Figure 21.1 Venn diagram representing the technological characteristics that are improved in foods by the use of food additives/ingredients.
The definition, general chemistry, classification and important sources of phytochemicals, as well as the effects of processing, are extensively outlined in the first part of this book. This chapter is related with the industrial applications of phytochemicals. The economic relevance of phytochemicals in food and other industries resides in their applicability to industry. An industrial application results of the transfer of a scientific knowledge into a technological use in order to control and/or modify an industrial process. The objective of this book chapter is to extensively review the use of phytochemicals in food industry. Phytochemicals are used in the food industry as food ingredients/additives and physico-chemical properties of phytochemicals determine how they are used in industry. For example, if a phytochemical is an antioxidant, this phytochemical could be potentially used to avoid undesirable oxidation of food products (fats or proteins). The use of phytochemicals in food industry is controlled by competent regulating bodies, in individual countries or economic areas. For example, the Food and Drug Administration (FDA) regulates the use of food additives in the United States of America while the European Food Safety Agency (EFSA) does so in all the European Union.
Phytochemicals are naturally occurring in fruits, vegetables and seaweeds and, as shown in previous chapters, the processing involved for their human consumption leads to a loss or degradations of these compounds in the foods, reducing some of their quality properties. Moreover, in most cases the waste or by-products of these processes is particularly rich in phytochemicals such as fruit juice production (apple pomace) or peeling (i.e. carrots and onions). Considerable efforts are carried out by research and development centres and food industry to find innovative ways to reduce this loss without jeopardising in other sensory attributes and/or their cost efficiency. The main trends in this sense have been: (1) the use of new or unique varieties, rich in a specific or family of phytochemicals, so when processed the overall content in phytochemicals is still high; (2) the use of new innovative ways of food processing that improve the contents of phytochemicals when compared with more traditional ways of food processing; and (3) to artificially enhance the food product in order to supplement or fortify the original products. In addition to this last trend, phytochemicals have been used not only to fortify, but also as food ingredients, in order to improve sensory attributes and shelf life of a given food product. As a result phytochemicals are in fact a broad group of compounds that present multiple physico-chemical properties, and consequently can be potentially employed for many industrial applications.
The use of a substance as a food additive must require that this substance is not-toxic at the recommended levels of use. This requirement also applies for both chronic or acute toxicity. As a consequence the use of food additives is, in most countries, regulated by a food safety authority. Hence additive regulations might differ in part from country to country. Although in some economic areas such as the European Union there has been a compliance of these regulations. Some of these regulations extend to countries that don’t belong to this economic area but have strong economic links with it (i.e. Switzerland, Norway, Iceland, Turkey and Australia). At the international level the Food and Agriculture Organization (FAO) of the United Nations and the World Health Organization (WHO) have a joint expert committee in food additives (JECFA). This committee evaluates safety of food additives advices on the standards, guidelines and codes of practice on the use of food additives. The classification of food additives can be done according to their technological use (Figure 21.1). More precisely they can be categorised according to their specific role or their chemical characteristics. Vitamins, minerals, amino acids and other phytochemicals can be used to improve nutritional profile of foodstuffs, but they contribute to this increase in nutritional value in a very different way (due to their chemical properties). Therefore vitamins, amino acids and minerals are normally included in food formulations to balance losses during processing. This is a common practice in fruit juices, canned vegetables, bakery products, snacks and milk. In the case of minerals, fortification is usually done in those that are more fully available such as iron or calcium; this is a common practice in products such as cereal based breakfasts (Belitz et al ., 2004; Poletti et al ., 2004; Akhtar and Ashgar, 2011). As shown in Figure 21.1 some additives might have multiple functions and they contribute to increase value in more than one way. Ascorbic acid is a strong antioxidant and at the same time can have some beneficial effects as a dough improver in baked products. Some amino acids have important biological value and also can contribute to a protein-rich kind of taste in foods.
Figure 21.1 Venn diagram representing the technological characteristics that are improved in foods by the use of food additives/ingredients.

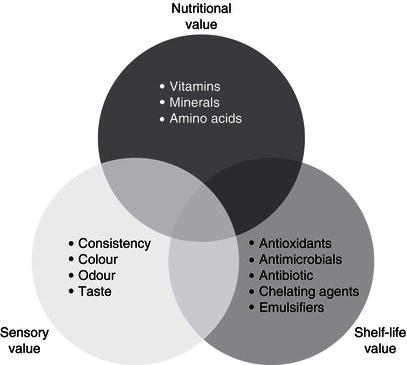
Flavourings are substances that are used as food additives to improve taste and/or smell of food. Fruit, vegetables, herbs and spices contain a great variety of volatile compounds many of which have flavouring properties (Figure 21.2). Chemical compounds such as carbonyl compounds, hydrocarbons esters pyranones, furanones, volatile sulphur compounds pyrazines, phenols and terpenes are common volatile compounds extracted from fruits and vegetables for their use as flavouring substances. In particular volatile sulphur compounds are common in vegetables of the Brassicacea (broccoli and cabbage) and Liliaceae (onion, garlic and leek) family. Pyrazines are contained in some spices and fruits of the Capsicum family (black pepper or chilli pepper). Terpenes (mono and sesquiterpenes) are present in fruits, vegetables and spices, for example β -caryophyllene is a volatile with flavouring properties present in many herbs (oregano, rosemary, black pepper). Many non-volatile phytochemicals can easily degrade to volatiles with flavouring properties, such as β -carotene into β -ionone or ferulic acid into vainillin.
Figure 21.2 Chemical structure of some phytochemicals often used as flavourings in the industry.
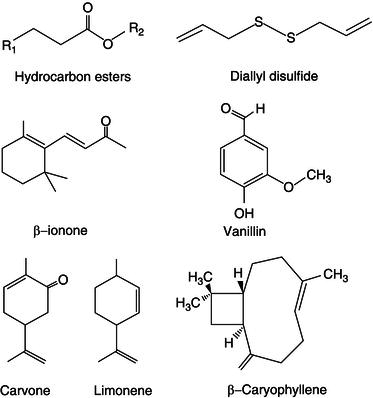
Plants and fruits are a great source of phytochemicals with sweetening properties (Figure 21.3). Many of these sweetening compounds are interestingly not sugars, but proteins (Gibbs et al., 1996). This is the case of several sweetening proteins (Monellin, Thaumatin, Brazzein, Pentadin, Miraculin and Mabilin) from plant origin that have been discovered in Africa and/or Asia. The pulp of the serendipity berry (Dioscorephyllum volkensii) is rich in Monellin a protein with a molecular weight of 10.5 kDa (Morris et al., 1973; Fan et al., 1993). Monellin is composed of two polypeptide chains that are not covalently bound and is 1500–2000 times sweeter than sucrose (Morris et al., 1973; Belitz et al., 2004).
Thaumatin (E-957) is obtained from Thaumatococcus danielii. Thaumatin contains two sweet proteins Thaumatin I and Thaumatin II and is approximately 2000 times sweeter than sucrose. Curculin from Curculingo latifolia and Miraculin from the fruit of Synsepalum dulcificum are both taste modifiers (Belitz et al., 2004). The mode of action seems to affect the taste buds, making sour or acid foods taste sweet. Brazzein and Pentadin are two sweetening proteins from the climbing plant Oubli (Pentadiplandra brazzeana) (Gao et al., 1999). Chemical stability of brazzein makes it a very interesting ingredient for food processing. Brazzein is stable over a broad pH range of 2.5–8 (Caldwell et al., 1998; Hellekant and Danilova, 2005) and heat stable at 98 °C for 2 hours (Ming and Hellekant, 1994; Assadi-Porter et al., 2000; Hellekant and Danilova, 2005).
Mabinlins are sweet-tasting proteins extracted from the seed of Mabinlang (Capparis masaikai Levl.), a Chinese plant from the region of Yunnan. The sweetness of mabinlin-2 is unchanged after 48 hours incubation at 80 °C (Kurihara, 1992; Liu et al., 1993) Mabinlin-3 and -4 sweetness stayed unchanged after 1 hour at 80 °C, while mabinlin-1 lost sweetness after 1 hour in the same conditions (Kurihara, 1992). Apart from sweetening proteins there are other compounds in plants that can be used as sweeteners. For example, Monatin isolated from the South African plant Sclerochiton ilicifoliu (Abraham et al., 2005). Glycyrrhizin is a sweet-tasting compound obtained from liquorice root. It is 30–50 times sweeter than sucrose. Glycyrrhizin is a triterpenoid saponin glycoside of glycyrrhizic acid (Belitz et al., 2004) and it loses its sweetening properties when hydrolysed. The sweet taste of glycyrrhizin is different from sugar but it lasts for longer in the mouth. It is also quite stable upon heating. However the use of glycyrrhizin is limited due to its cortisone (anti-inflammatory) like properties (Akamatsu et al., 1991).
Figure 21.3 Chemical structure of some phytochemicals often used as sweetening agents in the food industry.
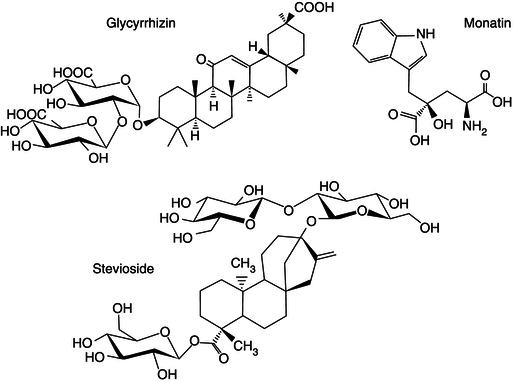
The leaves of stevia ( Stevia rebaudiana ) are rich ( ca 6% in mass) in non-protein sugar substitute sweetener substances known as steviosides and rebaudiosides. They are heat- stable, pH-stable and do not ferment. Steviosides are diterpene glycosides of steviol. Its use has been limited due to unclear toxic properties. Stevioside has in addition a bitter taste, the Rebaudiside A, which is steviol β -linked to a glucose ,and a glucose-glucose-glucose trisaccharide (also β -linked 2-1 and 3-1 with β -D-glucose) is less bitter and more polar.
A study performed in 1985 reported that steviol was mutagen (Pezzuto et al., 1985). However stevioside as a sweetener was evaluated by the Scientific Committee for Food (SCF) in 1984, 1989 and 1999. JECFA reviewed the safety of steviol glycosides (in 2000, 2005, 2006, 2007 and 2009) and established an ADI for steviol glycosides (expressed as steviol equivalents) of 4 mg/kg bw/day. In 2010 EFSA reviewed the use of stevioside glycosides as food additive establishing similar ADI levels as the JECFA (EFSA Journal, 2010, 8(4), 1537).
Many other phytochemicals are used as colouring substances in food processing (Figure 21.4). These colouring substances are used to adjust or correct food discoloration or colour change during processing or storage (Belitz et al., 2004). There are four main types of phytochemicals used as colouring substances, anthocyanins, betalains, chlorophylls and carotenoids. Anthocyanins, a class of flavonoids derived ultimately from phenylalanine, are water-soluble. Anthocyanins can provide a large range of colours (orange/red to violet/blue) as a function of their chemical structure and environment. Therefore slight modifications of their chemical structure may lead to colour changes, but their colour also depends on co-pigments, metal ions and pH. They are widely distributed in the plant kingdom and are industrially obtained by aqueous extraction of by-products such as fruit skins and peels (Schieber et al., 2001).
Figure 21.4 Chemical structure of some phytochemicals often used as colouring agents in the food industry.
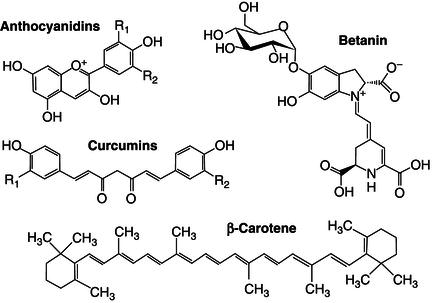
Betalains are nitrogen-containing water-soluble compounds derived from tyrosine that are found only in a limited number of plant lineages and present yellow-to-red colours. Some betalains have a stronger colouring capacity than anthocyanins and their colour exhibits higher pH stability (Stintzing and Carle, 2007; Tanaka et al., 2008; Azeredo, 2009). Betalains are obtained by aqueous extraction of beet roots and are constituted by betacyanins (red) and betaxanthines (yellow). Betanin represent 75–95% of main colouring principle. Interestingly anthocyanins and betalains are mutually exclusive and never have both been found in the same plant. Betalains and anthocyanins are used as colouring agents for fruit preparations, dairy products, ice creams, confectionery, pet-foods, soups, sauces, beverages and drinks.
On the other hand, most of the carotenes and carotenoids are lipid-soluble, yellow-to-red phytochemicals. Carotenes and carotenoids are a subclass of terpenoids, that often are obtained by solvent extraction of carrots (Daucus carota), oil palm fruit (Elaeis guinensis), sweet potato (Ipomea batatas), marigold (Tagetes erecta) and microalgae (Spirulina platensis). Carotenes and carotenoids are used in multiple industrial applications such as dairy (beverages, cream and dairy desserts), oils, fats and emulsions, fruit based products (spreads, desserts, canned fruits, pastry fillings), mustards, egg based products, soups and broths, edible coatings and cooked fish and fish products.
Chlorophylls and their derivatives are obtained by solvent extraction of grasses, alfalfa ( Mendicago sativa ), nettles ( Urticaceaes ) and other plants or algae materials. During the extraction of chlorophylls and their subsequent solvent removal, the naturally present coordinated magnesium may be wholly or partly removed from the chlorophylls to yield dark olive green pheophytins. In order to keep the bright green colour of chlorophylls the copper complexes of chlorophyll can be synthesised. Copper complexes of chlorophylls can be obtained by addition of an organic salt of copper. However, due to the toxicity of copper salts, its use is limited and maximum levels recommended in Codex Alimentarius of the GSFA (General Standard for Food Additives) are rarely above 500 mg/kg. Curcumin or turmeric yellow is obtained by solvent extraction of turmeric (ground rhizomes of Curcuma longa L.) and the extract is purified by crystallisation. This process eliminates the pungent and aromatic essential oil in turmeric, leaving deodorised turmeric which is used in dairy products and baked goods. Curcumin is relatively inexpensive and heat stable, but has poor light stability (Timberlake and Henry, 1986).
Antimicrobials are used for either killing or inhibiting the growth of microorganisms. A large range of antimicrobial substances are used in the food industry (Figure 21.5). Most of them are small organic acids that are chemically synthesised, such as benzoic, sorbic or propoinic acids (Cowan, 1999). However plants present a vast chemical collection of antimicrobial substances that are synthesised to protect themselves and that in recent years have been explored as antimicrobial additives by the food industry as an alternative to chemical synthesised compounds which are perceived by consumers as unhealthy. There are three main groups of antimicrobial compounds in plants, phenolic/polyphenols; terpenoids and alkaloids (Cowan, 1999; Tiwari et al ., 2009; Van Vuuren et al ., 2009; Tajkarimi et al ., 2010). Simple phenols and phenolic acids such as cinnamic or caffeic have shown strong antimicrobial properties against viruses, bacteria and fungi (Cowan, 1999). Catechol and pyrogallol, both hydroxilated phenols, have also shown strong toxicity towards microorganism and the number of hydroxyl groups on the phenol ring has been associated with their level of antimicrobial capacity. Flavones, flavonoids and flavonols are more complex phenolic structures, derived of the 2-phenyl-1,4 benzopyrone polyphenolic structure, and they are known to be synthesised by plants in response to microbial infection (Dixon et al ., 2006). Several flavonoids have shown to have strong antimicrobial properties (Proestos et al ., 2005).
Figure 21.5 Chemical structure of some phytochemicals responsible for antimicrobial properties in some plants.
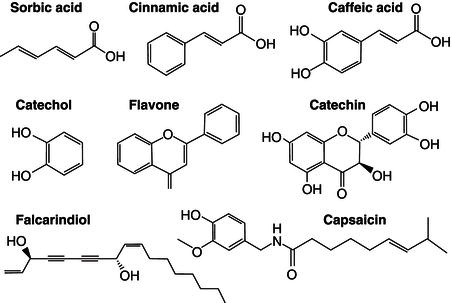
C-17 polyacetylenes, such as falcarinol and falcarindiol (commonly found in the Apiaceae family such as carrots, parsnips, celery and fennel), have antibacterial effects against various micro-organisms such as gram-positive bacteria ( Bacillus ssp., Staplylococcus ssp., Streptococcus s sp.) and gram-negative bacteria (Escherichia ssp., Pseudomonas ssp.) (Christensen et al., 2010). These polyacetylenes also present antimycobacterial effects, of which the most important seems to be the activity against M. tubercolosis (Kobaisy et al., 1997). These effects represent pharmacologically useful properties by which falcarinol and related polyacetylenes could have positive effects on human health and may be used to develop antibiotics (Christensen et al., 2010). Recently it was shown that falcarindiol strongly inhibited the growth of Micrococcus luteus and Bacillus cereus, with a minimum inhibitory concentration (MIC) value of 50 µg mL−1 (Meot-Duros et al., 2010).
Capsaicin, one of nature’s most pungent spices from plants from the capsicum genus (peppers), is known to have strong antimicrobial properties and is used traditionally to preserve foods (Belitz et al., 2004). Terpenoids or isoprenoids are the main constituent of essential oils, which are used due to their antimicrobial properties in food preservation (Cowan, 1999; Tiwari et al., 2009).
Essential oils, which are concentrated hydrophobic phases extracted from plants by distillation or solvent extraction, contain high amounts of some of the antimicrobial compounds already described (Van Vuuren et al ., 2009). Therefore essential oils do not have any specific chemical or pharmaceutical properties in common, although the individual properties of essential oils have been extensively studied (Sacchetti et al ., 2005). They are used for flavouring food and drinks but also in perfumes, cosmetics, soaps and cleaning products.
Figure 21.6 Chemical structure of some phytochemicals with antioxidant properties from different plant sources.
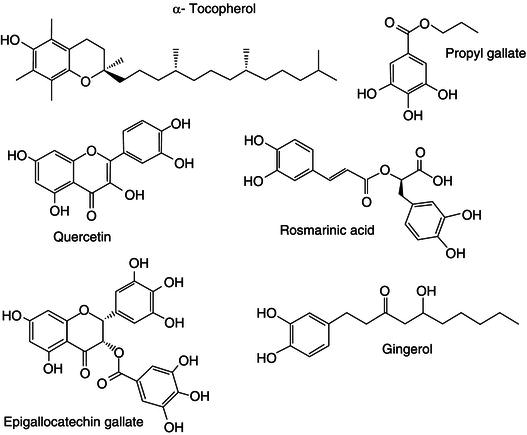
Many phytochemicals have antioxidant properties. Antioxidants are compounds capable of inhibiting oxidation of other molecules (Figure 21.6). Oxidation, from a chemical point of view, is a reaction where the oxidation state of a compound is increased. This often happens by loss of electrons. Consequently oxidation reactions can generate free radicals. Free radicals are known to initialise chain reactions that can interfere with a multitude of biological processes. Antioxidants are able to terminate with chain reactions by removing free radicals, inhibiting further oxidation reactions. This is achieved by oxidising themselves into stable radicals that do not continue reacting. Tocopherols (vitamin D) are obtained by the vacuum steam distillation of edible vegetable oil product. D- α -tocopherol is the most abundant. Tocopherols are used as antioxidants in butter oil (ghee), anhydrous milk fat, fat spreads, dairy fat spreads and blended spreads at a maximum level of 500 mg/kg. Ascorbates (vitamin C) are also used as strong antioxidants, however most of their production nowadays is chemically synthesised. Esters from gallic acid with different alkyl alcohols (propanol, octanol and lauryl-alcohol) although present in nuts and other plants are also chemically synthesised and used in the food industry as antioxidants. Gum guaiacum (E-314) is an extract from the tree species Guaiacum officinale that is used as antioxidant. Other plant extracts rich in phenolics and/or flavonoids, such as cathechins from tea ( Camellia sinensis ), gingerol from ginger ( Zingiber officinale ) have shown strong antioxidant capacity (Aruoma et al ., 1997; Ho et al ., 1997; Moure et al ., 2001). Also terpenes and terpenoids such as carnosolic acid and carnosol from herbs like sage ( Salvia officinalis ) and rosemary ( Rosmarinus officinalis ) have shown to be strong participants in antioxidant activity of extracts and essential oils from these plants (Lagouri et al ., 1995; Frankel et al ., 1996). More recently potato peels and sugar beet pulp extract (Mohdaly et al ., 2010), bran and stalks from cereals (Esposito et al ., 2005; Lai et al ., 2009; Lerma-Garcia et al ., 2009) and onion skins (Roldan et al ., 2008) have been largely explored as sources of antioxidants that could be used in industry (Shahidi and Wanasundara, 1994; Wanasundara and Shahidi, 1994; Chotimarkorn and Silalai, 2008; Lerma-Garcia et al ., 2009).
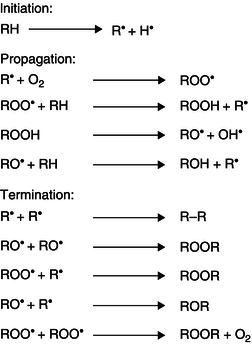
Rancidity or lipid oxidation can take place in three different ways:
These different types of rancidity can yield different products and therefore different sensory appreciation of the food products. For example, microbial rancidity takes place during the ageing of cheeses, developing flavours considered both desirable and/or unpleasant. However, chemical reactions such as hydrolytic or oxidative rancidity yield to mainly undesirable flavour products (Belitz et al ., 2004). Lipid oxidation requires oxygen to take place and can occur through enzymatic hydrolysis (lypoxygenases) or autoxidation. Autoxidation involves free-radical mediated reactions, often catalysed by the presence of metals or by the presence of light (UV) and irradiation. The mechanism of lipid autoxidation is complex and involves a vast number of interrelated reactions of intermediates. Model systems have been used in order to try and determine the mechanistic pathways of autoxidation (Shahidi and Zhong, 2005). The rate of autoxidation has been shown to depend on fatty acid composition, degree of insaturation, the presence of and activity of pro-and anti-oxidants, partial pressure of oxygen, the nature of the surface being exposed to oxygen and the storage conditions (Murado and Vazquez, 2010).
The autoxidation process can be explained more easily through a sequential free radical chain reaction mechanism. This chain reaction mechanism is constituted by three main steps: initiation, propagation and termination (Figure 21.7). The initiation step occurs when a hydrogen atom at α methylene group in double bond of the unsaturated fatty acid is removed to form an alkyl radical (R·). The initiation step is followed by the propagation step where the unstable alkyl radical generated in the initiation step reacts with oxygen (in triplet state) generating a peroxy free radical (Figure 21.8). The peroxy free radical continues reacting, propagating the chain reaction. The chain reaction finishes with the termination step, which occurs when the radicals formed during the propagation step react with other radicals generating stable products. The oxidation products generated (alkyl aldehydes) are responsible of the ‘off-taste’ or rancid flavours. The free radicals generated also react or damage other compounds including vitamins and proteins. The oxidation rate is affected by the number, position and geometry of the double bonds, the position of the fatty acid in the glycerol residue (those in positions 1 and 3 react more easily than those in position 2) and the temperature of the system (at higher temperatures, higher oxidation rates).
Figure 21.7 Elementary steps of the autoxidation of unsaturated lipids.
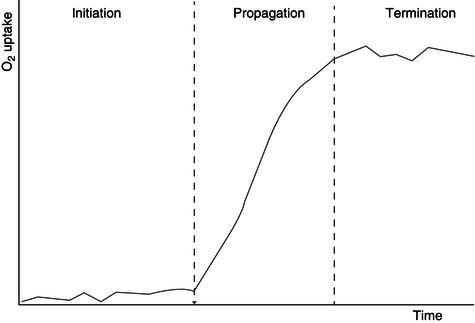
Figure 21.8 Elementary steps of the autoxidation of unsaturated lipids (details on the chain reaction mechanism are given).
The initiation or the rate of lipid oxidation of the propagation can be delayed or slowed down by the presence of antioxidants. Antioxidants work by either inhibiting the formation of free radical lipids in the initiation step or by interrupting propagation of free radical chain. Generally antioxidants are believed to intervene in the chain reaction by donating a hydrogen atom to the peroxy free radicals formed in the propagation step, giving as a result a peroxide and a rather stable radical. However, if this radical is able to trap a second peroxide radical; it is known to be an efficient inhibitor of the chain reaction. In the case of phenolics, flavonoids, ascorbic acid and tocopherols the free radical is stabilised through resonance delocalisation. Since the second half of the twentieth century, it is common practice to add synthetic antioxidants to fats and oils in order to stabilise them. Synthetic antioxidants used by industry include butylated hydroxyanisole (BHA, E-320), butylated hydroxytoluene (BHT, E-321), tertiary butylhydroquinone (TBHT, E-319) and propyl-gallate (E-310). The synthetic antioxidants are effective and cheap; however they are not well regarded by consumers who often prefer more natural products. Therefore research in this field has concentrated in the use of natural sources of antioxidants (spices, herbs, teas, seeds, cereals, grains, fruits and vegetables) as alternatives to the synthetic ones (Yanishlieva and Marinova, 2001). Moreover, mixtures of antioxidants can lead to synergism; so when two or more antioxidants are combined together their overall antioxidant capacity is statistically significantly higher than the addition of their individual antioxidants capacity. Substances such as ascorbyl palmitate, phospholipids or organic acids are known to have this reinforcing effect.
Figure 21.9 Increase of induction time of oxidation (measured as weight gain in %) by the addition of an antioxidant.
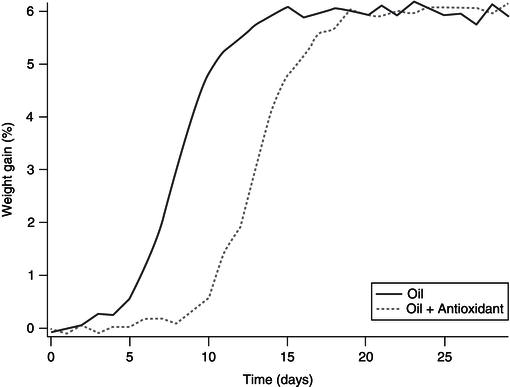
The effectiveness of the added antioxidant is estimated on the basis of the induction period (IP), usually this induction period is determined in time units. The comparison between the actions of various inhibitors in different lipid systems can be carried out by comparing the relative stabilisation factor (F), which is the ratio between the induction period with added antioxidants and the induction period of the control sample as shown in Figure 21.9. There have been many studies on the use of natural antioxidants for the stabilisation of edible oils. There is vast material written on the subject and Table 21.1 summarises the use of phytochemicals to the stabilisation of oil.
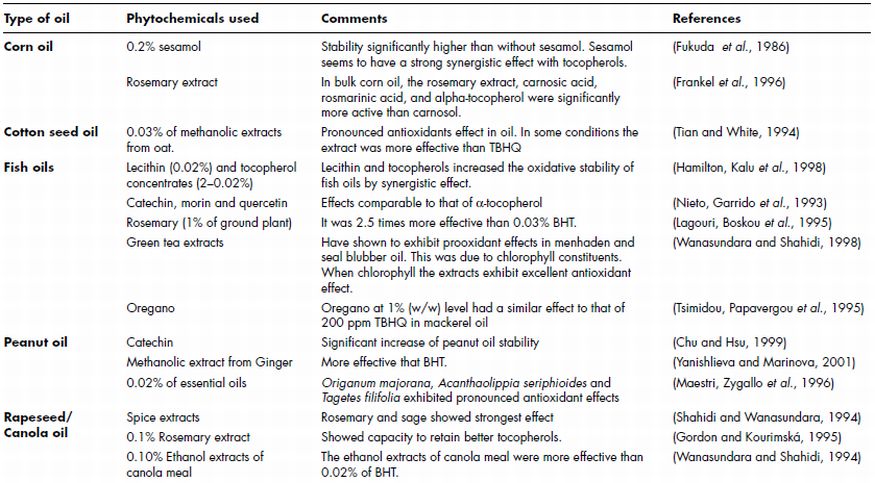
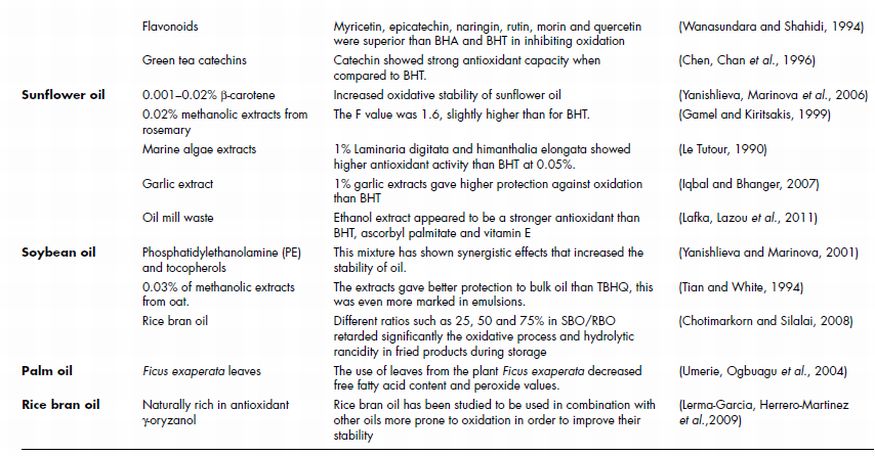
Table 21.1 Summary of studies using phytochemicals for the stabilisation of edible oils
Lipid oxidation during deep-frying takes place easily due to the high temperatures used (140–200 °C) and because the process is a semi-open system where the presence of oxygen from the atmosphere can be transferred at a high rate. Lipid oxidation has a major impact on the quality of the deep-fired product as well as the oil used for frying. Thus oxidation of fats is characterised by chemical changes such as a decrease of total unsaturated fatty acid content and the formation of polar and polymeric products (Yanishlieva and Marinova, 2001). During the frying process, drastic lipid oxidation takes place. Unpleasant flavours can be developed but also important nutrients from the oils can be degraded rapidly (such as tocopherols). Therefore there is an interest in use of antioxidants capable of resisting the frying process. Again extracts from plants have been used for this purpose. Studies on effect of changes in tocopherols in deep-fat-frying have shown that α -tocopherol is lost much faster than β -, γ - or δ -tocopherols, with a reduction of 50% α -tocopherol after four to five frying operations compared with values of about seven and seven to eight frying operations for β - and γ -tocopherol. The presence of rosemary extract in the frying oil has shown to have a marked reduction in the rate of loss of the tocopherols (Gordon and Kourimská, 1995).
There are potential uses of Pandan (Pandanus amaryllifolius) leaf extract in refined, blanched and deodorised palm oil. The extracts (optimum concentration 0.2%) significantly retarded oil oxidation and deterioration (p < 0.05), comparable to 0.02% BHT in tests such as peroxide value, anisidine value, iodine value, free fatty acid and oxidative stability index (OSI) (Nor et al., 2008).
Fried products are susceptible to changes over time in their sensory and nutritional quality as they have a layer of fat/oil covering them. This layer can degrade, taking into consideration that fried products often have a large contact surface and therefore are more prone to oxidation. However, some authors have considered the use of frying process to increase nutritional value (phytochemical content) of certain foods (Saguy and Dana, 2003). Frying has been shown to produce less deterioration of water-soluble nutrients and other phytochemicals (Saguy and Dana, 2003). This higher retention has been explained as being due to the fact that the temperature of frying a product is below 100 °C under the crust region, and the fact that water-soluble nutrients will not leach into the frying oil, which does happen when foods are boiled. However it does have an impact in lipid soluble nutrients. Some authors consider that the use of oils naturally or artificially enriched with phytochemicals could help to nutritionally improve some fried foods. Holland and co-workers studied the effect of oil uptake in French fries (Holland et al., 1991). Holland et al. reported that a portion of 100 g of French fries fired in vitamin E rich corn oil could represent 50% of recommended daily allowance (RDA) of vitamin E. Rossi et al. showed that the stability of vitamin E in vegetable oils during deep-fat-frying of French fries depends basically on two factors: (1) the fatty composition of the oil (in particular polyunsaturated fatty acid content) and (2) the type of vitamin E homologues (tocopherols and tocotrienols) present (Rossi et al., 2007). Another possibility for increasing the oxidative stability of fats and oils has been explored by enriching the food product in antioxidative phytochemicals before the frying step. This has been explored for dough-based fried products. Rice flour containing rice bran powder, which is rich in antioxidant γ -oryzanol (Lai et al., 2009), fried in soybean oil showed better PUFA’s stability and TBARS values were lower (Chotimarkorn and Silalai, 2008).
Recently the influence of microencapsulation and addition of the phenolic antioxidant caffeic acid on the storage stability of olive oil has been reported (Sun-Waterhouse et al., 2011). Olive oil in the absence or presence of 300 ppm caffeic acid and encapsulated in 1.5% w/w sodium alginate shells was compared. The addition of caffeic acid increased the stability and total phenolic content of the final oil product. Oxidation changes were generally slower in the encapsulated oil samples. Both encapsulation and addition of caffeic acid preserved unsaturated fatty acids including C18:1 (omega-9), C18:2 (omega-6) and C18:3 (omega-3). Oil encapsulation method using alginate microspheres was shown to be a feasible approach to increasing olive oil stability. In addition the presence of caffeic acid in the olive oil provides additional protection to the oil and also improves the nutritional value of the final oil product in terms of elevated total phenolic content and desired unsaturated fatty acids (Sun-Waterhouse et al., 2011).
Microencapsulation is a technique widely used in food manufacturing of powdered edible oil products (Gouin, 2004). Microencapsulation allows a prolonged stability over time by protecting core component oils from oxidation caused by light, moisture and oxygen (Heinzelmann and Franke, 1999; Suh et al., 2007; Velasco et al., 2009; Serfert et al., 2010). However, the outer lipid fraction in the surface of the microcapsules is exposed to oxidation during processing or storage (Velasco et al., 2009). In order to improve the stability of microcapsules two main strategies have been pursued: (1) minimising the lipid content on the surface of microcapsules by improving the microencapsulation efficiency (MEE) and (2) the addition of antioxidants to the lipid fraction to delay oxidative processes in the product. In this regard, the effect of microencapsulated γ -oryzanol as an antioxidant was evaluated during the heat treatment of animal fat lard (Suh et al., 2007).
The stability of microencapsulated fish oil and the effect of various antioxidant phytochemicals and relative humidity has been explored by means of PV and TBARS. Without antioxidants, the encapsulated fat was around ten times more stable against oxidation than non-encapsulated fat. Lipophilic antioxidants (such as tocopherols) seem to be more effective than amphiphilic antioxidants (ascorbyl palmitate). Antioxidants were shown to be more efficient at low relative humidity values (Baik et al., 2004).
Fish and flaxseed oils stability against oxidation has been shown to improve by microencapsulation in conjunction with antioxidants. Flaxseed oils with variable levels of vitamin E, rosmarinic acid in addition to carnosic acid for fish oils, were encapsulated and their oxidative stability was tested over time (Barrett et al., 2010). Stability for both fish oils was improved with encapsulation, most significantly for flaxseed oil rather than for fish oil. Fish oil encapsulated with antioxidants had improved stability, mostly significant with carnosic acid. Results were not so promising for flaxseed oils (Barrett et al., 2010).
Natural product extracts showed great potential for their application as antioxidants in microencapsulation of oil products in the food industry (Ahn et al., 2008). The use of natural plant extracts such as rosemary, broccoli sprout and citrus has been shown to effectively inhibit the lipid oxidation of microencapsulated high oleic sunflower oil. High microencapsulation efficiency was achieved using dextrin-coating method with milk protein isolates, soy lecithins and sodium triphosphate as supplements. Stability was tested by using Rancimat method, peroxide value (PV) p-anisidine value (ASV) and showed that induction period was significantly increased in presence of natural product extracts.
In this section we have detailed the stabilisation of fats, frying oils and fried products by using antioxidant phytochemicals. However, often fats are constituents of more complex foods or used for other uses rather than frying (such as sauces, emulsions, spreads/margarines). Oxidation in these products also occurs but it generally takes places at much lower rate due to structural constraints that lead to lower exposure of fat to oxygen (as happens by microencapsulation effect). Nevertheless some of these products are still sensible to oxidation and much effort has been made to avoid their spoilage during storage. The following section will consider the use of certain phytochemicals for the stabilisation of other food products different from fats and oils for frying.
The discoloration or colour change of some food products (in particular fruits and fruit derived products) is a major concern for the food industry (Wrolstad and Wen, 2001). Most of this discoloration and/or browning is caused by exposure to air and subsequent oxidation of colouring compounds into non-coloured compounds. Oxidation of fruits involves an enzyme-catalysed oxidation of phenolic compounds present in the fruit. Browning of a fruit typically occurs following a mechanical injury to the fruit, such as during the harvesting or processing of such foods. Traditionally sulfites have been used to inhibit the enzymatic oxidation and browning in ‘fresh-cut’ and processed fruits. Nevertheless, the increase in regulatory attention and consumer awareness of some risks associated with sulfites has drawn some attention to other anti-oxidation and anti-browning alternatives. Since a segment of the population is hypersensitive to sulfites, therefore, food processors prefer to avoid using sulfite compounds and it has been reported that sulfites can have an increased risk for asthmatic patients (Mathison et al., 1985; Bush et al., 1986). As in the case for other food additives, the food industry is particularly interested in the use of browning inhibitors from natural sources rather than synthetic. Other oxidation and browning inhibitors such as citric acid and phosphates in combination with ascorbic acid, however, are not sufficiently effective. The use of inhibitor 4-hexyl resorcinol is limited in the United States, Canada and some Latin American countries to use with shrimp (Montero et al., 2004). Even if 4-hexyl resorcinol was approved for use in other products it is not certain that it would be used by food processors due to be derived from a synthetic chemical rather than from a natural source (Guandalini et al., 1998; Son et al., 2001). The use of other natural inhibitors of polyphenol oxidases (PPOs) has been motivated by the need to replace sulfating agents in order to prevent or minimise the loss of fresh or processed foodstuffs (Billaud et al., 2003).
Some sulfur-containing substances such as N-acetylcysteine and reduced glutathione are natural compounds with antioxidant properties, and have been proposed as browning inhibitors to prevent darkening in apple, potato and fresh fruit juices (Friedman and Molnar-Perl, 1990; Molnar-Perl and Friedman, 1990; Friedman et al., 1992; Friedman and Bautista, 1995). Sulfur-containing anti-browning additives seem to react with o-quinones formed during the initial phase of enzymatic browning reactions to yield colourless addition products or to reduce o-quinones to diphenols (Richard-Forget et al., 1992). This means that the sulphur containing compounds are not PPO enzymes inhibitors per se, but that they intervene by conjugating with some primary oxidation products formed in this reaction (Richard-Forget et al., 1992; Billaud et al., 2003). Studies have revealed that different PPO enzymes from different plant sources react differently with the same sulphur-containing compound (Sapers and Miller, 1998; Billaud et al., 2003). Fresh-cut apples and pears dipped into an anti-browning solution containing N-acetylcysteine and/or glutathione have been shown to inhibit browning in comparison to non-dipped slices (Rojas-Graü et al., 2006). Pineapple juice has also been shown to inhibit browning and oxidation of fresh fruit (Lozano- De-Gonzalez et al., 1993). The anti-browning/antioxidant effectiveness of pineapple juice is, however, unacceptably variable for use in the food industry. The effectiveness of the pineapple juice as an anti-browning/antioxidising agent varies depending on the type, cultivar and where the pineapple was grown. Specific methods for making natural anti-browning/antioxidant compositions from pineapple juice and/or from pineapple processing plant waste streams have been reported and patented (Wrolstad and Wen, 2001). These compositions are known to comprise S-sinapyl-L-cysteine, N-L- δ -glutamyl-S-sinapyl-L-cysteine and S-sinapyl glutathione as active ingredients.
Onion has been found to have low molecular weight sulphur bioactive compounds capable of reducing enzymatic browning and/or oxidoreductase activity (Eissa et al., 2006). The PPO activities of avocado fruit were significantly reduced by the different onion by-products analysed (Roldan et al., 2008). In addition, some technological and stabilisation processes applied to onion may significantly influence their PPO inhibition capacity. Heated onion extracts have shown to be more effective in prevention of pear and banana browning than fresh onion extracts (Kim et al., 2005). The addition of heated onion extract exhibited stronger inhibitory effect on peach polyphenol oxidase activity than that of the fresh one. The retardation of peach juice browning by onion extract seemed to be caused by inhibition of peach PPO (Kim and Kim, 2007).
The positive effect of a temperature rise in onion extracts towards PPO inhibition of different fruits or vegetables has been widely studied (Ding et al ., 2002; Kim and Kim, 2007; Lee, 2007; Roldan et al ., 2008). For example, higher anti-browning activity was found in sterilised by-products than in pasteurised and frozen ones. On the other hand, sterilisation of sugar rich products (such as onion) induces the formation of caramelisation and Maillard reaction products with marked sensory properties that could influence final product quality. Therefore milder processes like pasteurisation have been suggested as a more suitable choice in order to develop a food ingredient with an interesting added anti-browning property (Roldan et al ., 2008). In addition the safety of the food ingredient could be maintained by the thermal treatment.
Although PPO normally are able to oxidise phenolic compounds, certain phenolic acids are able to inhibit PPO activity by binding to the active site of the enzyme (Janovitz-Klapp et al ., 1990). Kojic acid (5-hydroxy-2-(hydroxymethyl)-4-pyrone) is a phenolic acid from fungal origin produced by many species of Aspergillus and Penicillium that it has been suggested acts as an inhibitor to the PPO enzyme (Chen et al ., 1991; Iyidogan and BayIndIrlI, 2004). Chen et al . suggested that the mechanism action of Kojic acid is probably due to its capacity to interfer with the uptake of O 2 required for the enzyme reaction (Chen et al ., 1991). This reduces o-quinones to diphenols preventing the formation of melanin via polymerisation and/or by combination of the two previous processes (Chen et al ., 1991). Son et al . reported that the minimal concentration of kojic acid for effective anti-browning activity on fresh-cut apples is similar to the concentration for commercial anti-browning additives such as oxalic and cysteine (Son et al ., 2001). Other phenolic acids such as p -Coumaric acid, ferulic acid, cinnamic acid and gallic acid showed similar inhibitory activity to ascorbic acid, but chlorogenic acid and caffeic acid were much weaker ( p < 0.05) (Son et al ., 2001).
As mentioned already, the enzymatic browning is often inhibited by a direct immersion of the fruit pieces in an aqueous solution of anti-browning agents. On the other hand, the use of edible coatings as carriers of anti-browning agents for fresh-cut products has also been investigated (Baldwin et al., 1996; Rojas-Graü et al., 2006; Montero-Calderón et al., 2008; Oms-Oliu et al., 2008; Oms-Oliu et al., 2008; Rojas-Graü et al., 2008; Rojas-Graü et al., 2009; Oms-Oliu et al., 2010). Baldwin et al. (1996) reported improved browning inhibition on fresh-cut apples by ascorbic acid incorporated into an edible coating formulation when compared to the apples dipped directly into an aqueous solution of the very same compound (Baldwin et al., 1996). Other authors have reported improved browning inhibition in fresh-cut apples, pears and papayas coated with alginate and gellan based coatings with presence of thiol containing compounds N-acetylcysteine and glutathione (Rojas-Graü et al., 2006; Rojas-Graü et al., 2007; Tapia et al., 2007; Tapia et al., 2008). Some polysaccharide-based coatings (alginate based) are applied in a first step and the anti-browning agents are incorporated afterwards in a dipping solution containing calcium for cross-linking and instant gelation of the coating (Wong et al., 1994; Lee et al., 2003). Increased levels of vitamin C and total phenolic content were observed in pear wedges coated with alginate, gellan and pectin including N-acetylcysteine and glutathione compared with control samples (Oms-Oliu et al., 2008). Tapia and co-workers reported that the addition of ascorbic acid in alginate- and gellan-based coatings helped to preserve the natural ascorbic acid content of fresh-cut papaya (Tapia et al., 2008). The effect of application of a chitosan coated film on enzymatic browning of litchi (Litch chinensis Sonn.) fruit was studied by Zhang and Quantick (1997). It was reported that chitosan film coating delayed changes in contents of anthocyanins, flavonoids and total phenolics (Zhang and Quantick, 1997; Jiang et al., 2005). It also delayed the increase in polyphenol oxidase activity and partially inhibited the increase in peroxidase activity. These authors further reported that application of chitosan may form a layer of film on the outer pericarp surface, thus resulting in less browning. The mechanism of action of chitosan is unclear, but may involve adsorption of PPO, its substrates or products, or a combination of such processes.
The impact of protein oxidation on the quality of meat and meat products is manifested by the presence of a free radical chain similar to those described for lipids. Oxidative degradation of meat proteins involves the modification of amino acid side chains leading to the formation of carbonyl compounds (Xiong, 2000; Stadtman and Levine, 2003). The discolouration of raw burger patties is generally attributed to the oxidation of ferrous heme–iron (Fe 2+ ) into its ferric form (Fe 3+ ) in proteins induced by lipid products (Yin and Faustman, 1993). Oxymyoglobin is transformed into metmyoglobin and consequently the colour changes the characteristic bright red colour of fresh meat to brownish colour, often considered as undesirable (except for aged beef or game). The oxidising proteins could affect the tenderness of fresh pork during chill storage and protein oxidation has been suggested to likely impact certain sensory quality attributes such as colour, texture and flavour of cured products (Ventanas et al ., 2007). Other meat products such as burger patties are even more susceptible to oxidation as mincing and salt addition might promote oxidative reactions (Ladikos and Lougovois, 1990). Ganhao et al . (2010) reported significant increases of protein carbonyl during chill storage of burger patties (Ganhão et al ., 2010). In parallel, an intense loss of redness and increase in hardness was found to take place throughout the refrigerated storage. The effect of several fruit extracts and quercetin in these trends showed that most phenolic rich wild Mediterranean fruit extracts as well as quercetin reduced the formation of protein carbonyls and inhibited the colour and texture deterioration during refrigerated storage (Ganhão et al ., 2010). Yin and Cheng (2004) showed that isolated sulphur containing compounds from garlic (diallyl sulfide, diallyl disulfide, s-ethyl cysteine and n-acetyl cysteine) inhibited discoloration of ground beef. The exogenous addition of these garlic-derived compounds delayed oxymyoglobin formation significantly (Yin and Cheng, 2003; Sallam et al ., 2004; Bozin et al ., 2008). In addition, Yin and Cheng showed that these sulphur compounds significantly inhibited the growth of pathogenic bacteria such as Salmonella typhimurium , Escherichia coli O157:H7, Listeria monocytogenes , Staphyllococcus aureus and Campylobacter jejuni , suggesting that these compounds are interesting for microbiological safety and extending the shelf life of several food products (Yin and Cheng, 2003). The use of phytochemicals as antimicrobials to extend shelf life is considered in the section 21.4.4.2.
Antimicrobial compounds present in essential oils from certain plants can be used to extend the shelf life of foods (Holley and Patel, 2005; Gutierrez et al ., 2008; Gutierrez et al ., 2009; Tiwari et al ., 2009). These antimicrobials extend the shelf life by reducing microbial growth rate or viability (Tiwari et al ., 2009). When used as food additives, essential oils might sometimes only be effective in high concentrations (1–3%). These concentrations are often above those that are organoleptic acceptable (Lis-Balchin et al ., 1998; Arora and Kaur, 1999; Tajkarimi et al ., 2010). The presence of fat, carbohydrate, protein, salt and pH reaction influence the effectiveness of these agents in foods (Holley, 2005). There are many examples of antimicrobial inactivation of essential oils on foods in order to improve their shelf life. The use of essential oils as antimicrobials to improve shelf life were reviewed by Burt (2004) and later by Holley and co-workers (Holley and Patel, 2005) who highlighted two examples where spice/herbal materials have been successfully used as either a dip on poultry carcasses (Dickens and Ingram, 2001) or as a surface coating on salt water fish (Harpaz et al ., 2003). In particular some phytochemicals have shown strong antimicrobial properties and consequently their mechanism of action has been studied in more detail. This is the case for allyl isothiocyanate (AIT) commonly found in mustard and horseradish oil; diallyl sufide and diallyl disulfide from garlic and related alliums; eugenol from clove, carvone from spearmint; cinnemaldehyde from cinnamon and carvacrol and thymol from oregano and thyme, respectively.
Due to the great antimicrobial activity that garlic and onion possess, both vegetables could be used as natural preservatives, to control the microbial growth (Pszczola, 2002). Chemical characterisation sulphur compounds contained in garlic have allowed the statement that they are the main active antimicrobial agents (Tsao and Yin, 2001; Rose et al., 2005). However, other compounds such as proteins, saponins and phenolic compounds can also contribute to this activity (Griffiths et al., 2002). Garlic has been proven to inhibit the growth of grampositive, gram-negative and acid-fast bacteria, as well as toxin production. Bacteria against which garlic is effective include strains of Pseudomonas, Proteus, Escherichia coli, Staphylococcus aureus, Klebsiella, Salmonella, Micrococcus, Bacillus subtilis, Mycobacterium and Clostridium (Delaha and Garagusi, 1985), some of which are resistant to penicillin, streptomycin, doxycilline and cephalexin, among other antibiotics.
Allyl isothiocyanate is derived from the glucosinolate sinigrin found in plants of the family Brassicaceae. It is a well-recognised antimicrobial agent against a variety of organisms, including food-borne pathogens such as Escherichia coli O157:H7 (Luciano and Holley, 2009). Interestingly allyl isothiocyanate has been found to be generally more effective against gram-negative bacteria with less or no effect on LAB. AIT possesses strong antimicrobial activity against E. coli O157:H7 as well as V. parahaemolyticus in ground beef (200–300 ppm) after 21 days at 4 °C (Nadarajah et al., 2005). AIT, along with other thiocyanates, is known to react with thiols and sulphydryls as well as terminal amino acids, and these reactions may contribute to its loss from products during storage (Ward et al., 1998; Wang, 2003; Wang and Chen, 2010; Wang et al., 2010). In addition allyl isothiocyanate has been reported to have bactericidal effects against Helicobacter pylori, which has been investigated due to its association with infections and upper gastrointestinal diseases, such as chronic gastritis, peptic ulcer and gastric cancer (Shin et al., 2004).
The term nutraceutical was coined as a contraction of nutritional and pharmaceutical by DeFelice and the Foundation for Innovation medicine (Wildman, 2007). At the present time there is no universally accepted definition but the term has evolved since it was first coined and nowadays it is generally accepted that a nutraceutical is any substance that may be considered a food or part of a food and that provides medical or health benefits, including the prevention and treatment of disease. Such products may range from isolated nutrients, dietary, supplements and diets to genetically engineered foods, herbal products and processed foods such as cereals, soups and beverages (Wildman, 2007). On the other hand, functional foods are generally considered to be ‘foods or dietary components that may provide a health benefit beyond basic nutrition’ (Wildman, 2007).
Phytosterols are plant derived compounds with a similar chemical structure and function to cholesterol. Many clinical trials have reported a cause and effect relationship between the consumption of plant sterols and the reduction of blood cholesterol levels. Phytosterols inhibit the intestinal absorption of cholesterol. Some foods are naturally rich in phytosterols like unrefined vegetable oils, whole grains, nuts and legumes and some of these products have been used as ingredients for processed foods and beverages with added plant sterols or stanols. Many of these products are now available in many countries, and many countries allow health claims for such commercial products. Presently the EU regulatory authorities on food and feed safety have raised and registered 14 questions regarding the use of phytosterols. Ten of these questions have been assed and a scientific opinion has been published; in particular two questions regarding the use of health claims that phytosterols in functional foods reduce blood cholesterol levels have been approved (EFSA Journal, 2010, 8(10), 1813–1835). On the other hand, two regarding the effect of phytosterols on prostate cancer have been rejected (EFSA Journal, 2010, 8(10), 1813–1835). Three are still under consideration and one, regarding the use of phytosterols in low fat fermented milk product, has been withdrawn by the application (EFSA-Q-2008-3823).
Resveratrol is a phenolic compound commonly found in grapes, red wine and berries. Resveratrol has been considered among certain authors to be responsible of the ‘French paradox’: the fact that French nationals have lower incidence of heart disease than other Westerners despite high red wine consumption. However some other authors claim that resveratrol is not present in sufficient quantities in red wine to explain this paradox. Resveratrol is well-absorbed when taken orally, but is also rapidly metabolised and eliminated. Cellular and animal models have shown very remarkable results on the capacity of resveratrol to inhibit the growth of cancer cells and to increase lifespan of animal models, but to date little is known about the effect of resveratrol in humans. However this has not stopped the food industry from commercialising beverages and supplements rich in resveratrol and claiming its health benefits. Indeed there are four registered questions by the EU regulatory authorities in food and feed safety on the health claims of resveratrol. Two of them have been assessed and rejected on the basis that the data supplied by applications were insufficient to explain the cause and effect relationship between the consumption of resveratrol and health benefits. Both of the rejected questions assumed that the mechanism of action of resveratrol is based in its antioxidant activity, an assumption that EFSA scientific panel considers insufficient to justify several potential health benefits. The other two questions are still under consideration: one related to cardiovascular health. Nevertheless, in 2009, the Food and Drug Administration of the US considered the status of resveratrol and it was upgraded from GRAS (generally recognised as safe) status to novel food. On the other hand, the Danish Council for strategic research announced in February 2011 that it will commit € 2.47 million to a complete resveratrol study investigating multiple metabolic syndrome endpoints, including obesity, type-2 diabetes and osteoporosis, for the next five years.
Isoflavones are a class of phytoestrogens (estrogens from plant origin). Soy and soy products are comparatively rich sources of isoflavones in the human diet. It has been claimed that isoflavones are beneficial for the prevention of cardiovascular diseases, hormone associated cancers, osteoporosis, cognitive decline and the treatment of menopausal symptoms. However, in most cases mixed results have been reported. Some of the initial assumptions of the beneficial effects of isoflavones were based on the review of epidemiological studies (Clarkson, 2002; Messina et al., 2004; Cassidy and Hooper, 2006) and these assumptions were not verified with later clinical trials (Lichtenstein et al., 2002; Weggemans and Trautwein, 2003; Dewell et al., 2006; Brink et al., 2008). It has been suggested that differences in results obtained by several studies are due to the significant differences in bioavailability of isoflavones in function of the food matrix used for their delivery (de Pascual-Teresa et al., 2006).
Most of the nutraceutical applications of isoflavones or soy protein (rich in isoflavones) are related to dairy-like products such as milk-soy, soy drinks and beverages, yogurts and dairy desserts. At the present time there are 17 registered health claims applications that have been submitted to EFSA. Three have already been reviewed; two are related to bone health and the third to the antioxidant capacity of isoflavones. All three have been rejected by the scientific panel who considered that in the applications there was insufficient data to explain cause and effect relationship between isoflavones and the claimed health benefits (EFSA Journal, 2009, 7(9), 1267–1282; EFSA Journal, 2009, 7(9), 1270–1284; EFSA Journal 2010, 8(2), 1493–1515).
Polysaccharides of D-glucose, linked by β -glycosidic bonds or β -glucans, are a wide range of molecules of different molecular size. Therefore β -glucan rich fractions have significant differences in solubility and viscosity. β -glucans are found in the bran of many cereal grains and the cell wall of mushrooms. Most nutraceutical applications of β -glucans are oat based. Commonly a soluble fibre rich in β -glucans (above 30%) is introduced into a food formulation, such as smoothies, ready-meals, condiments, dressings and bakery products. Most of these products are low in fat and fine products that claim to be beneficial to heart health. There are 34 registered applications to be reviewed by EFSA which are related to β -glucans. Many scientific opinions have delivered; those relating the mechanism of action to β -glucans to their antioxidant capacity have been rejected. There are many still under revision and there is one, approved in December 2010, which links the consumption of oat β -glucans. The report from the scientific panel states that a cause and effect relationship has been established between the consumption of oat β -glucan and lowering of blood LDL-cholesterol concentrations. Therefore the scientific panel considered that ‘ Oat beta-glucan has been shown to lower/reduce blood cholesterol. Blood cholesterol lowering may reduce the risk of (coronary) heart disease’ (EFSA Journal 2010, 8(12), 1885–1900). This confirms earlier health claims from national food and safety authorities, such as the French and Swedish, that approved the claim of cholesterol lowering of oat β -glucan in 2009.
Nowadays there are a wide range of supplements rich in flavonoids from numerous plant extracts (tea, berries, grape and herbs) that can be easily found in so-called health stores. Also some drink and beverage companies are using flavonoids to target the healthy product market. Large worldwide producers of soft and fizzy drinks have been launching variations of their existing products but enriched with polyphenols. This is the case for Coca Cola company, that launched in October 2007 Diet Coke plus Antioxidants in the UK, a year later in France and has recently launched in Brazil. Some large drink and beverage companies have shown their interest in research on the bioavailability of polyphenols for this kind of drink (Borges et al., 2010).
The fortification or enrichment of polyphenols has been hindered by the chemical properties of some polyphenols, which are prone to interact with proteins (Labuckas et al ., 2008; Han et al ., 2011). These reactions can have significant effects in nutritional and sensory quality of the enriched products. However the use of polyphenols in cheese has been explored lately. Purified phenolic compounds such as catechin, epigallocatechin gallate (EGCG), tannic acid, homovanillic acid, hesperetin and natural extracts rich in polyphenols like grape, green tea or cranberry extract were added to a prepared cheese. They were shown to have an impact in the retention time of the cheese curds and their gel-formation behaviours. The authors observed that the effects were related to molecular properties and in particular hydrophobicity of phenolic compounds (Han et al ., 2011). There are at least 44 health claim applications for flavonoids registered by EFSA. All the claims that related their health benefits to antioxidant capacity have been rejected (as mentioned for many other phytochemicals). There are 18 applications still under revision and at least one application that has been withdrawn.
The cosmetic industry uses phytochemicals or extracts from phytochemical rich plants in order to improve the sensory attributes of the product and/or to improve the technical properties of a formulation. For example, polysaccharides are commonly used for stabilising emulsions, foams and gels in many cosmetic products. Phenolic compounds and some carotenoids are used in cosmetics that protect skin from UV-light. These compounds absorb in the UV region, creating the effective radiation filter for sunscreens. Some of the phytochemicals used in the cosmetic industry are triterpene saponins (Balandrin, 1996; Ceppi, 1998) and phenylethanoid glycosides. Triterpene saponins are triterpenes that belong to the group of saponins. The amphiphilic characteristics of these compounds makes them an important surface active compound potentially interesting for stabilising complex dispersed systems such as emulsions and foams (Sarnthein-Graf and La Mesa, 2004; Wang et al., 2005). In addition saponins in general have been shown to possess a vast array of chemical/biological properties and were reviewed by Francis et al. (2002).
Phenylethanoid glycosides are a type of phenylpropanoid and therefore they hold a series of antioxidant, antimicrobial and colour stabilising activities (inhibitory properties of tyrosinases) (Kurkin, 2003). In addition to these general properties phenylethanoids have shown great potential for commercial/industrial exploitation in the cosmetics industry (Kurkin, 2003; Fu et al., 2008). Due to their ability to inhibit the 5 α reductase enzyme the phenylethanoid glycosides present anti-seborrheic properties and therefore are of great use in the cosmetics industry for development of skin and hair care products (Korkina, 2007).
Some phytochemicals have been shown to present anti-nematode activities. Nematodes represent a serious threat for plants in agronomics. In recent years new methods of pest control using more environmentally friendly (or natural) pesticides have been proposed. Although these are never as effective as the synthetic ones, they could help to reduce the amount of less environmental friendly pesticides used in farming. Integrated pest management, transgenic plant resistance and biological control strategies are being investigated as methods of control (Ghisalberti and Atta, 2002). Glucosinolates, glucosinolate derived compounds, alkaloids, terpenes, phenylpropanoids and sesquiterpenes are some of the natural products from plant origin that have shown biopesticide properties (Ujváry and Robert, 2009; Gonzalez-Coloma et al., 2010; Oka, 2010).
Abraham, T.W., Cameron, D.C. et al. (2005) Beverage compositions comprising monatin and methods of making same U.S.P. Office. United States, Cargill, Inc.: 81.
Ahn, J.-H., Kim, Y.-P. et al. (2008) Antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil . Journal of Food Engineering, 84 (2), 327–334.
Akamatsu, H., Komura, J. et al. (1991) Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation . Planta Medica, 57 (2), 119–121.
Akhtar, S. and Ashgar, A. (2011) Mineral Fortification of whole wheat flour: an overview. In: V.R. Preedy, R.R. Watson and V.B. Patel (eds) Flour and Breads and their Fortification in Health and Disease Prevention, San Diego, Academic Press, pp. 263–271.
Arora, D.S. and Kaur, J. (1999) Antimicrobial activity of spices . International Journal of Antimicrobial Agents, 12 (3), 257–262.
Aruoma, O.I. and Spencer, J.P.E. et al. (1997) Characterization of food antioxidants, illustrated using commercial garlic and ginger preparations . Food Chemistry, 60 (2), 149–156.
Assadi-Porter, F.M., Aceti, D.J. et al. (2000) Efficient Production of Recombinant Brazzein, a Small, Heat-Stable, Sweet-Tasting Protein of Plant Origin . Archives of Biochemistry and Biophysics, 376 (2), 252–258.
Azeredo, H.M.C. (2009) Betalains: properties, sources, applications, and stability – a review . International Journal of Food Science and Technology, 44 (12), 2365–2376.
Baik, M., Suhendro, E. et al. (2004) Effects of antioxidants and humidity on the oxidative stability of microencapsulated fish oil . Journal of the American Oil Chemists’ Society, 81 (4), 355–360.
Balandrin, M.F. (1996) Commercial utilization of plant-derived saponins: an overview of medicinal, pharmaceutical, and industrial applications . Advances in Experimental Medicine and Biology, 404 , 1–14.
Baldwin, E.A., Nisperos, M.O. et al. (1996) Improving storage life of cut apple and potato with edible coating . Postharvest Biology and Technology, 9 (2), 151–163.
Barrett, A.H., Porter, W.L. et al. (2010) Effect of various antioxidants, antioxidant levels, and encapsulation on the stability of fish and flaxseed oils: assessment by fluorometric analysis. Journal of Food Processing and Preservation, no.
Belitz, H.–D., Grosch, W. et al. (eds) (2004) Food Chemistry, Berlin, Springer-Verlag.
Billaud, C., Roux, E. et al. (2003) Inhibitory effect of unheated and heated -glucose, -fructose and -cysteine solutions and Maillard reaction product model systems on polyphenoloxidase from apple. I. Enzymatic browning and enzyme activity inhibition using spectrophotometric and polarographic methods . Food Chemistry, 81 (1), 35–50.
Borges, G., Mullen, W. et al. (2010) Bioavailability of multiple components following acute ingestion of a polyphenol-rich juice drink . Molecular Nutrition and Food Research, 54 (S2): S268–S277.
Bozin, B., Mimica-Dukic, N. et al. (2008) Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chemistry, 111 (4), 925–929.
Brink, E., Coxam, V. et al. (2008) Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study . The American Journal of Clinical Nutrition, 87 (3), 761–770.
Burt, S. (2004) Essential oils: their antibacterial properties and potential applications in foods: A review . International Journal of Food Microbiology, 94 (3), 223–253.
Bush, R.K., Taylor, S.L. et al. (1986) Prevalence of sensitivity to sulfiting agents in asthmatic patients . The American Journal of Medicine, 81 (5), 816–820.
Caldwell, J.E., Abildgaard, F. et al. (1998) Solution structure of the thermostable sweet-tasting protein brazzein . Nature Structural Biology, 5 (6), 427–431.
Cassidy, A. and L. Hooper (2006) Phytoestrogens and cardiovascular disease . Journal of the British Menopause Society, 12 ( 2), 49–56.
Ceppi, P. (1998) Uso de las saponinas en cosemticos. Faculty of Engineering. Santiago, Chile, Catholic University of Chile. B.Sc. thesis.
Chen, J.S., Wei, C.-i. et al. (1991) Inhibitory effect of kojic acid on some plant and crustacean polyphenol oxidases . Journal of Agricultural and Food Chemistry, 39 (8), 1396–1401.
Chen, J.S., Wei, C.I. et al. (1991) Inhibition mechanism of kojic acid on polyphenol oxidase . Journal of Agricultural and Food Chemistry, 39 (11), 1897–1901.
Chotimarkorn, C. and Silalai, N. (2008a) Addition of rice bran oil to soybean oil during frying increases the oxidative stability of the fried dough from rice flour during storage . Food Research International, 41 (3), 308–317.
Chotimarkorn, C. and Silalai, N. (2008b) Oxidative stability of fried dough from rice flour containing rice bran powder during storage . LWT – Food Science and Technology, 41 (4), 561–568.
Christensen, L.P., Ronald Ross, W. et al . (2010) Bioactivity of Polyacetylenes in food plants. In: V.R. Preedy, R.R. Watson and V.B. Patel (eds) Bioactive Foods in Promoting Health , San Diego, Academic Press, pp. 285–306.
Clarkson, T.B. (2002) Soy, soy phytoestrogens and cardiovascular disease . The Journal of Nutrition, 132 (3), 566S–569S.
Cowan, M.M. (1999) Plant Products as Antimicrobial Agents . Clinical Microbiology Review , 12 (4), 564–582.
de Pascual-Teresa, S., Hallund, J. et al. (2006) Absorption of isoflavones in humans: effects of food matrix and processing . The Journal of Nutritional Biochemistry, 17 (4), 257–264.
Delaha, E.C. and Garagusi, V.F. (1985) Inhibition of mycobacteria by garlic extract (Allium sativum) . Antimicrob Agents Chemotherapy, 27 (4), 485–486.
Dewell, A., Hollenbeck, P.L.W. et al. (2006) A critical evaluation of the role of soy protein and isoflavone supplementation in the control of plasma cholesterol concentrations . Journal of Clinical Endocrinology and Metabolism, 91 (3), 772–780.
Dickens, J.A. and Ingram, K.D. (2001) Efficacy of an Herbal extract, at various concentrations, on the microbiological quality of broiler carcasses after simulated chilling . Journal of Applied Poultry Research, 10 (2), 194–198.
Ding, C.-K., Chachin, K. et al. (2002) Inhibition of loquat enzymatic browning by sulfhydryl compounds . Food Chemistry, 76 (2), 213–218.
Dixon, R.A., Dey, P.M. et al. (2006) Phytoalexins: Enzymology and Molecular Biology, New York, John Wiley & Sons, Inc.
Eissa, H.A., Fadel, H.H.M. et al. (2006) Thiol containing compounds as controlling agents of enzymatic browning in some apple products . Food Research International, 39 (8), 855–863.
Esposito, F., Arlotti, G. et al. (2005) Antioxidant activity and dietary fibre in durum wheat bran by-products . Food Research International, 38 (10), 1167–1173.
Fan, P., Bracken, C. et al. (1993) Structural characterization of monellin in the alcohol-denatured state by NMR: Evidence for .beta.-sheet to .alpha.-helix conversion . Biochemistry, 32 (6), 1573–1582.
Francis, G., Kerem, Z. et al. (2002) The biological action of saponins in animal systems: a review . British Journal of Nutrition, 88, 587–605.
Frankel, E.N., Huang, S.-W. et al. (1996) Antioxidant Activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion . Journal of Agricultural and Food Chemistry, 44 (1), 131–135.
Friedman, M. and Bautista, F.F. (1995) Inhibition of Polyphenol Oxidase by Thiols in the Absence and Presence of Potato Tissue Suspensions . Journal of Agricultural and Food Chemistry, 43 (1), 69–76.
Friedman, M. and Molnar-Perl I. (1990). “ Inhibition of browning by sulfur amino acids. 1. Heated amino acid-glucose systems .” Journal of Agricultural and Food Chemistry, 38 (8), 1642–1647.
Friedman, M., I. Molnár-Perl, et al. (1992). “ Browning prevention in fresh and dehydrated potatoes by SH-containing amino acids .” Food Addit Contam. 9 (5), 499–503.
Fu, G., H. Pang, et al. (2008). “ Naturally Occurring Phenylethanoid Glycosides, Potential Leads for New Therapeutics ” Curr Med Chem. 15 (2), 2592–2612.
Ganhão, R., D. Morcuende, et al. (2010). “ Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage .” Meat Science 85 (3), 402–409.
Gao, G.-H., J.-X. Dai, et al. (1999). “ Solution conformation of brazzein by 1 H nuclear magnetic resonance: resonance assignment and secondary structure .” International Journal of Biological Macromolecules 24 (4), 351–359.
Ghisalberti, E.L. and R. Atta (2002). Secondary metabolites with antinematodal activity. Studies in Natural Products Chemistry , Elsevier. Volume 26 , Part 7, 425–506.
Gibbs, B.F., I. Alli, et al. (1996). “ Sweet and taste-modifying proteins: A review .” Nutrition Research 16 (9), 1619–1630.
Gonzalez-Coloma, A., M. Reina, et al. (2010). Natural Product-Based Biopesticides for Insect Control. Comprehensive Natural Products II. Oxford, Elsevier: 237–268.
Gordon, M.H. and L. Kourimská (1995). “ Effect of antioxidants on losses of tocopherols during deep-fat frying .” Food Chemistry 52 (2), 175–177.
Gouin, S. (2004). “ Microencapsulation: industrial appraisal of existing technologies and trends .” Trends in Food Science & Technology NFIF part 2 15 (7–8), 330–347.
Griffiths, G., L. Trueman, et al. (2002). “ Onions—A global benefit to health .” Phytotherapy Research 16 (7), 603–615.
Guandalini, E., A. Ioppolo, et al. (1998). “ 4-hexylresorcinol as inhibitor of shrimp melanosis: Efficacy and residues studies; evaluation of possible toxic effect in a human intestinal in vitro model (caco-2); preliminary safety assessment .” Food Additives and Contaminants 15 (2), 171–180.
Gutierrez, J., C. Barry-Ryan, et al. (2008). “ The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients .” International Journal of Food Microbiology 124 (1), 91–97.
Gutierrez, J., C. Barry-Ryan, et al. (2009). “ Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components .” Food Microbiology 26 (2), 142–150.
Han, J., M. Britten, et al. (2011). “ Effect of polyphenolic ingredients on physical characteristics of cheese .” Food Research International 44 (1), 494–497.
Han, J., M. Britten, et al. (2011). “ Polyphenolic compounds as functional ingredients in cheese .” Food Chemistry 124 (4), 1589–1594.
Harpaz, S., L. Glatman, et al. (2003). “ Effects of Herbal Essential Oils Used To Extend the Shelf Life of Freshwater-Reared Asian Sea Bass Fish (Lates calcarifer) .” Journal of Food Protection 66 , 410–417.
Heinzelmann, K. and K. Franke (1999). “ Using freezing and drying techniques of emulsions for the microencapsulation of fish oil to improve oxidation stability .” Colloids and Surfaces B: Biointerfaces 12 (3–6), 223–229.
Hellekant, G. r. and V. Danilova (2005). “ Brazzein a Small, Sweet Protein, Discovery and Physiological Overview .” Chemical Senses 30 (suppl 1), i88–i89.
Ho, C.T., C.W. Chen, et al. (1997). Natural Antioxidants from tea. Natural Antioxidants, Chemistry, Health Effects and Applications. F. Shahidi. Champaign, Illinois, AOCS Press, 213–223.
Holland, B., A.A. Welch, et al. (1991). The composition of foods, Cambridge, UK: Royal Society of Chemistry., 10–21.
Holley, R.A. and D. Patel (2005). “ Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials .” Food Microbiology 22 (4), 273–292.
Iyidogan, N.F. and A. BayIndIrlI (2004). “ Effect of -cysteine, kojic acid and 4-hexylresorcinol combination on inhibition of enzymatic browning in Amasya apple juice .” Journal of Food Engineering 62 (3), 299–304.
Janovitz-Klapp, A.H., F.C. Richard, et al. (1990). “ Inhibition studies on apple polyphenol oxidase .” Journal of Agricultural and Food Chemistry 38 (4), 926–931.
Jiang, Y., J. Li, et al. (2005). “ Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature .” LWT – Food Science and Technology 38 (7), 757–761.
Kim, C.Y. and M.J. Kim (2007). “ Inhibition of Polyphenol Oxidase and Peach Juice Browning by Onion Extract .” Food Science and Biotechnology 16 (3), 421–425.
Kim, M.-J., C.Y. Kim, et al. (2005). “ Prevention of enzymatic browning of pear by onion extract .” Food Chemistry 89 (2), 181–184.
Kobaisy, M., Z. Abramowski, et al. (1997). “ Antimycobacterial Polyynes of Devil’s Club (Oplopanax horridus), a North American Native Medicinal Plant .” Journal of Natural Products 60 (11), 1210–1213.
Korkina, L.G. (2007). “ Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health .” Cellular and Molecular Biology 53 (1), 15–25.
Kurihara, Y. (1992). “ Characteristics of antisweet substances, sweet proteins, and sweetness-inducing proteins .” Critical Reviews in Food Science and Nutrition 32 (3), 231–252.
Kurkin, V.A. (2003). “ Phenylpropanoids from Medicinal Plants: Distribution, Classification, Structural Analysis, and Biological Activity .” Chemistry of Natural Compounds 39 (2), 123–153.
Labuckas, D.O., D.M. Maestri, et al. (2008). “ Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins .” Food Chemistry 107 (2), 607–612.
Ladikos, D. and V. Lougovois (1990). “ Lipid oxidation in muscle foods: A review .” Food Chemistry 35 (4), 295–314.
Lagouri, V., D. Boskou, et al. (1995). Screening for antioxidant activity of essential oils obtained from spices. Developments in Food Science, Elsevier. Volume 37 , Part 1 , 869–879.
Lai, P., K.Y. Li, et al. (2009). “ Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran .” Food Chemistry 117 (3), 538–544.
Lee, J.Y., H.J. Park, et al. (2003). “ Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents .” Lebensmittel-Wissenschaft und-Technologie 36 (3), 323–329.
Lee, M.–K. (2007). “ Inhibitory effect of banana polyphenol oxidase during ripening of banana by onion extract and Maillard reaction products .” Food Chemistry 102 (1), 146–149.
Lerma-Garcia, M.J., J.M. Herrero-Martinez, et al. (2009). “ Composition, industrial processing and applications of rice bran gamma-oryzanol .” Food Chemistry 115 (2), 389–404.
Lichtenstein, A.H., S.M. Jalbert, et al. (2002). “ Lipoprotein Response to Diets High in Soy or Animal Protein With and Without Isoflavones in Moderately Hypercholesterolemic Subjects .” Arterioscler Thromb Vasc Biol 22 (11), 1852–1858.
Lis-Balchin, M., G. Buchbauer, et al. (1998). “ Antimicrobial activity of Pelargonium essential oils added to a quiche-filling as a model food system .” Letters in Applied Microbiology 27 (4), 207–210.
Liu, X., S. Maeda, et al. (1993). “ Purification, complete amino acid sequence and structural characterization of the heat-stable sweet protein, mabinlin II .” European Journal of Biochemistry 211 (1–2), 281–287.
Lozano-De-Gonzalez, P.G., D.M. Barrett, et al. (1993). “ Enzymatic Browning Inhibited in Fresh and Dried Apple Rings by Pineapple Juice .” Journal of Food Science 58 (2), 399–404.
Luciano, F.B. and R.A. Holley (2009). “ Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7 .” International Journal of Food Microbiology 131 (2–3), 240–245.
Mathison, D.A., D.D. Stevenson, et al. (1985). “ Precipitating Factors in Asthma .” Chest 87 (1 Supplement), 50S–54S.
Meot-Duros, L., S. Cérantola, et al. (2010). “ New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract .” Food and Chemical Toxicology 48 (2), 553–557.
Messina, M., S. Ho, et al. (2004). “ Skeletal benefits of soy isoflavones: a review of the clinical trial and epidemiologic data .” Current Opinion in Clinical Nutrition & Metabolic Care 7 (6), 649–658.
Ming, D. and G. Hellekant (1994). “ Brazzein, a new high-potency thermostable sweet protein from Pentadiplandra brazzeana B .” FEBS Letters 355 (1), 106–108.
Mohdaly, A.A.A., M.A. Sarhan, et al. (2010). “ Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection .” Food Chemistry 123 (4), 1019–1026.
Molnar-Perl, I. and M. Friedman (1990). “ Inhibiton of browning by sulfur amino acids. 2. Fruit juices and protein-containing foods .” Journal of Agricultural and Food Chemistry 38 (8), 1648–1651.
Montero-Calderón, M., M.A. Rojas-Graü, et al . (2008). “ Effect of packaging conditions on quality and shelf-life of fresh-cut pineapple (Ananas comosus) .” Postharvest Biology and Technology 50 (2-3), 182–189.
Montero, P., O. Martínez-Álvarez, et al . (2004). “ Effectiveness of Onboard Application of 4-Hexylresorcinol in Inhibiting Melanosis in Shrimp (Parapenaeus longirostris) .” Journal of Food Science 69 (8), C643–C647.
Morris, J.A., R. Martenson, et al. (1973). “ Characterization of Monellin, a Protein That Tastes Sweet .” Journal of Biological Chemistry 248 (2), 534–539.
Moure, A., J.M. Cruz, et al. (2001). “ Natural antioxidants from residual sources .” Food Chemistry 72 (2), 145–171.
Murado, M.A. and J.A. Vazquez (2010). “ Mathematical Model for the Characterization and Objective Comparison of Antioxidant Activities .” Journal of Agricultural and Food Chemistry 58 (3), 1622–1629.
Nadarajah, D., J.H. Han, et al. (2005). “ Inactivation of Escherichia coli O157,H7 in packaged ground beef by allyl isothiocyanate .” International Journal of Food Microbiology 99 (3), 269–279.
Nor, F.M., S. Mohamed, et al. (2008). “ Antioxidative properties of Pandanus amaryllifolius leaf extracts in accelerated oxidation and deep frying studies .” Food Chemistry 110 (2), 319–327.
Oka, Y. (2010). “ Mechanisms of nematode suppression by organic soil amendments--A review .” Applied Soil Ecology 44 (2), 101–115.
Oms-Oliu, G., M.A. Rojas-Graü, et al. (2010). “ Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review .” Postharvest Biology and Technology 57 (3), 139–148.
Oms-Oliu, G., R. Soliva-Fortuny, et al. (2008). “ Physiological and microbiological changes in fresh-cut pears stored in high oxygen active packages compared with low oxygen active and passive modified atmosphere packaging .” Postharvest Biology and Technology 48 (2), 295–301.
Oms-Oliu, G., R. Soliva-Fortuny, et al . (2008). “ Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon .” LWT - Food Science and Technology 41 (10), 1862–1870.
Pezzuto, J.M., C.M. Compadre, et al. (1985). “ Metabolically activated steviol, the aglycone of stevioside, is mutagenic .” Proceedings of the National Academy of Sciences of the United States of America 82 (8), 2478–2482.
Poletti, S., W. Gruissem, et al. (2004). “ The nutritional fortification of cereals .” Current Opinion in Biotechnology 15 (2), 162–165.
Proestos, C., N. Chorianopoulos, et al. (2005). “ RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity .” Journal of Agricultural and Food Chemistry 53 (4), 1190–1195.
Pszczola, D.E. (2002). “Antimicrobials: setting up additional hurdles to ensure food safety.” Food and Technology (56), 99–107.
Richard-Forget, F.C., P.M. Goupy, et al. (1992). “ Cysteine as an inhibitor of enzymic browning. 2. Kinetic studies .” Journal of Agricultural and Food Chemistry 40 (11), 2108–2113.
Rojas-Graü, M.A., R. Grasa-Guillem, et al. (2007). “ Quality Changes in Fresh-Cut Fuji Apple as Affected by Ripeness Stage, Antibrowning Agents, and Storage Atmosphere .” Journal of Food Science 72 (1), S036–S043.
Rojas-Graü, M.A., A. Sobrino-López, et al. (2006). “ Browning Inhibition in Fresh-cut‘Fuji’Apple Slices by Natural Antibrowning Agents .” Journal of Food Science 71 (1), S59–S65.
Rojas-Graü, M.A., R. Soliva-Fortuny, et al. (2009). “ Edible coatings to incorporate active ingredients to fresh-cut fruits: a review .” Trends in Food Science & Technology 20 (10), 438–447.
Rojas-Graü, M.A., M.S. Tapia, et al. (2008). “ Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples .” LWT - Food Science and Technology 41 (1), 139–147.
Roldan, E., C. Sanchez-Moreno, et al. (2008). “ Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties .” Food Chemistry 108 (3), 907–916.
Rose, P., M. Whiteman, et al. (2005). “ Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents .” Natural Product Reports 22 (3), 351–368.
Rossi, M., C. Alamprese, et al. (2007). “ Tocopherols and tocotrienols as free radical-scavengers in refined vegetable oils and their stability during deep-fat frying .” Food Chemistry 102 (3), 812–817.
Sacchetti, G., S. Maietti, et al. (2005). “ Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods .” Food Chemistry 91 (4), 621–632.
Saguy, I.S. and D. Dana (2003). “ Integrated approach to deep fat frying: engineering, nutrition, health and consumer aspects .” Journal of Food Engineering 56 (2–3), 143–152.
Sallam, K.I., M. Ishioroshi, et al. (2004). “ Antioxidant and antimicrobial effects of garlic in chicken sausage .” Lebensmittel-Wissenschaft und-Technologie 37 (8), 849–855.
Sapers, G.M. and R.L. Miller (1998). “ Browning Inhibition in Fresh-Cut Pears .” Journal of Food Science 63 (2), 342–346.
Sarnthein-Graf, C. and C. La Mesa (2004). “ Association of saponins in water and water-gelatine mixtures .” Thermochimica Acta 418 (1-2), 79–84.
Schieber, A., F.C. Stintzing, et al. (2001). “ By-products of plant food processing as a source of functional compounds – recent developments .” Trends in Food Science & Technology 12 (11), 401–413.
Serfert, Y., S. Drusch, et al. (2010). “ Sensory odour profiling and lipid oxidation status of fish oil and microencapsulated fish oil .” Food Chemistry 123 (4), 968–975.
Shahidi, F. and U. Wanasundara (1994). Stabilization of Canola Oil by Natural Antioxidants . Lipids in Food Flavors, American Chemical Society. 558 , 301–314.
Shahidi, F. and Y. Zhong (2005). Lipid Oxidation: Measurement Methods. Bailey’s Industrial Oil & Fat Products. F. Shahidi. New Jersey, John Wiley & Sons Inc., 357–385.
Shin, I.S., H. Masuda, et al. (2004). “ Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori .” International Journal of Food Microbiology 94 (3), 255–261.
Son, S.M., K.D. Moon, et al. (2001). “ Inhibitory effects of various antibrowning agents on apple slices .” Food Chemistry 73 (1), 23–30.
Stadtman, E.R. and R.L. Levine (2003). “ Free radical-mediated oxidation of free amino acids and amino acid residues in proteins .” Amino Acids 25 (3), 207–218.
Stintzing, F.C. and R. Carle (2007). “ Betalains - emerging prospects for food scientists .” Trends in Food Science & Technology 18 (10), 514–525.
Suh, M.-H., S.-H. Yoo, et al. (2007). “ Antioxidative activity and structural stability of microencapsulated [gamma]-oryzanol in heat-treated lards .” Food Chemistry 100 (3), 1065–1070.
Sun-Waterhouse, D., J. Zhou, et al. (2011). “ Stability of encapsulated olive oil in the presence of caffeic acid .” Food Chemistry 126 (3), 1049–1056.
Tajkarimi, M.M., S.A. Ibrahim, et al. (2010). “ Antimicrobial herb and spice compounds in food .” Food Control 21 (9), 1199–1218.
Tanaka, Y., N. Sasaki, et al. (2008). “ Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids .” The Plant Journal 54 (4), 733–749.
Tapia, M.S., M.A. Rojas-Graü, et al. (2008). “ Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya .” Food Hydrocolloids 22 (8), 1493–1503.
Tapia, M.S., M.A. Rojas-Graü, et al. (2007). “ Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits .” Journal of Food Science 72 (4), E190-E196.
Timberlake, C.F. and B.S. Henry (1986). “ Plant pigments as natural food colours .” Endeavour 10 (1), 31–36.
Tiwari, B.K., V.P. Valdramidis, et al. (2009). “ Application of Natural Antimicrobials for Food Preservation .” Journal of Agricultural and Food Chemistry 57 (14), 5987–6000.
Tsao, S.-M. and M.-C. Yin (2001). “ In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils .” J Med Microbiol 50 (7), 646–649.
Ujváry, I. and K. Robert (2009). Pest Control Agents from Natural Products. Hayes’ Handbook of Pesticide Toxicology (Third Edition). New York, Academic Press, 119–229.
Van Vuuren, S.F., S. Suliman, et al. (2009). “ The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials .” Letters in Applied Microbiology 48 (4), 440–446.
Velasco, J., F. Holgado, et al. (2009). “ Influence of relative humidity on oxidation of the free and encapsulated oil fractions in freeze-dried microencapsulated oils .” Food Research International 42 (10), 1492–1500.
Ventanas, S., J. Ventanas, et al. (2007). “ Extensive feeding versus oleic acid and tocopherol enriched mixed diets for the production of Iberian dry-cured hams: Effect on chemical composition, oxidative status and sensory traits .” Meat Science 77 (2), 246–256.
Wanasundara, U. and F. Shahidi (1994). “ Canola extract as an alternative natural antioxidant for canola oil .” Journal of the American Oil Chemists’ Society 71 (8), 817–822.
Wang, C.Y. (2003). “ Maintaining postharvest quality of raspberries with natural volatile compounds .” International Journal of Food Science & Technology 38 (8), 869–875.
Wang, S.Y. and C.-T. Chen (2010). “ Effect of allyl isothiocyanate on antioxidant enzyme activities, flavonoids and post-harvest fruit quality of blueberries (Vaccinium corymbosum L., cv. Duke) .” Food Chemistry 122 (4), 1153–1158.
Wang, S.Y., C.-T. Chen, et al. (2010). “ Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries .” Food Chemistry 120 (1), 199–204.
Wang, Z.W., M.Y. Gu, et al. (2005). “ Surface Properties of Gleditsia Saponin and Synergisms of Its Binary System “ Journal of Dispersion Science and Technology 26 (3), 341–347.
Ward, S.M., P.J. Delaquis, et al. (1998). “ Inhibition of spoilage and pathogenic bacteria on agar and pre-cooked roast beef by volatile horseradish distillates .” Food Research International 31 (1), 19–26.
Weggemans, R.M. and E.A. Trautwein (2003). “ Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis .” Eur J Clin Nutr 57 (8), 940–946.
Wildman, R.E.C., Ed. (2007). Handbook of Nutraceuticals and Functional Foods Boca Raton, CRC Press.
Wong, D.W.S., S.J. Tillin, et al. (1994). “ Gas exchange in cut apples with bilayer coatings .” Journal of Agricultural and Food Chemistry 42 (10), 2278–2285.
Wrolstad, R.E. and L. Wen (2001). Natural antibrowning and antioxidant compositions and methods for making the same. United States, The State of Oregon Acting By and Through the State Board of Higher Education on Behalf of Oregon State University.
Xiong, X.L. (2000). Protein oxidation and implications for muscle foods quality. Antioxidants in muscle foods. C. Faustman and C.J. Lopez-Bote. New York, John Wiley & Sons Ltd., 85–111.
Yanishlieva, N.V. and E.M. Marinova (2001). “ Stabilisation of edible oils with natural antioxidants .” European Journal of Lipid Science and Technology 103 (11), 752–767.
Yin, M.-c. and W.-s. Cheng (2003). “ Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef .” Meat Science 63 (1), 23–28.
Yin, M.C. and C. Faustman (1993). “ Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model .” Journal of Agricultural and Food Chemistry 41 (6), 853–857.
Zhang, D. and P.C. Quantick (1997). “ Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit .” Postharvest Biology and Technology 12 (2), 195–202.