 CHAPTER 2
CHAPTER 2 
THE NATURE OF BONE
 CHAPTER 2
CHAPTER 2 
Bone is one of the hardest substances found in the human body, second only to the enamel of the teeth. However, bone is also a living tissue, undergoing constant change. It consists of cells embedded in an abundant, hard intercellular material. The two principal components of this material, collagen and calcium phosphate, distinguish bone from such other hard tissues as chitin, enamel, and shell. Bone tissue makes up the individual bones of the human skeletal system, as well as the skeletons of other vertebrates.
The functions of bone include (1) structural support for the mechanical action of soft tissues, such as the contraction of muscles and the expansion of lungs, (2) protection of soft organs and tissues, as by the skull, (3) provision of a protective site for specialized tissues such as the blood-forming system (bone marrow), and (4) a mineral reservoir, whereby the endocrine system (the group of ductless glands that secrete hormones) regulates the level of calcium and phosphate in the circulating body fluids.
In modern vertebrates, true bone is found only in animals capable of controlling the fluid and solute composition of their internal environment. With the emergence of terrestrial life-forms, the availability of calcium regulation became significant for proper bone formation and function. Along with the kidney and the various component glands of the endocrine system, bone has contributed to the evolutionary development of internal fluid homeostasis—the maintenance of a constant chemical composition. The structural rigidity of bone also afforded mechanical advantages, which are the most obvious features of the modern vertebrate skeleton.
The nonliving intercellular material of bone consists of an organic component called collagen (a fibrous protein arranged in long strands or bundles similar in structure and organization to the collagen of ligaments, tendons, and skin), with small amounts of protein polysaccharides, glycoaminoglycans (formerly known as mucopolysaccharides) chemically bound to protein and dispersed within and around the collagen fibre bundles, and an inorganic mineral component in the form of rod-shaped crystals. These crystals are arranged parallel with the long axes of collagen bundles and many actually lie in voids within the bundles themselves.
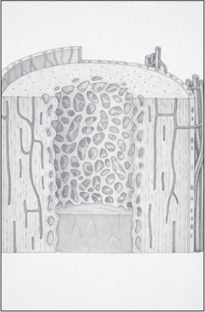
Cross-sectional view of a human long bone. Dorling Kindersley/Getty Images
Organic material comprises 50 percent of the volume and 30 percent of the dry weight of the intercellular composite, with minerals making up the remainder. The major minerals of the intercellular composite are calcium and phosphate. When first deposited, mineral is crystallographically amorphous, but with maturation it becomes typical of the apatite minerals, the major component being hydroxyapatite. Carbonate is also present and occurs in two distinct phases: calcium carbonate and a carbonate apatite. Except for that associated with its cellular elements, there is little free water in adult mammalian bone (approximately 8 percent of total volume). As a result, diffusion from surfaces into the interior of the intercellular substance occurs at the slow rates more typical of diffusion from surfaces of solids than within liquids.
The mineral crystals are responsible for hardness, rigidity, and the great compressive strength of bone, but they share with other crystalline materials a great weakness in tension, arising from the tendency for stress to concentrate about defects and for these defects to propagate. On the other hand, the collagen fibrils of bone possess high elasticity, little compressive strength, and considerable intrinsic tensile strength. The tensile strength of bone depends, however, not on collagen alone but on the intimate association of mineral with collagen, which confers on bone many of the general properties exhibited by two-phase materials such as fibre glass and bamboo. In such materials the dispersion of a rigid but brittle material in a matrix of quite different elasticity prevents the propagation of stress failure through the brittle material and therefore allows a closer approach to the theoretical limiting strength of single crystals.
The fine structure of bone has thus far frustrated attempts to determine the true strength of the mineral-matrix composite at the “unit” structural level. Compact (cortical) bone specimens have been found to have tensile strength in the range of 700–1,400 kg per square cm (10,000–20,000 lbs per square in) and compressive strengths in the range of 1,400–2,100 kg per square cm (20,000–30,000 lbs per square in). These values are of the same general order as for aluminum or mild steel, but bone has an advantage over such materials in that it is considerably lighter. The great strength of bone exists principally along its long axis and is roughly parallel both to the collagen fibre axis and to the long axis of the mineral crystals.
Although apparently stiff, bones exhibit a considerable degree of elasticity, which is important to the skeleton’s ability to withstand impact. Estimates of modulus of elasticity of bone samples are of the order of 420 to 700 kg per square cm (6,000 to 10,000 lbs per square in), a value much less than steel, for example, indicating the much greater elasticity of bone. Perfect elasticity exists with loads up to 30 to 40 percent of breaking strength; above this, “creep,” or gradual deformation, occurs, presumably along natural defects within the bony structure. The modulus of elasticity in bone is strikingly dependent upon the rate at which loads are applied, bones being stiffer during rapid deformation than during slow; this behaviour suggests an element of viscous flow during deformation.
As might be anticipated from consideration of the two-phase composition of bone, variation in the mineral-collagen ratio leads to changes in physical properties: less mineral tends ultimately to greater flexibility and more mineral to increased brittleness. Optimal ratios, as reflected in maximal tensile strength, are observed at an ash content of approximately 66 percent, a value that is characteristic of the weight-bearing bones of mammals.
Bone tissue is organized into a variety of shapes and configurations adapted to the function of each bone: broad, flat plates, such as the scapula, serve as anchors for large muscle masses, while hollow, thick-walled tubes, such as the femur, the radius, and the ulna, support weight or serve as a lever arm. These different types of bone are distinguished more by their external shape than by their basic structure.
All bones have an exterior layer called cortex that is smooth, compact, continuous, and of varying thickness. In its interior, bony tissue is arranged in a network of intersecting plates and spicules called trabeculae, which vary in amount in different bones and enclose spaces filled with blood vessels and marrow. This honeycombed bone is termed cancellous or trabecular. In mature bone, trabeculae are arranged in an orderly pattern that provides continuous units of bony tissue aligned parallel with the lines of major compressive or tensile force. Trabeculae thus provide a complex series of cross-braced interior struts arranged so as to provide maximal rigidity with minimal material.
Bones such as vertebrae, subject to primarily compressive or tensile forces, usually have thin cortices and provide necessary structural rigidity through trabeculae, whereas bones such as the femur, subject to prominent bending, shear, or torsional forces, usually have thick cortices, a tubular configuration, and a continuous cavity running through their centres (medullary cavity).
Long bones, distinctive of the body’s extremities, exhibit a number of common gross structural features. The central region of the bone (diaphysis) is the most clearly tubular. At one or commonly both ends, the diaphysis flares outward and assumes a predominantly cancellous internal structure. This region (metaphysis) functions to transfer loads from weight-bearing joint surfaces to the diaphysis. Finally, at the end of a long bone is a region known as an epiphysis.
In addition to the gross morphology of bones, there also exists numerous microscopic phenomena, including different types of cells and cellular arrangements. Investigation into the microscopic properties of bone has improved the scientific understanding of how bone forms and has revealed valuable information for medicine.
Compact bone, which is also called cortical bone, is dense and has a bony matrix that is solidly filled with organic ground substance and inorganic salts, leaving only tiny spaces (lacunae) that contain the osteocytes, or bone cells. Compact bone makes up 80 percent of the human skeleton; the remainder is cancellous bone. Both types are found in most bones. Compact bone forms a shell around cancellous bone and is the primary component of the long bones of the arm and leg and other bones, where its greater strength and rigidity are needed.
Mature compact bone is lamellar, or layered, in structure. It is permeated by an elaborate system of interconnecting vascular canals, the haversian systems, which contain the blood supply for the osteocytes; the bone is arranged in concentric layers around these canals, forming structural units called osteons. Immature compact bone does not contain osteons and has a woven structure. It forms around a framework of collagen fibres and is eventually replaced by mature bone in a remodeling process of bone resorption and new bone formation that creates the osteons.
Cancellous bone, which is also called trabecular (or spongy) bone, is light and porous and encloses numerous large spaces, which is what gives it the honeycombed appearance discussed previously. The bone matrix, or framework, is organized into a three-dimensional latticework of bony processes, the trabeculae, arranged along lines of stress. The spaces between are often filled with marrow.
Cancellous bone makes up about 20 percent of the human skeleton, providing structural support and flexibility without the weight of compact bone. It is found in most areas of bone that are not subject to great mechanical stress. It makes up much of the enlarged ends (epiphyses) of the long bones and is the major component of the ribs, the shoulder blades, the flat bones of the skull, and a variety of short, flat bones elsewhere in the skeleton. The open structure of cancellous bone enables it to dampen sudden stresses, as in load transmission through the joints. Varying proportions of space to bone are found in different bones according to the need for strength or flexibility.
Cancellous bone can develop into compact bone through the action of bone-forming cells called osteoblasts. It is in this manner that all long bones develop in the embryo. The osteoblasts deposit new bone matrix in layers around the trabeculae, which thus enlarge at the expense of the spaces between them. Eventually the spaces are eliminated, and immature compact bone is produced.
Each epiphysis at the end of a long bone ossifies separately from the bone shaft but becomes fixed to the shaft when full growth is attained. The epiphysis is made of cancellous bone covered by a thin layer of compact bone. Prior to full skeletal maturity the epiphysis is separated from the metaphysis by a cartilaginous plate called the growth plate or physis; in bones with complex articulations (such as the humerus at its lower end) or bones with multiple protuberances (such as the femur at its upper end) there may be several separate epiphyses, each with its own growth plate.
The chief structural unit of compact bone is the osteon, which consists of concentric bone layers called lamellae. These layers surround a long hollow passageway—the Haversian canal (named for Clopton Havers, a 17th-century English physician). The Haversian canal contains small blood vessels responsible for the blood supply to osteocytes (individual bone cells). Osteons are several millimetres long and about 0.2 millimetre (0.008 inch) in diameter. They tend to run parallel to the long axis of a bone.
Osteons are formations characteristic of mature bone and take shape during the process of bone remodeling, or renewal. New bone may also take this structure as it forms, in which case the structure is called a primary osteon. The process of the formation of osteons and their accompanying Haversian canals begins when immature woven bone and primary osteons are destroyed by large cells called osteoclasts, which hollow out a channel through the bone, usually following existing blood vessels. Layers of bone-forming cells, or osteoblasts, follow the osteoclasts and lay down new bone on the sides of the channel. The layers of bone built up in this way slowly narrow the channel until a tunnel not much larger than the central blood vessel remains. The blood supply for the osteocytes then passes through these channels, the Haversian canals. The spaces between adjacent osteons are filled with interstitial lamellae, layers of bone that are often remnants of previous Haversian systems. Transverse vessels, which run perpendicular to the long axis of the cortex, are called Volkmann canals. Volkmann canals connect adjacent osteons and also connect the blood vessels of the Haversian canals with the periosteum, the tissue covering the bone’s outer surface.
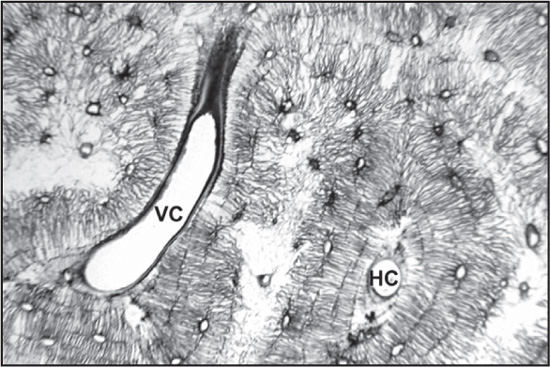
The osteon units of bone are made up of Haversian canals (HC) and Volkmann canals (VC), which run perpendicular to the long axes of osteons and connect adjacent Haversian canals. Uniformed Services University of the Health Sciences (USUHS)
The cells that lie within the substance of fully formed bone are known as osteocytes. Each cell occupies a small chamber called a lacuna, which is contained in the calcified matrix of bone. Osteocytes derive from osteoblasts, or bone-forming cells, and are essentially osteoblasts surrounded by the products they secreted. Cytoplasmic processes of the osteocyte extend away from the cell toward other osteocytes in small channels called canaliculi. By means of these canaliculi, nutrients and waste products are exchanged to maintain the viability of the osteocyte.
The osteocyte is capable of bone deposition and resorption. It also is involved in bone remodeling by transmitting signals to other osteocytes in response to even slight deformations of bone caused by muscular activity. In this way, bone becomes stronger if additional stress is placed on it (for example, by frequent exercise or physical exertion) and weaker if it is relieved of stress (for example, by inactivity). The osteocyte may aid in calcium removal from bone when the body’s calcium level drops too low.
Osteoclasts are large multinucleated cells responsible for the dissolution and absorption of bone. Bone is a dynamic tissue that is continuously being broken down and restructured in response to such influences as structural stress and the body’s requirement for calcium. The osteoclasts are the mediators of the continuous destruction of bone. Osteoclasts occupy small depressions on the bone’s surface, called Howship lacunae. The lacunae are thought to be caused by erosion of the bone by the osteoclasts’ enzymes. Osteoclasts are formed by the fusion of many cells derived from circulating monocytes in the blood. These, in turn, are derived from the bone marrow. Osteoclasts may have as many as 200 nuclei, although most have only 5 to 20. The side of the cell closest to the bone contains many small projections (microvilli) that extend into the bone’s surface, forming a ruffled, or brush, border that is the cell’s active region. Osteoclasts produce a number of enzymes, chief among them acid phosphatase, that dissolve both the organic collagen and the inorganic calcium and phosphorus of the bone. Mineralized bone is first broken into fragments; the osteoclast then engulfs the fragments and digests them within cytoplasmic vacuoles. Calcium and phosphorus liberated by the breakdown of the mineralized bone are released into the bloodstream. Unmineralized bone (osteoid) is protected against osteoclastic resorption.
The large cells responsible for the synthesis and mineralization of bone during both initial bone formation and later bone remodeling are called osteoblasts. Osteoblasts form a closely packed sheet on the surface of the bone, from which cellular processes extend through the developing bone. They arise from the differentiation of osteogenic cells in the periosteum, the tissue that covers the outer surface of the bone, and in the endosteum of the marrow cavity. This cell differentiation requires a regular supply of blood, without which cartilage-forming chondroblasts, rather than osteoblasts, are formed. The osteoblasts produce many cell products, including the enzymes alkaline phosphatase and collagenase, growth factors, hormones such as osteocalcin, and collagen, part of the organic unmineralized component of the bone called osteoid. Eventually the osteoblast is surrounded by the growing bone matrix, and, as the material calcifies, the cell is trapped in a space called a lacuna. Thus entrapped, it becomes an osteocyte, or bone cell. Osteocytes communicate with each other as well as with free bone surfaces via extensive cytoplasmic processes that occupy long, meandering channels (canaliculi) through the bone matrix.
Bone marrow, also called myeloid tissue, is a soft, gelatinous tissue that fills the cavities of the bones. Bone marrow is either red or yellow, depending upon the preponderance of hematopoietic (red) or fatty (yellow) tissue. In humans the red bone marrow forms all of the blood cells with the exception of the lymphocytes, which are produced in the marrow and reach their mature form in the lymphoid organs. Red bone marrow also contributes, along with the liver and spleen, to the destruction of old red blood cells.
Yellow bone marrow serves primarily as a storehouse for fats but may be converted to red marrow under certain conditions, such as severe blood loss or fever. At birth and until about the age of seven, all human marrow is red, as the need for new blood formation is high. Thereafter, fat tissue gradually replaces the red marrow, which in adults is found only in the vertebrae, hips, breastbone, ribs, and skull and at the ends of the long bones of the arm and leg. Other cancellous bones and the central cavities of the long bones are filled with yellow marrow.
Red marrow consists of a delicate, highly vascular fibrous tissue containing stem cells, which differentiate into various blood cells. The new blood cells are released into the sinusoids, large thin-walled vessels that drain into the veins of the bone. In mammals, blood formation in adults takes place predominantly in the marrow. In lower vertebrates a number of other tissues may also produce blood cells, including the liver and the spleen.
Because the white blood cells produced in the bone marrow are involved in the body’s immune defenses, marrow transplants have been used to treat certain types of immune deficiency and hematological disorders, especially leukemia. The sensitivity of marrow to damage by radiation therapy and some anticancer drugs accounts for the tendency of these treatments to impair immunity and blood production.
Examination of the bone marrow is helpful in diagnosing certain diseases, especially those related to blood and blood-forming organs, because it provides information on iron stores and blood production.
In a typical long bone, blood is supplied by three separate systems: a nutrient artery, periosteal vessels, and epiphyseal vessels. The diaphysis and metaphysis are nourished primarily by the nutrient artery, which passes through the cortex into the medullary cavity and then ramifies outward through Haversian and Volkmann canals to supply the cortex. Extensive vessels in the periosteum, the membrane surrounding the bone, supply the superficial layers of the cortex and connect with the nutrient-artery system. In the event of obstruction of the nutrient artery, periosteal vessels are capable of meeting the needs of both systems. The epiphyses are supplied by a separate system that consists of a ring of arteries entering the bone along a circular band between the growth plate and the joint capsule. In the adult these vessels become connected to the other two systems at the metaphyseal-epiphyseal junction, but while the growth plate is open there is no such connection, and the epiphyseal vessels are the sole source of nutrition for the growing cartilage; therefore, they are essential for skeletal growth.
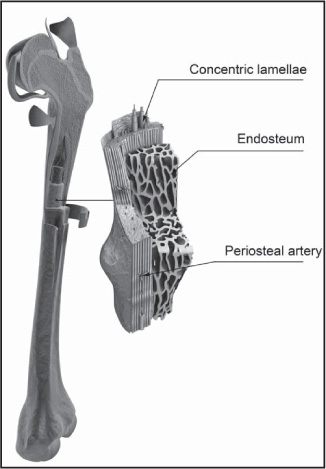
Internal view of a femur bone. 3D4Medical.com/Getty Images
Drainage of blood is by a system of veins that runs parallel with the arterial supply and by veins leaving the cortical periosteum through muscle insertions. Muscle contraction milks blood outward, giving rise to a centrifugal pattern of flow from the axial nutrient artery through the cortex and out through muscle attachments.
Whereas renewal in tissues such as muscle occurs largely at a molecular level, renewal of bone occurs at a tissue level and is similar to the remodeling of buildings in that local removal (resorption) of old bone must precede new bone deposition. Remodeling is most vigorous during the years of active growth, when deposition predominates over resorption. Thereafter remodeling gradually declines, in humans until about age 35, after which its rate remains unchanged or increases slightly. From the fourth decade on, resorption exceeds formation, resulting in an approximate 10 percent loss in bone mass per decade, equivalent to a daily loss of 15 to 30 mg of calcium.
Except for the addition of the ossification mechanisms within cartilage, growth and development involve exactly the same type of remodeling as that in the adult skeleton. Both require continuous, probably irreversible differentiation of osteoclasts and osteoblasts, the former from circulating monocytes in the blood and the latter from the undifferentiated bone mesenchyme. The life span of osteoclasts is from a few hours to at most a few days, while that of osteoblasts is a few days to at most a few weeks.
Resorption is produced by clusters of osteoclasts that either erode free bone surfaces or form “cutting cones” that tunnel through compact bone and create the cylindrical cavities that may be subsequently filled by osteons. Osteoclastic cells secrete enzymes and hydrogen ions onto the bone surface, dissolving the mineral and digesting the matrix at virtually the same moment. The process is associated with locally augmented blood flow and with a greater surface acidity than elsewhere in bone, despite the fact that the process of dissolving apatite consumes hydrogen ions. Resorption is usually a much more rapid process than formation. Osteoclastic cutting cones have been observed to advance at rates up to 500 micrometres, or microns, per day (1 micron = 1 × 10-6 metre).
Bone is formed on previously resorbed surfaces by deposition of an unmineralized protein matrix material (osteoid) and its subsequent mineralization. Osteoblasts elaborate matrix is a continuous membrane covering the surface on which they are working at a linear rate that varies with both age and species but which in large adult mammals is on the order of one micron per day. The unmineralized matrix constitutes an osteoid seam or border, averaging 6 to 10 microns in thickness during active bone formation. The biochemical and physical sequence of events that prepare matrix for mineralization includes intracellular biosynthesis of collagen by osteoblasts, extrusion of collagen extracellularly in soluble form, maturation or polymerization of collagen into an array of fibrils (in random orientation in rapidly deposited bone, in a highly ordered regular pattern in slowly formed lamellar bone), binding of calcium to collagen fibrils, and formation of protein-glycoaminoglycan complexes.
Mineralization itself depends upon the establishment of crystal nuclei within the matrix; this process requires 5 to 10 days and is under the control of the osteoblast. A suitable nucleating configuration is established, and, once nuclei reach a critical size, further mineralization proceeds spontaneously in the presence of usual body fluid calcium and phosphorus concentrations. Other collagenous tissues, such as dermis, tendon, and ligament, do not normally calcify, even though bathed by the same body fluids as bone. Although extracellular fluid is a highly supersaturated solution with respect to hydroxylapatite, calcium and phosphorus will not spontaneously precipitate in this crystalline form at normal physiological pH, so one and the same fluid is indefinitely stable in non-bone-forming regions yet richly supports mineralization in the presence of suitable crystal nuclei. Mineral movement into new bone is initially rapid and in compact bone is known to reach approximately 70 percent of full mineralization within a few hours after matrix nucleation. This mineral deposition involves replacement of the water that occupied half the original matrix volume. As water content decreases, further mineral diffusion is impeded; and the final mineralization occurs progressively more slowly over a period of many weeks. In normal adult humans, new bone formation takes up about 400 mg of calcium per day, an amount approximately equal to that in the circulating blood.
Osteocytes, once thought of as resting cells, are now recognized to be metabolically active and to possess, at least in latent form, the ability to resorb and re-form bone on their lacunar walls. Although osteocytes constitute only a small fraction of total bone volume, they are so arranged within bone, and the network of their protoplasmic extensions is so extensive, that there is essentially no volume of bony material situated more than a fraction of a micron from a cell or its processes. Of the more than 1,200 square metres (1,435 square yards) of anatomic surface within the skeleton of an adult man, about 99 percent is accounted for by the lacunar and canalicular surfaces. Resorption and deposition on this surface serve both to regulate plasma calcium concentration and to renew bony material. This renewal may be particularly important because all composite materials change in their physical properties with time. It is not known whether bone properties change sufficiently to have biological consequence, but, to the extent that such change does occur, renewal around osteocytes would provide for the physical maintenance of bone structural material.
The remodeling of bone is a continuous process of synthesis and destruction that gives bone its mature structure and maintains normal calcium levels in the body. Destruction, or resorption, of bone by osteoclasts releases calcium into the bloodstream to meet the body’s metabolic needs and simultaneously allows the bone—which is inhibited by its inorganic component from growing by cell division like other tissues—to alter in size and shape as it grows to adult proportions. Osteoclasts act on the inner surfaces of bones, in the marrow cavity and the spaces of cancellous bone, to widen these cavities. They also act on the outer surfaces to reduce bony processes, such as the epiphyseal swellings at the ends of the long bones of the arm and leg. Osteoclast activity takes place behind the epiphyseal growth zone to reduce former swellings to the width of the lengthening shaft. Within the bone, osteoclastic destruction helps to convert immature bone (called woven bone) into mature compact bone by clearing long tubular spaces that will serve as centres for the development of osteons.
While the osteoclasts resorb bone at various sites, osteoblasts make new bone to maintain the skeletal structure. During childhood, bone formation outpaces destruction as growth proceeds. After skeletal maturity is reached, the two processes maintain an approximate balance.
Ossification, the process by which new bone is produced, begins about the third month of fetal life in humans and is completed by late adolescence. The process takes two general forms, one for compact bone and the other for cancellous bone.
Bone of the first type begins in the embryonic skeleton with a cartilage model, which is gradually replaced by bone. Osteoblasts secrete a matrix material called osteoid, a gelatinous substance made up of collagen (a fibrous protein), and mucopolysaccharide, an organic glue. Soon after the osteoid is laid down, inorganic salts are deposited in it to form the hardened material recognized as mineralized bone. The cartilage cells die out and are replaced by osteoblasts clustered in ossification centres. Bone formation proceeds outward from these centres. This replacement of cartilage by bone is known as endochondral ossification. Most short bones have a single ossification centre near the middle of the bone; long bones of the arms and legs typically have three, one at the centre of the bone and one at each end. Ossification of long bones proceeds until only a thin strip of cartilage remains at either end. This cartilage, called the epiphyseal plate, persists until the bone reaches its full adult length and is then replaced with bone.
The flat bones of the skull are not preformed in cartilage like compact bone but begin as fibrous membranes consisting largely of collagen and blood vessels. Osteoblasts secrete the osteoid into this membrane to form a spongelike network of bony processes called trabeculae. The new bone formation radiates outward from ossification centres in the membrane. This process is called intermembranous ossification. There are several ossification centres in the skull. At birth, bone formation is incomplete, and soft spots can be felt between these centres. The lines where the new bone from adjacent centres meets form cranial sutures visible on the surface of the adult skull.
Both endochondral and intermembranous ossification produce immature bone, which undergoes bone remodeling to produce mature bone.
As important as the structural properties of bone is the role bone plays in the maintenance of the ionic composition of the blood and interstitial fluids of the body. All vertebrates possessing true bone exhibit body-fluid calcium ion concentrations of approximately 50 mg per litre (1.25 millimoles) and phosphorus concentrations in the range of 30–100 mg per litre (1–3 millimoles). These levels, particularly those of calcium, are extremely important for the maintenance of normal neuromuscular function, interneuronal transmission, cell membrane integrity and permeability, and blood coagulation. The rigid constancy with which calcium levels are maintained, both in the individual and throughout all higher vertebrate classes, attests to the biological importance of such regulation. Approximately 99 percent of total body calcium and 85 percent of total body phosphorus reside in the mineral deposits of bone; thus, bone is quantitatively in a position to mediate adjustments in concentration of these two ions in the circulating body fluids. Such adjustments are provided by three hormonal control loops (control systems with feedback) and by at least three locally acting mechanisms. The hormonal loops involve parathyroid hormone (PTH), calcitonin (CT), and vitamin D and are concerned exclusively with regulation of calcium ion and phosphorus ion concentrations.
Parathyroid hormone and vitamin D act to elevate ionized calcium levels in body fluids, and calcitonin (from the ultimobranchial body or C cells of the thyroid gland) acts to depress them. The secretion of each hormone is controlled by the level of calcium ion in the circulating blood. At normal calcium concentrations, there are low levels of secretion of all three hormones. When the blood levels of ionized calcium decline, there is an almost immediate increase in parathyroid hormone synthesis and secretion. Parathyroid hormone has three principal actions in maintaining blood calcium concentrations. It directly stimulates the kidneys to enhance the tubular reabsorption of calcium from the ultrafiltrate that would otherwise be excreted into the urine. It also stimulates the kidney to activate the major circulating form of vitamin D to calcitrial. Calcitrial enters the circulation and travels to the small intestine where it acts to increase the absorption efficiency of dietary calcium into the bloodstream.
Parathyroid hormone and calcitrial can also stimulate osteoblasts to produce osteoclast differentiation factor (ODF). Osteoblasts that have ODF on their surfaces can interact with the precursor cells of osteoclasts (monocytes) to induce them to become mature osteoclasts. The osteoclasts in turn release hydrochloric acid and enzymes into the mineralized bone and release calcium and phosphorus into the circulation. Thus, when there is inadequate dietary calcium to satisfy the body’s calcium needs, both parathyroid hormone and calcitrial work in concert on osteoblasts to recruit precursors of osteoclasts to become mature osteoclasts. When the body’s calcium needs are satisfied by adequate dietary intake of calcium, both parathyroid hormone and calcitrial act on osteoblasts to increase their activity, resulting in increased bone formation and mineralization. Calcitonin is the only hormone that interacts directly on osteoclasts, which have a receptor for it. It decreases mature osteoclastic activity, thereby inhibiting their function.
Parathyroid hormone and calcitrial also are important in maintaining serum phosphorus levels. Parathyroid hormone interferes with renal tubular phosphorus reabsorption, causing an enhanced renal excretion of phosphorus. This mechanism, which serves to lower levels of phosphorus in the bloodstream, is significant because high phosphate levels inhibit and low levels enhance osteoclastic reabsorption. The calcium ion itself has similar effects on the osteoclastic process: high levels inhibit and low levels enhance the effect of systemically acting agents such as parathyroid hormone. On the other hand, parathyroid hormone stimulates the production of calcitrial, which in turn stimulates the small intestine to increase its efficacy of absorption of dietary phosphorus.
A deficiency in vitamin D results in poor mineralization of the skeleton, causing rickets in children and osteomalacia in adults. Mineralization defects are due to the decrease in the efficiency of intestinal calcium absorption, which results in a decrease in ionized calcium concentrations in blood. This results in an increase in parathyroid hormone in the circulation, which increases serum calcium and decreases serum phosphorus because of the enhanced excretion of phosphorus into the urine.
The exact function of calcitonin is not fully understood. However, it can offset elevations in high calcium ion levels by decreasing osteoclast activity, resulting in inhibition of bone absorption.
In the language of control mechanics, remodeling depends upon two control loops with negative feedback. The first one is a homeostatic loop involving the effects of parathyroid hormone and calcitonin on resorption. The second one is a mechanical loop that brings about changes in skeletal mass and arrangement to meet changing structural needs. The parathyroid hormone-calcitonin loop is basically a systemic process, and the mechanical loop is local. However, the two loops interact significantly at the level of the cells that act as intermediaries in both processes. A large number of other factors, including minerals in the diet, hormonal balance, disease, and aging, have important effects on the skeleton that interact with the control system.
The controls exerted by mechanical forces, recognized for over a century, have been formulated as Wolff’s law: “Every change in the function of a bone is followed by certain definite changes in its internal architecture and its external conformation.” Of the many theories proposed to explain how mechanical forces communicate with the cells responsible for bone formation and resorption, the most appealing has been postulation of induced local electrical fields that mediate this information exchange. Many crystalline or semicrystalline materials, including both bone collagen and its associated mineral, exhibit piezoelectric properties. Deformation of macroscopic units of bone by mechanical force produces a charge in the millivolt range and current flow on the order of 10-15 ampere; both voltage and current flow are proportional to the applied force. Regions under tension act as anode and compressed regions as cathode. Currents of this magnitude are capable of aligning collagen fibrils as they aggregate from the solution phase. The negative feedback characteristic of this mechanism lies in the fact that bone accumulates about the cathodal region of this system, hence reducing the electrical effects produced by an applied force.
The mechanisms by which the bone mesenchyme responds to mechanical stimuli (whether or not mediated by electrical signals) are uncertain. In general, heavy usage leads to heavy bone, and disuse, as in immobilization associated with injury or severe disease, results in decreased bone mass and increased excretion of calcium, phosphorus, and nitrogen. The cellular response, however, is complex. In broad outline it appears that the local expression of decreased stress is an increase in bone resorption coupled variably with a smaller and secondary increase in bone formation, whereas increased stress appears to be accompanied by a decrease in bone resorption coupled also with a smaller and probably secondary increase in bone formation. The decrease in resorption represents a decreased sensitivity to systemic stimuli, such as parathyroid hormone, and reflects an interaction between hormonal and physical forces at the cellular level. Parathyroid hormone is the major determinant of all remodeling, structural as well as homeostatic; mechanical forces are the major determinant of where that remodeling occurs.
One of the most arresting features of skeletal remodeling is the tendency for rates of bone resorption and bone formation to change in the same direction. Three mechanisms for this coupling can be identified. The first is homeostatic and rises from the mineral demand created by formation of crystal nuclei in the bone matrix. Unless the calcium demands of increased bone formation can be met by some other source (such as an increase in calcium in the diet), they will inevitably lead to increased parathyroid hormone secretion and bone resorption. Since the level of parathyroid hormone is a principal determinant of bone resorption, it follows that high levels of formation tend to produce high levels of resorption (and vice versa). A second mechanism is the mechanical force–piezoelectric system discussed earlier. Local bone resorption, by reducing structural volume, concentrates applied forces in the remaining bone; this leads to increased strain and presumably increases the stimulus for local bone repair. A third mechanism is inferred from the observation in adult animals that the induction of specialized bony cells from the mesenchyme proceeds in a predetermined sequence—first osteoclasts and then osteoblasts—so that, even on free surfaces, resorption usually precedes formation.
In mammals studied prior to skeletal maturity, administration of estrogens produces an accelerated appearance of ossification centres, a slowing in growth of cartilage and bone, and fusion of the epiphyses. The result is an adult skeleton smaller than normal. In older mammals, estrogens in certain dosages and schedules of administration may inhibit trabecular bone resorption. In postmenopausal women, administration of estrogen suppresses bone resorption and produces a transient decrease in serum calcium and phosphorus and in renal reabsorption of phosphorus, as well as positive calcium balance—effects that help to stabilize the total skeletal bone mass.
In mammals, including humans, just prior to sexual maturity, the growth spurt occurring in males is attributable principally to the growth-promoting action of the male sex hormone testosterone. When administered, testosterone and related steroids stimulate linear growth for a limited period. Ultimately, however, particularly if they are given in large doses, they suppress bone growth as the result of hastened skeletal development and premature epiphyseal closure. Studies have indicated that testosterone derivatives administered to adult mammals suppress the turnover and resorption of bone and increase the retention of nitrogen, phosphorus, and calcium.
The influence of the adrenal corticosteroid hormones on bone is varied, but the principal result is slowing of growth in the young and decrease in bone mass in the adult. In Cushing syndrome, in which there is abnormally high secretion of corticosteroids, bone loss to the point of fractures often occurs. Cortisol in high concentration suppresses protein and mucopolysaccharide synthesis, with inhibition of bone matrix formation and of incorporation of nucleosides into bone cells. Cortisol also inhibits intestinal calcium absorption, which in turn causes increases in parathyroid hormone production and the rate of bone resorption.
Lack of the internal secretion of the thyroid gland results in retardation of skeletal growth and development. Action of this hormone to facilitate growth and skeletal maturation is probably indirect, through its general effects on cell metabolism. Thyroid hormone in excess leads in the young to premature appearance of ossification centres and closure of the epiphyses and in adults to increased bone-cell metabolism. Commonly, in the hyperthyroid adult, bone resorption predominates over increased bone formation with resultant loss of bone mass.
The anterior lobe of the pituitary gland secretes a hormone essential for growth and development of the skeleton. This effect of the hormone is indirect and is mediated by insulin-like growth factor 1 (IGF-1), a substance produced in the liver in response to stimulation by the growth hormone. The extent to which growth hormone is involved in skeletal remodeling in the adult is not known, but excessive elaboration of the hormone after maturity leads to distorted enlargement of all bones in the condition known as acromegaly. Excessive elaboration of growth hormone prior to epiphyseal closure leads to gigantism. Studies of the administration of growth hormone to humans have indicated marked species specificity; growth in hypopituitary dwarfs is stimulated only by human or primate growth hormone. The principal metabolic effects of the hormone in humans are retention of nitrogen and increased turnover of calcium, resulting in increases both in intestinal calcium absorption and in urinary calcium excretion.
Insulin participates in the regulation of bone growth. It may enhance or even be necessary for the effect of growth hormone on bone. Insulin has been found to stimulate growth and epiphyseal widening in rats whose pituitaries have been removed and to promote chondroitin sulfate synthesis in cartilage and bone and the transport of amino acids and nucleosides into bone.
The most significant nutritional influence on bone is the availability of calcium. The close relationship between bone and calcium is indicated by the principal processes of calcium metabolism. Bone contains 99 percent of the calcium in the body and can behave as an adequate buffer for maintenance of a constant level of freely moving calcium in soft tissues, extracellular fluid, and blood. The free-calcium concentration in this pool must be kept within fairly narrow limits (50–65 mg per litre of extracellular fluid) to maintain the constant internal environment necessary for neuromuscular irritability, blood clotting, muscle contractility, and cardiac function. Calcium leaves the pool by way of bone formation, by such routes as the urine, feces, and sweat, and periodically by way of lactation and transplacental movement. Calcium enters the pool by the mechanism of bone resorption and by absorption from dietary calcium in the upper intestinal tract.
The significance with respect to bone of adequate availability of calcium to animals or humans is that the mechanical strength of bone is proportional to its mineral content. All of the other components of bone, organic and inorganic, are of course also essential for bone integrity, but the importance of availability of structural materials is most easily illustrated by consideration of calcium balance (dietary intake versus excretory output). If intake of calcium is limited, maintenance of normal levels of extracellular and soft tissue calcium in the face of mandatory daily losses from this pool by various excretory routes requires that calcium be mined from its storage depot, bone. Abundant mineral intake then tends to preserve bone mass, and an increase of positivity of calcium balance has been shown to suppress resorption of bone.
The Food and Nutrition Board of the U.S. Institute of Medicine of the National Academies has recommended 1,000 mg of calcium daily for adults age 19 and older and 800 to 1,300 mg for children age 4 to 18. The usual daily intake of calcium in the diet, however, is between 400 and 600 mg, about 150 to 250 mg from green vegetables and the remainder usually from milk and milk products. Daily urinary excretion of calcium is normally from 50 to 150 mg in females and 50 to 300 mg in males. Fecal excretion of calcium is much larger than urinary excretion; most of the calcium in the feces is unabsorbed dietary calcium. Heavy sweating can result in a loss of more than 200 mg per day. Calcium absorption varies depending on previous and current levels of calcium intake and type of diet. Approximately 30 percent of dietary calcium is absorbed when there is adequate vitamin D intake.
The other principal mineral constituent of bone is phosphorus, which is abundantly available in milk, meat, and other protein-rich foods. The recommended daily intake of phosphorus is 700 mg daily for adults, 1,250 mg daily for adolescents, and 500 mg daily for children up to age eight. A prolonged dietary deficiency in phosphorus or marked loss of phosphorus in the urine can result in rickets in children and osteomalacia in adults. The skeleton also serves as a storage reservoir for magnesium. Magnesium deficiency can result in neuromuscular dysfunction similar to a calcium deficiency. Magnesium is critically important for the regulation of parathyroid hormone.
Fluoride, an element of proven value and safety in prevention of dental cavities when provided in drinking water at concentrations of one part per million, is absorbed into the bone lattice structure as well as into enamel and produces a larger crystal more resistant to resorption. Amounts 10 or more times that normally taken in fluoridated drinking water have been noted to cause abnormalities of bone collagen synthesis. Extremely large dosages in humans produce the denser but irregularly structured and brittle bone of fluorosis.
The function of vitamin A remains to be clarified, but it is apparently necessary for proliferation of cartilage and bone growth. Without vitamin A, bone remodeling is also impaired and bones develop in abnormal shapes. Excessive amounts of the vitamin result in thinning of cortical bone and fracture. Ascorbic acid, or vitamin C, is essential for intracellular formation of collagen and for hydroxylation of proline. In scurvy, a disease caused by vitamin C deficiency, the collagen matrix of bone is either partially or completely unable to calcify.
Vitamin D has several complex physiologic actions that affect calcium, phosphorus, and bone metabolism. A form of vitamin D called calcitrial increases the efficiency of intestinal calcium absorption and also interacts directly with osteoblasts to increase osteoblast function. At times when dietary calcium is inadequate, calcitrial will stimulate osteoblasts to increase osteoclast differentiation factor (ODF) on their surface, which in turn mobilizes osteoclast mesenchymal cells to become mature osteoclasts. Thus, the major function of vitamin D is to maintain serum levels of calcium by increasing absorption of dietary calcium in the intestine. At times of increased need, such as during pregnancy, lactation, and adolescent growth, circulating levels of calcitrial are increased, resulting in an increase of up to 80 percent in the efficiency of intestinal calcium absorption. In vitamin D deficiency, parathyroid hormone levels are elevated, causing an increased loss of phosphorus into the urine.
Other nutritional factors include protein, which, as an essential component of the matrix of bone, must be provided by a combination of dietary intake and conversion from other tissues. Changes in acid-base balance also have an influence on the skeleton—acidosis in various clinical disorders and ingestion of acid salts being accompanied by mineral loss.