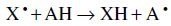
Phytochemicals in fruits, vegetables and cereals have attracted a great deal of attention mainly concentrated on their role in preventing diseases caused as a result of oxidative stress. Oxidative stress, which releases free oxygen radicals in the body, has been implicated in a number of disorders including cardiovascular malfunction, cataracts, cancers, rheumatism and many other auto-immune diseases besides ageing.
These phytochemicals act as antioxidants, scavenge free radicals and may inhibit cell death or apoptosis. Epidemiological studies have shown that there may be significant positive associations between intake of fruits and vegetables or cereals and reduced rate of heart disease mortality, common cancers and other degenerative diseases as well as ageing (Steinmetz and Potter, 1996; Joseph et al., 1999; Dillard and German, 2000; Prior and Cao, 2000). The most thoroughly investigated dietary components in fruits, vegetables, cereals or legumes acting as antioxidants are fibre, carotenoids, polyphenols, flavonoids, conjugated isomers of linoleic acid, epigallocatechin, gallate, soya protein, isoflavanones, vitamins A, B, C, E, tocopherols, calcium, selenium, chlorophyl, alipharin, sulphides, catechin, tetrahydrocurecumin, sesaminol, lignans, glutathione, uric acid, indoles, thiocyanates and protease inhibitors (Karakaya and Kavas, 1999). These compounds may act independently or in combination as anti-cancer or cardio-protective agents by a variety of mechanisms. The available scientific data indicates a protective role for fruits and vegetables against certain cancers including those of the pancreas, bladder and breast (American Institute of Cancer Research, 1997). This is attributed to the fact that these foods may provide an optimal mix of phytochemicals such as natural antioxidants, fibres and other bioactive compounds. In contrast, a recent report by the European Food Safety Authority (EFSA, 2010) has issued negative opinions on the actions of antioxidants in human health. The EFSA panel documented that the claimed effects refer to the protection of body cells and molecules (such as DNA, proteins and lipids) from oxidative damage, including UV-induced oxidative damage. The panel considered that the protection of molecules such as DNA, proteins and lipids from oxidative damage may be a beneficial physiological effect (EFSA, 2010). No human studies investigating the effects of the food(s)/food constituent(s) on reliable markers of oxidative damage to body cells or to molecules such as DNA, proteins and lipids have been provided in relation to any of the health claims evaluated in this opinion (EFSA, 2010). Nevertheless, we believe it is too early to make strong judgements about antioxidants and their biological properties.
The concept of antioxidant activity of unprocessed and processed foods is gaining significant momentum and emerging as an important parameter to assess the quality of the product. With the expansion of the global market and fierce competition amongst multinational companies, the parameter of antioxidant activity will soon secure its place in nutritional labelling with accompanying regulatory guidelines. In this context development of a practical method of determining the antioxidant activity for industrial use will become imperative. This will give a further boost to the exploitation of fruits and vegetables and development of nutraceuticals and beverages. Particular focus will be given to mechanisms and measurement of antioxidant activity by different assays.
According to its definition, an antioxidant should have a significantly lower concentration than the substrate in the antioxidant activity test. Depending on the type of reactive oxygen species (ROS) and target substrate, a certain antioxidant may play a completely different action or have a completely different role/performance. In line with this, some authors support the use of a selection of methods to measure the antioxidant activity (Yuan et al., 2005a, 2005b).
The choice of substrate is very important in an antioxidant activity test. Depending on the type of substrate and its amount/concentration, different results will be achieved. The application of tests in both aqueous and lypophilic phase systems has also been described as important, in order to study the relative bioactivity of an antioxidant. Because certain stable free radical methods (e.g. ABTS•+, DPPH etc.) generally do not include a substrate other than the stable free radical in question, they are considered artificial because they do not represent the real process in food samples (discussed in section 20.2.1).
The method generally used to determine total antioxidant activity is the Trolox equivalent antioxidant capacity (TEAC) assay, although total oxyradical scavenging capacity (TOSC) assay, oxygen radical absorbance capacity (ORAC) assay, 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay or ferric ion reducing antioxidant parameter (FRAP) assay can also be used.
Assays involving a biological substrate have the advantage of being closer to an in vivo situation, where both aqueous and a lipid phase are present and take into account the solubilities and partitioning between different phases. One of these assays measures the inhibition of ascorbate/iron induced lipid peroxidation of cell or liver microsomes (Plumb et al., 1996; Lana and Tijskens, 2006). Other assays that employ biological substrates include the inhibition of human LDL oxidation (Heinonnen et al., 1998a; Meyer et al., 1997, 1998) and the lecithin-liposome oxidation assay (Heinonnen et al., 1998a, 1998b; Frankel and Huang, 1997), both catalysed by copper. These models are important because LDL oxidation is related to coronary disease and liposome oxidation to food oxidation.
Assays for measurement of antioxidant activity may involve hydrogen atom transfer (HAT) or single electron transfer (SET). These two mechanisms generally occur simultaneously and the prevalence of one of them depends on the structure of the antioxidant and pH. The mechanism and antioxidant efficiency are mainly determined by two factors: the bond dissociation energy (BDE) and the ionisation potential (IP) (Prior et al., 2005; Karadag et al., 2009). HAT methods measure the capacity of an antioxidant (AH, a hydrogen donor) to quench free radicals by hydrogen donation.
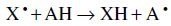
In HAT based assays, the reactivity is determined by the BDE of the H donating group of the antioxidant and it is higher for compounds with Δ BDE ≈ 10 kcal/mol and Δ IP ≤ 36 kcal/mol (Prior et al., 2005).
HAT assays depend on the solvent, pH and are affected by the presence of reducing agents such as metals. HAT reactions are generally quite fast and quantitation is derived from the kinetic curves (Karadag et al., 2009). HAT assays include the oxygen radical absorbance capacity (ORAC), the total peroxyl radical-trapping antioxidant parameter assay (TRAP) and the crocin-bleaching assay.
SET methods measure the capacity of a potential antioxidant to transfer one electron to reduce a compound:
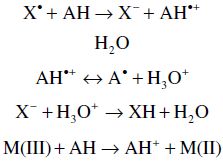
In the SET based assays, the reactivity is determined by the deprotonation and IP of the functional group. These assays are pH dependent (MacDonald-Wicks et al., 2006). The higher the pH, the lower IP values are and deprotonation increases. In compounds with Δ IP ≥ 45 kcal/mol, the major reaction mechanism is SET (Prior et al., 2005).
SET reactions are usually slow and negatively affected by trace components and contaminants, especially metals (Prior et al., 2005). Generally, these reactions measure the relative percent decrease in product instead of kinetics or total antioxidant capacity (Karadag et al., 2009).
SET assays include the ferric ion reducing antioxidant power (FRAP) and the copper reduction capacity assay. Trolox equivalent antioxidant capacity (TEAC) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays are usually classified as SET but both mechanisms may be used (Prior et al., 2005). HAT and SET are competitive reactions but it has been demonstrated that HAT is dominant in biological redox reactions (Karadag et al., 2009).
The Fremy’s radical assay is an indirect method to determine ‘chain-breaking antioxidant activity’ in food, and is based on the capability of the Fremy’s stable free radical to react with H-donors. The Fremy’s radical (potassium nitrosodisulfonate) is a specific oxidising salt which converts phenols into quinines (Zimmer et al., 1970). The concentration of the Fremy’s radical is monitored by ESR (electron spin resonance) spectroscopy. A low signal indicates the detection of low amounts of radicals and therefore an antioxidant and dominating pro-oxidant effect of the extracts (Summa et al., 2007).
In particular, the method was applied to wine (Burns et al., 2001), extracts made from cherry liqueur pomace (R φ dtjer et al., 2006), fruit juices (Gardner et al., 2000), coffee (Summa et al., 2007) and Scotch whiskeys (MacPhail et al., 1999). Gardner et al. (2000) has pointed out the advantages of this assay: it is very sensitive, allowing detection at a sub-micromolar level; analysis can be carried out on turbid or highly coloured solutions and radicals have well-defined spectra, allowing clear resolution from radical intermediates which may be formed during the oxidation process.
This method is a variant of the FRAP assay, using copper (Cu) instead of iron (Fe). It is based on the reduction of Cu (II) to Cu (I) by the action of the reductants (antioxidants) present in a sample (Prior et al., 2005; Huang et al., 2005). This method, however, has not been broadly used (MacDonald-Wicks et al., 2006).
The FOX assay measures the hydroperoxides (ROOHs), which are the initial stable products formed during peroxidation of unsaturated lipids such as fatty acids and cholesterol (Nourooz-Zadeh, 1999). The assay is based on the oxidation of ferrous (Fe2+) to ferric (Fe3+) ions by ROOHs under acidic conditions. FOX is a precise and simple method, but the amount of extract and the incubation time have to be adapted for each sample (Grau et al., 2000). FOX was also reported as being highly specific (Grau et al., 2000). Moreover, the FOX method is sensitive (measures concentrations of 5 μ M LOOH), inexpensive, rapid, not sensitive to ambient oxygen or light levels and it does not require special reaction conditions (DeLong et al., 2002). FOX assay has been applied, for instance, to lipoprotein and lipossomes (Jiang et al., 1991; Jiang et al., 1992), plasma (Nourooz-Zaheh et al., 1994; Nourooz-Zaheh et al., 1995), vegetable oils (Nourooz-Zaheh et al., 1995), soybean oils (Yildiz et al., 2003), plant tissue (DeLong et al., 2002), fried snacks (Navas et al., 2004) and dark chicken meat (Grau et al., 2000).
The ferric thiocyanate method determines the amount of peroxide at the initial stage of lipid peroxidation. The peroxide reacts with ferrous chloride (FeCl2) to give a ferric chloride dye which has a red colour. Recently, some studies have used this technique to evaluate the antioxidant activity in different matrices such as citrus by-products (Senevirathne et al., 2009); gingers (Ruslay et al., 2007); Malay traditional vegetables (Abas et al., 2006); rosemary extract, blackseed essential oil, carnosic acid, rosmarinic acid and sesamol (Erkan et al., 2008); an edible seaweed (Kappaphycus alvarezzi) (Kumar et al., 2008); sugar cane bagasse (Ou et al., 2009); hazelnut skin (Locatelli et al., 2010); wines, grape juices (Sanchez-Moreno et al., 1999), extracts from Platycodon grandiflorum A. De Condolle roots (plants used both as a herbal medicine and food in Asia) (Lee et al., 2004) and sweet potatoes (Huang et al., 2006).
The deoxyribose assay for detection of hydroxyl radical (•OH) scavenging activity described by Halliwell and co-workers (1987) was designed as a relatively simple and cheap spectrophotometric alternative to pulse radiolysis for the determination of the rate constants of •OH scavenging compounds reacting with hydroxyl radicals. The assay relies on the generation of •OH via the Fenton reaction which reacts with deoxyribose under neutral pH conditions, followed by degradation of the sugar molecule and the formation of malondialdehyde (MDA) with heating under acidic conditions. The MDA will yield a pink chromogen upon heating with 2-thiobarbituric acid which can then be detected at 532 nm. The rate of deoxyribose degradation in this assay is enhanced by the inclusion of ascorbic acid which reduces ferric to ferrous ions to facilitate the Fenton reaction. Antioxidant molecules which scavenge •OH will compete with deoxyribose and thereby decrease the final amount of the pink chromogen formed. Interestingly, EDTA itself is an •OH scavenger in this assay (Halliwell et al., 1987), therefore only those •OH which are not scavenged by EDTA go on to degrade the deoxyribose. It is important to note that the deoxyribose assay for •OH scavenging activity is sensitive to contamination by transition metal ions, resulting in high ‘blank’ or ‘control’ values, approximately A532 = 0.2 to 0.3 (Aruoma et al., 1987). Common sources of iron contamination may include the phosphate buffer, or other reagents (deoxyribose, ascorbate; Aruoma et al., 1987), thus reagents and water used in analyses must be treated with Chelex resin or otherwise deionised.
The deoxyribose assay can only be used to evaluate the •OH scavenging activity of polar antioxidants (Aruoma, 1994). Aqueous ethanol can be used to solubilise antioxidants under study as needed, keeping in mind that ethanol is an •OH scavenger itself (Halliwell et al., 1987). For example, Yuan and co-workers (2005a) used 0.1 % ethanol to solubilise Palmaria palmata extracts prior to assessing the •OH scavenging activities of these marine red algal samples; these workers corrected for the ethanol antioxidant activity by using an appropriate solvent ‘control’.
The 1,1-diphenyl-2-picrylhydrazyl (DPPH•) – which is also known as α , α -diphenyl- β -picrylhydrazyl, 2,2-diphenyl-1-picrylhydrazyl or 2, 2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl) – assay originally described by Blois (1958) was designed to take advantage of a common electron spin resonance reagent, a stable free radical with an odd, unpaired valence electron to study antioxidant activity. With its odd electron, DPPH• can be stabilised by accepting an electron or hydrogen radical from an antioxidant molecule such as a sulfhydryl group (Blois, 1958); ascorbic acid as a reducing agent; polyphenols; or more generically by an antioxidant (AH) or free radical (R•; Brand-Williams et al., 1995).
DPPH• is known for its deep violet colour and strong absorbance at 517 nm when dissolved in ethanol at concentrations between 1 mM and 22.5 μ M (Blois, 1958; Yen and Chen, 1995; Sharma and Bhat, 2009); this absorbance is decreased with the decolourisation of DPPH• which accompanies the pairing of the lone electron. The A517 of DPPH• is stable between pH 5 and 6.5, but is sensitive to highly alkaline conditions which can be buffered by acetate (Blois, 1958; Sharma and Bhat, 2009). The wide range of DPPH• concentrations used in the literature is no doubt related to the limited solubility of this stable free radical. Moreover, studies using DPPH• have varied widely not only in the solvent used to dissolve the stable free radical, but also the wavelength used to monitor the decolourisation of the stable free radical (Blois, 1958; Brand-Williams et al., 1995; Kitts et al., 2000; Sharma and Bhat, 2009; Shimada et al., 1992; Yan et al., 1998; Yen and Chen, 1995; Yuan et al., 2005a). When Sharma and Bhat (2009) measured the A517 of DPPH• over a wide range of concentrations (approximately 10–250 μ M) using different solvent systems, the A517 varied as follows: 60 % methanol was slightly > methanol > ethanol, thus, 517 nm may not have been the optimal wavelength to monitor the decolourisation of DPPH• in differing solvents. The peak intensity absorption characteristics of chromophores can be observed to be influenced by not only the structure of the molecule, but also solvent and vibrational effects. The choice of solvent may also have been influenced by the solubility of the antioxidant compounds or extracts under evaluation, since the DPPH• stable free radical scavenging methodology can be used to study both polar and non-polar antioxidants such as ascorbic acid and butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT), respectively. On the other hand, many studies of antioxidant molecules or plant extracts with the potential to be functional foods or nutraceuticals will use ethanol as the extraction medium and/or solvent as opposed to the notably toxic methanol. The impact of the choice of solvent is clearly demonstrated with BHT, which exhibited an EC50 of 60 μM when methanol was the solvent, but 9.7 μM when the solvent was 60 % methanol (Sharma and Bhat, 2009), thus the solubility of the antioxidant molecules in the chosen solvent system plays an important role in these studies.
Perhaps the variable of most interest and debate in attempting to compare and reconcile data from different laboratories and studies from the literature in the evaluation of antioxidant efficacy is the quantitation or expression of DPPH• stable free radical scavenging activity. The length of time that a sample is incubated with DPPH• and monitored by spectrophotometer is highly variable in the literature despite the in depth discussion of the importance of antioxidant kinetic behaviour by Brand-Williams and co-workers (1995). These workers identified three ranges of kinetic behaviour: rapid kinetics exhibited by ascorbic acid which reacts very quickly with DPPH• reaching a steady state plateau in 1 min. or less; intermediate kinetics exhibited by α -tocopherol which reached a steady state plateau between 5 and 30 min; and slow kinetics exhibited by a diversity of antioxidants including phenolic acids and complex marine algae/seaweed-derived extracts which only reached a steady state plateau after 1–6 h incubation (Yuan et al., 2005a).
On the other hand, many investigators have chosen a single time point to quantify the DPPH• stable free radical scavenging efficacy of the antioxidants under study, with the most common choice as 30 min (varies from 20 to 60 or 90 min). However, unless the antioxidants are screened and identified as having rapid or intermediate kinetics, the stable free radical scavenging activity can be underestimated (Brand-Williams et al., 1995; Sharma and Bhat, 2009). For example, Brand-Williams and co-workers (1995) observed EC50 values for BHT of 0.943 mol/L after 30 min incubation, but 0.189 mol/L after 240 min, a five-fold difference in antioxidant efficacy. Best practices likely reflect monitoring the decrease in DPPH• absorbance until a steady state plateau is reached, particularly since the majority of antioxidants appear to exhibit intermediate or slow reaction kinetics.
Azo compounds, or dyes, are distinguished by containing an azo group –N = N- within their structure and comprise a large class of synthetic organic dyes such as Congo red and Tartrazine; approximately 60–70 % of dyes used in the food and textile industries are azo dyes. Azo compounds are also regularly used as free radical initiators in the study of antioxidant compounds, and particularly the quantitation of lipid peroxidation in vitro and vivo, due to the predictable thermal decomposition of these compounds to yield N2 and two carbon radicals, R• (Niki, 1990). These radicals may then either react with each other to yield a stable non-radical end product (R-R), or react with molecular O2 to yield peroxyl radicals, ROO• which can then participate in the peroxidation of a polyunsaturated lipid emulsion model system. The structure or composition of R will determine not only the solubility of the azo compound, but also the kinetics of the decomposition. There have been two commonly used hydrophilic radical initiators in the recent literature: 2, 2 ′ -azo-bis-(2-amidipropropane hydrochloride) (ABAP) or 2,2 ′ -azo-bis(2-amidinopropane) dihydrochloride (AAPH). ABAP and AAPH are the same chemical, just differing with one HCl moiety present in the former and two HCl moieties in the latter. Due to its polarity, AAPH generates its radicals in the aqueous region of an oil-in-water emulsion used in studying lipid peroxidation; whereas, 2, 2 ′ -azobis(2,4-dimethylvaleronitrile) (AMVN) is a lipophilic radical initiator which generates its radicals within the lipid regions of emulsion, micelle droplets or membranes (Niki, 1990; Noguchi et al., 1998; Yuan et al., 2005b). More recently, Noguchi and co-workers (1998) described a novel lipophilic azo free radical initiator, 2,2 ′ -azobis (4-methoxy-2, 4-dimethylvaleronitrile) (MeO-AMVN). The decomposition of azo radical initiators is a function of mainly temperature, and to a lesser extent solvent and pH (Niki, 1990). Therefore, the rate of generation of radicals would be constant over the short term in the case of an accelerated lipid oxidation model using a free radical initiator and elevated temperature of incubation (Ng et al., 2000; Zhang and Omaye, 2001; Yuan et al., 2005b; Hu et al., 2007).
The solubility of the azo free radical initiator used is very important with respect to the polarity of the antioxidant(s) under study as well as the composition of the model system (Niki, 1990; Yuan et al., 2005b). For example, Niki (1990) discussed that if AAPH is used to generate free radicals in a liposome system with a lipophilic antioxidant such as α -tocopherol, care should be taken to sonicate multilamellar liposomes to yield unilamellar liposomes to facilitate the interaction of AAPH radicals with the antioxidant, which otherwise would not be possible if the antioxidant was located within the inner membranes of a multilamellar system. Similarly, Yuan and co-workers (2005b) reported a protective effect of red algal Palmaria palmata (dulse) extracts on lipid peroxidation in linoleic acid emulsions when AAPH was the free radical initiator, but the absence of a protective effect in the presence of AMVN; the lack of a protective effect of dulse extracts could be associated with the localisation of the free radicals within the lipid phase of the emulsion not in contact with the aqueous dulse extract constituents. Azo compounds are desirable for in vivo studies of lipid peroxidation or oxidative stress due to the spontaneous and known breakdown of these compounds under physiological conditions (Niki, 1990). Moreover, because of the ability of azo compounds to generate peroxyl radicals at a known and constant rate, they can also be used in other model systems to good effect, including as a free radical initiator in the scission of supercoiled plasmid pBR322 DNA (Hu et al., 2007) or in the oxygen radical absorbance capacity (ORAC) assay, to be discussed in section 20.2.8.
When originally developed, the ORAC assay was designed to evaluate the protective effect of antioxidant compounds against reactive oxygen species-mediated damage to the fluorescent indicator R- or β -phycoerythrin (PE); free radicals were derived from AAPH or •OH in the presence of Cu1 + /2 + and ascorbic acid (Dávalos, Gómez-Cordovés and Bartolomé, 2004). However, despite the linear, zero order kinetics exhibited by PE in the ORAC assay, the natural variability of this reagent and its instability to photobleaching (necessitating making a fresh PE solution daily) and interaction with polyphenols lead researchers to look for an alternate fluorescent indicator. Fluorescein was subsequently demonstrated to not only have excellent photostability within assay conditions, but also not to have any interactions with antioxidant molecules, such as polyphenols (Dávalos et al., 2004). The ORAC assay has gained great acceptance amongst the food science, functional food and nutraceutical research community, and indeed by marketers of such foods, due to its utility in analysing multiple samples quickly using 96-well microplates and the potential for automated (robotic) reagent handling (Dávalos et al., 2004). The ORAC assay is based on quantitation of antioxidant activity from the area under the curve calculated from the decay in fluorescence intensity when fluorescein is degraded by AAPH-derived peroxy radicals (Dávalos et al., 2004). Thus, one of the strengths of the ORAC methodology is that the antioxidant efficacy of compounds is monitored until exhaustion and the fluorescence returns to the baseline. The ORAC methodology was modified from the original to analyse both hydrophilic (H-ORACFL) and hydrophobic antioxidant molecules (L-ORACFL; Wu et al., 2004a, 2004b), with the total ORACFL activity of a food represented by the sum of the two. The H-ORACFL values were roughly ten-fold that of the corresponding L-ORACFL values as expected for fresh and dried fruits, vegetables, nuts, spices, cereals and infant foods.
The original total peroxyl radical trapping antioxidant parameter (TRAP) methodology described by Wayner and co-workers (1985) was designed to assess the capacity of plasma antioxidant constituents to quench or trap azo compound-derived peroxy radicals from the thermal decomposition of ABAP or AAPH as already discussed. TRAP assay conditions comprised monitoring the uptake of O2, using an oxygen electrode, by a test sample incubated at 37°C (Wayner et al., 1985). Subsequent modifications to the TRAP assay methodology favoured the use of fluorescent indicators and monitoring the degradation of these molecules, such as PE (Ghiselli et al., 1995), as in the ORAC assay in section 20.2.8. However, as described, the inherent variability of this naturally occurring pigment and its lack of photostability led to its replacement with other indicators such as the nonfluorescent 2,7-dichlorofluorescin-diacetate (DCFH-DA; Valkonen and Kuusi, 1997). In contrast to the ORAC assay, monitoring DCF fluorescence results in an initial lag phase whilst the endogenous antioxidants are depleted, followed by a rapid increase in fluorescence, representing a propagation phase. There is a second lag phase attributed to the effects of the addition of Trolox as an internal standard, and another propagation phase after the Trolox has been depleted. Valkonen and Kuusi (1997) reported lag phases, = 15 min, = 19.5 min and TRAP values of 1292 μ M for fresh plasma, whereas after storage at − 80°C, 2 mo., the corresponding values were 16.5 min., 23 min. and 1205 μ M, respectively.
Alho and Leinonen (1999) modified the TRAP methodology to incorporate chemiluminescence to measure the antioxidant capacities of human plasma and cerebrospinal fluid (CSF). The methodology was further modified to use the lipophilic azo compound AMVN to determine the TRAP activity of low density lipoprotein (LDL) as a measure of LDL oxidisability an indicator of the atherogenicity of these particles (Ahlo and Leinonen, 1999; Malminiemi et al., 2000). Thus, the TRAP assay has value as a clinical measure of overall antioxidant capacity of biological fluids, but has also been adapted for use in functional food and nutraceutical research.
The radical cation form of 2,2 ′ -azino-bis-(3-ethyl-benzthiazoline-6-sulfonic acid) (ABTS) is generated by oxidising ABTS with potassium persulfate to form ABTS• + , a blue-green chromophore with absorbance maxima at 415, 645, 734 and 815 nm (Pellegrini et al., 1999; Re et al., 1999). Similar to the DPPH• stable free radical scavenger in section assay 20.2.6, the evaluation of potential antioxidant activity using ABTS• + involves the decolourisation of the preformed cation radical by the antioxidant molecule donating an electron or hydrogen atom. Free radical scavenging by hydrophilic or lipophilic antioxidants is measured by monitoring the A734 until a steady state plateau is achieved, or by using a single time point measure (Hu and Kitts, 2000). Sample antioxidants can be tested for ABTS• + radical cation scavenging efficacy including phenolics, flavonoids and hydroxycinnamates solubilised in ethanol; anthocyanidins in acidic ethanol, pH 1.3; carotenoids (lycopene and β -carotene) dissolved in dichloromethane; α -tocopherol in ethanol and plasma antioxidants diluted with water (Pellegrini et al., 1999; Re et al., 1999).
Interestingly, the ABTS• + radical cation scavenging EC50 values for L-ascorbic acid, BHA and the red marine alga Palmaria palmata observed by Yuan and co-workers (2005a) were relatively similar to the corresponding results obtained for these antioxidants in the DPPH• free radical scavenger assay for both kinetics (rapid versus slow) as well as antioxidant efficacy. For example, the % inhibition of A734 values for L-ascorbic acid reached a steady state plateau within 1–2 min when incubated with ABTS• + , and similar kinetics were observed for % DPPH• quenching; the % inhibition of A734 values for BHA achieved a steady state plateau after 30–50 min with ABTS• + , and similarly with % DPPH• quenching; whereas the % inhibition of A734 values for the marine red alga Palmaria palmata extract never reached a steady state plateau even after 180 min incubation with ABTS• + , but did reach a steady state plateau after 50–60 min. for % DPPH• quenching. These differences in kinetic behaviour of antioxidant compounds in the DPPH• and ABTS• + free radical scavenging assay systems are thought to be related to the reaction stoichiometry of the number of electrons available to inactivate the free radicals (Koleva et al., 2002). Slow reacting compounds such as BHT, or the closely related BHA, and P. palmaria extracts herein are hypothesised to have a more complex reaction mechanism involving one or more secondary reactions in the quenching of the DPPH• (Koleva et al., 2002) and thereby also, ABTS• + free radicals.
The ferric reducing ability of plasma (FRAP) assay was designed as a simple, inexpensive method to quantify the collective non-enzymatic antioxidant capacity of biological fluids such as plasma, saliva, tears, urine and cerebrospinal fluid (Benzie and Strain, 1996, 1999). It was proposed that an assay such as this could evaluate the combined effect of plasma antioxidant constituents and thus directly measure the ‘total antioxidant power’ of a complex mixture with potential synergistic effects which would not be evident when assayed as single components (Benzie and Strain, 1999). The FRAP assay is based on the single electron transfer by an antioxidant to reduce the ferric to ferrous ion; when the ferric-tripyridyltriazine (Fe3+-TPTZ) complex is reduced to the ferrous counterpart, the complex absorbs at 593 nm with an intense blue colour. The time course of the assay will vary depending on the kinetics of the sample antioxidant being evaluated; for example, L-ascorbic acid and α -tocopherol exhibited rapid kinetics with a steady state plateau reached within 1 min, uric acid reached a steady state plateau after 3 min, whereas, bilirubin did not reach a steady state plateau after 8 min (Benzie and Strain, 1996). The importance of assay temperature is demonstrated in particular with uric acid which exhibits slower reaction kinetics at room temperature versus 37°C (Benzie and Strain, 1999).
A modification to the FRAP assay, named the FRASC assay, allows the dual measurements of ascorbic acid content and FRAP activity in one test system. For the FRASC assay, one sample aliquot is treated with ascorbate oxidase while its pair is left untreated (Benzie and Strain, 1997). A major criticism of the FRAP assay is the lack of physiological relevance of the reaction conditions at pH 3.6; however, the assay does provide a means to determine the potential reducing activity of complex biological fluids, as well as aqueous or ethanolic extracts of potential functional foods and nutraceuticals and solutions of purified antioxidant molecules.
The antioxidant activity of molecules is most often attributed to the ability to delay the onset of lipid autoxidation, or peroxidation, by scavenging reactive oxygen species (ROS) or the ability to act as chain-breaking antioxidants to inhibit the propagation phase of lipid autoxidation (Yuan et al., 2005a; Nawar, 1996). In vitro systems designed to study the efficacy of molecules to inhibit lipid peroxidation have often comprised emulsion (Yuan et al., 2005a, 2005b) or liposomal systems (Hu et al., 2007) to model tissue membranes or food systems. The lipids most often incorporated into these systems include linoleic acid (C18:2, ω -6) as well as ethyl linoleate since the majority of foods contain a variety of unsaturated fatty acids and associated esterified forms (Coupland et al., 1996).
The assays chosen to monitor lipid oxidation are of key importance since the data they provide are a function of the stage of the peroxidation process. For example, the alteration of unsaturated fatty acid bond geometry from the native non-conjugated isomer to the formation of conjugated dienes (CD):
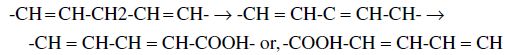
is associated with the resonance stabilisation and shift in double bond position with the formation of isomeric hydroperoxides during the early stages of lipid oxidation (Nawar, 1996). Thus, the UV-absorbance due to CD formation subsequently decreases as the hydroperoxides begin to decompose, prior to increasing once again as decomposition products begin to form (Puhl et al., 1994). On the other hand, 2-thiobarbituric acid reactive substances (TBARS) formation reflects primarily the production of scission and breakdown dialdehyde products such as MDA later in the reaction (Nawar, 1996).
Lipid emulsions and liposome preparations are well suited to the inclusion of pro-oxidants or free radical-generators such as the azo compounds AAPH and AMVN already discussed (Yuan et al., 2005b). When studying these systems, researchers must also be aware of the ‘polar paradox’ whereby hydrophilic compounds exhibit weak antioxidant activity in emulsions due to the dilution of these compounds in the aqueous phase; or conversely, lipophilic compounds exhibit strong antioxidant activity due to the concentration of the antioxidant at the lipid-air interface allowing strong protection of an emulsion against oxidation. On the other hand, the opposite antioxidant profile may be observed in bulk lipid or oil systems (Koleva et al., 2002). The solubilities of the free radical-generator and the antioxidant under study are also important variables in study design.
The main principle of these methods is based on the ability of luminol and related compounds to luminesce under the flux of free radicals (chemiluminescence, CL) (Roginsky and Lissi, 2005). CL is brought about due to a reaction of a free radical derived from luminol with active free radicals. CL can be easily recorded. The addition of an antioxidant compound, being a scavenger of an active free radical, results in CL quenching, commonly with a pronounced induction period (Roginsky and Lissi, 2005). The quantity of the tested antioxidant can be estimated from the duration of tIND. As a rule, antioxidant activity is given in Trolox equivalents. The attractive feature of CL methods is their productivity; commonly, one run normally takes a few minutes only; in addition, the assay can be easily automated. As for shortcomings of this group of methods, first of all, the mechanism for chemical processes resulting in CL is not known in detail. The latter may create problems with interpreting the data obtained. Different versions of this method differ in the type of active free radical produced and the way of free radical production as well as in details of the protocol. While the majority of assays have been developed for testing biologically relevant samples, they can be easily applied for food testing (Roginsky and Lissi, 2005). Parejo, Codina, Petrakis and Kefalas (2000) suggested inducing CL by reaction of Co2+ chelated by EDTA with H2O2. Although the authors suggested HO• as an active free radical, which attacks luminol, it is more realistic that O·-2 plays this role. The method was used to test red wines (Arnous et al., 2001). The method is well-instrumented and computerised and its capability was demonstrated by the example of testing several natural products including wines, tea and medicinal herb extracts. The evident advantage of the method is its very high productivity: the procedure takes commonly a couple of minutes only. At the same time, the kinetic theory of the process underlying the assay is really not suggested (Roginsky and Lissi, 2005).
The antioxidant content of food samples may be characterised by two independent parameters: antioxidant capacity and reactivity (Roginsky and Lissi, 2005). For individual antioxidants, this corresponds to the stoichiometric coefficient and the rate constant for reaction between antioxidants and highly reactive free radicals. There is no single robust answer to the question of which index of antioxidant activity is more relevant. The main attention is currently paid to determining antioxidant capacity. The absolute majority of the recently developed methods are designed to solve this major problem. Admittedly, the reactivity of food samples may be of interest under certain conditions. Meanwhile, the information on the reactivity of food and individual natural polyphenols is still rather poor and conflicting (Roginsky and Lissi, 2005).
It is well known that indirect methods (DPPH, ABTS•+) are used more frequently than direct methods (competitive crocin bleaching, competitive β -carotene bleaching). The question now arises as to which of the methods, direct or indirect, is better in principle. Each kind of method has both advantages and disadvantages. The direct methods are more adequate in principle, especially those based on the model of the chain controlled reaction. Besides, they are commonly more sensitive. The disadvantage of the direct methods is that most of them are rather time-consuming and their application requires significant experience in chemical kinetics (Roginsky and Lissi, 2005). As a consequence, direct methods are commonly not so suitable for routine testing of natural products.
As a rule, well-developed indirect methods, such as the DPPH and ABTS•+ assays, are more productive and easier in handling (Roginsky and Lissi, 2005). The crucial point concerning the application of indirect assays is their informative capability. The indirect methods commonly provide information on the capability of natural products to scavenge stable free radicals, for example, DPPH and ABTS•+. Undoubtedly, the best indirect methods as well as the Folin–Ciocalteu test allow the estimation of antioxidant activity to the first approximation (Roginsky and Lissi, 2005). However, it is questionable whether the raw data obtained with indirect methods give quantitative information on the capability of natural products to inhibit oxidative processes. To conclude, it should be remembered that the methods described here are intended for the determination of the antioxidant activity of food samples, that is, the antioxidative potential of food. As for the antioxidative action of food substituents in real biological systems, this will mainly depend also on their bioavailability and food antioxidants metabolism in vivo.
It should be noted that beneficial influence of many foodstuffs and beverages including fruits, vegetables, tea, red wine, coffee and cacao on human health has been recently recognised to originate from the chain-breaking antioxidant activity of natural polyphenols, a significant constituent of these products. For this reason, the dietary value of such products is determined to a large extent by their antioxidant activity. Although the kinetic approach provides the basis of the majority of these methods, only a few of them have been analysed from the viewpoint of chemical kinetics.
Different assays have been introduced to measure antioxidant activity of foods and biological samples. The concept of antioxidant activity first originated from chemistry and was later adapted to biology, medicine, epidemiology and nutrition (Pellegrini et al., 2003). It describes the ability of redox molecules in foods and biological systems to scavenge free radicals. This concept provides a broader picture of the antioxidants present in a biological sample as it considers the additive and synergistic effects of all antioxidants rather than the effect of single compounds, and may, therefore, be useful for study of the potential health benefits of antioxidants on oxidative stress-mediated diseases (Brighenti et al., 2005).
Recently, Floegel et al. (2011) evaluated the antioxidant activity of various fruits, vegetables and their products by ABTS, DPPH and ORAC assays. Their study showed that relative to DPPH assay, ABTS assay was more strongly correlated with ORAC from USDA database, phenolics and flavonoids content of the 50 most popular antioxidant-rich foods in the US diet. The results suggested that ABTS assay better reflects the antioxidant contents in a variety of foods than DPPH assay. It has been previously reported that antioxidant capacity determined by different in vitro assays give different values (Ou et al., 2002). Ou et al. (2002) conducted a large scale vegetable analysis using two different in vitro assays, FRAP and ORAC, and obtained very different antioxidant capacities from these methods. In their study, antioxidant capacities as determined by FRAP and ORAC assays were only weakly correlated. Pellegrini et al. (2003) reported that rankings of several fruits, vegetables and beverages differed based on antioxidant capacity measured by FRAP and ABTS assays suggesting that caution should be exercised when interpreting antioxidant capacities from different assays.
Xu and Chang (2008) studied the effect of soaking, boiling and steaming on antioxidant activities of cool season food legumes by two different methods (FRAP and ORAC). As compared to original unprocessed legumes, all processing steps caused significant (p < 0.05) decreases in total phenolic content, DPPH and ORAC values in all tested cool season food legumes (green pea, yellow pea, chickpea and lentil). In contrast, oxygen radical absorbance capacities were increased with the increase of pressure in both pressure boiling and pressure steaming treatments. TPC and DPPH were not parallel with ORAC in cases of pressure boiling and pressure steaming treatments. This phenomenon could be attributed to the increases or the formation (after high pressure heat treatments) of specific compounds, which could provide more hydrogen atom during oxidation–reduction reaction.
Gorinstein et al. (2010) recently reported a high correlation between polyphenols content in three exotic fruits and antioxidant capacities measured by ABTS, DPPH and FRAP assays. Similarily, Dudonné et al. (2009) reported a strong positive correlation between ABTS and DPPH assays with a Pearson correlation coefficient of r = 0.906 when used for 30 aqueous plant extracts.
By definition, the antioxidant activity is the capability of a compound (composition) to inhibit oxidative degradation, for example, lipid peroxidation. Phenolics are the main antioxidant components of foods (Roginsky and Lissi, 2005). Antioxidant activity of polyphenols is associated with various mechanisms of action, the elevated reactivity of phenolics towards active free radicals is considered as the most common principle mechanism. The authors would like to distinguish between the antioxidant activity and the reactivity. The antioxidant activity gives the information about the duration of antioxidative action; the reactivity characterises the starting dynamics of antioxidation at a certain concentration of an antioxidant or complex antioxidant mixture (Roginsky and Lissi, 2005).
Antioxidant activity may be a key parameter for both food science and technology and nutritional studies, and therefore there is presently a vital need to develop a standardised methodology to measure total antioxidant activity in plant foods. As discussed in the above sections, there are substantial differences in sample preparation, extraction of antioxidants (solvent, temperature etc.), selection of end-points and expression of research results, even for the same antioxidant assay, so that comparison between the values reported by different laboratories can be quite difficult.
Most original works and reviews on antioxidant activity focus mainly on the characteristics of the measurement procedure such as free radical generating system, redox interactions, molecular target, end-point, lipophilic and hydrophilic solubility etc. However, little attention has been paid to critical steps such as sample preparation (Luthria, 2006) or the procedure for extraction of antioxidants (Pellegrini et al., 2007).
It should be remembered that the already mentioned methods are intended for the determination of antioxidant activity of food sample per se, that is, the antioxidative potential of food. As for the antioxidative action of food substituents in real biological systems, this will depend also on their bioavailability and food antioxidants metabolism. The authors also strictly recommend complete standardisation of antioxidant assays as the results in the above studies can be confusing. Discussed here are different processing technologies and their impact on antioxidant activity of fruits, vegetables, juices, cereal, legumes, spices etc. It should be noted that the authors have tried to address all processing technologies and their effects on the antioxidant content. However, not all research studies have carried out two or more antioxidant assays with the aim to estimate their reliability and limitations. It is also very complicated and perplexing to correlate the data on antioxidant activity of natural products reported in various works and measured by various methods. These data are generally poorly repeatable, first of all, because natural products are hardly repeatable in principle (Roginsky and Lissi, 2005).
Some critical points to rememember while assessing antioxidant status of unprocessed and processed foods (Pérez-Jiménez et al., 2008):
The key nutritional role of plant produce is unquestionable, which is in part due to the presence of phytochemicals with various biological activities. These plant foods are a good source of major and minor (polyphenols, vitamins, carotenoids, glucosinolates minerals, etc.) compounds which may have important metabolic and/or physiological effects. More recent evidence provides potential information of their impact on health, so these secondary metabolites are currently marketed as functional foods and nutraceutical ingredients. The authors would like to highlight the fact that there are many methods used to determine total antioxidant activity, and it is important to point out that all of them have some limitations. It has been observed in previous studies that some antioxidant assay methods give different trends. For that reason multiple methods to generate an ‘antioxidant profile’ might be needed.
Abas, F., Lajis, N.H., Israf, D.A., Khozirah, S. and Kalsom, Y.U. (2006) Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables . Food Chemistry, 95 , 566–573.
Alho, H. and Leinonen, J. (1999) Total antioxidant activity measured by chemiluminescence methods . Methods in Enzymology, 299 , 3–15.
American Institute of Cancer Research. (1997) Food Nutrition, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute of Cancer Research.
Aramwit, P., Bang, N. and Srichana, T. (2010) The properties and stability of anthocyaninsin mulberry fruits . Food Research International, 43 , 1093−1097.
Arena, E., Fallico, B. and Maccarone, E. (2001) Evaluation of antioxidant capacity of blood orange juice as influenced by constituents, concentration process and storage . Food Chemistry, 74 , 423–427.
Arnous, A., Makris, D.P., and Kefalas, P. (2001) Effect of principle polyphenolic components in relation to antioxidant characteristics of aged red wines . Journal of Agriculture and Food Chemistry, 49 , 5736–5742.
Aruoma, O.I. (1994) Deoxyribose assay for detecting hydroxyl radicals . Methods in Enzymology 233 , 57–66.
Aruoma, O.I., Grootveld, M. and Halliwell, B. (1987) The role of iron in ascorbate-dependent deoxyribose degradation. Evidence consistent with a site-specific hydroxyl radical generation caused by iron ions bound to the deoxyribose molecule . Journal of Inorganic Biochemistry, 29 (4), 289–299.
Benkeblia, N. (2000) Phenylalanine ammonia-lyase, peroxidase, pyruvic acid and total phenolics variations in onion bulbs during long-term storage . Lebensmittel-Wissenschaft und-Technologie, 33 (2), 112–116.
Benzie, I.F.F. and Strain, J.J. (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration . Methods in Enzymology, 299 , 15–27.
Benzie, I.F.F. and Strain, J.J. (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay . Analytical Biochemistry, 239 (1), 70–76.
Benzie, I.F.F. and Strain, J.J. (1997) Simultaneous automated measurement of total ‘antioxidant’ (reducing) capacity and ascorbic acid concentration . Redox Report, 3 (4), 233–238.
Blois, M.S. (1958) Antioxidant determinations by the use of a stable free radical . Nature 181 (4617), 1199–1200.
Beyers, M. and Thomas, A.C. (1979) Irradiation of subtropical fruits. 4. Changes in certain nutrients present inmangoes, papayas, and litchis during canning, freezing, and irradiation . Journal of Agricultural and Food Chemistry, 27 , 48−51.
Brighenti, F., Valtueñ a, S., Pellegrini, N., Ardigo, D., Del Rio, D., Salvatore, S. et al. (2005) Total antioxidant capacity of the diet is inversely and independently related to plasma concentrations of highsensitive C-reactive protein in adult Italian subjects . British Journal of Nutrition, 93 (5), 619–625.
Brand-Williams, W., Cuvelier, M.E. and Berset, C. (1995) Use of a free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und-Technologie, 28 , 25–30.
Burns, J., Gardner, P.T., Matthews, D., Duthie, G.G., Lean, M.E.J. and Crozier, A. (2001) Extraction of phenolics and changes in antioxidant activity of red wines during vinification . Journal of Agricultural and Food Chemistry, 49 , 5797–5808.
Chu, Y.H., Chang, C.L. and Hsu, H.F. (2000) Flavonoid content of several vegetables and their antioxidant activity . Journal of the Science of Food and Agriculture, 80 , 561–566.
Coupland, J.N., Zhu, Z., Wan, H., McClements, D.J., Nawar, W.W. and Chinachoti, P. (1996) Droplet composition affects the rate of oxidation of emulsified ethyl linoleate . Journal of the American Oil Chemists Society, 73 (6), 795–801.
Dávalos, A., Gómez-Cordovés, C. and Bartolomé, B. (2004) Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay . Journal of Agricultural and Food Chemistry, 52 (1), 48–54.
DeRuiter, F.E. and Dwyer, J. (2002) Consumer acceptance of irradiated foods: Dawn of a new era? Food Service Technology, 2 , 47−58.
DeLong, J.M., Prange, R.K., Hodges, D.M., Forney, C.F., Bishop, M.C. and Quilliam, M. (2002) Using a modified ferrous oxidation- xylenol orange (FOX) assay for detection of lipid hydroperoxides in plant tissues . Journal of Agricultural and Food Chemistry, 50 , 248–254.
Del Pozo-Insfran, D., Balaban, M.O. and Talcott, S.T. (2006) Enhancing the retention of phytochemicals and organoleptic attributes in muscadine grape juice through a combined approach between dense phase CO 2 processing and copigmentation . Journal of Agricultural and Food Chemistry, 54 , 6705−6712.
Dillard, C.J. and German, J.B. (2000) Phytochemicals: nutraceuticals and human health . Journal of the Science of Food and Agriculture, 80 , 1744–1756.
Da Porto, C., Decorti, D. and Tubaro, F. (2010) Effects of continuous dense-phase CO 2 system on antioxidant capacity and volatile compounds of apple juice . International Journal of Food Science and Technology, DOI: 10.1111/j.1365-2621.2010.02339.x
Eberhardt, M.V., Lee, C.Y. and Liu, R.H. (2000) Antioxidant activity of fresh apples . Nature, 405 (6789), 903–904.
EFSA (2010) Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006 . EFSA Journal, 8 (2), 1489.
Erkan, N., Ayranci, G. and Yranci, E. (2008) Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol . Food Chemistry, 110 , 76–82.
Esterbauer, H., Lang, J., Zadravec, S. and Slater T.F. (1984) Detection of malonaldehyde by high-performance liquid chromatography . Methods in Enzymology, 105 , 319–328.
FAO/IAEA/WHO (1999) High-dose irradiation: Wholesomeness of food irradiated with doses above 10 kGy. Report of a joint FAO IAEA WHO study group. Rome: Food and Agriculture Organization of the United Nations.
Floegel, A., Kim, D.K., Chung. S.J., Koo, S.I. and Chun, O.K. (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, doi:10.1016/j.jfca.2011.01.008
Frankel, E.N., Huang, S.-W. and Aeschbach, R. (1997) Antioxidant activity of green teas in different lipid systems . JAOCS, 74 , 1309–1315.
Fraser, D. (1951) Bursting bacteria by release of gas pressure . Nature, 167 , 33−34.
Garcia-Gonzalez, L., Geeraerd, A.H., Spilimbergo, S., Elst, K., VanGinneken, L. and Debevere, J. (2007) High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future . International Journal of Food Microbiology, 117 (1), 1−28.
Gardner, P.T., White, T.A.C., McPhail, D.B. and Duthie, G.G. (2000) The relative contributions of vitamine C, carotenoids and phenolics to the antioxidant potential of fruit juices . Food Chemistry, 68 , 471–474.
Ghiselli, A., Serafini, M., Maiani, G., Azzini, E. and Ferro-Luzzi, A. (1995) A fluorescence-based method for measuring total plasma antioxidant capability . Free Radical Biology and Medicine, 18 (1), 29–36.
González-Aguilar, G.A., Villegas-Ochoa, M.A., Martίnez- Téllez, M.A., Gardea, A.A. and Ayala-Zavala, J.F. (2007) Improving antioxidant capacity of fresh-cut mangoes treated with UV-C . Journal of Food Science, 72 , S197–S202.
Gorinstein, S., Martín-Belloso, O., Park, Y.-S., Haruenkit, R., Lojek, A., Číž, M., Caspi, A., Libman, I. and Trakhtenberg, S. (2001) Comparison of some biochemical characteristics of different citrus fruits . Food Chemistry, 74 , 309–315.
Gorinstein, S., Haruenkit, R., Poovarodom, S., Vearasilp, S., Ruamsuke, P., Namiesnik, J., Leontowicz, M., Leontowicz, H., Suhaj, M. and Sheng, G.P. (2010) Some analytical assays for the determination of bioactivity of exotic fruits . Phytochemical Analysis, 21 , 355–362.
Grau, A., Codony, R., Rafecas, M., Barroeta, A.C. and Guardiola, F. (2000) Lipid hydroperoxide determination in dark chicken meat through a ferrous oxidation-xylenol Orange method . Journal of Agricultural and Food Chemistry, 48 , 4136–4143.
Granado, F., Olmedilla, B., Blanco, I. and Rojas-Hidalgo, E. (1992) Carotenoid composition in raw and cooked Spanish vegetables . Journal of Agricultural and Food Chemistry, 40 , 2135–2140.
Gunes, G., Blum, L.K. and Hotchkiss, J.H. (2005) Inactivation of yeasts in grape juice using a continuous dense phase carbon dioxide processing system . Journal of the Science of Food and Agriculture, 85 (14), 2362−2368.
Guderjan, M., Toepfl, S., Angersbach, A. and Knorr, D. (2005) Impact of pulsed electrifield treatment on the recovery and quality of plant oils . Journal of Food Engineering, 67 (3), 281−287.
Greefield, H. and Southgate, D.A.T. (1992) Food Composition Data Production, Management and Use. Elsevier Applied Science.
Halliwell, B., Gutteridge, J.M.C. and Aruoma, O.I. (1987) The deoxyribose method: a simple ‘test-tube’ assay for determination of rate constants for reactions of hydroxyl radicals . Analytical Biochemistry, 165 (1), 215–219.
Heinonnen, I. M., Meyer, A.S. and Frankel, E.N. (1998a) Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation . Journal of Agricultural and Food Chemistry, 46 , 4107–4112.
Heinonnen, M., Rein, D., Satué-Gracia, M., Huang, S.-W., German, J.B. and Frankel, E.N. (1998b) Effect of Protein on the antioxidant activity of phenolic compounds in a lecithin-lipossome oxidation system . Journal of Agricultural and Food Chemistry, 46 , 917–922.
Henríquez, C., Carrasco-Pozo, C., Gómez, M., Brunser, O. and Speisky, H. (2008) Slow and fast-reacting antioxidants from berries: their evaluation through the FRAP (ferric reducing antioxidant power) assay . Acta Horticulturae, 777 , 531–536.
Hu, C. and Kitts, D.D. (2000) Studies on the antioxidant activity of Echinacea root extract . Journal of Agricultural and Food Chemistry, 48 , 1466–1472.
Hu, C., Yuan, Y.V. and Kitts, D.D. (2007) Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro . Food and Chemical Toxicology, 45 (11), 2219–2227.
Huang, D., Ou, B. and Prior, R.L. (2005) The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry, 53 , 1841–1856.
Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J.A. and Deemer, E.K. (2002) Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β -cyclodextrin as the solubility enhancer . Journal of Agricultural and Food Chemistry, 50 (7), 1815–1821.
Huang, D.-J., Chen, H.-J., Hou, W.-C., Lin, C.-D. and Lin, Y.-H. (2006). Sweet potato (Ipomoea batatas [L.] Lam ‘Tainong 57”) storage root mucilage with antioxidant activities in vitro . Food Chemistry, 98 , 774–781.
Ismail, A., Marjan, Z.M. and Foong, C.W. (2004) Total antioxidant activity and phenolic content in selected vegetables . Food Chemistry, 87 , 581–586.
Ismail, A. and Lee, W.Y. (2004) Influence of cooking practice on antioxidant properties and phenolic content of selected vegetables . Asia Pacific Journal of Clinical Nutrition, 13 , S162–S165, (Suppl).
Jay, J.M., Loessner, M.J. and Golden, D.A. (2005) Modern Food Microbiology (7th edition), New York: Springer.
Jiang, Z.-Y., Hunt, J. V. and Wolff, S. P. (1992). Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein . Analytical biochemistry, 202 , 384–389.
Jiang, Z.-Y., Woollard, A.C.S. and Wolff, P. (1991) Lipid Hydroperoxide measurement by oxidation of Fe 2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method . Lipids, 26 , 853–856.
Joseph, J.A., Shukit-Hale, B., Denisova, N.A. et al. (1999) Reversal of age-related declines in neuronal signal transduction, cognitive, and motor behavioural de®cits with blue berry, spinach, or strawberry dietary supplementation . Journal of Neuroscience, 19 , 8114–8812.
Kabir, H. (1994) Fresh-cut vegetables. In: A.L. Brods and V.A. Herndon (eds), Modified Atmosphere Food Packaging, Institute of Packaging Professionals, pp. 155–160.
Karadag, A., Ozcelik, B. and Saner, S. (2009) Review of methods to determine antioxidant capacities . Food Analysis Methods, 2 , 41–60.
Karakaya, S. and Kavas, A. (1999). Antimutagenic activities of some foods . Journal of Agricultural Food Chemistry, 79 , 237–242.
Kaur, C. and Kapoor, H. (2001) Review: antioxidants in fruits and vegetables – the millennium’s health . International Journal of Food Science and Technology, 36 , 703–725.
Kitts, D.D., Wijewickreme, A.N. and Hu, C. (2000) Antioxidant properties of a North American ginseng extract . Molecular and Cellular Biochemistry, 203 , 1–10.
Koleva, I.I., van Beek, T.A., Linssen, J.P.H., de Groot, A. and Evstatieva, L.N. (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods . Phytochemical Analysis, 13 , 8–17.
Kumar, K.S., Ganesan, K. and Rao, P.V.S. (2008) Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty – an edible seaweed . Food Chemistry, 107 , 289–295.
Lafuente, M.T., Zacarías, L., Martínez-Téllez, M.A., Sánchez-Ballesta, M.T. and Granell, A. (2003) Phenylalanine ammonia-lyase and ethylene in relation to chilling injury as affected by fruit age in citrus . Postharvest Biology and Technology, 29 (3), 308–317.
Lee, J.-Y., Hwang, W.-I. and Lim, S.-T. (2004) Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A . De Candolle roots. Journal of Ethnopharmacology, 93 , 409–415.
Lee, S.K. and Kader, A.A. (2000) Preharvest and postharvest factors influencing vitamin C content of horticultural crops . Postharvest Biology and Technology, 20 , 207−220.
Locatelli, M., Travaglia, F., Coisson, J.D., Martelli, A., Stévigny, C. and Arlorio, M. (2010) Total antioxidant activity of hazelnut skin (Nocciola Piemonte PGI): Impact of different roasting conditions . Food Chemistry, 119 , 1647–1655.
Luthria, D. (2006) Significance of sample preparation in developing analytical methodologies for accurate estimation of bioactive compounds in functional foods . Journal of the Science of Food and Agriculture, 86 (14), 2266–2272.
MacDonald-Wicks, L.K., Wood, L.G. and Garg, M.L. (2006) Methodology for the determination of biological antioxidant capacity in vitro: a review . Journal of the Science of Food and Agriculture, 86 , 2046–2056.
MacPhail, D.B., Gardner, P.T., Duthie, G.G., Steele, G.M. and Reid, K. (1999) Assessement of the antioxidant potential of Scotch whiskeys by electron spin resonance spectroscopy: relationship to hydroxyl-containing aromatic components . Journal of Agricultural and Food Chemistry, 47 , 1937–1941.
Mahattanatawee, K., Manthey, J. A., Luzio, G., Talcott, S. T., Goodner, K., and Baldwin, E. A. (2006) Total antioxidant activity and fiber content of select Florida- Grown Tropical fruits . Journal of Agricultural and Food Chemistry, 54 , 7355–7363.
Malminiemi, K., Palomäki, A. and Malminiemi, O. (2000) Comparison of LDL trap assay to other tests of antioxidant capacity; effect of vitamin E and lovastatin treatment . Free Radical Research, 33 (5), 581–593.
Manvell, C. (1997) Minimal processing of food . Food Science and Technology Today, 11 , 107–111.
Meyer, A.S., Heinonen, M. and Frankel, E.N. (1998) Antioxidant interactions of catechin, cyaniding, caffeic acid, quercetin, and ellagic acid on human LDL oxidation . Food Chemistry, 61 , 71–75.
Meyer, A.S., Yi, O.-S., Pearson, D.A., Waterhouse, A.L. and Frankel, E.N. (1997) Inhibition of Human Low-Density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera) . Journal of Agricultural and Food Chemistr., 45 , 1638–1643.
Miller, N., Diplock, A. T., and Rice-Evans, C. (1995) Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storage . Journal of Agricultural and Food Chemistry, 43 , 1794–1801.
Musa, K.H., Abdullah, A., Jusoh, K. and Subramaniam, V. (2008) Antioxidant activity of pink-flesh guava (psidium guajava l.): effect of extraction techniques and solvents . Food Analytical Methods, 4 (1), 100–107, doi: 10.1007/s12161-010-9139-3.
Nawar, W.W. (1996) Lipids. In: Fennema, O.R. (ed.) Food Chemistry New York, Marcel Dekker Inc., pp. 225–319.
Navas, J. A., Tres, A., Codony, R., Boatella, J., Bou, R., and Guardiola, F. (2004). Modified ferrous oxidation-xylenol orange method to determine lipid hydroperoxides in fried snacks . European Journal of Lipid Science and Technology, 688–696.
Ng, T.B., Lui, F. and Wang, Z.T. (2000) Antioxidatve activity of natural products from plants . Life Sciences, 66 (8), 709–723.
Niki, E. (1990) Free radical initiators as as source of water- or lipid-soluble peroxyl radicals . Methods in Enzymology, 186 , 100–108.
Noguchi, N., Yamashita, H., Gotoh, N., Yamamoto, Y., Numano, R., and Niki, E. (1998) 2,2 ′ –Azobis (4-methoxy-2,4-dimethylvaleronitrile), a new lipid-soluble azo initiator: application to oxidations of lipids and low-density lipoprotein in solution and in aqueous dispersions . Free Radical Biology and Medicine, 24 (2), 259–268.
Nourooz-Zadeh, J. (1999) Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. In: H. Sies, Oxidants and Antioxidants, Methods in Enzymology series, London, Academic Press.
Nourooz-Zadeh, J., Tajaddini-Sarmadi, J., Birlouez-Aragon, I., and Wolff, S. P. (1995). Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay , Journal of Agricultural and Food Chemistry, 43 , 17–21.
Nourooz-Zadeh, J., Tajaddini-Sarmadi, J., and Wolff, S. P. (1994). Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjuction with triphenylphosphine , Analytical Biochemistry, 220 , 403–409.
Ou, B., Hampsch-Woodill, M. and Prior, R.L. (2001) Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe . Journal of Agricultural and Food Chemistry, 49 (10), 4619–4626.
Ou, B., Huang, D., Hampsch-Woodill, M., Flanagan, J.A. and Deemer, E.K. (2002) Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study . Journal of Agricultural and Food Chemistry, 50 , 3122–3128.
Ou, S.Y., Luo, Y.L., Huang, C.H., and Jackson, M. (2009) Production of coumaric acid from sugarcane bagasse . Innovative Food Science and Emerging Technologies, 10 , 253–259.
O’Beirne, D. and Francis, G.A. (2003) Reducing pathogen risk in MAP-prepared produce. In R. Ahvenainen (ed.) Novel Food Packaging Techniques, Cambridge, UK/Boca Raton, FL, Woodhead Publishing Limited/CRC Press LLC, pp. 231–232.
Parejo, I., Codina, C., Petrakis, C. and Kefalas, P. (2000) Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminal chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) freeradical assay . Journal of Pharmocological and Toxicological Methods, 44 , 3871–3880.
Pellegrini, N., Re, R., Yang, M. and Rice-Evans, C. (1999) Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2 ′ -Azinobis(3-ethylene-benzothiazoline-6-sulfonic acid radical cation decolorization assay . Methods in Enzymology, 299 , 379–389.
Pellegrini, N., Visioli, F., Buratti, S. and Brighenti, F. (2001) Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating . Journal of Agricultural and Food Chemistry, 49 , 2532–2538.
Pellegrini, N., Colombi, B., Salvatore, S., Brenna, O.V., Galaverna, G., Del Rio, D. et al. (2007) Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents . Journal of the Science of Food and Agriculture, 87 , 103–111.
Pellegrini, N., Serafini, M., Colombi, B., Del Rio, D., Salvatore, S., Bianchi, M. et al. (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays . Journal of Nutrition, 133 , 2812–2819.
Pietta, P., Simonetti, P. and Mauri, P. (1998). Antioxidant activity of selected medicinal plants . Journal of Agricultural and Food Chemistry, 46 , 4487–4490.
Polydera, A.C., Stoforos, N.G. and Taokis, P.S. (2004) The effect of storage on the antioxidant capacity of reconstituted orange juice which had been pasteurized by high pressure or heat . International Journal of Food Science and Technology, 39 , 789–791.
Piga, A., Agabbio, M., Gambella, F. and Nicoli, M.C. (2002) Retention of antioxidant activity in minimally processed mandarin and satuma fruits . Lebensmittel-Wissenschaft und-Technologie-Food Science and Technology, 35 , 344–347.
Pinilla, M.J., Plaza, L., Sánchez-Moreno, C., De Ancos, B. and Cano, M.P. (2005) Hydrophilic and lipophilic antioxidant capacities of commercial mediterranean vegetable soups (gazpachos) . Journal of Food Science, 70 , S60–S65.
Plaza, M.L., Ramirez-Rodrigues, M.M., Balaban, M.O., and Balaban, M.O. (2010) Quality improvement of guava puree by dense phase carbon dioxide (DP-CO2) pasteurization. (236–09). IFT Annual Meeting + Food Expo, Anaheim, CA., 20 July,
Plumb, G.W., Chambers, S.J., Lambert, N., Bartolomé, B., Heaney, R.K., Wanigatunga, S., Aruoma, O.I., Halliwell, B. and Williamson, G. (1996) Antioxidant actions of fruit, herb and spice extracts . Journal of Food Lipids, 3 , 171–188.
Prior, R.L., Wu, X. and Schaich, K. (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and Dietary Supplements . Journal of Agricultural and Food Chemistry, 53 , 4290–4302.
Prior, R.L. and Cao, G. (2000) Antioxidant phytochemicals in fruits and vegetables: diet and health implications . Horticulture Science, 35 , 588–592.
Puhl, H., Waeg, G. and Esterbauer, H. (1994) Methods to determine oxidation of low-density lipoproteins . Methods in Enzymology, 233 , 425–441.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. and Rice-Evans, C. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine, 26 (9–10), 1231–1237.
Rice-Evans, C.A. (2000) Measurement of total antioxidant activity as a marker of antioxidant status in vivo: Procedures and limitations . Free Radical Research, 33 (Suppl.), 559–566.
Rodtjer, A., Skibsted, L.H. and Andersen, M.L. (2006) Antioxidative and prooxidative effects of extracts made from cherry liqueur pomace . Food Chemistry, 99 , 6–14.
Roginsky, V. and Lissi, E.A. (2005) Review of methods to determine chain-breaking antioxidant activity in food . Food Chemistry, 92 , 235–254.
Ruslay, S., Abas, F., Shaari, K., Zainal, Z., Maulidiani, Sirat, H., Israf, D.A. and Lajis, N.H. (2007) Characterization of the components present in the active fractions of health gingers (Curcuma xanthrrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS . Food Chemistry, 104 , 1183–1191.
Sales, J.M and Resurreccion, A.V.A (2010a) Maximizing phenolics, antioxidants and sensory acceptance of UV and ultrasound-treatbed peanuts . LWT – Food Science and Technology, 43 , 1058–1066.
Salvini, S., Parpinel, M., Gnagnarella, P., Maisonneuve, P. and Turrini, A. (1998) in Banca Dati di Composizione degli Alimenti per Studi Epidemiologici in Italia. Milano, Istituto Europeo di Oncologia.
Salleh-Mack, S.Z. and Roberts, J.S. (2007) Ultrasound pasteurization: The effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922 . Ultrasonics Sonochemistry, 14 , 323−329.
Saxena, A., Maity, T., Raju, P.S. and Bawa, A.S. (2010) Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying . Food and Bioprocess Technology, DOI 10.1007/s11947-010-0409-2.
Sharma, O.P. and Bhat, T.K. (2009) DPPH antioxidant assay revisited . Food Chemistry, 113 , 1202–1205.
Shimada, K., Fujikawa, K., Yahara, K. and Nakamura, T. (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion . Journal of Agricultural and Food Chemistry, 40 (6), 945–948.
Shui, G. and Leong, L. P. (2006) Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals . Food Chemistry, 97 , 277–284.
Steinmetz, K.A. and Potter, J.D. (1996) Vegetable, fruit and cancer epidemiology . Cancer Causes and Control, 2 , 325–351.
Valkonen, M. and Kuusi, T. (1997) Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum . Journal of Lipid Research, 38 (4), 823–833.
Wang, H., Cao, G. and Prior, R.L. (1996) Total antioxidant capacity of fruits . Journal of Agricultural and Food Chemistry, 44 , 701–705.
Wayner, D.D.M., Burton, G.W., Ingold, K.U. and Locke, S. (1985) Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation . The important contribution made by plasma proteins. FEBS Letters, 187 (1), 33–37.
Wood, O.B. and Bruhn, C.M. (2000) Position of the American dietetic association: Food irradiation . Journal of the American Dietetic Association, 100 , 246−253.
Wu, X., Beecher, G.R., Holden, J.M., Haytowitz, D.B., Gebhardt, S.E. and Prior, R.L. (2004a) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States . Journal of Agricultural and Food Chemistry, 52 (12), 4026–4037.
Wu, X., Gu, L., Holden, J., Haytowitz, D.B., Gebhardt, S.E., Beecher, G. and Prior, R.L. (2004b) Development of a database for total antioxidant capacity in foods: a preliminary study . Journal of Food Composition and Analysis, 17 , 407–422.
Xue, J., Chen, L. and Wang, H. (2008) Degradation mechanism of Alizarin Red in hybridgas–liquid phase dielectric barrier discharge plasmas: Experimental and theoreticalexamination . Chemical Engineering Journal, 138 , 120−127.
Yan, X., Nagata, T. and Fan, X. (1998) Antioxidative activities in some common seaweeds . Plant Foods for Human Nutrition, 52 , 253–262.
Yen, G.C. and Lin, H.T. (1996) Comparison of high pressure treatment and thermal pasteurisation on the quality and shelf life of guava puree . International Journal of Food Science and Technology, 31 , 205–213.
Yen, G.-C. and Chen, H.-Y. (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity . Journal of Agricultural and Food Chemistry, 43 , 27–32.
Yildiz, G., Wehling, R.L. and Cuppett, S.L. (2003) Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils . JAOCS, 80 , 103–107.
Yoshida, Y., Shimakawa, S., Itoh, N. and Niki, E. (2003) Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain . Free Radical Research, 37 (8), 861–872.
Yuan, Y.V., Bone, D.E. and Carrington, M.F. (2005a) Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro . Food Chemistry, 91 (3), 485–494.
Yuan, Y.V., Carrington, M.F. and Walsh, N.A. (2005b) Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro . Food and Chemical Toxicology, 43 (7), 1073–1081.
Yuan, Y.V., Westcott, N.D., Hu, C. and Kitts, D.D. (2009) Mycosporine–like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick . Food Chemistry, 112 (2), 321–328.
Zepka, L.Q. and Mercadante, A.Z. (2009) Degradation compounds of carotenoids formed during heating of a simulated cashew apple juice . Food Chemistry, 117 , 28−34.
Zenker, M., Heinz, V. and Knorr, D. (2003) Application of ultrasound assisted thermalprocessing for preservation and quality retention of liquid foods . Journal of Food Protection, 66 , 1642−1649.
Zhang, L., Lu, Z.,Yu, Z. and Gao, X. (2005) Preservation of fresh-cut celery by treatment of ozonated water . Food Control, 16 (3), 279–283.
Zhang, P. and Omaye, S.T. (2001) β -Carotene: interactions with α -tocopherol and ascorbic acid in microsomal lipid peroxidation . Journal of Nutritional Biochemistry, 12 , 38–45.
Zhang, J., Davis, T.A., Matthews, M.A., Drews, M.J., LaBerge, M. and An, Y.H. (2006) Sterilization using high-pressure carbon dioxide . Journal of Supercritical Fluids, 38 (3), 354−372.
Zhang, D. and Hamauzu, Y. (2004) Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking . Food Chemistry, 88 (4), 503–509.
Zimmer, H., Lankin, D.C. and Horgan, S.W. (1970) Oxidations with potassium nitrodisulfonate (Fremy’s radical) . The Teuber reaction. Chemical Reviews, 2 , 229–246.