applications. Journal of Chromatography A, 1152(1–2), 234–246.
Mongkholkhajornsilp, D., Douglas, S., Douglas, P.L., Elkamel, A., Teppaitoon, W. and Pongamphai, S. (2005) Supercritical CO2 extraction of nimbin from neem seeds—a modelling study. Journal of Food Engineering, 71(4), 331–340.
Monrad, J.K., Howard, L.R., King, J.W., Srinivas, K. and Mauromoustakos, A. (2010a) Subcritical Solvent Extraction of Anthocyanins from Dried Red Grape Pomace. Journal of Agricultural and Food Chemistry, 58(5), 2862–2868.
Monrad, J.K., Howard, L.R., King, J.W., Srinivas, K. and Mauromoustakos, A. (2010b) Subcritical solvent extraction of procyanidins from dried red grape pomace. Journal of Agricultural and Food Chemistry, 58, 4014–4021.
Mukhopadhyay, M., Luthria, D.L. and Robbins, R.J. (2010) Optimization of extraction process for phenolic acids from black cohosh (Cimicifuga racemosa) by pressurized liquid extraction. Journal of the Science of Food and Agriculture, 86(1), 156–162.
Murga, R., Ruiz, R., Beltrán, S. and Cabezas, J.L. (2000) Extraction of Natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. Journal of Agricultural and Food Chemistry, 48(8), 3408–3412.
Murga, R., Sanz, M.T., Beltrán, S. and Cabezas, J.L. (2002) Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide. The Journal of Supercritical Fluids, 23(2), 113–121.
Nakada, M., Imai, M. and Suzuki, I. (2009) Impact of ethanol addition on the solubility of various soybean isoflavones in supercritical carbon dioxide and the effect of glycoside chain in isoflavones. Journal of Food Engineering, 95(4), 564–571.
Nobre, B.P., Palavra, A.n.F., Pessoa, F.L.P. and Mendes, R.L. (2009) Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chemistry, 116(3), 680–685.
Park, H.S., Choi, H.K., Lee, S.J., Park, K.W., Choi, S.G. and Kim, K.H. (2007a) Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. The Journal of Supercritical Fluids, 42(2), 205–211.
Park, H.S., Lee, H.J., Shin, M.H., Lee, K.W., Lee, H., Kim, Y.S., Kim, K.O. and Kim, K.H. (2007b) Effects of cosolvents on the decaffeination of green tea by supercritical carbon dioxide. Food Chemistry, 105(3), 1011–1017.
Patist, A. and Bates, D. (2008) Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innovative Food Science and Emerging Technologies, 9(2), 147–154.
Pferschy-Wenzig, E.M., Getzinger, V., Kunert, O., Woelkart, K., Zahrl, J. and Bauer, R. (2009) Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chemistry, 114(3), 1083–1090.
Pinelo, M., Arnous, A. and Meyer, A.S. (2006) Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends in Food Science and Technology, 17(11), 579–590.
Pinelo, M., Zornoza, B. and Meyer, A.S. (2008) Selective release of phenols from apple skin: Mass transfer kinetics during solvent and enzyme-assisted extraction. Separation and Purification Technology, 63(3), 620–627.
Pronyk, C. and Mazza, G. (2009) Design and scale-up of pressurized fluid extractors for food and bioproducts. Journal of Food Engineering, 95(2), 215–226.
Puértolas, E., Hernández-Orte, P., Saldaña, P.G., Álvarez, I. and Raso, J. (2010a) Improvement of winemaking process using pulsed electric fields at pilot-plant scale. Evolution of chromatic parameters and phenolic content of Cabernet Sauvignon red wines. Food Research International, 43(3), 761–766.
Puértolas, E., Saldaña, G., Condón, S., Álvarez, I. and Raso, J. (2010b) Evolution of polyphenolic compounds in red wine from Cabernet Sauvignon grapes processed by pulsed electric fields during aging in bottle. Food Chemistry, 119(3), 1063–1070.
Reverchon, E. (1996) Mathematical modeling of supercritical extraction of sage oil. AIChE Journal, 42(6), 1765–1771.
Reverchon, E. and De Marco, I. (2006) Supercritical fluid extraction and fractionation of natural matter. The Journal of Supercritical Fluids, 38(2), 146–166.
Richter, B.E., Jones, B.A., Ezzell, J.L., Porter, N.L., Avdalovic, N. and Pohl, C. (1996) Accelerated solvent extraction (ASE®): a technique for sample preparation. Analytical Chemistry, 68(6), 1033–1039.
Rodríguez-Meizoso, I., Jaime, L., Santoyo, S., Cifuentes, A., García-Blairsy Reina, Señoráns, F.J. and Ibáñez, E. (2008) Pressurized fluid extraction of bioactive compounds from phormidium species. Journal of Agricultural and Food Chemistry, 56(10), 3517–3523.
Rostagno, M.A., Palma, M. and Barroso, C.G. (2004) Pressurized liquid extraction of isoflavones from soybeans. Analytica Chimica Acta, 522(2), 169–177.
Rostagno, M.A., Araújo, J.M.A. and Sandi, D. (2002) Supercritical fluid extraction of isoflavones from soybean flour. Food Chemistry, 78(1), 111–117.
Rostagno, M.A., Palma, M. and Barroso, C.G. (2003) Ultrasound-assisted extraction of soy isoflavones. Journal of Chromatography A, 1012(2), 119–128.
Rozzi, N.L., Singh, R.K., Vierling, R.A. and Watkins, B.A. (2002) Supercritical fluid extraction of lycopene from tomato processing byproducts. Journal of Agricultural and Food Chemistry, 50(9), 2638–2643.
Runnqvist, H., Søren, A.B., Hansen, M., Styrishave, B., Halling-Sørensen, B. and Björklund, E. (2010) Determination of pharmaceuticals in environmental and biological matrices using pressurised liquid extraction–Are we developing sound extraction methods? Journal of Chromatography A, 1217(16), 2447–2470.
Sabio, E., Lozano, M., Montero de Espinosa, V., Mendes, R.L., Pereira, A.P., Palavra, P.F. and Coelho, J.A. (2003) Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Industrial and Engineering Chemistry Research, 42(25), 6641–6646.
Sahena, F., Zaidul, I.S.M., Jinap, S., Karim, A.A., Abbas, K.A., Norulaini, N.A.N/ and Omar, A.K.M. (2009) Application of supercritical CO2 in lipid extraction – A review. Journal of Food Engineering, 95(2), 240–253.
Saldaña, M.D.A., Sun, L., Guigard, S.E. and Temelli, F. (2006) Comparison of the solubility of [beta]-carotene in supercritical CO2 based on a binary and a multicomponent complex system. The Journal of Supercritical Fluids, 37(3), 342–349.
Sanal, I.S., Gnvenτ, A., SalgIn, Mehmetoglu, U. and ¦alImlI, A. (2004) Recycling of apricot pomace by supercritical CO2 extraction. The Journal of Supercritical Fluids, 32(1–3), 221–230.
Santamaria, R.I., Reyes-Duarte, M.D., Barzana, E., Fernando, D., Gama, F.M., Mota, M. and Lopez-Munguia, A. (2000) Selective enzyme-mediated extraction of capsaicinoids and carotenoids from chili guajillo puya (Capsicum annuum L.) using ethanol as solvent. Journal of Agricultural and Food Chemistry, 48(7), 3063–3067.
Sarmento, L.A.V., Machado, R.A.F., Petrus, J.C.C., Tamanini, T.R. and Bolzan, A. (2008) Extraction of polyphenols from cocoa seeds and concentration through polymeric membranes. The Journal of Supercritical Fluids, 45(1), 64–69.
Schilling, S., Alber, T., Toepfl, S., Neidhart, S., Knorr, D., Schieber, A. and Carle, R. (2007) Effects of pulsed electric field treatment of apple mash on juice yield and quality attributes of apple juices. Innovative Food Science and Emerging Technologies, 8(1), 127–134.
Schilling, S., Toepfl, S., Ludwig, M. Dietrich, H., Knorr, D., Neidhart, S., Schieber, A. and Carle, R. (2008) Comparative study of juice production by pulsed electric field treatment and enzymatic maceration of apple mash. European Food Research and Technology, 226(6), 1389–1398.
Serra, A.T., Seabra, I.J., Braga, M.E.M., Bronze, M.R., de Sousa, H.C. and Duarte, C.M.M. (2010) Processing cherries (Prunus avium) using supercritical fluid technology. Part 1– Recovery of extract fractions rich in bioactive compounds. The Journal of Supercritical Fluids, 55(1), 184–191.
Shi, J., Nawaz, H., Pohorly, J., Mittal, G., Kakuda, Y. and Jiang, Y. (2005) Extraction of Polyphenolics from Plant Material for Functional Foods Engineering and Technology. Food Reviews International 21(1), 139–166.
Shi, J., Yi, C., Xue, S.J., Jiang, Y., Ma, Y. and Li, D. (2009) Effects of modifiers on the profile of lycopene extracted from tomato skins by supercritical CO2. Journal of Food Engineering, 93(4), 431–436.
Smith, R.M. (2002) Extractions with superheated water. Journal of Chromatography A, 975(1), 31–46.
Soliva-Fortuny, R., Balasa, A., Knorr, D. and Martín-Belloso, O. (2009) Effects of pulsed electric fields on bioactive compounds in foods, a review. Trends in Food Science and Technology, 20(11–12), 544–556.
Sovová (2005) Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. The Journal of Supercritical Fluids, 33(1), 35–52.
Sowbhagya, H.B., Srinivas, P. and Krishnamurthy, N. (2010) Effect of enzymes on extraction of volatiles from celery seeds. Food Chemistry, 120(1), 230–234.
Srinivas, K., King, J.W., Monrad, J.K., Howard, L.R. and Hansen, C.M. (2009) Optimization of Subcritical Fluid Extraction of Bioactive Compounds Using Hansen Solubility Parameters. Journal of Food Science, 74(6), E342–E354.
Srinivas, K., King, J.W., Howard, L.R. and Monrad, J.K. (2010) Solubility of Gallic Acid, Catechin, and Protocatechuic Acid in Subcritical Water from (298.75 to 415.85) K. Journal of Chemical and Engineering Data: null.
Sun, M. and Temelli, F. (2006) Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. The Journal of Supercritical Fluids, 37(3), 397–408.
Tao, G., He, L., Liu, W.-S., Xu, L., Xiong, W., Wang, T. and Kou, Y. (2006) Preparation, characterization and application of amino acid-based green ionic liquids. Green Chemistry, 2006(8), 639–646.
Tao, G., He, L., Sun, N. and Kou, Y. (2005) New generation ionic liquids, cations derived from amino acids. Chemical Communications, (28), 3562–3564.
Taylor, L.T. (1996) Properties of supercritical fluids. In: L.T. Taylor (ed.) Supercritical Fluid Extraction. New York, John Wiley & Sons Inc., pp. 7–27.
Temelli, F. (2009) Perspectives on supercritical fluid processing of fats and oils. The Journal of Supercritical Fluids, 47(3), 583–590.
Tena, M.T., Valcárcel, M., Hidalgo, P.J. and Ubera, J.L. (1997) Supercritical Fluid Extraction of Natural Antioxidants from Rosemary, ΓÇë Comparison with Liquid Solvent Sonication. Analytical Chemistry, 69(3), 521–526.
Thuy Pham, T.P., Cho, C.W. and Yun, Y.S. (2010) Environmental fate and toxicity of ionic liquids: A review. Water Research, 44(2), 352–372.
Töpfl, S. (2006) Pulsed Electric Fields (PEF) for Permeabilization of Cell Membranes in Food and Bioprocessing Applications, Process and Equipment Design and Cost Analysis. Berlin, Technical University of Berlin.
Vági, E., Simándi, B., Vásárhelyiné, K/P., Daood, H., Kéry, Á., Doleschall, F. and Nagy, B. (2007) Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. The Journal of Supercritical Fluids, 40(2), 218–226.
Vasapollo, G., Longo, L., Rescio, L. and Ciurlia, L. (2004) Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. The Journal of Supercritical Fluids, 29(1–2), 87–96.
Vaughn Katherine, L.S., Clausen Edgar, C., King Jerry, W., Howard Luke, R. and Julie, C.D. (2008) Extraction conditions affecting supercritical fluid extraction (SFE) of lycopene from watermelon. Bioresource Technology, 99(16), 7835–7841.
Vega, P.J., Balaban, M.O., Sims, C.A., O’Keefe, S.F. and Cornell, J.A. (1996) Supercritical carbon dioxide extraction efficiency for carotenes from carrots by RSM. Journal of Food Science, 61(4), 757–759.
Vinatoru, M. (2001) An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonics Sonochemistry, 8(3), 303–313.
Virot, M., Tomao, V., Le Bourvellec, C., Renard, C.M.C.G. and Chemat, F. (2010) Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrasonics Sonochemistry, 17(6), 1066–1074.
Vorobiev, E. and Lebovka, N. (2010) Enhanced Extraction from Solid Foods and Biosuspensions by Pulsed Electrical Energy. Food Engineering Reviews, 2(2), 95–108.
Wakelyn, P.J. and Wan, P.J. (2001) Food industry-solvents for exrtacting vegetables oils. In: G. Wypych (ed.) Handbook of Solvents, Toronto, William Andrew Publishing; ChemTec Publishing, pp. 923–946.
Wang, L. and Weller, C.L. (2006) Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology, 17(6), 300–312.
Wang, W.C. and Sastry, S.K. (2002) Effects of moderate electrothermal treatments on juice yield from cellular tissue. Innovative Food Science and Emerging Technologies, 3(4), 371–377.
Wijngaard, H. and Brunton, N. (2009) The Optimization of Extraction of Antioxidants from Apple Pomace by Pressurized Liquids. Journal of Agricultural and Food Chemistry, 57(22), 10625–10631.
Xu, H., Zhang, Y. and He, C. (2007) Ultrasonically Assisted Extraction of Isoflavones from Stem of Pueraria lobata (Willd.) Ohwi and Its Mathematical Model. Chinese Journal of Chemical Engineering, 15(6), 861–867.
Zimmermann, U., Pilwat, G. and Riemann, F. (1974) Dielectric breakdown of cell membranes. Biophysical Journal, 14(11), 881–899.
Zosel, K., inventor. (1981) Process for the decaffeination of coffee. Germany Pat. No. US4260639.
Zuo, Y.B., Zeng, A.W., Yuan, X.G. and Yu, K.T. (2008) Extraction of soybean isoflavones from soybean meal with aqueous methanol modified supercritical carbon dioxide. Journal of Food Engineering, 89(4), 384–389.
* Sadly, Peter passed away in 2012