FIGURE 4.1. Age-at-death distributions of Velia Porta Marina (I–II cent. CE; N=297) and Isola Sacra (I–III cent. CE; N=526).
Bones, Teeth, and History
Alessandra Sperduti, Luca Bondioli, Oliver E. Craig, Tracy Prowse, & Peter Garnsey
Introduction
Human bones and teeth are the primary databank for biological anthropologists, but have aroused little interest among historians of antiquity. The beginnings of an explanation of this disparity are to be sought in the fact that human skeletal remains have no obvious relevance as a source of information for politics, political institutions, political thought, government, law, religion, warfare: in brief, for the traditional concerns of ancient historians. A second consideration is that biological anthropology is rooted in prehistory; its practitioners are characteristically involved in the exploration of the origins of humanity. Fortunately (for our present purposes), some anthropologists have allowed themselves to stray into historical periods, including the classical world of Greece and Rome. For example, the laboratory of bioarchaeology at the Museo Nazionale Preistorico Etnografico ‘Luigi Pigorini’ in Rome houses the skeletons of Isola Sacra, the burial ground of classical Rome’s harbor-town of Portus, and over the last three decades or so has produced a lengthy sequence of articles and dissertations deriving from the study of this large sample. In the meantime, historians of antiquity are showing increased interest in social, economic, and cultural history, and are displaying a new willingness to expose themselves to other disciplines, including the natural and social sciences. Thus, the time seems ripe for fruitful communication between historians and anthropologists. Specifically, health and demography (mortality, fertility, mobility) hold promise as fields for constructive dialogue and collaborative research. Initial contacts have already been made, though not always with happy results.
The challenge awaiting historians is to provide contextualization, to put the results of scientific analysis into a historical setting, and to bring other evidence to bear—while being fully conscious of the limitations of that other evidence. As regards the central question of health and well-being—how healthy were the Romans?—the anthropological evidence appears to have a clear advantage over the conventional source material. An enquiry as to health inevitably begins with diet. The question of what people normally ate and in what quantities is in fact impossible to answer from the traditional sources with respect to past societies, at least prior to the nineteenth century. This is because quantitative data is unobtainable from the conventional source material on food and diet. And yet, without such data, any historical account of food consumption can at best be only impressionistic. One can, of course, derive from a variety of sources, for the most part literary and archaeological, a list of foods that were in principle available to and utilized by inhabitants of a given society in the past, usually members of the upper classes, towards whom the evidence is skewed, and this has been achieved for Roman society.1 Such a catalogue is not without interest or utility, but historians of food should have higher ambitions, and should be prepared to ask what proportion of the diet came from what source, among any particular group of people, including groups representative of the mass of ordinary people, and not just the elite, in a given society. The best hope of progress in this area lies with the scientific analysis of human skeletal remains, which alone provide data which are quantitatively and qualitatively significant, and are also cross-class. In particular, through the analysis of the stable isotopes of carbon and nitrogen, one can move towards a reconstruction of cumulative diet, that is to say, diet over the last ten years of so of the life of an individual. Those (few) historians who are aware of stable isotope analysis are apt to regard it with skepticism and to underestimate its potential.
Meanwhile a number of historians have shown a tendency to progress from judgments about the diet of a people to conclusions (typically optimistic) about their health. In so doing they leave out of account the factor of disease. Diet and nutrition are not the same thing as health or nutritional status. Nutritional status equals nutrition minus the claims made by disease (and workload). Exposure to disease, endemic or episodic, undermines even the best of diets. While we are fully entitled to be enthusiastic about the impressive range and variety of foods consumed by the residents of an apartment block at Herculaneum, as revealed by the contents of their sewers, we should not forget that there are other variables to be brought into play in any judgment concerning health. On this particular issue, cooperation between historians and anthropologists can produce significant results. Historians have two main cards up their sleeve: historical demography and epigraphy. One theme of the historical demographers (whose focus of interest, inevitably, is on the early modern and modern historical periods) is the negative impact of urban concentrations on health, especially in prescientific societies. One thinks first of course of ancient Rome itself and its environs, but the Bay of Naples was also a significant center of population. At Herculaneum perhaps 4000 people were densely packed into a space of ca. 20 hectares, at ca.250 per hectare. High infant mortality implying low life expectancy at birth—something in the 20s, and maybe the lower 20s—can be assumed for urban agglomerations in Central Italy in the early Roman Empire, other things being equal. As it happens, on the basis of a remarkable inscription (of which significant fragments survive), one can surmise that the population of the town of Herculaneum was sustained at a stable level not by the natural reproduction of the existing inhabitants, but by the forced migration of slaves, a significant proportion of whom passed through manumission into the ranks of the free population. We now look to the anthropologists to fill out the picture of morbidity and mortality (and indeed geographical mobility) at Herculaneum through the various state-of-the-art techniques that they have developed and are developing—including the use of dental histology—to provide a close-grained map of the experience of stress at the most perilous stage of the life cycle, namely, early infancy.
In one particular area, related to health, historians have moved too fast, too soon, and with too little. Some economic historians who have become interested in health and nutrition are looking to bones to provide support for their thesis that the early Roman Empire witnessed substantial economic growth. Specifically, it has been asserted that the Romans enjoyed better health and nutrition than most Europeans up to the nineteenth and early twentieth century, and that this is reflected in their stature. In general, establishing a link-up between health status and economic development is not likely to be a straightforward procedure.2 As to stature in particular, it can be agreed—and has long been familiar to historical demographers—that heights (and weights, where available) are an important index of health and nutritional status (see Chapter 5). The problem is that the figures for stature that have been derived from the anthropological literature are estimates, arrived at by regression analysis from long-bone measurements. And anthropologists have used a variety of regression formulae, in the absence of any consensus as to which is the most appropriate. Worse still, many anthropologists in their publications have failed to provide the raw data (long-bone lengths), and even neglected to identify the regression formula they have applied.
This is one indication of the fact that it is not easy for historians to make constructive use of the work of anthropologists. If historians have been blind to the opportunities provided by anthropology, anthropologists have been uninterested in reaching out to historians. They have been preoccupied with developments within their own fast-evolving discipline, which have been debated at every turn. As will soon appear, trails have been identified as false, methodologies have been found wanting, and the best way to advance is disputed.
As indicated above, in recent years the idea that bones and teeth are an essential source of information for the historical reconstruction of ancient populations has begun to be taken up by archaeologists and historians. At the same time anthropologists are now becoming aware that the reconstruction of biocultural adaptations and lifestyles of past populations relies on integrated analyses, involving biological, ecological, historical, and archaeological evidence brought together in the collaborative work of researchers from various disciplines.3 More specifically, social institutions, subsistence strategies, and mortuary rites—and the ways they materialize in the bioarchaeological record—represent the main focus of current anthropological research. Nevertheless, data extraction and interpretation are not simple and straightforward processes: the community of the dead very rarely reflects that of the living,4 but more often is the outcome of the interplay of cultural, environmental, and biological phenomena that are not always quantifiable.
In fact, the quality and reliability of biocultural reconstructions from skeletal populations have been much debated over the last three decades among anthropologists, since the publication of two major (and now famous) contributions that provided a serious challenge to the assumption that a good basic skeletal biology is capable of reconstructing paleodemographic profiles and describing in detail the living conditions of ancient communities.5 Since the appearance of these papers, a new generation of studies, mostly focused on critical reassessment of both theoretical and methodological issues, has been launched, and today we can avail ourselves of an ever-increasing number of pertinent contributions in skeletal anthropology, acknowledging fundamental problems and pitfalls, and at the same time pointing to new and more promising approaches of analysis and interpretation.
Any anthropological intervention in cemetery contexts that is to contribute to an integrative, collaborative study with archaeologists and historians should be directed at asking the following, basic questions, of the buried individuals: Who were they? What was their physiological condition? What were their occupations? What did they eat? Where were they from?
Who Were They? The Basics: Sex, Age and Paleodemography
No anthropological analysis carried out on bones and teeth, whether simple or complex and based on advanced investigatory techniques, can neglect two fundamental items of data: the sex and the age at death of the individual under examination. At the same time, no reconstruction, if it is to have demographic significance, can fail to consider as a whole the demographic profile of the set of skeletal remains under scrutiny. For this reason, a large part of skeletal research, from its very beginnings, has been dedicated to the problem of how to arrive at best estimates of sex and age at death, and how to construct a coherent demographic picture out of the data on individuals.
SEX DETERMINATION
As regards the determination of sex from bone and tooth remains, every individual part of the skeleton has been subjected to analysis directed at the calculation of sexual dimorphism and its potential application for the determination of sex, on the basis of examination carried out on skeletal series whose sex and age is established. Numerous morphological and metric criteria have been proposed and published in the literature, together with geometric morphometric elaborations, mainly with reference to the skull and hip bone.6 Moreover, the ever more concrete possibility of isolating, amplifying and sequencing DNA extracted from ancient skeletons has made available an additional diagnostic tool, although the time and expense involved means that this is a procedure which is difficult to adopt on a large scale.
There are fundamental issues connected with sex-determination. In the front line are the morphological criteria, whether whole bones are involved (usually the skull and the hip bone), or parts thereof. At the same time, as the assessments are made on the basis of visual scoring, they require that the observer have received specialized training, and they are susceptible to subjective judgment, thus undermining intra-and-interobserver replicability.7 The metric criteria have certain advantages, in that they are applicable even to individuals presented in a highly fragmented state (as is the case with cremated skeletons); again, they are objective and are rigorously defined.8 Nevertheless, they often issue in a relatively high percentage of misclassifications, and are strongly dependent on the genetic background, not to mention the living conditions, of the population under examination. Numerous studies have demonstrated that metrical and morphological standards derived from a population of known sex are not equally reliable when applied to skeletal series of a different origin.9
Still, skeletal sex estimates can attain very high levels of consistency: from 90% for the cranium alone, to 95% for the pelvis alone, to 98% for both in combination or for the pelvis alone.10 A recent study has shown similar values for some postcranial metrical variables, whether used as a single measurement (epiphyseal breadth of long bones) or within multivariate functions.11 The calculation of volumes and areas from 3-D models of specific bones has obtained a very high reliability in sex-prediction.12 However, this procedure cannot be used routinely in archaeological contexts, since it requires bones that are perfectly intact.
The skeletal sexual dimorphism of subadult individuals has often been described, quantitatively and qualitatively. Nevertheless, few attempts have been made to set standards for sexing subadults.13 Essentially, attention has been directed toward the development of diagnostic criteria based on the dimensional variations of primary and secondary dentition and on morphological aspects of the pelvis and mandible. Notwithstanding the efforts dedicated to this subject, there is now a general consensus that the determination of sex in the prepubic period on the basis of skeletal morphology is unattainable. The only viable option is aDNA analysis. This has proved to be particularly useful in providing better definition of cases and behavioral patterns of infant disposal in ancient Roman communities.14
AGE-AT-DEATH DETERMINATION
Age at death assessment from bones and teeth is one of the most investigated and debated topics in skeletal biology. The search for the best and most trustworthy odontoskeletal characteristics for the determination of age at death, and their constant validation, goes back to the middle of the nineteenth century. To warrant serious consideration, an age-marker should satisfy the following criteria: strong correlation with the chronological age; progressive and unambiguously identifiable aging pattern; continuous change through an extended period in the life span; wide applicability; no, or little, influence from environmental factors (pathologies, nutrition, work-load etc.).15
The several aging criteria that have been formulated for infant and juvenile individuals are for the most part reliant on developmental stages in the growth of teeth (the stages of formation and eruption) and of bones (the fusion of bone-ends, general and specific dimensions).16 For adult skeletons, the indicators that are taken into account are mainly linked with postgrowth processes (mainly wear and physiological degeneration) of specific segments of the skeleton. Macroscopic morphological techniques are routinely adopted, followed by the application of radiological techniques and histological observations.17 Because the growth and maturation process is more regular and constant across populations than the degenerative phenomena, infants and subadults are more easily and precisely diagnosed for age at death than adults.18
Despite the plethora of works that have appeared in this research field, there is a widespread awareness that the criteria routinely applied may not ensure high levels of accuracy (in achieving a result that comes close to the chronological age) and precision.19 One major source of error in estimating age-at-death which is intrinsic and hard to control for, consists in the dissociation between the chronological and the biological age,20 the point being that the biological age is influenced by the genetic background as well as by environmental factors, such as type and level of physical activity, general health, nutrition, specific pathologies that may alter the aging rate of bones and teeth. Experience shows that the skeletal maturation and aging process are not constant, regular or homogeneous among different anatomical districts of a single individual,21 and may vary significantly across individuals and populations. Moreover, body size can influence age-at-death determination and result in misclassification.22
A second pitfall relates to the reference series: how far are criteria that are derived from a modern population, where sex and age are known, applicable to skeletal samples that come from different geographical regions and chronological settings? This issue has been investigated time and time again, with divergent results.23
A third issue was first raised by Bocquet-Appel and Masset in a famous paper. They argue that skeletal age-at-death estimates tend to mimic the age distribution of the reference sample by which the criteria were assessed, and that in consequence the mortality tables that are produced are merely “random fluctuations” and reflect “erroneous methodology.”24 Their paper has the merit of having launched a new generation of studies, mostly focused on the critical reassessment of both theoretical and methodological issues.25
The problem of the influence of the reference sample is partly overcome by a shift from single-traits to multiple-traits assessment procedures.26 Multifactorial standardized procedures for the determination of skeletal age at death include the “combined” method, the summary age method, and the transition analysis.27 The above-cited methods have had some (partial) success, but it should be acknowledged that they are not routinely used or usable, due to the incomplete preservation of skeletons. In general, research in this area suffers from inconsistency among researchers and across laboratories in the choice of aging techniques and age category definition.28 Add to this the differences between researchers in terms of the level of their individual experience, and we can see why outcomes can be divergent.29 The assertion of Maples is still valid,30 that the process of age determination is “an art, not a precise science.” How to react to this issue? At the least, we should continue to explore and test age indicators,31 with the end in view of making our analyses more scientific and less subjective, through constant checking and standardization of methods, and searching for quantifiable aging procedures.32
The determination of infant age at death is a specialist area in itself. Age-estimation is mostly based on the development and eruption of the primary and secondary dentition, following a number of standards derived from radiographic surveys of modern populations.33 If these estimates are imperfect, this is because of the intrinsic individual variability in the pattern of dental formation. In recent years advances in dental enamel and dentine histology are offering a more precise method of assessing the age at death of infants, calculated as the time that occurred from birth to form an incomplete deciduous tooth.34 Dental enamel is a highly mineralized tissue that is formed of hydroxyapatite prisms produced through a complex process (amelogenesis), which rhythmically deposits a protein matrix (during the so-called secretion phase), that becomes mature enamel in a second phase (the maturation phase). This process leaves unambiguous traces inside the enamel microstructure in the form of daily striations of the enamel prism (cross-striations) and bands through the enamel thickness (Retzius lines), that correspond to a fixed time interval (in the range of 6–11 days) for each individual. Moreover, when the amelogenesis is disturbed by a stressing agent, the Retzius lines become accentuated (more marked). The first accentuated Retzius line forms at birth. The transition from an intra- to extrauterine environment leaves its mark in deciduous teeth (and first permanent molars) as an accentuated enamel incremental ring called the neonatal line (NL).35 At birth all the deciduous teeth are present, and very often the first molar is already in the process of formation. Therefore the age at death of an infant can be determined by counting the time markers on the developing crowns from birth till death. Similarly, it is possible to estimate the dentine extension rates in the forming dental roots and extend the range of age at death estimation till the last moment of the first permanent molar formation (~9.5 years).36 Smith and co-authors, working with macaques, estimated that standard histological techniques yield an average 3.5% overestimate and a 7.2% average absolute difference from the known age.37 Therefore, dental histology methods offer a much more precise estimate of the infant age at death in comparison with the most widely used morphological methods, and should be used particularly when estimating mortality profiles and weaning-related studies.
DEMOGRAPHIC ESTIMATES
Skeletal data have the potential to provide valuable information on the structure, size, and biological dynamics of past human populations. At the same time their limitations are fully recognized and debated.38 In addition to the issues relating to age and sex determination discussed above, the data are problematic in other ways, both practical and theoretical. For example, partial recovery of the skeletal material, influence of selective funerary practices, and seasonality in the use of burial are all factors that may profoundly influence the structure of the sample, introducing biases that can affect the reconstruction of the characteristics of the population.
Wood and colleagues in their landmark paper of 1992 questioned the validity of paleodemographic and paleopathologial reconstruction by adding other sources of error: the nonstationary nature of populations, selective mortality and heterogeneity of death-risk among individuals from the same age group. For the calculation of demographic parameters, in fact, a sample must be considered as a single cohort of individuals with the same birth interval. Thus the reference population should be stationary and closed, that is, it should be characterized by the absence of migration flows and events (biological and/or cultural) that may have altered mortality/birth rates and growth. These conditions are difficult to observe in real life, and even if some scholars think that there was greater stability in past than in present communities,39 this is certainly not the case with many towns of the Roman period, for which large migration flows of people of both slave and free status are historically, archaeologically, and biologically attested.40
FIGURE 4.1. Age-at-death distributions of Velia Porta Marina (I–II cent. CE; N=297) and Isola Sacra (I–III cent. CE; N=526).
Thus far there have been very few paleodemographic studies of any substance of ancient Roman communities, and even fewer published in scientific journals.41 In some cases the skeletal series proved to be too biased to yield reasonable results—for example, Lucus Feroniae with its skewed sex ratio of 0.79 and virtual lack of infants aged 0–1, and Isola Sacra with its oversized class of young adult males.42
IS THERE HOPE?
A striking exception to this scenario, is Velia (second century CE, Campania, Italy). Almost 300 burials were excavated at Velia during the 2003–2006 field campaigns, in a necropolis immediately adjacent to the ancient port, just outside the south entrance to the city (Porta Marina Sud). The context of the burials and finds from the excavations indicate that the cemetery was used between the first and second centuries CE. As clearly indicated by the age at death distribution compared with Isola Sacra (Fig. 4.1), the proportion of infants aged 0–1, in Velia is more than 30%, and the whole subadult subsample almost reaches 60%. Velia demographic parameters fit the Western Female model no.1.43 As shown in Figure 4.2, the survivorship trend through ages in Velia follows the theoretical model, while that in Isola Sacra does not.
FIGURE 4.2. The survival trend through the age-cycle follows the theoretical model at Velia but not at Isola Sacra. The gray shading shows different levels of mean life expectancy at birth from e0=20 (lightest) to e0=80 (darkest).
As stated above, the age and sex structure of cemetery samples are not normally a reliable guide to the demographic parameters of the living community. Nevertheless, these same data are very useful for the detection of the conditions that have skewed the profile. Anomalous sex ratio and under-/over-representation of particular age classes have been variously interpreted as the outcome of selective burials, slavery, infanticide, warfare, epidemics, migration flows, and other dynamics of sample formation.44 As for the constant and ubiquitous lack of infant remains from archeological samples, contrary to the differential preservation hypothesis, Lewis suggests that “the absence of infant remains from cemetery sites at different periods is probably revealing more about their status within the society, than their ability to ‘dissolve in the ground’.”45
For the Roman world we have a clear case of how large-scale natural disasters can affect the demographic profile: the eruption of Vesuvius in 79 CE. The skeletal data from Pompeii show a lower proportion of deaths of adult males than of women and children, and this was to be expected.46 In the Herculaneum sample, however, the sex and age distribution is significantly different. Males outnumber females, and very young individuals are few. Further, if we set the demographic data and the provenance of the skeletons side by side, we obtain a very striking result. The skeletons were found mainly on the beach and within the fornici (vaults) set back from the beach. As Figure 4.3 shows, males predominate on the beach but are outnumbered in 7 out of 9 fornici. Conversely, infants and most of the juveniles were found in the fornici together with a large number of females. All this is suggestive of a pattern of social behavior, and the operation of a particular escape strategy, which remain to be uncovered and elucidated. In this case, furthermore, there is the additional informative but complicating factor, that the population of the town is known through epigraphy to have contained a substantial number of ex-slaves (and, a fortiori, slaves). This inevitably raises further issues, as to how representative the beach sample is of the population of the town as a whole, and as to how the peculiar demographic structure of the town influenced the distribution of individuals on the beach and in the fornici.47
In conclusion: we have spelled out the problems that beset paleodemography.48 At the same time, we firmly believe that age-and-death-profile analysis is the initial, fundamental, and mandatory phase of all anthropological research, for the following reasons. First, it is possible, as demonstrated by the case of Velia, to come across a cemetery sample that meets most of the requirements of a reliable mortality profile. Second, the very same deviations from the expected norm, which appear to undermine the credibility of paleodemographic estimates (in particular, the under- or over-representation of specific categories), can serve as a highly informative source of information as to specific events and patterns of social behavior that have produced the biases in particular samples. Third, if we hold back from describing a cemetery sample in terms of (at least) the sex ratio and gross age distribution, then we cannot proceed to consider other data relevant to the population in question, especially such data as are closely related to the sex and age of individuals, such as skeletal markers of work activities, specific and aspecific health indicators, pathological changes, diet.
For the Roman period, scholars have been collecting paleopathological data, and integrating them with evidence from archaeological and written sources, in order to contribute to the understanding of, first, the origin, diffusion, and evolution of diseases; second, the epidemiological aspects related to the presence and spreading of the diseases, mainly in their relation with sex, age, social standing, diet, growth, mortality, and occupation, and so on; and third, medical knowledge and skills in the treatment of the diseases. With regard to the last of these items, the most striking evidence of surgical expertise is provided by one of the oldest cases of amputation with individual recovery and survival.49
Researchers have also published epidemiological studies involving the systematic surveys of skeletal health markers on large Roman age samples,50 while others have sought to trace variations in health conditions through different historical ages,51 or between Rome and the periphery of the Empire.52 Some studies focus on selected issues, such as the presence of aspecific metabolic stress markers.53 Others investigate the correlation of diet with health or the osteopenic/osteoporotic processes through life.54
A number of scholarly contributions offer description and diagnosis of rare pathological conditions. Hyperostosis frontalis interna, pituitary gigantism, and dwarfism are hormonal diseases located in various Roman age skeletal series.55 Some data have been also gathered for specific infectious diseases. Rubini and colleagues isolated Mycobacterium leprae DNA from two skeletons showing several bony changes indicative of leprosy.56 Evidence of tuberculosis was found in Herculaneum and in a first-century CE necropolis in Rome.57 Among metabolic diseases, one case of scurvy and one of rickets have been described.58 In the Collatina necropolis (Rome, first century BCE to third century CE), a case of ankylosing spondylitis and a case of gout were found.59 These papers give valuable information in the fields of medical and disease history,60 but contribute little to an understanding of the overall health status of the ancient Romans. It is now acknowledged that historians and anthropologists have a common interest in the study, over a broad canvas, of the health and physical well-being of the inhabitants of past communities, as an index of their adaptability to environmental resources and constraints. An additional benefit of such research is that it can provide useful insight into past medical knowledge, social organization, and resource distribution.
It hardly needs emphasizing that skeletal data are meaningful only if discussed within a framework that brings together clinical, historical, and paleo-environmental evidence, and gives attention to their mutual influence. For instance, it is of interest to know how far ancient parasitoses may have influenced the distribution of settlements, productive activity, socioeconomic levels, and demographic trends in ancient populations.61
Paleopathological surveys, in past generations, have done useful groundwork in detecting, describing, and quantifying diseases, and setting them in their temporal context, through macroscopic, radiological, and microscopic analyses of bones and teeth.62 Similarly, the integration of skeletal with histopathological and biomolecular evidence is crucial to further significant advance on the scientific front.63 Improved techniques for aDNA detection and sequencing are now providing interesting results with regard to human hereditary diseases and ancient pathogens infections, Treponema pallidum (syphilis), Brucella sp. (brucellosis) Mycobacterium tuberculosis (tuberculosis) among the latter.64 Interest in pathogens is also focused on their evolutionary trajectories in the interaction with the human host.65 Besides DNA, other biomolecules (i.e., hemoglobins, human leukocyte antigens, hemozoin) are also providing further means of diagnosis. The search for ancient molecules extends beyond the teeth and the bone, even resorting to dental calculus and coprolites as sources of data.66
Furthermore, the field has been moving out of descriptive research on individual cases, into multidisciplinary approaches applied to whole populations, marshalling evidence from histology, anatomy, microbiology, physiology, biochemistry, medicine, archaeology, history, and ecology. At the same time, much effort has been dedicated to validating new research methods and addressing limitations in data assessment and interpretations.67
An example of how technical innovations, along with an integrated approach, are providing useful results, is the identification of malarial parasitosis in ancient bones, a subject of particular interest for the Roman period. Traditional means of diagnosis—that is, gross examination of bone manifestation of cribra orbitalia and porotic hyperostosis—have failed to provide concrete and secure evidence,68 in the main because they can in fact be associated with several different conditions, related to other kind of parasites, malnutrition, or inherited hemolytic anemias.69 Meanwhile, sequencing ancient DNA has provided some, but limited, results.70 Finally, a quicker and less expensive diagnostic method has been tested on skeletal material in the effort to identify the biomolecules related to malarial infection. Hemozoin, a plasmodium catabolite, is indeed currently used in clinical research as a malaria biomarker.71 Very recently this biomolecule was detected—with fluorescence microscopy—on the spongy bone tissue of a Late Roman Age sample of children,72 confirming the diagnosis of malaria previously obtained through the isolation of Plasmodium falciparum DNA.73
However, a few methodological pitfalls and theoretical limitations in paleopathological analyses should be acknowledged and addressed.
Issue 1. Diagnosis and etiology. The first constraint in the information potentiality of paleopathological studies is that only a limited number of diseases actually manifest on the skeleton and on the teeth. Moreover, as stressed by several authors, in a skeletal sample, unaffected individuals may actually represent the most affected group within the population, given the possibility that they represent those who have died in a short time period, before the disease could leave permanent markers on the bones. For specific conditions, mostly infections and epidemics, molecular data can make a decisive contribution. The presence of Yersinia pestis,74 and of other pathogens has been detected in skeletal series from particular depositional contexts (mass graves) and are characterized by biased demographic profiles, but without presenting any gross pathological changes.75 Still, even with these techniques, a false negative can occur, that is, the disease is present, but the tests fail to detect it.
Secondly, the bone tissue responds in a very homogeneous way to a broad spectrum of afflictions, and this complicates, or even prevents, differential diagnosis. Lesions on the visceral surface of the ribs, for instance, are generally linked to tuberculosis,76 but the association is not ubiquitous,77 and other pathological conditions come into the reckoning,78 like rickets. Furthermore, many diseases have a complex etiology. Since their mechanisms, as well as the role of every single causative factor, are not fully understood, nor weighted, we may fail to comprehend what we are really measuring in our surveys. In this respect, the case of osteoarthritis is emblematic. Osteoarthritis (or degenerative joint disease, DJD) is a multifactorial condition linked to hereditary factors, endocrine agents, age and sex, and nutrition, as well as functional stress, related to traumas, body weight, and articular loading and movements.79 Nevertheless, in bioanthropological studies, the analysis of DJD has been exclusively used to assess type and extent of working activities, ignoring a more complex interpretation, or even a simple association with the ageing process.
Thirdly, skeletal analysis may reveal co-occurrences of conditions whose significance is not always clear. For instance, a correlation between enamel hypoplasia and reduced longevity has been registered.80 However, several possible alternative explanations are available: repeated metabolic stress during growth may increase adult susceptibility to pathologies;81 hypoplasia and mortality may both be the effect of poor dietary and sanitary conditions, or of congenital individual frailty.82
Association between disease, mortality, and diet gives a multifaceted picture which lends itself to complex interpretation.83 For instance, in Velia, the presence of cribra orbitalia does not correlate with diets characterized by low protein content (Fig. 4.4). In the same sample, cases of diffuse idiopathic skeletal hyperostosis (DISH), a pathological condition characterized by a complex etiology, is more frequently associated with rich in proteins diets (Fig. 4.5).
Issue 2. Methodology. There is an absence of shared diagnostic standards. In order fruitfully to share and compare results, researchers should follow the same diagnostic criteria and adopt the same survey protocols. This procedure should be mandatory in the case of the most common and widespread afflictions (such as caries, degenerative joint diseases, traumas) or nonspecific stress indicators (enamel hypoplasia, cribra orbitalia, porotic hyperostosis, periosteal bone reaction). Lack of standardization in scoring the trait, but also in the presentation of results, one for all, the extension of the age classes, is a frustrating situation, experienced by anyone who has attempted to conduct meaningful comparisons with data from the literature.84
FIGURE 4.4. Nitrogen and carbon isotopic delta values and presence of cribra orbitalia in the adult sample from Velia (I–II cent. CE; N=74) do not show a significant correlation.
FIGURE 4.5. Nitrogen and carbon isotopic delta values and presence of diffuse idiopathic skeletal hyperostosis (DISH) in the adult sample from Velia (I–II cent. CE; N=85) show a positive correlation.
Even within the same laboratories, adopting the same protocol, the interobserver error may reach statistically significant levels.85 In this respect, Ortner and Buikstra and Ubelaker call for, and have been working toward, a more extensive standardization in data collection and diagnosis, associated with a more objective descriptive terminology.86
There is another aspect that should not be overlooked: since we are dealing with skeletal material of archaeological provenance, individuals are never complete. How is the partial recovery of bones to be reflected in the representation of pathologies? Boldsen and Milner discuss the high risk of obtain a “false negative” in fragmentary skeletons compared to those that are intact.87
In the specific case of the studies of osteoarthritis, the preservation of archaeological remains often precludes the recovery of complete joints. This consideration led us to investigate the effective informative value of the scoring procedure customarily adopted in the relevant literature. We used for this purpose the archaeological sample of Velia (Italy, first–second century CE) as our test case. In a sample of 103 adult individuals of both sexes, every single bone of the 6 major joint complexes (shoulder, elbow, wrist, hip, knee, and ankle) was scored separately for osteoarthritic changes. Our first objective was to evaluate the effective informative value of single bone elements within each articular complex. The results indicate that each joint shows to some extent intra-articular differences in DJD percentage, with a presence of what we have called a “bone leader” (a bone showing more DJD affection than the others within the same joint). The most frequently and most severely affected bones are: the scapula for the shoulder complex; the os coxale for the hip; the femur for the knee; and the tibia for the ankle.
A simulation was developed in order to quantify the influence of incomplete joints on the estimation of the mean scores of DJD simulating a set of hypothetical archaeological sub-samples (extracted from our sample with all complete joints) at different levels of preservation. This was achieved by simply randomly eliminating a given percentage of bones from the main sample. The preservation levels are expressed as the percentage of complete joints over the total number of joints scored, ranging between 90% and 10%. For each level, a computer routine eliminates in the data base the DJD score of at least one bone per joint, and the DJD prevalence was calculated again. For the prevalence calculation, a joint was considered affected if the score of any indicator (lipping, porosity, and eburnation) was greater than 1. The simulation was run 1000 times for each level of completeness resulting in a distribution of possible prevalence values.
FIGURE 4.6. Decreasing levels of bone representation affect the osteoarthritic frequency, with different grade of bias across the joints. For instance, with 100% of complete joints, the hip prevalence is higher than in the knee, while, at 70% of completeness the result is reversed.
The results show that for all the 6 joints analyzed, the decrease of joint completeness (from 90% to 10%) is associated with a constant and progressive lowering of the mean DJD prevalence. At the same time, they show a progressive increase of variability of the prevalence values (standard deviation and range values), as obtained by the 1000 simulation runs. This tendency is clearly illustrated in Figure 4.6 where the results of the joints are presented, with the kernel density estimates of the simulated distributions plotted for lipping.
The results confirm that most of the joints have a bone that is more susceptible to the mechanical stress, so that joint incompleteness strongly affects the results by underestimating the pathological conditions and by neglecting the differences across the joint which consistently occur.
Issue 3. Theoretical questions. Besides the methodological issues concerning diagnosis and compatibility in data collections, scholars are currently addressing the problem of translating the results into consistent and meaningful interpretations of past life conditions, through the development and implementation of new theories and approaches. Once again, we have to cite the “Osteological Paradox” of Wood and colleagues and the three key issues hindering our understanding of the past health: demographic nonstationarity, selective mortality, and hidden heterogeneity in the morbidity and mortality risk.88
Demographic nonstationarity. Among the demographic variables, migration has the effect of altering the pathological profile of a population, since the health status and life history of newcomers are not those of the host community but are the product of a different biocultural environment. There is no easy solution to this problem. It has to be accepted that the disease regime is likely to be highly complex in the case of communities where there is a considerable degree of population mobility, notably Rome itself, but also cities on or near the coast in, for example, Central/South Italy.
Hidden heterogeneity in the risk of disease and mortality. There is a plethora of factors influencing the probability of falling ill and dying, ranging from genetic background to acquired susceptibility. These factors, and their interaction, are nonquantifiable, except in rare cases. One promising approach is to single out smaller homogeneous groups within a broader population, possibly identified by age, sex, working conditions, or social status, and pathological afflictions.89 Individual life history, in so far as it can be reconstructed, should be helpful in tracing and identifying the sources of frailty. Another recommended line of action consists of analyzing the possible relationships between different health indicators, diet, and mortality.90
Selective mortality. In approaching once-living populations, we should always remember that we are dealing with the people who did not survive, and who are likely to embody higher frequencies of pathological afflictions and other skeletal signals related to harsher life-styles: growth disruption, poor nutritional intake, heavy working loads, amongst others. In some instances, skeletal series derived from catastrophic events can give us a glimpse of the living community, even if they certainly cannot represent an exact cross-section of the population. As stated above, the demographic parameters of the Herculaneum and Pompeii Vesuvius victims, rather than being a proxy of the city census,91 reflect behavioral responses to the crisis.92 Nevertheless, these samples provide us with an interesting opportunity to compare “living” Romans with dead ones. It is indeed rewarding to compare dietary habits as derived from cemetery samples (Isola Sacra and Velia) with those from the Herculaneum catastrophic assemblages.93 Preliminary finds suggest that the variability observable in mortuary samples might be inflated when set against the more homogeneous nutritional pattern of the “living” community of Herculaneum.
Conversely, also using a comparative perspective, stature (calculated using the Pearson’s regression formulae),94 shows a remarkable overlap among the samples; the implication is that this derived trait is less affected by the “Osteological Paradox” and more dependent on the homogeneous environmental conditions prevailing in central Italy under the Roman Empire.
An integrated perspective As suggested above, a way of confronting the challenge of the “Osteological Paradox,” specifically heterogeneity in the risk of disease and mortality, and selective mortality, is to isolate a subgroup within a given population and subject it to multivariate analysis. Infancy suggests itself as a promising candidate for such an approach and within it, more particularly, the weaning period in which the interplay of diet, disease, and mortality can be studied in depth by a combination of diverse methodologies.95 The timing and procedures of weaning influence infant mortality, the demographic structure of the population, and the health status of infants and adults. Weaning is a critical phase of childhood, and the levels of morbidity and mortality associated with it are an indirect measure of the nursing practices and sanitation levels of the community. In the case of weaning, we can study the interplay of diet, disease, and mortality by a combination of diverse methodologies. Specifically, one can combine the isotopic, mortality, and morbidity data for infants, with microscopic analysis of the dental enamel in infants, juveniles, and young adults (unfortunately older individuals can be included only with difficulty, because of dental wear).
Dental enamel histology (see above 2.2) produces more precise age determinations, and consequently results in a better definition of the weaning time, when studied through the change in trophic level, using the carbon and nitrogen stable isotopes.96 Moreover, laser ablation–inductively coupled plasma-mass spectrometry (LA-ICPMS) techniques can integrate the isotopic data with high resolution chronological variation of strontium concentration.97 Finally, the quantification and chronology of possibly weaning-related metabolic stress using the prevalence of enamel microdefects during growth,98 provides a means of measuring the weaning time frame.
Dental histology, infancy, and multivariate analysis all feature in the latest investigations of the effects of exposure to and consumption of lead in the Roman world. It is well known that lead was widely used in Roman society.99 In scholarly discussions the pendulum has swung, over time, from exaggerating, to underrating, the extent and the consequences of lead poisoning. A recent study of sediments from the harbor at Portus and the Tiber river concluded that Roman drinking water was polluted with lead, but not to a dangerous degree.100 The authors make no reference to an earlier study which showed persuasively that Pompeians must have taken in high levels of lead through the water supply: regular repair and maintenance of the water distribution system reduced the protective crust of sinter in the pipes within the city.101 The authors’ use of skeletal evidence in relation to Herculaneum—an excellent idea in principle, because what we really need to know is how far lead built up in the bodies of consumers—is less successful, in that they take over an earlier assessment of a skeletal sample,102 which ignored the postmortem alteration of bones. The way forward may lie with the examination of tooth enamel, which is less vulnerable to environmental contamination than are buried bones. In any case, it is clear that lead cannot yet be written off as a major health risk for Roman populations, while enamel lead concentration and isotopic ratio can contribute to the topic of mobility during the Roman Age.103
Economic activities within ancient communities and workload distribution across different social groups are important indicators of past biocultural adaptation to the environment. Complementing historical accounts and archaeological evidence, skeletons can be informative as to occupational tasks. The underlying assumption is that working activities are reflected in both skeletal morphology and pathology. Repeated gestures may be responsible for skeletal overloading and biomechanical bone response.104 A number of jobs or occupations may lead to specific pathologies, or may increase the risk of skeletal traumatism.105 Craft production involving the dentition as “third hand” leave permanent signs on teeth.106
Many odontoskeletal indicators of occupational activities are presented and discussed in the literature. In some cases there is corroboration from contemporary observations from epidemiology, sports medicine, or ethnography. In more detail, one can list among the markers of odontoskeletal activities the following: degenerative joint disease; enthesopathies; cross-sectional bone geometry, specific anatomical variants (such as Allen’s fossa on the femur or kneeling facets), extramasticatory unintentional modifications of teeth (that is dental grooves, notches, chipping and specific patterns of attrition).
This line of research, after an enthusiastic beginning, has to some extent lost its momentum. Meanwhile, the informative value of enthesopathic and osteoarthritic changes has come under critical scrutiny as they are skeletal markers, whose etiology is multifactorial and complex. Many researchers now reject the claim that a specific pattern of bone changes corresponds to a precise working activity.107 Conversely, cross-sectional bone geometry has proved to be a more reliable indicator, as is indicated by some clinical studies and bioarchaeological applications.108 Variation in the cross-sectional properties of long bones is related to mechanical stress induced by habitual body postures and movements. This approach has produced good results, especially in detecting asymmetric mechanical loads. But even with this approach the risk of misinterpretation is high if some important aspect of the individual condition and life history is neglected. For instance, Figure 4.7 shows an adult male femur, anatomically normal, from Velia, whose cortical thickness distribution is anomalous and resembles that of a great ape.109 The discrepancy between the external and internal (and virtually never accessible) morphology signals is striking but explicable in terms of the presence of a completely healed tibia fracture that drove the anomalous remodeling of the femur shaft bone.
In spite of the great interest aroused by the analysis of occupational stress markers on past populations, very little is known, in this respect, about the ancient Roman world.110 The most fully investigated topic is water-related activities, as detected at the two coastal towns of Portus and Velia, in the presence of external auditory exostosis, an ear pathology caused by frequent and prolonged contact of the auditory canal with cold water.111 These were port cities, and it was only to be expected that a certain proportion of their populations would have been engaged in aquatic activities, namely, the procurement and processing of marine foods, and, especially at Portus which was the entrepôt of Rome, the maritime trade, which employed sailors, divers, porters, and ship-and wharf-workers. The incidence of the pathology is high: at Portus, 21% among adult males (aged over 15) and at Velia 35.3% suffered from EAE, and these figures certainly underestimate the percentage of the populations regularly involved in aquatic activities. Significantly, the condition does not occur among females, which points clearly to an occupational divide between the sexes. Companion studies of diet through carbon and nitrogen stable isotope analysis highlight the contribution made by marine foods to the diet of both populations. In addition, within a subgroup of Velians, a conspicuously higher consumption of food of a higher trophic level, mostly marine in origin, and a higher incidence of both EAE and skull traumas, provide strong supporting evidence of their sphere of employment.
FIGURE 4.7. Cortical thickness of the femoral diaphysys of the individual Velia 70 (above left; right femur), compared with data from a reference collection (bottom left). The anomalous cortical pattern is explained by the presence of a healed fracture on the right tibia.
Of the many sources pertaining to diet in the Roman world, direct information from skeletal tissues in the form of stable isotopes is often thought to eclipse all. Theoretically, measurements of isotopes of the atoms of carbon, nitrogen, oxygen, and hydrogen preserved in the organic and mineral portion of bone and teeth directly reflect what an individual consumed over an extended period prior to death. The power of an isotope approach is therefore its ability to provide direct, deep, and broad dietary reconstructions, independent of other sources of evidence that often appear anecdotal in comparison. As such, stable isotope analysis has been widely applied to prehistoric human remains, transforming our understanding of dietary change through time.112 With such high credentials, it is apt to ask what exactly stable isotope analysis brings to the table in later periods—particularly one already richly furnished with historical accounts of food production and consumption, and extensive assemblages of food remains.
While there has been no shortage of stable isotope studies of Roman populations in Italy, Britain, and elsewhere,113 the impact of these studies is arguably lessened compared to prehistoric examples. Expectations too have been higher. After all, dietary reconstruction implies data that may be fruitfully compared with other premodern, developing, and modern societies and economies in order to properly contextualize the Roman diet. For example, we may want to know the proportion of proteins, lipids (fats), and carbohydrates in an individual’s diet or the contribution by weight of different foodstuffs. A second expectation is to understand how diets vary both within populations: for example, according to age, sex, and social standing, and between populations with different geographic, demographic, and social dimensions. Here, we will briefly review progress to each of the goals and attempt to define the limits of this technology in an attempt to curb expectations. Numerous reviews and texts describe both the method and the rationale for inferring diet from stable isotope data; the reader is directed to these.114
HOW ACCURATE ARE DIETARY RECONSTRUCTIONS USING STABLE ISOTOPES?
Most commonly, stable isotope analysis involves measuring the carbon (δ13C) and nitrogen (δ15N) stable isotopes in collagen, the major protein in bone and tooth dentine. Carbon isotopes have also been widely measured in the mineral part of bone (bioapatite). With these two (or three) measurements inferring precise dietary composition is as challenging as it may seem. Firstly, the sources of the different elements that are measured isotopically need to be considered. For carbon and nitrogen in collagen, these are largely derived from dietary proteins; however, an additional contribution of carbon from nonprotein sources (carbohydrate and perhaps lipids) has also been demonstrated through animal feeding experiments.115 Conversely, the carbon present in bone apatite is assumed to be derived equally from all dietary carbon although, in this case, alteration of the biomineral fraction of bone during burial will affect the δ13C values. Therefore, one needs to be cautious of bioapatite isotope data, without extensive tests to check the integrity of the biomineral, which are seldom carried out.116
Secondly, the carbon and nitrogen isotope values for the sources of protein, lipids, and carbohydrate in reference foodstuffs need to be determined. Collagen from animal bone found in association with the humans is the most obvious source for this information, provided this is available, which is not always the case in funerary contexts. Also, as the bones from animals were not actually consumed, corrections based on assumptions to the corresponding consumed tissues need to be made. Notably missing, however, are isotope values for carbohydrate and proteins in plants, values for lipids in meat and fish, and values for other foods of potential dietary significance, olive oil, garum etc. The third source of uncertainty is how the carbon and nitrogen isotope values change (fractionate) as they pass through the food chain, that is, from the foodstuff to its consumer. Standard values of enrichment of the heavier isotope are often assumed but rarely scrutinized and may even vary between the types of food consumed.117
The list of uncertainties and assumptions involved in accurately reconstructing diets is often seen as insurmountable and thus it is not often attempted. Researchers often prefer to discuss comparative isotope difference between individuals or describe diets in the broadest terms, for example as “predominantly” terrestrial or marine, with little attention to differentiating the protein or carbohydrate parts of diets. When investigating the diet at large coastal necropolises, such as Velia or Isola Sacra and at Herculaneum, this approach has been particularly problematic since the data show a large variation in δ15N encompassing expected ranges for consumption of both terrestrial foods at one extreme, and marine fish at the other, but the values δ13C are entirely consistent with terrestrial foods. These seemingly mixed signals have been interpreted as reflecting dietary complexity, where substantial amounts of cereal carbohydrate are combined with various amounts of protein from marine fish and terrestrial animal products.118 This interpretation is partially informed by our knowledge of the extent of grain supply and consumption in the Roman world, so it is not without some circularity. Indeed, similar isotope values from humans at a riverine hunter-gatherer site have been interpreted as reflecting a diet rich in freshwater fish,119 and in Rome itself, this interpretation has been proposed for humans from the catacombs of St. Callixtus.120
Radiocarbon dating of human bone has been used to investigate the fish contribution to diet, since carbon from a marine source contains a known amount of “old” carbon derived from the marine reservoir. The discrepancy in radiocarbon dates between bones that are assumed to be of the same age can therefore reveal the extent of fish consumption. This approach has been applied to humans from both Herculaneum and the catacomb of Sts. Peter and Marcellinus in Rome leading to an estimation of up to 30% marine carbon in diet, which is equivalent to about 30% marine foods by dry weight.121 At Herculaneum, it is estimated that the fish contribution to dietary protein, reflected in δ15N values, is almost double given its relatively high protein content. Consequently, here, it is estimated that the remainder of the bulk diet (50–70% by weight) consists almost entirely of cereal grain, consistent with estimates based on historical records.122 The degree of freshwater fish consumption is more difficult to assess using this approach. Consumers of freshwater fish will also incorporate “old” carbon in their bone collagen, but the reservoir age may vary greatly depending on the environment. Without knowledge of this “age,” estimates are fruitless.
For most cemeteries, the luxury of dating individuals of known age is not afforded. In these cases, another approach is to use a Bayesian statistical approach that is able to take into account the uncertainties in the assumptions mentioned above.123 Such models provide a range of estimations of the proportional contribution by weight of different foodstuffs sources to an individual’s diet. The efficacy of this approach was demonstrated at Herculaneum, where the model outputs supported the interpretation from the radiocarbon dating regarding the marine contribution.124 Nevertheless, as with all other approaches, without knowledge of the reference food ranges, and without isotopic discrimination of these, we will not be able reconstruct diet with any useful precision.
Looking to the horizon, analysis of different isotope systems, such as sulfur (δ34S), hydrogen (δD), or even oxygen (δ18O), in collagen hold some promise as additional variables for dietary discrimination. Perhaps more exciting, though, is the measurement of δ15N and δ13C in the individual amino acids that make up collagen. As individual amino acids are obtained from different dietary components (either through biosynthesis or routing) in fairly predictable ways, we should be able to reconstruct diet at much greater precision than with bulk analysis. This approach has already shown to be useful for quantifying the terrestrial and marine contributions to diet and, although more methodologically challenging, is surely set to transform the field of paleodietary study.125
WHAT DIFFERENCES EXIST IN DIETS IN THE ROMAN WORLD?
Even if we are unable to quantify the Roman diet with absolute certainty, absolute differences in isotope values between individuals still offer exceptional insights. Large Roman necropolises provide excellent contexts for such studies of intrapopulation dietary variability. Differences in carbon and nitrogen isotope values between fish and terrestrial foods mean that isotope differences are more evident at coastal sites compared to inland settings. At both Velia and Isola Sacra there are significant differences in δ15N values between males and females,126 reflecting increased access to higher trophic-level marine foods by males. These differences may reflect differential access to fish, meat and cereals at the household level or are perhaps related to occupation outside the home. In contrast, there is very little evidence that individuals afforded different burials had different diets, at least, at the Italian Imperial Roman sites.
Larger isotopic differences are noticeable between archaeological sites, but here caution needs to be exercised so that like may be compared with like. This is because the marine and terrestrial fauna available at different locations, and also perhaps the grain supplied, might also vary isotopically therefore influencing the values measured in humans. Such changes in the faunal baseline means that ideally the “isoscape” needs to be carefully defined by measuring animal bones and plant materials so that isotope values in humans can be meaningfully compared through space and time. Clearly, this is no easy task at complex urban centers such as Rome, where food may be supplied from a range of sources, not all of which are available for analysis. At Isola Sacra and Velia both the humans and the terrestrial fauna show significant differences between sites. However, when the faunal differences are taken into account, it becomes clear the individuals buried at Isola Sacra still generally show higher δ15N values reflecting higher consumption of marine fish than at Velia, which may be related to differences in the social standing and occupation of the populations that these cemeteries represent.
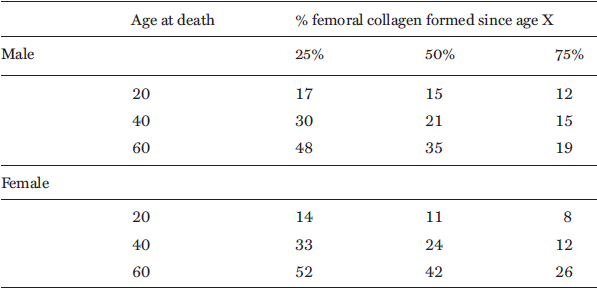
As mentioned in Section 3, the problem of demographic bias and the osteological paradox is still crucially important when interpreting stable isotope data in attritional death assemblages skewed to the frail, the old, and the young. As collagen is formed over a period of time prior to death, the data obtained from stable isotope analysis offers an integrated dietary signal over this period. Therefore, additional complexity relates to the time taken for collagen to “turn-over” in bone. The most detailed study of this phenomenon was carried out on femurs from individuals who acquired 14C into their bone during the nuclear bomb testing of the 1960s and 1970s.127 These bomb tracer experiments show differences in collagen turn-over between males and females, related to physiology. It was also seen that collagen is much more rapidly replaced in earlier than in later life (see Table 4.1). For example, a 60-year-old man still retains 25% of his collagen in his femur from before he was were 19 years old. Interestingly therefore, the sex-related differences noted above may relate to access to foods earlier in adolescence rather than in later life. Furthermore, that age-related isotopic differences should be observable at all would be remarkable given such an attenuated signal.
Nevertheless, at Isola Sacra, it has been observed that the collagen from older individuals is significantly enriched in 15N compared with younger,128 implying, at face value, greater access to fish with age. Alternatively, considering the rates of collagen turnover, it is perhaps more likely that individuals who had more access to fish over their life simply lived longer. Resolving these two interpretations, although far from trivial, has consequences for our interpretation of how food is distributed within a population and/or the effects of diet on health. Understanding both demography and tissue turnover holds the key. Two future approaches may help in this respect. First, by microsampling incrementally growing tissues that do not turn-over, such as tooth dentine, we may be able to more meaningfully compare diet across cohorts of children and adolescents.129 Hair keratin would be another useful tissue were it readily available. A second approach is to focus attention on catastrophic death assemblages, such as Herculaneum, Oplontis, and Pompeii, which record snapshots of living Roman populations avoiding many of the demographic issues that plague the field. One thing is clear, as the application of stable isotopes broadens, equal research effort needs to be directed to understanding some of these fundamental issues.
Table 4.1. Approximate collagen formation times in femoral bone based on the radiocarbon tracer experiments (Hedges et al. 2007)
Where Were They From?
Migration, the movement of individuals, families, and populations, was a feature of the Roman world. In addition to being one of the main demographic variables, migration has an obvious relevance to significant areas of enquiry such as slavery, the economy, and identity—not to mention the dissemination of cultural and religious practices and ideas.130 Historical, epigraphic, and archaeological sources are useful for the identification of both voluntary and involuntary migrants, although each kind of evidence has its limitations.131 Isotopic analysis, in addition to its role in the study of diets in the past, is an important method of investigating mobility of individuals and groups, providing key evidence for the presence of migrants at a site and information about their possible places of origin.
Isotopic analysis of oxygen (18O/16O) and strontium (87Sr/86Sr) in human bones and teeth is employed to investigate mobility and geographic origins in skeletal samples, because the ratios of these isotopes are primarily determined by characteristics of local water (for oxygen) and underlying geology (for strontium). Humans acquire these isotopic signatures during life by consuming local food and water. Thus, people born and raised in a given area will have a chemical “signature” in their tissues that is consistent with baseline δ18O and 87Sr/86Sr values for that region. The “delta” (δ) symbol is used to represent the ratio of a heavier (e.g., 18O) to lighter (e.g., 16O) isotope, measured in relation to an international standard. Strontium isotope values are usually represented as a ratio only, not with the delta symbol, because one of the isotopes (87Sr) is radiogenic, that is, it is the decay product of rubidium (87Rb). Carbon (δ13C) and nitrogen (δ15N) isotopes can be used together with oxygen and strontium isotopes to investigate mobility, based on the premise that recent immigrants to an area will display dietary signals that are different from the local isotopic signature.132 One limitation is that different diets may have similar isotopic values, so δ13C and δ15N values should not be used on their own to infer mobility.
Bones and teeth provide different information about mobility over the life course. Teeth retain isotopic information about diet from infancy through late childhood, while bones preserve the average isotopic signal from the last few decades of an adult’s life. Tooth enamel forms early in life, and once enamel mineralization is complete the isotopic signal remains unchanged. Permanent teeth start to form around birth, and the crown of the last tooth to erupt (the third molar) is complete by 12 to 16 years of age. Tooth enamel is also one of the hardest substances in the human body and preserves well in the archaeological record. Bone, in comparison, goes through rapid growth and development during childhood and adolescence, and once growth is complete it continues to remodel at a relatively constant rate, so it is a long-term signal. The rate of bone remodeling is not well understood, although it is estimated to be anywhere from 3–8% per year, and this rate decreases with advanced age.133 Other factors that can affect the in vivo isotopic signals of bone are chemical changes that occur in the burial environment (i.e., diagenesis). Strontium is particularly problematic since strontium ions readily substitute with calcium and other trace elements in archaeological soils. Thus, isotopic studies of strontium mainly use teeth, as this exchange occurs less readily, given that enamel is much harder and more chemically stable. There is also the possibility of postmortem alteration in the case of oxygen isotopes, but there are ways of detecting when the sample has been compromised by diagenetic changes.134
Oxygen in bones and teeth is largely determined by δ18O values in drinking water, which approximates δ18O in local precipitation, although a small portion of the signal can come from diet. Oxygen isotope composition of rainwater fluctuates in relation to local air temperature, humidity, distance from coastlines, latitude, and elevation.135 Oxygen consumed as drinking water is incorporated into the body’s tissues, although there is a slight offset between the values in drinking water and human tissues due to metabolic processes. Formulae exist to convert δ18O values in bones and teeth to drinking water δ18O values.136 These adjusted δ18O values can then be compared to published regional and global maps of annual average δ18O in modern precipitation, which show variability due to differences in climate and geography. Modern precipitation maps can be compared to δ18O data from Roman period skeletal samples, because temperature changes of global precipitation have been minimal over the past few millennia.
The strontium isotope signals in local food and water consumed by humans vary in relation to the 87Sr/86Sr values of the underlying bedrock.137 Generally very old geological formations (>100 mya) have higher 87Sr/86Sr ratios, while younger formations (<1–10 mya) have lower ratios.138 In contrast to oxygen, strontium undergoes little change as it passes through the food chain, and readily substitutes for calcium in the mineral portion of bones and teeth. If the underlying geology of the region is known, then regional maps of 87Sr/86Sr variation can be used to establish a local strontium range for comparison with human values. However, not all regions have detailed geological maps with known 87Sr/86Sr signals, and although strontium values are correlated with characteristics of the underlying bedrock, soil values can vary slightly due to factors such as soil transportation, which in turn affect the strontium signal of the foods consumed.139
IDENTIFYING MIGRANTS IN ROMAN ITALY
Oxygen isotope analysis of teeth from the inhabitants of Portus Romae reveals that approximately one-third of the individuals in the sample were not from Rome and its environs, including both males and females. Further, a comparison of early- and late-forming teeth from the same individuals suggested that some of these people migrated as children.140 A subsequent critique by Bruun, which raised concerns about the use of isotopes for studying human mobility in the Roman world, and underlined the limitations of the evidence, produced responses from Killgrove and Prowse.141 Bruun correctly noted that oxygen isotopes do not explain the varied motivations behind human mobility, as between voluntary migration of individuals and families, or involuntary mobility linked to slavery.142 It is clearly essential to integrate isotopic data with contextual information from historical, literary, and archaeological evidence if we are to try to understand why men, women, and/or children migrated.
Strontium and oxygen isotopic analysis of skeletal samples from two suburban Roman sites, Casal Bertone and Castellaccio Europarco, found proportionately fewer people born outside of Rome and its suburbs than at Portus, suggesting a lower rate of migration at the former sites; in addition, women and children were underrepresented in the suburban samples.143 We clearly need many more, and much larger, samples from Roman sites before we can attempt to estimate migration rates to Rome and its suburbs on the basis of skeletal evidence. As with other isotopic studies, the precise geographic origins of migrants to these places could not be identified because different regions have similar baseline values. It is likely enough that there was no standard pattern of migration nor source of migrants, even within the comparatively limited region of Rome and its environs; each cemetery sample will provide individualized histories of mobility, which can be explored in relation to age, gender, status, and other aspects of social identity.
Isotopic analyses of origins in Roman Italy to-date have for the most part studied samples from Rome itself or places in the region, where high levels of mobility are to be expected. Chenery and coworkers noted the need for studies on populations where lower levels of mobility can be anticipated.144 The rural Roman estate of Vagnari in southern Italy is one such place. Archaeological evidence from this site indicates the presence of a substantial settlement, probably an Imperial estate, along with a necropolis mainly in use from the first to third centuries CE.145 A preliminary study integrating oxygen isotope and ancient DNA (aDNA) analysis found evidence of a small number of non-locals.146 More recently, an integrated mortuary and δ18O analysis found that only 8% of the sample were nonlocal, but of those 5 individuals 2 were children under the age of 10 years and 1 was a 6-month-old infant, suggestive of mobility throughout the life course.147
LIMITATIONS AND PROSPECTS OF ISOTOPE ANALYSIS
Isotopes can be used to identify nonlocals in a skeletal sample, but they are limited in their ability to account for short-term residency or repeated movement, as people may live in multiple locations prior to death. Due to the slow turnover of bone, it will take years for the isotopic signature of the new location to be registered in bone. In oxygen isotope studies, there are a number of factors that may contribute to the consumption of water in areas that do not reflect the regional 18O/16O of rainwater. For example, Rome’s extensive aqueduct system brought in drinking water from topographically higher regions inland, an area where oxygen isotope composition of rain is significantly lighter.148 Food preparation activities, such as brewing, boiling, and stewing, may cause a slight increase in δ18O values of liquids consumed.149 Breastfeeding has also been shown to shift the isotopic signal towards heavier values in teeth that develop during this period of time. Breast milk is enriched in the heavier isotope (18O) in relation to the water consumed by the mother, so teeth forming during infancy will have slightly higher δ18O values.150 The isotope data can be adjusted to account for this effect, or teeth that formed after weaning can be used. A final limitation of oxygen isotope analysis is that it does not pinpoint a specific geographic location associated with a particular δ18O value. One possible way to deal with this issue is to use modern human values as “proxies” for local oxygen values,151 although obtaining sufficient modern samples for this type of analysis can be challenging. In any case, the integration of oxygen and strontium isotope data will improve our chances of identifying possible geographic origins.
With respect to strontium, Slovak and Paytan stressed that there must be sufficient geologic variability between regions under study in order to detect mobility.152 Further, if marine foods are a significant component of a population’s diet, the strontium values in teeth may reflect seawater 87Sr/86Sr values and confound the interpretations of mobility between coastal and inland regions.153 Killgrove and Montgomery have published estimates for strontium isotope ratios in and around the city of Rome based on geological maps of Italy, and Tafuri and coworkers for Neolithic sites in the Gargano region of southern Italy.154 There are, however, no large-scale regional studies of bio-available strontium in the Mediterranean region, with the exception of work done by Nafplioti for Greece.155 It is possible to have multiple regions with similar strontium isotope signals—hence the advantage of using oxygen and strontium together to better define possible geographic origins of migrants. Finally, regular food items may come from different regions (with different 87Sr/86Sr signals), so the strontium isotope signals in human tissues represent an average of these sources. To control for these issues with strontium, isotope studies use ‘bioavailable’ 87Sr/86Sr values from local, nonmigratory animals or modern plant remains from the same region represent local strontium values.156 It is assumed that these values reflect the average 87Sr/86Sr values of food available to humans in the region.157
The number of isotopic studies of mobility in the Roman world is rapidly increasing, particularly on samples from Roman Britain.158 More work is needed on comparable human isotope data from all geographic areas in the Mediterranean region and other areas of the Roman Empire, as well as more information on local drinking water values (for oxygen) and baseline strontium signatures from local plants and animals in order to refine the ranges for specific geographic areas. The integration of dietary (C, N) and mobility (O, Sr) isotopic data may help to further clarify the geographic origins of individuals.159 Lead (Pb) is also being used to identify migrants in Roman Britain in relation to the increased postconquest use of lead in both industrial and household contexts.160 Only a small number of studies have integrated isotopic and aDNA evidence to explore geographic origins and biological relationships,161 but this is another possible future avenue of research.
These are destructive analyses, even though the amounts needed for isotopic analysis are relatively small, so research questions should be carefully designed before testing takes place. If our ultimate aim is to understand how and why people (adult males, women, children, or families) migrated, then isotopic data needs to be interpreted within the social and historical framework obtained from literary, epigraphic, and archaeological evidence. Each kind of evidence has its own strengths and weaknesses, but when these apparently disparate elements and diverse methodologies are brought together, we gain a much more nuanced picture of human mobility in the past.
Conclusion
Bones and teeth are a uniquely valuable source of information on population structure, diet, disease, health, social behavior, migration. The data on offer are quantitatively impressive and cross-class, and there is more to come.
The data do not speak for themselves: they have to be interpreted. This statement, of course, applies also to other kinds of evidence from antiquity. There are however specific problems with the interpretation of skeletal data. The most vocal and trenchant criticisms have come from within the anthropological community itself. One famous article declared paleodemography defunct; another hammered some more nails into the coffin of paleodemography, and, into the bargain, proclaimed that a prominent, current approach to paleopathology is a false trail.
Biological anthropology is an imperfect science. It is, however, a science, and also one that is on the advance, constantly learning from its mistakes, and assessing and improving the analyses and methodologies that it employs. Is paleodemography dead? The report of its death is greatly exaggerated.162 True, one must lower one’s sights and have modest objectives. One cannot expect to arrive at a plausible reconstruction of the demographic shape of a community from a particular skeletal sample. This is not actually impossible, as the case of Velia in South Italy demonstrates; rather it is not normally possible. But if that is the case, why not? What are the factors that produce skewed profiles? A negative result of an experiment is not worthless, as any scientist knows; it may lead to productive enquiries and, eventually, plausible explanations. Meanwhile there are other dimensions of paleodemography that remain thus far relatively unexplored, such as the comparative demography of the dead of the cemeteries and the “living” victims of catastrophes, not to mention the bone evidence for a key demographic variable, migration.
The critics themselves hint at the way forward: isolate a particular age group, study it in depth, bring into play the latest techniques from a whole range of scientific disciplines, and make comparisons, ideally between communities from similar environments. Thus, paleopathology is moving away from a previous, narrow focus on individual cases of eccentric diseases, and on some specific but problematic markers of stress in the form of bone lesions, and advancing towards a broad reconstruction of the morbidity and mortality regime of whole communities.
Our discussion has highlighted an age cohort in the case of which promising research is emerging, and converging: infants and juveniles. Age at death is more easily diagnosed here than is the case with adults. Dental histology has made a breakthrough in enabling us to count the time-markers on the crowns as they develop from birth to death. In addition, state-of-the-art techniques make possible a close analysis of the impact of disease-induced stress, and the infusion of potentially toxic minerals, centering on, but extending beyond, the critical period of weaning.
Stable isotope analysis is a relatively new methodology, and it is all too easy to focus on its present limitations and deficiencies while failing to appreciate its singular contribution—in providing an integrated dietary signal over an extended period of time, or identifying the nonlocal element in a population—and underestimating its potential to reconstruct diets and uncover patterns of migration. We can expect steady progress to be made, for example, in quantifying the terrestrial and marine contributions to the diet, and in locating the geographic origins of migrants.
A multidisciplinary approach holds the key to further advances in our knowledge. On the scientific side, histology, microbiology, biochemical medicine, among other disciplines, are combining forces, for example, in the identification of infectious diseases, such as malarial parasitosis, in skeletal samples. Archaeology is the handmaid of anthropology. What of history? History at present stands on the sidelines, although historians and anthropologists have overlapping interests, most obviously in the areas of diet, health, the structure and movement of populations, and the urban environment. For historians (or archaeologists) to ignore, or write off, the contribution of biological anthropology and adjacent sciences to our knowledge of ancient society is not a sensible option.
Notes
1. André 1981.
2. Scheidel 2012.
3. Larsen 2006.
4. Bondioli and Sperduti 2011.
5. Bocquet-Appel and Masset 1982; Wood et al. 1992.
6. See, e.g., Ferembach et al. 1979; Buikstra and Mielke 1985; Buikstra and Ubelaker 1994, Krogman and İsçan 1986; İsçan and Kennedy 1989; White and Falkens 2000; Garvin 2012.
7. Walrath et al. 2004.
8. Weiss 1972.
9. Walker 2008; Guyomarc’h and Bruzek 2011; Garvin 2012.
10. Krogman and İsçan 1986; Rogers and Saunders 1994.
11. Spradley and Jantz 2011.
12. Lee et al. 2015.
13. See review in Sutter 2003.
14. Mays and Faerman 2001; Hassan et al. 2014. See Chapter 6.
15. Spirduso 1995; Schmitt et al. 2002; Cox 2000; Corsini et al. 2005.
16. For a review, see Schaefer et al. 2009.
17. Radiological: Macchiarelli et al. 1990. Histological: see synthesis in Kemkes-Grottenhaler 2002.
18. Klepinger 2006.
19. Soomer et al. 2003; Baccino et al. 1999; Garvin et al. 2012.
20. See Nawrocki 2010 for a full discussion.
21. Spirduso 1995.
22. Merritt 2015.
23. Katz and Suchey 1989; Schmitt 2004; Falys et al. 2006; Hens et al. 2008; Merritt 2014; Mays 2014.
24. Bocquet-Appel and Masset 1982, 329.
25. See, for instance, Gowland and Chamberlain 2002; Hoppa and Vaupel 2002; Soomer et al. 2003; Gowland 2007; Jackes 2011.
26. Kemkes-Grottenhaler 2002.
27. Acsàdi and Nemeskéri 1970 (combined); Lovejoy et al. 1985 (summary age); Boldsen et al. 2002 (transition).
28. Falys and Lewis 2011; Garvin and Passalacqua 2012.
29. Kimmerle et al. 2008.
30. Maples 1989, 323.
31. Falys and Prangle 2015; Tang et al. 2014.
32. Stoyanova et al., 2015.
33. See most recently AlQahtani 2009.
34. Antoine et al. 2009; Birch and Dean 2009; Mahoney 2011.
35. Sabel et al. 2008.
36. Macchiarelli et al. 2006; Dean 2009; Dean and Cole 2013.
37. Smith et al. 2006.
38. Bocquet-Appel and Masset 1982, 1985; Van Gerven and Armelagos 1983; Konigsberg and Frankenberg 1994, 2002; Bocquet-Appel and Masset 1996; Paine and Harpending 1998; Hoppa and Vaupel 2002; Jackes 2011.
39. Green et al. 1974; Johansson and Horowitz 1986.
40. McNeill 1979; Scheidel 2001a; Sperduti 1995; Paine and Storey 2006.
41. Farwell and Molleson 1993. Journals: Molleson 1989; Lewis 2010; Redfern and Dewitte 2011a.
42. Sperduti 1995.
43. Coale and Demeny 1983; on the viability of model life tables, see Scheidel 2001b; Hin 2013.
44. Mays and Faerman 2001; Hassan et al. 2014; Sperduti et al. in press.
45. Lewis 2011, 4.
46. Luongo et al. 2003; Frey et al. 2010.
47. Fattore et al. 2012.
48. Cf. Jackes 2011.
49. Weaver et al. 2000.
50. See for instance Bisel 1991; Capasso 2001; Paine et al. 2009; Gowland and Garnsey 2010; Petrone et al. 2011.
51. Cucina et al. 1998; Belcastro et al. 2007; Manzi et al. 1999.
52. Gowland and Redfern 2010.
53. Manzi et al. 1989; Ricci et al. 1997; Salvadei et al. 2001; Facchini et al. 2004; Fitz-Gerald et al. 2006.
54. Bisel 1988; Bonfiglioli et al. 2003; Prowse et al. 2008 (diet); Cho and Stout 2011; Beauchesne and Agarwal 2014 (processes).
55. Sperduti and Manzi 1990; Ottini et al. 2001; Lazer 2011; Minozzi et al. 2012a.
56. Rubini et al. 2014.
57. Capasso and Di Tota 1999 (Herculaneum); Canci et al. 2005 (Rome).
58. Lunardini et al. 2005; Minozzi et al. 2012b.
59. Lunardini et al. 2008 (spondylitis); Minozzi et al. 2013 (gout).
60. For a detailed description of orthopedic pathologies in Roman Imperial age, see Piccioli et al. 2015.
61. Sallares 2002.
62. Ortner and Putschar 1981.
63. Ragsdale and Lehmer 2012; Zuckerman et al. 2016.
64. For an updated review, see Spigelman 2012 and below, Chapter 6.
65. Bos et al. 2011; Schuenemann et al. 2011; Bouwman et al. 2012.
66. Jaeger and Iñiguez 2014; Warinner et al. 2014; Weyrich et al. 2015.
67. Ortner 1991; Wood et al. 1992; Zuckerman et al. 2012, 2015; DeWitte and Stojanowski 2015.
68. Walker et al. 2009; Setzer 2014.
69. Stuart-Macadam 1989, 1992.
70. Hawass et al. 2010; Sallares and Gomzi 2001; Marciniak et al. 2016. See below, Chapter 6.
71. Mirdha et al. 1999.
72. Lugnano in Teverina, Inwood, J., personal communication.
73. Abbott 2001; Sallares et al. 2004.
74. Drancourt et al. 1998; Zink et al. 2006; Bos et al. 2012; Wagner et al. 2014.
75. DeWitte and Wood 2008.
76. Santos and Roberts 2001.
77. Mays et al. 2001, 2002.
78. Molto 1990.
79. Hough 2001; Weiss and Jurmain 2007; Musumeci et al. 2015.
80. Palubeckaitė et al. 2002.
81. Duray 1996; Armelagos et al. 2009.
82. Rothman and Greenlander 1998.
83. Bondioli et al. 2016.
84. Bridges 1993.
85. Macchiarelli et al. 1994; Davis et al. 2013; Jacobi and Danforth 2002; Waldron and Rogers 1991.
86. Ortner 1991, 2012; Buikstra and Ubelaker 1994.
87. Boldsen and Milner 2012.
88. Wood, et al. 1992.
89. Storey 1997; DeWitte and Bekvalac 2010; Boldsen et al. 2015.
90. DeWitte 2014.
91. Capasso 2001; Lazer 2011.
92. Fattore et al. 2012.
93. Crowe et al. 2010; Craig et al. 2013 (Isola Sacra and Velia); Martyn et al. 2015 (Herculaneum).
94. See Giannecchini and Moggi-Cecchi 2008.
95. Sandberg et al. 2014.
96. For a review see Fulminante 2015.
97. Humphrey et al. 2007; Bondioli et al. 2011.
98. FitzGerald et al. 2006.
99. E.g., Retief and Cilliers 2005.
100. Delile et al. 2014.
101. Keenan-Jones et al. 2012.
102. Bisel and Bisel 2002.
103. Montgomery et al. 2010.
104. Ruff et al. 1984; Ruff 1987; Chenorkian et al.1990.
105. Crowe et al. 2010 (pathologies); Brasili et al. 2004; Kanz and Grosschmidt 2006; Van der Merwe et al. 2010 (traumatism).
106. Frayer and Russell 1987; Erdal 2008; Waters-Rist et al. 2010; Lorkiewicz 2010; Sperduti et al. 2011.
107. Jurmain et al. 2012; Villotte and Knüsel 2013.
108. Shaw and Stock 2009a, b (clinical studies); Maggiano et al. 2008; Sládek et al. 2007 (archaeology).
109. See Bondioli et al. 2010 for the methodology.
110. Farwell and Molleson 1993; Petrone 1993; Sperduti 1997; Catalano et al. 2010.
111. Manzi et al. 1991; Crowe et al. 2010; Sperduti et al. 2012. These articles also provide clinical and epidemiological references for the etiology of the pathology.
112. Richards et al. 2003; Richards et al. 2005; Richards et al. 2000.
113. Prowse et al. 2004; Craig et al. 2009; Craig et al. 2013; Müldner et al. 2011; Richards and Hedges 1998; Keenleyside et al. 2009.
114. E.g., Katzenberg 2000; Sealy 2001; van Klinken et al. 2000.
115. Hedges 2006.
116. Salesse et al. 2014.
117. O’Connell et al. 2012; Hedges and Reynard 2007.
118. Craig et al. 2009; Prowse et al. 2004; Craig et al. 2013.
119. Cook et al. 2001.
120. Rutgers et al. 2009.
121. Craig et al. 2013; Salesse et al. 2014.
122. Foxhall and Forbes 1982.
123. Fernandes et al. 2014.
124. Fernandes 2015.
125. Colonese et al. 2014; Naito et al. 2010.
126. Craig et al. 2009; Prowse et al. 2004.
127. Hedges et al. 2007.
128. Prowse et al. 2005.
129. Beaumont et al. 2013.
130. E.g., Noy 2000; Scheidel 2004, 2005, 2007; de Ligt and Northwood 2008; Eckardt 2010; Holleran 2011; de Ligt 2012; Hin 2013; de Ligt and Tacoma 2016.
131. Eckardt et al. 2014.
132. E.g., Dupras and Schwarcz 2001; Chenery et al. 2010, 2011; Müldner et al. 2011.
133. Parfitt 2004; Hedges et al. 2007; Stepańczak et al. 2014.
134. Hedges 2002.
135. Yurtsever and Gat 1981; Gat 1996.
136. Daux et al. 2008; Pollard et al. 2011a; Chenery et al. 2012.
137. Slovak and Paytan 2012.
138. Bentley 2006.
139. Chenery et al. 2010, 2011.
140. Prowse et al. 2007.
141. Bruun 2010; Killgrove 2010a; Prowse 2016.
142. Bruun 2010.
143. Killgrove and Montgomery 2016; cf. Killgrove 2010b; 2013.
144. Chenery et al. 2010.
145. Small 2011.
146. Prowse et al. 2010.
147. Prowse 2016.
148. Lightfoot et al. 2014.
149. Brettell et al. 2012; Lightfoot et al. 2014.
150. Roberts et al. 1998; Wright and Schwarcz 1998.
151. E.g., Prowse et al. 2007.
152. Slovak and Paytan 2012.
153. Prowse et al. 2007; Slovak and Paytan 2012.
154. Killgrove and Montgomery 2016; Tafuri and coworkers 2015.
155. Nafplioti 2011.
156. Bataille and Bowen 2012; Chenery et al. 2011.
157. For a detailed discussion of the use of strontium isotopes in archaeology, see Bentley 2006 and Slovak and Paytan 2012.
158. E.g., Evans et al. 2006; Chenery et al. 2010, 2011; Montgomery et al. 2010; Müldner et al. 2011; Eckardt et al. 2014.
159. E.g., Pollard et al. 2011b.
160. E.g., Montgomery et al. 2010; Shaw et al. 2016.
161. E.g., Prowse et al. 2010; Sofeso et al. 2012.
162. Mark Twain, adapted; see Van Gerven and Armelagos 1983.
References
Abbott, A. 2001. “Earliest malaria DNA found in Roman baby graveyard.” Nature 412 (6850): 847. DOI: 10.1038/35091226.
Acsàdi, G., and J. Nemeskéri. 1970. History of Human Life Span and Mortality. Budapest: Akadémiai Kiadò.
AlQahtani, S. J. 2009. Atlas of Human Tooth Development and Eruption. London: Queen Mary and Westfield College.
André, J. 1981. L’alimentation et la cuisine à Rome. Paris: Belles Lettres.
Antoine, D., S. Hillson, and M. C. Dean. 2009. “The developmental clock of dental enamel: a test for the periodicity of prism cross-striations in modern humans and an evaluation of the most likely sources of error in histological studies of this kind.” Journal of Anatomy 214: 45–55. DOI: 10.1111/j.1469–7580.2008.01010.x.
Armelagos, G., et al. 2009. “Enamel hypoplasia and early mortality: bioarcheological support for the Barker hypothesis.” Evolutionary Anthropology 18: 261–271. DOI: 10.1002/evan.20239.
Baccino, E., et al. 1999. “Evaluation of seven methods of estimating age at death from mature human skeletal remains.” Journal of Forensic Sciences 44: 931–936.
Bataille, C., and G. Bowen. 2012. “Mapping 87Sr/86Sr variations in bedrock and water for large scale provenance studies.” Chemical Geology 304–305: 39–52.
Beauchesne, P., and S. C. Agarwal. 2014. “Age-related cortical bone maintenance and Loss in Imperial Roman population.” International Journal Osteoarchaeology 24: 15–30. DOI: 10.1002/oa.1303.
Beaumont, J., et al. 2013. “Childhood diet: a closer examination of the evidence from dental tissues using stable isotope analysis of incremental human dentine.” Archaeometry 55 (2): 277–295. DOI: 10.1111/j.1475–4754.2012.00682.x.
Belcastro, G., et al. 2007. “Continuity or discontinuity of the life-style in central Italy during the Roman Imperial Age–Early Middle Ages transition: diet, health, and behavior.” American Journal of Physical Anthropology 132: 381–394. DOI: 10.1002/ajpa.20530.
Bentley, R. A. 2006. “Strontium isotopes from the earth to the archaeological skeleton: a review.” Journal of Archaeological Method and Theory 13: 135–187. DOI: 10.1007/s10816–006–9009-x.
Birch, W., and M. C. Dean. 2009. “Rates of enamel formation in human deciduous teeth.” In Comparative Dental Morphology: Frontiers of Oral Biology 13, eds. T. Koppe, G. Meyer, and K. W. Alt. Basel: Karger, 116–120. DOI: 10.1159/000242402.
Bisel, S. 1988. “Nutrition in 1st Century Herculaneum.” Anthropologie Brno 26: 61–66.
Bisel, S. 1991. “The human skeletons of Herculaneum.” International Journal of Anthropology 6: 1–20. DOI: 10.1007/BF02447284.
Bisel, S., and J. Bisel. 2002. “Health and Nutrition at Herculaneum: an examination of human skeletal remains.” In The Natural History of Pompeii, eds. W. F. Jashemski and F. G. Meyer. Cambridge: Cambridge University Press, 451–475.
Bocquet-Appel, J.-P., and C. Masset. 1982. “Farewell to paleodemography.” Journal of Human Evolution 11 (4): 321–333.
Bocquet-Appel, J.-P., and C. Masset. 1985 “Paleodemography: resurrection or ghost?” Journal of Human Evolution 14 (2): 107–111.
Bocquet-Appel, J.-P., and C. Masset. 1996. “Palaeodemography: expectancy and false hope.” American Journal of Physical Anthropology 89: 235–256. DOI: 10.1002/(SICI)1096–8644(199604)99:4<571::AID-AJPA4>3.0.CO;2-X.
Boldsen, J. L., and G. R. Milner. 2012. “An epidemiological approach to paleopathology.” In A Companion to Paleopathology, ed. A. L. Grauer Malden, MA: Wiley-Blackwell, 114–132. DOI: DOI: 10.1002/9781444345940.ch7.
Boldsen, J. L., G. R. Milner, and S. Weise. 2015. “Cranial vault trauma and selective mortality in medieval to early modern Denmark.” Proceedings of the National Academy of Sciences of the United States of America 112 (6): 1721–1726. DOI: 10.1073/pnas.1412511112.
Boldsen, J. L., et al. 2002. “Transitional analysis: a new method for estimating age from skeletons.” In Paleodemography: Age Distributions from Skeletal Samples, eds. R. D. Hoppa, and J. W. Vaupel. New York: Cambridge University Press, 73–106.
Bondioli, L., W. Müller, and P. F. Rossi. 2001. “Evaluation of secretion vs. maturation in human dental enamel from LA-ICPMS compositional profiles.” American Association of Physical Anthropologists Supplement 144 (S52): 93.
Bondioli, L., and A. Sperduti. 2011. “Comunità dei morti ed individui scheletrici: dallo studio di popolazioni alla ricostruzione della storia biologica individuale” In Dalla nascita alla morte: antropologia e archeologia a confronto. Atti dell’Incontro Internazionale di studi in onore di Claude Lévi-Strauss, ed. V. Nizzo. Roma: Museo Nazionale Preistorico Etnografico Luigi Pigorini, 431–460.
Bondioli L., et al. 2010. “Technical note: morphometric maps of long bone shafts and dental roots for imaging topographic thickness variation.” American Journal of Physical Anthropology 142: 328–334. DOI: 10.1002/ajpa.21271.
Bondioli, L., et al. 2016. “Diet and health in Central-Southern Italy during the Roman Imperial time.” ACTA IMEKO 5 (2): 19–25.
Bonfiglioli, B., P. Brasili, and M. G. Belcastro. 2003. “Dental-alveolar lesions and nutritional habits: paleopathology of Roman skeletons of a Roman Imperial Age population (1st–4th c.AD): Quadrelli (Molise, Italy).” Homo 54: 35–56.
Bos, K. I., et al. 2011. “A draft genome of Yersinia pestis from victims of the Black Death.” Nature 478 (7370): 505–510. DOI: 10.1038/nature10549.
Bos, K. I., et al. 2012. “Yersinia pestis: new evidence for an old infection.” PLoS ONE 7 (11): e49803. DOI: 10.1371/journal.pone.0049803.
Bouwman, A. S., et al. 2012. “Genotype of a historic strain of Mycobacterium tuberculosis.” Proceedings of the National Academy of Sciences of the United States of America 109 (45): 18511–18516. DOI: 10.1073/pnas.1209444109.
Brasili, P., E. Bianchi, and A. R. Ventrella. 2004. “Traumatic events and life-style in ancient Italian populations.” Collegium Antropologicum 28: 179–191.
Brettell, R., J. Montgomery, and J. Evans. 2012. “Brewing and stewing: the effect of culturally mediated behaviour on the oxygen isotope composition of ingested fluids and the implications for human provenance studies.” Journal of Analytic Atomic Spectrometry 27: 778–785. DOI: 10.1039/C2JA10335D.
Bridges, P. S. 1993. “The effect of variation in methodology on the outcome of osteoarthritic studies.” International Journal of Osteoarchaeology 3: 289–295. DOI: 10.1002/oa.1390030407.
Bruun, C. 2010. “Water, oxygen isotopes, and immigration to Ostia-Portus.” Journal of Roman Archaeology 23: 109–132. DOI: 0.1017/S1047759400002324.
Buikstra, J. E., and J. H. Mielke. 1985. “Demography, diet and health.” In The Analysis of Prehistoric Diets, eds. R. I. Gilbert and J. H. Mielke. Orlando: Academic Press, 359–422.
Buikstra, J. E., and D. H. Ubelaker. 1994. Standards for Data Collection from Human Skeletal Remains. Fayetteville: Arkansas Archeological Survey Research.
Canci, A., et al. 2005. “A case of healing spinal infection from Classical Rome.” International Journal of Osteoarchaeology 15: 77–83. DOI: 10.1002/oa.734.
Capasso, L. 2001. I fuggiaschi di Ercolano. Rome: L’Erma Di Bretschneider.
Capasso, L., and G. Di Tota. 1999. “Tuberculosis in Herculaneum (79 AD).” In Tuberculosis: Past and Present, eds. G. Palfi et al. Golden Book Publishers and Tuberculosis Foundation: Budapest, 463–467.
Catalano, P., et al. 2010. “Health status and life style in Castel Malnome (Rome, I–II cent. A. D.).” Medicina nei Secoli 22: 11–28.
Chenery, C., H. Eckardt, and G. Müldner. 2011. “Cosmopolitan Catterick? Isotopic evidence for population mobility on Rome’s northern frontier.” Journal of Archaeological Science 38 1525–1536. DOI: 10.1016/j.jas.2011.02.018.
Chenery, C., et al. 2010. “Strontium and stable isotope evidence for diet and mobility in Roman Glouchester, UK.” Journal of Archaeological Science 37: 150–163. DOI: 10.1016/j.jas.2009.09.025.
Chenery, C. A., et al. 2012. “The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite.” Rapid Communication in Mass Spectrometry 26: 309–319. DOI: 10.1002/rcm.5331.
Chenorkian, R., et al. 1990. “Pour une archéologie du geste.” Travaux du Lapmo: 147–151.
Cho, H., and S. D. Stout. 2011. “Age-associated bone loss and intraskeletal variability in the Imperial Romans.” Journal of Anthropological Science 89: 109–125. DOI: 10.4436/jass.89007.
Coale, A. J., and P. Demeny. 1983. Regional Model Life Tables and Stable Populations. Princeton: Princeton University Press.
Colonese, A. C., et al. 2014. “Long-Term Resilience of Late Holocene Coastal Subsistence System in Southeastern South America.” PLoS ONE 9 (4): e93854. DOI: 10.1371/journal.pone.0093854.
Cook, G. T., et al. 2011. “A freshwater diet-derived C-14 reservoir effect at the stone age sites in the iron gates gorge.” Radiocarbon 43 (2): 453–460. DOI: 10.2458/azu_js_rc.43.3985.
Corsini, M., A. Schmitt, and J. Bruzek. 2005. “Ageing process variability on the human skeleton: artificial network as an appropriate tool for age at death assessment.” Forensic Science International 148: 163–167. DOI: 10.1016/j.forsciint.2004.05.008.
Cox, M. 2000. “Ageing adults from the skeleton.” In Human Osteology in Archaeology and Forensic Science, eds. M. Cox and S. Mays. London: Greenwich Medical Media, 61–81.
Craig, O. E., et al. 2009. “Stable isotopic evidence for diet at the imperial Roman coastal site of Velia (1st and 2nd Centuries AD) in Southern Italy.” American Journal of Physical Anthropology 139: 572–583. DOI: 10.1002/ajpa.21021.
Craig, O. E., et al. 2013. “Evaluating marine diets through radiocarbon dating and stable isotope analysis of victims of the AD 79 eruption of Vesuvius.” American Journal of Physical Anthropology 152: 345–352. DOI: 10.1002/ajpa.22352.
Crowe, F., et al. 2010. “Water-Related occupations and diet in two Roman coastal communities (Italy, first to third century AD): Correlation between stable carbon and nitrogen isotope values and auricular exostosis prevalence.” American Journal of Physical Anthropology 142: 355–366. DOI: 10.1002/ajpa.21229.
Cucina, A., D. Mancinelli, and A. Coppa. 1998. “Demography, nutrition and stress in the Italian Peninsula from the Copper Age to the Roman Imperial Age.” Rivista di Antropologia 76: 135–138.
Daux, V., et al. 2008. “Oxygen isotope fractionation between human phosphate and water revisited.” Journal of Human Evolution 55 (6): 1138–1147. DOI: 10.1016/j.jhevol.2008.06.006.
Davis, C. B., et al. 2013. “Patterns of interobserver error in the scoring of entheseal changes.” International Journal of Osteoarchaeology 23: 147–151. DOI: 10.1002/oa.2277.
Dean, M. C. 2009. “Extension rates and growth in tooth height of modern human and fossil hominin canines and molars.” In Frontiers of Oral Biology: Interdisciplinary Dental Morphology, 13, eds. T. Koppe, G. Meyer, and K.W. Alt. Karger: Basel, 68–73. DOI: 10.1159/000242394.
Dean, M.C., and T. J. Cole. 2013. “Human life history evolution explains dissociation between the timing of tooth eruption and peak rates of root growth.” PLoS ONE 8 (1): e54534. DOI: 10.1371/journal.pone.0054534.
Delile, H., et al. 2014. “Lead in ancient Rome’s city waters.” Proceedings of National Academy of Sciences of the United States of America 111 (18): 6594–6599. DOI: 10.1073/pnas.1400097111.
DeWitte, S. N. 2014. “Differential survival among individuals with active and healed periosteal new bone formation.” International Journal of Paleopathology 7: 38–44. DOI: 10.1016/j.ijpp.2014.06.001.
DeWitte, S. N., and J. Bekvalac. 2010, “Oral health and frailty in the medieval English cemetery of St Mary Graces.” American Journal of Physical Anthropology 142: 341–354. DOI: 10.1002/ajpa.21228.
DeWitte, S. N., and C. M. Stojanowski. 2015. “The Osteological Paradox 20 years later: past perspectives, future directions.” Journal of Archaeological Research. DOI 10.1007/s10814–015–9084–1.
DeWitte, S. N., and J. W. Wood. 2008. “Selectivity of Black Death mortality with respect to pre-existing health.” Proceedings of the National Academy of Sciences of the United States of America 105 (5): 1436–1441. DOI: 10.1073/pnas.0705460105.
Drancourt, M., et al. 1998. “Detection of 400-year-old Yersinia pestis DNA in human dental pulp: an approach to the diagnosis of ancient septicemia.” Proceedings of the National Academy of Sciences of the United States of America 95 (21): 12637–12640. DOI: 10.1073/pnas.95.21.12637.
Dupras, T. L., and H.P. Schwarcz. 2001. “Strangers in a strange land: Stable isotope evidence for human migration in the Dakhleh Oasis, Egypt.” Journal of Archaeological Science 28: 1199–1208. DOI: 10.1006/jasc.2001.0640.
Duray, S. 1996. “Dental indicators of stress and reduced age at death in prehistoric native Americans.” American Journal of Physical Anthropology 99, 275–286. DOI: 10.1002/(SICI)1096–8644(199602)99:2<275::AID-AJPA5>3.0.CO;2-Y.
Eckardt, H., ed. 2010. Roman Diasporas: Archaeological Approaches to Mobility and Diversity in the Roman Empire. Portsmouth, RI: Journal of Roman Archaeology. Supplementary volume 78.
Eckardt, H., G. Mülnder, and M. Lewis. 2014. “People on the move in Roman Britain.” World Archaeology 46 (4): 534–550. DOI: 10.1080/00438243.2014.931821.
Erdal, Y.S. 2008. “Occlusal grooves in anterior dentition among Kovuklukaya inhabitants (Sinop, Northern Anatolia, 10th Century AD).” International Journal of Osteoarchaeology 18: 152–166. DOI: 10.1002/oa.925.
Evans, J., N. Stoodley, and C. Chenery. 2006. “A strontium and oxygen isotope assessment of a possible fourth century immigrant population in a Hampshire cemetery, England.” Journal of Archaeological Science 33: 265–272. DOI: 10.1016/j.jas.2005.07.011.
Facchini, F., E. Rastelli, and P. Brasili. 2004. “Cribra orbitalia and cribra cranii in Roman skeletal remains from the Ravenna area and Rimini (I–IV Century AD).” International Journal of Osteoarchaeology 14: 126–136. DOI: 10.1002/oa.717.
Falys, C. G., H. Schutkowski, and D. Weston. 2006. “Auricular surface ageing: worse than expected? A test of the revised method on a documented historic skeletal assemblage.” American Journal of Physical Anthropology 130: 508–513. DOI: 10.1002/ajpa.20382.
Falys, C. G., and M. E. Lewis. 2011. “Proposing a way forward: a review of standardisation in the use of age categories and ageing techniques in osteological analysis (2004–2009).” International Journal of Osteoarchaeology 21: 704–716. DOI: 10.1002/oa.1179.
Falys, C. G., and D. Prangle. 2015. “Estimating age of mature adults from the degeneration of the sternal end of the clavicle.” American Journal of Physical Anthropology 156: 203–214. DOI: 10.1002/ajpa.22639.
Farwell, D. E., and T. I. Molleson. 1993. Excavations at Poundbury 1966–80. Volume II: The Cemeteries. Dorchester: Dorset Natural History and Archaeology Society Monograph 11.
Fattore, L., et al. 2012. “The Roman skeletal remains from Herculaneum: new evidence from the excavation of the fornici 7, 8, 9, 10, and 11.” American Association of Physical Anthropologists Suppl. 81st Annual Meeting. Portland, Oregon: 11–14, April 2012. 142 DOI 10.1002/ajpa.22032.
Ferembach, D., I. E., Schwidetzky, and M. Stloukal. 1979. “Raccomandazioni per la determinazione dell’età e del sesso sullo scheletro.” Rivista di Antropologia 60: 5–51.
Fernandes, R. 2015. “A simple(r) model to predict the source of dietary carbon in individual consumers.” Archaeometry: 500–512. DOI: 10.1111/arcm.12193.
Fernandes, R., et al. 2014. “Food reconstruction using isotopic transferred signals (FRUITS): a bayesian model for diet reconstruction.” PLoS ONE 9 (2): e87436. DOI: 10.1371/journal.pone.0087436.
FitzGerald, C., et al. 2006. “Health of infants in an Imperial Roman skeletal sample: perspective from dental microstructure.” American Journal of Physical Anthropology 130: 179–189. DOI: 10.1002/ajpa.20275.
Foxhall, L., and H. A. Forbes. 1982. “Sitometria: The role of grain as a staple food in classical antiquity.” Chiron 12: 41–90.
Frayer, D. W., and M. D. Russell. 1987. “Artificial grooves in the Krapina Neanderthal teeth.” American Journal of Physical Anthropology 74: 393–405. DOI: 10.1002/ajpa.1330740311.
Frey, B. S., D. A. Savage, and B. Torgler. 2010. “Interaction of natural survival instincts and internalized social norms exploring the Titanic and Lusitania disasters.” Proceedings of the National Academy of Sciences of the United States of America 16 (11): 4862–4865. DOI: 10.1073/pnas.0911303107.
Fulminante, F. 2015. “Infant feeding practices in Europe and the Mediterranean from prehistory to the Middle Ages: a comparison between the historical sources and bioarchaeology.” Childhood in the Past 8: 24–47. DOI: 10.1179/1758571615Z.00000000026.
Garvin, H. M. 2012. “Adult sex determination: methods and application.” In A Companion to Forensic Anthropology, ed. D. C. Dirkmaat. Malden, MA: Wiley-Blackwell, 239–247. DOI: 10.1002/9781118255377.ch12.
Garvin, H. M., and N. V. Passalacqua. 2012. “Current practices by forensic anthropologists in adult skeletal age estimation.” Journal of Forensic Sciences 57: 427–433. DOI: 10.1111/j.1556–4029.2011.01979.x.
Garvin, H. M., et al. 2012. “Developments in forensic anthropology: age at-death estimation.” In A Companion to Forensic Anthropology, ed. D.C. Dirkmaat. Malden, MA: Wiley-Blackwell, 202–223. DOI: 10.1002/9781118255377.ch10.
Gat, J. R. 1996. “Oxygen and hydrogen isotopes in the hydrologic cycle.” Annual Review of Earth and Planetary Sciences 24: 225–262. DOI: 10.1146/annurev.earth.24.1.225.
Giannecchini, M., and J. Moggi-Cecchi. 2008. “Stature in archeological samples from central Italy: methodological issues and diachronic changes.” American Journal of Physical Anthropology 135: 284–292. DOI: 10.1002/ajpa.20742.
Gowland, R. L. 2007. “Age, ageism and osteological bias: the evidence from late Roman Britain.” Journal of Roman Archaeology Supplementary 65: 153–169.
Gowland, R. L., and A. T. Chamberlain. 2002. “A Bayesian approach to ageing perinatal skeletal remains: implications for the evidence of infanticide in Roman Britain.” Journal of Archaeological Science 29: 677–685. DOI: 10.1006/jasc.2001.0776.
Gowland, R. L., and P. Garnsey. 2010. “Skeletal evidence for health, nutritional status and malaria in Rome and the Empire.” In Roman Diasporas: Archaeological Approaches to Mobility and Diversity in the Roman Empire, ed. H. Eckhardt. Portsmouth, RI, Journal of Roman Archaeology, 131–156.
Gowland, R. L., and R. C. Redfern. 2010. “Childhood health in the Roman world: perspectives from the centre and margin of the Empire.” Childhood in the Past 3: 15–42. DOI: 10.1179/cip.2010.3.1.15.
Green, S., S. Green, and G. Armelagos. 1974. “Settlement and mortality of the Christian site (1050 A.D.—1300 A.D.) of Meinarti (Sudan).” Journal of Human Evolution 3 (4): 297–316. DOI: 10.1016/0047–2484(74)90024–4.
Guyomarc’h, P., and J. Bruzek. 2011. “Accuracy and reliability in sex determination from skulls: a comparison of Fordisc3.0 and the discriminant function analysis.” Forensic Science International 208: 180–e1. DOI: 10.1016/j.forsciint.2011.03.011.
Hassan, N.A-M., et al. 2014. “Ancient DNA study of the remains of putative infanticide victims from the Yewden Roman villa site at Hambleden, England.” Journal Archaeological Science 43: 192–197. DOI: 10.1016/j.jas.2013.12.017.
Hawass, Z., et al. 2010. “Ancestry and pathology in King Tutankhamun’s family.” The Journal of the American Medical Association 303: 638–647. DOI: 10.1001/jama.2010.121.
Hedges, R.E.M. 2002. “Bone diagenesis: an overview of process.” Archaeometry 44 (3): 319–328. DOI: 10.1111/1475–4754.00064.
Hedges, R.E.M. 2006. “Where does our protein carbon come from?” British Journal of Nutrition 95: 1031–1032. DOI: 10.1079/BJN20061782.
Hedges, R.E.M., and L. M. Reynard. 2007. “Nitrogen isotopes and the trophic level of humans in archaeology.” Journal of Archaeological Science 34: 1240–1251. DOI: 10.1016/j.jas.2006.10.015.
Hedges, R.E.M., et al. 2007. “Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements.” American Journal of Physical Anthropology 133: 808–816. DOI: 10.1002/ajpa.20598.
Hens, S. M., E. Rastelli, and G. Belcastro. 2008. “Age estimation from the human os coxa: a test on a documented Italian collection.” Journal of Forensic Sciences 53: 1040–1043. DOI: 10.1111/j.1556–4029.2008.00818.x.
Hin, S. 2013. The Demography of Roman Italy: Population Dynamics in an Ancient Conquest Society, 201 BCE–14 CE. Cambridge: Cambridge University Press.
Holleran, C. 2011. “Migration and the urban economy of Rome.” In Demography and the Graeco-Roman World: New Insights and Approaches, eds. C. Holleran and A. Pudsey. Cambridge: Cambridge University Press, 155–180.
Hoppa, R. D., and J. W. Vaupel. 2002. Paleodemography: Age Distributions from Skeletal Samples. Cambridge: Cambridge University Press.
Hough, A. J. 2001. “Pathology of osteoarthritis.” In Arthritis and Allied Conditions, ed. W. J. Koopman. Philadelphia: Lea and Febiger, 2167–2194.
Humphrey, L. T., et al. 2007. “Unlocking evidence of early diet from tooth enamel.” Proceedings of the National Academy of Sciences of the United States of America 105 (19): 6834–6839. DOI: 10.1073/pnas.0711513105.
İsçan, M. Y., and K. A. Kennedy. 1989. Reconstruction of Life from the Skeleton. New York: Alan R. Liss.
Jackes, M. 2011. “Representativeness and bias in archaeological skeletal samples.” In Social Bioarchaeology, eds. S. C. Agarwal, and B. A. Glencross. Chichester: Blackwell, 107–146. DOI: 10.1002/9781444390537.ch5.
Jacobi, K. P., and M. E. Danforth. 2002. “Analysis of interobserver scoring patterns in porotic hyperostosis and cribra orbitalia.” International Journal of Osteoarchaeology 12: 248–258. DOI: 10.1002/oa.619.
Jaeger, L. H., and A. M. Iñiguez. 2014. “Molecular paleoparasitological hybridization approach as effective tool for diagnosing human intestinal parasites from scarce archaeological remains.” PLoS ONE 9 (8): e105910. DOI: 10.1371/journal.pone.0105910.
Johansson, S. R., and S. Horowitz. 1986. “Estimating mortality in skeletal populations: influence of the growth rate on the interpretation of levels of trends during the transition to agriculture.” American Journal of Physical Anthropology 71: 233–250. DOI: 10.1002/ajpa.1330710211.
Jurmain, R., et al. 2012. “Bioarchaeology’s Holy Grail: the reconstruction of activity.” In A Companion to Paleopathology, ed. A. L. Grauer. New York: Wiley-Blackwell, 531–552. DOI: 10.1002/9781444345940.ch29.
Kanz, F., and K. Grosschmidt. 2006. “Head injuries of Roman gladiators.” Forensic Science International 13: 207–216. DOI: 10.1016/j.forsciint.2005.10.010.
Katz, D., and J. M. Suchey. 1989. “Race differences in pubic synphyseal aging patterns in the male.” American Journal of Physical Anthropology 80: 167–172. DOI: 10.1002/ajpa.1330800204.
Katzenberg, M. A. 2000. “Stable isotope analysis: a tool for studying past diet, demography and history.” In Biological Anthropology of the Human Skeleton, eds. M. A. Katzenberg and S. R. Saunders. New York: Wiley-Liss, 305–328. DOI: 10.1002/9780470245842.ch13.
Keenan-Jones, D., J. Hellstrom, and R. Drysdale. 2012. “Lead contamination in the drinking water of Pompeii.” In Art, Industry and Infrastructure in Roman Pompeii, eds. E. Poehler, M. Flohr, and K. Cole. Oxford: Oxford University Press, 130–181.
Keenleyside, A., et al. 2009. “Stable isotopic evidence for diet in a Roman and Late Roman population from Leptiminus, Tunisia.” Journal of Archaelogical Science 36: 51–63. DOI: 10.1016/j.jas.2008.07.008.
Kemkes-Grottenthaler, A. 2002. “Aging through the ages: historical perspectives on age indicator methods.” In Paleodemography: Age Distributions from Skeletal Samples, eds. R. D. Hoppa and J. W. Vaupel. Cambridge: Cambridge University Press, 48–72.
Killgrove, K. 2010a. “Response to C. Bruun’s ‘Water, oxygen isotopes, and immigration to Ostia-Portus.’” Journal of Roman Archaeology 23: 133–136. DOI: 10.1017/S1047759400002336.
Killgrove, K. 2010b. “Identifying immigrants to Imperial Rome using strontium isotope analysis.” In Roman Diasporas: Archaeological Approaches to Mobility and Diversity in the Roman Empire, ed. H. Eckardt. Portsmouth, RI: Journal of Roman Archaeology, Supplementary volume 78, 157–174.
Killgrove, K., and J. Montgomery. 2016. “All roads lead to Rome: exploring human migration to the eternal city through biochemistry of skeletons from two Imperial-era cemeteries (1st–3rd c AD).” PLoS ONE 11 (2): e0147585. DOI: 10.1371/journal.pone.0147585.
Kimmerle, E. H., D. A. Prince, and G. E. Berg. 2008. “Inter-observer variation in methodologies involving the pubic symphysis, sternal ribs, and teeth.” Journal of Forensic Sciences 53: 594–600. DOI: 10.1111/j.1556–4029.2008.00715.x.
Klepinger, L. L. 2006. Fundamentals of Forensic Anthropology. Wiley-Liss: New Jersey.
Konigsberg, L. W. and S. R. Frankenberg, 1994. “Paleodemography: ‘Not quite dead.’” Evolutionary Anthropology 3: 92–105. DOI: 10.1002/evan.1360030306.
Konigsberg, L. W., and S. R. Frankenberg. 2002. “Deconstructing death in paleodemography.” American Journal of Physical Anthropology 117: 297–309. DOI: 10.1002/ajpa.10039.
Krogman, W. M., and M. Y. İsçan. 1986. The Human Skeleton in Forensic Medicine. Springfield, IL: Charles C. Thomas.
Larsen, C. S. 2006. “The changing face of bioarchaeology: an interdisciplinary science.” In Bioarchaeology: The Contextual Analysis of Human Remains, eds. J. E. Buikstra and L. A. Beck. Burlington, MA: Academic Press, 359–374.
Lazer E. 2011. Resurrecting Pompeii. Abingdon, Oxon, and New York: Routledge. DOI: 10.3764/ajaonline1161.
Lee, U. Y., I. B. Kim, and D. S. Kwak. 2015. “Sex determination using discriminant analysis of upper and lower extremity bones: new approach using the volume and surface area of digital model.” Forensic Science International 253: 135.e1–4. DOI: 10.1016/j.forsciint.2015.05.017.
Lewis, M. E. 2010. “Life and death in a civitas capital: metabolic disease and trauma in the children from Late Roman Dorchester, Dorset.” American Journal of Physical Anthropology 143: 405–416. DOI: 10.1002/ajpa.21239.
Lewis, M. E. 2011. “The osteology of infancy and childhood: misconceptions and potential.” In Rethinking The Little Ancestor: New Perspectives on the Archaeology of Infancy and Childhood, eds. M. Lally and A. Moore. Oxford: Archaeopress, 1–13.
Lightfoot, E., M. Slaus, and T. C. O’Connell. 2014. “Water consumption in Iron Age, Roman and early medieval Croatia.” American Journal of Physical Anthropology 154: 535–543. DOI: 10.1002/ajpa.22544.
Ligt, L. de. 2012. Peasants, Citizens and Soldiers: Studies in the Demographic History of Roman Italy 225 BC–AD 100. Cambridge: Cambridge University Press.
Ligt, L. de, and S. Northwood, eds. 2008. People, Land and Politics: Demographic Developments and the Transformation of Roman Italy, 300 BC–AD 14. Leiden: Brill.
Ligt, L. de, and L. E. Tacoma, eds. 2016. Migration and Mobility in the Early Roman Empire. Leiden: Brill.
Lorkiewicz, W. 2011. “Nonalimentary tooth use in the Neolithic population of the Lengyel culture in central Poland (4600–4000 BC).” American Journal of Physical Anthropology 144: 538–551. DOI: 10.1002/ajpa.21435.
Lovejoy C. O., et al. 1985. “Multifactorial determination of skeletal age at death: a method and blind test of its accuracy.” American Journal of Physical Anthropology 68: 1–14. DOI: 10.1002/ajpa.1330680102.
Lunardini, A., et al. 2005. “A severe case of rickets in the Roman Imperial Age (I–II century A.D.).” Journal of Paleopathology 17: 137–143.
Lunardini, A., et al. 2008. “A severe case of ankylosing spondylitis from the Roman Imperial Age (I–II century A.D.).” Journal of Paleopathology 20: 29–35.
Luongo, G., et al. 2003. “Impact of the AD 79 explosive eruption on Pompeii. II. Causes of death of the inhabitants inferred by stratigraphic analysis and areal distribution of the human casualties.” Journal of Volcanology and Geothermal Research 126: 169–200. DOI: 10.1016/S0377–0273(03)00147–1.
Macchiarelli, R., A. Sperduti, and L. Bondioli. 1990. “L’indagine radiografica dello scheletro nella attribuzione dell’età alla morte. II. Analisi sperimentale dei corpi vertebrali.” Rivista di Antropologia 68: 103–127.
Macchiarelli, R., et al. 1994. “Intra- and interobserver concordance in scoring Harris lines: a test on bone sections and radiographs.” American Journal of Physical Anthropology 95: 77–83. DOI: 10.1002/ajpa.1330950107.
Macchiarelli, R., et al. 2006. “How Neanderthal molar teeth grew.” Nature 444 (7120): 748–751. DOI: 10.1038/nature05314.
Maggiano, I. S., et al. 2008. “Cross-sectional analysis of long bones, occupational activities and long-distance trade of the Classic Maya from Xcambó: archaeological and osteological evidence.” American Journal of Physical Anthropology 136: 470–477. DOI: 10.1002/ajpa.20830.
Mahoney, P. 2011. “Human deciduous mandibular molar incremental enamel development.” American Journal of Physical Anthropology 144: 204–214. DOI: 10.1002/ajpa.21386.
Manzi, G., A. Sperduti, and P. Passarello. 1991. “Behavior-induced auditory exostoses in imperial Roman society: evidence from coeval urban and rural communities near Rome.” American Journal of Physical Anthropology 85: 253–260. DOI: 10.1002/ajpa.1330850303.
Manzi, G., et al. 1989. “Linee di Harris e ipoplasia dello smalto nei resti scheletrici delle popolazioni umane di Isola Sacra e Lucus Feroniae (Roma, I–III sec. d.C.).” Rivista di Antropologia 67: 129–148.
Manzi, G., et al. 1999. “Discontinuity of life conditions at the transition from the Roman Imperial age and early Middle ages: example from central Italy evaluated by pathological dental alveolar lesions.” American Journal of Human Biology 11 (3): 327–341. DOI: 10.1002/(SICI)1520–6300(1999)11:3<327::AID-AJHB5>3.0.CO;2-M.
Maples, W. R. 1989. “The practical application of age-estimation techniques.” In Age Markers in the Human Skeleton, ed. M. Y. İşcan. Springfield, IL: Charles C. Thomas, 319–324.
Marciniak, S., et al. 2016. “Plasmodium falciparum malaria in 1st–2nd century CE southern Italy.” Current Biology 26: 1220–1222.
Martyn, R.E.V., et al. 2015. “Capturing Roman dietary variability in the catastrophic death assemblage at Herculaneum, AD 79.” United Kingdom Archaeological Science, Environmental and Archaeological Science Conference. Durham, UK, April 2015: 8–11.
Mays, S. 2014. “A test of a recently devised method of estimating skeletal age at death using features of the adult acetabulum.” Journal of Forensic Sciences 59: 184–187. DOI: 10.1111/1556–4029.12293.
Mays, S., and M. Faerman. 2001. “Sex identification in some putative infanticide victims from Roman Britain using ancient DNA.” Journal of Archaeological Science 28: 555–559. DOI: 10.1006/jasc.2001.0616.
Mays, S., E. Fysh, and G. M. Taylor. 2002. “Investigation of the link between visceral surface rib lesions and tuberculosis in a medieval skeletal series from England using ancient DNA.” American Journal of Physical Anthropology 119: 27–36. DOI: 10.1002/ajpa.10099.
Mays, S., et al. 2001. “Paleopathological and biomolecular study of tuberculosis in a medieval skeletal collection from England.” American Journal of Physical Anthropology 114: 298–311. DOI: 10.1002/ajpa.1042.
McNeill, W. H. 1979. “Historical patterns of migration.” Current Anthropology 20: 95–102. DOI: 10.1086/202206.
Merritt, C. E. 2014. “A test of Hartnett’s revisions to the pubic symphysis and fourth rib methods on a modern sample.” Journal of Forensic Sciences 59: 703–711. DOI: 10.1111/1556–4029.12380.
Merritt, C. E. 2015. “The influence of body size on adult skeletal age estimation methods.” American Journal of Physical Anthropology 156: 35–57. DOI: 10.1002/ajpa.22626.
Minozzi, S., et al. 2012a. “Pituitary disease from the past: a rare case of gigantism in skeletal remains from the Roman Imperial Age.” The Journal of Clinical Endocrinology and Metabolism 97: 4302–4303. DOI: 10.1210/jc.2012–2726.
Minozzi, S., et al. 2012b. “Palaeopathology of human remains from the Roman Imperial Age.” Pathobiology 79: 268–283. DOI: 10.1159/000338097.
Minozzi, S., et al. 2013. “A case of gout from Imperial Rome (1st–2nd Century AD).” Journal of Clinical Research & Bioethics 4: 162. DOI: 10.4172/2155–9627.1000162.
Mirdha, B. R., et al. 1999. “Bone marrow examination for identifying malaria in fever of unknown origin.” Journal Association of Physicians of India 47: 177–179.
Molleson, T. I. 1989. “Social implications of mortality patterns of juveniles from Poundbury Camp, Romano-British Cemetery.” Anthropologischer Anzeiger 47: 27–38.
Molto, J. E. 1990. “Differential diagnosis of rib lesions: a case study from Middle Woodland, Southern Ontario. Circa 230 AD.” American Journal of Physical Anthropology 83: 439–447. DOI: 10.1002/ajpa.1330830405.
Montgomery, J., et al. 2010. “‘Gleaming white and deadly’: the use of lead to track human exposure and geographic origins in the Roman period in Britain.” In Roman Diasporas: Archaeological Approaches to Mobility and Diversity in the Roman Empire, ed. H. Eckardt. Portsmouth, RI: Journal of Roman Archaeology, Supplementary volume 78, 199–226.
Müldner, G., C. Chenery, and H. Eckardt. 2011. “The ‘Headless Romans:’: multi-isotope investigations of an unusual burial ground from Roman Britain.” Journal of Archaeological Science 38: 280–290. DOI: 0.1016/j.jas.2010.09.003.
Musumeci, I., et al. 2015. “Osteoarthritis in the XXIst Century: risk factors and behaviours that influence disease onset and progression.” International Journal of Molecular Sciences 16: 6093–6112. DOI: 10.3390/ijms16036093.
Nafplioti, A. 2011. “Tracing population mobility in the Aegean using isotope geochemistry: a first map of local biologically available 87Sr/86Sr signatures.” Journal of Archaeological Science 38: 1560–1570. DOI: 10.1016/j.jas.2011.02.021.
Naito, Y. I., et al. 2010. “Quantitative evaluation of marine protein contribution in ancient diets based on nitrogen isotope ratios of individual amino acids in bone collagen: an investigation at the Kitakogane Jomon site.” American Journal of Physical Anthropology 143: 31–40. DOI: 10.1002/ajpa.21287.
Nawrocki, S. P. 2010. “The nature and sources of error in the estimation of age at death from the skeleton.” In Age Estimation of the Human Skeleton, eds. K. Lathamand and M. Finnegan. Springfield, IL: Charles C. Thomas, 79–101.
Noy, D. 2000. Foreigners at Rome: Citizens and Strangers. London: Duckworth.
O’Connell, T. C., et al. 2012. “The diet-body offset in human nitrogen isotopic values: a controlled dietary study.” American Journal of Physical Anthropology 149: 426–434. DOI: 10.1002/ajpa.22140.
Ortner, D. J. 1991. “Theoretical and methodological issues in paleopathology.” In Human Paleopathology: Current Syntheses and Future Options, eds. D. Ortner and A. Aufderheide. Washington, DC: Smithsonian Institution Press, 5–11.
Ortner, D. J. 2012. “Differential diagnosis and issues in disease classification.” In A Companion to Paleopathology, ed. A. L. Grauer. Malden, MA: Wiley-Blackwell, 250–267. DOI: 10.1002/9781444345940.ch14.
Ortner, D. J., and G. J. Putschar. 1981. Identification of Pathological Conditions in Human Skeletal Remains. Washington, D.C.: Smithsonian Institution Press.
Ottini, L., et al. 2001. “A subject with abnormally short stature from Imperial Rome.” Journal of Endocrinological Investigation 24: 546–548. DOI: 10.1007/BF03343890.
Paine, R. R., and H. C. Harpending. 1998. “Effect of sample bias on palaeodemographic fertility estimates.” American Journal of Physical Anthropology 105: 231–240. DOI: 10.1002/(SICI)1096–8644(199802)105:2<231::AID-AJPA9>3.0.CO;2-X.
Paine, R. R., and G. R. Storey. 2008. “Epidemics, age at death and mortality in Ancient Rome.” In Urbanism in the Preindustrial World, ed. G. R. Storey. Tuscaloosa: University of Alabama Press, 69–85.
Paine, R. R., et al. 2009. “A health assessment for Imperial Roman burials recovered from the necropolis of San Donato and Bivio CH, Urbino, Italy.” Journal of Anthropological Science 87: 193–210.
Palubeckaitė, Ž., R. Jankauskas, and J. Boldsen. 2002. “Enamel hypoplasia in Danish and Lithuanian Late Medieval / Early Modern samples: a possible reflection of child morbidity and mortality patterns.” International Journal of Osteoarchaeology 12: 189–201. DOI: 10.1002/oa.607.
Parfitt, A. 2004. “What is the normal rate of bone remodeling?” Bone 35: 1–3. DOI: 10.1016/j.bone.2004.03.022.
Petrone, P. P. 1993. “Schiavitù, stress da attività lavorativa, malnutrizione: condizioni socioculturali quali principali cause di morbilità e mortalità in popolazioni di età imperiale dell’area flegrea (Napoli, Campania).” Abstracts X Congresso Antropologi Italiani: 18.
Petrone, P., et al. 2011. “Enduring fluoride health hazard for the Vesuvius area population: the case of AD 79 Herculaneum.” PLoS ONE 6 (6): e21085. DOI: 10.1371/journal.pone.0021085.
Piccioli, A., V. Gazzaniga, and P. Catalano, eds. 2015. Bones: Orthopaedic Pathologies in Roman Imperial Age. New York: Springer International Publishing.
Pollard, A. M., et al. 2011a. “Technical note: some observations on the conversion of dental enamel δ18Op values to δ18Ow to determine human mobility.” American Journal of Physical Anthropology 145: 499–504. DOI: 10.1002/ajpa.21524.
Pollard, A. M., et al. 2011b. “’These boots were made for walking’: the isotopic analysis of a C4 Roman inhumation from Gravesend, Kent, UK.” American Journal of Physical Anthropology 146: 446–456. DOI: 10.1002/ajpa.21602.
Prowse, T. L. 2016. “Isotopes and mobility in the ancient Roman world.” In Approaches to Migration in the Early Roman Empire, eds. L. de Ligt and L. E. Tacoma. Leiden: Brill Publishers, 205–233. DOI: 10.1163/9789004307377_011.
Prowse, T. L., et al. 2004. “Isotopic paleodiet studies of skeletons from the imperial Roman-age cemetery of Isola Sacra, Rome, Italy.” Journal of Archaeological Science 31: 259–272. DOI: 10.1016/j.jas.2003.08.008.
Prowse, T. L., et al. 2005. “Isotopic evidence for age-related variation in diet from Isola Sacra, Italy.” American Journal of Physical Anthropology 128: 2–13. DOI: 10.1002/ajpa.20094.
Prowse, T. L., et al. 2007. “Isotopic evidence for age-related immigration to Imperial Rome.” American Journal Physical Anthropology 132: 510–519. DOI: 10.1002/ajpa.20541.
Prowse, T. L., et al. 2008. “Isotopic and dental evidence for infant and young child feeding practices in an imperial Roman skeletal sample.” American Journal Physical Anthropology 137: 294–308. DOI: 10.1002/ajpa.20870.
Prowse, T. L et al. 2010. “Stable isotope and ancient DNA evidence for geographic origins at the site of Vagnari (2nd–4th centuries AD), Italy.” In Roman Diasporas: Archaeological Approaches to Mobility and Diversity in the Roman Empire, ed. H. Eckardt. Portsmouth, RI: Journal of Roman Archaeology, 175–198.
Ragsdale, B. D., and L. M. Lehmer. 2012. “A knowledge of bone at the cellular (histological) level is essential to paleopathology.” In A Companion to Paleopathology, ed. A. L. Grauer. Malden, MA: Wiley-Blackwell, 227–249. DOI: 10.1002/9781444345940.ch13.
Redfern, R. C., and S. N. DeWitte. 2011a. “A new approach to the study of Romanization in Britain: a regional perspective of cultural change in late Iron Age and Roman Dorset using the Siler and Gompertz-Makeham models of mortality.” American Journal of Physical Anthropology 144: 269–285. DOI: 10.1002/ajpa.21400.
Redfern, R. C., and S. N. DeWitte. 2011b. “Status and health in Roman Dorset: the effect of status on risk of mortality in post-conquest populations.” American Journal of Physical Anthropology 146: 197–208. DOI: 10.1002/ajpa.21563.
Retief, F. P., and L. Cilliers. 2005. “Lead poisoning in ancient Rome.” Acta Theologica Supplementum 7: 147–164. DOI: /10.4314/actat.v26i2.52570.
Ricci, R., et al. 1997. “Pattern of porotic hyperostosis and quality of life in a II century A.D. farm near Rome.” Rivista di Antropologia 75: 117–128.
Richards, M. P., and R.E.M. Hedges. 1998. “Stable isotope analysis reveals variations in human diet at the Poundbury camp cemetery site.” Journal of Archaeological Science 25: 1247–1252. DOI: 10.1006/jasc.1998.0307.
Richards, M. P., R. J. Schulting, and R.E.M. Hedges. 2003. “Sharp shift in diet at onset of Neolithic.” Nature 425 (6956): 366–366. DOI: 10.1038/425366a.
Richards, M. P., et al. 2000. “Neanderthal diet at Vindija and Neanderthal predation: the evidence from stable isotopes.” Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7663–7666. DOI: 10.1073/pnas.120178997.
Richards, M. P., et al. 2005. “Isotope evidence for the intensive use of marine foods by Late Upper Palaeolithic humans.” Journal of Human Evolution 49 (3): 390–394. DOI: 10.1016/j.jhevol.2005.05.002.
Roberts, S. B., et al. 1988. “Effect of weaning on accuracy of double labeled water method in infants.” American Journal of Physiology 254: 622–627.
Rogers, T., and S. Saunders. 1994. “Accuracy of sex determination using morphological traits of the human pelvis.” Journal of Forensic Sciences 39: 1047–1056. DOI: 10.1016/1353–1131(95)90091–8.
Rothman, K., and S. Greenlander. 1998. Modern Epidemiology. Philadelphia: Lippincott–Raven.
Rubini, M., et al. 2014. “Paleopathological and molecular study on two cases of ancient childhood leprosy from the Roman and Byzantine empires.” International Journal of Osteoarcheology 24: 570–582. DOI: 10.1002/oa.2242.
Ruff, C. B. 1987. “Sexual dimorphism in human lower limb bone structure: relationship to subsistence strategy and sexual division of labour.” Journal of Human Evolution 16 (5): 391–416. DOI: 10.1016/0047–2484(87)90069–8.
Ruff, C. B., C. S. Larsen, and W. C. Hayes. 1984. “Structural changes in the femur with the transition to agriculture on the Georgia coast.” American Journal Physical Anthropology 64: 125–136. DOI: 10.1002/ajpa.1330640205.
Rutgers, L. V., et al. 2009. “Stable isotope data from the early Christian catacombs of ancient Rome: new insights into the dietary habits of Rome’s early Christians.” Journal of Archaeological Science 36: 1127–1134. DOI: 10.1016/j.jas.2008.12.015.
Sabel, N., et al. 2008. “Neonatal lines in the enamel of primary teeth: a morphological and scanning electron microscopic investigation.” Archives of Oral Biology 53: 954–963. DOI: 10.1016/j.archoralbio.2008.05.003.
Salesse, K., et al. 2014. “Variability of bone preservation in a confined environment: the case of the catacomb of Sts Peter and Marcellinus (Rome, Italy).” Palaeogeography Palaeoclimatology Palaeoecology 416: 43–54. DOI: 10.1016/j.palaeo.2014.07.021.
Sallares, R. 2002. Malaria and Rome: A History of Malaria in Ancient Italy. Oxford: Oxford University Press. DOI: 10.1093/acprof:oso/9780199248506.001.0001.
Sallares, R., A. Bouwman, and C. Anderung. 2004. “The spread of malaria to Southern Europe in antiquity: new approaches to old problems.” Medical History 48: 311–328.
Sallares, R., and R. Gomzi. 2001. “Biomolecular archaeology of malaria.” Ancient Biomolecules 3: 195–213.
Salvadei, L., F. Ricci, and G. Manzi. 2001. “Porotic hyperostosis as a marker of health and nutritional conditions during childhood: studies at the transition between Imperial Rome and the early Middle Ages.” American Journal of Human Biology 13 (6): 709–717. DOI: 10.1002/ajhb.1115.
Sandberg, P. A., et al. 2014. “Intra-tooth stable isotope analysis of dentine: a step toward addressing selective mortality in the reconstruction of life history in the archaeological record.” American Journal of Physical Anthropology 155: 281–293. DOI: 10.1002/ajpa.22600.
Santos, A. L., and C. A. Roberts. 2001. “A picture of tuberculosis in young Portuguese people in the early 20th century: a multidisciplinary study of the skeletal and historical evidence.” American Journal of Physical Anthropology 115: 38–49. DOI: 10.1002/ajpa.1054.
Schaefer, M., S. M. Black, and L. Scheuer. 2009. Juvenile osteology: a laboratory and field manual. Amsterdam: Academic.
Scheidel, W. 2001a. “Progress and problems in Roman demography.” In Debating Roman Demography, ed. W. Scheidel. Leiden: Brill, 1–81.
Scheidel, W. 2001b. “Roman age structure: evidence and models.” Journal of Roman Studies 91: 1–26. DOI: 10.1017/S0075435800015811.
Scheidel, W. 2004. “Human mobility in Roman Italy, I: The free population.” The Journal of Roman Studies 94: 1–26. DOI: 10.2307/4135008.
Scheidel, W. 2005. “Human mobility in Roman Italy, II: The slave population.” The Journal of Roman Studies 95: 64–79. DOI: 0.3815/000000005784016270.
Scheidel, W. 2007. “A model of real income growth in Roman Italy.” Historia: Zeitschrift für Alte Geschichte 56: 322–346. DOI: 10.2307/25598399.
Scheidel, W. 2012. “Physical well-being.” In The Cambridge Companion to the Roman Economy, ed. W. Scheidel. Cambridge: Cambridge University Press, 321–333. DOI: 10.1017/CCO9781139030199.020.
Schmitt, A. 2004. “Age-at-death assessment using the os pubis and the auricular surface of the ilium: a test on an identified Asian sample.” International Journal of Osteoarchaeology 14: 1–6. DOI: 10.1002/oa.693.
Schmitt, A., et al. 2002. “Variability of the pattern of aging on the human skeleton: evidence from bone indicators and implications on age at death estimation.” Journal of Forensic Sciences 47: 1203–1209. DOI: 10.1520/JFS15551J.
Schuenemann, V. J., et al. 2011. “Targeted enrichment of ancient pathogens yielding the pPCP1 plasmid of Yersinia pestis from victims of the Black Death.” Proceedings of the National Academy of Sciences of the United States of America 108 (38): 746–752. DOI: 10.1073/pnas.1105107108.
Schuenemann, V. J., et al. 2013. “Genome-wide comparison of medieval and modern Mycobacterium leprae.” Science 341 (6142): 179–183. DOI: 10.1126/science.1238286.
Sealy, J. 2001. “Body tissue chemistry and palaeodiet.” In Handbook of Archaeological Sciences, eds. D. R. Brothwell and A. M. Pollard. Chichester: John Wiley, 269–279.
Setzer, T. J. 2014. “Malaria detection in the field of paleopathology: a meta-analysis of the state of the art.” Acta Tropica 140: 97–104. DOI: 10.1016/j.actatropica.2014.08.010.
Shaw, C. N., and J. T. Stock. 2009a. “Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern athletes.” American Journal of Physical Anthropology 140, 160–172. DOI: 10.1002/ajpa.21063.
Shaw, C. N., and J. T. Stock. 2009b. “Intensity, repetitiveness, and directionality of habitual adolescent mobility patterns influence the tibial diaphysis morphology of athletes.” American Journal of Physical Anthropology 140: 149–159. DOI: 10.1002/ajpa.21064.
Shaw, H., et al. 2016. “Identifying migrants in Roman London using lead and strontium stable isotopes.” Journal of Archaeological Science 66: 57–68. DOI: 10.1016/j.jas.2015.12.001.
Sládek, V., et al. 2007. “Human manipulative behavior in the central European late eneolithic and early Bronze Age: humeral bilateral asymmetry.” American Journal of Physical Anthropology 133: 669–681. DOI: 10.1002/ajpa.20551.
Slovak, N. M., and A. Paytan. 2012. “Applications of Sr isotopes in archaeology.” In Handbook of Environmental Isotope Geochemistry, Advances in Isotope Geochemistry, ed. M. Baskaran. Berlin: Springer-Verlag, 743–768. DOI: 10.1007/978–3–642–10637–8_35.
Small, A. M. 2011. Vagnari: The Village, the Industries, the Imperial Property. Bari: Edipuglia.
Smith, T. M., D. J. Reid, and J. E. Sirianni. 2006. “The accuracy of histological assessments of dental development and age at death.” Journal of Anatomy 208: 125–138. DOI: 10.1111/j.1469–7580.2006.00500.x.
Sofeso, C., et al. 2012. “Verifying archaeological hypotheses: investigations on origin and genealogical lineages of a privileged society in Upper Bavaria from Imperial Roman times (Erding, Kletthamer Feld).” In Population Dynamics in Prehistory and Early History: New Approaches Using Stable Isotopes and Genetics, eds. E. Kaiser, J. Burger, and W. Schier. Berlin: De Gruyter, 113–130. DOI: 10.1515/9783110266306.113.
Soomer, H., et al. 2003. “Reliability and validity of eight dental age estimation methods for adults.” Journal of Forensic Sciences 48: 149–152.
Sperduti, A. 1995. “I resti scheletrici umani nella necropoli di eta’ romano-imperiale di Isola Sacra (1–3 sec. d.C.). Analisi paleodemografica.” PhD Dissertation, Università di Roma.
Sperduti, A. 1997. “Life conditions of a Roman Imperial Age population: occupational stress markers and working activities in Lucus Feroniae (Rome, I–II cent. AD).” Human Evolution 12 (4): 253–267. DOI: 10.1007/BF02438179.
Sperduti, A., L. Bondioli, and P. Garnsey. 2012. “Skeletal evidence for occupational structure and diet at the coastal towns of Portus and Velia (Central Italy, I–III cent. AD).” In More Than Just Numbers? The Role of Science in Roman archaeology, ed. I. Schrüfer-Kolb. Portsmouth, RI: Journal of Roman Archaeology, Supplementary volume 91, 53–70.
Sperduti, A., and G. Manzi. 1990. “Hyperostosis frontalis interna in a cranial sample from the Roman population of Portus.” Rivista di Antropologia 68: 279–286.
Sperduti, A., et al. 2011. “Non-masticatory tooth wear at Gricignano d’Aversa, Italy (2500–1750 BCE): the importance of macro- and microscopic analysis.” American Association of Physical Anthropologists 80th Annual Meeting. Minneapolis, Minnesota: 12–16 April 2011, 281.
Sperduti, A., et al. in press. “Differential burial treatment of newborn infants from Late Roman Age: children and dogs depositions at Peltuinum.” In Archeologia e Antropologia della Morte, Atti del III Incontro Internazionale di Studi di Antropologia e Archeologia a confronto, Vol. 1, ed. V. Nizzo. Rome: Editorial Service System.
Spigelman, M., D. H. Shin, and G.K.B. Gal. 2012. “The promise, the problems, and the future of DNA analysis in paleopathology studies.” In A Companion to Paleopathology, ed. A. L. Grauer. Malden, MA: Wiley-Blackwell, 133–151. DOI: 10.1002/9781444345940.ch8.
Spirduso, W. W. 1995. Physical dimensions of aging. Champaign, IL: Human Kinetics.
Stepańczak, B., K. Szostek, and J. Pawlyta. 2014. “The human bone oxygen isotope ratio changes with aging.” Geochronometria 41 (20): 147–159. DOI: 10.2478/s13386–013–0146–1.
Storey, R. 1997. “Individual frailty, children of privilege, and stress in Late Classic Copan.” In Bones of the Maya, eds. S. Whittington and D. Reed. Tuscaloosa: University of Alabama Press, 116–126.
Stoyanova, D., B.F.D. Algee-Hewitt, and D. E. Slice. 2015. “An enhanced computational method for age-at-death estimation based on the pubic symphysis using 3D laser scans and thin plate splines.” American Journal of Physical Anthropology 158: 431–40. DOI: 10.1002/ajpa.22797.
Spradley, M. K., and R. L. Jantz. 2011. “Sex estimation in forensic anthropology: skull versus postcranial elements.” Journal of Forensic Sciences 56: 289–296. DOI: 10.1111/j.1556–4029.2010.01635.x.
Stuart-Macadam, P. 1989. “Nutritional deficiency diseases: a survey of scurvy, rickets, and iron deficiency anemia.” In Reconstruction of Life from the Skeleton, eds. M. Y. İşcan and K.A.R. Kennedy. New York: Alan R. Liss, 201–222.
Stuart-Macadam, P. 1992. “Anemia in past human populations.” In Diet, Demography, and Disease: Changing Perspectives on Anemia, eds. P. Stuart-Macadam and S. Kent. New York: Aldine de Gruyter, 151–170.
Sutter, R. C. 2003. “Nonmetric subadult skeletal sexing traits: I. A blind test of the accuracy of eight previously proposed methods using prehistoric known-sex mummies from Northern Chile.” Journal of Forensic Sciences 48: 927–935.
Tafuri, M. A, et al. 2016. “Life and death in Neolithic southeastern Italy: the strontium isotope evidence.” International Journal of Osteoarcheology DOI: 10.1002/oa.2516.
Tang, N., D. Antoine, and S. Hillson. 2014. “Application of the Bang and Ramm age at death estimation method to two known-age archaeological assemblages.” American Journal of Physical Anthropology 155: 332–351. DOI: 10.1002/ajpa.22566.
Van der Merwe, A. E., M. Steyn, and E. N. L’Abbé. 2010. “Trauma and amputations in 19th century miners from Kimberley, South Africa.” International Journal of Osteoarcheology 20: 291–306. DOI: 10.1002/oa.1035.
Van Gerven, D. P., and G. J. Armelagos. 1983. “‘Farewell to paleodemography?’ Rumors of its death have been greatly exaggerated.” Journal of Human Evolution 12 (4): 353–360. DOI: 10.1016/S0047–2484(83)80162–6.
van Klinken, G. J., M. P. Richards, and R.E.M. Hedges. 2000. “An overview of causes for stable isotopic variations in past human environmental, ecophysiological, and cultural effects.” In Biogeochemical Approaches to Paleodietary Analysis, eds. S. H. Ambrose and M. A. Katzenberg New York: Kluwer Academic/Plenum, 39–58. DOI: 10.1007/0–306–47194–9_3.
Villotte, S., and C. J. Knüsel. 2013. “Understanding entheseal changes: definition and life course changes.” International Journal of Osteoarcheology 23: 135–146. DOI: 10.1002/oa.2289.
Wagner, D., et al. 2014. “Yersinia pestis and the plague of Justinian 541–543 AD: a genomic analysis.” The Lancet Infectious Diseases 14: 319–326. DOI: 10.1016/S1473–3099(13)70323–2.
Waldron, T., and J. Rogers. 1991. “Inter-observer variation in coding osteoarthritis in human skeletal remains.” International Journal of Osteoarcheology 1: 49–56. DOI: 10.1002/oa.1390010107.
Walker, P. L. 2008. “Sexing skulls using discriminant function analysis of visually assessed traits.” American Journal of Physical Anthropology 136: 39–50. DOI: 10.1002/ajpa.20776.
Walker, P. L., et al. 2009. “The causes of porotic hyperostosis and cribra orbitalia: a reappraisal of the iron-deficiency-anemia hypothesis.” American Journal of Physical Anthropology 139: 109–125. DOI: 10.1002/ajpa.21031.
Walrath, D. E., P. Turner, and J. Bruzek. 2004. “Reliability test of the visual assessment of cranial traits for sex determination.” American Journal of Physical Anthropology 125: 132–137. DOI: 10.1002/ajpa.10373.
Warinner, C., et al. 2014. “Pathogens and host immunity in the ancient human oral cavity.” Nature Genetics 46 (4): 336–344. DOI: 10.1038/ng.2906.
Waters-Rist, A. et al. 2010. “Activity-induced dental modification in Holocene Siberian hunter-fisher-gatherers.” American Journal of Physical Anthropology 143: 266–278. DOI: 10.1002/ajpa.21313.
Weaver, D. S., et al. 2000. “A surgical amputation in 2nd century Rome.” Lancet 356 (9230), 686. DOI: 10.1016/S0140–6736(05)73840-X.
Weiss, E., and R. Jurmain. 2007. “Osteoarthritis revisited: a contemporary review of aetiology.” International Journal of Osteoarcheology 17: 437–450. DOI: 10.1002/oa.889.
Weiss K. M. 1972. “On the systematic bias in skeletal sexing.” American Journal of Physical Anthropology 37: 239–250. DOI: 10.1002/ajpa.1330370208.
Weyrich, L. S., K. Dobney, and A. Cooper. 2015. “Ancient DNA analysis of dental calculus.” Journal of Human Evolution 79: 119–124. DOI: 10.1016/j.jhevol.2014.06.018.
White, T. D., and P. A. Folkens. 2005. The Human Bone Manual. Boston: Elsevier Academic Press.
Wood, J. W., et al. 1992. “The Osteological Paradox: Problems of inferring prehistoric health from skeletal samples.” Current Anthropology 33: 343–358. DOI: 10.1086/204084.
Wright, L., and H. P. Schwarcz. 1998. “Stable carbon and oxygen isotopes in human tooth enamel: identifying breastfeeding and weaning in prehistory.” American Journal of Physical Anthropology 106: 1–18. DOI: 10.1002/(SICI)1096–8644(199805) 106:1<1::AID-AJPA1>3.0.CO;2-W.
Yurtsever, Y., and J. R. Gat. 1981. “Atmospheric Waters.” In Stable Isotope Hydrology: Deuterium and Oxygen-18 in the Water Cycle, eds. J. Gat and R. Gonfiantini. Vienna: International Atomic Energy Agency, 103–142.
Zink, A. R., et al. 2006. “Molecular analysis of ancient microbial infections.” FEMS Microbiology Letters 213: 141–147. DOI: 10.1111/j.1574–6968.2002.tb11298.x.
Zuckerman, M. K., K. N. Harper, and G. J. Armelagos. 2016. “Adapt or die: three case studies in which the failure to adopt advances from other fields has compromised paleopathology.” International Journal of Osteoarchaeology 26: 375–383. DOI: 10.1002/oa.2426.
Zuckerman, M. K., B. L. Turner, and G. J. Armelagos. 2012. “Evolutionary thought in paleopathology and the rise of the biocultural approach.” In A Companion to Paleopathology, ed. A. L. Grauer. Malden MA: Wiley-Blackwell, 34–57. DOI: 10.1002/9781444345940.ch3.